Abstract
Introduction:
Tobacco cessation treatments have not been evaluated among Alaska Native (AN) adolescents. This pilot study evaluated the feasibility and the potential efficacy of a targeted cessation intervention for AN youth using a group randomized design.
Methods:
Eight villages in western Alaska were randomly assigned to receive the intervention (n = 4 villages) or a delayed treatment control condition (written materials only; n = 4 villages). Ten adolescents aged 12–17 years were targeted from each village with a planned enrollment of 80. The intervention was held over a weekend, and youth traveled from their villages to quit tobacco use with other teens. The intervention comprised 8hr of group-based counseling. Talking circles, personal stories from elders, and recreational activities were included to enhance cultural acceptability and participation. Newsletters were mailed weekly for 5-weeks postprogram. Assessments were conducted at baseline, week 6 (end-of-treatment), and 6 months. Self-reported tobacco abstinence was confirmed with salivary cotinine.
Results:
Recruitment targets were met in the intervention (41 enrolled) but not in control villages (27 enrolled). All intervention participants attended the weekend program. Retention was high; 98% of intervention and 86% of control participants completed 6-month follow-up. The 7-day point-prevalence self-reported tobacco abstinence rates for intervention and control participants were 10% (4/41) and 0% (0/27) at both week 6 and 6 months (p = .15). Only 1 adolescent in the intervention condition was biochemically confirmed abstinent at week 6 and none at 6 months.
Conclusion:
The intensive individual-focused intervention used in this study was feasible but not effective for tobacco cessation among AN youth. Alternative approaches are warranted.
INTRODUCTION
Reducing tobacco use among American Indian (AI) and Alaska Native (AN) youth is a national priority (Cox, Okuyemi, Choi, & Ahluwalia, 2011; Fernander, Resnicow, Viswanath, & Pérez-Stable, 2011; Fiore et al., 2008). Among AI/AN U.S. youth 12–17 years of age, the prevalence of cigarette smoking (37%) and smokeless tobacco (ST) use (17%) is highest when compared with White (22%, 12%), Hispanic (10%, 5%), Black (9%, 3%), or Asians (7%, 3%; Centers for Disease Control [CDC], 2012). Adverse health risks of cigarette smoking and ST use have been documented among adolescents including respiratory illnesses and pre-cancerous oral lesions, respectively (US Department of Health and Human Services [USDHHS], 2012).
Behavioral counseling is recommended as an evidence-based treatment for adolescent smokers (Fiore et al., 2008), but intervention reach is an issue. With some exceptions (Peterson et al., 2009), recruitment is a primary barrier to evaluation of adolescent cessation programs (Gray et al., 2011; Whittaker et al., 2011). Consequently, increasing attention has focused on identifying adolescent preferences to enhance program receptivity (Dalum, Schaalma, & Kok, 2012).
Adolescent tobacco use treatment research has not adequately addressed diverse populations or health disparities (Kong, Singh, & Krishnan-Sarin, 2012; USDHHS, 2012), and only a few pilot studies targeted AI youth (Bowen, Henderson, Harvill, & Buchwald, 2012; Horn et al., 2005; Taualii, Bush, Bowen, & Forquera, 2010). Testing effective behavioral counseling approaches in diverse adolescent populations is essential as the Food and Drug Administration has not approved the use of pharmacotherapy for tobacco cessation due to the lack of efficacy in this age group (Fiore et al., 2008).
This study builds on a successful 13-year collaboration and partnership with ANs residing in the Yukon-Kuskokwim (Y-K) Delta region of Western Alaska (see Patten, 2012 for review; Renner et al., 2013). Interventions to reduce tobacco use among pregnant women and adolescents were an important community need as indicated by AN leadership, focus groups, and individual interviews with key informants (Renner et al., 2004). Since 2000, a team of scientists and community experts has worked together to address this community need.
In the Y-K Delta region, 29% of 11–14 year olds and 63% of 15–18 year olds reported current ST use or cigarette smoking (Angstman et al., 2007). The most common form of ST used by youth and other AN people of the region is Iqmik, a mixture of tobacco leaves and fungus ash (Renner et al., 2005). The addition of ash raises the pH of the tobacco and increases the amount of free (nonionized) nicotine available for absorption (Hearn et al., 2013; Renner et al., 2005), which likely contributes to addiction. Iqmik also contains high levels of known carcinogenic tobacco-specific nitrosamines (Hearn et al., 2013). Nonetheless, Iqmik is thought to be less harmful than other forms of tobacco use because it contains mostly “natural” ingredients, for example, ash (Renner et al., 2004).
In a prior study, we developed and pretested a targeted, behavioral tobacco cessation intervention for AN youth in the region (Patten et al., 2013). This study reports on a pilot evaluation of the intervention using a group randomized design. It was hypothesized that the program would be (a) feasible as indicated by the recruitment and retention rates, and treatment acceptability ratings; and (b) associated with higher tobacco abstinence rates at 6-month follow-up compared to a control condition.
METHODS
This study was approved by the Alaska Area Institutional Review Board, the Mayo Clinic Institutional Review Board, and the Yukon-Kuskokwim Health Corporation (YKHC) Human Studies Committee and Board of Directors.
Study Setting
The Y-K Delta region in Western Alaska has a total population of 25,000. Bethel (population 6,000) is the hub of the 56 villages comprising the region. The geography and climate of the region pose severe transportation limitations. No road system connects the villages, and thus, people travel by small airplane, boat, or snow machine. Approximately, 94% of the population outside of Bethel are AN (Yupik or Cupik ethnicity), and are fairly homogenous with respect to language and culture (Alaska Humanities Forum, 2003). The Y-K Delta Regional Hospital (YKDRH) in Bethel provides health care for AN residents.
