Abstract
Use of adoptive T-cell therapy (ACT) is increasing; however, T-cell therapy can result in severe toxicity. Consequently, several suicide-gene strategies that allow selective destruction of the infused T cells have been described. We compared effectiveness of four such strategies in vitro in Epstein Barr virus (EBV)-cytotoxic T lymphocytes (CTLs). Herpes simplex virus thymidine kinase (HSV-TK), human inducible caspase 9 (iCasp9), mutant human thymidylate kinase (mTMPK), and human CD20 codon optimized genes were cloned in frame with 2A-truncated codon optimized CD34 (dCD34) in a retroviral vector. Codon-optimization considerably improved CD20 expression. EBV-CTLs could be efficiently transduced in all constructs, with transgene expression similar to the control vector containing dCD34 alone. Expression was maintained for prolonged cultures. Expression of the suicide genes was not associated with alterations in immunophenotype, proliferation, or function of CTLs. Activation of HSV-TK, iCasp9, and CD20 ultimately resulted in equally effective destruction of transduced T cells. However, while iCasp9 and CD20 effected immediate cell-death induction, HSV-TK-expressing T cells required 3 days of exposure to ganciclovir to reach full effect. mTMPK-transduced cells showed lower T-cell killing all time points. Our results suggest that the faster activity of iCasp9 might be advantageous in treating certain types of acutely life-threatening toxicity. Codon-optimized CD20 has potential as a suicide gene.
Marin and colleagues compare the effectiveness of four suicide-gene strategies in a model in vitro T-cell system. Herpes simplex virus thymidine kinase (HSV-TK), human inducible caspase 9 (iCasp9), mutant human thymidylate kinase (mTMPK), and human CD20 codon-optimized genes were cloned in frame with 2A-truncated codon optimized CD34 in a retroviral vector. Suicide-gene activation ultimately resulted in equally effective destruction of transduced T cells, with iCasp9 and CD20 displaying the most immediate induction of cell death.
Introduction
Adoptive T-cell therapy (ACT) is a modality whose scope and application is expanding. T-cell therapy has long been used in allogeneic haematopoietic stem cell transplantation (HSCT), where donor lymphocyte infusion (DLI) results in rejection of allogeneic antigens expressed by malignant cells. Ex vivo selected and expanded T cells are effective in treating opportunistic CMV infection (Peggs et al., 2003), Epstein Barr virus (EBV)-driven lymphomas (Savoldo et al., 2001), and some carcinomas (Turcotte and Rosenberg, 2011). More recently, genetic engineering of T cells with either T-cell receptors (TCRs) (Stauss et al., 2007) or chimeric antigen receptors (CARs) (Pule et al., 2003), has expanded the application of ACT to treat a wider range of malignancies (Morgan et al., 2006; Pule et al., 2008; Brentjens et al., 2011; Porter et al., 2011; Rosenberg, 2011). Unlike small molecules or protein therapeutics, infused T cells have the capacity to engraft and survive indefinitely (Louis et al., 2011), conferring the potential for chronic or hyper-acute toxicity. Graft-versus host disease (GVHD), for instance, is a common life-threatening complication of DLI. Serious toxicities have recently been observed in studies of engineered T cells (Lamers et al., 2006; Brentjens et al. 2010; Morgan et al., 2010), and other toxicities suggested by animal models (Bendle et al., 2010). In addition, malignancy subsequent to uncontrolled proliferation of infused T cells is a concern, particularly when infused T cells have been engineered with integrating vectors. Consequently, a number of “suicide-gene” strategies that allow selective destruction of administered T cells in the face of unacceptable toxicity have been developed. Here, we compare four such strategies.
Several suicide-gene approaches have been described. The herpes symplex virus thymidine kinase (HSV-TK) is the best characterized (Bonini et al., 1997). The expression of this gene renders T cells susceptible to the anti-herpetic agent ganciclovir. HSV-TK is currently under evaluation in a phase III clinical trial in patients undergoing haploidentical HSCT and appears effective (Bordignon et al,. 2012). However the immunogenicity of this viral protein limits its use to clinical settings such as haploidentical HSCT, where the recipient is profoundly immunosuppressed (Riddell et al., 1996). Further, HSV-TK precludes the use of ganciclovir for treating an eventual CMV reactivation, a not uncommon complication in these settings. More recently, an inducible caspase 9 (iCasp9) has been constructed (Straathof et al., 2005) by fusing an FKBP12 domain modified to bind to a homodimeric small molecule (Clackson et al., 1998) with the proteolytic domain of caspase 9. The triggering small molecule appears to be otherwise biologically inert (Iuliucci et al., 2001), and recent early clinical data shows promise (Di Stasi et al., 2011). iCasp9 is likely nonimmunogenic since, apart from a single amino acid substitution in the FKBP and a short junctional sequence, it is a self-protein. Transgenic expression of the B-cell antigen CD20 by T cells has also been proposed as a suicide-gene strategy (Introna et al., 2000; Serafini et al., 2004). T-cells could by selectively destroyed by administration of the highly lytic and easily available therapeutic antibody rituximab. The main disadvantage of this strategy is the concomitant transient depletion of the patient's B-cell compartment. Clinical experience with this system is currently lacking. Finally, Sato et al. (2007) recently proposed the use of a mutated human thymidilate kinase (mTMPK) enzyme as a suicide gene. TMPK-mediated phosphorylation is the rate-limiting step in the activation of the anti-retroviral agent azidothymidine (Zidovudine, AZT). TMPK mediates this phosphorylation only at a low enzymatic efficiency, but a single amino acid substitution in TMPK enhances this sufficiently to confer susceptibility in expressing cells at therapeutic doses of this agent.
In this study, we codon-optimized HSV-TK, iCasp9, CD20, and mTMPK and cloned them into the same retroviral vector. Using the foot-and-mouth disease 2A peptide (Donnelly et al., 2001), these suicide genes were co-expressed with truncated CD34, which we used as a marker/selection gene. We transduced EBV-specific T-cells, a convenient model T-cell system, with these vectors and compared expression and function in vitro after triggering with the corresponding activating agent. All four suicide genes were stably expressed at high levels in T-cells and did not affect T-cell phenotype or function. iCasp9 and CD20 show rapid and efficient destruction of expressing T cells. HSV-TK ultimately showed equivalent effectiveness but slower activity. mTMPK in contrast is less effective than the other strategies. These in vitro results indicate that HSV-TK, iCasp9, and CD20 have equal activity, but iCasp9 may be more suitable if rapid activity is required. Further, our data suggest that codon-optimized CD20 shows promise as a suicide gene.
