Abstract
Background
Natural killer (NK) cells are a critical component of the host innate immune system. We investigated whether alcohol impairs NK cell function, particularly production of CC chemokines induced by interleukin (IL)-2, the natural ligands for CCR5 receptor.
Methods
Primary NK cells and NK cell line (YTS) were cultured with or without alcohol (10 to 80 mM) for three hours. The culture supernatants were then harvested and used to treat human peripheral blood monocyte-derived macrophages and a HeLa cell line, which expresses CD4, CCR5, and CXCR4 receptors (MAGI cells). CC chemokine expression by YTS and primary NK cells treated with or without alcohol was analyzed with the real-time RT-PCR and ELISA. [Ca2+]i and Western blot assays were used to determine calcium-mediated intracellular signaling pathway and NF-κB p65 expression. HIV strains (Bal and UG024) were used to infect macrophages and MAGI cells. In addition, ADA (macrophage-tropic strain) and murine leukemia virus (MLV) envelope-pseudotyped HIV infection was carried out in macrophages. HIV infectivity was determined by HIV reverse transcriptase (RT) and β-galactosidase activity assays.
Results
Alcohol inhibited IL-2–induced CC chemokine (CCL3 and CCL4) expression by NK cells. Functional tests demonstrated that this reduced expression of CC chemokines was associated with diminished anti-HIV ability of NK cells. Alcohol also reduced the ability of NK cells to response to CCL3-mediated chemotaxis. Alcohol inhibited IL-2–induced NF-κB p65 protein expression and calcium mobilization by NK cells.
Conclusions
Alcohol, through the inhibition of IL-2–induced NF-κB p65 protein expression and intra-cellular calcium mobilization, suppressed NK cell production of CC chemokines. This suppression of CC chemokine production was associated with diminished anti-HIV activity of NK cells. Thus, by inhibiting NK cell–mediated innate immunity against HIV, alcohol consumption may have a cofactor role in the immunopathogenesis of HIV disease.
Keywords: Alcohol, Natural Killer Cell, HIV, CC Chemokines
INTRODUCTION
Alcohol is the most commonly used and abused drug in the United States. Approximately 14 million individuals in the United States suffer from alcohol abuse and dependence (Isaki and Kresina, 2000). The effect of alcohol consumption on the immune system has been extensively studied. Alcohol consumption induces a variety of immune alterations that lead to increased host vulnerability to infectious diseases (Cook, 1998; Nelson and Kolls, 2002). Alcohol causes a decrease in T- and B-lymphocyte functions, altering immunoglobulin production and secretion of cytokines (Cook, 1998; Nelson and Kolls, 2002; Seitz et al., 1998). Alcohol also affects host innate immunity that contributes to nonspecific host immune protection against a variety of pathogens (Szabo, 1999). Increased alcohol consumption is associated with reduced numbers and function of circulating NK cells (Wu et al., 1994), a key element of host innate immune system. Alcohol has the ability to decrease both innate and IL-2–stimulated cytolytic activity of NK cells (Dokur et al., 2004; Dokur et al., 2003; Gallucci and Meadows, 1996). This inhibition of NK cytolytic activity is associated with decreased expression of perforin, granzyme A, and B in response to IL-2 or poly I: C stimulation (Collier and Pruett, 2000; Spitzer and Meadows, 1999).
NK cells are critical in host innate immunity against HIV infection. Although direct NK cell–mediated cytotoxicity has a significant role in controlling viral infections, the antiviral chemokines such as CC chemokine, produced by NK cells, are also important in the control of HIV infection (Fehniger et al., 1998; Oliva et al., 1998). NK cells derived from HIV-infected subjects are an important source of CC chemokines, the natural ligands for CCR5 (the main coreceptor for HIV entry into macrophages) (Alkhatib et al., 1996; Choe et al., 1996). NK cells derived from HIV-infected subjects secrete CC chemokines that suppress HIV infection in vitro (Kottilil et al., 2003). Although many studies have demonstrated that alcohol suppressed NK cell cytolytic activity (Arjona et al., 2004; Boyadjieva et al., 2004; Dokur et al., 2003), there is little information about whether alcohol has the ability to impair the noncytolytic anti-HIV ability of NK cells. This noncytolytic anti-HIV ability of NK cells is mediated predominantly by secretion of CC chemokines (Kottilil et al., 2003; Mavilio et al., 2005). In the present study, we investigated whether alcohol impairs the ability of NK cells to produce CC chemokines, thus inhibiting NK cell–mediated anti-HIV activity in macrophages.
MATERIALS AND METHODS
Cells
NK cells were isolated from peripheral blood of three adult healthy donors. Studies were approved by the Institutional Review Board of the Children’s Hospital of Philadelphia, and informed consent was obtained from all the subjects. NK cells were enriched by immunomagnetic negative selection (Miltenyi Biotec, Auburn, CA) (Li et al., 2004). The purity (% of CD56+CD3−) of primary NK cells measured by fluorescence-activated cell sorting (FACS) analysis was greater than 98%. Monocytes were purified as described previously (Hassan et al., 1986). In brief, heparinized blood was separated by centrifugation over Lymphocyte Separation Medium (Organon Tekniks Corporation, Durham, NC) at 400 to 500g for 45 minutes. The mononuclear cell layer was collected and incubated with DMEM in a 2% gelatin-coated flask for 45 minutes at 37°C, followed by the removal of the nonadherent cells with DMEM. Adherent monocytes were detached with 10 mM of ethylenediamine-tetraacetic acid (EDTA). Freshly isolated monocytes (98.5% purity) were plated in 48-well culture plates (5 × 105 cells/well) in DMEM with 10% fetal calf serum (FCS). Monocyte-derived macrophages are monocytes that are maintained in vitro cultures for seven days. The human NK cell line (YTS) is a subclone of YT lymphoid cell line derived from a patient with NK cell leukemia (Cohen et al., 1999). MHC class I negative human EBV B-lymphoblastoid cell line (721.221) (Shimizu and DeMars, 1989) was used in the experiments as the NK target cells (kindly provided by Dr. Jordan S. Orange). Multinuclear-activation galactosidase indicator (MAGI) cells refer to the Hela cell line that stably expresses CD4, CXCR4, and CCR5 receptors and contains single integrated copy of the β-galactosidase gene driven by a truncated HIV-1 long terminal repeat (LTR) (Kimpton and Emerman, 1992). MAGI cells were obtained from the AIDS Research and Reference Reagent Program (National Institutes of Health, Bethesda, MD). Jurkat (human T-cell line) was obtained from American Type Culture Collection (Manassas, VA).
Reagents
Alcohol, 4-methylpyrazole (4-MP), cyanamide, phosphatase inhibitor cocktail I, phosphatase inhibitor cocktail II, Phenylmethylsulphonylfluoride, phorbol 12-myristate 13-acetate (PMA), and aprotinin were purchased from Sigma-Aldrich (St. Louis, MO). DEAE-dextran was purchased from Pharmacia Inc. (Piscataway, NJ). IL-2 was purchased from Roche Applied Science (Indianapolis, IN). CCL3 (MIP-1α) was purchased from R&D Systems (Minneapolis, MN). Rabbit polyclonal antibody against actin was purchased from Sigma-Aldrich Co. (St. Louis, MO). Mouse monoclonal antibody against NF-κB p65 was purchased from BD PharMingen (San Diego, CA). Phycoerythrin (PE)-conjugated mouse antihuman CCR1, CCR5 antibodies, and PE-conjugated anti mouse IgG2a antibody were purchased from BD PharMingen (San Diego, CA). ELISA kits for CCL3 and CCL4 (MIP-1α and MIP-1β) were purchased from Endogen, Inc. (Cambridge, MA). Alcohol concentration in the supernatants of alcohol-treated NK cell cultures was measured by the Ethanol UV-method Kit (R-Biopharm Inc., SouthMarshall, MI), and the assay was performed according to the protocol of the manufacturer.