Study Design
A group randomized design was used with village as the unit of assignment. Eight villages were matched as closely as possible for village size and distance from Bethel, and randomly assigned to receive the intervention (n = 4 villages) or to a delayed treatment control condition (n = 4 villages). Population size of Y-K Delta villages ranges from 23 to 1,093 (average 386; U.S. Bureau of the Census, 2010). Village size for this pilot ranged from 418 to 1,093 (average 734). The M population size for intervention villages was 780 (SD = 298, median = 804) and 688 (SD = 100, median = 674) for control villages; p = .58. Village distance from Bethel ranges from 29 to 152 (average 93) air miles. Average distance from Bethel was 115 (SD = 40, median = 120) air miles for intervention villages and 70 (SD = 47, median = 70) for control villages; p = .20.
Study staff attempted to recruit 10 adolescent participants from each village (Lancaster, Dodd, & Williamson, 2004; Rounsaville, Carroll, & Onken, 2001) for a projected total of 80. Intervention programs were held with adolescents from two villages at a time for a projected maximum attendance of 20 youth. Intervention group size was based on feasibility considerations (e.g., staffing), and to permit full application of the program elements including group discussions and individual time with youth.
Assessments were conducted in the intervention and control villages at baseline, week-6 (end-of-treatment), and 6-months follow-up. Six months postenrollment, all participants from control villages were offered the intervention. Adolescents and their parents were told which condition their village was assigned during the consent process. Control village participants were informed they would have the opportunity to participate in the intervention after the final assessment irrespective of their tobacco use status.
Participants
All recruitment activities took place during the school year and incorporated “lessons learned” during Phase 1 of the research (Patten et al., 2013). The study coordinator traveled to each village to meet with community members, including village health clinic staff and school officials. The study coordinator contacted each school administrator to introduce the study and to offer a brief educational presentation about tobacco. One school administrator declined due to time constraints, but all others participated. Each village tribal council was also contacted to inform them about the study.
Study flyers were displayed in the village schools, health clinics, grocery stores, and community halls. Flyers included the study coordinator’s cell phone number, which allowed for receiving text messages from interested adolescents, which increased enrollment in our prior work. Moreover, teens were encouraged to quit with friends and/or siblings, and to tell others about the study.
Interested adolescents and parents met with the study coordinator at the village health clinic, where they were provided with information about the study. Written informed consent was obtained from the parent, and written assent was procured from the teen to conduct the screening procedures.
Eligibility criteria were as follows: (a) AN ethnicity; (b) between 12 and 17 years of age; (c) provided written assent; (d) parent provided written consent; (e) self-reported daily use of Iqmik, commercial ST, and/or cigarette smoking in the last 7 days with current tobacco use verified with a NicALert salivary cotinine test strip value of >0 (Cooke et al., 2008); (f) willing to make a quit attempt; and (g) had access to a working telephone. Exclusionary criteria were as follows: (a) adolescent would potentially pose harm to self or other group participants, and/or disrupt the group process based on parental/teacher/self-report or behavior at screening (i.e., intoxication); (b) Patient Health Questionnaire (PHQ-2; Kroenke, Spitzer, & Williams, 2003) score of ≥2; or (c) current (past 3 months) participation in any pharmacological or behavioral tobacco treatment.
Procedures
A culturally appropriate baseline interview, developed in prior work (Patten et al., 2009), was conducted in-person by the study coordinator in the adolescent’s village lasting about 90min. The study coordinator conducted telephone assessments, approximately 15-min, at 6-weeks, and at 6-months postenrollment. A letter was mailed reminding participants about the follow-up call. The study coordinator traveled to the village of participants reporting tobacco abstinence to collect a saliva specimen for cotinine analysis. After completing the assessment, participants received a $40 gift card at 6-weeks follow-up and a $60 gift card at 6-months follow-up.
Interventions
After the baseline assessment, adolescents in both study conditions received culturally and youth-specific, written self-help materials for quitting tobacco.
Intervention Villages
The Bethel-based group intervention program was held on a weekend with two overnight stays and scheduled within 1 month of the adolescent’s study enrollment. The study coordinator arranged the adolescents’ travel to Bethel via a small, chartered airplane, and contacted the parents and teens by mail and telephone to confirm the travel arrangements. It was expected that adolescents would stop using all forms of tobacco at the point of commencement of the program. The program and housing for the teens took place at a vocational training center that had instructional rooms and dormitories. All meals and snacks were provided.
The intervention was developed based on the literature (Fiore et al., 2008) and a social-cognitive theoretical framework (Bandura, 2004). Recruitment and retention are challenges to determining the efficacy of youth cessation interventions as described previously. Thus, to enhance program “participation” and “cultural acceptability,” focus groups were held with adolescents teen tobacco users in the region (Patten et al., 2009), and an AN teen advisory group provided feedback on the intervention (Patten et al., 2013).
The updated USPHS Clinical Practice Guideline on treatment of tobacco use concluded that behavioral counseling increases tobacco cessation among adolescents, and therefore recommended that adolescents be provided with counseling interventions to aid them in quitting (Fiore et al., 2008). The recommendations were based on an analysis of seven studies comparing counseling to usual care. The counseling content of the interventions involved efforts to enhance motivation, establish rapport, set goals, promote problem-solving and skill training, and prevent relapse. Usual care included brief advice, self-help pamphlets, reading materials, or a referral. The odds ratio for the effect of counseling versus usual care was 1.8 (95% confidence interval 1.1–3.0), with an estimated 6-month abstinence rate of 11.6% (7.5%–17.5%) versus 6.7%. Thus, the use of counseling approximately doubled the long-term abstinence rates when compared to usual care or no treatment. Consistent with a more recent meta-analysis of 24 adolescent tobacco use intervention trials (Grimshaw & Stanton, 2010), there was insufficient evidence to recommend widespread implementation of any one counseling technique (Fiore et al., 2008).
There are few trials of pharmacological interventions (nicotine replacement and bupropion) and none demonstrated effectiveness for adolescent smokers (Fiore et al., 2008; Grimshaw & Stanton, 2010). Medications were not recommended as a component of adolescent tobacco use interventions in the updated Clinical Practice Guidelines (Fiore et al., 2008) and thus were not included in our treatment developed for AN youth. Moreover, use of traditional medicines was not reported as a potentially acceptable approach to tobacco cessation in the AN community (Renner et al., 2004) and given the lack of evidence, it was not explored.
