Introduction
Mantle cell lymphoma (MCL) is an aggressive B-cell non-Hodgkin lymphoma considered incurable using conventional chemotherapeutic approaches. Emerging clinical data suggest considerable clinical heterogeneity, with some patients showing chronic/indolent course, while others may have a more fulminant course and very short survival akin to the acute leukemias. In the following review, we will attempt to highlight the epidemiology, prognosis, and management of this protean and challenging condition.
Diagnosis
MCL cells are typically described as being slightly increased in size with an indented nucleus lacking nucleoli. The classic immunophenotype includes positivity for CD5, CD19, CD20, and sIgM [Fig 1a]. Differentiating from marginal zone lymphoma and chronic lymphocytic leukemia may be challenging, though MCL is more likely to have higher expression of CD20, is typically negative for CD11c. Although CD23 is also typically negative, positive cases may be seen in up to 25% of cases1,2. MCL is more specifically identified by the presence of the translocation t(11;14)(q13;q32), which juxtaposes the cyclin D1 gene to the immunoglobulin locus. Rare cyclin D1 negative variants do exist, which may be characterized by increased expression of cyclin D2. However, additional confirmatory testing is needed in such situations, as the specificity for cyclin D2 immunostaining in MCL is low. Although methodology continues to be refined, dual color FISH remains the most sensitive method for detection at this time [Fig 1b].1,3
Fig 1.
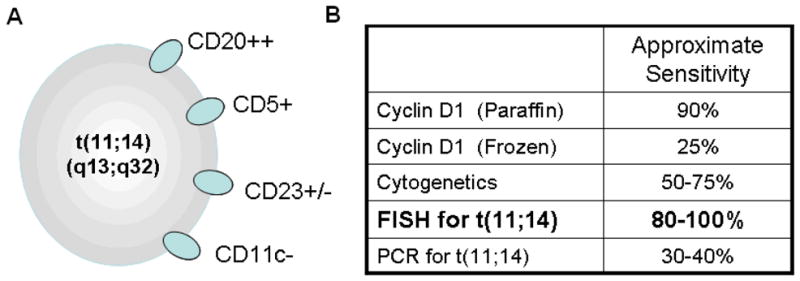
(a): Classical immunophenotype for MCL shows high CD20 expression in conjunction with CD5. CD23 is typically negative, though can be dimly positive in up to 25% of cases. CD11c is very seldom positive, and may provide better diagnostic utility than CD23.1,2 (b) The translocation between 11q13 and 14q32 is defines the vast majority of cases, and is best identified by dual color FISH techniques.3,98,99,100
Epidemiology and Etiology
MCL accounts for 2–10% of the non-Hodgkin lymphomas, with a recently reported incidence rate of 0.51–0.55 per 100,000 persons. In general, patients are typically Caucasian (~2:1), male (~2.5:1), elderly (median age of onset: 68 years), and usually present with extensive disease, including widespread lymphadenopathy, bone marrow involvement, splenomegaly, circulating tumor cells, and bowel infiltration.4 US cancer registry data suggest an increase in the incidence of MCL between 1992 and 20075,6. Interestingly, this is coupled with a decrease in the number of cases of CLL, suggesting that improvements in diagnosis have facilitated better differentiation between these two entities7. This may similarly help to explain improvements in survival if patients are being diagnosed in earlier more indolent phases.
While some non-Hodgkin lymphomas have been found to be related to specific inherited, environmental, or infectious exposures, no strong and consistent relationship has been made for MCL. Weaker associations have been noted with exposure to European strains of Borellia burgdoferi (OR 3.5, 95% CI 1.8–7.4)8, family history of hematologic malignancy (OR 2.0, 95%CI 1.2–3.0)9, and genetic polymorphisms in the pro-inflammatory cytokine IL-10 (OR 1.3 per allele, p for trend=.04)10. Never-the-less, observed restrictions in immunoglobulin diversity suggest there may be as yet unidentified exposures that play a critical role in the development, and potentially maintenance, of this malignancy11,12.
Indolent MCL
Retrospective data demonstrate that up to 30% of patients with MCL may have an indolent presentation, with no acute indication for therapy13,14. These patients were identified by studying time to treatment, with indolent patients showing a delay of approximately one year before initiation of therapy. Notably, once patients require therapy, their outcome does not appear to be appreciably different from those with more classic presentations of disease13,14. This may suggest a progression from indolent to aggressive disease akin to what is seen in multiple myeloma, where patients may progress from an indolent asymptomatic stage (smoldering myeloma) to more fulminant disease.
It is important to distinguish indolent MCL from ‘in situ’ MCL. The natural history of in situ MCL is not clearly delineated. Most series, including our own [Kubal T, unpublished observations], have only identified cases of in situ MCL retrospectively after patients’ present with more aggressive disease15,16,17. This may suggest that a cohort of patients with in situ disease may exist that do not ultimately develop disease. Among those who do progress to aggressive disease, latency periods of up to 12 years have been noted, suggesting that these patients may be closely monitored without introduction of therapy18.
Attempts to prospectively identify patients with indolent MCL have proven difficult. Clinically, such patients often present with mild lymphocytosis and splenomegaly, with bone marrow and/or gastrointestinal involvement. However, some patients will also present with slowly progressive lymphadenopathy14. The use of PET-CT has helped to identify highly proliferative, so called blastoid variants, of MCL, which will typically show maximum standard uptake values in excess of 14. However, PET-CT has not been shown to reliably facilitate identification of indolent variants19. Ki-67, an obligate marker of proliferation, has been shown to correlate with prognosis, but is limited by significant inter-observer variability in measurement20,21. Current research using quantitative image analysis to account for this is ongoing, but is not yet routinely available.
Molecular exploration has identified two potential candidates for discrimination of indolent cases: SOX11 and HDAC11. SOX11, a DNA binding protein important in cell ontogeny, is commonly and aberrantly expressed in MCL. Emerging data suggest that indolent cases may lack expression of this protein22. Mechanistically, however, enforced suppression of SOX11 in cell culture increased proliferative rates, suggesting that further confirmation by other groups will be needed before any definitive conclusions can be reached in this regards23. Finally, work by our group suggests that expression of the histone deaceytlase, HDAC11, may correlate with prognosis. Specifically, blastoid MCL demonstrated very high expression of this enzyme (~15–20 fold), while classic MCL had an intermediate level of expression (~10 fold), and indolent MCL had only a minimal elevation (~2–3 fold)24,25. Further work to better clarify the role and mechanism of HDAC11’s influence on proliferation is ongoing.
Prognosis
The prognosis in MCL appears to be improving. This is likely a reflection of both earlier identification of more indolent cases, as well as the application of modern therapeutic modalities, including rituximab and autologous transplantation26,27. Characterization of prognosis using algorithms tailored to either indolent lymphomas (the Follicular Lymphoma International Prognostic Index) or aggressive lymphomas (the International Prognostic Index) fail to effectively stratify patients into distinct subgroups. This has culminated in the development of a MCL specific algorithm – the Mantle Cell Lymphoma International Prognostic Index, or MIPI. This algorithm relies both on measures of chemotherapy tolerance (age, performance status) and indirect measures of disease activity (white blood cell count, LDH) to stratify patients into low, intermediate, and high risk groups. With a median followup of 32 months, those in the low risk group an estimated 5 year survival of 60%, while those in the intermediate and high risk groups had a median survival of 51 and 29 months, respectively28,29,30.
