Abstract
Substance P (SP), the most extensively studied and potent member of the tachykinin family, is a major modulator of inflammation and immunomodulatory activities within the central and peripheral nervous systems. We have examined the gene expression of SP and its receptor in a human neuronal cell line (NT2-N). Using reverse transcribed polymerase chain reaction (RT-PCR), the four isoforms of preprotachykinin-A gene transcripts (α, β, γ, and δ) were detected in the NT2-N. We also identified the presence of mRNA for neurokinin-1 receptor (NK-1R), a primary receptor for SP, in the NT2-N cells. Concomitant with NT2 cell differentiation into neurons, SP and NK-1R mRNA expression increased consistently. Intracellular SP and cell membrane NK-1R immunoreactivity were all observed in NT2-N cells. Most importantly, we demonstrated that SP and NK-1R presented in NT2-N cells are functionally involved in the regulation of macrophage inflammatory protein 1 beta (MIP-1β), an important β-chemokine participating in the activation and directional migration of immune cells to sites of central nervous systems (CNS) inflammation. Thus, SP and its receptor may play an important role in modulation of neuronal functions related to regulation of immune activities within the CNS. The NT2-N cell line is well suited for in vitro investigations of the SP-NK-1R pathway in immune responses and inflammation in the CNS.
Keywords: Substance P, NK-1R, real-time RT-PCR, MIP-1β
Substance P (SP) is an 11 amino acid neuropeptide that is widely distributed in the central and peripheral nervous systems and has a well-established role as a neurotransmitter. SP also is an important modulator of neuroimmunoregulation. SP, at nanomolar concentration, effectively activates the transcription factor nuclear factor kappa B (NF-κB; Lieb et al., 1997). SP stimulates human peripheral blood monocytes to produce inflammatory cytokines including interleukin 1 (IL-1), IL-6, IL-12, and tumor necrosis factor alpha (TNF-α; Lotz et al., 1988; Lee et al., 1994; Ho et al., 1996; Kincy-Cain and Bost, 1997). We have demonstrated that SP enhances TNF-α and IL-10 production by monocytes/macrophages isolated from both adult human peripheral and placental cord blood (Lee et al., 1994; Ho et al., 1996, 1998). SP also induces IL-1 (Martin et al., 1992) and IL-6 (Gitter et al., 1994) secretion by astrocytes and enhances the secretion of TNF-α from neuroglial cells stimulated with lipopolysaccharide (Luber-Narod et al., 1994). SP exerts its biological activities upon binding to a G protein-coupled receptor of the neurokinin (NK) receptor family (Regoli et al., 1989; Gerard et al., 1991). SP has a preferential affinity for the neurokinin 1 receptor (NK-1R). Gene-target disruption of the NK-1R protected the lung from immune complex injury (Bozic et al., 1996). Multiple cell types (neurons, astrocytes, and oligodendrocytes) in the brain express NK-1 receptors and may therefore be the potential sites for action of a SP receptor antagonist. SP is characterized almost exclusively as a peptide of neuronal origin (Pernow, 1983; Nakanishi, 1987). We recently showed that microglia derived from human fetal brain express SP and its receptor (Lai et al., 2000). SP receptor (NK-1R) binding sites are highly expressed by glia in vivo after neuronal injury (Mantyh et al., 1989). In addition, NK-1R is expressed in areas of the brain that are implicated in multiple sclerosis (MS; Kostyk et al., 1989). Furthermore, SP-immunoreactive astrocytes have been described within the glial scars in multiple sclerosis brain tissue (Kostyk et al., 1989). Thus, SP and its receptor are indeed involved in inflammation and neurological disorders within the central nervous systems (CNS).
Retinoic acid (RA) treatment of NT-era2/cl.D1 (NT2) cells, a human teratocarcinoma cell line, yields 95% pure cultures of terminally differentiated neuronal cells, designated NT2-N (Pleasure et al., 1992). NT2-N cells morphologically resemble primary neuronal cultures from rodents and, like primary neurons, have processes that differentiate into axons and dendrites (Andrews, 1984). In addition, NT2-N cells express a variety of neuronal markers including many neuronal cytoskeletal proteins, secretory markers, and surface markers (Pleasure et al., 1992). RA-induced NT2-N cells express neuronal N-methyl-D-aspartate (NMDA) and non-NMDA glutamate receptors concomitant with their differentiation into neuron-like cells (Younkin et al., 1993). Immature human NT2 cells grafted into mouse brain differentiated into neuronal and glia cells (Ferrari et al., 2000). In addition to susceptibility to glutamate-induced neurotoxicity, the molecular and physiological characteristics of these human glutamate receptors on NT2-N are nearly identical to those of central nervous system neurons, as demonstrated by the polymerase chain reaction and patch-clamp recordings (Younkin et al., 1993). NT2-N cells express functional neurotransmitters such as γ-aminobutyric acid (GABA) (Guillemain et al., 2000) and inducible δ and μ opioid receptor mRNA and protein (Beczkowska et al., 1997). Since SP is a neurotransmitter and regulator of the immune response in the CNS, it is important to understand the role of SP in biological processes in the CNS. In the present study, we examined whether NT2-N cells express SP and SP receptors, and whether SP and SP receptors expressed by NT2-N cells have functional activity.
