Abstract
Background
Acute and chronic alcohol abuse impairs various functions of the immune system and thus has been implicated as a cofactor in HIV infection. The mechanisms by which alcohol affects the function of human immune cells that are the targets for HIV are unknown.
Methods
Human blood monocyte-derived macrophages (MDM) were incubated with or without alcohol (10–40 mM) for 24 hr and then infected with HIV for 24 hr. Culture supernatants were harvested for HIV reverse transcription assay. HIV entry receptor (CCR5, CD4, and CXCR4) expression was determined by reverse transcription–polymerase chain reaction and flow cytometry assays. β-Chemokines were analyzed using enzyme-linked immunosorbent assay. Different HIV strains (Bal, SF-162, 89.6, and UG024) were used for infection experiments. In addition, ADA (macrophage-tropic strain) and murine leukemia virus envelope-pseudotyped HIV infection was carried out.
Results
Although alcohol had little effect on HIV T-lymphocyte–tropic strain infection, it significantly enhanced HIV R5 strain infection in MDM. The enhancing effect of alcohol on the HIV R5 strain was further evidenced by the observation that the R5 (ADA) strain envelope-pseudotyped HIV infection is markedly increased by alcohol, whereas murine leukemia virus envelope-pseudotyped HIV infection was not affected. Alcohol significantly up-regulated CCR5 receptor expression and inhibited the endogenous production of β-chemokines by MDM.
Conclusion
Alcohol, through the down-regulation of β-chemokine production and the up-regulation of CCR5 receptor expression, enhances HIV R5 strain infection of MDM and may have an important role as a cofactor in the progression of HIV disease.
Keywords: Alcohol, HIV, β-Chemokines, CCR5, Macrophage
Alcohol is one of the most commonly used and abused drugs. Chronic and acute alcohol administration is associated with alterations in specific and nonspecific immunity. Alcohol abuse significantly affects morbidity and mortality from infectious diseases. Alcohol-associated alterations in immune function include changes in cell number (Saad and Jerrells, 1991) and cytokine production (Na and Seelig, 1994). Acute or chronic alcohol consumption also modulates the function of macrophages, including the impairment of microbicidal activity and the suppression of superoxide anion production in pulmonary macrophages (D’Souza et al., 1996). Dysregulation of the production of HIV-inducing cytokines such as tumor necrosis factor-alpha (TNF-α), interleukin-1 (IL-1), and IL-6 may be potential mechanisms responsible for alcohol-induced HIV infection of human immune cells. Chronic alcohol consumption, particularly alcoholic liver disease, is associated with increased levels of circulating TNF-α, IL-1, and IL-6 (Deviere et al., 1989; Khoruts et al., 1991; Lin et al., 1998; McClain and Cohen, 1989). These cytokines promote HIV replication in latently infected cells (Folks et al., 1987). Another possibility is that alcohol directly affects HIV transcription and/or activates HIV promoter long terminal repeat (LTR). Dong et al. (2000) reported that ethanol enhances TNF-α–induced activation of NF-κB and HIV LTR in CD4+ Jurkat T lymphocytes. Thus, it is reasonable to assume that alcohol acts as a cofactor for HIV infection. This hypothesis has been tested in both in vitro and in vivo model systems. Alcohol augmented HIV replication in peripheral blood mononuclear cells in vitro (Bagasra et al., 1996, 1989, 1993). In an animal model, chronic alcohol consumption accentuated the severity of murine retrovirus infection (Lee et al., 1999). Early clinical studies regarding the acceleration of HIV disease in alcohol abusers were inconclusive (MacGregor and Louria, 1997). Lake-Bakkar and Grimson (1996) reported that in a cross-sectional study of HIV disease in intravenous drug users, the relative risk of AIDS was 3.8 times higher in heavier drinkers than in moderate drinkers. Pol et al. (1996) showed that HIV-infected alcohol abusers had a 41% increase in the number of CD4 cells after cessation of alcohol use, whereas only a 15% increase was seen in uninfected control subjects who stopped consuming alcohol. Fong et al. (1994) reported a single alcoholic patient who progressed to AIDS 3 months after HIV seroconversion. Thus, this data suggested that alcohol use potentiates HIV disease progression.
The mononuclear phagocyte system, which includes circulating monocytes, resident macrophages, and activated macrophages, is a major cell system in host immune and inflammatory responses. Macrophages play a central role in the immunopathogenesis of HIV disease, because they are the primary target cells for HIV infection. Monocytes/macrophages are involved in HIV infection during all stages of disease in that they serve as major target cells, reservoirs, vehicles to other tissues, and transmitters of the virus to CD4+ T cells (Levy, 1993). There is little information with respect to the effect of alcohol on HIV infection of macrophages. Thus, we examined the impact of alcohol on HIV replication in acutely infected human blood monocyte-derived macrophage (MDM). We also investigated possible mechanisms whereby alcohol exerts its effect on HIV replication in these cells.
