Abstract
Introduction:
Smoking prevalence is 49% among Medicaid enrollees in Ohio. The objective of this pilot project was to test a comprehensive tobacco dependence treatment program targeting rural Medicaid-enrolled smokers for both physician-level and smoker-level outcomes.
Methods:
Using a group-randomized trial design, intervention group physicians (n = 4) were exposed to systems-level changes in their clinics, and smokers in these clinics were offered 12 weeks of telephone cessation counseling. Control group physicians (n = 4) were given the clinician’s version of the U.S. Public Health Serivce (USPHS) Clinical Practice Guideline, and smokers in these clinics were given information about the Ohio Tobacco Quitline. Physician-level and smoker-level outcomes were assessed at 1 week and 3 months, respectively. Costs per quit were estimated.
Results:
A total of 214 Medicaid smokers were enrolled. At 1 week, there were no reported differences in rates of being asked about tobacco use (68% intervention, 58% control) or advised to quit (69% intervention, 63% control). However, 30% of intervention and 56% of control smokers reported receiving a prescription for pharmacotherapy (p < .01). At 3 months, there were no differences in quit attempts (58% intervention, 64% control), use of pharmacotherapy (34% intervention, 46% control), or abstinence (24% intervention, 16% control for self-reported abstinence; 11% intervention, 3.5% control for cotinine-confirmed abstinence). The intervention group proved more cost-effective at achieving confirmed quits ($6,800 vs. $9,700).
Conclusions:
We found few differences in outcomes between physicians exposed to a brief intervention and physicians who were intensively trained. Future studies should examine how tobacco dependence treatment can be further expanded in Medicaid programs.
INTRODUCTION
The Affordable Care Act (ACA) expands Medicaid to individuals whose family incomes are up to 133% of the federal poverty level, which translates to an additional 15.9–22.8 million Medicaid recipients if all states participate (Holahan & Headen, 2010). The ACA mandates state Medicaid programs to cover tobacco dependence treatment for pregnant women and, for states that choose to expand Medicaid, include cessation pharmacotherapy options for all enrollees (Mann, 2011). These provisions could have a major impact on the rate at which tobacco dependence treatment is delivered in Medicaid programs, given the estimated 34% smoking prevalence among Medicaid enrollees (Schiller, Lucas, Ward, & Peregoy, 2012). In Ohio, the smoking prevalence among adults enrolled in Medicaid is even higher, at 49% (OMAS, 2012).
The Clinical Practice Guideline Treating Tobacco Use and Dependence recommends strategies for healthcare systems that can promote cessation of tobacco use (Fiore et al., 2008a). These strategies include, among others, implementation of tobacco user identification systems, education of physicians to deliver brief tobacco cessation counseling, provision of feedback to physicians on their performance, and inclusion of dedicated staff. While over 75% of state Medicaid programs cover cessation pharmacotherapy, less than 30% have incorporated the systems strategies (Bellows, McMenamin, & Halpin, 2007). This prescription benefit is highly underutilized, with some reports suggesting that less than 2% of enrollees have filled a prescription (Burns & Fiore, 2001). Less than half of Medicaid enrollees and only slightly over half of physicians appear to even be aware of this benefit (McMenamin, Halpin, & Bellows, 2006; McMenamin, Halpin, Ibrahim, & Orleans, 2004; Murphy, Mahoney, Hyland, Higbee, & Cummings, 2005). Compared to the general population of smokers, Medicaid smokers are less likely to have ever tried pharmacotherapy (Murphy et al., 2005), but in one study awareness of the benefit was associated with a fourfold increase in utilization (McMenamin et al., 2006).
Few studies have examined the effect of tobacco dependence treatment programs among Medicaid smokers. The objective of the current study was to test an intervention targeting both rural physicians and their Medicaid patients. Our hypothesis was that both a systems-level approach at the provider-level, and counseling at the smoker-level, would be more effective than a usual care approach at promoting brief counseling among physicians and cessation among smokers. In an exploratory aim, we examined the costs of the intervention and calculated the cost-per-quit estimates in each arm of the study. The rationale for testing a comprehensive intervention that included both system-level changes and individual-level counseling was that such a model could be incorporated into state Medicaid systems. Physicians could ask, advise, and assist smokers who are interested in quitting by writing a prescription for pharmacotherapy and referring to counseling. A second reason for testing this comprehensive intervention was that it fits with the Chronic Care Model of disease management (Wagner, 1998; Wagner, Austin, & Von Korff, 1996). Proponents of this model argue that outcomes will only improve if health care systems redesign themselves to deliver comprehensive care to patients with chronic conditions, such as tobacco dependence (Carlini, Schauer, Zbikowski, & Thompson, 2010).