MATERIALS AND METHODS
Cell Cultures
Microglia, monocytes/macrophages (MDMs), and peripheral blood lymphocytes (PBLs) were purified as described previously (Lai et al., 2002). Ntera2/c1.D1 (NT2) cells were originally provided by Dr. Virginia Lee (University of Pennsylvania, Philadelphia, PA) and were cultured and differentiated into neurons as described previously (Pleasure et al., 1992; Younkin et al., 1993). Cells were plated at a density of 2.3 × 106 per T75 flask and fed twice weekly with Dulbecco’s Modified Eagle’s Medium (DMEM)-high glucose (HG; GIBCO, Grand Island, NY) with 10% fetal bovine serum (FBS; Hyclone, Logan, UT), 100 IU/ml penicillin, 100 μg/ml streptomycin (GIBCO), and 10 μM retinoic acid (Sigma, St. Louis, MO) for up to 6 weeks. The cells were then split 1:4 and grown for 2 more days in identical medium without retinoic acid. Neuronal cells growing atop a monolayer of nonneuronal cells were dislodged with trypsin and plated in 12-well plates (Falcon; Becton Dickinson, Lincoln Park, NJ) at 1.0 × 106 cells for RNA studies, in 24-well plates (Falcon; Becton Dickinson) at 0.5 × 106 cells for immunoreactivity assays.
RNA Extraction
Total RNA was extracted from the cultured cells at four time points (days 2, 14, 28, and 42) during NT2 differentiation into NT2-N using Tri-Reagent (Molecular Research Center, Cincinnati, OH). In brief, the total RNA was extracted by a single step, guanidium thiocyanate-phenol-chloroform extraction. After centrifugation at 13,000 × g for 15 min, the RNA-containing aqueous phase was precipitated in isopropanol. RNA precipitates were then washed once in 75% ethanol and resuspended in 30 μl of RNase-free water.
Reverse Transcription
Total RNA (1 μg) was subjected to reverse transcription using Reverse Transcription System (Promega, Madison, WI) with specific primers (antisense) for SP and NK-1R for 1 hr at 42°C. The reaction was terminated by incubating the reaction mixture at 99°C for 5 min. One-tenth of the resulting cDNA was used as a template for polymerase chain reaction (PCR) amplification. The specific primers (HSP5: 5′-GCATTGCACTCCTTTCATAAGCCA-3′ and HSPR2: 5′-CTGCTGGATAAACTT-CTTCAGGTAG-3′) were used in reverse transcription for SP and NK-1R mRNA, respectively.
PCR Analysis
The PCR amplification of SP and NK-1R cDNA has been described previously (Ho et al., 1997; Lai et al., 1998a, b; 1999). Briefly, PCR amplification was performed with 2 μl of the cDNA for 40 cycles with AmpliTaq Gold (Perkin Elmer, Norwalk, CT) in a GeneAmp PCR System 2400 (Perkin Elmer). The PCR reaction mixture contained 0.2 mM of dNTPs, 20 pM of each of two primers, and 1.5 units of AmpliTaq Gold in 1× reaction buffer. Since all four isoforms of preprotachykinin-A (PPT-A) mRNA transcripts share Exon 3 (Lai et al., 1999), we designed a primer HSP3 (5′-CAGCATCCCGTTTGCC-3′, antisense) to paired with HSP4 (5′-GACAGCGACCAGATCAAGGAGGAA-3′, sense) to amplify a single cDNA fragment that represents a total SP from all four isoforms of PPT-A mRNA transcripts (Lai et al., 1999). The PCR cycle consisted of heat activation of AmpliTaq Gold for 9 min at 95°C followed by 40 cycles of 95°C for 30 sec, 48°C for 30 sec, 72°C for 30 sec, and further elongation at 72°C for 7 min. We also designed an additional primer HSP7 (5′-CTTTCATAA-GCCATTTTGTGAGAGA-3′, antisense) to pair with the primer HSP4. This primer pair amplifies only α and δ fragments at sizes of 197 and 152 bp, respectively (Lai et al., 1999). The PCR cycle condition used for this pair of primers was as follows: 95°C 9 min followed by 40 cycles of 95°C 30 sec, 55°C 30 sec, 72°C 30 sec, and further elongation at 72°C for 7 min. In order to amplify only β and γ isoforms, another primer HSP67 (5′-CTTTCATAAGCCACAG-AATTTAAA-3′, antisense) derived from the junction of exons 6 and 7 was designed to pair with HSP4, resulting in PCR amplified products of 251 bp (β) and 206 bp (γ), respectively (Lai et al., 1999). The PCR cycle condition for this primer pair was the same as that for primer pair of HSP4/HSP3. The NK-1R primer pair (sense: 5′-AGGACAGTGACGAACTATTTTCTGG-3′; antisense: 5′-CTGCTGGATAAAACTTCTTCAGGTAG-3′) corresponds to +190 and +831 on mRNA NK-1R sequences with a predicted amplification size of 640 bp based on the published cDNA sequence for human NK-1R (Rameshwar and Gascon, 1995; Ho et al., 1997; Lai et al., 1999). The PCR cycle condition was as follows: 95°C 9 min followed by 40 cycles of 95°C 30 sec, 55°C 30 sec, 72°C 45 sec and further elongation at 72°C for 7 min. The primer pair for MIP-1β was 5′-CCAAACCAAAAGAAGCAAGC-3′ (sense) and 5′-AGAAACAGTGACAGT GGACC-3′ (antisense) and the PCR cycle condition was as follows: 94°C 9 min followed by 35 cycles of 94°C 30 sec, 55°C 30 sec, 72°C 30 sec, and further elongation at 72°C for 7 min. One-fourth of each PCR amplified cDNA product (12.5 μl) was electrophoresed on either 2% (for NK-1R) or 4% (for SP) ethidium bromide-stained agarose gels. The primers described above were synthesized by Integrated DNA Technologies, Inc. (Coralville, IA).