MATERIALS AND METHODS
Monocytes and Macrophages
Peripheral blood was obtained from six healthy adult donors without known history of drug abuse in the previous 6 months. Heparinized blood samples were identified as HIV antibody negative by anonymous testing by the enzyme-linked immunosorbent assay (ELISA) method (Coulter Immunology, Hialeah, FL). Informed consent was obtained, and the Institutional Research Board of our institution approved this study. Monocytes were purified according to our previously described technique (Hassan et al., 1986). In brief, heparinized blood was separated by centrifugation over Lymphocyte Separation Medium (Organon Teknika Corp., Durham, NC) at 400 to 500 × g for 45 min. The mononuclear cell layer was collected and incubated with Dulbecco’s modified eagle medium (DMEM; Life Technologies, Grand Island, NY) in a 2% gelatin-coated flask for 45 min at 37°C. Nonadherent cells were then removed by DMEM wash. Adherent monocytes were detached with 10 mM ethylenediami-netetraacetic acid. After the initial purification, >97% of the cells were monocytes, as determined by nonspecific esterase staining and flow cytometry analysis using monoclonal antibody (Leu-M3) against CD14, a mark for monocytes and macrophages. Freshly isolated monocytes were plated in 48-well culture plates at a density of 5 × 105 cells/well in DMEM containing 10% fetal calf serum. MDM refer to 7-day-cultured monocytes in vitro. Monocyte and MDM viability was monitored by trypan blue exclusion and maintenance of cell adherence. All media and reagents used for monocyte/macrophage culture were endotoxin-free as demonstrated by the limulus amebocyte lysate assay.
Reagents
Fluorescein (FITC)-conjugated anti-CD4, anti-CD14 antibodies, and IgG2a were obtained from PharMingen (San Diego, CA). FITC-conjugated anti-CCR5 and anti-CXCR4 antibodies were obtained from R&D Systems (Minneapolis, MN). Alcohol was purchased from Aaper Alcohol and Chemical Company (Shelbyville, KY).
HIV Strains
On the basis of their differential use of the major HIV coreceptors (CCR5 and CXCR4), HIV isolates have been referred to as R5, X4, or R5X4 strains (Berger et al., 1998). The macrophage-tropic R5 strains (Bal and SF162), dual tropic R5X4 strain (89.6), and T-cell–tropic X4 strain (UG024) were obtained from the AIDS Research and Reference Reagent Program, National Institutes of Health (Bethesda, MD). HIV Bal strain was initially isolated from human infant lung tissue. Primary macrophage-tropic strain (SF-162) was initially isolated from the cerebrospinal fluid of a patient with AIDS.
RT Assay
HIV reverse transcription (RT) activity was determined based on the technique of Willey et al. (1988) with modifications. In brief, 10 μl of collected culture supernatants was added to a cocktail containing poly (A), oligo (dT) (Pharmacia Inc., Piscataway, NJ), MgCl2, and 32P dTTP (Amersham Corp., Arlington Heights, IL) and incubated for 20 hr at 37°C. Then, 30 μl of the cocktail was spotted onto DE81 paper, dried, and washed 5 times with 2× saline-sodium citrate buffer and once with 95% ethanol. The filter paper was then air-dried. Radioactivity was counted in a liquid scintillation counter (Packard Instrument Inc., Palo Alto, CA).
Flow Cytometry
To determine the expression of CD14, CD4, CXCR4, and CCR5 receptors on alcohol-treated monocytes/macrophages, we incubated cells with or without alcohol (40 mM) for 24 hr. The cells were removed from the culture plate with a rubber policeman and then resuspended in 100 μl of phosphate-buffered saline (PBS). After incubation with 20 μl of FITC-conjugated antibodies against CD4, CXCR4, or CCR5 reagents for 45 min at 4°C, the cells were washed twice with PBS and fixed with 1% paraformaldehyde in PBS. FITC-conjugated control IgG was isotype-matched for the antibodies described above. Fluorescence was analyzed on an EPICS-elite flow cytometer (Beckman Coulter Electronics, Hialeah, FL).
ELISA for β-Chemokines
β-Chemokine ELISA kits for macrophage inflammatory protein-1α (MIP-1α) and MIP-1β were purchased from Endogen, Inc. (Cambridge, MA). The assay was performed as instructed in the protocol provided by the manufacturer. In brief, 50 μl of supernatants was added to antibody-coated wells and incubated for 1 hr at room temperature. The plates were washed with the provided buffer solution and incubated with 100 μl of biotinylated antibody reagent for 1 hr at room temperature. The plate was washed again, treated with 100 μl of prepared streptavidin–horseradish peroxidase solution, and incubated for 30 min at room temperature. After an additional wash, 100 μl of TMB (3,3′,5,5′-tetramethyl benzidine dihydrochloride) substrate solution was added to each well, and color was allowed to develop at room temperature for 30 min. The reaction was stopped by the addition of 100 μl of stop solution to each well. The plate was read on a microplate reader (ELX800; Bio-Tek Instruments, Inc., Winooski, VT).
RNA Extraction and RT
Total cellular RNA was isolated from MDM (106 cells) using Tri-reagent (Molecular Research Center, Cincinnati, OH). In brief, the total RNA was extracted by a single-step, guanidium thiocyanate-phenol-chloroform extraction. Tri-reagent (0.5 ml) was added to each well (5 × 105 MDM), and the cell lysates were then pooled from two wells of the MDM cultures. After centrifugation at 13,000 × g for 15 min at 4°C, the RNA-containing aqueous phase was precipitated in isopropanol. RNA precipitates were then washed once in 75% ethanol and resuspended in 30 μl of RNase-free water. One microgram of total RNA was subjected to RT using the RT system (Promega, Madison, WI) with specific primers (antisense) for the HIV gag gene (see below for primer sequences) and HIV receptor (CD4, CCR5, and CXCR4; see below for primer sequences) genes for 1 hr at 42°C. The reaction was terminated by incubating the reaction mixture at 99°C for 5 min and then kept at 4°C. The resulting cDNA was then used as a template for PCR amplification or real-time polymerase chain reaction (PCR) quantification.
