Abstract
BACKGROUND
Bortezomib is active for newly diagnosed and relapsed multiple myeloma, and has synergistic activity with melphalan. We conducted a randomized trial to determine the safety and efficacy of adding bortezomib to a preparative regimen of arsenic trioxide (ATO), ascorbic acid (AA) and melphalan.
METHODS
Among 60 patients enrolled between October 2006 and September 2007, 58 received autologous transplantation with a preparative regimen of melphalan 200 mg/m2 IV, AA 1000 mg/day IV × 7 days and ATO 0.25 mg/kg IV × 7 days. Patients were randomized to receive no bortezomib (group 1), bortezomib 1 mg/m2 × 3 doses (group 2), and bortezomib 1.5 mg/m2 × 3 doses (group 3). Primary endpoints were complete response (CR), grade 4 toxicity, and 90-day treatment-related mortality (TRM). Secondary endpoints were progression-free (PFS) and overall survival (OS).
RESULTS
Median follow-up in all surviving patients was 36 months (range 20–43). CR rates in groups 1, 2 and 3 were 20%, 10% and 10%. Grade 3–4 non-hematologic toxicities and TRM were comparable. Median OS has not been reached in the groups, while median PFS was 17.8, 17.4 and 20.7 months, respectively. PFS and OS were significantly shorter in patients with high-risk cytogenetics (p0.016 and 0.0001) and relapsed disease (p=0.0001 and 0.0001) regardless of the treatment group.
CONCLUSION
Adding bortezomib to a preparative regimen of ATO, AA and high dose melphalan is safe and well tolerated in patients with multiple myeloma. There was no significant improvement in CR rate, PFS or OS in the bortezomib groups.
Keywords: Autologous transplant, Myeloma, Bortezomib, Arsenic Trioxide, Melphalan
INTRODUCTION
Multiple myeloma (MM) is the second most common haematological malignancy and is responsible for approximately 1 per cent of all cancer related deaths in western countries1. Current treatment options include induction therapy followed by consolidation with high-dose chemotherapy and autologous hematopoietic stem cell transplantation (auto-HCT) in eligible patients2. At least 2 randomized trials have shown that auto-HCT results in a significantly prolonged event-free and overall survival when compared to conventional chemotherapy in patients with MM 3, 4. Although auto-HCT is safe with a transplant-related mortality (TRM) of <2%, it is associated with a continuing risk of disease relapse5. Attempts at improving outcomes with more intensive regimens have not been successful 5–8. Thus further exploration of novel preparative regimens is warranted.
Bortezomib, a protease inhibitor, is an active agent in newly diagnosed as well as relapsed and refractory multiple myeloma. Several large clinical trials have demonstrated the safety and efficacy of bortezomib in multiple myeloma 9–11. In-vitro exposure of melphalan resistant myeloma cell lines to noncytotoxic concentrations of bortezomib increased sensitivity to melphalan 12, 13. In one preclinical study, subtoxic concentrations of bortezomib potently sensitized MM cell lines and patient cells to the DNA-damaging activity of melphalan, including cells resistant to melphalan13. In addition, combining bortezomib with oral melphalan showed acceptable toxicity and encouraging responses in patients resistant to either agent alone 14.
Bortezomib has been used in combination with high-dose melphalan as part of conditioning regimens in at least two clinical trials14, 15. We have previously demonstrated the feasibility of combining arsenic trioxide (ATO) with ascorbic acid (AA) and high-dose melphalan (MAC) in relapsed multiple myeloma 16. This combination was well tolerated and resulted in a response rate of 87%. Based on these data, we conducted a randomized phase II clinical trial assessing the safety and efficacy of bortezomib in combination with high-dose melphalan, AA and ATO. We postulated that the combination of these anti-myeloma drugs would improve the CR rate, a surrogate for improved disease-free and overall survival.
METHODS
Patients
Patients were enrolled from October 2006 to September 2007. The inclusion criteria were a diagnosis of multiple myeloma, age up to 75 years, Zubrod performance status <2, left ventricular ejection fraction >40% with no uncontrolled arrhythmia or unstable cardiac disease, corrected QT interval less than 470 milliseconds, adequate pulmonary function test results with no symptomatic pulmonary disease; serum bilirubin <2 X upper limit of normal, and serum glutamic pyruvic transaminase <4 X upper limit of normal with no evidence of chronic active hepatitis or cirrhosis. Other inclusion criteria were the absence of effusions or ascites >1L prior to drainage, negative human immunodeficiency virus testing, a negative pregnancy test in a woman with child bearing potential and the willingness of the patient or guardian to provide informed consent.
Treatment Plan
Peripheral blood stem cells (PBSC) were mobilized and collected following granulocyte colony stimulating factor (G-CSF) alone, or chemotherapy+G-CSF according to standard institutional guidelines. Patients were randomized to one of the three treatment groups in a 1:1:1 ratio. Table 1 depicts the treatment schema. All patients received ATO 0.25 mg/kg IV on days −9 to −3 over 2 hours, AA 1000 mg IV on days −9 to −3 over 30 minutes, and melphalan 100 mg/m2 IV on days −4 and −3 over 30 minutes. The dose of ATO was administered after melphalan in all patients. Group 1 did not receive bortezomib and served as a control. Patients received supportive care according to established departmental guidelines. Patients received G-CSF, 5 μg /kg/day from day +1 until the absolute neutrophil count (ANC) was 0.5 × 109/L for 2 consecutive days. Oral levofloxacin, acyclovir, and fluconazole were given for the duration of neutropenia. Blood products were given for hemoglobin <8 g/dL and platelets <20 × 109/L.
