Abstract
Dilated cardiomyopathy is a frequent cause of heart failure and death. Atrial natriuretic peptide (ANP) is a biomarker of dilated cardiomyopathy, but there is controversy whether ANP modulates the development of heart failure. Therefore we examined whether ANP affects heart failure, cardiac remodeling, function and survival in a well-characterized, transgenic model of dilated cardiomyopathy. Mice with dilated cardiomyopathy with normal ANP levels survived longer than mice with partial ANP (p<0.01) or full ANP deficiency (p<0.001). In dilated cardiomyopathy mice, ANP protected against the development of heart failure as indicated by reduced lung water, alveolar congestion, pleural effusions etc. ANP improved systolic function and reduced cardiomegaly. Pathologic cardiac remodeling was diminished in mice with normal ANP as indicated by decreased ventricular interstitial and perivascular fibrosis. Mice with dilated cardiomyopathy and normal ANP levels had better systolic function (p<0.001) than mice with dilated cardiomyopathy and ANP-deficiency. Dilated cardiomyopathy was associated with diminished cardiac transcripts for natriuretic peptide receptors A and B in mice with normal ANP and ANP-deficiency but transcripts for natriuretic peptide receptor C and CNP were selectively altered in mice with dilated cardiomyopathy and ANP-deficiency. Taken together, these data indicate that ANP has potent effects in experimental dilated cardiomyopathy that reduce the development of heart failure, prevent pathologic remodeling, preserve systolic function and reduce mortality. Despite the apparent overlap in physiologic function between the natriuretic peptides, these data suggest that the role of ANP in dilated cardiomyopathy and heart failure is not compensated physiologically by other natriuretic peptides.
Keywords: ANP, dilated cardiomyopathy, heart failure, natriuretic peptides
The natriuretic peptides (NP) are valuable biomarkers for detecting and monitoring heart failure (HF). Atrial natriuretic peptide (ANP) and B-type natriuretic peptide (BNP), the two major cardiac synthesized NPs, have potent natriuretic, diuretic, vasodilator and renin- and aldosterone-suppressing activities 1. In volume or pressure overload mouse models, genetic deletion of ANP results in exaggerated cardiac hypertrophy and fibrosis2, 3. However, the effects of ANP on dilated cardiomyopathy (DCM), a distinctly different process, are unknown. In patients with DCM rising ANP levels are correlated with increasing severity of cardiac dysfunction4. Yet, despite high ANP levels, patients with HF paradoxically retain salt and water, which suggests that ANP may have limited effect on modulating the pathophysiology of HF 4. Clinical studies have not provided conclusive insights as infusions of ANP either improves cGMP levels or has no effects 5,6. Thus, it is unclear whether ANP is a modulator, or simply a biomarker of the development and progression of HF in DCM.
We assessed the role of ANP in modulating the progression of HF in a well-established transgenic mouse model of DCM characterized by progressive HF and death due to the specific action of a cardiac-targeted, dominant-negative cAMP response element-binding protein (CREB) transcription factor 7,8.
Methods
Experimental details are found in the online data supplement (please see http://hyper.ahajournals.org).
Results
ANP affects mortality in mice with DCM
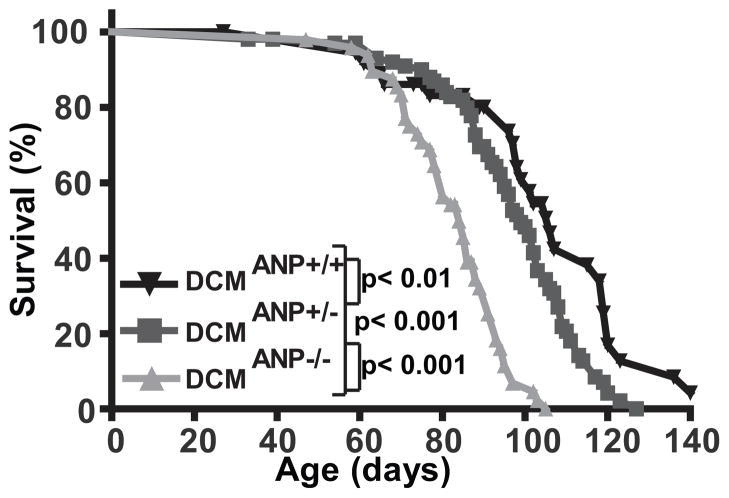
To examine whether ANP affects the development and progression of DCM, we monitored the survival of DCM mice with normal ANP levels and or ANP deficiency due to gene deletion (Figure 1). By comparison to DCM mice with normal ANP levels (DCMANP+/+), mice with partial ANP deficiency (DCMANP+/−) had an accelerated mortality (median survival 106 vs. 96 days, p<0.01). Mice with complete deficiency of ANP (DCMANP−/−) had a significantly reduced survival (median survival 84 days) by comparison to DCMANP+/− mice with partial ANP deficiency (p<0.001) or DCM mice with normal ANP levels (p<0.001). These data indicated that mortality in DCM mice was modulated by ANP levels in a gene-dose related fashion.