Direct Sequencing of PCR-Amplified Products
In order to further confirm the identity of PCR-amplified products of PPT-A gene, a cycle sequencing protocol was used. PCR-amplified SP fragments separated on 4% NuSieve 3:1 agarose gels were isolated and purified using Wizard PCR Preps DNA Purification System (Promega). The purified fragments with the size of 251, 206, 197, and 152 base pairs were then subjected to direct sequencing analysis. The direct sequencing was done by The Nucleic Acid/Protein Core Facility at The Children’s Hospital of Philadelphia. The sequences of each PPT-A mRNA isoform were compared for homology with the published human PPT-A gene sequence (Chiwakata et al., 1991; Ho et al., 1997; Lai et al., 1998a, b).
Real-Time RT-PCR for Quantification of SP, NK-1R, and MIP-1β mRNA
We have recently developed a highly sensitive and specific real-time reverse transcribed polymerase chain reaction (RT-PCR) assay for quantitatively measuring SP mRNA (Lai et al., 2002). The PCR primers and the MB probes used were designed with Primer Express software (PE Applied Biosystems) and were synthesized by Integrated DNA Technologies, Inc. The HSP4-HSP3′ primer pair (HSP4, 5′-CGACCAGATCAAGGAGGAACTG-3′; HSP3′, 5′-CAGCATCCCGTTTGCC-CATT-3′), which is specific for a 121-bp fragment of the SP transcript, was described previously (Lai et al., 1999), but with modification by the addition of four additional nucleotides at the 3′ end of primer HSP3 (underlined) because of the design obtained with the Primer Express software. The sequences of the primers and the MB probe were selected from the sequences of exons 2 and 3 of the PPT-A gene (Lai et al., 1998b, 2002) so that they amplify a single cDNA fragment that represents the total SP from transcripts of all four isoforms of PPT-A mRNA (Lai et al., 1999). The sequence of the MB probe specific for SP was designed to be perfectly complementary to the target sequence in exon 3 of the SP gene (Lai et al., 1999). The primer pairs specific for MIP-1β and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) are 5′-GCTGCTCAGAGACAGGA-AGTCTT-3′(sense), 5′-ACAGGAACTGCGGAGAGGAGT-3′ (antisense), and 5′-GGTGGTCT-CCTCTGACTTCAACA-3′ (sense), 5′-GTTGCTGTAGCCA-AATTCGTTGT-3′ (antisense), respectively. The sequences of the MB probes for MIP-1β and GAPDH were designed to be complementary to the sequences between the sequences of each primer in the primer pairs for MIP-1β and GAPDH, respectively. The following are the sequences of the three MB probes: SP, 5′-FAM-GCGAGCA-GAATCGCCCGGAGACCCAAG-CGCTCGC-DABCYL-3′; MIP-1β, 5′-FAM-GCGAGCCCCGGATGCTTCTCCATG-AGACAC-AGCTCGC-DABCYL-3′; GAPDH, 5′-FAM-GCGAGCCTGGCATTGCCCTCAACGACCAC-GCTCGC-DAB-CYL-3′. The underlined sequences are the stem sequences, FAM is 6-carboxyfluorescein, and DABCYL is 4-(4′-dimethylaminophenylaso) benzoic acid. The stem sequences were selected such that they would not complement the sequences within the loop region. Also, the lengths of the MB probes were designed such that the annealing temperature is slightly higher than those for the PCR primers. The MB probes were labeled at the 5′ end with FAM and the quencher DAB-CYL at the 3′ end. The primers and MB probes were resuspended in TE (Tris-EDTA) buffer and stored at −30°C. The real-time PCR for the quantification of NK-1R mRNA was performed in the ABI PRISM 7000 Sequence Detection Systems using the Brilliant SYBR Green QPCP Master Mix (STRATAGENE, La Jolla, CA) as instructed by the manufacturer. The primer pair is the same as stated above.
Immunohistochemical Staining of SP in NT2-N
VECTASTAIN® ABC peroxidase system (Vector Laboratories, Inc., Burlingame, CA), rabbit anti-human SP antibody (Sigma) were used to stain the NT2-N maintained in the 24-well culture plates. Briefly, terminally differentiated NT2-N neurons were rinsed in phosphate-buffered saline (PBS), fixed in ice-cold methanol for 8 min, rinsed again with PBS, then incubated for 30 min in 0.3% H2O2-containing PBS and 20 min in diluted normal blocking serum. The cells were rinsed again with PBS and exposed to rabbit anti-human SP antiserum (1:1,000 in 0.5% bovine serum albumin [BSA]-containing PBS) at room temperature for 30 min. Normal rabbit serum (1:1,000 in 0.5% BSA-containing PBS) severed as negative controls for SP staining, respectively. Incubation with diluted biotinylated secondary antibody solution, followed by the avidin-biotin horseradish peroxidase complex each for 30 min at room temperature with rinses in PBS between incubations. After completion of the last incubation, peroxidase substrate (DAB, Vector Laboratories, Inc.) was added in order to develop a brown visible precipitate for photomicroscopy examination under a microscope (Zeiss, Germany) equipped with camera (Zeiss).
Immunofluorescent Staining of NK-1R in NT2-N
NT2 aggregate cultures were set up on glass coverslips in a 24-well culture plate. Terminally differentiated NT2-N neurons were fixed with cold methanol (−20°C) for 8 min, and then pretreated with a blocking solution (Minimal Essential Medium containing 15 mM HEPES buffer, 10% fetal bovine serum, and 0.05% sodium azide) for 10 min. Coverslips were then incubated with rabbit anti-human NK-1 receptor (SP receptor; Novus Biologicals, Inc., Littleton, CO) diluted 1:100 in blocking solution for 30 min at room temperature. Coverslips were then treated with biotinylated donkey anti-rabbit immunoglobulins (Jackson ImmunoResearch Labs Inc, West Grove, PA, and 1:100) for 30 min at room temperature followed by fluorescein-congugated streptavidin (Jackson ImmnunoResearch, 1:100) for 20 min at room temperature. Coverslips were washed between steps with PBS and postfixed with cold methanol for 8 min then mounted in vectorshield (Vector Laboratories, Inc.). Samples were viewed and scanned with a Leica TCS SP2 spectral confocal microscope.