Table 1.
Treatment Schema
| Group1 | Group2 | Group3 | |
|---|---|---|---|
| Melphalan | 100 mg/m2 IV Days −4, −3 |
100 mg/m2 IV Days −4, −3 |
100 mg/m2 IV Days −4, −3 |
| Ascorbic Acid | 1000 mg IV Days −9 to −3 |
1000 mg IV Days −9 to −3 |
1000 mg IV Days −9 to −3 |
| Arsenic Trioxide | 0.25 mg/kg Days −9 to −3 |
0.25 mg/kg Days −9 to −3 |
0.25 mg/kg Days −9 to −3 |
| Bortezomib | None | 1 mg/m2 Days −9, −6, −3 |
1.5 mg/m2 Days −9, −6, −3 |
Response Criteria
The primary endpoints were CR, time to grade 4 toxicity, and death. The secondary endpoints were response rate, progression-free survival (PFS), and overall survival (OS). Toxicity was graded according to National Cancer Institute Common Terminology Criteria (version 3.1; Bethesda, MD). Engraftment was defined as an ANC of 0.5 × 109/L for 2 consecutive days. Response was defined according to the criteria of the International Myeloma Working Group (IMWG) 17. PFS was the time from the day of auto-HCT to progression or time last known alive. OS was time from the day of auto-HCT to death or time last known alive.
Prognostic factors
High-risk cytogenetics and molecular characteristics were defined as: del13 or hypodiploidy on conventional cytogenetics, and t(4;14), t(14;16) or del17p on conventional cytogenetics or fluorescence in situ hybridization (FISH)18.
Statistical Analyses
Patient characteristics were summarized using the mean and standard deviation (SD) for numerical valued variables and frequencies with percentages for categorical variables. Differences in the distributions of patient characteristics between the three treatment groups and associations between variables were assessed using the Kruskal-Wallis test for numerical variables 19 or generalized Fisher exact tests 20, 21 for categorical variables. Unadjusted probabilities of OS and PFS time were estimated using the method of Kaplan and Meier (KM) 22. The log-rank test 23 was used to compare unadjusted OS and PFS between subgroups. Bayesian regression models 24 were fit to assess the joint effects of patient covariates and treatment arms on both OS and PFS, with the best fitting model chosen for each outcome from the exponential, Weibull, log logistic, log normal and gamma distributions using the Bayesian Information Criterion (BIC) 25. In each multivariate regression model, the covariates included treatment arm, Gender, Race, Age, Disease Stage, Diagnostic Status (defined by the three subgroups: 1st Remission Consolidation without progressive disease (PD) as a baseline group, No 1st Remission Consolidation without PD, and PD), Cytogenetics (high or standard risk) 26, and prior Response Category (PD, PR/VGPR, SD). For each model fit, we assumed that each parameter in the linear term followed a non-informative normal prior with mean 0 and variance 10,000. All frequentist statistical analyses were carried out in R and Bayesian computations in Open BUGS version 3.1.2 27
RESULTS
Patients
From October 2006 to September 2007, 60 patients were randomly assigned to 3 treatment groups with twenty patients enrolled in each group. Table 2 summarizes the characteristics of the 60 patients enrolled on this study, 58 of whom went on to receive auto-HCT. One patient in treatment group 3 developed deep venous thrombosis before completing the preparative regimen and was withdrawn from the study. A second patient in group 1 developed pneumonia and sepsis before receiving high-dose melphalan and died 2 months later of multi-organ failure. The mean (SD) age of patients at transplant was 60 (6.5), 57 (6.1) and 63 (7.9) in treatment groups 1, 2, and 3, respectively. The patients were well matched clinically, while high-risk cytogenetic abnormalities were present in 1 patient (5%) in group 1, 4 patients (15%) in group 2, and 4 patients (20%) in group 3. Fifteen patients (25%) had relapsed disease prior to auto-HCT (6, 6, and 3 in groups 1, 2 and 3, respectively), and six had received a prior auto -HCT. The median interval between diagnosis and auto-HCT was 12, 9 and 10 months in groups 1, 2 and 3, respectively. Sixteen patients (6, 6, and 4 in groups 1, 2, and 3) had >10% plasma cells in the BM at auto-HCT.
Table 2.