Importantly, the MIPI is prognostic for overall survival, not chemotherapy response or progression free survival. Further, the MIPI has only been validated when calculated prior to first therapy. Ki-67, when ≥30% may be associated with inferior progression free survival, though, as mentioned above is limited by poor inter-observer agreement, particularly around this threshold21. A study by the GOELAMS found that PET-CT might predict for worse event free survival when using a cut-off of SUV values of 6, but sample size was small, and these data await confirmation31. Further, as with Ki-67, PET-CT is also limited by inter-observer variability32.
First Line Treatment
Therapeutic strategies in MCL have evolved over the past decade in an effort to improve the depth and duration of remission. These approaches can be thought of in two broad contexts: treatment of the young/fit individual and treatment of the old and/or infirm individual. Among young patients with limited comorbidity, the emphasis has been on increased treatment intensity, coupling targeted agents to aggressive chemotherapeutic regimens to improve the depth of response in anticipation of autologous stem cell transplantation. Alternatively, in older and infirm patients, an emphasis has been placed on de-intensification of therapy, using targeted agents to either replace high dose therapy, and/or as a form of maintenance to prolong remission duration. These approaches are described further below.
Treatment of Indolent Disease
MCL in its indolent phases can be observed without therapy without any apparent detriment to survival. One clinical presentation includes asymptomatic lymphocytosis, splenomegaly without lymphadenopathy. Alternatively, some patients might present with asymptomatic, slowly progressive, non-bulky adenopathy33,34. Either group of patients can be followed clinically without introduction of therapy until development of symptoms, rapidly growing lymphadenopathy, cytopenias due to splenenomegaly or progression in bone marrow. However, some patients may be uncomfortable with watchful waiting and/or present with fatigue related to disease. In such cases rituximab can be administered as a single agent, given once weekly for four weeks, and followed with maintenance dosing once every two months for two years, akin to follicular lymphoma35,36,37,38,39. While rare infections, as well as infusion reactions, have been reported in conjunction with rituximab, overall there would appear to be a favorable benefit to toxicity ratio with this approach39.
Some patients with similar presentation may progress to a leukemic form of disease, with marked elevation in the white blood cell count (>100 k/μl), high LDH, progressive splenomegaly, and decline in the hemoglobin and/or platelet count. Leukemic MCL is frequently associated with p53 mutation and/or MDM2 overexpression (which binds active p53), and accordingly shows low response to standard chemotherapies. Such patients may benefit from palliative splenectomy, which has been shown to forestall the introduction of chemotherapy for as long as four years40,41,42.
Treatment of Limited Stage Disease
Up to 6–8% of patients with MCL may present with stage I or II disease, with limited data on treatment outcome6. The largest retrospective analysis in this regards included 26 patients with nonbulky stage I and IIA disease treated with chemotherapy with or without involved field radiotherapy. Receipt of radiation was associated with a significant improvement in progression free survival (5 year PFS 68% vs 11%), with no patients demonstrating progression beyond six years43. For stage I MCL, our practice has been to recommend involved field radiation in accordance to NCCN guidelines recommendation.44
Evolution of Treatment for Advanced Disease
Formal randomized comparisons of chemotherapeutic approaches are uncommon given relative infrequency of MCL relative to other non-Hodgkin lymphomas. One of the earliest trials conducted by the EORTC retrospectively compared two highly aggressive regimens, CHVmP-VB (cyclophosphamide, doxorubicin, teniposide, prednisone, vincristine, and bleomycin) with ProMACE-MOPP (doxorubicin, cyclophosphamide, etoposide, mechlorethamine, vincristine, procarbazine, and prednisone) in intermediate and high grade MCL. While CHMmp-VB demonstrated a slight improvement in CR rate (57%) and PFS (21 months) compared to ProMACE-MOPP, other trials using CHOP alone demonstrated lower toxicity and similar time to treatment failure (~2 years) despite lower CR rates (15%)45,46,47.
The introduction of rituximab to the therapeutic armamentarium was explored in several phase 2 studies, and ultimately in a pivotal randomized comparison of R-CHOP with CHOP36,37,38,48,49. This trial demonstrated a significant improvement in response rates (94% vs 75%, p=.0054) and CR rate (34% v 7%, P=.00024) in the R-CHOP arm. Interestingly, median time to treatment failure was also improved in the R-CHOP arm (21 months v 14 months, p=.0131), while progression free survival and overall survival were not improved. In this respect, it is important to mention that time to treatment failure was measured from the time of treatment initiation, while progression free survival was measured from the time of treatment completion among responders only (those with CR or PR). This may suggest that the primary benefit for rituximab was in improving chemotherapy response in otherwise refractory patients.
Evolution of therapy for the Young
Cytarabine was the next drug to be incorporated into the therapeutic paradigm for MCL. Initial data came from a small French trial in which patients without CR after four cycles of CHOP were subsequently treated with DHAP (dexamethasone, high-dose cytarabine, and cisplatin). Among 25 patients who failed to obtain CR, DHAP was able to produce CR in 84% and median progression free survival of 51 months50. This culminated in the development of regimens alternating cycles of CHOP-like chemotherapy with high-dose cytarabine given together with rituximab. Indeed, a recent comparison of R-CHOP (six cycles) versus R-CHOP/R-DHAP (given sequentially for 3 cycles each), demonstrated that the cytarabine containing arm was able to produce a higher rate of complete molecular response. Interestingly, while molecular response did not correlate with radiologic response, those with molecular response demonstrated an improved progression free survival51. One regimen in particular, R-HyperCVAD/MA has shown significant promise in MCL52,53. Although this regimen was complicated by significant toxicity, particularly for those over age 65, the CR rate was 87% with and overall response rate 97%. Unlike early applications of high dose multi-agent chemotherapy, this approach was associated with a prolonged time to treatment failure (median 4.6 years) and overall survival when compared to historical controls (64% at 10 years with median followup duration of 8.3 years). Notably, time to treatment failure was far worse among those over 65y, with only 16% still in remission, and 33% alive at 8 years (versus 46% and 68%, respectively, among those under 65y, p<.05). This may be attributed to decreased compliance with prescribed therapy related to increased toxicity in this subgroup.
Early data with R-CHOP based inductions suggested that remission data could be prolonged using consolidation with high dose therapy and autologous stem cell transplantation49,54. Further, outcomes appeared to be best among those transplanted in first complete remission55,56. These data prompted further study of high dose cytarabine containing regimens administered in conjunction with autologous stem cell transplant57. Pivotal phase II data were reported by the Nordic group in 2008, in which patients were treated with alternating cycles of Maxi-CHOP (similar to HyperCVAD) and high dose cytarabine for a total of six cycles, followed by high dose conditioning using either BEAM or BEAC and autologous transplant. Rituximab was also administered during cycles 4–6, and in the event of molecular relapse post-transplantation. This approach yielded a CR rate of 54% with an overall response rate of 96%. With median followup duration of 3.8 years, the 4 year event free survival rate was 63%58. A similar approach was explored by the CALGB, utilizing an induction of R-CHOP and methotrexate for 2–3 cycles, followed by one cycle of rituximab, high dose cytarabine, and etoposide, BEC conditioning and autologous transplant. All patients went on to receive an additional two doses of rituximab at weeks 6 and 7 after transplantation. With a median followup of 4.7 years, this study demonstrated a 5 year PFS of 56% (95% CI, 43% to 68%), and 5 year OS of 64% (95% CI, 50% to 75%). Unlike the Nordic study, which was limited to those age 65 and under, this study allowed patients up to age 69 to be enrolled (median age 57 years). Although responses were not stratified by age, those with high risk MIPI had a significantly lower survival than those with low or intermediate risk disease (33% vs 75%, p<.03)59. Indeed, significant grade 3–4 toxicity was observed in both trials, largely related to neutropenic fever and infection58,59.