NK Cell Supernatant Preparation and Alcohol Treatment
YTS cells plated in T75 culture flasks (107 cells) containing RPMI plus 10% FCS were incubated for three hours with or without alcohol (80 mM) in the presence of IL-2 (50 IU/ml). The concentration (80 mM) of alcohol that we selected is based on our earlier studies (Wang et al., 2002; Zhang et al., 2003b) and others (Ahluwalia et al., 2000; Szabo et al., 2001). This concentration of alcohol is achievable in vivo and is equivalent to a blood alcohol level of 0.3 g/dl. Purified NK cells from human blood were seeded in 24-well plates (106 cells/well) in RPMI with 10% FCS, supplemented with IL-2 (50 IU/ml) and treated with or without alcohol (80 mM) for three hours. Supernatants from the NK cell cultures were then collected. The supernatants collected from YTS cell cultures treated with alcohol are designated A-NK SN; the supernatants collected from YTS cell cultures without alcohol treatment are designated NK SN. During the process of preparing NK supernatants, we intentionally exposed NK supernatants (both alcohol-treated and non–alcohol-treated) in the tissue culture hood for 15 minutes to evaporate residual alcohol. In the culture dish, the residual alcohol levels were measured by the Ethanol UV-method Kit (R-Biopharm Inc.). To determine whether treatment with alcohol suppresses the anti-HIV ability of NK cells, MDM were first incubated with NK SN or A-NK SN for two hours (25%, vol/vol), followed by infection with HIV strain (Bal or UG024) for additional 24 hours. The cells were then washed three times to remove input virus and cultured for seven days. Culture supernatants were collected for HIV RT activity at day four and day seven after HIV infection. All supernatants were filtered through 0.22-μm filters and stored at −70°C in aliquots.
Virus Stocks
The macrophage-tropic (M-tropic) R5 strain (Bal), and T lymphocyte-tropic (T-tropic) X4 strain (UG024) were obtained from the AIDS Research and Reference Reagent Program (NIH).
HIV RT and MAGI Assays
HIV RT activity was determined based on the technique of Willey et al. (Willey et al., 1988). Culture supernatants (10 μl) were added to a cocktail containing poly (A), oligo (dT) (Pharmacia Inc.), MgCl2 and 32P dTTP (Amersham Corp., Arlington Heights, IL) and incubated for 20 hours at 37°C. The cocktail (30 μl) was spotted onto DE81 paper, dried, and washed five times with 2x saline-sodium citrate buffer (SSC) and once with 95% ethanol. The filter paper was then air-dried, and radioactivity was counted in a liquid scintillation counter (Packard Instrument Inc., Palo Alto, CA). MAGI assay was performed as described (Kimpton and Emerman, 1992). The MAGI cells were plated in 24-well plates (4 × 104 cells/well) in DMEM with 10% FCS the day before infection. The cells (about 20% confluent 24 hours after plated) were infected with HIV Bal strain (p24 50 ng/ml) or UG024 strain (p24 50 ng/ml) in the presence of DEAE-dextran (20 μg/ml). DMEM (1 ml) with 10% FCS was then added to each well. After 48 hours, the cells were fixed at room temperature with a fixative (1% formaldehyde plus 0.2% glutaraldehyde in phosphate-buffered saline [PBS]) for five minutes. After washing three times with PBS, the cells were incubated for 50 minutes at 37°C in 500 μl of a solution of 4 mM potassium ferrocyanide, 4 mM potassium ricyanide, 2 mM MgCl2, and 0.4 mg of X-Gal per mL. The reaction was stopped by removing the staining solution and washing the cells twice with PBS. The amounts of β-galactosidase activity in culture supernatants of the MAGI cells were measured by the Tropix Gal-Screen System purchased from Applied Biosystems (Bedford, MA), and the assay was performed according to the manufacturer’s protocol.
Pseudotyped Reporter Virus Entry Assay
HIV virions pseudotyped with the envelope (Env) from the macrophage-tropic strain ADA (CCR5 receptor dependent) or from amphotropic murine leukemia virus (MLV) (HIV entry receptor independent) were used to study the impact of alcohol on HIV entry. The plasmids encoding HIV ADA or MLV Env were provided by John Moore (Aaron Diamond AIDS Research Center, New York, NY). The Env-deleted luciferase reporter gene containing plasmid (PNL-Luc-E-R+) was co-transfected into 293T cells along with the plasmids encoding the ADA or MLV Env genes as described (Connor et al., 1995; Lai et al., 2001). Supernatants were collected as virus stock 48 hours after transfection. All virus stocks were assayed for p24 antigen and stored at −70°C as cell-free virus after filtration through a 0.22-μm filter. Macrophages derived from three different donors were plated in 48-well plates (5 × 105 cells/well) were first treated with NK SN or A-NK SN (25% vol/vol) for two hours. The cells were then infected with the pseudotyped HIV (p24 20 ng/ml) for 24 hours. At 72 hours after infection, the cells were lysed in 150 μl of 1x Reporter Lysis Buffer (Promega Corp.). Lysate (20 μl) was mixed with 100 μl of luciferase substrate (Promega Corp., Madison, WI) and luciferase activity was then determined in a TD-20/20 Luminometer (Turner Designs, Sunnyvale, CA). Data were presented in relative light units (RLU).
Measurements of [Ca2+]i
YTS cells (2 × 105 cells) were plated on Poly-D-Lysine (Sigma) coated glass cover slips (Fisher Scientific, Pittsburgh, PA). Cells were first incubated with or without (control) alcohol (80 mM) for three hours. After treatment, the cells were washed twice by Hank’s Balanced Salt Solution (HBSS) buffer. The cells were then loaded with 2 μM fura-2 acetoxymethylester (Molecular Probes, Eugene, OR) and 0.2 mg/ml pluronic F-127 (Molecular Probes) in 3 ml HBSS supplemented with 1% FCS and 1.25 mM CaCl2 for 30 minutes at 37°C. The cells were then stimulated with 721.221 cells (4 × 106 cells/100 μl) supplied with HBSS with 1% FCS and 1.25 mM CaCl2 at 37°C, and excitation was performed at 334 and 380 nm with two narrow band-pass filters. The emitted fluorescence was filtered (520 nm), captured with an Attofluor CCD video camera (512 × 480 pixel resolution), digitized (256 gray levels), and analyzed with Atto fluor Ratio Vision (Version 6.00) software (Atto Instrument, Rockville, MD). The intracellular Ca2+ mobilization ([Ca2+]i) was calculated by comparing the ratio of fluorescence at each pixel to an in vitro 2-point calibration curve. The [Ca2+]i presented is that obtained by averaging the values of all pixels of a cell body. Data points were collected at intervals of five seconds. Activated YTS cells represents the percentage of ratio of YTS cells that response to target cell–induced calcium mobilization to total YTS cells loaded.