Descriptive Statistics for Categorical Variables. Each cell contains the number and % in each of the variable’s subgroups
| Variable | Group1 | Group2 | Group3 | Total | P-Value | |
|---|---|---|---|---|---|---|
| Gender | Female | 12 (60%) | 9 (45%) | 6 (30%) | 27 (45%) | 0.184 |
| Male | 8 (40%) | 11 (55%) | 14 (70%) | 33 (55%) | ||
| Race | Asian | 0 (0%) | 1 (5%) | 1 (5%) | 2 (3%) | 0.201 |
| Black | 1 (5%) | 2 (10%) | 5 (25%) | 8 (13%) | ||
| Mixed | 1 (5%) | 4 (20%) | 3 (15%) | 8 (13%) | ||
| Other | 0 (0%) | 1 (5%) | 0 (0%) | 1 (2%) | ||
| White | 18 (90%) | 12 (60%) | 11 (55%) | 41 (68%) | ||
| Stage (ISS) | I | 7 (35%) | 2 (10%) | 8 (40%) | 17 (28%) | 0.401 |
| II | 4 (20%) | 5 (25%) | 5 (25%) | 14 (23%) | ||
| III | 5 (25%) | 6 (30%) | 3 (15%) | 14 (23%) | ||
| Unknown | 4 (20%) | 7 (35%) | 4 (20%) | 15 (25%) | ||
| High Risk Cytogenetics | No | 19 (95%) | 16 (80%) | 13 (65%) | 51 (85%) | 0.166 |
| Yes | 1 (5%) | 4 (20%) | 4 (20%) | 9 (15%) | ||
| Disease Status at Transplant | 1st remission | 14 (70%) | 14 (70%) | 17 (85%) | 45 (75%) | 0.499 |
| Relapsed | 6 (30%) | 6 (30%) | 3 (15%) | 15 (25%) | ||
| Response to last Rx before Transplant | PD | 3 (15%) | 2 (10%) | 0 (0%) | 5 (8%) | 0.292 |
| PR/VGPR | 16 (80%) | 14 (70%) | 18 (90%) | 48 (80%) | ||
| SD | 1 (5%) | 4 (20%) | 2 (10%) | 7 (12%) | ||
| BM Plasma Cells | >10% | 6 (30%) | 6 (30%) | 4 (20%) | 16 (27%) | 0.99 |
| <10% | 14(70%) | 14 (70%) | 16 (80%) | 43 (73%) |
ISS: International Staging System, PD: Progressive disease, PR: Partial response VGPR: Very good partial response, SD: Stable disease, BM: bone marrow Rx: treatment
Stem Cell Mobilization and Engraftment
Forty-nine patients (81%) were mobilized with G-CSF, 10μg/kg/day, or pegylated G-CSF alone, given subcutaneously. Eleven patients (18%) with high tumor burden received chemotherapy+G-CSF, 10 μg/kg/day using the modified CVAD regimen (cyclophosphamide, vincristine, adriamycin and dexamethasone) to achieve cytoreduction and mobilization 28. The median CD34 cell dose in groups 1, 2, and 3 was 4.2, 4.1 and 3.6 × 106/kg, respectively. All patients engrafted with a median time to neutrophil engraftment (ANC >500/dL) of 10 days in each group, with no engraftment failures or delays in any of the 3 cohorts. Median time to platelet count of >20×109/L was 10 (7–17), 11 (7–31), and 10 (5–17) days, respectively, for the three groups.
Induction Therapy
In the 45 newly diagnosed patients who proceeded to auto-HCT to consolidate the initial response, these induction regimens were used: thalidomide + dexamethasone: 14; lenalidomide + dexamethasone: 12; bortezomib + dexamethasone: 14 (10 patients had thalidomide and 2 had lenalidomide added to the combination); other: 5. None of the patients was in CR prior to auto HCT due to the existing institutional practice of delaying the auto-HCT till relapse in patients achieving a CR to induction. In the 15 patients with relapsed disease at auto HCT, the initial induction therapies were: thalidomide + dexamethasone 6; bortezomib + dexamethasone 4 (3 patients had thalidomide and 1 had lenalidomide added to the combination); vincristine + doxorubicin + dexamethasone 2; others 3. Six patients with relapsed disease also received fractionated cyclophosphamide as salvage before proceeding to auto HCT.
Treatment-Related Mortality and Adverse Events
One patient in group 1 died of treatment-related causes within 90 days with an overall TRM of 1.6%, and rates of 5, 0 and 0% in groups 1, 2 and 3, respectively, with no significant difference between the 3 groups (p=1.0). There was Grade 3–4 toxicities were similar, and seen in 10 patients (50%) in group 1 (mucositis in 3, tachycardia in 2, chest pain in 1, venous thrombosis in 1, dyspnea in 1, acute renal failure in 1, and pleural effusion in 1); 10 patients (50%) in group 2 (mucositis in 2, tachycardia in 1, hypotension in 1, pneumonia in 1, and pulmonary edema in 1); and 12 patients (60%) in group 3 (left ventricular dysfunction in 1, chest pain in 1, hypertension in 1, pulmonary edema in 2, pleural effusion in 1, dyspnea in 1, mucositis in 1, intestinal obstruction in 1, and elevated transaminases in 1) (Table 3). The most common adverse events were fluid retention (78%), nausea (65%), and diarrhea (63%). Grade 1–2 weight gain due to fluid retention was seen in 75%, 70% and 90% of patients in groups 1, 2 and 3.
Table 3.