This has prompted further study of alternative approaches to induction, as well as additional retrospective evaluation of high-dose cytarabine containing regimens. One alternative approach utilized a sequential regimen of R-CHOP for 4 cycles, followed by R-ICE (rituximab, ifosfamide, carboplatin, and etoposide) for 2–3 cycles, and autologous transplant with BEAM conditioning. With 4.8 years of median followup, the authors observed a median PFS of 5 years and 5 year OS of 76%. Toxicity data were not presented in detail, however, despite inclusion of patients over the age of 60y (29% of cohort), this variable did not correlate with survival. Rather, proliferative index, as measured by Ki-67, was the only variable to be associated with this outcome.21 A more recent retrospective analysis from National Cancer Center Network’s (NCCN) Non-Hodgkin Lymphoma Database reviewed outcomes among patients receiving R-CHOP or R-HyperCVAD/MA with or without autologous stem cell transplant. With a median followup of 33 months, this study demonstrated similar 3 year PFS for the R-HyperCVAD/MA (58%, 95% CI: 44–69%), R-HyperCVAD/MA+transplant (55%, 95% CI: 22–79%), and R-CHOP+transplant (56%, 95% CI: 33–74%) arms, while the R-CHOP arm was the only to demonstrate inferior 3 year PFS (18%, 95% CI: 6–36%). Three year OS data were also compared, and showed no significant differences between R-CHOP (69%, 95% CI: 46–83%), R-CHOP+transplant (87%, 95% CI: 64–95%), and R-HyperCVAD/MA (85%, 95% CI: 74–92%). Data were not mature enough to allow comparison with R-HyperCVAD/MA+transplant. Also confirmed was a higher rate of complications and an decreased ability to tolerate all six prescribed cycles among those receiving R-HyperCVAD/MA +/− transplant.60 Together these data suggest that it may be possible to achieve a prolonged remission with less intensive therapy when coupled to autologous transplant.51 This may, in fact, be related to increasing depth of response, as measured by molecular response, when transplant is coupled to a less intensive induction. Alternatively, it may be premature to withhold autologous transplant even after more intensive therapy, as further differences may emerge with time.61
Evolution of therapy for the Elderly
While intensification of therapy to improve remission depth/duration has been the paradigm for young and fit patients, the approach in elderly patients has instead emphasized the addition, and increasingly the substitution, of novel agents with prolonged maintenance approaches to improve outcomes.
Important in this regards are seminal data presented at 2009 American Society of Hematology annual meeting comparing R-CHOP with R-bendamustine. Among 93 patients with MCL (with a median age of 70), R-bendamustine was shown to improve CR rates (39.6% v 30%, p<.05) as well as progression free survival (33 v 23 months, p<.05). Perhaps more relevant to this population, R-bendamustine was also associated with significantly less toxicity, including hematologic/infectious toxicity, alopecia, stomatitis, and parethesias62. Although final published data are still pending, the significance of these findings has propelled R-bendmustine to the front-line in many patients (including younger patients). Ongoing cooperative group trials in older and younger patients are further evaluating the role of R-bendamustine.
Another promising approach includes the combination of cladribine and rituximab. This was initially explored by the North Central Cancer Treatment Group in an elderly cohort of 29 patients with untreated MCL (median age 70). This regimen was associated with an overall response rate of 66% (52% CR). The median PFS for all patients was ~1 year. However, among those who obtained CR the duration of response was prolonged, with only 20% relapsing after a median followup of 21.5 months. Further, this regimen was associated with a 2 year survival of 78%, despite a lack of autologous transplant consolidation63. Given the strength of these data, a followup trial was performed with cladribine and rituximab. Importantly, this trial administered rituximab once per week for the first cycle, after which rituximab was given once with each additional cycle. Additionally, rituximab was continued in the majority of responders as a maintenance therapy. In this study of 31 untreated patients, an improved overall response rate (87%) and complete response rate (61%) were observed. After a median followup of 32.5 months, the median PFS and OS were 37.5 and 85 months, respectively. Among those who obtained a complete response, only 1 has relapsed after median followup of 23 months64.
Given the importance of rituximab in the induction setting, current studies are attempting to address the importance of this agent when administered as maintenance following chemotherapy, in lieu of autologous stem cell transplantation. Two trials recently examined this approach. The first trial explored the role of rituximab maintenance administered for two years to those with a CR or PR following modified R-HyperCVAD (in which high-dose cytarabine and methotrexate were omitted). Among 22 patients with newly diagnosed MCL, the median age was 63, and ranged from 40–81 years. Despite omission of high dose cytarabine, the overall response rate after induction was 77% (64% CR). With a median followup of 62 months, the median progression free survival in this cohort was 38 months, and the median overall survival 70 months65. This is surprisingly similar to the aforementioned data using rituximab and cladribine with rituximab maintenance64. A subsequent trial was recently performed by the European MCL Network and presented at the 2011 annual meeting of the American Society of Hematology. This trial recruited only patients over the age of 60 considered ineligible for more intensive approaches. In this trial, rituximab or interferon alpha maintenance was administered until progression in those with either a CR or PR following standard R-CHOP or R-FC induction. The overall response rate following R-CHOP was 87% (CR 50%). With a median followup of 38 months, the median remission duration in those randomized to rituximab maintenance is 56 months, compared to 26 months with interferon (p=.01). In addition rituximab maintenance resulted in an overall survival benefit in those patients that were initially treated with R-CHOP (4 year OS 87% vs 57%, p<.01)66. These outcome data are surprisingly similar to the more intensive approaches described for younger patients.
Relapsed Disease
Unfortunately, despite intensive and/or prolonged chemotherapeutic approaches, MCL remains incurable in the absence of allogeneic transplantation. This approach, however, is encumbered by significant toxicity, and is likely best tailored to young, fit patients with chemo-sensitive disease.67 Although a complete review of treatment approaches in this setting is beyond the scope of this review, a few are worthy of mention.
Lenalidomide, a second generation immunomodulatory agent derived from thalidomide is showing increasing promise in this malignancy. Lenalidomide as a single agent appears to show response rates as high as 53% with a median progression free survival of nearly six months68. Perhaps reflective of its immune-potentiating effects, when coupled to rituximab, the median progression free survival increases to approximately 14 months69,70. This is quite a dramatic response in the relapsed and refractory setting, and has prompted the development of phase II studies to study the role of this combination as a front-line regimen for patients considered ineligible for more intensive approaches (clinicaltrials.gov identifier NCT01472562).
Bortezomib has similarly shown promise in the relapsed and refractory patients, and was FDA approved for use in this setting. Response rates and median progression free survival mirror that seen with single agent lenalidomide, hovering around 50% and 6 months, respectively71,72,73,74. Interestingly, however, among responding patients, the median progression free survival is quite prolonged (10–24 months). These data have fueled numerous phase 1 and 2 studies in which bortezomib has been coupled to cytotoxic backbones – including bendamustine75, gemcitabine76, CHOP77,78, and HyperCVAD79, among others. Perhaps the most compelling data in this regards comes from a small phase II trial investigating the combination of bortezomib with rituximab and dexamethasone in 16 heavily pretreated patients. This combination produced an overall response of 81.3% with a CR rate of 43.8% with median progression free and overall survival of 12.1 and 38.6 months, respectively. Further, of the seven patients who obtained a CR, five sustained this response beyond 48 months in absence of further therapy80.
Several preclinical studies have suggested aberrant signaling via the AKT-MTOR pathway might play a dominant role in driving proliferation in MCL81. Attempts to inhibit this pathway with Temsirolimus, an inhibitor of the MTORC1 complex, however, have proven only moderately successful with response rates of approximately 20%, and a corresponding progression free survival of approximately 5 months82.