Based on preferences from AN youth (Patten et al., 2009), we chose to use a group counseling format. Duration of counseling was 8hr total. Consistent with prior counseling interventions (Fiore et al., 2008), session topics covered reasons for tobacco use, triggers to use tobacco, problem-solving skills and coping strategies, and preventing relapse. Counselors and teen advisors role-played situations involving social influence, such as when a family member or friend offers the adolescent tobacco. Each adolescent had the opportunity to observe behaviors modeled by others (attention processes) and to enact behavior (retention processes; Bandura, 2004). Volunteer speakers such as a dentist from the YKDRH talked with teens about the health effects of tobacco use. Abstinence from all tobacco products was emphasized as the treatment goal.
To enhance “cultural acceptability,” elders and AN teen advisors provided intra-treatment support for quitting and shared their personal stories with participants in the form of talking circles (Pelusi & Krebs, 2005). These individuals shared how tobacco had affected their family and community and why quitting is important. To enhance “program participation,” recreational activities such as games, basketball, and movies were provided.
After the program, as part of the intervention, teens were mailed weekly newsletters for 5 weeks. The colorful newsletters summarized what was learned during the intervention, along with featured tips for managing cravings and personal quit stories from people throughout the region.
Program Staff
Three to six counselors staffed each program, depending on the group size. Counselors were certified tobacco treatment specialists and/or individuals with a behavioral/social science degree. Counselors were trained on the manual-based intervention using didactics, role-plays, and mock sessions (Patten et al., 2013). They completed a checklist indicating topics covered for each session to document adherence.
Six AN teen advisors (three males and three females) and three elder speakers (two males and one female) from the Bethel area also attended the program. Requirements for being a teen advisor included no current tobacco, alcohol or drug use, and good standing in school. Teen advisors received training on group facilitation and techniques for supporting participants. They were given $25 gift cards for their assistance at each program.
The elders were identified by word-of-mouth. Two elders had previously used Iqmik and one had smoked cigarettes. Elders were required to undergo a criminal background check by the State of Alaska. They received a $100 honorarium for facilitating each program, and all transportation and lodging expenses were provided. Elders stayed overnight in a hotel or other location separate from the youth.
Many YKDRH employees volunteered to speak including a physician, physician assistant, substance abuse counselor, dentist, and dental hygienist. With the exception of a physician, all other YKDRH volunteer speakers were the same for all programs. Teen advisors, elders, and YKDRH volunteers were required to complete a 45-min Health Insurance Portability and Accountability Act (HIPAA) privacy and confidentiality training and pass a paper-and-pencil test covering course content.
Control Villages
No additional intervention was given beyond providing written self-help materials. However, 6 months after enrollment, all control participants were offered the intervention described previously. If the adolescent wished to participate at that time, the study coordinator obtained their written assent and written consent from a parent/guardian.
Measures
Baseline Characteristics
A baseline interview, developed in prior work (Patten et al., 2009), assessed age, ethnicity, language, education, and home restrictions on tobacco use. Adolescents were administered the Contemplation Ladder (Biener & Abrams, 1991) to assess readiness to quit; the seven-item adolescent version of the Fagerström Tolerance Questionnaire (FTQ; Cohen, Myers, & Kelly, 2002; Prokhorov et al., 2000) if they smoked cigarettes; and the nine-item FTQ-ST (Thomas et al., 2006) if they used ST.
Social Support
We used a nine-item researcher generated scale adapted from the Adolescent Social Support Scale (Harter, 1985). At baseline and week 6, adolescents were asked how much support they had received over the past month for quitting tobacco from close friends, peers, parents, siblings, other family members, elders, school principal, and their teacher. Response options were not much support (1), some support (2), or a great deal of support (3).
Feasibility Measures
Participant recruitment data included the number of subjects screened in each village, the number excluded for each of the study eligibility criteria, and the number of eligible adolescents who agreed to participate. Retention was based on the proportion of enrolled adolescents completing follow-up assessments.
Tobacco Use
Self-reported tobacco use status during the previous 7- and 30-day periods was obtained at each assessment (CDC, 2012; Mermelstein et al., 2002). At follow-up, for adolescents self-reporting no tobacco use in the past 7-day period, a saliva specimen was collected and mailed to Mayo Clinic, Rochester, MN laboratories for analysis of cotinine (Benowitz et al., 2002). Participants self-reporting no tobacco use in the last 7 days confirmed with a cotinine concentration of ≤15ng/mL (Hughes et al., 2003) were classified as nontobacco users. Also assessed was the number of days any tobacco was used during the past 30-day period (CDC, 2012; Mermelstein et al., 2002).
Treatment Use and Acceptability Measures
Attendance at the intervention program was documented by study staff. At week 6, all participants were asked the extent they had read and the helpfulness of the written materials, helpfulness of the overall program, and if they would recommend the program to another teen. Intervention village participants only were asked about the helpfulness of the mailed newsletters and the written materials provided to their parents.
Statistical Methods
Participant characteristics and outcomes were compared between intervention and control participants using generalized estimating equations to account for potential clustering within villages. Normal, binomial, and multinomial link functions were used depending on the variable being analyzed. Village characteristics were compared using a two-sample t-test (rank sum). p Values less than .05 were considered statistically significant.
Dissemination of Study Results
A power point presentation was created describing the study results and approved by the YKHC Human Studies Committee. Each of the eight villages, along with the two villages participating in the pre-testing phase (Patten et al., 2013) was mailed a paper copy of the power point presentation along with a cover letter. Study staff made up to three electronic mail and telephone contacts with the village tribal council and school administrators offering to share the results in their village.