Chemotaxis Assay
Chemotaxis was performed using 96-well microplate ChemoTX system (Neuroprobe, Cabin John, MD), 3.25-mm-diameter, 5-μM pore polycarbonate membranes were placed over the wells of a corresponding 96-well tissue culture plate. The chemoattractant (CCL3, 80 ng/ml) was diluted in chemotaxis buffer (RPMI with 20 mM HEPES, pH 7.4) and plated in the lower wells. The YTS cell suspension (105 cells/200 μl) was preincubated for two hours in the presence of different concentrations of alcohol (10 to 80 mM) or without alcohol at all were added to the upper wells and the plate was then incubated at 37°C for three hours. The cells that migrated to the lower wells were enumerated by light microscopy. The results were presented by chemotactic index (CI), the ratio of the number of cells migrating toward CCL3 to the number of cells migrating toward the medium alone.
Flow Cytometry
To determine whether alcohol affects the expression of CCR1 and CCR5 on the surface of NK cells, YTS cells were incubated with or without alcohol (80 mM) in the presence or the absence of IL-2 (50 IU/ml) for three hours. The cells were removed from the culture plate and then resuspended in 100 μl of PBS. After incubation with 10 μl of PE-conjugated mouse anti-human CCR1 or CCR5 for one hour at room temperature in the dark, the cells were washed twice with PBS and fixed with 300 μl of 1% paraformaldehyde in PBS. Isotope-matched, PE-conjugated anti-mouse IgG antibody was used as a control. Fluorescence was analyzed on an EPICS-elite flow cytometer (Beckman Coulter Electronics, Hialeah, FL).
RT-PCR and Real-Time RT-PCR
Total RNA was extracted from NK cells, using Tri-Reagent (Molecular Research Center, Cincinnati, OH) as described (Zhang et al., 2003a). Total cellular RNA (1 μg) was subjected to reverse transcription using the reverse transcription system from Promega, with specific primers (anti-sense) for CCL3, CCL4, CCR1, CCR5, and β-actin gene for one hour at 42°C. The reaction was terminated by incubating the reaction mixture at 99°C for five minutes and then kept at 4°C. The cDNA served as a template for PCR amplification. CCR1 gene primer: 5′-TTT TAA GGC CCA GTG GGA GTT CAC TCA CCG-3′ (sense) and 5′-TGG TAT AGC CAC ATG CCT TTG AAA CAG CTG C-3′ (antisense). CCR5 gene primers: 5′-CAA AAA GAA GGT CTT CAT TAC ACC-3′ (sense) and 5′-CCT GTG CCT CTT CTT CTC ATT TCG-3′ (antisense). β-actin gene primers: 5′-ATG TGG CAC CAC ACC TTC TAC AAT GAG CTG CG-3′ (sense) and 5′-CGT CAT ACT CCT GCT TGC TGA TCC ACA TCT GC-3′ (antisense). The thermal cycling conditions were designed as follows: 95°C, eight minutes; followed by 40 cycles of 95°C, 30 seconds; 55°C, 30 seconds; 72°C, 30 seconds; and elongation at 72°C for seven minutes. After PCR amplification, the samples were electrophoresed on ethidium bromide–stained 3% NuSieve 3:1 agarose gel (FMC BioProducts, Portland, ME).
Real-time RT-PCR was performed with ABI Prism 7700 Sequence Detection System (Perkin Elmer, Boston, MA). For CCL3 mRNA amplification, the primers: 5′-GCT GAC TAC TTT GAG ACG AGC-3′ (sense) and 5′-CCA GTC CAT AGA AGA GGT AGC-3′ (antisense) and the brilliant SYBR Green QPCR Master Mix (Stratagene, La Jolla, CA) were used to quantify CCL3 mRNA according to the manufacturer’s instructions. For CCL4 gene amplification, the reaction mixture contained 0.25 mM dNTPs, AmpliTaq Gold (1.5 U), 5 mM MgCl2, 50 pM of each of the two primers listed as follow: 5′-GCT GCT CAG AGA CAG GAA GTC TT-3′ (sense), 5′-ACA GGA ACT GCG GAG AGG AGT-3′(antisense), 20 pM of the molecular beacon probe: 5′-GCG AGC CCC GGA TGC TTC TCC ATG AGA CAC AGC TCG C-3′ labeled with 6-carboxyfluorescein (FAM) (a fluorophore) at the 5′ end and 4-(4′-dimethylaminophenylaso) benzoic acid (DABCYL) (a quencher) at the 3′ end. The cycle conditions were set as follows: 95°C for 10 minutes followed by 40 cycles of 95°C for 15 seconds and 60°C for one minute. A known amount of plasmid encoded with CCL4 cDNA was used as a standard control. All controls and samples were run in triplicate in the same plate. Glyceraldehyde-3-phosphate dehydrogenase mRNA levels of the samples in the same plate were analyzed by real-time RT-PCR to normalize the mRNA contents among the samples tested.
Western Blot for NF-κB p65
Cell nuclear extracts were obtained from YTS cell culture using ice-cold lysis buffer (50 mM of Tris-HCl [pH 7.4], 150 mM NaCl2, 1% Triton X-100, 1 mM EGTA, 0.1% SDS, 1% NP-40, 10 mg/ml PMSF, aprotinin, phosphatase inhibitor cocktail I 1:100, phosphatase inhibitor cocktail II 1:100) for 20 minutes. The protein concentration of each sample was assayed using a DC protein assay kit (Bio-Rad, Hercules, CA) standardized to BSA, according to manufacturer’s protocol. For electrophoresis, 50 μg of protein was loaded on a 10% Bis-Tris SDS polyacrylamide gel. Protein was electrotransferred to a nitrocellulose membrane and then blocked with 5% nonfat dry milk in PBS with 0.05% Tween. After blocking, the membrane was incubated with mouse monoclonal antibody against NF-κB p65 (1:250) for 2 hours followed by goat antimouse IgG, HRP-coupled secondary antibody at a dilution of 1: 10,000 for one hour. The bound antibodies were developed using SuperSignal West Pico Chemiluminescent Substrate (Pierce, Rockford, IL), according to the manufacturer’s instruction. Prestained molecular mass markers (Bio-Rad) were used to determine molecular weight of immunoreactive bands.
Statistical Analysis
All variables were tested in triplicate, and experiments were repeated at least three times. Triplicate wells had variability of less than 15%. One-way ANOVA was used to test for differences in means, and a post hoc t test was used for comparisons. The differences were considered significant at a value of p < 0.05.
RESULTS
Alcohol Inhibits IL-2–Induced CC Chemokine Production
We examined whether alcohol has the ability to inhibit the ability of NK cells to produce CC chemokines (CCL3 and 4). Although alcohol had no effect on the basal levels of CCL3 and 4 expression (data not shown), alcohol suppressed IL-2–induced CCL3 and 4 expressions at both mRNA and protein levels (Fig. 1). Similar results were observed in the experiments with freshly isolated human peripheral blood NK cells (Fig. 2), although the exact of alcohol inhibition varied in the cells isolated from different donors (Fig. 2).
Fig. 1.