METHODS
Setting
This study was conducted in the Appalachian region of Ohio. The area is largely white, rural, and experiences high rates of poverty (25% vs. 23% in Ohio), uninsured adults (16% vs. 14% in Ohio), Medicaid coverage (11% vs. 9% in Ohio), and adult smoking particularly among Medicaid enrollees (51% among Medicaid enrollees in Appalachia) (OMAS, 2012).
Participants
Both primary care physicians and Medicaid-enrolled smokers were participants in the study.
Physicians
Eight primary care clinics that had at least 100 Medicaid visits per month were invited to participate in the study. From each clinic, the physician with the highest patient volume per day was invited to enroll in the study. If he/she refused (n = 1), then another physician was selected based on their interest in participating. Of the eight eligible physicians, four were randomized to the intervention arm and four to a usual care control arm.
Smokers
From each clinic, the goal was to enroll 30 patients. A research interviewer, not affiliated with the clinics, visited the clinics on the days that Medicaid patients were on the clinic schedule to enroll the patients as they came in for their appointment. All Medicaid patients who arrived for an appointment were approached and invited to participate in the study. Eligible patients were adults age 18 years or older who reported daily smoking, were enrolled in Medicaid at the time of the appointment, and willing and able to provide consent. Motivation to quit smoking was not a criterion for enrollment, as the goal was to recruit a representative sample of Medicaid-enrolled smokers who visit their physician. Study participants completed the consent form and baseline questionnaire in the waiting room, before they saw the physician.
Procedures
Following recruitment and completion of the consent document, physician participants were given a baseline questionnaire. At the end of recruitment in their clinic, physicians completed a follow-up questionnaire. Physicians received $500 for completing the study.
Smoker participants who were consented to the study completed questionnaires at baseline and 1-week. Smokers from the intervention clinics who wished to quit were offered 12 weeks of telephone counseling by a trained Tobacco Treatment Specialist, whereas control clinic participants were encouraged to call the Ohio Tobacco Quitline if they wanted to try to quit smoking. At 3 months an interviewer, not involved in the intervention, called participants and asked questions about smoking status and use of resources to quit smoking since enrollment. Smokers received $25 for completing the baseline questionnaire, a $10 store card for completing the 1-week questionnaire, and a $20 store card for completing the 3-month questionnaire.
Intervention Arm
The intervention arm included both the systems-level recommendations from the Clinical Practice Guideline, as well as the counseling recommendation for smokers who wished to quit (Fiore et al., 2008a). The intervention had four components: (a) implementation of a user identification system; (b) physician education and feedback; (c) inclusion of dedicated clinic staff; and (d) telephone counseling for smokers who wish to make a quit attempt. No new user identification system was necessary because all clinics had one in place at the start of the study (control and intervention).
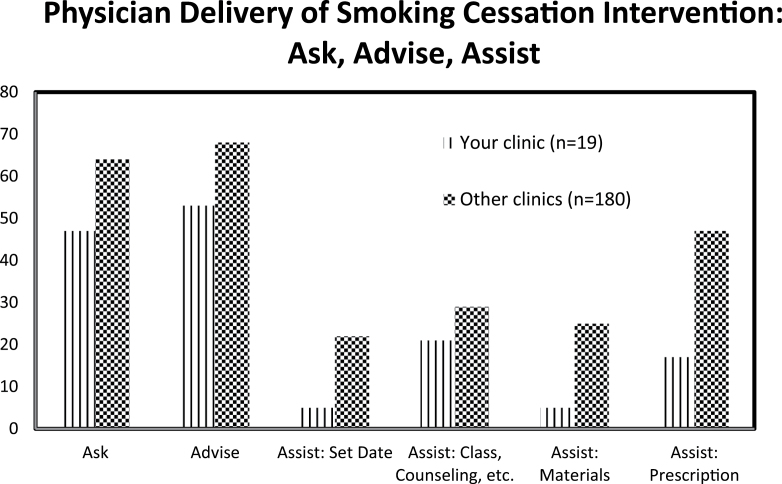
The physician education and feedback components were delivered in two parts. A physician member of the research team delivered the educational session, which was a 2-hr session created using slides and videos available from Rx for Change (2010) and the Center for Tobacco Research and Intervention (2010). The session included an introduction to the five As, a discussion of how to incorporate brief counseling into clinical practice, and a presentation of pharmacotherapy options, including information about the options covered by Ohio Medicaid. Physicians were given binders for their exam rooms that were filled with cessation-related educational handouts. Feedback was in the form of a report. After every 10 patients, physicians received a one-page document that compared their delivery of brief counseling to that of the other physicians in the study. The information for the feedback report was taken from the 1-week survey conducted with the smokers enrolled in the study. An example graph can be found in Figure 1.