RESULTS
Feasibility of Recruitment
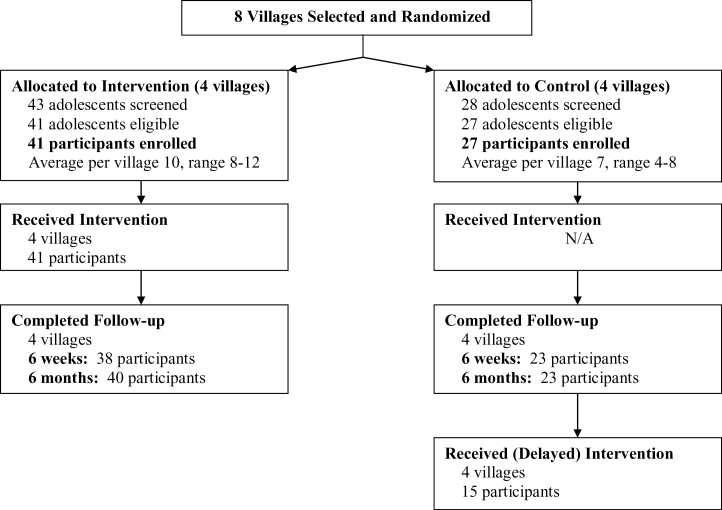
Figure 1 summarizes participant recruitment, treatment completion, and follow-up information. Of the teens screened for eligibility, 95% (41/43) from intervention villages and 96% (27/28) from control villages were eligible. Reasons for exclusion were nontobacco user, current use of pharmacological tobacco cessation treatment or PHQ-2 score >2. Of the 68 teens eligible, the enrollment rate was 100%. Recruitment took place over a total of 39 days, averaging 5 days per village. The average number of days to recruit participants in intervention villages was 4, compared to 6 days for control villages.
Figure 1.
Participant recruitment and follow-up.
Participants
Table 1 compares baseline characteristics between study groups. Participants ranged in age from 12 to 17 years and about half (53%) were female. Participants from intervention villages were significantly different from control villages on some baseline characteristics. They were more likely to be of Yupik ethnicity (p = .003), report more smokers in their home (p = .039), and to have used commercial ST in the past 30-day (p = .003) and 7-day periods (p = .002). Control village participants were also less likely to have a home smoking ban (p < .001); and among ST users, their M FTQ-ST score was lower (p = .02).
Table 1.
Participant Baseline Demographic and Tobacco Use Characteristics (N = 68)
| Intervention (N = 41) | Control (N = 27) | p value* | |
|---|---|---|---|
| n (%) | n (%) | ||
| Female gender | 19 (46.3) | 17 (63.0) | .39 |
| Age in years | |||
| M ± SD | 14.5 ± 1.4 | 14.3 ± 1.7 | .70 |
| Range | 12–17 | 12–17 | |
| Yupik ethnicity | 30 (73.2) | 27 (100.0) | .0033 |
| Cupik ethnicity | 12 (29.3) | 0 (0.0) | .0020 |
| Current or highest grade in school completed | .88 | ||
| <6th | 0 (0.0) | 5 (18.5) | |
| 6th–8th grade | 25 (61.0) | 9 (33.3) | |
| 9th–12th grade | 16 (39.0) | 13 (48.1) | |
| Yupik language | |||
| Spoken | 23 (56.1) | 22 (81.5) | .45 |
| Written | 19 (46.3) | 17 (63.0) | .48 |
| Cupik language | |||
| Spoken | 7 (17.1) | 0 (0.0) | .0234 |
| Written | 5 (12.2) | 0 (0.0) | .0594 |
| One or more smokers in the home (excluding self) | 20 (48.8) | 21 (77.8) | .039 |
| One or more chewers in the home (excluding self) | 39 (95.1) | 26 (96.3) | .80 |
| Age first tried tobacco | |||
| M ± SD | 9.8 ± 2.3 | 9.0 ± 2.8 | .32 |
| Range | 5–13 | 4–15 | |
| Current tobacco use: past 30 days | |||
| Cigarettes | 8 (19.5) | 8 (29.6) | .65 |
| Commercial chew | 10 (24.4) | 18 (66.7) | .003 |
| Iqmik | 39 (95.1) | 25 (92.6) | .44 |
| Number of days using tobacco: past 30 days | |||
| M ± SD | 27.1 ± 5.3 | 27.0 ± 4.9 | .97 |
| Range | 14–30 | 8–30 | |
| Current tobacco use: past 7 days | |||
| Cigarettes | 5 (12.2) | 5 (18.5) | .65 |
| Commercial chew | 0 (0.0) | 6 (22.2) | .0016 |
| Iqmik | 39 (95.1) | 24 (88.9) | .27 |
| Amount of tobacco used per day: past 7 days, M ± SD | |||
| Cigarettes (n = 10) | 2.6 ± 1.5 | 5.0 ± 5.6 | .12 |
| Commercial ST (n = 6) | 0 | 2.3 ± 0.8 | – |
| Iqmik (n = 63) | 5.1 ± 3.1 | 3.2 ± 1.8 | .031 |
| Prior stop attempt | 36 (87.8) | 26 (96.3) | .20 |
| Smoking ban in the home | 27 (65.9) | 3 (11.1) | <.001 |
| Iqmik use ban in the home | 2 (4.9) | 0 (0.0) | .26 |
| Contemplation ladder | .32 | ||
| 0–3 (low) | 2 (4.8) | 1 (3.7) | |
| 4–6 (medium) | 30 (73.2) | 16 (59.3) | |
| 7–10 (high) | 9 (22.0) | 10 (37.0) | |
| FTQ,a smokers only (n = 10) | .14 | ||
| M ± SD | 1.8 ± 1.1 | 2.8 ± 1.3 | |
| Range | 1–3 | 2–5 | |
| FTQ-ST,b Iqmik /ST users only (n = 65) | .02 | ||
| M ± SD | 3.6 ± 1.4 | 2.9 ± 1.1 | |
| Range | 1–7 | 1–6 | |
| Social support for quitting tobaccoc | |||
| M ± SD | 14.9 ± 3.5 | 15.2 ± 3.7 | .77 |
| Range | 9–25.0 | 9–26 | |
Note. FTQ = Fagerström Tolerance Questionnaire; FTQ-ST = Fagerström Tolerance Questionnaire-Smokeless Tobacco.
aThe FTQ has a scoring range of 0–9; scores of 6 or more indicate severe dependence.
bPotential scores range from 0 to 13 with a cutoff score of ≥6 indicating severe dependence.
cScores can range from 9 to 27.