Grade III–IV Adverse Events
| Group 1 | Group 2 | Group 3 | |
|---|---|---|---|
|
| |||
| Total grade 3 and 4: | 25 | 18 | 16 |
| Cardiac: | |||
| Grade 3 | 5 | 1 | 4 |
| Grade 4 | 1 | 1 | 0 |
| GI: | |||
| Grade 3 | 4 | 4 | 2 |
| Pulmonary: | |||
| Grade 3 | 1 | 2 | 4 |
| Grade 4 | 2 | 0 | 0 |
| Infection: | |||
| Grade 3 | 3 | 3 | 0 |
| Grade 4 | 0 | 1 | 0 |
| Neutropenic Fever: | |||
| Grade 3 | 5 | 6 | 5 |
| Other | |||
| Grade3 | 2 | 0 | 0 |
| Grade4 | 2 | 0 | 1 |
Response
CR rates at 100 days after auto-HCT in random groups 1, 2 and 3 were 20%, 10% and 10%, respectively. The combined CR + very good partial response (VGPR) rates at 100 days in groups 1, 2, and 3 were 60%, 60%, and 65%, respectively. The overall response rate (ORR) at 100 days was 85%, 90%, and 95% for groups 1, 2, and 3, respectively. Taken together, there was no significant difference in response rates between the 3 groups.
Maintenance
Only 10 of the 60 patients (4 in group1, 2 in group 2 and 4 in group 3) on the study received post-transplant maintenance therapy. Four patients received lenalidomide, 4 received thalidomide, 1 received curcumin and 1 received dexamethasone. There was no significant difference in PFS or OS in patients who received maintenance therapy (p=0.16 and 0.17, respectively).
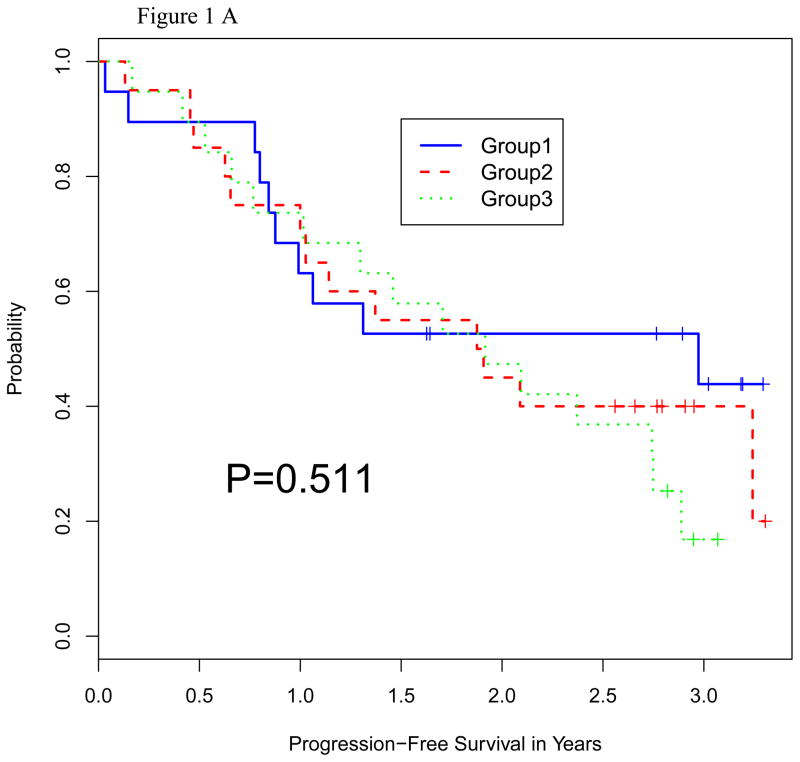
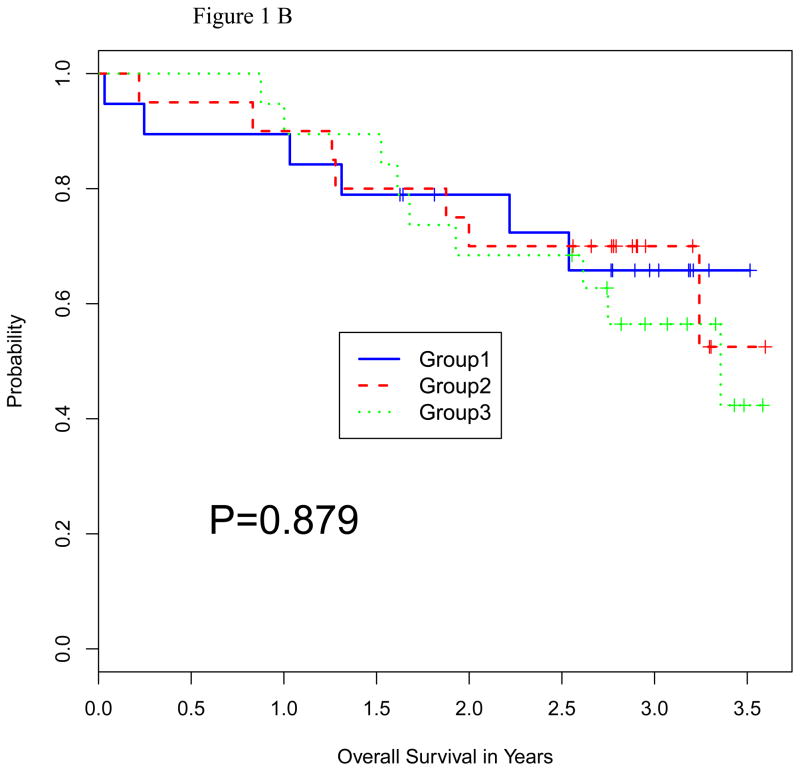
Survival
The median follow up in all surviving patients was 36 months (range 20–43). The median PFS times were 19.5, 17.2, and 20.5 months, respectively (Figure 1A). The median OS has not been reached in any of the 3 groups (Figure 1B). There was no significant difference in PFS or OS between the 3 treatment groups (P = 0.51 and 0.88, respectively).