Figure 1.
Example physician feedback report.
At each clinic, an “office champion” was identified and regularly engaged throughout the recruitment period in order to assure that smoking cessation remained a high priority in the clinical practice (Fiore et al., 2008a). This person was a staff member from the office, and in most cases this person was a nurse who was a strong proponent of smoking cessation.
All smokers recruited from intervention clinics were given information on the Ohio Medicaid pharmacotherapy formulary at baseline by the interviewer, following completion of the questionnaire. Also, smokers who expressed a desire to quit smoking during the 1-week call were offered 12 weeks of telephone counseling from a trained tobacco treatment specialist who was not affiliated with any of the physician offices. The nurse then scheduled the weekly calls and made the calls to participants enrolled in counseling. A tobacco dependence treatment protocol manual was followed, with topics that included self-monitoring use; side effects of pharmacotherapy; integration of cessation into daily living; coping with triggers; withdrawal; mood management; social support; and reinforcement of motivational messages.
Control Arm
Physicians in the control condition were given the USPHS Clinical Practice Guideline’s Quick Reference Guide for Physicians that included basic information on the five As as well as pharmacotherapy (Fiore et al., 2008b), information on the Ohio Tobacco Quitline, and the same one-page handout on the pharmacotherapy options available through the Medicaid formulary. Smokers in the control clinics were given a brochure on the Ohio Tobacco Quitline and information on the Medicaid pharmacotherapy formulary options by the interviewer, following completion of the questionnaire. During the 1-week call, if the participant reported receiving a prescription for pharmacotherapy, the nurse reviewed the medication (how to take it, side effects, etc.) and reminded the person to call the Ohio Tobacco Quitline for counseling.
Measures
Smoker Measures
Smokers were asked to complete questionnaires at baseline, 1 week, and 3 months post-enrollment. The baseline questionnaire included sections on demographics, health, provider interactions, and smoking history. The baseline demographic variables included gender, age, education, income, and marital status. The health measures included self-rated health, the Beck Depression Inventory (BDI) (Beck, Ward, Mendelson, Mock, & Erbaugh, 1961), and the Perceived Stress Scale (Cohen, Kamarck, & Mermelstein, 1983). The provider-related question asked whether the patient had ever been advised to quit by a healthcare provider. The smoking-related questions included the Fagerström Test of Nicotine Dependence (FTND; Heatherton, Kozlowski, Frecker, & Fagerstrom, 1991), the Decisional Balance Scale (Velicer, DiClemente, Prochaska, & Brandenburg, 1985), previous quit attempts, and a rating of the confidence in one’s ability to quit smoking (on a 1–10 scale). The decisional balance scale includes items rating both the pros and cons of smoking; the difference between the pros and cons is one’s decisional balance, with a positive score indicating more pros associated with smoking.
One week after the visit, a research nurse contacted all smokers by phone and asked questions about the baseline visit with the physician, including whether the physician: (a) asked about tobacco use; (b) advised them to quit smoking; (c) offered to help with cessation by suggesting a quit date, providing informational handouts, suggesting a call to the quit line; and (d) offered help by writing a prescription for pharmacotherapy. Participants were also asked about their motivation to quit smoking in the next 6 months.
At 3 months post-enrollment, quit attempts, use of pharmacotherapy, and abstinence were measured. Participants were asked whether they had not smoked for 24hr or more in a serious attempt to quit and whether they had used pharmacotherapy. Point prevalence abstinence was measured by asking participants if they had smoked at all in the past 7 days. All self-reported abstainers were mailed a saliva collection kit. The interviewer called these individuals to guide them through the collection process. Those who returned a sample received a $10 store card. Salivary cotinine was measured and abstainers were those with a concentration of 14ng/ml or lower. Following conventions for smoking cessation trials (Lichtenstein & Glasgow, 1992), we categorized participants as smokers if they could not be reached for the three month survey. We additionally classified non-responders as not having had a quit attempt or used pharmacotherapy. For cotinine-confirmed abstinence, self-reported quitters who failed to return a saliva sample were counted as smokers. The two participants who indicated they were using NRT at the time of the saliva collection were not included in the cotinine-confirmed analysis because we could not confirm if a positive cotinine concentration was due to NRT or due to smoking.
Statistical Analysis
This study was largely designed as a pilot test of an intervention that could be delivered to a larger number of primary care clinics. Therefore, the study was not powered to test for differences in abstinence rates; rather, the sample size was based on the quit attempts outcome. Using data from another published study, we assumed that the 24-hr quit attempt rate would be 13% in the control arm and 35% in the intervention arm (Kreuter, Chheda, & Bull, 2000). From our preliminary work we estimated that the intraclass correlation coefficient would be .03. Assuming that the loss to follow-up rate would be 10–15%, we had approximately 66% power to detect a significant difference between the two arms.