*Generalized estimating equations to account for potential clustering within villages.
Study Retention
Ninety-three percent (38/41) of intervention and 86% (23/27) of control village participants completed the 6-week assessment (p = .32); see Figure 1. At 6 months, retention rates were 98% (40/41) and 86% (23/27), respectively, p = .056.
Treatment Use and Acceptability
All intervention participants attended the weekend program (Figure 1). Table 2 displays treatment use and acceptability ratings by study condition at the 6-week assessment. Only about half of adolescents in each study condition had read most/all of the written materials. Seventy-nine percent of intervention participants and 56% of control participants indicated they would probably or definitely recommend their respective cessation program to another teen, p = .069.
Table 2.
Treatment Use and Acceptability Ratings at End-of-Treatment (Week 6; N = 61)a
| Intervention (N = 38) | Control (N = 23) | p value* | |
|---|---|---|---|
| n (%) | n (%) | ||
| Helpfulness of program in becoming tobacco-free | .11 | ||
| Not at all helpful | 5 (13) | 4 (17) | |
| A little helpful | 10 (26) | 9 (39) | |
| Somewhat helpful | 8 (21) | 7 (30) | |
| Very helpful | 15 (39) | 3 (13) | |
| Helpfulness of program in understanding tobacco health effects | .050 | ||
| Not at all helpful | 3 (8) | 6 (26) | |
| A little helpful | 8 (21) | 6 (26) | |
| Somewhat helpful | 8 (21) | 6 (26) | |
| Very helpful | 19 (50) | 5 (22) | |
| Recommend program to another teen | .069 | ||
| Definitely would not | 0 (0) | 1 (4) | |
| Probably would not | 1 (3) | 1 (4) | |
| Maybe | 7 (18) | 8 (35) | |
| Probably | 12 (32) | 6 (26) | |
| Definitely | 18 (47) | 7 (30) | |
| Helpfulness of written materials | .069 | ||
| Not at all helpful | 4 (11) | 7 (30) | |
| Somewhat | 15 (39) | 10 (43) | |
| Could be better | 13 (34) | 0 (0) | |
| Very helpful | 6 (16) | 6 (26) | |
| Amount of written materials read | .81 | ||
| None of it | 2 (5) | 4 (17) | |
| Some of it | 14 (37) | 7 (30) | |
| Most of it | 12 (32) | 4 (17) | |
| All of it | 10 (26) | 8 (35) | |
| Intervention group only | |||
| Helpfulness of mailed newsletters | |||
| Not at all helpful | 5 (13) | N/A | – |
| A little helpful | 9 (24) | ||
| Somewhat helpful | 17 (45) | ||
| Very helpful | 7 (18) | ||
Note. a N reflects those completing the week-6 assessment.
*Generalized estimating equations to account for potential clustering within villages.
At 6-months postenrollment, all control participants were offered the intervention but only about half participated (55%; 15/27); see Figure 1.
Tobacco Use Outcomes
The 30-day point-prevalence self-reported tobacco abstinence rates for intervention and control participants were 7% (3/41) and 0% (0/27) at week 6 (p = .27); and 10% (4/41) and 0% (0/27) at 6 months (p = .15). The 7-day point-prevalence self-reported tobacco abstinence rates for intervention and control participants were 10% (4/41) and 0% (0/27) at both assessments (p = .15). Only one adolescent in the intervention condition had biochemically confirmed 7-day point-prevalence abstinence at week 6 and none at 6 months.
At week 6, two control and no intervention village participants indicated NRT use. At 6 months, one intervention but no control participants reported nicotine replacement theory (NRT) use. No other concomitant treatments for tobacco cessation were reported by any participants at either time point.
Table 3 displays change from baseline in number of days that tobacco use was reported in the past 30-day period. Intervention village participants were significantly (p < .001) more likely to report a decrease from baseline in the number of days using tobacco at week 6, but no significant differences were detected at 6 months.
Table 3.
Change From Baseline to Week-6 (End-of-Treatment) and 6-Month Follow-Up for Tobacco Use Frequency
| Measure | Baseline | Week 6 | 6 months | |||
|---|---|---|---|---|---|---|
| Intervention (N = 41) | Control (N = 27) | Intervention (N = 35) | Control (N = 23) | Intervention (N = 33) | Control (N = 18) | |
| Number of days using tobacco: past 30 days | ||||||
| M ± SD | 27.1 ± 5.3 | 27.0 ± 4.9 | 16.4 ± 10.0 | 22.8 ± 8.4 | 19.3±9.7 | 20.8±10.1 |
| Range | 14–30 | 8–30 | 2–30 | 6–30 | 1–30 | 4–30 |
| Change from baseline | – | – | −10.8 ± 9.6 | −4.3±8.1 | −7.8±9.7 | −5.6±12.2 |
| Change from baseline imputed | – | – | −11.4±10.6 | −3.6±7.6 | −8.7±10.7 | −3.7±10.3 |
Note. For each analysis at week 6, the change from baseline was significantly different between groups (p < .001 in each case). For the nonimputed analysis at week 26, there was no difference between groups (p = .30); there was also no difference for the imputed analysis at week 26 (p = .078). These analyses were performed using generalized estimating equations to account for potential clustering within villages. The baseline value for number of days using tobacco in the past 30 days is included as a covariate in the analysis.
For the days using tobacco imputed, subjects who indicated not using in the past 30 days at week 6 (3 subjects) and week 26 (4 subjects) were assigned a value of 0; subjects with missing values were assigned their baseline value.
Changes in Social Support
After controlling for the baseline score, there was no significant difference in M ± SD social support scores between the intervention (13.0±3.0) and control (13.8±4.0) conditions at week 6, p = .18.
