Abstract
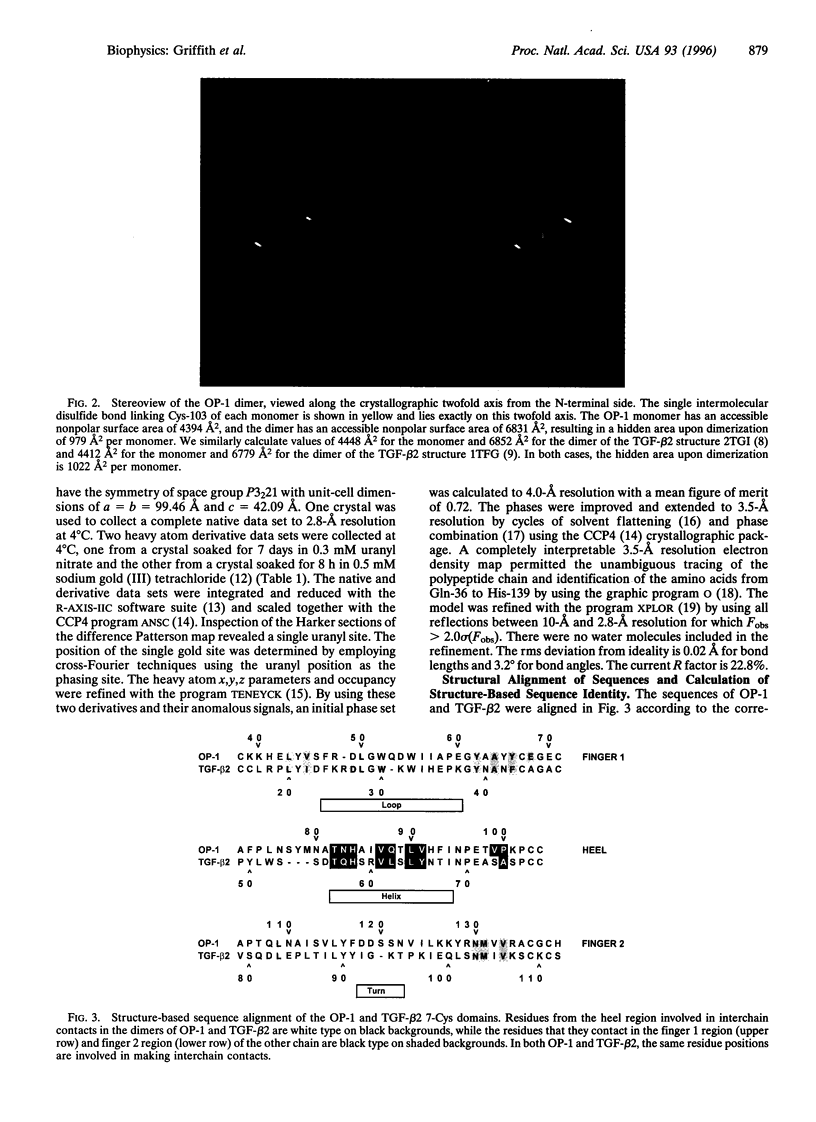
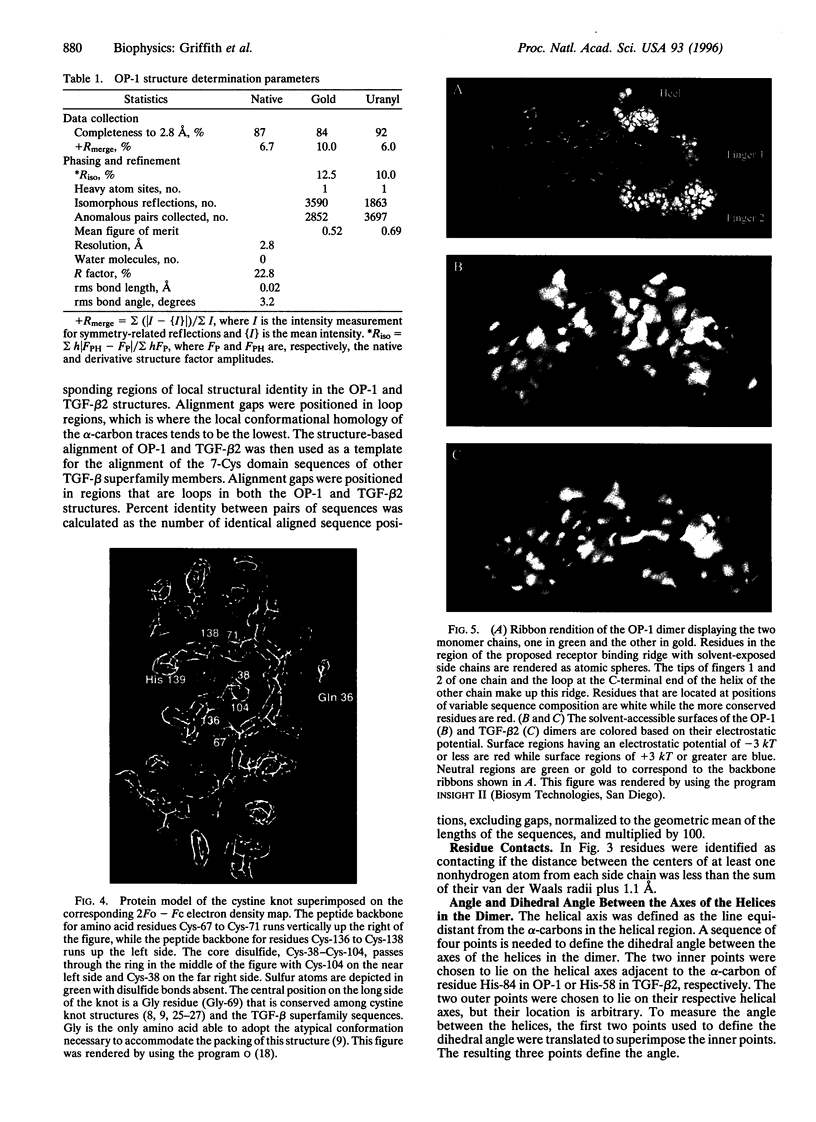
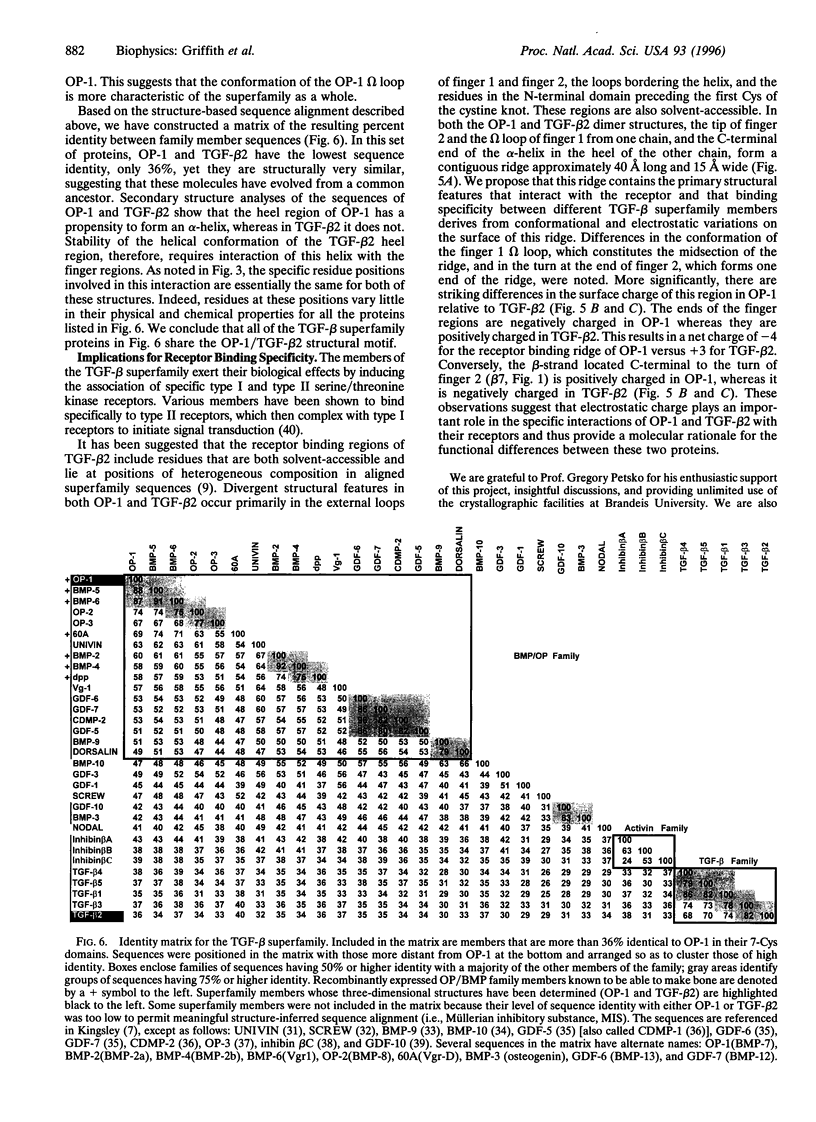
We report the three-dimensional structure of osteogenic protein 1 (OP-1, also known as bone morphogenetic protein 7) to 2.8-A resolution. OP-1 is a member of the transforming growth factor beta (TGF-beta) superfamily of proteins and is able to induce new bone formation in vivo. Members of this superfamily share sequence similarity in their C-terminal regions and are implicated in embryonic development and adult tissue repair. Our crystal structure makes possible the structural comparison between two members of the TGF-beta superfamily. We find that although there is limited sequence identity between OP-1 and TGF-beta 2, they share a common polypeptide fold. These results establish a basis for proposing the OP-1/TGF-beta 2 fold as the primary structural motif for the TGF-beta superfamily as a whole. Detailed comparison of the OP-1 and TGF-beta 2 structures has revealed striking differences that provide insights into how these growth factors interact with their receptors.
Full text
PDF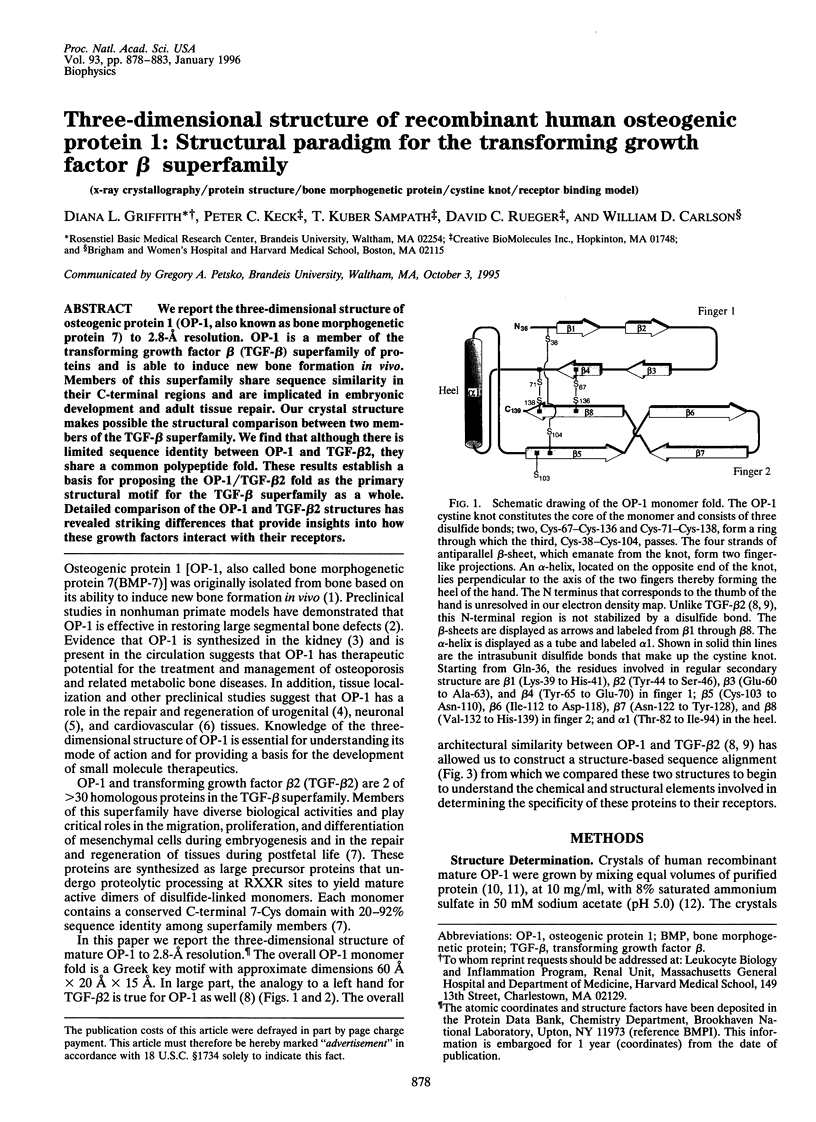


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arora K., Levine M. S., O'Connor M. B. The screw gene encodes a ubiquitously expressed member of the TGF-beta family required for specification of dorsal cell fates in the Drosophila embryo. Genes Dev. 1994 Nov 1;8(21):2588–2601. doi: 10.1101/gad.8.21.2588. [DOI] [PubMed] [Google Scholar]