Material and Methods
Cells
The EBV-producing marmoset B-cell line (B95-8), used as a source of EBV supernatant for transformation of human B cells, was purchased from “Istituto Zooprofilattico di Brescia” and maintained in RPMI-1640 (Lonza, Bergamo, Italy) supplemented with 10% fetal calf serum (FCS), l-glutamine, and antibiotics (complete RPMI). The human renal epithelial cell line 293T was purchased from American Type Culture Collection (ATCC; Teddington, Middlesex, United Kingdom) and was maintained in DMEM high-glucose (Lonza), supplemented with 10% FCS, L-glutamine, and antibiotics.
Generation of EBV-CTLs
Briefly, peripheral blood mononuclear cells (PBMCs) of healthy donors were obtained after centrifugation of fresh blood on a density gradient using Ficoll-Hypaque (Pharmacia LKB, Uppsala, Sweden). To generate EBV-transformed B-cell lines (LCLs), PBMCs (5×106) were incubated with 50 μl of concentrated supernatant from the EBV producer cell line B95-8 for 60 min. The cells were then plated at 106 cells per well in a round-bottomed 96-well plate in complete RPMI advanced medium (Lonza, Italy), added with 1 μg/ml of cyclosporin A (Sandoz Pharmaceuticals, Washington, DC). Cells were fed weekly until LCLs were established. EBV-CTL were expanded by co-culture of PBMCs (2×106) with 5×104 γ-irradiated (40 Gy) autologous LCLs. Starting on day 11, the responder cells were weekly restimulated with irradiated LCLs at a responder:stimulator ratio of 4:1. Two weekly doses of IL-2 (40 IU/ml) were added from 3rd stimulation.
FACS analysis
Aliquots of cells were analyzed for the expression of various surface markers using fluorescein isothiocyanate (FITC)-anti-CD8 (Becton Dickinson [BD], Milan, Italy), phycoerythrin (PE)-anti-CD4 (BD), PE-anti-CD56 (BD), and peridinin-chlorophyll-protein complex (PerCP)-anti-CD3 (BD). Transduction efficiency has been evaluated using a PE-anti-CD34 (BD). A FACScalibur (BD) flow cytometer device was used to analyze the samples.
Plasmid construction
We used the retroviral vector SFG (Rivière et al., 1995) for all experimental work detailed. To generate truncated CD34, we cloned open-reading-frame up until amino acid 335 from HSC cDNA into SFG. Human CD20 cDNA has been obtained from the LTR-CD20-LTR plasmid, (generous gift of Martino Introna, Ospedali Riuniti Bergamo, Italy). Human thymidylate kinase (mTMPK) containing the F105Y mutation was cloned from cDNA generated from healthy donor-derived PBMCs with mutations incorporated using overlapping primers. Herpes-symplex virus thymdine kinase (HSV-TK) cDNA was obtained from the MSCV-TK39-I-dNGFR vector (generous gift of Waseem Qasim, Institute of Child Health, London). iCasp9 was derived from lymphocyte cDNA with mutations and linker sequences introduced by overlapping PCR precisely as described (Clackson et al., 1998). Each suicide gene was cloned in frame with dCD34 by PCR with identical kozak sequences, separated by the foot and mouth disease 2A peptide TaV, to allow for 1:1 expression of suicide and marker genes. Subsequently, all of these constructs and dCD34 were codon optimized. Briefly, optimal coding sequences were computationally designed by minimizing a weighted penalty score for human codon usage, GC content, localized repeats, hairpins, and cryptic splice sites or promoter sequences. Overlapping oligonucleotides coding for these sequences were synthesized and subsequently assembled by PCR. All oligonucleotides were purchased from IDTDNA (Coralsville, Iowa). Sequences of all constructs were confirmed by capillary sequencing.
Retrovirus production and retroviral transduction of cells
The retroviral supernatant was produced by Mirus-mediated (TransIT®-LT1 Transfection Reagent, Tema Ricerca, Italy) cotransfection of 293T cells with the Moloney murine leukemia virus (MoMLV) gag-pol expression plasmid pEQ-PAM3(-E), the RD114 env expression plasmid pRDF (kindly provided by Dr. Yasu Takeuchi, Cancer Research Technology, London, United Kingdom), and the retroviral vectors carrying, respectively, dCD34, HSV-TK-2A-dCD34, iCasp9-2A-dCD34, mTMPK-2A-dCD34, and CD20-2A-dCD34. Supernatants containing retrovirus were harvested 48 hours and 72 hours after transfection, immediately frozen in dry ice, and stored at −80°C until further use. 293T cells were used to titrate virus concentration, and 0.5×106 EBV-CTLs were transduced 1 day after the 3rd LCL stimulation by resuspending cells in 2.5 ml of thawed viral supernatant in RetroNectin-coated (TaKaRa BioEurope, Gennevilliers, France) 14-mL polypropylene centrifuge tubes (Euroclone, Milan, Italy). The procedure was repeated the following day. The cells were then incubated for 72 hours in a humidified incubator at 37°C with 5% CO2.
Immunomagnetic selection of CD34+ EBV-CTLs
The day of the 5th stimulation with LCLs, transduced EBV-CTLs were immunoselected by passage through a separation column (25 MS for MiniMACS, Miltenyi Biotec, Bologna, Italy) with anti-CD34 microbeads (Miltenyi Biotec) according to the manufacturer instructions. The positive fraction was kept in culture for further functional analysis.
Cytotoxicity assay
The cytotoxicity of EBV-CTLs against allogenic and autologous LCLs was evaluated as previously described (Marin et al., 2010) three days after the 6th LCL stimulation with a standard 4-hour 51Chromium-release cytotoxicity assay. Radioactivity was detected by a β-scintillation counter (PerkinElmer Life Science, Boston, MA) as counts per minutes (CPM), and the percentage of specific lysis was calculated as previously described (Marin et al., 2010). Experiments were performed in triplicates.
IFN-γ release detection
For cytokine detection, three days after the 6th LCL stimulation, 1×105 EBV-CTLs were stimulated with γ-irradiated autologous LCL cells at 4:1 E:T ratio for 48 hours. Levels of IFN-γ in culture supernatants were determined by the human IFN-γ ELISA set (Becton Dickinson, BD, Italy).
Analysis of suicide genes activity
Three days after the 6th LCL stimulation, 1×105 transduced EBV-CTLs, diluted 1:1 with untransduced EBV-CTLs to normalize the effect of residual untransduced cell growth in the transduced population, were seeded in the presence of IL-2 at 40 IU/ml and medium alone or, respectively, ganciclovir (GCV; Roche, Monza, Italy) for HSV-TK-2A-dCD34, chemical inducer of dimerization (CID) (AP20187; ARIAD Pharmaceuticals, Cambridge, MA) for iCasp9-2A-dCD34, azidothymidine (AZT; GlaxoSmithKline, Verona, Italy) for mTMPK-2A-dCD34, rabbit complement (AbD Serotech, Oxford, United Kingdom), and rituximab (Roche) for CD20-2A-dCD34-transduced EBV-CTLs at the indicated concentrations. After 1, 4, and 7 days of incubation, cells were harvested and stained with anti-CD34 antibody. Elimination of transduced cells was then evaluated by flow cytometry analysis enumerating % residual CD34+ cells, and survival has been calculated by the ratio of residual CD34+ events in presence of the drug versus medium alone. In selected experiments, 1×106 transduced EBV-CTLs were exposed to a single dose of drug for 24 hours, washed, and then maintained in culture by weekly stimulation with autologous LCLs and without further addition of the drug. Viable cells were enumerated every three days. Control EBV-CTLs were maintained in culture without exposure to the drug.