Effect of alcohol on CCL3 and CCL4 expression in YTS cells. YTS cells were incubated with or without alcohol and/or IL-2 at indicated concentrations for three hours. Total cellular RNA extracted from the cell cultures was subjected to the real-time RT-PCR for CCL3 and CCL4 mRNA (A and B). Protein levels of CCL3 and CCL4 in supernatants from YTS cell cultures treated with or without alcohol for three hours were measured by ELISA (C and D). Data shown are mean ± SD of triplicate cultures, and the experiment was repeated three times with similar results. *p < 0.05; **p < 0.01.
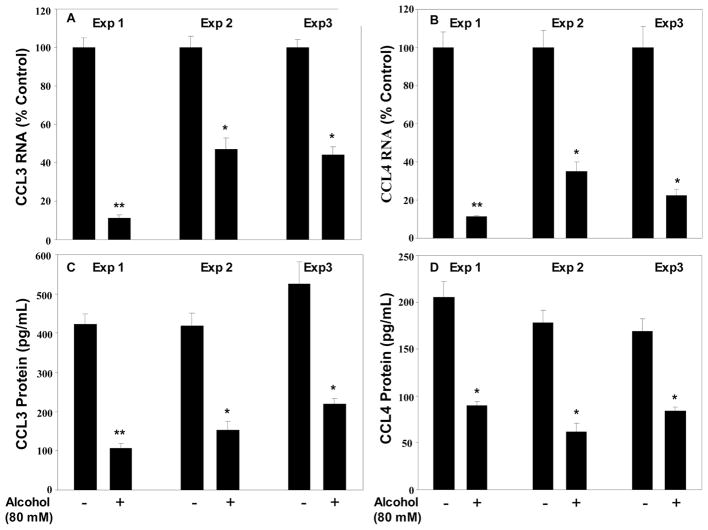
Fig. 2.
Effect of alcohol on CCL3 and CCL4 mRNA expression in primary NK cells. Primary NK cells isolated from three donors (indicated as experiments 1, 2, and 3) were incubated with or without alcohol (80 mM) for three hours. Total cellular RNA extracted from the cell cultures was subjected to the real-time RT-PCR for CCL3 (A) and CCL4 (B) mRNA. Protein levels of CCL3 and CCL4 in supernatants from primary NK cell cultures treated with or without alcohol for three hours were determined by ELISA (C and D). Data shown are mean ± SD of triplicate cultures, and the experiment was repeated three times with similar results. *p < 0.05; **p < 0.01
Alcohol Inhibits Chemotactic Activity
Since CC chemokines have a crucial role in NK cell chemotaxis (Loetscher et al., 1996; Salazar–Mather et al., 1998), we investigated whether alcohol affects NK cell migration toward CCL3, a major NK cell chemoattractant. Alcohol-treated (40 to 80 mM) YTS cells showed reduced ability to mobilize toward CCL3 (Fig. 3). We then investigated whether alcohol suppresses chemokine receptor (CCR1 and CCR5) expression, which may contribute to alcohol-mediated inhibition of NK cell chemotaxis. Alcohol had no effect on CCR1/CCR5 expression in YTS cells (data not shown).
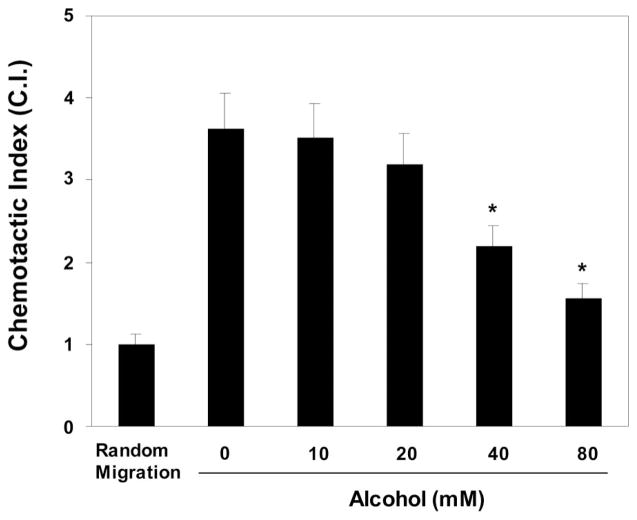
Fig. 3.
Effect of alcohol on chemotactic activity of NK cells CCL3 (80 ng/ml) was plated in the lower wells. YTS cell suspension (105 cells 200 μl), preincubated for three hours with or without alcohol (80 mM) at indicated concentrations, was added to the upper wells, and the plate was then incubated at 37°C for three hours. Cells that migrated to the lower wells were enumerated by a light microscopy. The results were presented by chemotactic index (CI), the ratio of the number of YTS cells migrating toward CCL3 to the number of cells migrating toward chemotaxis buffer, which is defined as 1.0 (random migration). Data shown are mean ± SD of triplicate wells, and the experiment was repeated three times with similar results (*p < 0.05).
Effect of Alcohol on Intracellular Ca2+ Mobilization
Intracellular Ca2+ mobilization ([Ca2+]i) is a primary regulator of numerous cellular process, including synthesis of cytokines and chemokines. We determined whether alcohol has a negative impact on this critical process in NK cells. We first determined whether treatment with alcohol affects NK cell activation (the cells that response to target cell-induced Ca2+ mobilization), the prerequisite for Ca2+ mobilization. Preincubation of YTS cells with alcohol (80 mM) significantly reduced the number of activated NK cells by 70% (Fig. 4A). In addition, alcohol suppressed intracellular Ca2+ mobilization by the activated NK cells (Fig. 4B).
Fig. 4.
Effect of alcohol on [Ca2+]i in response to target cells in YTS cells. YTS cells (2 × 105 cells/slip) pretreated with or without alcohol (80 mM) were plated on Poly-D-Lysine–coated cover slips, then loaded with 2 μM fura-2 acetoxymethylester and 0.2 ng/ml pluronic F-127 for 30 minutes at 37°C. Cells were then stimulated with 721.221 cells (4 × 106). The number of activated NK cells (A) and [Ca2+]i mobilization (B) were calculated as described in the Materials and Methods section. Results shown are mean ± SD of triplicate wells, and the experiment was repeated three times with similar results. **p < 0.01.
Alcohol Suppresses NF-κB p65 Expression
Since NF-κB p65 has a critical role in the activation of NF-κB that is involved in IL-2–induced CC chemokine gene expression, we examined the potential effect of alcohol on NF-κB p65 expression in YTS cells. The addition of alcohol to YTS cell cultures inhibited IL-2–induced expression of NF-κB p65 protein by 42% (Fig. 5).
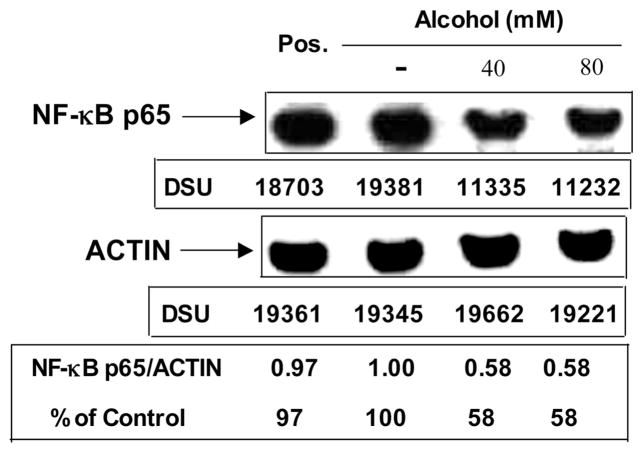
Fig. 5.