Figure 1.
Figure 1A: Progression-Free Survival by Random Group
Figure 1B: Overall Survival by Random Group
Prognostic factors
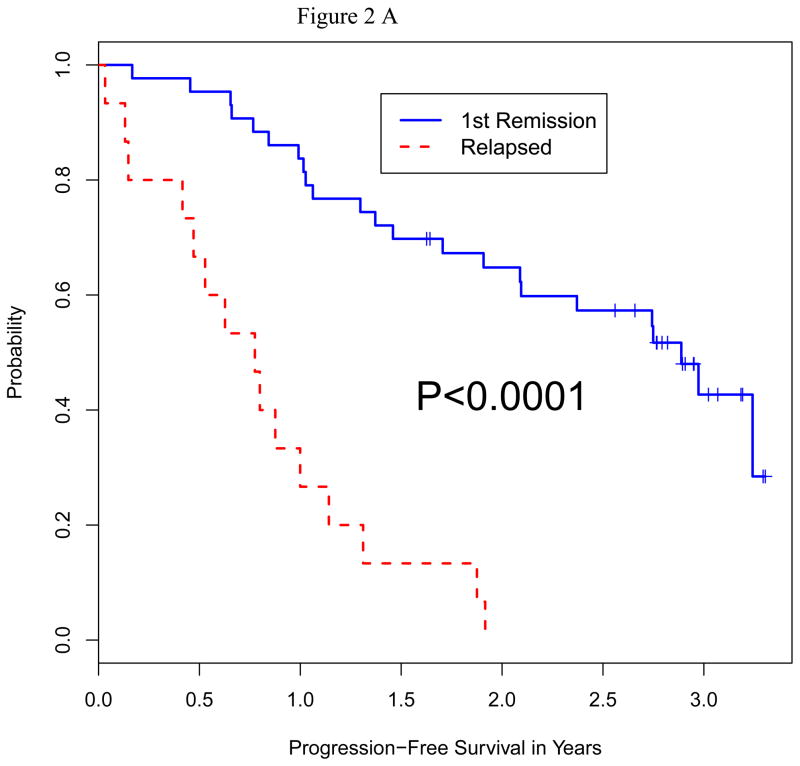
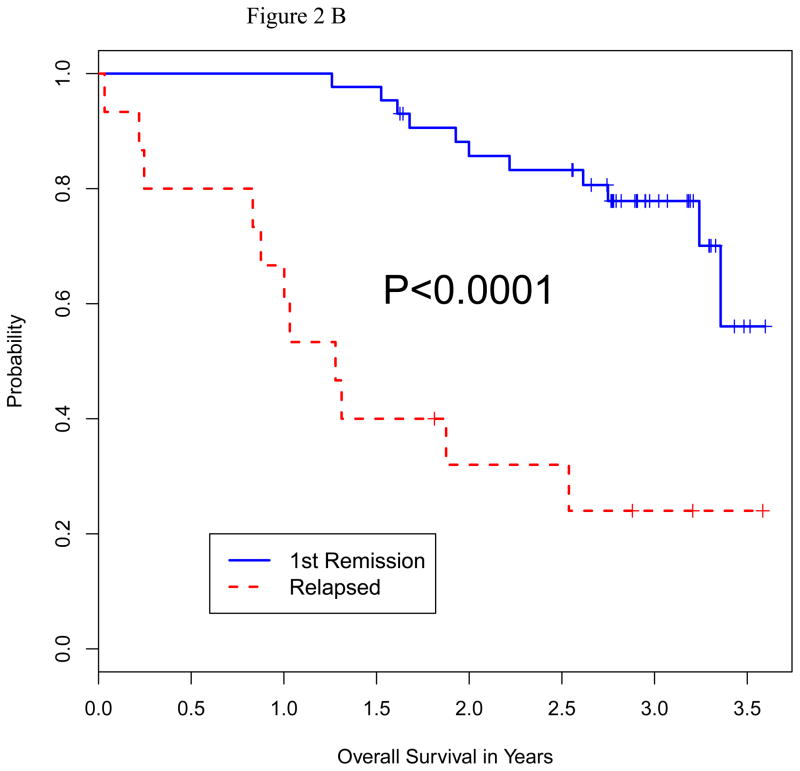
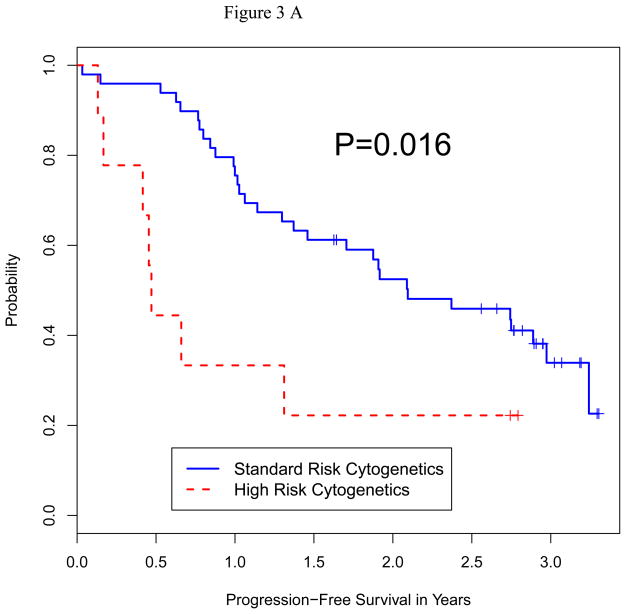
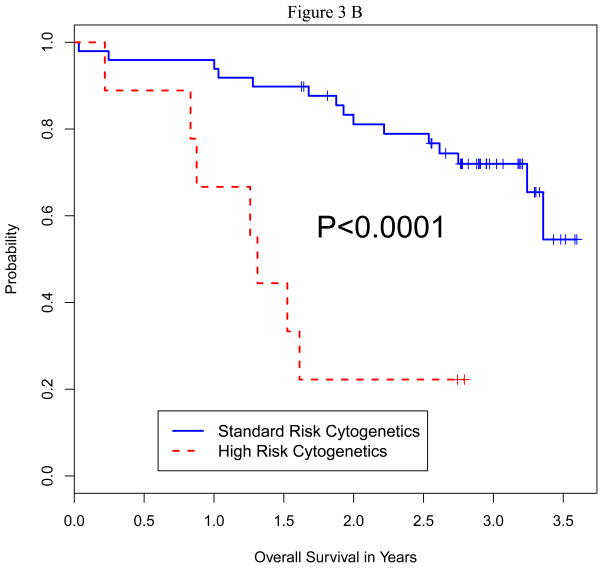
We analyzed the impact of a number of patient characteristics on PFS and OS, including age, serum albumin, beta-2 microglobulin, lactate dehydrogenase (LDH) level, disease stage, remission vs. relapsed disease at auto-HCT, high-risk chromosomal abnormalities, prior autologous transplant, and plasmacytosis (>10%) on bone marrow biopsy prior to auto-HCT. On multivariate analysis for both PFS and OS, only high-risk cytogenetics and relapsed disease at auto-HCT emerged as predictors of poor outcome (Table 4). Fifty-one patients had standard risk cytogenetics and 9 had high-risk based on published criteria 26. Median PFS in high-risk vs. standard-risk cytogentics was 5.7 vs. 21.8 months (p=0.016), and median OS in high-risk vs. standard-risk was 15.9 months vs. not reached (p=0.0001) (Figure 2A and B). Furthermore, median PFS (p=0.0001) and median OS (p=0.0001) were significantly shorter in patients with relapsed disease (Figure 3A and B). There was no significant difference in terms of response rate or PFS between growth factor vs. chemo+growth factor mobilization. Thirty-five of the 60 patients had used bortezomib as part of their induction or salvage therapy. There was no significant difference in CR+VGPR, PFS or OS between those who received bortezomib before auto-HCT vs. those who did not.