- Bernstein F. C., Koetzle T. F., Williams G. J., Meyer E. F., Jr, Brice M. D., Rodgers J. R., Kennard O., Shimanouchi T., Tasumi M. The Protein Data Bank: a computer-based archival file for macromolecular structures. J Mol Biol. 1977 May 25;112(3):535–542. doi: 10.1016/s0022-2836(77)80200-3. [DOI] [PubMed] [Google Scholar]
- Brünger A. T., Kuriyan J., Karplus M. Crystallographic R factor refinement by molecular dynamics. Science. 1987 Jan 23;235(4787):458–460. doi: 10.1126/science.235.4787.458. [DOI] [PubMed] [Google Scholar]
- Chang S. C., Hoang B., Thomas J. T., Vukicevic S., Luyten F. P., Ryba N. J., Kozak C. A., Reddi A. H., Moos M., Jr Cartilage-derived morphogenetic proteins. New members of the transforming growth factor-beta superfamily predominantly expressed in long bones during human embryonic development. J Biol Chem. 1994 Nov 11;269(45):28227–28234. [PubMed] [Google Scholar]
- Cook S. D., Wolfe M. W., Salkeld S. L., Rueger D. C. Effect of recombinant human osteogenic protein-1 on healing of segmental defects in non-human primates. J Bone Joint Surg Am. 1995 May;77(5):734–750. doi: 10.2106/00004623-199505000-00010. [DOI] [PubMed] [Google Scholar]
- Cunningham N. S., Jenkins N. A., Gilbert D. J., Copeland N. G., Reddi A. H., Lee S. J. Growth/differentiation factor-10: a new member of the transforming growth factor-beta superfamily related to bone morphogenetic protein-3. Growth Factors. 1995;12(2):99–109. doi: 10.3109/08977199509028956. [DOI] [PubMed] [Google Scholar]
- Daopin S., Piez K. A., Ogawa Y., Davies D. R. Crystal structure of transforming growth factor-beta 2: an unusual fold for the superfamily. Science. 1992 Jul 17;257(5068):369–373. doi: 10.1126/science.1631557. [DOI] [PubMed] [Google Scholar]