Analysis of annexin V/7AAD staining after triggering suicide-genes
Three days after the sixth LCL stimulation, 1×105–transduced EBV-CTLs were incubated with the corresponding drugs at indicated concentrations. After 30 minutes, 2 hours, 8 hours, and 24 hours, cells were harvested and washed with cold Dulbecco's phosphate buffered saline (Euroclone). Cells were stained with annexin-V and 7-amino-actinomycin D (GFP-Certified™ Apoptosis/Necrosis detection kit for microscopy and flow cytometry; ENZO Life Sciences, Farmingdale, NY) for 15 minutes then kept on ice, according to the manufacturer's instructions. Within 1 hour after staining, cells were analyzed by ELISA.
Antibody-dependent cellular cytotoxicity assay
Autologous natural killer (NK) cells were isolated from PBMCs using CD56 positive selection (CD56 MicroBead Kits from Miltenyi Biotech). Cells were cultured in RPMI advanced medium (Lonza) supplemented with 10% FBS, L-glutamine, antibiotics, and 1,000 U/ml IL-2 for 7–12 days before usage, as previously described (Vogler et al., 2010). Three days after the sixth LCL stimulation, NK cells were added to gene-modified EBV-CTLs at 3:1 effector:target ratio in the presence or absence of 10 μg/ml rituximab. After incubation for 24 hours, cells were harvested and stained with (PE)-anti-CD34 (BD) and (PerCP)-anti-CD3 antibodies (BD). Elimination of transduced cells was then evaluated by flow cytometry analysis enumerating residual CD34+/CD3+cells and survival has been calculated by the ratio of residual CD34+/CD3+ events in presence of NK cells and rituximab versus medium alone.
Statistical analysis
Means values±SD are given unless otherwise stated. The results were compared by using the paired Student t test. A p value ≤0.05 was considered to be significant.
Results
EBV-CTLs could be efficiently and stably transduced with suicide-gene-containing constructs
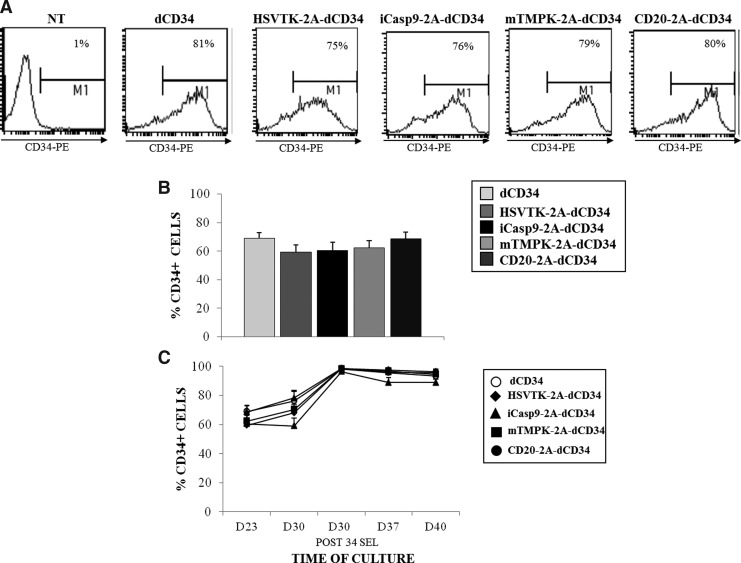
EBV-CTLs were obtained from five healthy donors and transduced with retroviral vectors coding for codon optimized HSV-TK-2A-dCD34, iCasp9-2A-dCD34, mTMPK-2A-dCD34, and CD20-2A-dCD34 (Fig. 1), with a mean % of CD34 expression, determined the day of the fourth LCL restimulation, respectively of 59%±5%, 60%±7%, 62%±5%, and 68%±5%, similar to what was obtained with a retroviral construct coding for dCD34 alone (mean % of CD34 expression, 69%±4%) (Fig. 2A and B). The day of the fifth LCL restimulation, transduced EBV-CTLs were efficiently enriched for CD34 expression up to 99% of CD34+ cells (Fig. 2C), and the expression of the marker gene was stable in culture up to day 40, which corresponds to three days after the sixth restimulation (Fig. 2C). In our experience, EBV-CTLs are highly sensitive to toxic transgenes. This prolonged and stable expression at high levels for protracted periods of culture indicates little basal toxicity of these transgenes.
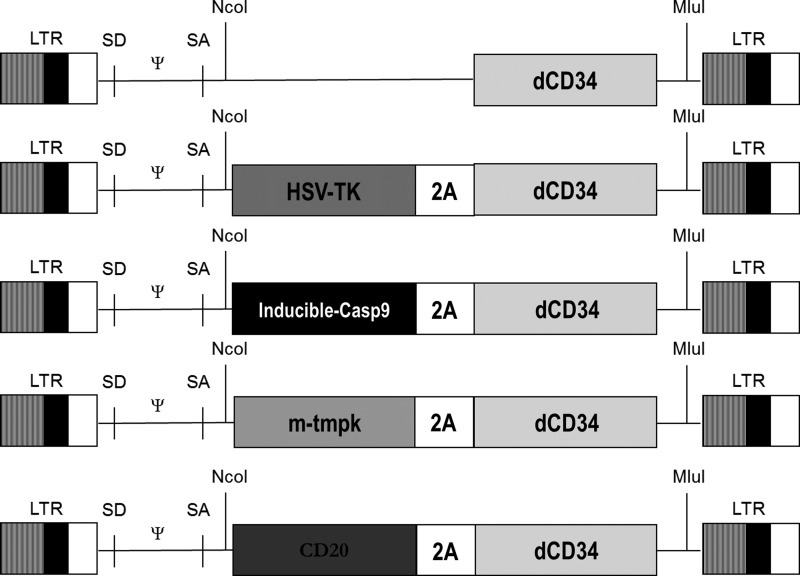
FIG. 1.
Schematic representation of the retroviral vectors used in the study. Each suicide gene was cloned in frame with the foot and mouth disease 2A peptide and truncated CD34 as a selectable marker gene to allow 1:1 expression of the two proteins.
FIG. 2.