ELISA for MIP-1β
Enzymed-linked immunosorbent assay (ELISA) kits for MIP-1β was purchased from Endogen, Inc. (Cambridge, MA). The assay was performed as instructed in the protocol provided by the manufacturer. In brief, 50 μl of supernatants was added to antibody-coated wells and incubated for 1 hr at room temperature. The plate was washed with the provided buffer solution and incubated with 100 μl of biotinylated antibody reagent for 1 hr at room temperature. The plate was washed again, treated with 100 μl of prepared Streptavidin-horseradish peroxidase (HRP) solution, and incubated for 30 min at room temperature. After an additional wash, 100 μl of 3,3′,5,5′-tetramethylbenzidine (TMB) substrate solution was added to each well, and color was allowed to develop at room temperature for 30 min. The reaction was stopped by the addition of 100 μl of stop solution to each well. The plate was read on a microplate reader (ELX800, Bio-Tek Instruments, Inc., Winooski, VT).
RESULTS
Expression of SP and NK-1R in NT2-N
We first investigated whether the terminally retinoic acid-differentiated NT2-N contains mRNA transcripts for SP and its receptor (NK-1R). NT2-N cells express mRNA for both SP (Fig. 1A) and NK-1R (Fig. 1B) as detected by RT-PCR. Since this RT-PCR analysis with specific primer pair distinguishes the four known alternatively spliced products (α, β, γ, and δ mRNA transcripts) of the PPT-A gene (Lai et al., 1998b, 1999, 2000), we then examined whether all four isoforms of the transcripts (α, β, γ, and δ) of SP mRNA are expressed by NT2-N. The NT2-N expressed all four PPT-A transcripts (Fig. 2), which were confirmed by sequence analysis of the RT-PCR amplified PPT-A α, β, γ, and δ transcripts from NT2-N (data not shown). The specificity of the RT-PCR-amplified NK-1R mRNA was also confirmed by sequence analysis (data not shown).
Fig. 1.

Reverse transcribed-polymerase chain reaction (RT-PCR) amplification of preprotachykinin-A (PPT-A) gene transcript (A) with the primer pair of HSP4/HSP3 and neurokinin-1 receptor (NK-1R) gene transcript (B) of human neuronal cells (NT2-N; 4 weeks after retinoic acid [RA] treatment). Sizes are estimated from DNA Ladder (100-bp fragments) coelectrophoresesed as markers. Marker,100-bp fragments of DNA ladder; −, negative control in which template was omitted; +, human brain tissue as positive control; NT2-N; RT-PCR-amplified PPT-A transcript (A) or NK-1R gene transcript (B) of NT2-N.
Fig. 2.

RT-PCR amplification of PPT-A transcripts of NT2-N (4 weeks after RA treatment) with the primer pairs of HSP4/HSP67 and HSP4/HSP7. Sizes are estimated from DNA Ladder (100-bp fragments) coelectrophoresesed as markers. Marker, 100-bp fragments of DNA ladder as markers; NT2-N, RT-PCR-amplified PPT-A transcripts of NT2-N using primer pairs of HSP4/HSP67 (β and γ isoforms) or HSP4/HSP7 (α and δ isoforms), respectively. RNA transcripts derived from α and δ, γ, and β isoform-containing plasmids are used as positive controls as indicated.
Expression of SP and NK-1R During NT2 Cell Differentiation
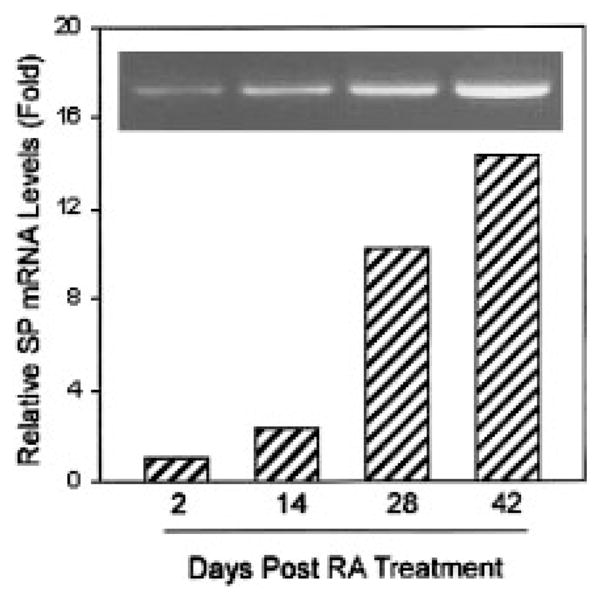
In order to determine whether the cell differentiation affects SP and NK-1R mRNA expression in NT2-N, cellular RNA was extracted at four different time points (dasy 2, 24, 28, and 42) during NT2 differentiation into NT2-N. We observed a consistent increase of SP mRNA expression during the cell differentiation as demonstrated by the specific PCR-amplified product visualized on ethidium bromide-stained agarose gels (Fig. 3, inset) and by real-time RT-PCR assay (Fig. 3). There is also increased expression of NK-1R mRNA in NT2-N cells in comparison to undifferentiated cells (NT2) during NT2 cell differentiation into NT2-N (Fig. 4).