Table 4.
Multivariate Analysis of Prognostic Factors for PFS and OS
| Variable | OS
|
PFS
|
||||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | 95% CI | Pr (Beneficial Effect) | Mean | SD | 95% CI | Pr (Beneficial Effect) | |
|
|
|
|||||||
| Intercept | 1.02 | 0.34 | 0.38, 1.68 | -- | 0.89 | 0.33 | 0.25, 1.55 | -- |
| Age | −0.008 | 0.02 | −0.06, 0.04 | 0.381 | −0.02 | 0.02 | −0.07, 0.03 | 0.215 |
| Race=Non-white | 0.11 | 0.33 | −0.52, 0.76 | 0.627 | 0.19 | 0.35 | −0.48, 0.88 | 0.701 |
| Bortezomib (1mg) vs. No Botezomib. | 0.14 | 0.36 | −0.57, 0.82 | 0.653 | 0.05 | 0.35 | −0.64, 0.72 | 0.56 |
| Bortezomib (1.5mg) vs. No Botezomib | 0.21 | 0.38 | −0.53, 0.95 | 0.716 | 0.1 | 0.38 | −0.64, 0.84 | 0.602 |
| Gender=Male | −0.05 | 0.32 | −0.68, 0.57 | 0.439 | −0.1 | 0.31 | −0.72, 0.51 | 0.378 |
| Stage II vs. I | 0.01 | 0.41 | −0.79, 0.82 | 0.514 | 0.17 | 0.42 | −0.64, 0.97 | 0.651 |
| Stage III vs. I | −0.15 | 0.43 | −1.01, 0.68 | 0.366 | −0.28 | 0.44 | −1.16, 0.58 | 0.266 |
| Stage Unknown vs. I | −0.25 | 0.41 | −1.02, 0.55 | 0.267 | −0.5 | 0.41 | −1.29, 0.30 | 0.11 |
| HR Cytogenetics | −0.71 | 0.44 | −1.54, 0.18 | 0.058 | −0.98 | 0.43 | −1.79, −0.10 | 0.016 |
| Maintenance Therapy Before Transplant | 0.07 | 0.42 | −0.71, 0.94 | 0.555 | 0.14 | 0.43 | −0.66, 1.02 | 0.618 |
| Chemo-Mobilization Before Transplant | 0.08 | 0.38 | −0.64, 0.85 | 0.571 | −0.14 | 0.38 | −0.84, 0.63 | 0.351 |
HR: high-risk
Figure 2.