Epstein-Barr virus–specific cytotoxic T lymphocytes (EBV-CTL) could be efficiently and stably transduced with suicide-gene-containing constructs. (A) The expression of the marker gene dCD34 on the surface of EBV-CTLs was evaluated by flow cytometry with a phycoerythrin (PE)-conjugate anti-human CD34 the day of fourth LCL restimulation. A representative experiment is shown. (B) Mean±SD of five separate experiments indicating the transduction efficiency with dCD34- or suicide-gene 2A-dCD34 retroviral vectors. (C) Stability of the expression of the marker gene dCD34 was evaluated over time in culture at the indicated time point by flow cytometry. Data shown are mean±SD of five separate experiments.
Suicide-gene expression in EBV-CTLs does not alter their phenotype and function
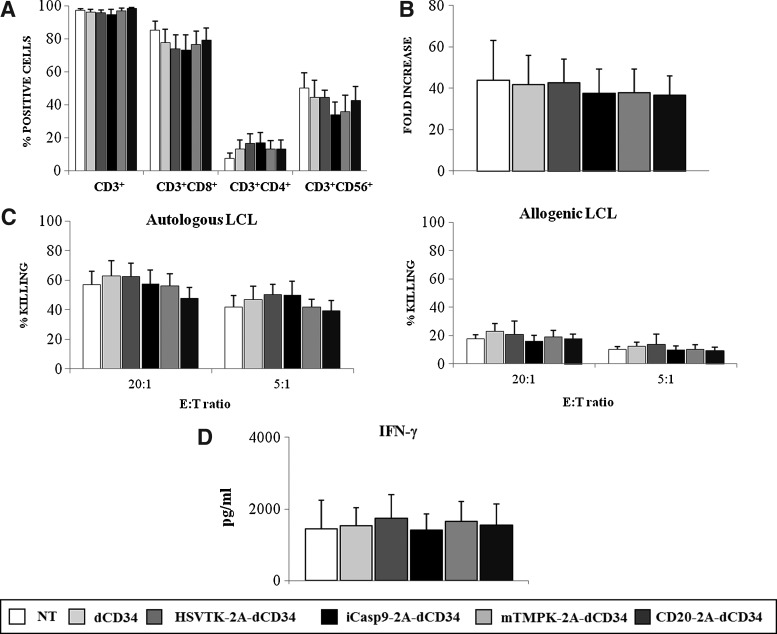
To ensure that neither the transduction process nor the expression of the suicide genes was affecting EBV-CTLs native properties, we compared immunophenotype, expansion, capacity to kill autologous and allogeneic LCLs, and secretion of IFN-γ after LCL stimulation of nontransduced and dCD34, HSV-TK-2A-dCD34, iCasp9-2A-dCD34, mTMPK-2A-dCD34, and CD20-2A-dCD34-transduced EBV-CTLs. As shown in Figure 3A, percentages of CD3+ cells, CD3+CD8+ cells, CD3+CD4+ cells, and CD3+CD56+ cells assessed 3 days after the sixth LCL stimulation were equal between nontransduced and transduced EBV-CTLs, with a typical selective enrichment in CD3+CD8+ cells. Similarly, the expansion rate determined at the same time point was analogous (Fig. 3B), with a mean fold increase of 44±19 (n=5) for nontransduced (NT) cells, 42±14 (n=5) for dCD34, 43±12 (n=5) for HSV-TK-2A-dCD34, 38±12 (n=5) for iCasp9-2A-dCD34, 38±11 (n=5) for mTMPK-2A-dCD34, and 37±9 (n=5) for CD20-2A-dCD34-transduced EBV-CTLs. When the capacity to recognize and kill autologous and allogeneic LCL targets was quantified, it was shown that transduced and nontransduced EBV-CTLs have the same levels of killing efficiency at both E:T ratios analyzed, with a mean lysis of autologous LCLs at E:T ratio of 5:1 of 45% (n=5) (Fig. 3C), and a mean lysis of allogeneic LCLs at E:T ratio of 5:1 of 12% (n=5) (Fig. 3C). In an analogous way, as indicated in Figure 3D, the secretion of IFN-γ after stimulation with γ-irradiated autologous LCLs was similar between nontransduced and transduced EBV-CTLs, with a mean IFN-γ release of 1448 pg/ml±788 pg/ml (n=3) for nontransduced cells, 1525 pg/ml±517 pg/ml (n=3) for dCD34, 1743 pg/ml±657 pg/ml (n=3) for HSV-TK-2A-dCD34, 1414 pg/ml±447 pg/ml (n=3) for iCasp9-2A-dCD34, 1660 pg/ml±555 pg/ml (n=3) for mTMPK-2A-dCD34, and 1550 pg/ml±579 pg/ml (n=3) for CD20-2A-dCD34-transduced EBV-CTLs (Fig. 3D). None of the four suicide genes affected phenotype, proliferation, or function of EBV-CTLs cultures.
FIG. 3.
Suicide gene expression in EBV-CTLs does not alter their phenotype and function. (A) The expression of CD3 along with CD8, CD4, and CD56 on the surface of EBV-CTLs was evaluated 3 days after the sixth autologous LCL restimulation. Data shown are mean±SD of five separate experiments. (B) Expansion of EBV-CTLs was calculated and expressed as the fold increase in cell number at 14 days after transduction versus the day of transduction. Data shown are mean±SD of five separate experiments. (C) Cytotoxicity of EBV-CTLs against autologous LCL (left panel) and allogeneic LCL (right panel) was evaluated by a standard 4-hour 51Chr-release assay 3 days after sixth autologous LCL restimulation at E:T ratios of 20:1 and 5:1. Data shown are mean±SD of five separate experiments, respectively. (D) EBV-CTLs were stimulated with γ-irradiated autologous LCL at E:T ratio of 4:1 for 48 hours and IFN-γ release in the supernatant was detected by flow cytometry. Data shown are mean±SD of three independent experiments.