Descriptive statistics were calculated for each study arm. The intervention and control arms were compared with respect to the 1-week brief counseling and 3-month cessation-related outcomes using a mixed logistic regression model with a random effect that accounted for the clustered data at the clinic level. Variables whose distributions differed between groups at baseline were included to adjust for any confounding.
The secondary analyses included examining the effect of time on the brief counseling outcomes, the effect of motivation to quit smoking on outcomes, calls to the quit line, and abstinence rates among intervention participants who participated in telephone counseling. The data were analyzed using mixed logistic regression models containing random effects for clinic. A cost analysis was performed as well. All costs associated with the intervention were examined. The cost analysis was based on a payer perspective rather than the broader societal perspective. This payer perspective excluded costs purely attributable to the research side of the project and also excluded costs incurred by the smokers and providers participating in the intervention. The analysis included costs associated with the time to perform the counseling calls, recruitment expenditures, physician and smoker participation incentives, and pharmacotherapy costs. Time spent supervising project staff was apportioned across the intervention and control groups. Supervision costs were a necessary component of the project, but these costs are dependent on the credentials of the project manager.
RESULTS
A total of 229 smokers were approached and invited to participate in the study and 214 were enrolled (93% participation rate), with 115 in the control and 99 in the intervention arm. Two intervention clinics and one control clinic were unable to recruit the targeted 30 smokers. One-week surveys were completed on 197 participants (93% retention) and the 3-month surveys on 182 participants (85% retention).
Table 1 contains information on the characteristics of the patients enrolled in each arm. While randomization was used at the clinic level, there was obvious imbalance with respect to age, gender, Medicaid plan type, and nicotine dependence level. These variables were included in adjusted models that compared the two arms.
Table 1.
Baseline Characteristics of the 214 Individuals Who Enrolled in the Smoking Cessation Study
| Characteristic | Intervention (n = 99) | Control (n = 115) |
|---|---|---|
| Demographics | ||
| Age (mean ± SD) | 37.6±13.9 | 47.5±13.1 |
| Female gender (n (%)) | 78 (79%) | 69 (60%) |
| Education (n (%)) | ||
| Less than high school (HS) | 33 (33%) | 44 (38%) |
| HS diploma or GED | 42 (42%) | 47 (41%) |
| >HS diploma | 24 (24%) | 24 (21%) |
| White race (n (%)) | 94 (95%) | 113 (97%) |
| Marital status (n (%)) | ||
| Never married | 33 (34%) | 33 (29%) |
| Married/member of couple | 36 (37%) | 41 (36%) |
| Divorced/widowed/separated | 28 (29%) | 41 (36%) |
| Medicaid plan (n (%)) | ||
| Caresource | 34 (37%) | 42 (38%) |
| Unison | 16 (18%) | 22 (20%) |
| Molina | 37 (41%) | 26 (23%) |
| Fee for service | 4 (4%) | 21 (19%) |
| Health-related factors | ||
| BDI score (mean ± SD) | 12.9±5.9 | 14±7.4 |
| PSS score (mean ± SD) | 12±3.1 | 11.5±3.5 |
| Self-reported health fair/poor (n (%)) | 44 (45%) | 67 (58%) |
| Smoking-related factors | ||
| Fagerström score (mean ± SD) | 4.7±2.3 | 5.7±2.3 |
| Heavy smoking index (mean ± SD) | 3.2±1.5 | 3.8±1.5 |
| Decisional balance (pros − cons of smoking) (mean ± SD) | 0.9±4.4 | 0.6±4.2 |
| Ever tried to quit (n (%)) | 81 (82%) | 94 (82%) |
| Confidence in quitting smoking (1–10 scale) (mean ± SD) | 5.2±2.9 | 5.0±3.1 |
The brief counseling outcome data, measured from the 1-week surveys, are presented in Table 2. Across clinics, there were significant differences in proportion of patients who stated that the doctor asked about smoking (p = .02), prescribed pharmacotherapy (p = .01), and provided other assistance (p = .01) (data not shown). Clinic was adjusted for all comparisons using random clinic effects. Intervention and control clinics were similar with respect to percentage of smokers who reported being asked about tobacco use (68% of intervention and 58% of control) and percentage of smokers who reported being advised to quit (69% of intervention and 63% of control) but differed with respect to percentage of smokers who reported receiving a prescription for pharmacotherapy (30% of intervention and 56% of control, p < .01). This difference was largely due to the differential prescribing practices of NRT products (15% intervention vs. 42% control) rather than varenicline (10% vs. 13%) or bupropion (5% in each arm) (data not shown). In a secondary analysis, we found that these brief counseling outcomes did not change over time (p > .1) (data not shown).