- Gilson M. K., Honig B. H. Calculation of electrostatic potentials in an enzyme active site. Nature. 1987 Nov 5;330(6143):84–86. doi: 10.1038/330084a0. [DOI] [PubMed] [Google Scholar]
- Griffith D. L., Oppermann H., Rueger D. C., Sampath T. K., Tucker R. F., Carlson W. D. Crystallization and preliminary crystallographic data of recombinant human osteogenic protein-1 (hOP-1). J Mol Biol. 1994 Dec 16;244(5):657–658. doi: 10.1006/jmbi.1994.1761. [DOI] [PubMed] [Google Scholar]
- H tten G., Neidhardt H., Schneider C., Pohl J. Cloning of a new member of the TGF-beta family: a putative new activin beta C chain. Biochem Biophys Res Commun. 1995 Jan 17;206(2):608–613. doi: 10.1006/bbrc.1995.1086. [DOI] [PubMed] [Google Scholar]
- Helder M. N., Ozkaynak E., Sampath K. T., Luyten F. P., Latin V., Oppermann H., Vukicevic S. Expression pattern of osteogenic protein-1 (bone morphogenetic protein-7) in human and mouse development. J Histochem Cytochem. 1995 Oct;43(10):1035–1044. doi: 10.1177/43.10.7560881. [DOI] [PubMed] [Google Scholar]
- Jones T. A., Zou J. Y., Cowan S. W., Kjeldgaard M. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr A. 1991 Mar 1;47(Pt 2):110–119. doi: 10.1107/s0108767390010224. [DOI] [PubMed] [Google Scholar]
- Kingsley D. M. The TGF-beta superfamily: new members, new receptors, and new genetic tests of function in different organisms. Genes Dev. 1994 Jan;8(2):133–146. doi: 10.1101/gad.8.2.133. [DOI] [PubMed] [Google Scholar]
- Lapthorn A. J., Harris D. C., Littlejohn A., Lustbader J. W., Canfield R. E., Machin K. J., Morgan F. J., Isaacs N. W. Crystal structure of human chorionic gonadotropin. Nature. 1994 Jun 9;369(6480):455–461. doi: 10.1038/369455a0. [DOI] [PubMed] [Google Scholar]