Several malignancies have shown significant disruption of gene expression as a consequence of aberrant epigenetic modification. Histone deacetylases, in particular, appear to play an important role in immunological escape, proliferation, and autocrine cytokine signaling which may be important in maintaining the microenvironmental niche and facilitating drug resistance83,84,85,86,87. As a single agent, the nonspecific histone deacetylase inhibitor vorinostat failed to show significant response in relapsed/refractory MCL, however, one of the nine treated patients maintained stable disease for over 2 years88. Building on this approach is an ongoing study evaluating the efficacy of vorinostat given in conjunction with velcade in relapsed/refractory MCL, with early data showing a 47% overall response rate89. Of particular interest are isotype specific HDAC inhibitors, including those targeting HDAC6, which may contribute significantly the malignant phenotype of MCL86.
Emerging Agents
Several drugs currently being explored in phase I and II studies are showing exquisite promise in MCL. One particularly promising approach has focused on disruption of B-cell receptor downstream signaling. Two agents are notable in this regards: PCI37625 and CAL-101. Administration of PCI37625 leads to targeted inhibition of Bruton’s Tyrosine Kinase, and so far is showing an overall response rate of 67%90. CAL-101 is targeted to the specific isoform of Phosphatidylinositol 3-kinase delta, and has shown an overall response rate of 62%91. Interestingly, both agents are associated with lymphocytosis, suggesting that inhibition of this pathway may be associated with disruption of microenvironment, perhaps via secondary influences on CXCR4 signaling92,93.
The failure of temsirolimus to produce dramatic responses has fostered further preclinical study showing that MCL may escape MTORC1 inhibition via increasing signaling through MTORC2 and phopshorylation of AKT, a survival pathway94. This has led to the development of several dual MTORC inhibitors, including OSI-027 and PP242, both of which are currently in phase I clinical testing.
Given that over-expression cyclinD1 is central to pathogenesis of mantle cell lymphoma, current studies are also underway to inhibit this molecule. Interestingly targeted disruption of cyclinD1 was associated with increased signaling via cyclinD2 and cyclinD3 in preclinical models95. Therefore research has been focused on inhibiting downstream targets of cyclinD1. One such novel agent is PD0332991, which targets cyclin dependent kinases 4 and 6 to effectively inhibit proliferation in MCL. Clinical studies using this as a single agent have shown an overall response rate of 18% in relapsed cases. Although this response rate was lower than expected, subsequent pharmacodynamic studies demonstrated significant inhibition of cell cycling96. This has fueled an alternative approach, currently under study, in which PD0332991 is administered to coordinate cell cycling to facilitate enhanced sensitivity to the agent bortezomib97.
Conclusions
MCL is a protean disease with both indolent and highly aggressive presentations, which must be reconciled with host factors, including patient age and comorbid conditions, to appropriately tailor therapy. Although increases in treatment intensity and duration have yielded significant benefit for many with MCL, the disease continues to be characterized by a pattern of continuous relapse. Novel approaches are beginning to show promise in these settings, with many exciting agents currently poised to make significant additional impact.
Fig 2.
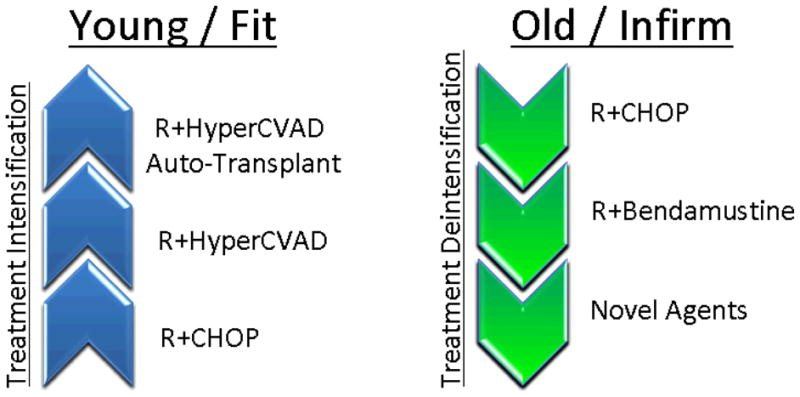
Treatment approaches in MCL. Treatment in the young has steadily progressed with regards to intensity with the goal of molecular eradication of disease. Treatment in the elderly, alternatively has stressed treatment tolerance with the application of novel agents for maintenance and in relapsed settings.
References
- 1.Klapper W. Histopathology of mantle cell lymphoma. Semin Hematol. 2011;48(3):148–154. doi: 10.1053/j.seminhematol.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 2.Kraus TS, Sillings CN, Saxe DF, Li S, Jaye DL. The role of CD11c expression in the diagnosis of mantle cell lymphoma. Am J Clin Pathol. 2010;134(2):271–277. doi: 10.1309/AJCPOGCI3DAXVUMI. [DOI] [PubMed] [Google Scholar]
- 3.Pott C. Minimal residual disease detection in mantle cell lymphoma: technical aspects and clinical relevance. Semin Hematol. 2011;48(3):172–184. doi: 10.1053/j.seminhematol.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 4.Dreyling M, Hiddemann W. Current treatment standards and emerging strategies in mantle cell lymphoma. Hematology Am Soc Hematol Educ Program. 2009:542–551. doi: 10.1182/asheducation-2009.1.542. [DOI] [PubMed] [Google Scholar]
- 5.Zhou Y, Wang H, Fang W, et al. Incidence trends of mantle cell lymphoma in the United States between 1992 and 2004. Cancer. 2008;113(4):791–798. doi: 10.1002/cncr.23608. [DOI] [PubMed] [Google Scholar]
- 6.Chandran R, Gardiner SK, Simon M, Spurgeon SE. Survival trends in Mantle cell lymphoma in the United States over sixteen years 1992–2007. Leukemia & Lymphoma. 2012:1–18. doi: 10.3109/10428194.2012.656628. [DOI] [PubMed] [Google Scholar]
- 7.Morton LM, Wang SS, Devesa SS, et al. Lymphoma incidence patterns by WHO subtype in the United States, 1992–2001. Blood. 2006;107(1):265–276. doi: 10.1182/blood-2005-06-2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schöllkopf C, Melbye M, Munksgaard L, et al. Borrelia infection and risk of non-Hodgkin lymphoma. Blood. 2008;111(12):5524–5529. doi: 10.1182/blood-2007-08-109611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang S, Slager S, Brennan P, et al. Family history of hematopoietic malignancies and risk of non-Hodgkin lymphoma (NHL): a pooled analysis of 10 211 cases and 11 905 controls from the International Lymphoma Epidemiology Consortium (InterLymph) Blood. 2007;109(8):3479–88. doi: 10.1182/blood-2006-06-031948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Skibola CF, Bracci PM, Nieters A, et al. Tumor Necrosis Factor (TNF) and Lymphotoxin-alpha (LTA) Polymorphisms and Risk of Non-Hodgkin Lymphoma in the InterLymph Consortium. American journal of epidemiology. 2010;171(3):267. doi: 10.1093/aje/kwp383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hadzidimitriou A, Agathangelidis A, Darzentas N, et al. Is there a role for antigen selection in mantle cell lymphoma? Immunogenetic support from a series of 807 cases. Blood. 2011;118(11):3088–3095. doi: 10.1182/blood-2011-03-343434. [DOI] [PubMed] [Google Scholar]