Dissemination of Study Results
Three of the 10 villages approached requested a presentation in-person by study staff. For one village, the school administrator with whom the presentation was arranged did not show. When results were shared with the two remaining villages, many ideas were generated for future youth tobacco control efforts. Study staff met with the tribal council and school administrator in one of these villages. Ideas generated here were that enforcement of tobacco policies for youth is essential. In addition, changing social norms around tobacco use in general would be considered most helpful. Media could be used to promote youth cessation especially local radio stations and television public service announcements. There was less enthusiasm for intervening with families, but elders were seen as a potential credible source for youth. For example, media advertisements highlighting elders who could reinforce the idea those parents should not be giving youth tobacco. Also, those working in tobacco control could have a presence at ongoing community events such as cultural heritage days.
In the second village, study staff met with the entire school board via teleconference. Feedback from this village centered on the need to target younger elementary school-age children with a focus on prevention and cessation. School settings were not seen as an optimal place to intervene. Instead, focusing on the family unit was viewed as most helpful for prevention efforts, that is, targeting parents and siblings. In addition, there is an opportunity for health aides and physicians to address tobacco use among children, for example, at Well-Child Assessment visits. Moreover, it would be useful to have a wellness coach or someone not local but residing in their village that a young person could talk with. Likewise, having someone from their village trained as a wellness coach and coming back to the village might be effective.
DISCUSSION
This study piloted a new approach to tobacco cessation among AN youth and addressed an important gap in the field. Some pilot prevention/cessations studies targeted AI adolescents, but to our knowledge, no prior investigation evaluated a targeted tobacco cessation intervention for AN youth (Fiore et al., 2008; USDHHS, 2012). Strengths of this study are the intervention was based on the scientific literature for youth cessation treatment and an iterative developmental process involving substantial community input. As expected, feasibility of this approach was demonstrated by our ability to recruit participants from the intervention villages and the excellent retention rates in both study conditions. However, despite an intensive culturally appropriate intervention received by youth, contrary to our hypothesis, the tobacco abstinence rates were disappointing. Nonetheless, intervention participants were more likely to report a reduction in the frequency of their tobacco use, at least in the short term, indicating that the treatment may have enhanced receptivity for behavior change.
The results demonstrate it is feasible to conduct a group randomized trial in the community. However, as with many cluster randomized trials (Murray, Varnell, & Blitstein, 2004), recruitment targets were not reached for control villages, and it took longer to recruit those who did enroll. This was despite the fact that participants were aware they could receive the intervention after the 6-month assessment. It is possible adolescents from the control villages were less likely to enroll because of the delay in receiving an intervention. Treatment acceptability ratings at week 6 indicate the program offered to control villages (written materials only) was not likely sufficient for helping adolescents become tobacco-free. Indeed, only about half of participants in either study group reported they had read most or all of the written materials.
Nearly all participants used Iqmik and many also used some other form of tobacco (Table 1) resulting in a high-risk profile of tobacco consumption and addiction (Renner et al., 2013; Rosendahl, Galanti, & Gilljam, 2008), thus representing a challenging group from a public health perspective. There are barriers to decreasing tobacco use disparities among AN youth including inadequate social or family pressure to not use or quit (Patten et al., 2009; Renner et al., 2004). Our intervention program was primarily individual focused. Indeed, compared with the control group, the intervention did not appear to differentially alter youth perceptions of social support at the end-of-treatment. Prior research on the etiology of adolescent tobacco use including AI/AN youth (Yu, 2011) suggest that multiple domains (individual, familial, social, community) should be considered in future approaches.
From a study design perspective, the villages were well matched on population size and geographic distance from Bethel. However, future work will need to take into account other characteristics as stratification factors, such as ethnicity and types of tobacco used, because intervention and control villages differed on these factors. Another design issue is the lower rate of recruitment for control villages. Offering an active control treatment at the time of enrollment may enhance the participation rates.
Our dissemination efforts reached only two villages to obtain feedback. Although cost was prohibitive for this pilot trial, future studies might consider other dissemination strategies including media. Nonetheless, interesting insights were gained from our community discussions that could guide future youth tobacco control efforts in the region. Community-focused interventions targeting social norms, including social marketing or social network strategies, might be effective (Phua, 2013). Elders may be credible spokespersons for such efforts. Although opinions on the potential role of family members were mixed, there is growing evidence on the use of family-based programs to prevent tobacco initiation among children and adolescents (Nilsson, Stenlund, Bergstrom, Weinehall, & Janlert, 2006; Rosen et al., 2011; Thomas, Baker, & Lorenzetti, 2007). Other possible approaches are health coaching for prevention and cessation (Werch et al., 2011), and beginning efforts at the elementary school-age to prevent initiation and/or progression to nicotine dependence. Future studies could also measure AN culture-specific protective factors as potential outcomes such as, the degree of connectedness with one’s family, community, and natural environment (Mohatt, Fok, Burket, Henry, & Allen, 2011).
FUNDING
This research was supported by the National Institutes of Health and National Institute on Drug Abuse (R01 DA025156 to CAP).
DECLARATION OF INTERESTS
None declared.
ACKNOWLEDGMENTS
MH is now with the Department of Psychiatry and Psychology and the Behavioral Health Research Program, Mayo Clinic. The authors would like to acknowledge the contributions of the elders, teen advisors, and numerous YKDRH employee volunteers who shared information and their personal stories and experiences with the youth participants. The team also acknowledges Dr. J. Klejka, Mr. G. Peltola, and the YKHC Board for their continued support of the team’s work on reducing tobacco use in the region.