- Lee B., Richards F. M. The interpretation of protein structures: estimation of static accessibility. J Mol Biol. 1971 Feb 14;55(3):379–400. doi: 10.1016/0022-2836(71)90324-x. [DOI] [PubMed] [Google Scholar]
- Lefer A. M., Tsao P. S., Ma X. L., Sampath T. K. Anti-ischaemic and endothelial protective actions of recombinant human osteogenic protein (hOP-1). J Mol Cell Cardiol. 1992 Jun;24(6):585–593. doi: 10.1016/0022-2828(92)91043-5. [DOI] [PubMed] [Google Scholar]
- Leszczynski J. F., Rose G. D. Loops in globular proteins: a novel category of secondary structure. Science. 1986 Nov 14;234(4778):849–855. doi: 10.1126/science.3775366. [DOI] [PubMed] [Google Scholar]
- Massagué J., Attisano L., Wrana J. L. The TGF-beta family and its composite receptors. Trends Cell Biol. 1994 May;4(5):172–178. doi: 10.1016/0962-8924(94)90202-x. [DOI] [PubMed] [Google Scholar]
- McDonald N. Q., Hendrickson W. A. A structural superfamily of growth factors containing a cystine knot motif. Cell. 1993 May 7;73(3):421–424. doi: 10.1016/0092-8674(93)90127-c. [DOI] [PubMed] [Google Scholar]
- McDonald N. Q., Lapatto R., Murray-Rust J., Gunning J., Wlodawer A., Blundell T. L. New protein fold revealed by a 2.3-A resolution crystal structure of nerve growth factor. Nature. 1991 Dec 5;354(6352):411–414. doi: 10.1038/354411a0. [DOI] [PubMed] [Google Scholar]
- Oefner C., D'Arcy A., Winkler F. K., Eggimann B., Hosang M. Crystal structure of human platelet-derived growth factor BB. EMBO J. 1992 Nov;11(11):3921–3926. doi: 10.1002/j.1460-2075.1992.tb05485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozkaynak E., Rueger