PCR Analysis
PCR amplification of HIV receptor (CD4, CCR5, and CXCR4) cDNA was performed with a GeneAmp PCR System 2400 (PerkinElmer-Cetus, Norwalk, CT). The specific oligonucleotide primers for these receptors are listed as follows: gag gene primers: 5′-GGTACATCAGGCCATATCACC-3′ (sense) and 5′-TGACATGCTGTCATCATTTCTTC-3′ (antisense); CD4 gene primers: 5′-GTGAACCTGGTGGTGATGAGAGC-3′ (sense) and 5′-GGCTACATGTCTTCTGAAACCGGTG-3′ (antisense); CCR5 gene primers: 5′-CAAAAAGAAGGTCTTCATTACACC-3′ (sense) and 5′-CCTGTGCCTCTTCTTCTCATTTCG-3′ (antisense); CXCR4 gene primers: 5′-GACCGCTACCTGGCCATT-3′ (sense) and 5′-GTTGTAGGGC-AGCCAGCA-3′ (antisense); β-actin gene primers: 5′-ATGTGGCA-CCACACCTTCTACAATGAGCTGCG-3′ (sense) and 5′-CGTCAT-ACTCCT-GCTTGCTGATCCACATCTGC-3′ (antisense). β-actin was used as a control to monitor the amount and integrity of RNA in each sample. The oligonucleotide primers were synthesized by Integrated DNA Technologies, Inc. (Coralville, IA). The PCR reaction mixture contained 0.2 mM dNTPs, 20 pM of each of two primers, and 1.5 units of AmpliTaq Gold in 1× reaction buffer (PerkinElmer-Cetus). Each PCR amplification consisted of heat activation of AmpliTaq Gold for 9 min at 94°C, followed by 35 cycles of 94°C for 30 sec, 50°C for 30 sec, and 72°C for 45 sec, and further elongation at 72°C for 7 min. PCR-amplified products were electrophoresed on ethidium bromide–stained 3% NuSieve 3:1 agarose gel (FMC BioProducts, Rockland, ME).
Real-Time PCR
Real-time PCR was performed with one tenth of cDNA derived from 1 μg of RNA extracted from MDM using ABI Prism 7700 Sequence Detection System (PerkinElmer). For CCR5 receptor gene expression, the same primer pair used for conventional RT-PCR as described above was used for real-time PCR. The molecular beacon probe for CCR5 gene is as follows: 5′-GCGAGTCCTGCGGCTGCTTGTCATGGTCCTCGC-3′. The cycle conditions were 95°C for 10 min followed by 45 cycles of 95°C for 15 sec and 60°C for 1 min. All controls and samples were run in duplicate in the same plate. The measurement of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA levels in the samples by real-time PCR performed on the same plate was used as a control to normalize the mRNA contents among the samples tested.
Pseudotyped Reporter Virus Entry Assay
Recombinant luciferase encoding HIV virions pseudotyped with Env from the macrophage-tropic ADA (CCR5 receptor dependent) or amphotropic murine leukemia virus (MLV; HIV entry receptor independent) were used to study HIV infection of MDM incubated with or without alcohol. John Moore (Aaron Diamond AIDS Research Center, New York, NY) provided the plasmids encoding HIV ADA or MLV Env. The Env-deleted luciferase reporter plasmid PNL-Luc-E-R+ was cotransfected into 293T cells along with plasmids encoding the ADA or MLV Env genes as described (Connor et al., 1995). Supernatants were collected as virus stock 48 hr later. All virus stocks were assayed for p24 antigen and stored at −70°C as cell-free virus after filtration through a 0.22-μm pore size filter. Seven-day-cultured MDM in 48-well plates (5 × 105 cells/well) were incubated with or without alcohol (10 –40 mM) for 24 hr and then infected for 24 hr with 20 ng of P24 Gag antigen equivalent of each pseudotyped HIV per well in the presence of polybrene (4 μg/ml). At 72 hr after infection, the cells were lysed in 150 μl of 1× reporter lysis buffer (Promega). Lysate (50 μl) was mixed with an equal volume of luciferase substrate (Promega), and luciferase activity was then determined in a Wallac Trilux Microbeta luminometer (Wallac, Turku, Finland). Data were presented in relative light units.