Figure 1. DCM mice with partial or complete ANP deficiency have an accelerated mortality.
Kaplan-Meier survival curves of DCMANP−/− (n=46), DCMANP-/+ (n=67) and DCMANP+/+ (n=26) mice were assessed by log-rank test. ***p<0.001, **p<0.01.
ANP modulates the development of HF
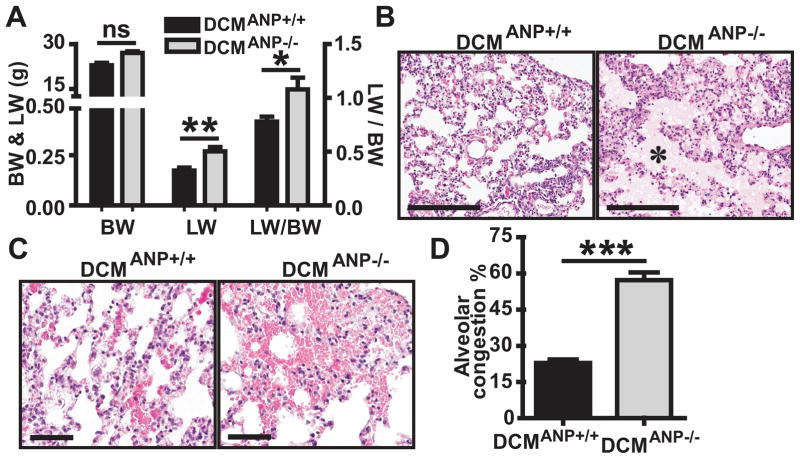
If ANP modulates survival in DCM mice, the presence or absence of ANP may affect the development and progression of HF. Chest radiographs showed that DCMANP+/+ mice had cardiomegaly (enlarged heart silhouettes) and mild lung edema without pleural effusions (Supplemental Figure S1). In contrast, DCMANP−/− mice had cardiomegaly with pronounced bilateral lung edema and pleural effusion (Figure S1). A post mortem analysis of DCMANP−/− mice showed severe lung congestion and pleural effusions. DCMANP−/− mice had greater lung fluid accumulation as indicated by higher total lung wet weight (LW, P<0.05) and the LW to body weight ratio (LW/BW, p<0.05) than DCMANP+/+ mice (n = 12–23 each group) (Figure 2A). Lung histology analysis showed that DCMANP−/− mice had more severe congestion in the interstitial and intra-alveolar space (Figure 2B, pink area, asterisks), and spotty hemorrhagic lesions in alveolar capillaries (Figure 2C). The total alveolar area filled with congestion was significantly increased in DCMANP−/− mice by comparison to DCMANP+/+ mice (p<0.001, n=3–4 each group) (Figure 2D). Renal function may affect salt and water retention associated with pleural effusions and pulmonary edema; creatinine levels were statistically higher in the DCMANP−/− mice (p<0.001) although blood urea nitrogen (BUN) and creatinine levels were within the normal range in both groups (Figure S2).
Figure 2. Enhanced heart dysfunction and pulmonary congestion in DCMANP−/− mice.
A. Lung wet weight (LW), body weight (BW) and LW:BW ratio (n = 12–23 in each group). B&C. Histological evaluation of lung congestion by H&E stain. B. DCMANP+/+ mice have mild interstitial edema while DCMANP−/− mice show severe interstitial and intra-alveolar edema (pink area, asterisks); C. DCMANP−/− mice have more punctate hemorrhagic lesions in alveolar capillaries than DCMANP+/+ mice. Scale bars=200μm. D. Quantitative analysis of total alveolar congestion area per 20× field. Results are means of averages of 12 randomly selected fields from 3 to 4 mice in each group. ***p<0.001, **p<0.01, *p<0.05, nsp>0.05.