Fig. 3.

RT-PCR analysis and real-time RT-PCR quantification of Substance P (SP) gene expression during NT2 differentiation into NT2-N. RA-treated NT2 cells at different time points as indicated were selected to evaluate SP mRNA expression and quantification by RT-PCR (inset) and real-time RT-PCR, respectively. The SP mRNA levels were normalized on the basis of the SP mRNA/glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA ratio obtained by the real-time RT-PCR.
Fig. 4.
RT-PCR analysis and real-time RT-PCR quantification of NK-1R mRNA expression during NT2 differentiation into NT2-N. The same RNA samples used in Figure 3 were also analyzed for NK-1R gene expression by RT-PCR (inset) and real-time RT-PCR (SYBR Green). The NK-1R mRNA levels were normalized on the basis of the NK-1R mRNA/GAPDH mRNA ratio obtained by the real-time RT-PCR.
Quantification of SP mRNA in NT2-N Cells and Human Immune Cells
Since we previously demonstrated that human immune cells (monocytes/macrophages, PBLs, and microglia) express SP and NK-1R, we quantitatively compared SP expression in NT2-N cells with that in the human immune cells. We therefore measured the SP mRNA levels in NT2-N cells and human immune cells (PBLs, monocytes/macrophages, and microglia) using SP real-time RT-PCR assay that we have recently developed (Lai et al., 2002). As expected, the NT2-N cells express the highest levels of SP mRNA among the cells tested (data not shown).
NT2-N Cells Express Functional SP and NK-1R
In order to determine whether NT2-N cells express functional SP and NK-1R, we performed immunohistochemical and immunofluroscent staining of the NT2-N cells using specific anti-human SP and NK-1R antibodies, respectively. NT2-N cells have intracellular immunoreactivity with anti-SP antibody (Fig. 5A) and the membrane staining with anti-NK-1R antibody (Fig. 6A). The specificity for SP and NK-1R staining was confirmed by the absence of immunoreactivity in the NT2-N cells incubated with the control antibodies (Figs. 5B, 6B).
Fig. 5.

Immunohistochemical staining of the intracellular SP in the NT2-N cells using anti-SP antibody. The NT2-N cells (4 weeks after RA treatment) were stained with either rabbit anti-human SP antiserum (A) or normal rabbit serum (B). The magnification is ×400.
Fig. 6.

Immunofluorescent staining of the membrane SP receptor (NK-1R) on the NT2-N cells using anti-NK-1R antibody. The NT2-N cells (4 weeks after RA treatment) were stained with either a rabbit anti-human NK-1R antibody (A) or normal rabbit IgG (B). The magnification is ×400.
Induction of MIP-1β Production by SP in NT2-N
Cells within the human central nervous system including neurons constitutively express or can be induced to express several chemokines including MIP-1β under both physiological and pathological conditions (Xia et al., 1998). In order to further determine whether NT2-N cells possess functional SP receptors, we investigated whether SP has the ability to regulate β-chemokine (MIP-1β) expression in these cells. We demonstrated that NT2-N cells express MIP-1β at both mRNA and protein levels (Fig. 7). We also observed that NT2-N cells treated with exogenous SP showed elevated expression of MIP-1β in comparison to the control, as demonstrated by quantitative real-time RT-PCR and ELISA assays (Fig. 7), indicating that SP receptors expressed by NT2-N cells are functionally active.
Fig. 7.

Effect of SP on macrophage inflammatory protein 1 beta (MIP-1β) expression in NT2-N cells. A: Effect of SP on MIP-1β mRNA expression. NT2-N cells were incubated with SP (10−8 M to 10−6 M) for 3 hr and total RNA was isolated and subjected to RT-PCR and electrophoresis (inset) and real-time PCR to quantify MIP-1β mRNA. The quantitative data are expressed as mean of triplicate cultures of MIP-1β mRNA copy number per 103 copies of GAPDH mRNA. B: Effect of SP on MIP-1β protein production in NT2-N cells. NT2-N cells were incubated with SP (10−8 M to 10−6 M) for 24 hr, MIP-1β protein in the supernatants were then assayed using enzyme-linked immunosorbent assay (ELISA). The data shown are presented as the mean ± S.D. of triplicate cultures and are representative of three independent experiments (*P < 0.05).
DISCUSSION
There is substantial evidence supporting that SP is a major mediator of neurogenic inflammation and immunomodulatory activities in the CNS. Activation or damage of neurons can lead to changes in neuropeptide biosynthesis that results from induction of neuropeptide gene expression (Harrison and Geppetti, 2001). In this investigation, we have provided compelling evidence that human NT2-N cells express SP and SP receptor NK-1R. In comparison to human immune cells (microglia, MDMs, and PBLs), NT2-N cells expressed the highest levels SP mRNA (data not shown), indicating that a neuronally differentiated, teratocarcinoma-derived cell line has more SP mRNA than do isolated primary human immune cells. Sequencing of SP- and NK-1R-encoding mRNA isolated from the NT2-N has confirmed the identity of this SP mRNA to that found in human brain tissue, suggesting that the SP produced from NT2-N may have biological function that is similar to in vivo role of SP and SP receptor. This assumption is supported by our observations that both SP and NK-1R immunoreactivity was detected either intracellularly or on the cell membrane of NT2-N (Figs. 5 and 6), and that SP up-regulated MIP-1β expression in NT2-N cells (Fig. 7). Coughlan et al. (2000) recently demonstrated that NT2-N cells not only express multiple functional chemokine receptors such as CCR2, CXCR3, and CXCR4, but also produce chemokine MCP-1 (monocyte chemoattractant protein-1). The chemokines play an important role in the activation and directional migration of immune cells to sites of CNS inflammation. Thus, SP-mediated up-regulation of β-chemokine in NT2-N cells provide additional evidence that SP plays an important role in neurogenic inflammation and immunoregulation in the CNS.