Dose-response curves for the codon-optimized sequences were determined
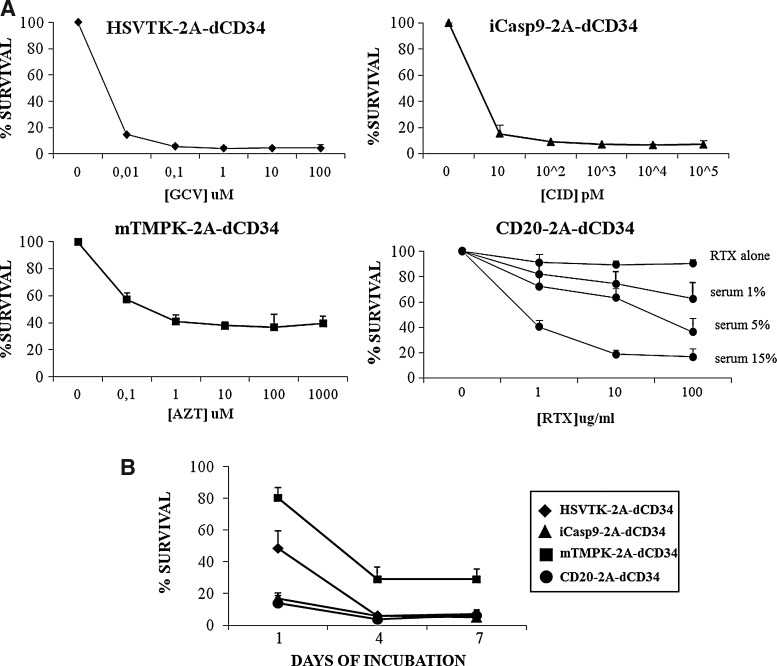
Since increased expression afforded by codon-optimization may have altered dose response, we determined the optimal concentration of each activating agent for suicide-gene triggering. We incubated suicide-gene-transduced EBV-CTLs 3 days after the sixth LCL restimulation with different doses of GCV for HSV-TK-2A-dCD34, CID for iCasp9-2A-dCD34, AZT for mTMPK-2A-dCD34, and rituximab+complement for CD20-2A-dCD34. After 1 day for iCasp9-2A-dCD34 and CD20-2A-dCD34 and 4 days for HSV-TK-2A-dCD34 and mTMPK-2A-dCD34, we have enumerated residual CD34+ cells by flow cytometry. As shown in Figure 4A, we determined optimal concentrations to be 10 μM for GCV, 10 nM for CID, 100 μM for AZT, and 10 μg/ml for rituximab+15% rabbit complement according to what was described in the literature (Rettig et al., 2003; Serafini et al., 2004; Straathof et al., 2005; Sato et al., 2007). Nontransduced and dCD34-transduced EBV-CTLs were not sensitive to any of these agents (see Supplementary Material, available online at www.liebertonline.com/hgtb).
FIG. 4.
iCasp9 and CD20 display the higher efficiency in shorter time points after treatment with their corresponding activating agent. (A) Optimal concentration of chemical inducer of dimerization (CID), rituximab + complement, ganciclovir (GCV), and azidothymidine (AZT) were determined by analyzing survival % of iCasp9-2A-dCD34, CD20-2A-dCD34, HSV-TK-2A-dCD34, and mTMPK-2A-dCD34-transduced EBV-CTLs after incubation for respectively 1 day or 4 days with increasing concentrations of the corresponding activating agent. Data shown are mean±SD of three independent experiments. (B) Suicide-gene-transduced EBV-CTLs were incubated with the corresponding activating agent at the optimal concentration determined above, and after 1 day, 4 days, or 7 days residual CD34+ cells were quantified by flow cytometry. Data shown are mean±SD of five independent experiments.
While HSV-TK, iCasp9, and CD20 are equally effective, iCasp9 and CD20 act more rapidly
Using the above concentrations of activating agents, we then evaluated the efficiency of each suicide gene at different time points (e.g., after 1 day, 4 days, or 7 days of co-incubation) by analyzing residual viable CD34+ cells using flow cytometry. To generate a convenient internal control of nontransduced cells, we diluted transduced cells 1:1 with nontransduced cells. iCasp9-2A-dCD34-transduced EBV-CTLs and CD20-2A-dCD34-expressing EBV-CTLS showed higher efficiency of cell-death induction after 1 day of incubation with the corresponding agent (Fig. 4B), with a mean survival of 15%±4% (n=5), and a mean survival of 14%±5% (n=5), compared to a mean survival of 80%±7% (n=5; p≤0.05) for mTMPK-2A-dCD34 and a mean survival of 48%±11% (n=5; p≤0.05) for HSV-TK-2A-dCD34-expressing cells. At longer time points, HSV-TK-2A-dCD34-transduced EBV-CTLs exposed to GCV display similar survival rates as iCasp9-2A-dCD34- and CD20-2A-dCD34-transduced cells, with a mean survival of 6%±1% (n=5) after 4 days, compared to a mean survival of 6%±2% (n=5) respectively for iCap9 and 4%±1% (n=5) for CD20 after 4 days; the same results were observed after 7 days (Fig. 4B). mTMPK showed significantly lower efficacy at all time points, with no more than 70% of cell-death induction in AZT-treated mTMPK-2A-dCD34-transduced cells.
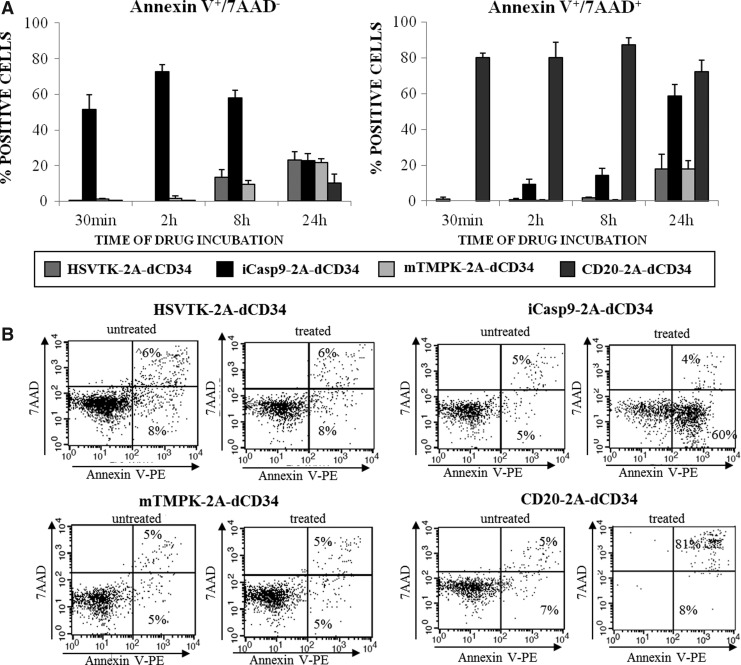
iCasp9 results in rapid Annexin-V positivity after activation, while CD20 results in rapid annexin-V/7AAD positivity
To further assess the mechanisms of cell-death triggering of each suicide gene and to compare their activity, we analyzed Annexin-V/7AAD (Schmid et al., 1992) staining after 30 minutes, 2 hours, 8 hours, and 24 hours of incubation with the corresponding activating agent. As indicated in Figure 5A and in the representative experiment shown in Figure 5B, just after 30 minutes of incubation with CID, iCasp9 expression in EBV-CTLs resulted in a high proportion of Annexin V+/7AAD- cells, with a mean % Annexin V+-7AAD- cells after CID treatment of 51%±8% (n=3) compared to a mean % of Annexin V+-7AAD- cells of 0.2% (±0.2%, n=3; p≤0.05) in GCV-treated HSV-TK-2A-dCD34 cells, 1% (±0.5%, n=3; p≤0.05) in AZT-treated-mTMPK-2A-dCD34 cells, and 0.2 % (±0.2%, n=3; p≤0.05) in rituximab+complement-treated CD20-2A-dCD34 cells. At the same time point, rituximab+complement-treated CD20-2A-dCD34-transduced EBV-CTLs resulted in even higher proportion of T cells becoming Annexin-V positive, and concomitantly 7AAD positive (mean % Annexin V+-7AAD+ cells, 80 %±2 %; n=3; p≤0.005) compared to the other suicide genes, where the rate of Annexin V+-7AAD+ cells was null. This likely represents the complement-mediated membrane perforation of CD20-expressing T cells.