ANP levels affect progression of left ventricular dysfunction
The increased lung congestion or pulmonary edema found in DCMANP−/− mice may signify severe left ventricle dysfunction. Therefore, cardiac function was assessed by echocardiography. When compared to DCMANP+/+ mice, DCMANP−/− showed greater left ventricular dilation in both diastole (LVIDd, p<0.001) and systole (LVIDs p<0.001) (Figure 3A–C), suggesting that DCMANP−/− mice had worse heart dilatation. There was no significant difference between DCMANP−/− and DCMANP+/+ mice in myocardial measures such as thickness of the interventricular septum or posterior wall (p>0.05, Figure 3D&E). However, DCMANP−/− mice had marked decreases in LV systolic function as assessed by ejection fraction (P<0.001) and fractional shortening (P<0.001) when compared to DCMANP+/+ mice (Figure 3F&G).
Figure 3. Echocardiographic findings in DCMANP+/+ and DCMANP−/− mice.
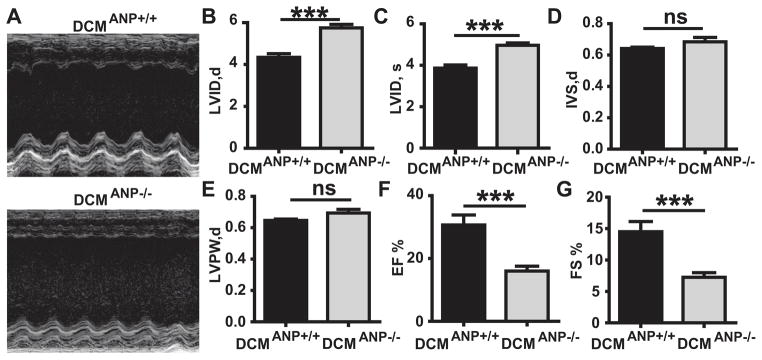
A. Representative M-mode images. B. LVID,d, left ventricular internal dimension in diastole. C. LVID,s, left ventricular internal dimension in systole. D. IVS,d, interventricular septal thickness in diastole. E. LVPW,d, left ventricular posterior wall thickness in diastole. F. EF %, ejection fraction. G. FS %, fractional shortening. ***p<0.001, nsp>0.05, n = 9 each group.
ANP affects cardiac remodeling
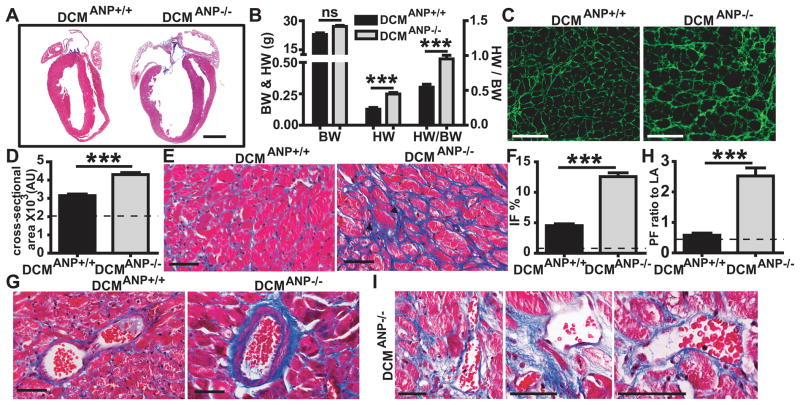
Autopsy and histologic studies showed that DCMANP−/− mice had more severe heart enlargement than DCMANP+/+ littermate controls (Figure 4A). By comparison to DCMANP+/+ mice, left atrial enlargement was more marked in DCMANP−/− mice. Heart weight (p<0.01) and heart weight:body weight ratio was significantly greater in DCMANP−/− mice than in DCMANP+/+ mice (p<0.05, n=12–23 each group, Figure 4B). Since eccentric hypertrophy may be a feature of DCM, we examined whether ANP deficiency affected cardiomyocyte hypertrophy by WGA staining 7. The cardiomyocyte cross-sectional area was significantly increased in DCMANP−/− vs DCMANP+/+ mice (p<0.001) (Figure 4C & D).
Figure 4. Pathologic cardiac remodeling in DCMANP−/− mice.
A. Representative longitudinal sections of hearts from DCMANP+/+ and DCMANP−/− mice (12 weeks old). Severe four-chamber dilation was frequently observed in DCMANP−/− mice. Scale bars=2 mm. B. Heart weight (HW), body weight (BW) and HW/BW ratio (n= 12–23 in each group). C & D. Representative wheat germ agglutinin (WGA) stain (20×) and quantitation of cardiomycyte cross-sectional area. Results are averages of 100–150 cardiomyocytes from 4–6 mice each group. E & G. Representative Masson’s trichrome stain showing interstitial fibrosis and peri-vascular fibrosis. F & H. Quantitative analysis of interstitial fibrosis (IF, % of total area) and perivascular fibrosis (PF, ratio to lumen area). A total of 12–15 fields (40×) or 12–15 arteries (40×) from left ventricle per heart were measured. Results are means of averages of 3–6 mice for each group. I. Intense perivascular fibrosis involving cardiac veins and various sizes of coronary arteries. C,E,G & I Scale bars=100μm. Dashed lines represent normal reference value from WT mice. ***p<0.001, nsp>0.05.