- 12.Thelander EF, Rosenquist R. Molecular genetic characterization reveals new subsets of mantle cell lymphoma. Leukemia & Lymphoma. 2008;49(6):1042–1049. doi: 10.1080/10428190801947559. [DOI] [PubMed] [Google Scholar]
- 13.Martin P, Chadburn A, Christos P, et al. Outcome of Deferred Initial Therapy in Mantle-Cell Lymphoma. Journal of Clinical Oncology. 2009;27:1209–1213. doi: 10.1200/JCO.2008.19.6121. [DOI] [PubMed] [Google Scholar]
- 14.Martin P, Leonard J. Is there a role for “watch and wait” in patients with mantle cell lymphoma? Semin Hematol. 2011;48(3):189–93. doi: 10.1053/j.seminhematol.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 15.Racke F, Simpson S, Christian B, et al. Evidence of Long Latency Periods Prior to Development of Mantle Cell Lymphoma. ASH Annual Meeting Abstracts. 2010;116(21):323. [Google Scholar]
- 16.Aqel N, Barker F, Patel K, Naresh KN. In-situ mantle cell lymphoma-a report of two cases. Histopathology. 2007;52(2):256–260. doi: 10.1111/j.1365-2559.2007.02906.x. [DOI] [PubMed] [Google Scholar]
- 17.Carvajal-Cuenca A, Sua LF, Silva NM, et al. In situ mantle cell lymphoma: clinical implications of an incidental finding with indolent clinical behavior. [Accessed December 3, 2011];Haematologica. 2011 doi: 10.3324/haematol.2011.052621. Available at: http://www.haematologica.org/content/early/2011/10/12/haematol.2011.052621.abstract. [DOI] [PMC free article] [PubMed]
- 18.Christian B, Zhao W, Hamadani M, et al. Mantle Cell Lymphoma 12 Years After Allogeneic Bone Marrow Transplantation Occurring Simultaneously in Recipient and Donor. Journal of Clinical Oncology. 2010;28(31):e629. doi: 10.1200/JCO.2010.29.8992. [DOI] [PubMed] [Google Scholar]
- 19.Brepoels L, Stroobants S, De Wever W, et al. Positron emission tomography in mantle cell lymphoma. Leukemia & Lymphoma. 2008;49(9):1693–1701. doi: 10.1080/10428190802216707. [DOI] [PubMed] [Google Scholar]
- 20.Klapper W, Hoster E, et al. for the European MCL Network. Ki-67 as a prognostic marker in mantle cell lymphoma—consensus guidelines of the pathology panel of the European MCL Network. Journal of Hematopathology. 2009;2(2):103–111. doi: 10.1007/s12308-009-0036-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schaffel R, Hedvat C, Teruya-Feldstein J, et al. Prognostic impact of proliferative index determined by quantitative image analysis and the International Prognostic Index in patients with mantle cell lymphoma. Annals of Oncology. 2010;21(1):133. doi: 10.1093/annonc/mdp495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fernandez V, Salamero O, Espinet B, et al. Genomic and Gene Expression Profiling Defines Indolent Forms of Mantle Cell Lymphoma. Cancer Research. 2010;70(4):1408–1418. doi: 10.1158/0008-5472.CAN-09-3419. [DOI] [PubMed] [Google Scholar]
- 23.Gustavsson E, Sernbo S, Andersson E, et al. SOX11 expression correlates to promoter methylation and regulates tumor growth in hematopoietic malignancies. Molecular cancer. 2010;9(1):187. doi: 10.1186/1476-4598-9-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shah BD, Villagra A, Merino O, et al. HDAC Profiling In Mantle Cell Lymphoma Unveils HDAC11 and HDAC10 as Potential Molecular Targets. ASH Annual Meeting Abstracts. 2010;116(21):2506. [Google Scholar]
- 25.Sahakian E, Shah BD, Powers J, et al. The Opposing Role of Histone Deacetylase 10 (HDAC10) and HDAC11 in Proliferation/Survival of Mantle Cell Lymphoma (MCL) and Chronic Lymphocytic Leukemia (CLL) ASH Annual Meeting Abstracts. 2011;118(21):1363. [Google Scholar]
- 26.Herrmann A, Hoster E, Zwingers T, et al. Improvement of Overall Survival in Advanced Stage Mantle Cell Lymphoma. Journal of Clinical Oncology. 2008;27:511–518. doi: 10.1200/JCO.2008.16.8435. [DOI] [PubMed] [Google Scholar]
- 27.Abrahamsson A, Dahle N, Jerkeman M. Marked improvement of overall survival in mantle cell lymphoma: a population based study from the Swedish Lymphoma Registry. Leukemia & Lymphoma. 2011;52(10):1929–1935. doi: 10.3109/10428194.2011.587560. [DOI] [PubMed] [Google Scholar]
- 28.Hoster E, Dreyling M, Klapper W, et al. A new prognostic index (MIPI) for patients with advanced-stage mantle cell lymphoma. doi: 10.1182/blood-2007-06-095331. [DOI] [PubMed] [Google Scholar]; Hoster E, Dreyling M, Klapper W, Gisselbrecht C, van Hoof A, Kluin-Nelemans HC, Pfreundschuh M, Reiser M, Metzner B, Einsele H, Peter N, Jung W, Wörmann B, Ludwig WD, Dührsen U, Eimermacher H, Wandt H, Hasford J, Hiddemann W, Unterhalt M German Low Grade Lymphoma Study Group (GLSG) European Mantle Cell Lymphoma Network. Blood. 2008;111(2):558–65. doi: 10.1182/blood-2007-06-095331. [DOI] [PubMed] [Google Scholar]
- 29.Geisler CH, Kolstad A, Laurell A, et al. The Mantle Cell Lymphoma International Prognostic Index (MIPI) is superior to the International Prognostic Index (IPI) in predicting survival following intensive first-line immunochemotherapy and autologous stem cell transplantation (ASCT) Blood. 2010;115(8):1530. doi: 10.1182/blood-2009-08-236570. [DOI] [PubMed] [Google Scholar]
- 30.Hoster E. Prognostic relevance of clinical risk factors in mantle cell lymphoma. Semin Hematol. 2011;48(3):185–8. doi: 10.1053/j.seminhematol.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 31.Bodet-Milin C, Touzeau C, Leux C, et al. Prognostic impact of 18F-fluoro-deoxyglucose positron emission tomography in untreated mantle cell lymphoma: a retrospective study from the GOELAMS group. European Journal of Nuclear Medicine and Molecular Imaging. 2010;37:1633–1642. doi: 10.1007/s00259-010-1469-2. [DOI] [PubMed] [Google Scholar]
- 32.Meignan M, Itti E, Bardet S, et al. Development and application of a real-time on-line blinded independent central review of interim PET scans to determine treatment allocation in lymphoma trials. Journal of Clinical Oncology. 2009;27(16):2739. doi: 10.1200/JCO.2009.22.4089. [DOI] [PubMed] [Google Scholar]
- 33.Thieblemont C, Houlgatte R, Felman P, et al. Indolent Mantle Cell Lymphoma (MCL): A Retrospective Detailed Clinical and Morphological Analysis of 21 Patients, with Histological, Cytological, Cytogenetic, Interphase Genetic, Immunoglobulin Gene, and Gene Expression Profiling Analysis. ASH Annual Meeting Abstracts. 2008;112(11):1780. [Google Scholar]
- 34.Nodit L, Bahler DW, Jacobs SA, Locker J, Swerdlow SH. Indolent mantle cell lymphoma with nodal involvement and mutated immunoglobulin heavy chain genes. Hum Pathol. 2003;34(10):1030–1034. doi: 10.1053/s0046-8177(03)00410-6. [DOI] [PubMed] [Google Scholar]