Effect of alcohol on IL-2–induced NF-κB p65 expression in YTS cells. Equal amounts (50 μg) of total nuclear proteins extracted from YTS cells pre-treated with or without alcohol (40 to 80 mM) for three hours then stimulated with IL-2 (50 IU/ml) for 30 minutes were subjected to Western blot assay, using the antibody specific to NF-κB p65 (1:250) and actin (1:3000). Pos. (positive control): Jurkat cells stimulated with PMA (2 ng/ml). Arrowheads indicate the position of NF-κB p65 (65KD) or actin (42KD). Insets below the panels show the signal intensities of protein bands of the representative blot expressed as densitometry scanning units (DSU). Results shown are representative of two independent experiments.
Effect of Alcohol on Anti-HIV Activity of NK Cells
Because CC chemokines compete with HIV for the CCR5 receptor and inhibit HIV R5 strain infection of macrophages, we examined whether alcohol-mediated suppression of CC chemokine expression affects the anti-HIV ability of NK cells. Supernatants derived from control NK cell cultures (without alcohol treatment) significantly inhibited HIV Bal infection of macrophages (Fig. 6). In contrast, supernatants from alcohol-treated NK cells had negligible anti-HIV activity in macrophages (Fig. 6). Treatment with supernatants from non–alcohol-treated NK cells inhibited HIV ADA Env-pseudotyped virus infection of macrophages (Fig. 7). This anti-HIV ability, however, was reduced in macrophages treated with supernatants from alcohol-treated NK cells (Fig. 7). Supernatants from NK cell cultures treated with or without alcohol had no significant effect on MLV Env-pseudotyped virus infection of macrophages (Fig. 7).
Fig. 6.
Effect of alcohol on noncytolytic anti-HIV ability of NK cells. Macrophages were incubated in media conditioned with supernatants from NK cell cultures treated with (A-NK SN) or without alcohol (80 mM) (NK SN) for two hours and then infected by HIV Bal strain (p24 50 ng/ml) for additional two hours. Culture supernatants were collected for HIV RT assay at days four and seven postinfection. Results shown are mean ± SD of triplicate cultures, and the experiment was repeated three times with similar results. **p < 0.01
Fig. 7.
Effect of NK SN on pseudotyped HIV infection of macrophages. Macrophages were incubated with media conditioned (25% vol/vol) with A-NK SN or NK SN for two hours and then infected with luciferase-encoding HIV pseudotyped with either ADA Env or MLV Env for 24 hours. Luciferase activity was measured in the cell lysates 72 hours after infection. Results shown are mean ± SD of triplicate cultures, and the experiment was repeated three times with similar results. **p < 0.01
To further determine that NK cell supernatant–mediated anti-HIV activity is at the HIV entry level, we used the MAGI assay, in which the target cells are the Hela cells stably transfected with the genes encoding CD4, CXCR4, and CCR5 receptors and harboring an integrated copy of the β-galactosidase gene (MAGI cells) under transcriptional control of a truncated HIV LTR (Kimpton and Emerman, 1992). HIV Bal infection of MAGI cells resulted in the activation of HIV LTR, as evidenced by appearance of the blue color cells (Fig. 8A) and β-galactosidase activity (Fig. 8D). Supernatants from control NK cell cultures (incubated in the absence of alcohol) inhibited HIV Bal-mediated LTR activation, as demonstrated by decreased number of the blue color cells (Fig. 8B) and diminished β-galactosidase activity (Fig. 8D), whereas supernatants from alcohol-treated NK cell cultures had little effect on HIV Bal infection–mediated LTR activation in MAGI cells (Fig. 8C, D). Supernatants from NK cell cultures treated with or without alcohol had no impact on HIV X4 strain (UG024) infection of MAGI cells (data not shown).
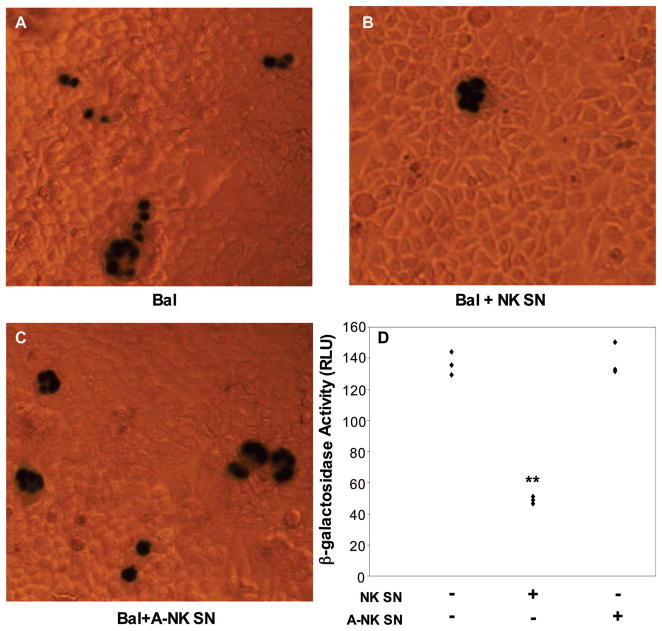
Fig. 8.
MAGI analysis of alcohol effect on NK cell-mediated anti-HIV activity. Cells were cultured in media conditioned with supernatants from either alcohol-treated (80 mM) NK cell cultures (A-NK SN) or from non–alcohol-treated NK cell cultures (NK SN) for two hours and then infected with HIV Bal (p24 50 ng/ml) strains. Cells were then fixed and stained (see Material and Methods) 48 hours after infection. A, HIV Bal infection of untreated cells; B, HIV Bal infection of NK SN–treated cells; C, HIV Bal infection of A-NK SN–treated cells; D, Quantification of β-galactosidase activity (RLU) in cell cultures shown in A, B, and C. Results shown are mean ± SD of triplicate wells, and the experiment was repeated three times with similar results. **p < 0.01
DISCUSSION
Although evidence obtained from both clinical and experimental studies implicates the involvement of alcohol in the immunopathogenesis of HIV disease, the mechanism(s) involved in such association remains to be determined. Since host innate immune response has a critical role in the immunopathogenesis of HIV disease, we determined whether alcohol has the ability to impair the anti-HIV functions of the NK cells, a critical component of host innate immune system against viral infections. NK cells represent 10% to 15% of circulating lymphocytes and are mediators of both innate and adaptive immunity. NK cells also are an important component of the early response against viral infections (Orange et al., 2002). Several studies have correlated high frequency of NK cells or NK cell activity with reduced susceptibility of individuals to HIV infection (Hu et al., 1995; Mavilio et al., 2003; Mavilio et al., 2005; Scott–Algara and Paul, 2002). Loss of NK cell activity has been correlated with HIV disease progression, particularly in individuals with opportunistic infections (Douglas et al., 2003; Jacobs et al., 2005; Kottilil, 2003; Kottilil et al., 2003). There are at least two different mechanisms involved in NK cell–mediated anti-HIV activity: To inhibit infection through the production of CC chemokines that inhibit HIV entry into immune cells and to eliminate viral replication through the lysis of infected cells. Since there was no correlation between NK cell-mediated cyto-toxicity and plasma HIV viremia, NK cell–mediated non-cytolytic anti-HIV ability has been considered to be a critical defense mechanism against HIV infection (Bernstein et al., 2004; Kottilil et al., 2004; Oliva et al., 1998). In the present study, we investigated whether alcohol has the ability to interfere with the latter function of NK cells.