Fibrosis often plays a critical role in pathological remodeling in cardiomyopathy. When compared to DCMANP+/+ mice, DCMANP−/− mice heart had more extensive interstitial fibrosis between bundles of myocytes (Figure 4E & F, p<0.001). DCMANP−/− mouse hearts showed areas consistent with fibrotic scarring in the left ventricle (Figure 4E arrow head), which have been associated with focal cardiomyocyte degeneration. Perivascular fibrosis was also more marked in DCMANP−/− vs. DCM ANP+/+ hearts (Figure 4 G & H, p<0.001). There was extensive perivascular fibrosis in the cardiac veins and in different sized arteries (conductive arteries, prearterioles, and arterioles) 9 in the heart of DCMANP−/− mice (Figure 4I). In addition, DCMANP−/− hearts had more severe atrial fibrosis, especially of the left atrium, than DCMANP+/+ mice (Figure 4A).
Effects of ANP deficiency on NP cardiac gene expression and cGMP levels
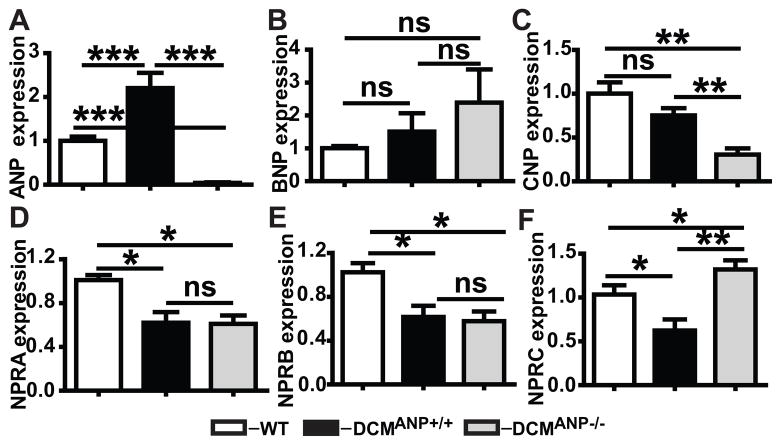
We have previously shown that ANP levels were increased in DCMANP+/+ mice by comparison to mice without DCM 8. As expected there were no detectable transcripts for ANP in DCMANP−/− mice (Figure 5A). There was a trend towards increased BNP expression in DCMANP−/− mice, but the expression levels were not statistically different from DCMANP+/+ mice (Figure 5B, p=0.67). Cardiac transcripts for CNP were significantly lower in DCMANP−/− mice than in DCMANP+/+ mice (Figure 5C, p<0.01), though both showed a trend to lower expression than WT mice. Cardiac expression of the NP receptors NPRA and NPRB were significantly decreased in both DCMANP+/+ and DCMANP−/− mice by comparison to WT mice (NPRA, p<0.01 vs WT; NPRB, p<0.05 vs WT, n=5 each group) but they did not differ from each other (p>0.05, n=5 each group, Figure 5D & E). Similarly cardiac expression of NPRC was lower in DCMANP+/+ (p<0.01 vs WT) but not in DCMANP−/− mice (p>0.05 vs. WT) (Figure 5F). However, cardiac levels of NPRC were two times higher in DCMANP−/− mice vs. DCMANP+/+ mice (p<0.01, n=5 each group) (Figure 5F). There was no significant difference between DCMANP+/+ mice and DCMANP−/− in plasma levels of angiotensin II, renin activity or aldosterone (Figures S3–S5); however levels of cortisol were 33% higher in DCMANP−/− mice (Figure S6, p<0.05). Blood levels of cGMP were significantly higher in DCMANP+/+ vs. DCMANP−/− mice (Figure S7, p<0.01) consistent with higher cardiac transcripts of ANP.
Figure 5. Changes in NPs and NP-receptors in WT, DCMANP+/+ and DCMANP−/− mice.
A–C. Relative cardiac expression of ANP, BNP and CNP. D–F. Relative cardiac expression of NPRA, NPRB and NPRC. Transcripts are means of averages of triplicate measures in 5 mice in each group assessed by qRT-PCR. ***p<0.001, **p<0.01, nsp>0.05.