FIG. 5.
iCasp9 induction by CID results in high and fast Annexin V positivity, while CD20 triggering by rituximab and complement results in rapid and strong Annexin V/7AAD positivity. Suicide-gene-transduced EBV-CTLs were treated with the corresponding activating agent at the optimal concentration determined as indicated in the text and in Figure 4A. After 30 minutes, 2 hours, 8 hours, and 24 hours, cells were stained with Annexin V and 7AAD to determine, by flow cytometric analysis, the rate of Annexin V+-7AAD- cells and Annexin V+-7AAD+ cell induction. (A) Mean±SD of three independent experiments are shown (*p≤0.05; **p≤0.005). (B) One representative experiment relative to the 30-minute time point is shown.
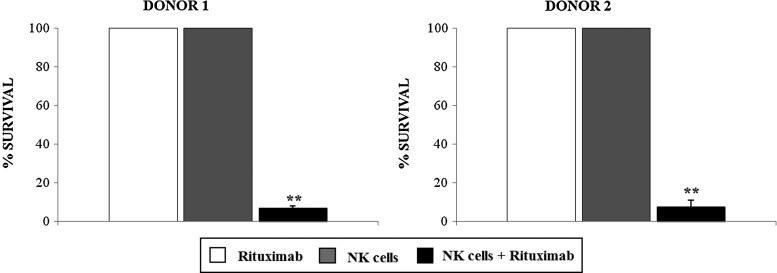
As well as complement-mediated lysis, rituximab acts by triggering antibody-dependent cellular cytotoxicity (ADCC) (Weiner et al., 2010). Hence we evaluated ADCC of CD20-2A-dCD34-expressing EBV-CTLs after incubation with autologous NK cells and rituximab, as previously described (Vogler et al., 2010). As shown in Figure 6, after 24 hours incubation with autologous NK cells at an effector:target ratio of 3:1 in the presence of 10 mg/ml rituximab, CD20 expression resulted in cell-killing equivalent to that seen with CDC: a mean survival of CD20-2A-dCD34-transduced EBV-CTLs of 7% for donor 1 and 8% for donor 2 respectively (p≤0.005, compared to treatment with NK cells alone or rituximab alone, which had no impact on CD20-2A-dCD34-expressing cell survival).
FIG. 6.
CD20-2A-dCD34-transduced EBV-CTLs could be efficiently killed by antibody-dependent cellular cytotoxicity (ADCC). Autologous natural killer (NK) cells were generated from two EBV-CTL donors, and ADCC was tested in vitro by incubating for 24 hours expanded autologous NK cells with CD20-2A-dCD34-expressing cells at an effector:target ratio of 3:1 in the presence of 10 μg/ml rituximab. Mean±SD of two independent experiments are shown (**p≤0.005).
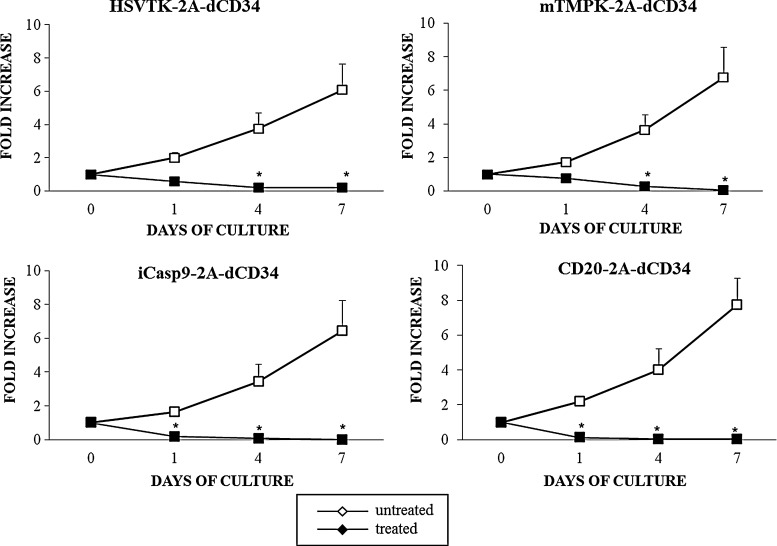
iCasp9 and CD20 are effective with only a brief exposure to the activating agent
To study if the rapid induction of Annexin-V positivity by CID and rituximab correlates with actual destruction of the suicide-gene-transduced T cells, we have exposed HSV-TK-2A-dCD34, iCasp9-2A-dCD34, mTMPK-2A-dCD34, and CD20-2A-dCD34-transduced EBV-CTLs to a single dose respectively of GCV, CID, AZT, or rituximab+complement for 24 hours. After extensive washing to remove any residual drug, we have kept cells in culture with IL-2 and restimulated them with autologous LCLs 4 days after treatment. As indicated in Figure 7, the drug treatment resulted in loss of culture for all four suicide-gene-expressing cells. Notably, iCasp9 and CD20 showed the more prominent effect only 1 day after treatment (mean fold increase, 0.2±0.04; n=5 for CID-treated iCasp9-2A-dCD34-expressing cells and mean fold increase, 0.1±0.04; n=5 for rituximab+complement-treated CD20-2A-dCD34-expressing cells compared to a mean fold increase of 0.6±0.06 (n=5; p≤0.005) for GCV-treated HSV-TK-2A-dCD34-expressing cells, and a mean fold increase of 0.8±0.09 (n=5; p≤0.005) for AZT-treated mTMPK-2A-dCD34-expressing cells.
FIG. 7.
Activation of iCasp9 and CD20 suicide genes induces the faster abrogation of in vitro expansion of EBV-CTLs. Suicide-gene-transduced EBV-CTLs were treated with the corresponding activating agent at the optimal concentration determined as indicated in the text and in Figure 4A. After 24 hours, cells were extensively washed to remove residual activating agents and kept in culture in the presence of IL-2. Four days after treatment, cells were restimulated with autologous LCLs and kept in culture in the presence of IL-2. Alive cells were counted every 3 days by Trypan-Blue exclusion, and the expansion rate of cells was calculated as the fold increase in cell number after 1, 4, and 7 days of culture vs. day of treatment. Fold increase of both treated and untreated cells are indicated. Data shown are mean±SD of five independent experiments (*p≤0.05; **p≤0.005).