D. C., Drier E. A., Corbett C., Ridge R. J., Sampath T. K., Oppermann H. OP-1 cDNA encodes an osteogenic protein in the TGF-beta family. EMBO J. 1990 Jul;9(7):2085–2093. doi: 10.1002/j.1460-2075.1990.tb07376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozkaynak E., Schnegelsberg P. N., Oppermann H. Murine osteogenic protein (OP-1): high levels of mRNA in kidney. Biochem Biophys Res Commun. 1991 Aug 30;179(1):116–123. doi: 10.1016/0006-291x(91)91342-a. [DOI] [PubMed] [Google Scholar]
- Perides G., Jensen F. E., Edgecomb P., Rueger D. C., Charness M. E. Neuroprotective effect of human osteogenic protein-1 in a rat model of cerebral hypoxia/ischemia. Neurosci Lett. 1995 Feb 24;187(1):21–24. doi: 10.1016/0304-3940(95)11327-s. [DOI] [PubMed] [Google Scholar]
- Sampath T. K., Coughlin J. E., Whetstone R. M., Banach D., Corbett C., Ridge R. J., Ozkaynak E., Oppermann H., Rueger D. C. Bovine osteogenic protein is composed of dimers of OP-1 and BMP-2A, two members of the transforming growth factor-beta superfamily. J Biol Chem. 1990 Aug 5;265(22):13198–13205. [PubMed] [Google Scholar]
- Sampath T. K., Maliakal J. C., Hauschka P. V., Jones W. K., Sasak H., Tucker R. F., White K. H., Coughlin J. E., Tucker M. M., Pang R. H. Recombinant human osteogenic protein-1 (hOP-1) induces new bone formation in vivo with a specific activity comparable with natural bovine osteogenic protein and stimulates osteoblast proliferation and differentiation in vitro. J Biol Chem. 1992 Oct 5;267(28):20352–20362. [PubMed] [Google Scholar]