Discussion
Chronic heart failure (HF) is increasing in prevalence. Both ANP and BNP are potent biomarkers for predicting mortality in patients with chronic HF 10. High levels of ANP are correlated with progressive HF but it remains unclear whether ANP is protective or merely serves as a biomarker in HF, particularly in conditions such as DCM 4. The paradox of high ANP levels and progressive HF has been attributed to: 1) incomplete processing of pro-ANP to the active form; 2) the inability of current immunologic assays to distinguish between functional ANP and dysfunctional or non-functional ANP-related peptides; 3) NPR-A/B receptor downregulation or clearance; 4) diminished kidney responsiveness and, other mechanisms 11. Experimental data indicates that ANP plays a key role in modulating the hypertrophic response of cardiomyocytes in normal hearts to increased pressure overload (e.g. transverse aortic constriction) and in volume overload (aortocaval fistula) 2, 3. Human studies, have largely focused on short term treatment of patients with impaired systolic function and show that brief ANP infusions do improve natriuresis in some but not all subjects; however, there is no data that they modify cardiac structure, function or survival 5. Thus, to our knowledge these are the first data to show that ANP plays a key role in the progression of pre-existing DCM by modifying systolic function, cardiac fibrosis, heart failure and mortality.
ANP has an anti-hypertrophic effect on cardiomyocytes both in vitro and in vivo 1. We found that ANP deficiency significantly affected cardiac remodeling. DCMANP−/− mice hearts were greater in mass and in mass normalized to body weight. There was an increase in cardiomyocyte cross-sectional area in DCMANP−/− vs. DCMANP+/+ mice. In contrast to models of pressure overload, the increased cardiomyocyte hypertrophy in this study was not associated with protective or compensatory effects, as DCMANP−/− mice showed greater impairment of systolic function, with lower EFs and, greater ventricular dilation with larger LVIDd and LVIDs. Moreover, DCMANP−/− mice did not show typical hypertrophic increases in the interventricular septum or posterior wall compared to DCMANP+/+ mice. Thus, the increased cardiac mass and worsened systolic function may be due to the fact that DCMANP−/− mice developed more extensive interstitial and perivascular fibrosis in the ventricles and atria, than that found in DCM ANP+/+ mice or, in ANP−/− mice (data not shown). ANP inhibits cardiac fibroblast-mediated collagen synthesis and may protect hearts from pathological remodeling 12. As such, ANP deficiency may have additive or synergistic effects on promoting fibrosis in the setting of a preexisting cardiomyopathy, such as DCM. Although plasma renin activity, angiotensin II and aldosterone levels were not significantly different between DCMANP−/− and DCMANP+/+ mice, ANP may still affect aldosterone and angiotensin II-induced fibrosis at the tissue level 13, 14. The finding of elevated cortisol levels in the DCMANP−/− mice underscores the potential pathogenic role of enhanced mineralocorticoid receptor activity in fibrosis 13. Extensive interstitial fibrosis can cause ventricular stiffness, abnormal electrical behavior of the myocardium and fatal arrythmias 15. There is also a clear link between interstitial fibrosis and ventricular dysfunction 16. DCMANP−/− hearts also showed extensive fibrosis in the left atrium, which is also associated with HF 17. There were areas of microscopic scar formation noted in DCMANP−/− hearts that may indicate cardiomyocyte loss 18. There was also extensive perivascular fibrosis of coronary vessels, particularly small coronary arteries and arterioles in DCMANP−/− mice (Figure 4I). Severe perivascular fibrosis can lead to structural remodeling and luminal narrowing of intramural coronary arteries and arterioles, which may be associated with impaired coronary blood flow 19. In patients with DCM, coronary microvascular dysfunction has been associated with advanced HF and increased risk of death 20.
In addition to acting on cardiac tissue through autocrine and paracrine mechanisms, ANP enhances natriuresis and modulates renal function. While ANP-deficient mice have normal blood pressures under normal salt diets, they may develop hypertension under high salt diets mediated by angiotensin II 21. Since these mice were not on high salt diets and, there were not significant differences in angiotensin II levels between the groups, this may not have contributed to the adverse outcomes in DCMANP−/− mice. In the present study, no physiologically significant differences were observed between DCMANP−/− vs DCMANP+/+ mice in indices of renal function, sodium or electrolyte or levels. Still DCMANP−/− mice showed objective evidence of more severe HF as indicated by chest radiography, increased lung fluid and extensive alveolar congestion.