Alcohol Treatment and HIV Infection
MDM maintained in culture for 7 days (5 × 105 cells/well in 48-well plates) were incubated either with alcohol (10 –40 mM) or in the absence of alcohol for 24 hr before infection with different HIV strains (BAL, SF162, 89.6, UG024). The cells were infected with equal quantities of cell-free HIV based on p24 protein content (20 ng/5 × 105 cells) for 24 hr at 37°C in the presence or absence of alcohol. The cells were then washed three times with DMEM to remove unabsorbed virus, and fresh medium containing alcohol was added to cell cultures. The final wash was tested for viral RT activity and shown to be free of residual inoculum. Untreated cells served as controls. The cell cultures were replaced with fresh medium containing alcohol every 4 days after infection. Supernatants were collected for HIV RT activity assay 8 days after infection. For HIV gag gene expression, total cellular RNA was extracted from MDM 72 hr after infection. To minimize alcohol evaporation, which influences alcohol concentration, we maintained alcohol-treated cells in the plates sealed with PARAFILM (American National Can, Greenwich, CT). Moreover, for avoiding evaporated alcohol contamination of control culture plates, alcohol-treated and control plates were cultured in different incubators.
Statistical Analysis
Where appropriate, data were expressed as mean ± standard error of the meant (SEM). For comparison of means of two groups, statistical significance was assessed by Student’s t test, and significance was defined as p < 0.05.
RESULTS
Effect of Alcohol on HIV Infection
For determining whether alcohol affects HIV infection of human blood MDM, 7-day-cultured MDM were incubated with or without alcohol at different concentrations (10 –40 mM) for 24 hr and then challenged with HIV Bal strain for 24 hr. The addition of alcohol to the cell cultures significantly increased HIV RT activity of alcohol-treated cells in comparison with the controls (Fig. 1A). MDM in the additional wells of the same experiments were also collected for RNA extraction 72 hr after infection and subjected to RT-PCR analysis using a specific primer pair for the HIV gag gene. Increased expression of the HIV gag gene mRNA was observed in alcohol-treated MDM (10 –40 mM) in comparison with untreated MDM (Fig. 1B). For determining whether there is a differential effect of alcohol on HIV infection with different strains (R5, X4, and R5X4), 7-day-cultured MDM were incubated with or without alcohol (40 mM) for 24 hr and infected with R5 strains (Bal and SF-162), R5X4 strain (89.6), or X4 strain (UG024). Because some of the T-cell–tropic strains can infect macrophages with much lower efficiency than macrophage-tropic strains, we used a T-tropic strain of HIV (UG024) to infect macrophages in these experiments. We demonstrated that the HIV UG024 strain had the ability to infect macrophages as demonstrated by RT activity (Fig. 2). Alcohol significantly enhanced HIV R5 strain infection, whereas R5X4 and X4 strain infection of MDM cultures were not affected (Fig. 2).
Fig. 1.
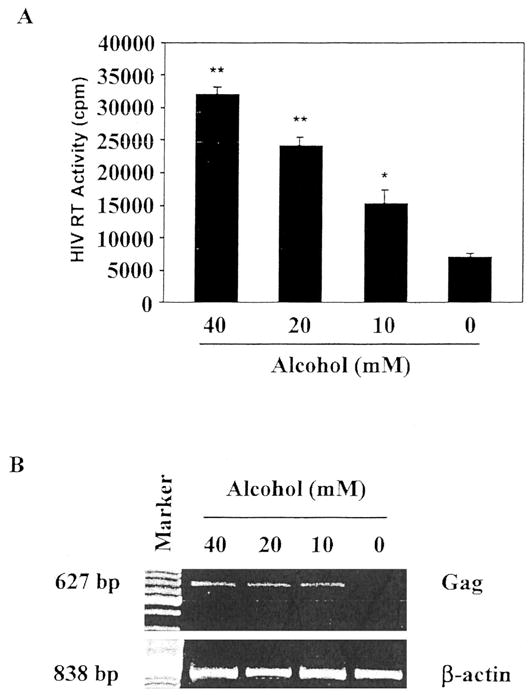
(A) Effect of alcohol on HIV Bal strain infection of MDM. Seven-day-cultured MDM were incubated with alcohol at the concentrations as indicated for 24 hr and then challenged with HIV Bal strain for 24 hr in the presence of alcohol. Cultures without the addition of alcohol served as the control. Fresh medium containing alcohol was added every 4 days. Day 8 culture supernatants were collected for HIV RT assay. The results shown are mean ± SEM of triplicate cultures and are representative of experiments using cells from three different donors (**p < 0.01, *p < 0.05, alcohol versus control). (B) Effect of alcohol on HIV Bal gag gene expression in MDM. MDM was treated with or without alcohol at the indicated concentrations for 24 hr and then infected with HIV Bal strain. Total cellular RNA was extracted from the cell culture 72 hr after infection and then subjected to RT-PCR. The data shown are representative of three independent experiments.
Fig. 2.