REFERENCES
- Alaska Humanities Forum. (2003). A survey on native perspectives on Alaska issues. Educational bulletin: Smoking cessation during pregnancy No. 260. Anchorage, AK: Retrieved from www.firstalaskans.org/index.cfm?fa=documents_overview&doctype=30 [Google Scholar]
- Angstman S., Patten C. A., Renner C. C., Simon A., Thomas J. L., Hurt R. D, … Offord K. P. (2007). Tobacco and other substance use among Alaska Native youth in western Alaska. American Journal of Health Behavior, 31, 249–260 [DOI] [PubMed] [Google Scholar]
- Bandura A. (2004). Health promotion by social cognitive means. Health Education & Behavior, 31, 143–164. 10.1177/1090198104263660 [DOI] [PubMed] [Google Scholar]
- Benowitz N. L., Ahijevych K., Jarvis M., Hall S., LeHouezec J., Hansson A, … Velicer W. F.(2002). Biochemical verification of tobacco use and cessation. Nicotine & Tobacco Research, 4, 149–159. 10.1177/109019810426366012028847 [Google Scholar]
- Biener L., Abrams D. B. (1991). The Contemplation Ladder: Validation of a measure of readiness to consider smoking cessation. Health Psychology, 10, 360–365 [DOI] [PubMed] [Google Scholar]
- Bowen D. J., Henderson P. N., Harvill J., Buchwald D. (2012). Short-term effects of a smoking prevention website in American Indian youth. Journal of Medical Internet Research, 14, e81. 10.2196/jmir.1682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control (CDC). (2012). Youth online: High school 2009 Youth Risk Behavioral Survey data. Retrieved from http://apps.nccd.cdc.gov/youthonline/app/Default.aspx
- Cohen L. M., Myers M. G., Kelly J. F. (2002). Assessment of nicotine dependence among substance abusing adolescent smokers: A comparison of the DSM-IV criteria and the modified Fagerström Tolerance Questionnaire. Journal of Psychopathology and Behavioral Assessment, 24, 225–233 [Google Scholar]
- Cooke F., Bullen C., Whittaker R., McRobbie H., Chen M. H., Walker N. (2008). Diagnostic accuracy of NicAlert cotinine test strips in saliva for verifying smoking status. Nicotine & Tobacco Research, 10, 607–612. 10.1080/14622200801978680 [DOI] [PubMed] [Google Scholar]
- Cox L. S., Okuyemi K., Choi W. S., Ahluwalia J. S. (2011). A review of tobacco use treatments in U.S. ethnic minority populations. American Journal of Health Promotion, 25, S11–S30. 10.4278/ajhp.100610-LIT-177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalum P., Schaalma H., Kok G. (2012). The development of an adolescent smoking cessation intervention–an Intervention Mapping approach to planning. Health Education Research, 27, 172–181. 10.1093/her/cyr044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernander A., Resnicow K., Viswanath K., Pérez-Stable E. J. (2011). Cigarette smoking interventions among diverse populations. American Journal of Health Promotion, 25, S1–S4. 10.4278/ajhp.25.5.c1 [DOI] [PubMed] [Google Scholar]
- Fiore M. C., Jaen C. R., Baker T. B., Bailey W. C., Benowitz N., Curry S. J. (2008). Treating tobacco use and dependence: 2008 update. Clinical Practice Guideline. Rockville, MD: U.S. Department of Health and Human Services and Public Health Service [Google Scholar]
- Gray K. M., Carpenter M. J., Baker N. L., Hartwell K. J., Lewis A. L., Hiott D. W, … Upadhyaya H. P. (2011). Bupropion SR and contingency management for adolescent smoking cessation. Journal of Substance Abuse Treatment, 40, 77–86. 10.1016/j.jsat.2010.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimshaw G., Stanton A. (2010). Tobacco cessation interventions for young people. Cochrane Database of Systematic Reviews, 1, 1–58 [Google Scholar]
- Harter S. (1985). Manual for the social support scale for children. Denver: University of Denver Press [Google Scholar]
- Hearn B. A., Renner C. C., Ding Y. S., Vaughan-Watson C., Stanfill S. B., Zhang L, … Watson C. H. (2013). Chemical analysis of Alaskan Iq’mik smokeless tobacco. Nicotine & Tobacco Research, 15, 1283–1288. 10.1177/1090198104263660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn K., McGloin T., Dino G., Manzo K., McCracken L., Shorty L, … Noerachmanto N. (2005). Quit and reduction rates for a pilot study of the American Indian Not On Tobacco (N-O-T) program. Preventing Chronic Disease, 2, A13 Retrieved from www.cdc/gov/issues/2005/oct/05_0001.htm [PMC free article] [PubMed] [Google Scholar]
- Hughes J. R., Keely J. P., Niaura R. S., Ossip-Klein D. J., Richmond R. L., Swan G. E. (2003). Measures of abstinence in clinical trials: Issues and recommendations. Nicotine & Tobacco Research, 5, 13–25. 10.1177/1090198104263660 [PubMed] [Google Scholar]
- Kong G., Singh N., Krishnan-Sarin S. (2012). A review of culturally targeted/tailored tobacco prevention and cessation interventions for minority adolescents. Nicotine & Tobacco Research, 14, 1394–1406. 10.1093/ntr/nts118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke K., Spitzer R. L., Williams J. B. (2003). The Patient Health Questionnaire-2: Validity of a two-item depression screener. Medical Care, 41, 1284–1292. 10.1097/01.MLR.0000093487.78664.3C [DOI] [PubMed] [Google Scholar]
- Lancaster G. A., Dodd S., Williamson P. R. (2004). Design and analysis of pilot studies: Recommendations for good practice. Journal of Evaluation in Clinical Practice, 10, 307–312. 10.1111/j.2002.384.doc.x [DOI] [PubMed] [Google Scholar]
- Mermelstein R., Colby S. M., Patten C., Prokhorov A., Brown R., Myers M, … McDonald P. (2002). Methodological issues in measuring treatment outcome in adolescent smoking cessation studies. Nicotine & Tobacco Research, 4, 395–403. 10.1080/1462220021000018470 [DOI] [PubMed] [Google Scholar]