The tachykinins in mammals are the products of two genes, preprotachykinin-A (PPT-A) and PPT-B. SP is translated from the PPT-A gene, which contains seven exons that undergo differential splicing to yield α, β, γ, and δ PPT-A mRNAs with each mRNA differing in exon usage corresponding to the protein coding region (Takeda et al., 1990) as a result of alternative splicing (Nawa et al., 1984; Kawaguchi et al., 1986; Krause et al., 1987; Lai et al., 1998b). SP precursor sequences are encoded by all four PPT-A mRNAs. The α and δ forms codes for the SP precursor, whereas the β and γ forms code for both the SP and substance K (neurokinin A) precursors (Krause et al., 1987; Carter and Krause, 1990). Thus, multiple tachykinin peptides with related biological activities can be derived from the SP gene. Neuronal tissue processes all three PPT mRNAs from the same gene (Krause et al., 1987; Carter and Krause, 1990). We have now demonstrated that human NT2-N cells contain all four mRNA transcripts (α, β, γ, and δ) of PPT-A gene (Fig. 2). It is important to know whether the NT2-N cells possess other biological properties that are similar to the neurons in human CNS. We showed that the RT-PCR-amplified products of SP mRNA isolated from NT2-N have the same size as those found in human neural tissue as demonstrated by ethidium bromide staining and direct DNA sequencing. Among the four isoforms of PPT-A gene transcripts, α, β, and δ transcripts are predominant isoforms expressed in NT2-N cells, which are similar (sizes) to those observed in human CNS tissue (Fig. 2). Although the biological significance of the existence of differential splicing of these mRNA transcripts is unknown, the high levels of α, β, and δ transcript expression suggest that these mRNA transcripts are the most relevant for SP-mediated biological activities in NT2-N cells. MacDonald et al. (1988, 1989) reported that the relative amounts of α, β, and γ-PPT mRNA in rat brain remain fairly constant, with γ-PPT predominant (80% of total PPT mRNA) and α-PPT mRNA in very low amounts (<1%). We, however, observed that γ-PPT transcript in NT2-N cells was not a dominant transcript. The difference between our observation and others may be due to the differences between passaged, transformed cell line in vitro and measurements of endogenous SP levels in vivo. Nawa et al. (1984) reported that relative amounts of α and β-PPT mRNA in bovine nervous system and peripheral tissues vary, suggesting these mRNA transcripts are regulated in a tissue-specific manner. Although the levels of these mRNA transcripts vary, each of these PPT mRNAs are translatable in vitro (MacDonald et al., 1988, 1989). Thus, it will be of importance for future studies to determine whether the products of each of these transcripts in NT2-N have differential function in regulation of biological activities within the CNS.
Since SP expression and metabolism are dynamic, changing processes (Black et al., 1984) and there is an autocrine regulation pathway of SP (Pascual and Bost, 1990; Castagliuolo et al., 1997; Cioni et al., 1998), our future studies will focus on factors that regulate SP expression in CNS cells. Whether neuronal release of SP is inducible and whether SP-induced MIP-1β expression in NT2-N cells have a role in recruitment of immune cells to inflammatory sites in the CNS is unknown; our data, however, provide a novel basis for future studies on the role of SP in neuroimmune responses within the CNS. Further studies are required to elucidate the precise mechanism(s) whereby SP-NK-1R pathway participates in inflammatory processes in the CNS.
Acknowledgments
This investigation was supported by grants from the National Institutes of Health (DA 12815 to W.Z.H., MH 49981 and AA 13547 to S.D.D., and NS 25044 to D.E.P. and funds from the National Multiple Sclerosis Society (to W.Z.H.) and Muscular Dystrophy Association (to D.E.P.).
References
- Andrews PW. Retinoic acid induces neuronal differentiation of a cloned human embryonal carcinoma cell line in vitro. Dev Biol. 1984;103:285–293. doi: 10.1016/0012-1606(84)90316-6. [DOI] [PubMed] [Google Scholar]
- Beczkowska IW, Gracy KN, Pickel VM, Inturrisi CE. Inducible expression of N-methyl-D-aspartate receptor, and delta and mu opioid receptor messenger RNAs and protein in the NT2-N human cell line. Neuroscience. 1997;79:855–862. doi: 10.1016/s0306-4522(97)00034-1. [DOI] [PubMed] [Google Scholar]
- Black IB, Adler JE, Dreyfus CF, Jonakait GM, Katz DM, LaGamma EF, Markey KM. Neurotransmitter plasticity at the molecular level. Science. 1984;225:1266–1270. doi: 10.1126/science.6147894. [DOI] [PubMed] [Google Scholar]
- Bozic CR, Lu B, Hopken UE, Gerard C, Gerard NP. Neurogenic amplification of immune complex inflammation. Science. 1996;273:1722–1725. doi: 10.1126/science.273.5282.1722. [DOI] [PubMed] [Google Scholar]