Table 2.
Results From the 1-Week Questionnaire on the Physician-Level Outcomes of Brief Counseling
| Intervention (n = 88) | Control (n = 109) | Crude OR (95% CI) | Adjusted OR a (95% CI) | |
|---|---|---|---|---|
| Asked if patient smokes | 60 (68%) | 63 (58%) | 1.54 (0.50, 4.77) | 1.25 (0.45, 3.48) |
| Advised patient to quit | 61 (69%) | 69 (63%) | 1.32 (0.42, 4.10) | 1.35 (0.32, 5.78) |
| Prescribed pharmacotherapy* | 26 (30%) | 60 (56%) | 0.33 (0.16, 0.70) | 0.30 (0.12, 0.73) |
| Helped set quit date | 17 (19%) | 23 (21%) | 0.90 (0.29, 2.86) | 0.75 (0.25, 2.21) |
| Recommended counseling | 27 (31%) | 28 (26%) | 1.32 (0.45, 3.90) | 1.23 (0.45, 3.39) |
| Provided other materials | 23 (26%) | 24 (22%) | 1.13 (0.33, 3.89) | 0.72 (0.21, 2.53) |
Note. OR = odds ratio; CI = confidence interval.
aAdjusted for age, gender, Medicaid plan, baseline Fagerström score; n = 79 intervention, 103 control (102 in control for pharmacotherapy analysis).
*p ≤ .01 (unadjusted analysis); p = .02 (adjusted analysis); n = 108 in control arm.
Table 3 contains the 3-month cessation-related outcome data. At the end of the study period, 58% of intervention and 64% of control participants reported at least one quit attempt, 34% of intervention and 46% of control participants reported using pharmacotherapy, and 24% of intervention and 16% of control participants self-reported abstinence (all not significantly different). Of the self-reported abstainers, only 55% returned a saliva sample and 68% of the samples confirmed abstinence. The cotinine-confirmed abstinence rates were 11% in the intervention and 3.5% in the control groups (results not significant).
Table 3.
Results From the 3-Month Questionnaire on Smoker-Level Outcomes
| Intervention (n = 99) | Control (n = 115) | Crude OR (95% CI) | Adjusted OR a (95% CI) | |
|---|---|---|---|---|
| Attempted to quitb | 57 (57.6%) | 73 (63.5%) | 0.76 (0.22, 2.64) | 0.65 (0.23, 1.84) |
| Used pharmacotherapyb | 34 (34.3%) | 53 (46.1%) | 0.61 (0.30, 1.24) | 0.60 (0.27, 1.35) |
| Self-reported abstinenceb | 24 (24.2%) | 18 (15.7%) | 1.68 (0.63, 4.44) | 1.13 (0.38, 3.37) |
| Cotinine-confirmed abstinencec | 11 (11)% | 4 (3.5)% | 3.37 (0.64, 17.67) | d |
Note. OR = odds ratio; CI = confidence interval.
aAdjusted for age, gender, Medicaid plan, baseline Fagerström score; n = 90 intervention, 108 control.
bIndividuals who did not complete the 3-month questionnaire were assumed to have no quit attempts, to have not used pharmacotherapy, and to not be abstinent.
cTwo control participants excluded from analysis because they were on a nicotine inhaler at time of measurement.
dAdjusted analysis not performed due to the low event rate.
In a secondary analysis, we examined the relationship between motivation to quit smoking and quit attempts (the sample size was too small to examine abstinence). During the 1-week call, 48% of intervention and 43% of control participants stated that they were seriously considering quitting in the next 6 months. Among those planning to quit in 6 months, 63% and 66% had at least one quit attempt in the intervention and control arms, respectively (results not significant).
In other secondary analyses, we examined use of the quit line and abstinence rates among intervention participants who enrolled in telephone counseling. Four controls called the quit line, one of which was a self-reported (but not a confirmed) quitter. No intervention participants called the quit line. In the intervention arm, 59 participants enrolled in telephone counseling (59.5%) and they received an average of 6.5 calls (range 1–12). Of these 59, 16 (27%) quit smoking according to self-report and 9 (15%) were confirmed quitters. There appeared to be a dose-response relationship between counseling and abstinence. Based on self-reported data, the odds of quitting increased 28% per counseling call (p = .03); however, using cotinine-confirmed abstinence, the odds increased by only 20% per counseling call (p = .14).
The cost analysis suggested that while total costs for the intervention group were higher than the control group, the intervention group proved more cost effective at achieving confirmed quits. Total costs for the intervention and control groups totaled $75,000 and $58,100, respectively. These costs imply a cost per self-reported quit of $3,100 in the intervention group and $3,200 in the control group. While the two groups showed no difference in cost per self-reported quit, cost per cotinine-confirmed quit was lower for the intervention group ($6,800 per confirmed quit for intervention vs. $9,700 per confirmed quit for the control group). The higher cost effectiveness for confirmed quits is due to the greater difference between intervention and control for confirmed quits.