NK cells are an important source of CC chemokines that suppress HIV entry and replication (Kottilil et al., 2003; Oliva et al., 1998). Our data demonstrated that the ability of NK cells (both NK cell line and primary NK cells) to produce CC chemokines under stimulation of IL-2 is inhibited by alcohol, which was evidenced by decreased expression of CC chemokines at both mRNA and protein levels. This alcohol-mediated reduction of IL-2–induced CC chemokines was associated with compromised anti-HIV activity of NK cells, since supernatants from alcohol-treated NK cell cultures had reduced anti-HIV activity in macrophages than those from non–alcohol-treated NK cell cultures. Our earlier study (Wang et al., 2002) showed that alcohol, through upregulation of CCR5 and downregulation of CC chemokine production, potentiated HIV infection of macrophages. To exclude the possibility that residual alcohol in supernatants from alcohol-treated NK cell cultures had direct effect on HIV infection of macrophages, we measured residual alcohol concentrations in the supernatants from alcohol-treated NK cell cultures. We showed that the levels of residual alcohol in the supernatants were 1.6 mM or lower (data not shown). Based on our earlier study (Wang et al., 2002) and others (Chen et al., 1998; Dong et al., 2000), alcohol at the concentration of 10 mM or lower had no impact on the immune cell function and on HIV infection of macrophages and T lymphocytes. We selected alcohol concentrations ranging from 10 to 80 mM, which is based on our earlier studies (Wang et al., 2002; Zhang et al., 2003b) and other studies (Lee et al., 2004; Saeed et al., 2004; Zhao et al., 2004). Although alcohol at the concentration of 80 mM had the highest impact, alcohol at 40 mM, a pertinent concentration to the majority of persons who drink, also had significant effect on the NK cell functions. The inhibitory effect of alcohol is not mediated specifically through its metabolite, acetaldehyde, because 4-MP or cyanamide, the specific inhibitors of alcohol metabolism, could not block alcohol action on CC chemokine expression in NK cells (data not shown). These data suggest one possibility that NK cells do not have the mechanism necessary to metabolize alcohol.
Chemokines mobilize the intracellular calcium in various cell types (Maghazachi, 1999). CC chemokines induce various intracellular signaling pathways in NK cells, including mobilization of intracellular calcium (Maghazachi, 2000). Since we observed that alcohol suppressed CC chemokine production by NK cells, we hypothesized that alcohol inhibits calcium mobilization in NK cells. The data that treatment with alcohol suppressed calcium mobilization support our hypothesis. The mechanism of this suppression, however, remains to be determined. A recent study showed that alcohol has the ability to directly inhibit the function of voltage-dependent Ca2+ channels in T-tubule membrane (Oz et al., 2005). This study provides a reasonable mechanism for our observation. Further studies are needed to determine the precise mechanism responsible for alcohol-mediated inhibition of calcium mobilization in NK cells.
An intact chemotactic response is vital for leukocyte trafficking and host defense against viral infections. In addition to the suppression of CC chemokine production, alcohol also reduced the ability of NK cells to response to CCL3-mediated chemotaxis. This alcohol-mediated reduction of NK cell chemotaxis to CCL3 is not due to the inhibition of expression of CCR1 and CCR5, the primary receptors for CCL3, since alcohol had no effect on the expression of those receptors by NK cells (data not shown). This finding indicated that alcohol might affect the function of CCR1 or CCR5 function. Our future studies will determine how alcohol affects the function of CCR1 and CCR5. Since CC chemokines induce the release of granule enzymes from both cloned and blood NK cells (Loetscher et al., 1996), they are capable of augmenting human NK-activated killer cell or antibody-dependent cell cytotoxicity-specific cytolytic responses (Taub et al., 1996). Thus, our finding that alcohol suppresses CC chemokine expression by NK cells suggests that alcohol not only directly inhibits noncytolytic anti-HIV ability of NK cells but also may have a role in suppressing NK cell–mediated anti-HIV cytolytic effect.
NF-κB is an important inflammatory signal related to expression of cytokines and chemokines. One of the target genes of NF-κB is the proinflammatory chemokine CCL3 (Brueckmann et al., 2004). Thus, the activation of NF-κB leads to an increase in CCL3 expression. We examined whether alcohol affects NF-κB activation in NK cells. We demonstrated that alcohol inhibited IL-2–induced expression of NF-κB p65, an essential protein for NF-κB activation. This finding differs with the study showing that alcohol enhances TNF-α–mediated NF-κB activation in CD4+ Jurkat T cells (Dong et al., 2000). This discrepancy between our observation and others may be due to the differences in the different target cells (NK cells vs T cells) and NF-κB inducers (IL-2 vs TNF-α). Further studies are needed to determine the precise role of alcohol in modulation of NF-κB activity in NK cells.
In conclusion, our study demonstrated that alcohol, through the inhibition of IL-2–induced NF-κB p65 protein expression and intracellular calcium mobilization, suppressed NK cell production of CC chemokines. This suppression of CC chemokine production was associated with diminished anti-HIV ability of NK cells. These findings support the notion that alcohol has the ability to suppress NK cell–mediated innate immunity against HIV infection. Further studies are needed to determine the in vivo role of alcohol in compromising NK cell function and promoting HIV disease.
Acknowledgments
This investigation was supported by grants from the National Institutes of Health (DA12815 and DA16022 to W-ZH; AA13547 to SDD).
We thank Dr. Jordan Orange (The Children’s Hospital of Philadelphia, University of Pennsylvania, Philadelphia, PA) for generously providing the 721.221 cell line.