- Schlunegger M. P., Grütter M. G. An unusual feature revealed by the crystal structure at 2.2 A resolution of human transforming growth factor-beta 2. Nature. 1992 Jul 30;358(6385):430–434. doi: 10.1038/358430a0. [DOI] [PubMed] [Google Scholar]
- Sibanda B. L., Thornton J. M. Conformation of beta hairpins in protein structures: classification and diversity in homologous structures. Methods Enzymol. 1991;202:59–82. doi: 10.1016/0076-6879(91)02007-v. [DOI] [PubMed] [Google Scholar]
- Stenzel P., Angerer L. M., Smith B. J., Angerer R. C., Vale W. W. The univin gene encodes a member of the transforming growth factor-beta superfamily with restricted expression in the sea urchin embryo. Dev Biol. 1994 Nov;166(1):149–158. doi: 10.1006/dbio.1994.1303. [DOI] [PubMed] [Google Scholar]
- Storm E. E., Huynh T. V., Copeland N. G., Jenkins N. A., Kingsley D. M., Lee S. J. Limb alterations in brachypodism mice due to mutations in a new member of the TGF beta-superfamily. Nature. 1994 Apr 14;368(6472):639–643. doi: 10.1038/368639a0. [DOI] [PubMed] [Google Scholar]
- TenEyck L. F., Arnone A. Three-dimensional Fourier synthesis of human deoxyhemoglobin at 2-5 A resolution I. X-ray analysis. J Mol Biol. 1976 Jan 5;100(1):3–11. doi: 10.1016/s0022-2836(76)80029-0. [DOI] [PubMed] [Google Scholar]
- Wang B. C. Resolution of phase ambiguity in macromolecular crystallography. Methods Enzymol. 1985;115:90–112. doi: 10.1016/0076-6879(85)15009-3. [DOI] [PubMed] [Google Scholar]
- Wu H., Lustbader J. W., Liu Y., Canfield R. E., Hendrickson W. A. Structure of human chorionic gonadotropin at 2.6 A resolution from MAD analysis of the selenomethionyl protein. Structure. 1994 Jun 15;2(6):545–558. doi: 10.1016/s0969-2126(00)00054-x. [DOI] [PubMed] [Google Scholar]