DISCUSSION
In this group of Medicaid smokers who were not necessarily motivated to quit, we found few differences in the outcomes between physicians who received only a brief introduction to the USPHS Clinical Practice Guideline versus physicians who received a more intensive intervention. Specifically, in both arms well over half of the smokers reported being asked about smoking, roughly two-thirds reported being advised to quit smoking, with no difference between intervention and control arms. Moreover, during the 3-month follow-up period, well over half of participants in each arm made a serious attempt to quit smoking. Perhaps a more important finding was that over 1 in 7 smokers were self-reported abstainers at 3 months. However, when we examined the continine-confirmed abstinence rate, there were fewer abstainers at 3 months.
Interestingly, while a significantly higher percentage of smokers in the control arm received a prescription for pharmacotherapy at the baseline visit, the two groups used pharmacotherapy at a similar rate during the 3-month study period. In fact, in the intervention group, while 30% reported having a prescription at the 1-week call, 34% reported using pharmacotherapy at 3 months. The reverse was true for control participants (56% at 1 week vs. 46% at 3 months). The self-reported and cotinine-confirmed abstinence rates were higher in the intervention arm, which could be attributed to the offer of 3 months of counseling by a tobacco treatment specialist. Over half of intervention clinic participants completed at least some of the counseling calls, an important finding given that these were smokers who were not recruited based on a desire to quit. Moreover, counseling was associated with an increase in self-reported abstinence. Taken together, these findings imply that pharmacotherapy alone will not promote abstinence; it is important to offer supportive counseling to improve cessation rates. This finding is consistent with the meta-analysis results reported in the Guideline, which suggest that the odds of cessation increase when counseling is added to pharmacotherapy (Fiore et al., 2008a).
It is unclear why control participants received more prescriptions for pharmacotherapy, but we offer two possible explanations. First, just prior to the start of the study, Ohio Medicaid created one formulary versus allowing each managed care plan to set its own. In this study, we clarified the options in both arms. Perhaps the control physicians were able to focus on this important information whereas the intervention physicians received a comprehensive training and did not only focus on the formulary options. Second, in the intervention we spent a lot of time discussing the importance of assessing a smoker’s willingness to quit and that physicians should not write a prescription unless the smoker was interested in quitting. While control physicians received the brief version of the Guideline, without a clear and focused explanation about why a physician should assess, it is possible that they wrote prescriptions without considering a smoker’s interest in quitting
National data suggest that 11% of Medicaid expenditures are due to smoking (Armour, Finkelstein, & Fiebelkorn, 2009). Brief tobacco dependence treatment interventions are one of the top three most valuable clinical prevention services (Maciosek et al., 2006). Solberg, Maciosek, Edwards, Khanchandani, and Goodman (2006) examined repeated tobacco-use screening and intervention and concluded that while this is a highly cost-effective clinical service, absolute cessation rates are not high because of an overall low adherence. Thus, improved adherence could lead to greater gains in cost effectiveness of repeated screening and brief intervention. Our finding of cost per self-reported quit of $3,100–$3,200 is in line with other studies that have reported costs per quit ranging from a few hundred to a few thousand dollars (Cromwell, Bartosch, Fiore, Hasselblad, & Baker, 1997; Feenstra, Hamberg-van Reenen, Hoogenveen, & Rutten-van Molken, 2005; Fiore et al., 2008a; Halpin, McMenamin, Rideout, & Boyce-Smith, 2006). The wide range in estimates is likely due to differences in smoker-level motivation, populations studied, and rigor of interventions.
With Medicaid expansion, State Medicaid programs will now have increased opportunities to provide tobacco dependence treatment. In 2009, only five states covered counseling and all recommended pharmacotherapies (MMWR, 2010). Data from Massachusetts have clearly demonstrated that counseling and pharmacotherapy can be an effective combination for Medicaid programs: smoking prevalence decreased from 38.3% to 28.3% in the 2-year period after the benefit was offered (Land et al., 2010) and for every $1 invested in the benefit, $3 was saved with a return on investment of $2 (Richard, West, & Ku, 2012). With Medicaid expansion, it will be increasingly important to examine innovative ways in which tobacco dependence treatment can be incorporated into state programs.