- Mohatt N. V., Fok C. C., Burket R., Henry D., Allen J. (2011). Assessment of awareness of connectedness as a culturally-based protective factor for Alaska Native youth. Cultural Diversity & Ethnic Minority Psychology, 17, 444–455. 10.1037/a0025456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray D. M., Varnell S. P., Blitstein J. L. (2004). Design and analysis of group-randomized trials: A review of recent methodological developments. American Journal of Public Health, 94, 423–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson M., Stenlund H., Bergström E., Weinehall L., Janlert U. (2006). It takes two: Reducing adolescent smoking uptake through sustainable adolescent-adult partnership. The Journal of Adolescent Health, 39, 880–886. 10.1016/j.jadohealth.2006.07.004 [DOI] [PubMed] [Google Scholar]
- Patten C. A. (2012). Tobacco cessation intervention during pregnancy among Alaska Native women. Journal of Cancer Education, 27, S86–S90. 10.1007/s13187-012-0317-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patten C. A., Enoch C., Renner C. C., Offord K. P., Nevak C., Kelley S, … Kaur J. (2009). Focus groups of Alaska Native adolescent tobacco users: Preferences for tobacco cessation interventions and barriers to participation. Health Education & Behavior, 36, 711–723. 10.1177/1090198104263660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patten C. A., Fadahunsi O., Hanza M., Smith C. M., Hughes C. A., Brockman T. A, … Offord K. (2013). Development of a tobacco cessation intervention for Alaska Native youth. Addiction Research & Theory, 21, 273–284. 10.1177/1090198104263660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelusi J., Krebs L. U. (2005). Understanding cancer: Understanding the stories of life and living. Journal of Cancer Education, 20, 12–16 [DOI] [PubMed] [Google Scholar]
- Peterson A. V., Jr, Kealey K. A., Mann S. L., Marek P. M., Ludman E. J., Liu J., Bricker J. B. (2009). Group-randomized trial of a proactive, personalized telephone counseling intervention for adolescent smoking cessation. Journal of the National Cancer Institute, 101, 1378–1392. 10.1093/jnci/djp317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phua J. J. (2013). The reference group perspective for smoking cessation: An examination of the influence of social norms and social identification with reference groups on smoking cessation self-efficacy. Psychology of Addictive Behaviors, 27, 102–112. 10.1037/a0029130 [DOI] [PubMed] [Google Scholar]
- Prokhorov A. V., De Moor C., Pallonen U. E., Hudmon K. S., Koehly L., Hu S. (2000). Validation of the modified Fagerström tolerance questionnaire with salivary cotinine among adolescents. Addictive Behaviors, 25, 429–433 [DOI] [PubMed] [Google Scholar]
- Renner C. C., Enoch C., Patten C. A., Ebbert J. O., Hurt R. D., Moyer T. P., Provost E. M.(2005). Iqmik: A form of smokeless tobacco used among Alaska natives. American Journal of Health Behavior, 29, 588–594 [DOI] [PubMed] [Google Scholar]
- Renner C. C., Lanier A. P., Lindgren B., Jensen J., Patten C. A., Parascandola M, … Hatsukami D. K.(2013). Tobacco use among southwestern Alaska Native people. Nicotine & Tobacco Research, 15, 401–406. 10.1093/ntr/nts137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renner C. C., Patten C. A., Enoch C., Petraitis J., Offord K. P., Angstman S, … Hurt R. D. (2004). Focus groups of Y-K Delta Alaska Natives: Attitudes toward tobacco use and tobacco dependence interventions. Preventive Medicine, 38, 421–431. 10.1177/1090198104263660 [DOI] [PubMed] [Google Scholar]
- Rosen L. J., Guttman N., Hovell M. F., Noach M. B., Winickoff J. P., Tchernokovski S, … Zucker D. M. (2011). Development, design, and conceptual issues of project zero exposure: A program to protect young children from tobacco smoke exposure. BMC Public Health, 11, 508. 10.1186/1471-2458-11-508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosendahl K. I., Galanti M. R., Gilljam H. (2008). Trajectories of smokeless tobacco use and of cigarette smoking in a cohort of Swedish adolescents: Differences and implications. Nicotine & Tobacco Research, 10, 1021–1027. 10.1080/14622200802097522 [DOI] [PubMed] [Google Scholar]
- Rounsaville B. J., Carroll K. M., Onken L. S. (2001). A stage model of behavioral therapies research: Getting started and moving on from stage I. Clinical Psychology: Science and Practice, 8, 133–142 [Google Scholar]
- Taualii M., Bush N., Bowen D. J., Forquera R. (2010). Adaptation of a smoking cessation and prevention website for urban American Indian/Alaska Native youth. Journal of Cancer Education, 25, 23–31. 10.1007/s13187-009-0004-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J. L., Ebbert J. O., Patten C. A., Dale L. C., Bronars C. A., Schroeder D. R. (2006). Measuring nicotine dependence among smokeless tobacco users. Addictive Behaviors, 31, 1511–1521. 10.1177/1090198104263660 [DOI] [PubMed] [Google Scholar]
- Thomas R. E., Baker P. R. A., Lorenzetti D. (2007). Family-based programmes for preventing smoking by children and adolescents; Art No: CD004493. Cochrane Database of Systematic Reviews. 10.1002/14651858.CD004493,pub2 [DOI] [PubMed] [Google Scholar]
- U.S. Bureau of the Census. (2010). Population census 2010. Retrieved from http://www.census.gov
- U.S. Department of Health and Human Services (USDHHS). (2012). Preventing tobacco use among youth and young adults: A report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office of Smoking and Health [Google Scholar]
- Werch C. E., Bian H., Carlson J. M., Moore M. J., Diclemente C. C., Huang I. C, … Pokorny S. B. (2011). Brief integrative multiple behavior intervention effects and mediators for adolescents. Journal of Behavioral Medicine, 34, 3–12. 10.1007/s10865-010-9281-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittaker R., Dorey E., Bramley D., Bullen C., Denny S., Elley C. R, … Salmon P. (2011). A theory-based video messaging mobile phone intervention for smoking cessation: Randomized controlled trial. Journal of Medical Internet Research, 13, e10. 10.2196/jmir.1553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M. (2011). Tobacco use among American Indian or Alaska Native middle- and high-school students in the United States. Nicotine & Tobacco Research, 13, 173–181. 10.1093/ntr/ntq233 [DOI] [PubMed] [Google Scholar]