- 35.Nabhan C, Smith SM, Kahl BS. Maintenance Rituximab in Follicular Non-Hodgkin Lymphoma: Facts and Controversies. Leukemia & Lymphoma. 2011:1–22. doi: 10.3109/10428194.2011.628061. [DOI] [PubMed] [Google Scholar]
- 36.Ghielmini M. Effect of Single-Agent Rituximab Given at the Standard Schedule or As Prolonged Treatment in Patients With Mantle Cell Lymphoma: A Study of the Swiss Group for Clinical Cancer Research (SAKK) Journal of Clinical Oncology. 2005;23(4):705–711. doi: 10.1200/JCO.2005.04.164. [DOI] [PubMed] [Google Scholar]
- 37.Foran JM, Rohatiner AZS, Cunningham D, et al. European phase II study of rituximab (chimeric anti-CD20 monoclonal antibody) for patients with newly diagnosed mantle-cell lymphoma and previously treated mantle-cell lymphoma, immunocytoma, and small B-cell lymphocytic lymphoma. Journal of Clinical Oncology. 2000;18(2):317. doi: 10.1200/JCO.2000.18.2.317. [DOI] [PubMed] [Google Scholar]
- 38.Coiffier B, Haioun C, Ketterer N, et al. Rituximab (anti-CD20 monoclonal antibody) for the treatment of patients with relapsing or refractory aggressive lymphoma: a multicenter phase II study. Blood. 1998;92(6):1927–1932. [PubMed] [Google Scholar]
- 39.Seggewiss R, Ho AD, Kraemer A. Remarkable response to rituximab in a patient with atypical CD20 ++ mantle cell lymphoma of the bone marrow leading to severe pancytopenia. Annals of Hematology. 2004;83(5):316–318. doi: 10.1007/s00277-003-0793-z. [DOI] [PubMed] [Google Scholar]
- 40.Angelopoulou MK, Siakantariz MP, Vassilakopoulos TP, et al. The splenic form of mantle cell lymphoma. European journal of haematology. 2002;68(1):12–21. doi: 10.1034/j.1600-0609.2002.00551.x. [DOI] [PubMed] [Google Scholar]
- 41.Matutes E, Parry-Jones N, Brito-Babapulle V, et al. The Leukemic Presentation of Mantle-cell Lymphoma: Disease Features and Prognostic Factors in 58 Patients. Leukemia & Lymphoma. 2004;45(10):2007–2015. doi: 10.1080/10428190410001723331. [DOI] [PubMed] [Google Scholar]
- 42.Solenthaler M, Matutes E, Brito-Babapulle V, Morilla R, Catovsky D. p53 and mdm2 in mantle cell lymphoma in leukemic phase. haematologica. 2002;87(11):1141–1150. [PubMed] [Google Scholar]
- 43.Leitch H, Gascoyne R, Chhanabhai M, et al. Limited-stage mantle-cell lymphoma. Annals of oncology. 2003;14(10):1555. doi: 10.1093/annonc/mdg414. [DOI] [PubMed] [Google Scholar]
- 44.Zelenetz A, et al. NCCN Clinical Practice Guidelines in Oncology, Non-Hodgkin’s Lymphomas. 2012 doi: 10.6004/jnccn.2010.0021. Available at: www.nccn.org. [DOI] [PubMed]
- 45.Teodorovic I, Pittaluga S, Kluin-Nelemans J, et al. Efficacy of four different regimens in 64 mantle-cell lymphoma cases: clinicopathologic comparison with 498 other non-Hodgkin’s lymphoma subtypes. European Organization for the Research and Treatment of Cancer Lymphoma Cooperative Group. Journal of clinical oncology. 1995;13(11):2819–2826. doi: 10.1200/JCO.1995.13.11.2819. [DOI] [PubMed] [Google Scholar]
- 46.Fisher RI, Dahlberg S, Nathwani B, et al. A clinical analysis of two indolent lymphoma entities: mantle cell lymphoma and marginal zone lymphoma (including the mucosa-associated lymphoid tissue and monocytoid B-cell subcategories): a Southwest Oncology Group study. Blood. 1995;85(4):1075. [PubMed] [Google Scholar]
- 47.Nickenig C, Dreyling M, Hoster E, et al. Combined cyclophosphamide, vincristine, doxorubicin, and prednisone (CHOP) improves response rates but not survival and has lower hematologic toxicity compared with combined mitoxantrone, chlorambucil, and prednisone (MCP) in follicular and mantle cell lymphomas. Cancer. 2006;107(5):1014–1022. doi: 10.1002/cncr.22093. [DOI] [PubMed] [Google Scholar]
- 48.Borgerding A, Hasenkamp J, Glaß B, Wulf G, Trümper L. Rituximab retherapy in patients with relapsed aggressive B cell and mantle cell lymphoma. Annals of Hematology. 2009;89(3):283–289. doi: 10.1007/s00277-009-0820-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lenz G. Immunochemotherapy With Rituximab and Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone Significantly Improves Response and Time to Treatment Failure, But Not Long-Term Outcome in Patients With Previously Untreated Mantle Cell Lymphoma: Results of a Prospective Randomized Trial of the German Low Grade Lymphoma Study Group (GLSG) Journal of Clinical Oncology. 2005;23:1984–1992. doi: 10.1200/JCO.2005.08.133. [DOI] [PubMed] [Google Scholar]
- 50.Lefrere F, Delmer A, Levy V, et al. Sequential chemotherapy regimens followed by high-dose therapy with stem cell transplantation in mantle cell lymphoma: an update of a prospective study. haematologica. 2004;89(10):1275–1276. [PubMed] [Google Scholar]
- 51.Pott C, Hoster E, Beldjord K, et al. R-CHOP/R-DHAP Compared to R-CHOP Induction Followed by High Dose Therapy with Autologous Stem Cell Transplantation Induces Higher Rates of Molecular Remission In MCL: Results of the MCL Younger Intergroup Trial of the European MCL Network. ASH Annual Meeting Abstracts. 2010;116(21):965. [Google Scholar]
- 52.Romaguera JE. High Rate of Durable Remissions After Treatment of Newly Diagnosed Aggressive Mantle-Cell Lymphoma With Rituximab Plus Hyper-CVAD Alternating With Rituximab Plus High-Dose Methotrexate and Cytarabine. Journal of Clinical Oncology. 2005;23(28):7013–7023. doi: 10.1200/JCO.2005.01.1825. [DOI] [PubMed] [Google Scholar]
- 53.Romaguera JE, Fayad LE, Feng L, et al. Ten-year follow-up after intense chemoimmunotherapy with Rituximab-HyperCVAD alternating with Rituximab-high dose methotrexate/cytarabine (R-MA) and without stem cell transplantation in patients with untreated aggressive mantle cell lymphoma. British journal of haematology. 2010;150(2):200–208. doi: 10.1111/j.1365-2141.2010.08228.x. [DOI] [PubMed] [Google Scholar]
- 54.Dreyling M, Lenz G, Hoster E, et al. Early consolidation by myeloablative radiochemotherapy followed by autologous stem cell transplantation in first remission significantly prolongs progression-free survival in mantle-cell lymphoma: results of a prospective randomized trial of the European MCL Network. Blood. 2005;105(7):2677–2684. doi: 10.1182/blood-2004-10-3883. [DOI] [PubMed] [Google Scholar]
- 55.Vandenberghe E, Ruiz de Elvira C, Loberiza FR, et al. Outcome of autologous transplantation for mantle cell lymphoma: a study by the European Blood and Bone Marrow Transplant and Autologous Blood and Marrow Transplant Registries. British journal of haematology. 2003;120(5):793–800. doi: 10.1046/j.1365-2141.2003.04140.x. [DOI] [PubMed] [Google Scholar]