We analyzed cardiac transcripts to determine how ANP deficiency in DCM affected other key members of the NP system (ANP, BNP, CNP and their receptors NPRA, NPRB and NPRC) which are believed to be regulated by LV wall stress in HF 1, 22. Transcript analyses of DCMANP+/+ hearts showed that ANP levels were significantly increased, BNP levels trended toward elevation, and CNP levels were unchanged to decreased when compared to WT controls. In contrast, in DCMANP−/− mice, transcripts for ANP were undetectable, there was a trend towards increased BNP transcripts and CNP transcripts were significantly diminished. The NPs exert their biological effects through specific receptors, NPRA (ANP and BNP) and NPRB (CNP), that increase intracellular cyclic GMP (cGMP) 23. NPRA downregulation occurs in HF and impairs the beneficial effects of NPs 24,1. We found that NPRA and NPRB were significantly and comparably downregulated in DCMANP+/+ mice and DCMANP−/− mice vs wild-type littermates. Unexpectedly NPRC levels were higher in DCMANP−/− mice than DCMANP+/+ mice. NPRC is considered a physiological clearance receptor for all three NPs 25 which suggests that NP clearance may be accelerated and contribute to functionally reduced level of NPs in DCMANP−/− mice, though definitive data is lacking. Taken together, the changes in transcript expression do not reflect an obvious pattern of coordination between members of the NP system to compensate for the loss of ANP activity. This failure of compensation is further supported by the finding that plasma cGMP levels were lower in DCMANP−/− mice when compared to DCMANP+/+ mice (Figure 5H). While it has been difficult to assign unique molecular functions to ANP and BNP at a cellular level, DCMANP−/− mice showed severe structural and functional impairments, indicating that ANP plays an essential role which was not compensated by the activity or function of other members of the NP system.
CNP transcripts were particularly diminished in DCMANP−/− mice. This may reflect the direct effect of ANP deficiency as ANP enhances the production and secretion of CNP in cell culture 26. CNP is not stored in granules and activity appears dependent on transcriptional regulation 22. CNP potently inhibits cardiac fibroblast proliferation and collagen synthesis and, has been shown to play a significant role in modulating fibrosis in vivo 27, 28. CNP also regulates the coronary circulation or microcirculation 29 which suggests that it may affect myocardial blood supply in DCMANP−/− mice. Thus, low CNP levels may also have affected cardiac function and remodeling in the DCMANP−/− mice 27.
In conclusion, these data suggest that ANP affects cardiac remodeling, function, HF progression and survival in the setting of DCM. Surprisingly, ANP deficiency is not adequately complemented by changes in the activity of other components of the NP system. Further studies are necessary to understand the functional inter-relationships of the NP and NPRs and how they act coordinately in processes such as cardiomyopathy.
Perspectives
ANP is a biomarker for predicting mortality in patients with chronic heart failure (HF). Still is unclear whether ANP functionally modulates the development of HF, especially in the setting of dilated cardiomyopathy (DCM). DCM mice with partial or complete ANP deficiency have a gene-does dependent accelerated mortality. ANP deficiency is associated with cardiac dilatation, hypertrophy, fibrosis, and impaired heart function. These data suggest that in addition to its value as a biomarker, ANP affects the progression of cardiac fibrosis, systolic dysfunction, heart failure and death in DCM.
Supplementary Material
Novelty and Significance.
What Is New?
-
In experimental dilated cardiomyopathy we found that:
partial or complete ANP deficiency accelerates mortality
ANP deficiency worsens heart function and worsens heart failure
ANP deficiency adversely affects cardiac remodeling by increasing both interstitial and peri-vascular fibrosis
ANP deficiency affects cardiac transcript expression of other natriuretic peptides and receptors but is not functionally compensated by other members of the natriuretic peptide family
What Is Relevant?
Beyond its role in natriuresis and vascular dilation, ANP appears to affect cardiac function, remodeling, heart failure development and survival in dilated cardiomyopathy
Summary
In addition to its value as a biomarker, ANP appears to play a critical, irreplaceable role in modulating cardiac function, myocardial fibrosis, heart failure and survival in experimental dilated cardiomyopathy.
Acknowledgments
We gratefully acknowledge the initial contributions of Rachel McNamee.
Sources of Funding
Grants: NIH (HL58496, HL78562; PI-Reed; HL115036 PI-Gladysheva); Scientist Development Grant from the American Heart Association (0835376N, Gladysheva).