Discussion
We have compared CD20, HSV-TK, mTMPK, and iCasp9 suicide-gene strategies in vitro using EBV-CTLs as a model T-cell system. After codon optimization, all suicide genes could be expressed at high levels. Expression was maintained through prolonged periods in culture. While high levels of expression are typically required for effective function, a concern with high expression is basal toxicity or alteration of T-cell function (Clackson et al., 1998). We found neither change in immunophenotype nor functionality of these cells as determined by expansion rate, capacity to kill autologous and allogeneic LCLs, and IFN-γ release after stimulation with autologous LCLs with any of the four suicide genes.
Next, we studied function of these suicide genes in response to their respective activation agent: GCV, CID, AZT, and rituximab with complement. Both iCasp9 and CD20 expression resulted in rapid activity as compared with HSV-TK and mTMPK as determined by AnnexinV/7AAD staining and the proliferative capacity of residual cells after brief exposure to activator. This is not surprising since caspase 9 directly activates the apoptosis pathway and rituximab induces direct cell lysis through complement, while HSV-TK and mTMPK both require incorporation of nucleotide analogues by DNA polymerase to act. A fast activation would be desirable in a clinical scenario where the toxicity induced by the T-cells is rapidly fatal, such as off-target effects on vital tissues like pulmonary or cardiac toxicity, or to resolve a T-cell-induced cytokine storm (Heslop, 2010).
After longer exposure to the activating agent, CD20, iCasp9, and HSV-TK all were equally efficient in destruction of expressing T cells. mTMPK however, only demonstrated a moderate activity, which we believe is not sufficient for clinical effectiveness. Codon-optimization was crucial for efficient expression and subsequent activity of CD20. Mean fluorescent intensity (MFI) of CD20 detected on EBV-CTLs transduced with our CD20-2A-dCD34 construct was initially low, before codon-optimization, and the initial results were largely disappointing (Supplementary Material), thus representing a possible explanation for the lack of efficacy registered in our experiments in the initial phase. This confirms the same finding by Grez et. al (Vogler et al., 2010). In our hands, codon optimization also contributed to a more stable and consistent expression of the dCD34 marker gene in iCasp-2A-dCD34-transduced EBV-CTLs, whether or not resulting into any improvement in either expression or function of the other suicide gene constructs (Supplementary Material).
An optimal suicide-gene strategy should be rapidly and highly effective, biologically inert, and nonimmunogenic. HSV-TK has a long heritage and considerable clinical experience with its use. However, it has well-documented immunogenicity (Berger et al., 2006), which likely limits its use to patients who are profoundly immunosuppressed. Based on the potential for immunogenicity and our data above, iCasp9 and CD20 have advantages over HSV-TK. Recent clinical data shows that iCasp9 is highly effectively in selective destruction of DLI, effectively treating GVHD in the haploidentical HSCT setting (Di Stasi et al., 2011). There is no such clinical data for the CD20 strategy, making iCasp9 the favored strategy at present. Further, use of iCasp9 relies on a small chemical dimerizer, which appears to be otherwise biologically inert, while rituximab administration would result in the concomitant depletion of normal B-cells. However, the codon-optimized CD20 strategy may be worth consideration: use of iCasp9 requires provision of a proprietary small molecule at clinical grade, while rituximab and new, more effective anti-CD20 mAbs (Alduaij and Illidge, 2011) are widely available. Further, with a decade of clinical experience with rituxumab, it is clear that B-cell depletion is very well tolerated. Finally, CD20 expression can readily be detected on the T-cell surface with simultaneous utility as marker/sort gene.
We compared four suicide-gene strategies: HSV-TK, iCasp9, mTMPK, and CD20. iCasp9 and CD20 expression could induce rapid and effective destruction of T cells. HSV-TK was equally effective, but only after longer exposure to its activator. iCasp9 is clearly the suicide gene of choice given its rapid effectiveness and recent encouraging clinical data. Codon-optimized CD20 appeared equally effective in vitro and deserves further exploration.
Supplementary Material
Acknowledgments
This work was supported by grants from: STREP 2006 (6th framework; LSHC-CT-2006-037381): “Chimaeric T cells for the treatment of paediatric cancers (Childhope),” see www.childhope.eu/; AIRC 2007 (4069): “The use of chimeric T-cell receptors (ChTCRs) for the therapy of haematological high-risk diseases”; AIRC 2007 (4636): “Childhood ALL: from clinical studies to research questions to understand molecular history and pathogenesis”; AIRC molecular clinical oncology 5 per mille, “Innate immunity in cancer. Molecular targeting and cellular therapy,” 9962; the “Progetto Integrato Oncologia 2006,” Ministero della Salute – Direzione Generale della Ricerca Scientifica e Tecnologica; the Fondazione “Matilde Tettamanti,” the “Comitato Stefano Verri” and the “Comitato Maria Letizia Verga.” A clinician-scientist fellowship from the UK Medical Research Council supported MP.
Contributions
VM, EC, AB, EB, and MP designed the research, critically analyzed the data, and wrote the article. VM, EC, ST, and IP performed the experiments. BP performed the cloning of the constructs.
Disclosure Statement
The authors reported no potential conflict of interest.