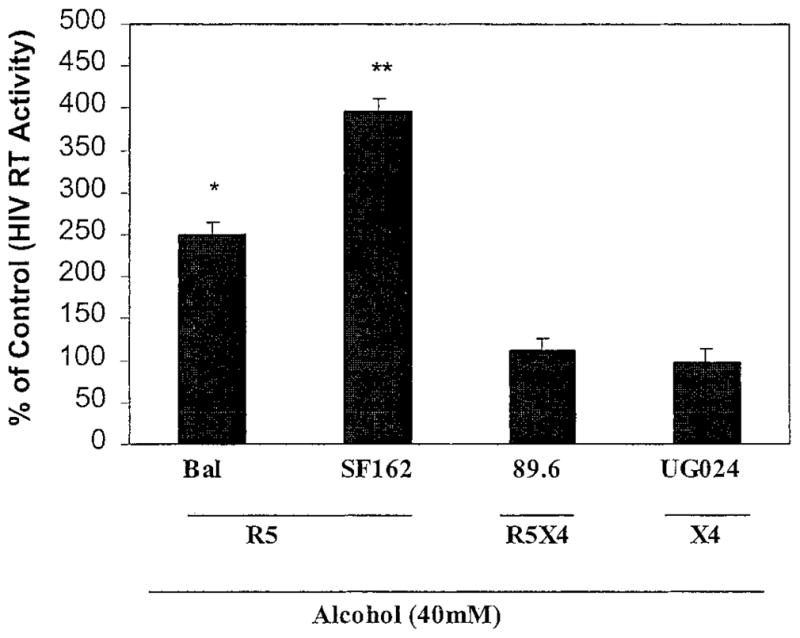
Effect of alcohol on different strains of HIV infection of MDM. Different HIV strains were used to infect 7-day-cultured MDM with or without alcohol pretreatment (40 mM). HIV RT activity in the alcohol-treated and HIV-infected MDM was expressed as a percentage of that of untreated and HIV (corresponding strains)-infected MDM controls (without alcohol treatment), which were defined as 100%. R5, CCR5 tropic strains; X4, CXCR4 tropic strain; R5X4, dual tropic strain. The data shown are mean ± SEM of triplicate cultures and are representative of experiments using cells from three different donors (**p < 0.01, *p < 0.05, alcohol versus control).
Effect of Alcohol on Pseudotyped HIV Infection
Because alcohol enhanced only HIV R5 strain infection of MDM, we hypothesized that alcohol enhances HIV infection of MDM by affecting viral entry. To prove this hypothesis, we examined the effect of alcohol on ADA (CCR5-dependent and macrophage-tropic strain) Env- or MLV (HIV receptor independent) Env-pseudotyped HIV infection of MDM. This pseudotyped HIV genome that encodes a luciferase reporter gene allows a quantitative measure of the levels of single round infection (Deng et al., 1996). MDM incubated with or without alcohol (10 –40 mM) were infected with recombinant luciferase-encoding HIV particles pseudotyped with either ADA Env or MLV Env in the presence of polybrene (4 μg/ml). The cells were lysed and subjected to luciferase activity determination 72 hr after infection as described in “MATERIALS AND METHODS.” When MDM was infected with the ADA Env-pseudotyped virus, a significant increase (3.71-, 3.48-, and 2.31-fold at 40, 20, 10 mM, respectively) in luciferase activity was observed in the alcohol-treated MDM as compared with the untreated MDM (Fig. 3). Alcohol, however, did not affect MLV Env-pseudotyped HIV infection of MDM (Fig. 3).
Fig. 3.
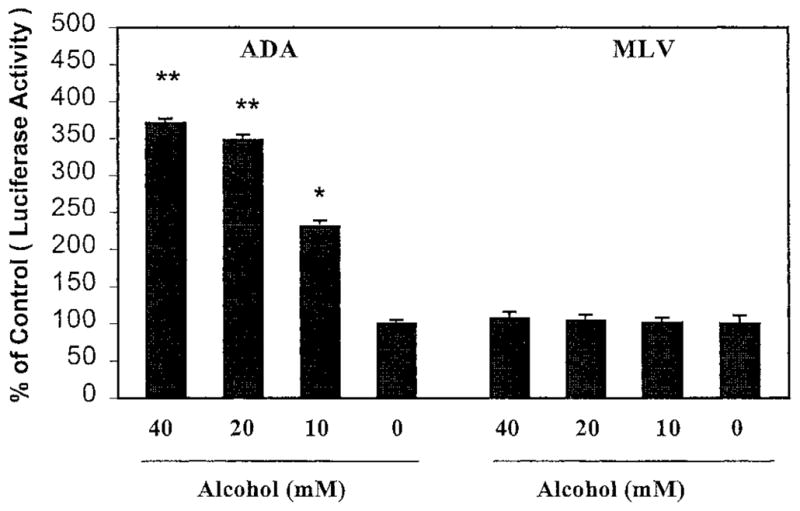
Effect of alcohol on pseudotyped HIV infection of MDM. Seven-day-cultured MDM were treated with alcohol at the concentrations as indicated for 24 hr and then challenged with recombinant luciferase-encoding HIV pseudotyped with either ADA Env or MLV Env for 24 hr. Luciferase activity was quantified in the cell lysates 72 hr after infection. The data are expressed as relative light units of alcohol-treated cells to that of controls incubated without alcohol. The results shown are mean ± SEM of triplicate cultures and are representative of experiments using cells from three different donors (**p < 0.01, *p < 0.05, alcohol versus control).
Effect of Alcohol on HIV Entry Receptor Expression
Because the infection of the HIV R5 strains but not the X4 strain was enhanced by alcohol (Fig. 2) and alcohol promoted ADA Env-pseudotyped HIV but not MLV Env-pseudotyped HIV infection of MDM (Fig. 3), we thus hypothesized that alcohol up-regulated the expression of the CCR5 receptor, a primary coreceptor for HIV R5 strain entry into macrophages. We examined the effect of alcohol on the expression of HIV entry receptors (CD4, CCR5, and CXCR4) in MDM. Among these receptors, only CCR5 receptor expression was up-regulated by alcohol (Figs. 4 and 5). To determine further the specificity of the alcohol effect, we also studied the effect of alcohol on expression of CD14, a marker of macrophages (Figs. 4 and 5). When treated with alcohol, MDM increased CCR5 mRNA expression compared with the control (7.2-, 2.17-, and 1.71-fold at 40, 20, and 10 mM, respectively; Fig. 4B). CD4 (a primary receptor for HIV) and CXCR4 (a primary coreceptor for HIV X4 strain entry into CD4+ T lymphocytes) were not affected by the alcohol treatment (Figs. 4 and 5).