- 56.Andersen NS, Pedersen L, Elonen E, et al. Primary treatment with autologous stem cell transplantation in mantle cell lymphoma: outcome related to remission pretransplant. European journal of haematology. 2003;71(2):73–80. doi: 10.1034/j.1600-0609.2003.00093.x. [DOI] [PubMed] [Google Scholar]
- 57.Ritchie DS, Seymour JF, Grigg AP, et al. The hyper-CVAD–rituximab chemotherapy programme followed by high-dose busulfan, melphalan and autologous stem cell transplantation produces excellent event-free survival in patients with previously untreated mantle cell lymphoma. Annals of Hematology. 2006;86(2):101–105. doi: 10.1007/s00277-006-0193-2. [DOI] [PubMed] [Google Scholar]
- 58.Geisler CH, Kolstad A, Laurell A, et al. Long-term progression-free survival of mantle cell lymphoma after intensive front-line immunochemotherapy with in vivo–purged stem cell rescue: a nonrandomized phase 2 multicenter study by the Nordic Lymphoma Group. Blood. 2008;112(7):2687. doi: 10.1182/blood-2008-03-147025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Damon LE, Johnson JL, Niedzwiecki D, et al. Immunochemotherapy and Autologous Stem-Cell Transplantation for Untreated Patients With Mantle-Cell Lymphoma: CALGB 59909. JCO. 2009;27(36):6101–6108. doi: 10.1200/JCO.2009.22.2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Anonymous. Comparative Outcome of Initial Therapy for Younger Patients with Mantle Cell Lymphoma: An Analysis from the NCCN NHL Database. Blood. 2012;119(9):2093–2099. doi: 10.1182/blood-2011-07-369629. [DOI] [PubMed] [Google Scholar]
- 61.Ahmadi T, McQuade J, Porter D, et al. Potential prolongation of PFS in mantle cell lymphoma after R-HyperCVAD: auto-SCT consolidation or rituximab maintenance. [Accessed December 3, 2011];Bone Marrow Transplant. 2011 doi: 10.1038/bmt.2011.218. Available at: http://dx.doi.org/10.1038/bmt.2011.218. [DOI] [PubMed]
- 62.Rummel MJ, Niederle N, Maschmeyer G, et al. Bendamustine Plus Rituximab Is Superior in Respect of Progression Free Survival and CR Rate When Compared to CHOP Plus Rituximab as First-Line Treatment of Patients with Advanced Follicular, Indolent, and Mantle Cell Lymphomas: Final Results of a Randomized Phase III Study of the StiL (Study Group Indolent Lymphomas, Germany) ASH Annual Meeting Abstracts. 2009;114(22):405. [Google Scholar]
- 63.Inwards DJ, Fishkin PAS, Hillman DW, et al. Long_term results of the treatment of patients with mantle cell lymphoma with cladribine (2_CDA) alone (95_80_53) or 2_CDA and rituximab (N0189) in the North Central Cancer Treatment Group. Cancer. 2008;113(1):108–116. doi: 10.1002/cncr.23537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Spurgeon SE, Pindyck T, Okada C, et al. Cladribine plus rituximab is an effective therapy for newly diagnosed mantle cell lymphoma. Leukemia & Lymphoma. 2011;52(8):1488–1494. doi: 10.3109/10428194.2011.575489. [DOI] [PubMed] [Google Scholar]
- 65.Kenkre VP, Long WL, Eickhoff JC, et al. Maintenance rituximab following induction chemo-immunotherapy for mantle cell lymphoma: long-term follow-up of a pilot study from the Wisconsin Oncology Network. Leukemia & Lymphoma. 2011;52(9):1675–1680. doi: 10.3109/10428194.2011.580404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kluin-Nelemans Jc, Hoster E, Walewski J, et al. R-CHOP Versus R-FC Followed by Maintenance with Rituximab Versus Interferon-Alfa: Outcome of the First Randomized Trial for Elderly Patients with Mantle Cell Lymphoma. ASH Annual Meeting Abstracts. 2011;118(21):439. [Google Scholar]
- 67.Le Gouill S, Mohty M, Guillaume T, Gastinne T, Moreau P. Allogeneic stem cell transplantation in mantle cell lymphoma: where are we now and which way should we go? Semin Hematol. 2011;48(3):227–239. doi: 10.1053/j.seminhematol.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 68.Habermann TM, Lossos IS, Justice G, et al. Lenalidomide oral monotherapy produces a high response rate in patients with relapsed or refractory mantle cell lymphoma. British journal of haematology. 2009;145(3):344–349. doi: 10.1111/j.1365-2141.2009.07626.x. [DOI] [PubMed] [Google Scholar]
- 69.Wang L, Fayad L, Hagemeister FB, et al. A Phase I/II Study of Lenalidomide in Combination with Rituximab in Relapsed/Refractory Mantle Cell Lymphoma. ASH Annual Meeting Abstracts. 2009;114(22):2719. [Google Scholar]
- 70.Zhang L, Qian Z, Cai Z, et al. Synergistic antitumor effects of lenalidomide and rituximab on mantle cell lymphoma in vitro and in vivo. American journal of hematology. 2009;84(9):553–559. doi: 10.1002/ajh.21468. [DOI] [PubMed] [Google Scholar]
- 71.Belch A, Kouroukis C, Crump M, et al. A phase II study of bortezomib in mantle cell lymphoma: the National Cancer Institute of Canada Clinical Trials Group trial IND. 150. Annals of oncology. 2007;18(1):116. doi: 10.1093/annonc/mdl316. [DOI] [PubMed] [Google Scholar]
- 72.Fisher RI, Bernstein SH, Kahl BS, et al. Multicenter Phase II Study of Bortezomib in Patients With Relapsed or Refractory Mantle Cell Lymphoma. Journal of Clinical Oncology. 2006;24(30):4867–4874. doi: 10.1200/JCO.2006.07.9665. [DOI] [PubMed] [Google Scholar]
- 73.O’Connor OA. Phase II Clinical Experience With the Novel Proteasome Inhibitor Bortezomib in Patients With Indolent Non-Hodgkin’s Lymphoma and Mantle Cell Lymphoma. Journal of Clinical Oncology. 2005;23(4):676–684. doi: 10.1200/JCO.2005.02.050. [DOI] [PubMed] [Google Scholar]
- 74.Goy A. Phase II Study of Proteasome Inhibitor Bortezomib in Relapsed or Refractory B-Cell Non-Hodgkin’s Lymphoma. Journal of Clinical Oncology. 2005;23(4):667–675. doi: 10.1200/JCO.2005.03.108. [DOI] [PubMed] [Google Scholar]
- 75.Friedberg JW, Vose JM, Kelly JL, et al. The combination of bendamustine, bortezomib, and rituximab for patients with relapsed/refractory indolent and mantle cell non-Hodgkin lymphoma. Blood. 2011;117(10):2807–2812. doi: 10.1182/blood-2010-11-314708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kouroukis CT, Fernandez LAV, Crump M, et al. A phase II study of bortezomib and gemcitabine in relapsed mantle cell lymphoma from the National Cancer Institute of Canada Clinical Trials Group (IND 172) Leukemia & Lymphoma. 2011;52(3):394–399. doi: 10.3109/10428194.2010.546015. [DOI] [PubMed] [Google Scholar]
- 77.Ruan J, Martin P, Furman RR, et al. Bortezomib Plus CHOP-Rituximab for Previously Untreated Diffuse Large B-Cell Lymphoma and Mantle Cell Lymphoma. Journal of Clinical Oncology. 2010;29(6):690–697. doi: 10.1200/JCO.2010.31.1142. [DOI] [PubMed] [Google Scholar]