Footnotes
Disclosures
None
References
- 1.Nishikimi T, Maeda N, Matsuoka H. The role of natriuretic peptides in cardioprotection. Cardiovasc Res. 2006;69:318–328. doi: 10.1016/j.cardiores.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Mori T, Chen YF, Feng JA, Hayashi T, Oparil S, Perry GJ. Volume overload results in exaggerated cardiac hypertrophy in the atrial natriuretic peptide knockout mouse. Cardiovasc Res. 2004;61:771–779. doi: 10.1016/j.cardiores.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 3.Franco V, Chen YF, Oparil S, Feng JA, Wang D, Hage F, Perry G. Atrial natriuretic peptide dose-dependently inhibits pressure overload-induced cardiac remodeling. Hypertension. 2004;44:746–750. doi: 10.1161/01.HYP.0000144801.09557.4c. [DOI] [PubMed] [Google Scholar]
- 4.Gottlieb SS, Kukin ML, Ahern D, Packer M. Prognostic importance of atrial natriuretic peptide in patients with chronic heart failure. J Am Coll Cardiol. 1989;13:1534–1539. doi: 10.1016/0735-1097(89)90344-6. [DOI] [PubMed] [Google Scholar]
- 5.Hata N, Seino Y, Tsutamoto T, Hiramitsu S, Kaneko N, Yoshikawa T, Yokoyama H, Tanaka K, Mizuno K, Nejima J, Kinoshita M. Effects of carperitide on the long-term prognosis of patients with acute decompensated chronic heart failure: The protect multicenter randomized controlled study. Circ J. 2008;72:1787–1793. doi: 10.1253/circj.cj-08-0130. [DOI] [PubMed] [Google Scholar]
- 6.Kohzuki M, Hodsman GP, Harrison RW, Western PS, Johnston CI. Atrial natriuretic peptide infusion in chronic heart failure in the rat. J Cardiovasc Pharmacol. 1989;13 (Suppl 6):S43–46. [PubMed] [Google Scholar]
- 7.Fentzke RC, Korcarz CE, Lang RM, Lin H, Leiden JM. Dilated cardiomyopathy in transgenic mice expressing a dominant-negative creb transcription factor in the heart. The Journal of clinical investigation. 1998;101:2415–2426. doi: 10.1172/JCI2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gladysheva IP, Wang D, McNamee RA, Houng AK, Mohamad AA, Fan TM, Reed GL. Corin overexpression improves cardiac function, heart failure, and survival in mice with dilated cardiomyopathy. Hypertension. 2013;61:327–332. doi: 10.1161/HYPERTENSIONAHA.112.193631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Camici PG, Crea F. Coronary microvascular dysfunction. N Engl J Med. 2007;356:830–840. doi: 10.1056/NEJMra061889. [DOI] [PubMed] [Google Scholar]
- 10.von Haehling S, Jankowska EA, Morgenthaler NG, Vassanelli C, Zanolla L, Rozentryt P, Filippatos GS, Doehner W, Koehler F, Papassotiriou J, Kremastinos DT, Banasiak W, Struck J, Ponikowski P, Bergmann A, Anker SD. Comparison of midregional pro-atrial natriuretic peptide with n-terminal pro-b-type natriuretic peptide in predicting survival in patients with chronic heart failure. J Am Coll Cardiol. 2007;50:1973–1980. doi: 10.1016/j.jacc.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 11.Ibebuogu UN, Gladysheva IP, Houng AK, Reed GL. Decompensated heart failure is associated with reduced corin levels and decreased cleavage of pro-atrial natriuretic peptide. Circ Heart Fail. 2011;4:114–120. doi: 10.1161/CIRCHEARTFAILURE.109.895581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Redondo J, Bishop JE, Wilkins MR. Effect of atrial natriuretic peptide and cyclic gmp phosphodiesterase inhibition on collagen synthesis by adult cardiac fibroblasts. Br J Pharmacol. 1998;124:1455–1462. doi: 10.1038/sj.bjp.0701994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Messaoudi S, Azibani F, Delcayre C, Jaisser F. Aldosterone, mineralocorticoid receptor, and heart failure. Mol Cell Endocrinol. 2012;350:266–272. doi: 10.1016/j.mce.2011.06.038. [DOI] [PubMed] [Google Scholar]
- 14.Holtwick R, Baba HA, Ehler E, Risse D, Vobeta M, Gehrmann J, Pierkes M, Kuhn M. Left but not right cardiac hypertrophy in atrial natriuretic peptide receptor-deficient mice is prevented by angiotensin type 1 receptor antagonist losartan. J Cardiovasc Pharmacol. 2002;40:725–734. doi: 10.1097/00005344-200211000-00010. [DOI] [PubMed] [Google Scholar]