Fig. 4.

Effect of alcohol on CCR5 mRNA expression in MDM. Seven-day-cultured MDM were incubated with or without alcohol at the indicated concentrations for 4 hr. Total cellular RNA was extracted from the cell cultures and subjected to RT-PCR (A) or real-time PCR (B). (A) The amplified PCR products (189 bp for CCR5) were visualized on an ethidium bromide–stained 3% agarose gel. β-actin was used to monitor the amount and integrity of RNA in each sample. (B) CCR5 cDNA transcribed from the total RNA was also subjected to real-time PCR for quantification of CCR5 mRNA copy numbers. CCR5 mRNA copy numbers were normalized with GAPDH mRNA. The results are expressed as CCR5 mRNA copies per 100,000 GAPDH mRNA. Data shown are mean ± SEM of duplicate cultures and are representative of experiments using cells from six different donors (**p < 0.01, *p < 0.05, alcohol versus control).
Fig. 5.

Effect of alcohol on HIV entry receptor expression on MDM. Seven-day-cultured MDM were incubated with or without alcohol at 40 mM for 24 hr. (A) Expression of CCR5, CD4, CXCR4, and CD14 receptors on MDM was determined by flow cytometry. The results shown are the percentage of MDM positive for the receptors analyzed and are representative of six donors. The data shown are presented as the mean ± SEM of triplicate cultures (*p < 0.05, alcohol versus control). (B) Expression of CCR5 receptor on MDM was analyzed by flow cytometry. The shaded histogram represents control staining with isotype-match antibody (IgG 2a). The open histogram represents CCR5 expression using FITC-conjugated anti-CCR5 antibody. The results shown are percentage of CCR5-positive cells and are representative of six donors.
Effect of Alcohol on β-Chemokine Expression
Because β-chemokines, natural ligands for the CCR5 receptor, have been identified as the HIV suppressive factors (Cocchi et al., 1995), we determined whether alcohol affected β-chemokine (MIP-1α and MIP-1β) production by MDM. Alcohol significantly inhibited the production of MIP-1α and MIP-1β in MDM (Fig. 6).
Fig. 6.

Effect of alcohol on β-chemokine production in MDM. Seven-day-cultured MDM were incubated with or without alcohol at indicated concentrations for 24 hr. Culture supernatants were collected 24 hr posttreatment for analysis of MIP-1α (A) and MIP-1β (B) production as determined by ELISA. The data shown are presented as the mean ± SEM of triplicate cultures (**p < 0.01, *p < 0.05, alcohol versus control) and are representative of experiments using cells from six donors.
DISCUSSION
Because alcohol abuse is common among HIV-infected individuals and sexually active age groups at risk for HIV infection, it has been proposed that the modulatory effects of alcohol on the immune system may play a role not only in increased susceptibility to initial HIV infection but also in the rapid progression of HIV disease toward AIDS (Saravolatz et al., 1990). Although increasing evidence that demonstrates the immunologic abnormalities as a result of alcohol use is emerging (Szabo, 1999), current understanding of the impact of the immunosuppressive effects of alcohol on HIV infection in human immune cells is limited. The administration of alcohol led to increased TNF-α–stimulated HIV LTR activation in T cells (Dong et al., 2000). Bagasra et al. (1989) reported increased HIV p24 levels in vitro in infected peripheral blood mononuclear cells isolated from individuals after a one-dose acute alcohol infusion or binge drinking. The CD4+ T-cell line (CEM), when exposed to alcohol and infected with HIV, produced significantly higher levels of P24 antigen compared with cells that were not exposed to alcohol (Saravolatz et al., 1990). Alcohol also decreased macrophage function during HIV infection (Chen et al., 1998). Although the clinical significance of these observations needs further investigation, these data indicate that alcohol use is likely to increase immune cell susceptibility to HIV infection.
There are several potential mechanisms by which alcohol may effectively enhance HIV infection (Lin et al., 1998; Mandrekar et al., 1997). First, alcohol may affect HIV infection and replication indirectly by up-regulation of cytokine (TNF-α, IL-1, and IL-6) expression. Alcohol modulated production of these cytokines in monocyte and lymphocyte cultures (McClain and Cohen, 1989; Nair et al., 1994). These cytokines induce HIV replication from a quiescent state (Folks et al., 1987). A second possibility involves modulation of β-chemokine production and its receptor (such as CCR5) expression. In the present study, we analyzed the impact of alcohol on HIV infection of human blood-isolated mononuclear phagocytes. Our experiments have demonstrated that (1) alcohol significantly enhanced HIV R5 strain infection of human blood MDM (Figs. 1–3); (2) alcohol up-regulated expression of the CCR5 receptor (Figs. 4 and 5), a primary coreceptor for HIV R5 strain entry into macrophages; and (3) alcohol significantly inhibits MIP-1α and MIP-1β expression (Fig. 6). Our finding that alcohol suppressed β-chemokine production is supported by a recent report that demonstrated that alcohol potently inhibited MIP-1α and monocyte chemotactic protein-1 production by Kupffer cells (residing macrophages in the liver) in the presence of HIV-1 gp120 and LPS treatment (Bautista, 2001).