References
- Ahluwalia B, Wesley B, Adeyiga O, Smith DM, Da-Silva A, Rajguru S. Alcohol modulates cytokine secretion and synthesis in human fetus: an in vivo and in vitro study. Alcohol. 2000;21:207–213. doi: 10.1016/s0741-8329(00)00076-8. [DOI] [PubMed] [Google Scholar]
- Alkhatib G, Combadiere C, Broder CC, Feng Y, Kennedy PE, Murphy PM, Berger EA. CC CKR5: a RANTES, MIP-1alpha, MIP-1beta receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- Arjona A, Boyadjieva N, Sarkar DK. Circadian rhythms of granzyme B, perforin, IFN-gamma, and NK cell cytolytic activity in the spleen: effects of chronic ethanol. J Immunol. 2004;172:2811–2817. doi: 10.4049/jimmunol.172.5.2811. [DOI] [PubMed] [Google Scholar]
- Bernstein HB, Kinter AL, Jackson R, Fauci AS, Kottilil S, Shin K, Planta M, McLaughlin M, Hallahan CW, Ghany M, Chun TW, Sneller MC. Neonatal natural killer cells produce chemokines and suppress HIV replication in vitro Expression of chemokine and inhibitory receptors on natural killer cells: effect of immune activation and HIV viremia. AIDS Res Hum Retroviruses. 2004;20:1189–1195. doi: 10.1089/aid.2004.20.1189. [DOI] [PubMed] [Google Scholar]
- Boyadjieva NI, Chaturvedi K, Poplawski MM, Sarkar DK. Opioid antagonist naltrexone disrupts feedback interaction between mu and delta opioid receptors in splenocytes to prevent alcohol inhibition of NK cell function. J Immunol. 2004;173:42–49. doi: 10.4049/jimmunol.173.1.42. [DOI] [PubMed] [Google Scholar]
- Brueckmann M, Hoffmann U, Dvortsak E, Lang S, Kaden JJ, Borggrefe M, Haase KK. Drotrecogin alfa (activated) inhibits NF-kappa B activation and MIP-1-alpha release from isolated mononuclear cells of patients with severe sepsis. Inflamm Res. 2004;53:528–533. doi: 10.1007/s00011-004-1291-z. [DOI] [PubMed] [Google Scholar]
- Chen H, George I, Sperber K. Effect of ethanol on monocytic function in human immunodeficiency virus type 1 infection. Clin Diagn Lab Immunol. 1998;5:790–798. doi: 10.1128/cdli.5.6.790-798.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe H, Farzan M, Sun Y, Sullivan N, Rollins B, Ponath PD, Wu L, Mackay CR, LaRosa G, Newman W, Gerard N, Gerard C, Sodroski J. The beta-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell. 1996;85:1135–1148. doi: 10.1016/s0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- Cohen GB, Gandhi RT, Davis DM, Mandelboim O, Chen BK, Strominger JL, Baltimore D. The selective downregulation of class I major histocompatibility complex proteins by HIV-1 protects HIV-infected cells from NK cells. Immunity. 1999;10:661–671. doi: 10.1016/s1074-7613(00)80065-5. [DOI] [PubMed] [Google Scholar]
- Collier SD, Pruett SB. Mechanisms of suppression of poly I:C-induced activation of NK cells by ethanol. Alcohol. 2000;21:87–95. doi: 10.1016/s0741-8329(00)00087-2. [DOI] [PubMed] [Google Scholar]
- Connor RI, Chen BK, Choe S, Landau NR. Vpr is required for efficient replication of human immunodeficiency virus type-1 in mono-nuclear phagocytes. Virology. 1995;206:935–944. doi: 10.1006/viro.1995.1016. [DOI] [PubMed] [Google Scholar]
- Cook RT. Alcohol abuse, alcoholism, and damage to the immune system–a review. Alcohol Clin Exp Res. 1998;22:1927–1942. [PubMed] [Google Scholar]
- Dokur M, Boyadjieva NI, Advis JP, Sarkar DK. Modulation of hypothalamic beta-endorphin-regulated expression of natural killer cell cytolytic activity regulatory factors by ethanol in male Fischer-344 rats. Alcohol Clin Exp Res. 2004;28:1180–1186. doi: 10.1097/01.alc.0000134222.20309.71. [DOI] [PubMed] [Google Scholar]
- Dokur M, Boyadjieva NI, Sarkar DK. Reduction of perforin, granzyme B, and cytokine interferon gamma by ethanol in male Fischer 344 rats. Alcohol Clin Exp Res. 2003;27:670–676. doi: 10.1097/01.ALC.0000060528.53113.5C. [DOI] [PubMed] [Google Scholar]
- Dong Q, Kelkar S, Xiao Y, Joshi-Barve S, McClain CJ, Barve SS. Ethanol enhances TNF-alpha-inducible NFkappaB activation and HIV-1-LTR transcription in CD4+ Jurkat T lymphocytes. J Lab Clin Med. 2000;136:333–343. doi: 10.1067/mlc.2000.110104. [DOI] [PubMed] [Google Scholar]
- Douglas SD, Camarca M, Xu J, Durako S, Murphy D, Moscicki B, Wilson CM. The relationships between substance abuse, psychosocial variables, and natural killer cell enumeration and function in HIV-infected and high-risk uninfected adolescents. AIDS Res Hum Retroviruses. 2003;19:399–408. doi: 10.1089/088922203765551746. [DOI] [PubMed] [Google Scholar]
- Fehniger TA, Herbein G, Yu H, Para MI, Bernstein ZP, O’Brien WA, Caligiuri MA. Natural killer cells from HIV-1+ patients produce C-C chemokines and inhibit HIV-1 infection. J Immunol. 1998;161:6433–6438. [PubMed] [Google Scholar]
- Gallucci RM, Meadows GG. Ethanol consumption suppresses the IL2-induced proliferation of NK cells. Toxicol Appl Pharmacol. 1996;138:90–97. doi: 10.1006/taap.1996.0102. [DOI] [PubMed] [Google Scholar]
- Hassan NF, Campbell DE, Douglas SD. Purification of human monocytes on gelatin-coated surfaces. J Immunol Methods. 1986;95:273–276. doi: 10.1016/0022-1759(86)90415-1. [DOI] [PubMed] [Google Scholar]
- Hu PF, Hultin LE, Hultin P, Hausner MA, Hirji K, Jewett A, Bonavida B, Detels R, Giorgi JV. Natural killer cell immunodeficiency in HIV disease is manifest by profoundly decreased numbers of CD16+CD56+ cells and expansion of a population of CD16dimCD56− cells with low lytic activity. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;10:331–340. [PubMed] [Google Scholar]
- Isaki L, Kresina TF. Directions for biomedical research in alcohol and HIV: where are we now and where can we go? AIDS Res Hum Retroviruses. 2000;16:1197–1207. doi: 10.1089/08892220050116961. [DOI] [PubMed] [Google Scholar]
- Jacobs R, Heiken H, Schmidt RE. Mutual interference of HIV and natural killer cell-mediated immune response. Mol Immunol. 2005;42:239–249. doi: 10.1016/j.molimm.2004.06.021. [DOI] [PubMed] [Google Scholar]
- Kimpton J, Emerman M. Detection of replication-competent and pseudotyped human immunodeficiency virus with a sensitive cell line on the basis of activation of an integrated beta-galactosidase gene. J Virol. 1992;66:2232–2239. doi: 10.1128/jvi.66.4.2232-2239.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kottilil S. Natural killer cells in HIV-1 infection: role of NK cell-mediated non-cytolytic mechanisms in pathogenesis of HIV-1 infection. Indian J Exp Biol. 2003;41:1219–1225. [PubMed] [Google Scholar]
- Kottilil S, Chun TW, Moir S, Liu S, McLaughlin M, Hallahan CW, Maldarelli F, Corey L, Fauci AS. Innate immunity in human immunodeficiency virus infection: effect of viremia on natural killer cell function. J Infect Dis. 2003;187:1038–1045. doi: 10.1086/368222. [DOI] [PubMed] [Google Scholar]
- Kottilil S, Shin K, Planta M, McLaughlin M, Hallahan CW, Ghany M, Chun TW, Sneller MC, Fauci AS. Expression of chemokine and inhibitory receptors on natural killer cells: effect of immune activation and HIV viremia. J Infect Dis. 2004;189:1193–1198. doi: 10.1086/382090. [DOI] [PubMed] [Google Scholar]