Our study is not without limitations. First, recruitment goals were not achieved and there was imbalance between the two arms. Second, we studied Medicaid patients and providers in the rural Appalachian region of Ohio and it is not clear if the results can be generalized to other rural areas or urban Medicaid smokers, or to non-white Medicaid smokers. Third, only about half of the self-reported quitters returned a saliva sample which greatly impacted cotinine-confirmed quit rates. Fourth, we did not collect information about why participants were at the doctor the day they enrolled in the study. Finally, the fact that control participants received a 1-week call from the nurse, and those who had received a prescription were given additional information about the medication, could have impacted the study results since this call could be considered a “counseling” call.
In conclusion, we found that exposing providers to even a brief intervention to promote adoption of tobacco dependence treatment practices results in positive outcomes among their Medicaid-enrolled smokers. Future studies should examine how tobacco dependence treatment programs, including reimbursement for counseling and coverage of pharmacotherapy, can be further expanded in state Medicaid programs. Given that Medicaid expansion will cover millions of new individuals, many of whom will be smokers, tobacco dependence programs will be an essential part of cost-savings measures adopted in state Medicaid programs.
FUNDING
This work was funded by an R21 from the National Cancer Institute (R21 CA141603-01, “Examining the effect of a provider-delivered intervention among Medicaid smokers”), by The Ohio State University Clinical and Translational Science Award (UL1RR025755 from the National Center for Research Resources), and by The Ohio State University Comprehensive Cancer Center’s Cancer Control Program Pilot Funding mechanism.
DECLARATION OF INTERESTS
None declared.
REFERENCES
- Armour B. S., Finkelstein E. A., Fiebelkorn I. C. (2009). State-level Medicaid expenditures attributable to smoking. Preventive Chronic Disease, 6, A84 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/19527585 [PMC free article] [PubMed] [Google Scholar]
- Beck A. T., Ward C. H., Mendelson M., Mock J., Erbaugh J. (1961). An inventory for measuring depression. Archives of General Psychiatry, 4, 561–571 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/13688369 [DOI] [PubMed] [Google Scholar]
- Bellows N. M., McMenamin S. B., Halpin H. A. (2007). Adoption of system strategies for tobacco cessation by state medicaid programs. Medical Care, 45, 350–356. 10.1097/01.mlr.0000254610.90363.dc [DOI] [PubMed] [Google Scholar]
- Burns M. E., Fiore M. C. (2001). Under-use of tobacco dependence treatment among Wisconsin’s fee-for-service Medicaid recipients. Wisconsin Medical Journal, 100, 54–58 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11491035 [PubMed] [Google Scholar]
- Carlini B. H., Schauer G., Zbikowski S., Thompson J. (2010). Using the chronic care model to address tobacco in health care delivery organizations: A pilot experience in Washington state. Health Promotion Practice, 11, 685–693. 10.1177/1524839908328999 [DOI] [PubMed] [Google Scholar]
- Center for Tobacco Research and Intervention. (2010). Retrieved from http://www.ctri.wisc.edu/Researchers/researchers.htm
- Cohen S., Kamarck T., Mermelstein R. (1983). A global measure of perceived stress. Journal of Health amd Social Behavior, 24, 385–396 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/6668417 [PubMed] [Google Scholar]
- Cromwell J., Bartosch W. J., Fiore M. C., Hasselblad V., Baker T. (1997). Cost-effectiveness of the clinical practice recommendations in the AHCPR guideline for smoking cessation. Agency for Health Care Policy and Research. The Journal of the American Medical Association, 278, 1759–1766 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9388153 [PubMed] [Google Scholar]
- Feenstra T. L., Hamberg-van Reenen H. H., Hoogenveen R. T., Rutten-van Molken M. P. (2005). Cost-effectiveness of face-to-face smoking cessation interventions: A dynamic modeling study. Value in Health, 8, 178–190. 10.1111/j.1524-4733.2005.04008.x [DOI] [PubMed] [Google Scholar]
- Fiore M. C., Jaén C. R., Baker T. B., Bailey W. C., Benowitz N. L., Curry S. L., … Wewers M. E. (2008a). Tobacco Use and Dependence: 2008 Update. Clinical Practice Guideline.
- Fiore M. C., Jaén C. R., Baker T. B., Bailey W. C., Benowitz N. L., Curry S. L., … Wewers M. E. (2008b). Treating Tobacco Use and Dependence: 2008 Update. Quick Reference Guide for Clinicians. Clinical Practice Guideline.