Because β-chemokines interfere with HIV infection of MDM by competing for the CCR5 receptor, a primary coreceptor for HIV entry into macrophages, alcohol-mediated up-regulation of the CCR5 receptor and down-regulation of β-chemokine production by MDM are important factors responsible for alcohol’s effect on HIV macrophage-tropic strain infection of MDM in vitro. This hypothesis is further supported by the findings that alcohol significantly enhanced the replication of HIV R5 strains (Bal and SF162) but not X4 strain (UG024; Fig. 2) and that only ADA (macrophage-tropic strain) Env-pseudotyped HIV infection of MDM was potentiated by alcohol (Fig. 3). Thus, these data provide a possible mechanism whereby alcohol potentiates HIV infection of human blood mononuclear phagocytes in vitro. These observations may be relevant to the in vivo effects of alcohol as an important cofactor in HIV/AIDS.
Taken together, our current data demonstrating that alcohol has the ability to enhance acute HIV infection of human blood mononuclear phagocyte, along with other reports, support the need for further mechanistic as well as epidemiologic investigations on the role of alcohol abuse in HIV/AIDS.
Acknowledgments
This study was supported by NIH-AA 13547, NIH-MH49981, and NIH-DA 12815.
We thank Dr. Donald E. Campbell and the flow cytometry core at Joseph Stokes Jr. Research Institute for the analysis of HIV entry receptor expression.
References
- Bagasra O, Bachman SE, Jew L, Tawadros R, Cater J, Boden G, Ryan I, Pomerantz RJ. Increased human immunodeficiency virus type 1 replication in human peripheral blood mononuclear cells induced by ethanol: potential immunopathogenic mechanisms. J Infect Dis. 1996;173:550–558. doi: 10.1093/infdis/173.3.550. [DOI] [PubMed] [Google Scholar]
- Bagasra O, Kajdacsy-Balla A, Lischner HW. Effects of alcohol ingestion on in vitro susceptibility of peripheral blood mononuclear cells to infection with HIV and of selected T-cell functions. Alcohol Clin Exp Res. 1989;13:636–643. doi: 10.1111/j.1530-0277.1989.tb00396.x. [DOI] [PubMed] [Google Scholar]
- Bagasra O, Kajdacsy-Balla A, Lischner HW, Pomerantz RJ. Alcohol intake increases human immunodeficiency virus type 1 replication in human peripheral blood mononuclear cells. J Infect Dis. 1993;167:789–797. doi: 10.1093/infdis/167.4.789. [DOI] [PubMed] [Google Scholar]
- Bautista AP. Acute alcohol intoxication and endotoxemia desensitize HIV-1 gp120-induced CC-chemokine production by Kupffer cells. Life Sci. 2001;68:1939–1949. doi: 10.1016/s0024-3205(01)00986-9. [DOI] [PubMed] [Google Scholar]
- Berger EA, Doms RW, Fenyo EM, Korber BT, Littman DR, Moore JP, Sattentau QJ, Schuitemaker H, Sodroski J, Weiss RA. A new classification for HIV-1. Nature. 1998;391:240. doi: 10.1038/34571. [DOI] [PubMed] [Google Scholar]
- Chen H, George I, Sperber K. Effect of ethanol on monocytic function in human immunodeficiency virus type 1 infection. Clin Diagn Lab Immunol. 1998;5:790–798. doi: 10.1128/cdli.5.6.790-798.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocchi F, DeVico AL, Garzino-Demo A, Arya SK, Gallo RC, Lusso P. Identification of RANTES, MIP-1 alpha, and MIP-1 beta as the major HIV-suppressive factors produced by CD8+ T cells. Science. 1995;270:1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- Connor RI, Chen BK, Choe S, Landau NR. Vpr is required for efficient replication of human immunodeficiency virus type-1 in mono-nuclear phagocytes. Virology. 1995;206:935–944. doi: 10.1006/viro.1995.1016. [DOI] [PubMed] [Google Scholar]
- Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton RE, Hill CM, Davis CB, Peiper SC, Schall TJ, Littman DR, Landau NR. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- Deviere J, Content J, Denys C, Vandenbussche P, Schandene L, Wybran J, Dupont E. High interleukin-6 serum levels and increased production by leucocytes in alcoholic liver cirrhosis. Correlation with IgA serum levels and lymphokines production. Clin Exp Immunol. 1989;77:221–225. [PMC free article] [PubMed] [Google Scholar]
- Dong Q, Kelkar S, Xiao Y, Joshi-Barve S, McClain CJ, Barve SS. Ethanol enhances TNF-alpha-inducible NFκB activation and HIV-1-LTR transcription in CD4+ Jurkat T lymphocytes. J Lab Clin Med. 2000;136:333–343. doi: 10.1067/mlc.2000.110104. [DOI] [PubMed] [Google Scholar]