- Lai JP, Ho WZ, Zhan GX, Yi Y, Collman RG, Douglas SD. Substance P antagonist (CP-96,345) inhibits HIV-1 replication in human mononuclear phagocytes. Proc Natl Acad Sci U S A. 2001;98:3970–3975. doi: 10.1073/pnas.071052298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Jeong J, Son E, Mosa A, Cho GJ, Choi WS, Ha JH, Kim IK, Lee MG, Kim CY, Suk K. Ethanol selectively modulates inflammatory activation signaling of brain microglia. J Neuroimmunol. 2004;156:88–95. doi: 10.1016/j.jneuroim.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Li Y, Zhang T, Ho C, Orange JS, Douglas SD, Ho WZ. Natural killer cells inhibit hepatitis C virus expression. J Leukoc Biol. 2004;76:1171–1179. doi: 10.1189/jlb.0604372. [DOI] [PubMed] [Google Scholar]
- Loetscher P, Seitz M, Clark-Lewis I, Baggiolini M, Moser B. Activation of NK cells by CC chemokines. Chemotaxis, Ca2+ mobilization, and enzyme release. J Immunol. 1996;156:322–327. [PubMed] [Google Scholar]
- Maghazachi AA. Intracellular signalling pathways induced by chemokines in natural killer cells. Cell Signal. 1999;11:385–390. doi: 10.1016/s0898-6568(99)00008-x. [DOI] [PubMed] [Google Scholar]
- Maghazachi AA. Chemokines, G proteins and natural killer cells. Arch Immunol Ther Exp (Warsz) 2000;48:65–72. [PubMed] [Google Scholar]
- Mavilio D, Benjamin J, Daucher M, Lombardo G, Kottilil S, Planta MA, Marcenaro E, Bottino C, Moretta L, Moretta A, Fauci AS. Natural killer cells in HIV-1 infection: dichotomous effects of viremia on inhibitory and activating receptors and their functional correlates. Proc Natl Acad Sci U S A. 2003;100:15011–15016. doi: 10.1073/pnas.2336091100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavilio D, Lombardo G, Benjamin J, Kim D, Follman D, Marcenaro E, O’Shea MA, Kinter A, Kovacs C, Moretta A, Fauci AS. Characterization of CD56−/CD16+ natural killer (NK) cells: A highly dysfunctional NK subset expanded in HIV-infected viremic individuals. Proc Natl Acad Sci U S A. 2005;102:2886–2891. doi: 10.1073/pnas.0409872102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson S, Kolls JK. Alcohol, host defense and society. Nat Rev Immunol. 2002;2:205–209. doi: 10.1038/nri744. [DOI] [PubMed] [Google Scholar]
- Oliva A, Kinter AL, Vaccarezza M, Rubbert A, Catanzaro A, Moir S, Monaco J, Ehler L, Mizell S, Jackson R, Li Y, Romano JW, Fauci AS. Natural killer cells from human immunodeficiency virus (HIV)-infected individuals are an important source of CC-chemokines and suppress HIV-1 entry and replication in vitro. J Clin Invest. 1998;102:223–231. doi: 10.1172/JCI2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orange JS, Fassett MS, Koopman LA, Boyson JE, Strominger JL. Viral evasion of natural killer cells. Nat Immunol. 2002;3:1006–1012. doi: 10.1038/ni1102-1006. [DOI] [PubMed] [Google Scholar]
- Oz M, Alptekin A, Tchugunova Y, Dinc M. Effects of saturated long-chain N-acylethanolamines on voltage-dependent Ca2+ fluxes in rabbit T-tubule membranes. Arch Biochem Biophys. 2005;434:344–351. doi: 10.1016/j.abb.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Saeed RW, Varma S, Peng T, Tracey KJ, Sherry B, Metz CN. Ethanol blocks leukocyte recruitment and endothelial cell activation in vivo and in vitro. J Immunol. 2004;173:6376–6383. doi: 10.4049/jimmunol.173.10.6376. [DOI] [PubMed] [Google Scholar]
- Salazar-Mather TP, Orange JS, Biron CA. Early murine cytomegalovirus (MCMV) infection induces liver natural killer (NK) cell inflammation and protection through macrophage inflammatory protein 1alpha (MIP-1alpha)-dependent pathways. J Exp Med. 1998;187:1–14. doi: 10.1084/jem.187.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott-Algara D, Paul P. NK cells and HIV infection: lessons from other viruses. Curr Mol Med. 2002;2:757–768. doi: 10.2174/1566524023361781. [DOI] [PubMed] [Google Scholar]
- Seitz HK, Poschl G, Simanowski UA. Alcohol and cancer. Recent Dev Alcohol. 1998;14:67–95. doi: 10.1007/0-306-47148-5_4. [DOI] [PubMed] [Google Scholar]
- Shimizu Y, DeMars R. Production of human cells expressing individual transferred HLA-A,-B,-C genes using an HLA-A,-B,-C null human cell line. J Immunol. 1989;142:3320–3328. [PubMed] [Google Scholar]
- Spitzer JH, Meadows GG. Modulation of perforin, granzyme A, and granzyme B in murine natural killer (NK), IL2 stimulated NK, and lymphokine-activated killer cells by alcohol consumption. Cell Immunol. 1999;194:205–212. doi: 10.1006/cimm.1999.1511. [DOI] [PubMed] [Google Scholar]
- Szabo G. Consequences of alcohol consumption on host defense. Alcohol Alcohol. 1999;34:830–841. doi: 10.1093/alcalc/34.6.830. [DOI] [PubMed] [Google Scholar]
- Szabo G, Catalano D, Bellerose G, Mandrekar P. Interferon alpha and alcohol augment nuclear regulatory factor-kappaB activation in HepG2 cells, and interferon alpha increases pro-inflammatory cytokine production. Alcohol Clin Exp Res. 2001;25:1188–1197. [PubMed] [Google Scholar]
- Taub DD, Ortaldo JR, Turcovski-Corrales SM, Key ML, Longo DL, Murphy WJ. Beta chemokines costimulate lymphocyte cytolysis, proliferation, and lymphokine production. J Leukoc Biol. 1996;59:81–89. doi: 10.1002/jlb.59.1.81. [DOI] [PubMed] [Google Scholar]
- Wang X, Douglas SD, Metzger DS, Guo CJ, Li Y, O’Brien CP, Song L, Davis-Vogal A, Ho WZ. Alcohol potentiates HIV-1 infection of human blood mononuclear phagocytes. Alcohol Clin Exp Res. 2002;26:1880–1886. doi: 10.1097/01.ALC.0000042148.50808.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willey RL, Smith DH, Lasky LA, Theodore TS, Earl PL, Moss B, Capon DJ, Martin MA. In vitro mutagenesis identifies a region within the envelope gene of the human immunodeficiency virus that is critical for infectivity. J Virol. 1988;62:139–147. doi: 10.1128/jvi.62.1.139-147.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu WJ, Wolcott RM, Pruett SB. Ethanol decreases the number and activity of splenic natural killer cells in a mouse model for binge drinking. J Pharmacol Exp Ther. 1994;271:722–729. [PubMed] [Google Scholar]
- Zhang T, Guo CJ, Li Y, Douglas SD, Qi XX, Song L, Ho WZ. Interleukin-1beta induces macrophage inflammatory protein-1beta expression in human hepatocytes. Cell Immunol. 2003a;226:45–53. doi: 10.1016/j.cellimm.2003.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Li Y, Lai JP, Douglas SD, Metzger DS, O’Brien CP, Ho WZ. Alcohol potentiates hepatitis C virus replicon expression. Hepatology. 2003b;38:57–65. doi: 10.1053/jhep.2003.50295. [DOI] [PubMed] [Google Scholar]
- Zhao XJ, Oliver P, Song K, Schurr J, Zhang Z, Kolls JK. Chronic ethanol enhances ectodomain shedding in T cells and monocytes. Alcohol Clin Exp Res. 2004;28:1399–1407. doi: 10.1097/01.alc.0000139819.46514.06. [DOI] [PubMed] [Google Scholar]