- 78.Ribrag V, Gisselbrecht C, Haioun C, et al. Efficacy and toxicity of 2 schedules of frontline rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone plus bortezomib in patients with B-cell lymphoma. Cancer. 2009;115(19):4540–4546. doi: 10.1002/cncr.24518. [DOI] [PubMed] [Google Scholar]
- 79.Chang JE, Peterson C, Choi S, et al. VcR-CVAD induction chemotherapy followed by maintenance rituximab in mantle cell lymphoma: a Wisconsin Oncology Network study. British Journal of Haematology. 2011 doi: 10.1111/j.1365-2141.2011.08820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lamm W, Kaufmann H, Raderer M, et al. Bortezomib combined with rituximab and dexamethasone is an active regimen for patients with relapsed and chemotherapy-refractory mantle cell lymphoma. Haematologica. 2011;96(7):1008–1014. doi: 10.3324/haematol.2011.041392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Coiffier B, Ribrag V. Exploring mammalian target of rapamycin (mTOR) inhibition for treatment of mantle cell lymphoma and other hematologic malignancies. Leukemia and Lymphoma. 2009:1–15. doi: 10.3109/10428190903207548. [DOI] [PubMed] [Google Scholar]
- 82.Hess G, Herbrecht R, Romaguera J, et al. Phase III Study to Evaluate Temsirolimus Compared With Investigator’s Choice Therapy for the Treatment of Relapsed or Refractory Mantle Cell Lymphoma. Journal of Clinical Oncology. 2009;27(23):3822–3829. doi: 10.1200/JCO.2008.20.7977. [DOI] [PubMed] [Google Scholar]
- 83.Zhang X, Chen X, Lin J, et al. Myc represses miR-15a/miR-16-1 expression through recruitment of HDAC3 in mantle cell and other non-Hodgkin B-cell lymphomas. Oncogene. 2011 doi: 10.1038/onc.2011.470. [DOI] [PMC free article] [PubMed]
- 84.Cheng F, Wang H, Woan K, et al. Targeting Histone Deacetylase 6 (HDAC6) as a Novel Approach to Overcome T-Cell Anergy. ASH Annual Meeting Abstracts. 2010;116(21):1483. [Google Scholar]
- 85.Cheng F, Wang Z, Wang H, et al. Epigenetic Modulation of STAT3 by Histone Deacetylase 6 (HDAC6) Regulates IL-10 Gene Expression and Immune Tolerance Mediated by Antigen-Presenting Cells (APCs) ASH Annual Meeting Abstracts. 2011;118(21):519. [Google Scholar]
- 86.Villagra A, Cheng F, Wang H-W, et al. The histone deacetylase HDAC11 regulates the expression of interleukin 10 and immune tolerance. Nature Immunology. 2008;10(1):92–100. doi: 10.1038/ni.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Alas S, Bonavida B. Rituximab inactivates signal transducer and activation of transcription 3 (STAT3) activity in B-non-Hodgkin’s lymphoma through inhibition of the interleukin 10 autocrine/paracrine loop and results in down-regulation of Bcl-2 and sensitization to cytotoxic drugs. Cancer research. 2001;61(13):5137. [PubMed] [Google Scholar]
- 88.Kirschbaum M, Frankel P, Popplewell L, et al. Phase II Study of Vorinostat for Treatment of Relapsed or Refractory Indolent Non-Hodgkin’s Lymphoma and Mantle Cell Lymphoma. Journal of Clinical Oncology. 2011;29(9):1198–1203. doi: 10.1200/JCO.2010.32.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Holkova B, Perkins EB, Sokol L, et al. A Phase II Trial of Bortezomib and Vorinostat in Mantle Cell Lymphoma and Diffuse Large B-Cell Lymphoma. ASH Annual Meeting Abstracts. 2011;118(21):779. doi: 10.1016/j.clml.2018.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang L, Martin P, Blum KA, et al. The Bruton’s Tyrosine Kinase Inhibitor PCI-32765 Is Highly Active As Single-Agent Therapy in Previously-Treated Mantle Cell Lymphoma (MCL): Preliminary Results of a Phase II Trial. ASH Annual Meeting Abstracts. 2011;118(21):442. [Google Scholar]
- 91.Kahl B, Byrd JC, Flinn IW, et al. Clinical Safety and Activity In a Phase 1 Study of CAL-101, An Isoform-Selective Inhibitor of Phosphatidylinositol 3-Kinase P110{delta}, In Patients with Relapsed or Refractory Non-Hodgkin Lymphoma. ASH Annual Meeting Abstracts. 2010;116(21):1777. [Google Scholar]
- 92.Hoellenriegel J, Meadows S, Sivina M, et al. The phosphoinositide 3′-kinase delta inhibitor, CAL-101, inhibits B-cell receptor signaling and chemokine networks in chronic lymphocytic leukemia. doi: 10.1182/blood-2011-05-352492. [DOI] [PMC free article] [PubMed] [Google Scholar]; Hoellenriegel J, Meadows SA, Sivina M, Wierda WG, Kantarjian H, Keating MJ, Giese N, O’Brien S, Yu A, Miller LL, Lannutti BJ, Burger JA. Blood. 2011;118(13):3603–12. doi: 10.1182/blood-2011-05-352492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chang BY, Francesco M, Magadala P, et al. Egress of CD19+CD5+ Cells Into Peripheral Blood Following Treatment with the Bruton Tyrosine Kinase Inhibitor, PCI-32765, in Mantle Cell Lymphoma Patients. ASH Annual Meeting Abstracts. 2011;118(21):954. doi: 10.1182/blood-2013-02-482125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gupta M, Wahner Hendrickson AE, Yun SS, et al. Dual mTORC1/mTORC2 inhibition diminishes Akt activation and induces Puma-dependent apoptosis in lymphoid malignancies. [Accessed December 3, 2011];Blood. 2011 doi: 10.1182/blood-2011-04-346601. Available at: http://bloodjournal.hematologylibrary.org/content/early/2011/11/10/blood-2011-04-346601.abstract. [DOI] [PMC free article] [PubMed]
- 95.Tchakarska G, Le Lan-Leguen A, Roth L, Sola B. The targeting of the sole cyclin D1 is not adequate for mantle cell lymphoma and myeloma therapies. haematologica. 2009;94(12):1781–1782. doi: 10.3324/haematol.2009.011460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Leonard JP, LaCasce A, Smith MR, et al. Cdk4/6 Inhibitor PD 0332991 Demonstrates Cell Cycle Inhibition Via FLT-PET Imaging and Tissue Analysis in Patients with Recurrent Mantle Cell Lymphoma. ASH Annual Meeting Abstracts. 2008;112(11):264. [Google Scholar]
- 97.Menu E, Garcia J, Huang X, et al. A novel therapeutic combination using PD 0332991 and bortezomib: study in the 5T33MM myeloma model. Cancer research. 2008;68(14):5519. doi: 10.1158/0008-5472.CAN-07-6404. [DOI] [PubMed] [Google Scholar]
- 98.Hankin RC, Hunter SV. Mantle cell lymphoma. Arch Pathol Lab Med. 1999;123(12):1182–1188. doi: 10.5858/1999-123-1182-MCL. [DOI] [PubMed] [Google Scholar]
- 99.Li JY, Gaillard F, Moreau A, et al. Detection of translocation t(11;14)(q13;q32) in mantle cell lymphoma by fluorescence in situ hybridization. Am J Pathol. 1999;154(5):1449–1452. doi: 10.1016/S0002-9440(10)65399-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Belaud-Rotureau M-A, Parrens M, Dubus P, et al. A comparative analysis of FISH, RT-PCR, PCR, and immunohistochemistry for the diagnosis of mantle cell lymphomas. Mod Pathol. 2002;15(5):517–525. doi: 10.1038/modpathol.3880556. [DOI] [PubMed] [Google Scholar]