- Carter MS, Krause JE. Structure, expression, and some regulatory mechanisms of the rat preprotachykinin gene encoding substance P, neurokinin A, neuropeptide K, and neuropeptide gamma. J Neurosci. 1990;10:2203–2214. doi: 10.1523/JNEUROSCI.10-07-02203.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castagliuolo I, Keates AC, Qiu B, Kelly CP, Nikulasson S, Leeman SE, Pothoulakis C. Increased substance P responses in dorsal root ganglia and intestinal macrophages during Clostridium difficile toxin A enteritis in rats. Proc Natl Acad Sci USA. 1997;94:4788–4793. doi: 10.1073/pnas.94.9.4788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiwakata C, Brackmann B, Hunt N, Davidoff M, Schulze W, Ivell R. Tachykinin (substance-P) gene expression in Leydig cells of the human and mouse testis. Endocrinology. 1991;128:2441–2448. doi: 10.1210/endo-128-5-2441. [DOI] [PubMed] [Google Scholar]
- Cioni C, Renzi D, Calabro A, Annunziata P. Enhanced secretion of substance P by cytokine-stimulated rat brain endothelium cultures. J Neuroimmunol. 1998;84:76–85. doi: 10.1016/s0165-5728(97)00235-x. [DOI] [PubMed] [Google Scholar]
- Coughlan CM, McManus CM, Sharron M, Gao Z, Murphy D, Jaffer S, Choe W, Chen W, Hesselgesser J, Gaylord H, Kalyuzhny A, Lee VM, Wolf B, Doms RW, Kolson DL. Expression of multiple functional chemokine receptors and monocyte chemoattractant protein-1 in human neurons. Neuroscience. 2000;97:591–600. doi: 10.1016/s0306-4522(00)00024-5. [DOI] [PubMed] [Google Scholar]
- Ferrari A, Ehler E, Nitsch RM, Gotz J. Immature human NT2 cells grafted into mouse brain differentiate into neuronal and glial cell types. FEBS Lett. 2000;486:121–125. doi: 10.1016/s0014-5793(00)02251-1. [DOI] [PubMed] [Google Scholar]
- Gerard NP, Garraway LA, Eddy RL, Jr, Shows TB, Iijima H, Paquet JL, Gerard C. Human substance P receptor (NK-1): organization of the gene, chromosome localization, and functional expression of cDNA clones. Biochemistry (Mosc) 1991;30:10640–10646. doi: 10.1021/bi00108a006. [DOI] [PubMed] [Google Scholar]
- Gitter BD, Regoli D, Howbert JJ, Glasebrook AL, Waters DC. Interleukin-6 secretion from human astrocytoma cells induced by substance P. J Neuroimmunol. 1994;51:101–108. doi: 10.1016/0165-5728(94)90134-1. [DOI] [PubMed] [Google Scholar]
- Guillemain I, Alonso G, Patey G, Privat A, Chaudieu I. Human NT2 neurons express a large variety of neurotransmission phenotypes in vitro. J Comp Neurol. 2000;422:380–395. doi: 10.1002/1096-9861(20000703)422:3<380::aid-cne5>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Harrison S, Geppetti P. Substance p. Int J Biochem Cell Biol. 2001;33:555–576. doi: 10.1016/s1357-2725(01)00031-0. [DOI] [PubMed] [Google Scholar]
- Ho WZ, Kaufman D, Uvaydova M, Douglas SD. Substance P augments interleukin-10 and tumor necrosis factor-alpha release by human cord blood monocytes and macrophages. J Neuroimmunol. 1996;71:73–80. doi: 10.1016/s0165-5728(96)00132-4. [DOI] [PubMed] [Google Scholar]
- Ho WZ, Lai JP, Zhu XH, Uvaydova M, Douglas SD. Human monocytes and macrophages express substance P and neurokinin-1 receptor. J Immunol. 1997;159:5654–5660. [PubMed] [Google Scholar]
- Ho WZ, Stavropoulos G, Lai JP, Hu BF, Magafa V, Anagnostides S, Douglas SD. Substance P C-terminal octapeptide analogues augment tumor necrosis factor-alpha release by human blood monocytes and macrophages. J Neuroimmunol. 1998;82:126–132. doi: 10.1016/s0165-5728(97)00175-6. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Hoshimaru M, Nawa H, Nakanishi S. Sequence analysis of cloned cDNA for rat substance P precursor: existence of a third substance P precursor. Biochem Biophys Res Commun. 1986;139:1040–1046. doi: 10.1016/s0006-291x(86)80282-0. [DOI] [PubMed] [Google Scholar]
- Kincy-Cain T, Bost KL. Substance P-induced IL-12 production by murine macrophages. J Immunol. 1997;158:2334–2339. [PubMed] [Google Scholar]
- Kostyk SK, Kowall NW, Hauser SL. Substance P immunoreactive astrocytes are present in multiple sclerosis plaques. Brain Res. 1989;504:284–288. doi: 10.1016/0006-8993(89)91369-3. [DOI] [PubMed] [Google Scholar]
- Krause JE, Chirgwin JM, Carter MS, Xu ZS, Hershey AD. Three rat preprotachykinin mRNAs encode the neuropeptides substance P and neurokinin A. Proc Natl Acad Sci USA. 1987;84:881–885. doi: 10.1073/pnas.84.3.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai JP, Douglas SD, Ho WZ. Human lymphocytes express substance P and its receptor. J Neuroimmunol. 1998a;86:80–86. doi: 10.1016/s0165-5728(98)00025-3. [DOI] [PubMed] [Google Scholar]
- Lai JP, Douglas SD, Rappaport E, Wu JM, Ho WZ. Identification of a delta isoform of preprotachykinin mRNA in human mononuclear phagocytes and lymphocytes. J Neuroimmunol. 1998b;91:121–128. doi: 10.1016/s0165-5728(98)00170-2. [DOI] [PubMed] [Google Scholar]
- Lai JP, Douglas SD, Zhao M, Ho WZ. Quantification of substance P mRNA in human mononuclear phagocytes and lymphocytes using a mimic-based RT-PCR. J Immunol Methods. 1999;230:149–157. doi: 10.1016/s0022-1759(99)00120-9. [DOI] [PubMed] [Google Scholar]