- Halpin H. A., McMenamin S. B., Rideout J., Boyce-Smith G. (2006). The costs and effectiveness of different benefit designs for treating tobacco dependence: Results from a randomized trial. Inquiry, 43, 54–65 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/16838818 [DOI] [PubMed] [Google Scholar]
- Heatherton T. F., Kozlowski L. T., Frecker R. C., Fagerstrom K. O. (1991). The Fagerstrom Test for Nicotine Dependence: A revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addiction, 86, 1119–1127 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/1932883 [DOI] [PubMed] [Google Scholar]
- Holahan J., Headen I. (May 2010). Medicaid coverage and spending in health reform: National and state‐by‐state results for adults at or below 133% FPL. The Kaiser Commission on Medicaid and the Uninsured. Retrieved January 2014, from http://kaiserfamilyfoundation.files.wordpress.com/2013/01/medicaid-coverage-and-spending-in-health-reform-national-and-state-by-state-results-for-adults-at-or-below-133-fpl.pdf
- Kreuter M. W., Chheda S. G., Bull F. C. (2000). How does physician advice influence patient behavior? Evidence for a priming effect. Archives of Family Medicine, 9, 426–433 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10810947 [DOI] [PubMed] [Google Scholar]
- Land T., Warner D., Paskowsky M., Cammaerts A., Wetherell L., Kaufmann R., … Keithly L. (2010). Medicaid coverage for tobacco dependence treatments in Massachusetts and associated decreases in smoking prevalence. PLoS ONE, 5, e9770. 10.1371/journal.pone.0009770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenstein E., Glasgow R. E. (1992). Smoking cessation: What have we learned over the past decade? Journal of Consulting and Clinical Psychology, 60, 518–527 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/1506500 [DOI] [PubMed] [Google Scholar]
- Maciosek M. V., Coffield A. B., Edwards N. M., Flottemesch T. J., Goodman M. J., Solberg L. I. (2006). Priorities among effective clinical preventive services: Results of a systematic review and analysis. American Journal of Preventive Medicine, 31, 52–61. 10.1016/j.amepre.2006.03.012 [DOI] [PubMed] [Google Scholar]
- Mann C. (2011). New Medicaid tobacco cessation services Retrieved from http://downloads.cms.gov/cmsgov/archived-downloads/SMDL/downloads/SMD11-007.pdf
- McMenamin S. B., Halpin H. A., Bellows N. M. (2006). Knowledge of Medicaid coverage and effectiveness of smoking treatments. American Journal of Preventive Medicine, 31, 369–374. 10.1016/j.amepre.2006.07.015 [DOI] [PubMed] [Google Scholar]
- McMenamin S. B., Halpin H. A., Ibrahim J. K., Orleans C. T. (2004). Physician and enrollee knowledge of Medicaid coverage for tobacco dependence treatments. American Journal of Preventive Medicine, 26, 99–104 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/14751319 [DOI] [PubMed] [Google Scholar]
- MMWR. (2010). State medicaid coverage for tobacco-dependence treatments—United States, 2009. MMWR Morbidity and Mortality Weekly Report, 59, 1340–1343 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/20966897 [PubMed] [Google Scholar]
- Murphy J. M., Mahoney M. C., Hyland A. J., Higbee C., Cummings K. M. (2005). Disparity in the use of smoking cessation pharmacotherapy among Medicaid and general population smokers. Journal of Public Health Management and Practice, 11, 341–345 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/15958934 [DOI] [PubMed] [Google Scholar]
- OMAS. (2012). Ohio Medicaid Assessment Survey 2012. Retrieved from http://grc.osu.edu/omas/Data
- Richard P., West K., Ku L. (2012). The return on investment of a Medicaid tobacco cessation program in Massachusetts. PLoS ONE, 7, e29665. 10.1371/journal.pone.0029665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rx for Change. (2010). Retrieved from http://rxforchange.ucsf.edu/
- Schiller J. S., Lucas J. W., Ward B. W., Peregoy J. A. (2012). Summary health statistics for U.S. adults: National Health Interview Survey, 2010. Vital and Health Statistics Series, 10, 1–207 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/22834228 [PubMed] [Google Scholar]
- Solberg L. I., Maciosek M. V., Edwards N. M., Khanchandani H. S., Goodman M. J. (2006). Repeated tobacco-use screening and intervention in clinical practice: Health impact and cost effectiveness. American Journal of Preventive Medicine, 31, 62–71. 000000010.1016/j.amepre.2006.03.013 [DOI] [PubMed] [Google Scholar]
- Velicer W. F., DiClemente C. C., Prochaska J. O., Brandenburg N. (1985). Decisional balance measure for assessing and predicting smoking status. Journal of Personality and Social Psychology, 48, 1279–1289 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/ 3998990 [DOI] [PubMed] [Google Scholar]
- Wagner E. H. (1998). Chronic disease management: What will it take to improve care for chronic illness? Effective Clinical Practice, 1, 2–4 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10345255 [PubMed] [Google Scholar]
- Wagner E. H., Austin B. T., Von Korff M. (1996). Organizing care for patients with chronic illness. The Milbank Quarterly, 74, 511–544 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/8941260 [PubMed] [Google Scholar]