Figure 2A: Progression-Free Survival by Cytogenetic Status at Auto HCT
Figure 2B: Overall Survival by Cytogenetic Status at Auto HCT
Figure 3.
Figure 3A: Progression-Free Survival by Disease Status at Auto HCT
Figure 3B: Overall Survival by Disease Status at Auto HCT
DISCUSSION
We report here the results of the first randomized phase II trial incorporating the protease inhibitor bortezomib to the conditioning regimen for auto-HCT in multiple myeloma. Melphalan 200 mg/m2 is considered the standard conditioning regimen. Attempts to intensify this regimen have not been successful due to increased toxicity 5–8, 16. Based on anti-myeloma activity exhibited by bortezomib in combination with alkylating agents, we designed this randomized phase II trial to test the safety and efficacy of bortezomib in combination with high-dose melphalan 12–15, 29. Patients in the three randomized groups were comparable. There were no patients in CR at the time of auto-HCT, although 80% had at least a PR. There was no difference in time to neutrophil or platelet engraftment, and comparable grade 3–4 toxicities between the three treatment groups. TRM was 1.6% with only one death in group 1. With a median follow-up of 36 months, the primary endpoint of CR was achieved in 20%, 10% and 10% of patients in groups 1, 2, and 3, respectively. There was no significant improvement in median PFS or OS in the bortezomib-containing groups. Patients with relapsed disease and high-risk cytogenetic abnormalities had significantly shorter PFS and OS.
Historical data from our institution suggests a median PFS of 21 months following an auto-HCT performed immediately after induction therapy 6. The PFS in relapsed patients undergoing auto-HCT at our institution is approximately 12 months 30. The lack of benefit in bortezomib treated groups in this study may be attributed to several factors, such as the possibility that concomitant administration of ascorbic acid may have compromised the efficacy of bortezomib, as reported by several investigators 31, 32. Also, the inclusion of a large number (25%) of patients with relapsed disease, who are known to have worse outcome, likely contributed as well 30.
There are two prior clinical trials that studied the incorporation of bortezomib to a melphan-based conditioning regimen for myeloma 15, 29. The first study was a single arm trial by the IFM group with a matched-historical control design 15. The conditioning regimen included bortezomib 1 mg/m2 given on days −6, −3, +1 and +4, with melphalan 200 mg/m2 given on day −2 and the autologous cell reinfusion on day 0. Fifty-four patients were treated in that trial with a median age of 58(40–65) years. Fifty-four percent of patients had induction therapy with vincristine, adriamycin and dexamethasone (VAD), 33% with bortezomib and dexamethasone, and 13% had more than two lines of therapy. Following the auto-HCT with the bortezomib-melphalan conditioning, 32% achieved a CR and 70% were in a CR+VGPR at 3 months post auto-SCT. In matched control analysis with patients from the IFM 2005-01 trial of high-dose melphalan only, CR was higher in the bortezomib-melphalan vs. melphalan only group (35% vs. 11%; p=.001), regardless of induction therapy. Compared to the IFM study, more patients (25%) in our trial had relapsed disease, and no patient was in a CR at auto-HCT. Although the CR rate was lower in our study, perhaps due to the inclusion of patients with more advanced disease, the CR+VGPR rates of >60% were not much different from 70% in the IFM trial. Although the IFM study did report cytogenetic abnormalities, no information was available regarding the outcome of high-risk patients.
The second study evaluated the synergy between bortezomib and melphalan in the high dose setting, the maximum tolerated dose of bortezomib in combination with melphalan and the timing of administration of bortezomib (before or after melphalan) 29. Only patients with <VGPR following one or more induction regimens were enrolled, and randomized to receive a single escalating dose of bortezomib (1.0, 1.3, or 1.6 mg/m2) either 24 hours before or 24 hours after high-dose melphalan. Bone marrow was collected before the initiation of therapy and at the time of transplant to evaluate the sequence associated with maximal plasma cell apoptosis. Among 39 randomized patients, 19 received bortezomib before and 20 after melphalan. Toxicity and post-transplant hematopoietic recovery were similar in both arms. The overall response rate was 87%, with 51% achieving a VGPR or better. Pharmacodynamic studies showed greater plasma cell apoptosis among patients who received bortezomib following melphalan. Unlike the IFM study, patients >65 were included, patients were heavily pre-treated, including with prior autologous transplant, and no patients were in CR or VGPR at auto-HCT. The TRM was 5% with one death due to severe ileus and another due to Para influenza infection. The median time to engraftment of neutrophils and platelets was 12 and 16 days, respectively. With a median follow-up of 17.3 months, the ORR was 87%, with 72% achieving a >VGPR post-transplant. In patients with prior exposure to bortezomib, 57% achieved a >VGPR post-transplant. Although the dose and frequency of bortezomib in this trial was different from ours, the patient population was relatively comparable. Like our study, the trial by Lonial et al. also included heavily pre-treated patients none of whom was in CR prior to transplant. The CR+VGPR rates were comparable to our trial. Even with the inclusion of patients with advanced disease, the PFS was 15.3 months, which was comparable to a PFS of 17–20 months seen in our trial.