- Lai JP, Zhan GX, Campbell DE, Douglas SD, Ho WZ. Detection of substance P and its receptor in human fetal microglia. Neuroscience. 2000;101:1137–1144. doi: 10.1016/s0306-4522(00)00398-5. [DOI] [PubMed] [Google Scholar]
- Lai JP, Douglas SD, Shaheen F, Pleasure DE, Ho WZ. Quantification of substance P mRNA in human immune cells by real-time reverse transcriptase PCR assay. Clin Diagn Lab Immunol. 2002;9:138–143. doi: 10.1128/CDLI.9.1.138-143.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HR, Ho WZ, Douglas SD. Substance P augments tumor necrosis factor release in human monocyte-derived macrophages. Clin Diagn Lab Immunol. 1994;1:419–423. doi: 10.1128/cdli.1.4.419-423.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieb K, Fiebich BL, Berger M, Bauer J, Schulze-Osthoff K. The neuropeptide substance P activates transcription factor NF-kappa B and kappa B-dependent gene expression in human astrocytoma cells. J Immunol. 1997;159:4952–4958. [PubMed] [Google Scholar]
- Lotz M, Vaughan JH, Carson DA. Effect of neuropeptides on production of inflammatory cytokines by human monocytes. Science. 1988;241:1218–1221. doi: 10.1126/science.2457950. [DOI] [PubMed] [Google Scholar]
- Luber-Narod J, Kage R, Leeman SE. Substance P enhances the secretion of tumor necrosis factor-alpha from neuroglial cells stimulated with lipopolysaccharide. J Immunol. 1994;152:819–824. [PubMed] [Google Scholar]
- MacDonald MR, McCourt DW, Krause JE. Posttranslational processing of alpha-, beta-, and gamma-preprotachykinins. Cell-free translation and early posttranslational processing events. J Biol Chem. 1988;263:15176–15183. [PubMed] [Google Scholar]
- MacDonald MR, Takeda J, Rice CM, Krause JE. Multiple tachykinins are produced and secreted upon post-translational processing of the three substance P precursor proteins, alpha-, beta-, and gamma-preprotachykinin. Expression of the preprotachykinins in AtT-20 cells infected with vaccinia virus recombinants. J Biol Chem. 1989;264:15578–15592. [PubMed] [Google Scholar]
- Mantyh PW, Johnson DJ, Boehmer CG, Catton MD, Vinters HV, Maggio JE, Too HP, Vigna SR. Substance P receptor binding sites are expressed by glia in vivo after neuronal injury. Proc Natl Acad Sci USA. 1989;86:5193–5197. doi: 10.1073/pnas.86.13.5193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin FC, Charles AC, Sanderson MJ, Merrill JE. Substance P stimulates IL-1 production by astrocytes via intracellular calcium. Brain Res. 1992;599:13–18. doi: 10.1016/0006-8993(92)90846-2. [DOI] [PubMed] [Google Scholar]
- Nakanishi S. Substance P precursor and kininogen: their structures, gene organizations, and regulation. Physiol Rev. 1987;67:1117–1142. doi: 10.1152/physrev.1987.67.4.1117. [DOI] [PubMed] [Google Scholar]
- Nawa H, Kotani H, Nakanishi S. Tissue-specific generation of two preprotachykinin mRNAs from one gene by alternative RNA splicing. Nature. 1984;312:729–734. doi: 10.1038/312729a0. [DOI] [PubMed] [Google Scholar]
- Pascual DW, Bost KL. Substance P production by P388D1 macrophages: a possible autocrine function for this neuropeptide. Immunology. 1990;71:52–56. [PMC free article] [PubMed] [Google Scholar]
- Pernow B. Substance P. Pharmacol Rev. 1983;35:85–141. [PubMed] [Google Scholar]
- Pleasure SJ, Page C, Lee VM. Pure, postmitotic, polarized human neurons derived from NTera 2 cells provide a system for expressing exogenous proteins in terminally differentiated neurons. J Neurosci. 1992;12:1802–1815. doi: 10.1523/JNEUROSCI.12-05-01802.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rameshwar P, Gascon P. Substance P (SP) mediates production of stem cell factor and interleukin-1 in bone marrow stroma: potential autoregulatory role for these cytokines in SP receptor expression and induction. Blood. 1995;86:482–490. [PubMed] [Google Scholar]
- Regoli D, Drapeau G, Dion S, D’Orleans-Juste P. Receptors for substance P and related neurokinins. Pharmacology. 1989;38:1–15. doi: 10.1159/000138512. [DOI] [PubMed] [Google Scholar]
- Takeda Y, Takeda J, Smart BM, Krause JE. Regional distribution of neuropeptide gamma and other tachykinin peptides derived from the substance P gene in the rat. Regul Pept. 1990;28:323–333. doi: 10.1016/0167-0115(90)90030-z. [DOI] [PubMed] [Google Scholar]
- Xia MQ, Qin SX, Wu LJ, Mackay CR, Hyman BT. Immunohistochemical study of the beta-chemokine receptors CCR3 and CCR5 and their ligands in normal and Alzheimer’s disease brains. Am J Pathol. 1998;153:31–37. doi: 10.1016/s0002-9440(10)65542-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younkin DP, Tang CM, Hardy M, Reddy UR, Shi QY, Pleasure SJ, Lee VM, Pleasure D. Inducible expression of neuronal glutamate receptor channels in the NT2 human cell line. Proc Natl Acad Sci USA. 1993;90:2174–2178. doi: 10.1073/pnas.90.6.2174. [DOI] [PMC free article] [PubMed] [Google Scholar]