References
- Alduaij W. Illidge T.M. The future of anti-CD20 monoclonal antibodies: are we making progress? Blood. 2011;117:2993–3001. doi: 10.1182/blood-2010-07-298356. [DOI] [PubMed] [Google Scholar]
- Bendle G.M. Linnemann C. Hooijkaas A.I., et al. Lethal graft-versus-host disease in mouse models of T cell receptor gene therapy. Nat. Med. 2010;16:565–570. doi: 10.1038/nm.2128. [DOI] [PubMed] [Google Scholar]
- Berger C. Flowers M.E. Warren E.H. Riddell S.R. Analysis of transgene-specific immune responses that limit the in vivo persistence of adoptively transferred HSV-TK-modified donor T cells after allogeneic hematopoietic cell transplantation. Blood. 2006;107:2294–2302. doi: 10.1182/blood-2005-08-3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonini C. Ferrari G. Verzeletti S., et al. HSV-TK gene transfer into donor lymphocytes for control of allogeneic graft-versus-leukemia. Science. 1997;276:1719–24. doi: 10.1126/science.276.5319.1719. [DOI] [PubMed] [Google Scholar]
- Bordignon C. Bonini C. Sarina B, et al. Randomised Phase III trial of haploidentical HCT with or without an add-back strategy of HSV-TK donor lymphocytes in patients with high-risk acute leukaemia. J. Clin. Oncol. 2012;30(suppl; abstr 6541)) [Google Scholar]
- Brentjens R. Yeh R. Bernal Y., et al. Treatment of chronic lymphocytic leukemia with genetically targeted autologous T cells: case report of an unforeseen adverse event in a phase I clinical trial. Mol. Ther. 2010;18:666–668. doi: 10.1038/mt.2010.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brentjens R.J. Rivière I. Park J.H., et al. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood. 2011;118:4817–28. doi: 10.1182/blood-2011-04-348540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clackson T. Yang W. Rozamus L.W., et al. Redesigning an FKBP-ligand interface to generate chemical dimerizers with novel specificity. Proc. Natl. Acad. Sci. U.S.A. 1998;95:10437–42. doi: 10.1073/pnas.95.18.10437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Stasi A. Tey S.K. Dotti G., et al. Inducible apoptosis as a safety switch for adoptive cell therapy. N. Engl. J. Med. 2011;365:1673–83. doi: 10.1056/NEJMoa1106152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly M.L. Hughes L.E. Luke G, et al. The 'cleavage' activities of foot-and-mouth disease virus 2A site-directed mutants and naturally occurring '2A-like' sequences. J. Gen. Virol. 2001;82:1027–41. doi: 10.1099/0022-1317-82-5-1027. [DOI] [PubMed] [Google Scholar]
- Heslop H.E. Safer CARS. Mol. Ther. 2010;18:661–662. doi: 10.1038/mt.2010.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Introna M. Barbui A.M. Bambacioni F., et al. Genetic modification of human T cells with CD20: a strategy to purify and lyse transduced cells with anti-CD20 antibodies. Hum. Gene Ther. 2000;11:611–620. doi: 10.1089/10430340050015798. [DOI] [PubMed] [Google Scholar]
- Iuliucci J.D. Oliver S.D. Morley S., et al. Intravenous safety and pharmacokinetics of a novel dimerizer drug, AP1903, in healthy volunteers. J. Clin. Pharmacol. 2001;41:870–9. doi: 10.1177/00912700122010771. [DOI] [PubMed] [Google Scholar]
- Lamers C.H. Sleijfer S. Vulto A.G., et al. Treatment of metastatic renal cell carcinoma with autologous T-lymphocytes genetically retargeted against carbonic anhydrase IX: first clinical experience. J. Clin. Oncol. 2006;24:e20–2. doi: 10.1200/JCO.2006.05.9964. [DOI] [PubMed] [Google Scholar]
- Louis C.U. Savoldo B. Dotti G., et al. Antitumor activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma. Blood. 2011;118:6050–6. doi: 10.1182/blood-2011-05-354449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin V. Pizzitola I. Agostoni V., et al. Cytokine-induced killer cells for cell therapy of acute myeloid leukemia: improvement of their immune activity by expression of CD33-specific chimeric receptors. Haematologica. 2010;95:2144–52. doi: 10.3324/haematol.2010.026310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan R.A. Dudley M.E. Wunderlich J.R., et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–9. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan R.A. Yang J.C. Kitano M., et al. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol. Ther. 2010;18:843–851. doi: 10.1038/mt.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peggs K.S. Verfuerth S. Pizzey A., et al. Adoptive cellular therapy for early cytomegalovirus infection after allogeneic stem-cell transplantation with virus-specific T-cell lines. Lancet. 2003;362:13757. doi: 10.1016/S0140-6736(03)14634-X. [DOI] [PubMed] [Google Scholar]
- Porter D.L. Levine B.L. Kalos M., et al. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N. Engl. J. Med. 2011;365:725–33. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pule M. Finney H. Lawson A. Artificial T-cell receptors. Cytotherapy. 2003;5:211–26. doi: 10.1080/14653240310001488. [DOI] [PubMed] [Google Scholar]
- Pule M.A. Savoldo B. Myers G.D., et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat. Med. 2008;14:1264–70. doi: 10.1038/nm.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettig M.P. Ritchey J.K. Meyerrose T.E., et al. Transduction and selection of human T cells with novel CD34/thymidine kinase chimeric suicide genes for the treatment of graft-versus-host disease. Mol. Ther. 2003;8:29–41. doi: 10.1016/s1525-0016(03)00142-4. [DOI] [PubMed] [Google Scholar]
- Riddell S.R. Elliott M. Lewinsohn D.A., et al. T-cell mediated rejection of gene-modified HIV-specific cytotoxic T lymphocytes in HIV-infected patients. Nat. Med. 1996;2:216–23. doi: 10.1038/nm0296-216. [DOI] [PubMed] [Google Scholar]
- Rivière I. Brose K. Mulligan R.C. Effects of retroviral vector design on expression of human adenosine deaminase in murine bone marrow transplant recipients engrafted with genetically modified cells. Proc. Natl. Acad. Sci. U.S.A. 1995;92:6733–7. doi: 10.1073/pnas.92.15.6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg S.A. Cell transfer immunotherapy for metastatic solid cancer–what clinicians need to know. Nat. Rev. Clin. Oncol. 2011;8:577–85. doi: 10.1038/nrclinonc.2011.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savoldo B. Goss J. Liu Z., et al. Generation of autologous Epstein-Barr virus-specific cytotoxic T cells for adoptive immunotherapy in solid organ transplant recipients. Transplantation. 2001;72:1078–86. doi: 10.1097/00007890-200109270-00017. [DOI] [PubMed] [Google Scholar]
- Sato T. Neschadim A. Konrad M., et al. Engineered human tmpk/AZT as a novel enzyme/prodrug axis for suicide gene therapy. Mol. Ther. 2007;15:962–970. doi: 10.1038/mt.sj.6300122. [DOI] [PubMed] [Google Scholar]
- Schmid I. Uittenbogaart C.H. Krall W.J., et al. Dead cell discrimination with 7-amino-actinomycin D in combination with dual color immunofluorescence in single laser flow cytometry. Cytometry. 1992;13:204–208. doi: 10.1002/cyto.990130216. [DOI] [PubMed] [Google Scholar]
- Serafini M. Manganini M. Borleri G., et al. Characterization of CD20-transduced T lymphocytes as an alternative suicide gene therapy approach for the treatment of graft-versus-host disease. Hum. Gene Ther. 2004;15(6):3–76. doi: 10.1089/10430340460732463. [DOI] [PubMed] [Google Scholar]
- Stauss H.J. Cesco-Gaspere M. Thomas S., et al. Monoclonal T-cell receptors: new reagents for cancer therapy. Mol. Ther. 2007;15:1744–50. doi: 10.1038/sj.mt.6300216. [DOI] [PubMed] [Google Scholar]
- Straathof K.C. Pulè M.A. Yotnda P., et al. An inducible caspase 9 safety switch for T-cell therapy. Blood. 2005;105:4247–4254. doi: 10.1182/blood-2004-11-4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turcotte S. Rosenberg S.A. Immunotherapy for metastatic solid cancers. Adv. Surg. 2011;45:341–60. doi: 10.1016/j.yasu.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogler I. Newrzela S. Hartmann S., et al. An improved bicistronic CD20/tCD34 vector for efficient purification and in vivo depletion of gene-modified T cells for adoptive immunotherapy. Mol. Ther. 2010;18:1330–1338. doi: 10.1038/mt.2010.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner G.J. Rituximab: mechanism of action. Semin Hematol. 2010;47:115–23. doi: 10.1053/j.seminhematol.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.