- D’Souza NB, Nelson S, Summer WR, Deaciuc IV. Alcohol modulates alveolar macrophage tumor necrosis factor-alpha, superoxide anion, and nitric oxide secretion in the rat. Alcohol Clin Exp Res. 1996;20:156–163. doi: 10.1111/j.1530-0277.1996.tb01059.x. [DOI] [PubMed] [Google Scholar]
- Folks TM, Justement J, Kinter A, Dinarello CA, Fauci AS. Cytokine-induced expression of HIV-1 in a chronically infected promonocyte cell line. Science. 1987;238:800–802. doi: 10.1126/science.3313729. [DOI] [PubMed] [Google Scholar]
- Fong IW, Read S, Wainberg MA, Chia WK, Major C. Alcoholism and rapid progression to AIDS after seroconversion. Clin Infect Dis. 1994;19:337–338. doi: 10.1093/clinids/19.2.337. [DOI] [PubMed] [Google Scholar]
- Hassan NF, Campbell DE, Douglas SD. Purification of human monocytes on gelatin-coated surfaces. J Immunol Methods. 1986;95:273–276. doi: 10.1016/0022-1759(86)90415-1. [DOI] [PubMed] [Google Scholar]
- Khoruts A, Stahnke L, McClain CJ, Logan G, Allen JI. Circulating tumor necrosis factor, interleukin-1 and interleukin-6 concentrations in chronic alcoholic patients. Hepatology. 1991;13:267–276. [PubMed] [Google Scholar]
- Lake-Bakaar G, Grimson R. Alcohol abuse and stage of HIV disease in intravenous drug abusers. J R Soc Med. 1996;89:389–392. doi: 10.1177/014107689608900709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Sepulveda RT, Jiang S, Zhang Z, Inserra P, Zhang Y, Hosseini S, Watson RR. Immune dysfunction during alcohol consumption and murine AIDS: the protective role of dehydroepiandrosterone sulfate. Alcohol Clin Exp Res. 1999;23:856–862. [PubMed] [Google Scholar]
- Levy JA. Pathogenesis of human immunodeficiency virus infection. Microbiol Rev. 1993;57:183–289. doi: 10.1128/mr.57.1.183-289.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HZ, Yang SQ, Zeldin G, Diehl AM. Chronic ethanol consumption induces the production of tumor necrosis factor-alpha and related cytokines in liver and adipose tissue. Alcohol Clin Exp Res. 1998;22:231S–237S. doi: 10.1097/00000374-199805001-00004. [DOI] [PubMed] [Google Scholar]
- MacGregor RR, Louria DB. Alcohol and infection. Curr Clin Top Infect Dis. 1997;17:291–315. [PubMed] [Google Scholar]
- Mandrekar P, Catalano D, Szabo G. Alcohol-induced regulation of nuclear regulatory factor-kappa beta in human monocytes. Alcohol Clin Exp Res. 1997;21:988–994. [PubMed] [Google Scholar]
- McClain CJ, Cohen DA. Increased tumor necrosis factor production by monocytes in alcoholic hepatitis. Hepatology. 1989;9:349–351. doi: 10.1002/hep.1840090302. [DOI] [PubMed] [Google Scholar]
- Na HR, Seelig LL., Jr Effect of maternal ethanol consumption on in vitro tumor necrosis factor, interleukin-6 and interleukin-2 production by rat milk and blood leukocytes. Alcohol Clin Exp Res. 1994;18:398–402. doi: 10.1111/j.1530-0277.1994.tb00032.x. [DOI] [PubMed] [Google Scholar]
- Nair MP, Kumar NM, Kronfol ZA, Saravolatz LA, Pottathil R, Greden JF, Schwartz SA. Selective effect of alcohol on cellular immune responses of lymphocytes from AIDS patients. Alcohol. 1994;11:85–90. doi: 10.1016/0741-8329(94)90048-5. [DOI] [PubMed] [Google Scholar]
- Pol S, Artru P, Thepot V, Berthelot P, Nalpas B. Improvement of the CD4 cell count after alcohol withdrawal in HIV-positive alcoholic patients. AIDS. 1996;10:1293–1294. doi: 10.1097/00002030-199609000-00019. [DOI] [PubMed] [Google Scholar]
- Saad AJ, Jerrells TR. Flow cytometric and immunohistochemical evaluation of ethanol-induced changes in splenic and thymic lymphoid cell populations. Alcohol Clin Exp Res. 1991;15:796–803. doi: 10.1111/j.1530-0277.1991.tb00603.x. [DOI] [PubMed] [Google Scholar]
- Saravolatz LD, Cerra RF, Pohlod DJ, Smereck S. The effect of alcohol on HIV infection in vitro. Prog Clin Biol Res. 1990;325:267–271. [PubMed] [Google Scholar]
- Szabo G. Consequences of alcohol consumption on host defense. Alcohol Alcohol. 1999;34:830–841. doi: 10.1093/alcalc/34.6.830. [DOI] [PubMed] [Google Scholar]
- Willey RL, Smith DH, Lasky LA, Theodore TS, Earl PL, Moss B, Capon DJ, Martin MA. In vitro mutagenesis identifies a region within the envelope gene of the human immunodeficiency virus that is critical for infectivity. J Virol. 1988;62:139–147. doi: 10.1128/jvi.62.1.139-147.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]