- 15.Mandinov L, Eberli FR, Seiler C, Hess OM. Diastolic heart failure. Cardiovasc Res. 2000;45:813–825. doi: 10.1016/s0008-6363(99)00399-5. [DOI] [PubMed] [Google Scholar]
- 16.Hamid T, Gu Y, Ortines RV, Bhattacharya C, Wang G, Xuan YT, Prabhu SD. Divergent tumor necrosis factor receptor-related remodeling responses in heart failure: Role of nuclear factor-kappab and inflammatory activation. Circulation. 2009;119:1386–1397. doi: 10.1161/CIRCULATIONAHA.108.802918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kostin S, Klein G, Szalay Z, Hein S, Bauer EP, Schaper J. Structural correlate of atrial fibrillation in human patients. Cardiovasc Res. 2002;54:361–379. doi: 10.1016/s0008-6363(02)00273-0. [DOI] [PubMed] [Google Scholar]
- 18.Hanna N, Cardin S, Leung TK, Nattel S. Differences in atrial versus ventricular remodeling in dogs with ventricular tachypacing-induced congestive heart failure. Cardiovasc Res. 2004;63:236–244. doi: 10.1016/j.cardiores.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 19.Dai Z, Aoki T, Fukumoto Y, Shimokawa H. Coronary perivascular fibrosis is associated with impairment of coronary blood flow in patients with non-ischemic heart failure. J Cardiol. 2012;60:416–421. doi: 10.1016/j.jjcc.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 20.Neglia D, Michelassi C, Trivieri MG, Sambuceti G, Giorgetti A, Pratali L, Gallopin M, Salvadori P, Sorace O, Carpeggiani C, Poddighe R, L’Abbate A, Parodi O. Prognostic role of myocardial blood flow impairment in idiopathic left ventricular dysfunction. Circulation. 2002;105:186–193. doi: 10.1161/hc0202.102119. [DOI] [PubMed] [Google Scholar]
- 21.Melo LG, Veress AT, Chong CK, Ackermann U, Sonnenberg H. Salt-sensitive hypertension in anp knockout mice is prevented by at1 receptor antagonist losartan. Am J Physiol. 1999;277:R624–630. doi: 10.1152/ajpregu.1999.277.3.R624. [DOI] [PubMed] [Google Scholar]
- 22.Potter LR, Abbey-Hosch S, Dickey DM. Natriuretic peptides, their receptors, and cyclic guanosine monophosphate-dependent signaling functions. Endocr Rev. 2006;27:47–72. doi: 10.1210/er.2005-0014. [DOI] [PubMed] [Google Scholar]
- 23.Garbers DL, Lowe DG. Guanylyl cyclase receptors. J Biol Chem. 1994;269:30741–30744. [PubMed] [Google Scholar]
- 24.Tsutamoto T, Kanamori T, Morigami N, Sugimoto Y, Yamaoka O, Kinoshita M. Possibility of downregulation of atrial natriuretic peptide receptor coupled to guanylate cyclase in peripheral vascular beds of patients with chronic severe heart failure. Circulation. 1993;87:70–75. doi: 10.1161/01.cir.87.1.70. [DOI] [PubMed] [Google Scholar]
- 25.Koller KJ, Goeddel DV. Molecular biology of the natriuretic peptides and their receptors. Circulation. 1992;86:1081–1088. doi: 10.1161/01.cir.86.4.1081. [DOI] [PubMed] [Google Scholar]
- 26.Nazario B, Hu RM, Pedram A, Prins B, Levin ER. Atrial and brain natriuretic peptides stimulate the production and secretion of c-type natriuretic peptide from bovine aortic endothelial cells. J Clin Invest. 1995;95:1151–1157. doi: 10.1172/JCI117763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Horio T, Tokudome T, Maki T, Yoshihara F, Suga S, Nishikimi T, Kojima M, Kawano Y, Kangawa K. Gene expression, secretion, and autocrine action of c-type natriuretic peptide in cultured adult rat cardiac fibroblasts. Endocrinology. 2003;144:2279–2284. doi: 10.1210/en.2003-0128. [DOI] [PubMed] [Google Scholar]
- 28.Soeki T, Kishimoto I, Okumura H, Tokudome T, Horio T, Mori K, Kangawa K. C-type natriuretic peptide, a novel antifibrotic and antihypertrophic agent, prevents cardiac remodeling after myocardial infarction. J Am Coll Cardiol. 2005;45:608–616. doi: 10.1016/j.jacc.2004.10.067. [DOI] [PubMed] [Google Scholar]
- 29.Wright RS, Wei CM, Kim CH, Kinoshita M, Matsuda Y, Aarhus LL, Burnett JC, Jr, Miller WL. C-type natriuretic peptide-mediated coronary vasodilation: Role of the coronary nitric oxide and particulate guanylate cyclase systems. J Am Coll Cardiol. 1996;28:1031–1038. doi: 10.1016/s0735-1097(96)00241-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.