We conclude that adding bortezomib to a preparative regimen of ATO, AA and high dose melphalan is safe and well tolerated in patients with multiple myeloma with no increase in grade 3–4 toxicity or TRM. However, there was no significant improvement in CR, PFS or OS at either dose level (1 mg/m2 or 1.5 mg/m2) in this group of patients. A prospective study with bortezomib and high-dose melphalan without AA and ATO, which excludes patients with relapsed disease, may better define the role of bortezomib in this setting.
Acknowledgments
Funding Sources: M.D. Anderson Cancer Center
Footnotes
Financial disclosure: None
Contributor Information
Manish Sharma, Email: Manish.Sharma@tuhs.temple.edu.
Hassan Khan, Email: drhasankhan@gmail.com.
Peter F Thall, Email: rex@mdanderson.org.
Robert Z Orlowski, Email: rorlowsk@mdanderson.org.
Roland L Bassett, Jr, Email: rlbasset@mdanderson.org.
Nina Shah, Email: nshah@mdanderson.org.
Qaiser Bashir, Email: qbashir@mdanderson.org.
Simrit Parmar, Email: sparmar@mdanderson.org.
Michael Wang, Email: miwang@mdanderson.org.
Jatin J Shah, Email: jjshah@mdanderson.org.
Chitra M Hosing, Email: cmhosing@mdanderson.org.
Uday R Popat, Email: upopat@mdanderson.org.
Sergio A Giralt, Email: GiraltS@mskcc.org.
Richard E Champlin, Email: rchampli@mdanderson.org.
Muzaffar H Qazilbash, Email: mqazilba@mdanderson.org.
References
- 1.Kyle RA, Rajkumar SV. Multiple myeloma. N Engl J Med. 2004;351(18):1860–73. doi: 10.1056/NEJMra041875. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15509819. [DOI] [PubMed] [Google Scholar]
- 2.Kyle RA, Rajkumar SV. Treatment of multiple myeloma: a comprehensive review. Clin Lymphoma Myeloma. 2009;9(4):278–88. doi: 10.3816/CLM.2009.n.056. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19717377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Attal M, Harousseau JL, Stoppa AM, Sotto JJ, Fuzibet JG, Rossi JF, et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Francais du Myelome. N Engl J Med. 1996;335(2):91–7. doi: 10.1056/NEJM199607113350204. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=8649495. [DOI] [PubMed] [Google Scholar]
- 4.Child JA, Morgan GJ, Davies FE, Owen RG, Bell SE, Hawkins K, et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med. 2003;348(19):1875–83. doi: 10.1056/NEJMoa022340. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12736280. [DOI] [PubMed] [Google Scholar]
- 5.Kazmi SM, Saliba RM, Donato M, Wang M, Hosing C, Qureshi S, et al. Phase II trial of high-dose topotecan, melphalan and CY with autologous stem cell support for multiple myeloma. Bone Marrow Transplant. 46(4):510–5. doi: 10.1038/bmt.2010.160. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=20581887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anagnostopoulos A, Aleman A, Ayers G, Donato M, Champlin R, Weber D, et al. Comparison of high-dose melphalan with a more intensive regimen of thiotepa, busulfan, and cyclophosphamide for patients with multiple myeloma. Cancer. 2004;100(12):2607–12. doi: 10.1002/cncr.20294. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15197803. [DOI] [PubMed] [Google Scholar]
- 7.Moreau P, Facon T, Attal M, Hulin C, Michallet M, Maloisel F, et al. Comparison of 200 mg/m(2) melphalan and 8 Gy total body irradiation plus 140 mg/m(2) melphalan as conditioning regimens for peripheral blood stem cell transplantation in patients with newly diagnosed multiple myeloma: final analysis of the Intergroupe Francophone du Myelome 9502 randomized trial. Blood. 2002;99(3):731–5. doi: 10.1182/blood.v99.3.731. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11806971. [DOI] [PubMed] [Google Scholar]
- 8.Christoforidou AV, Saliba RM, Williams P, Qazilbash M, Roden L, Aleman A, et al. Results of a retrospective single institution analysis of targeted skeletal radiotherapy with (166)Holmium-DOTMP as conditioning regimen for autologous stem cell transplant for patients with multiple myeloma. Impact on transplant outcomes. Biol Blood Marrow Transplant. 2007;13(5):543–9. doi: 10.1016/j.bbmt.2006.12.448. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17448913. [DOI] [PubMed] [Google Scholar]
- 9.Richardson PG, Barlogie B, Berenson J, Singhal S, Jagannath S, Irwin D, et al. A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med. 2003;348(26):2609–17. doi: 10.1056/NEJMoa030288. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12826635. [DOI] [PubMed] [Google Scholar]
- 10.Richardson PG, Sonneveld P, Schuster MW, Irwin D, Stadtmauer EA, Facon T, et al. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med. 2005;352(24):2487–98. doi: 10.1056/NEJMoa043445. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15958804. [DOI] [PubMed] [Google Scholar]
- 11.Richardson PG, Weller E, Jagannath S, Avigan DE, Alsina M, Schlossman RL, et al. Multicenter, phase I, dose-escalation trial of lenalidomide plus bortezomib for relapsed and relapsed/refractory multiple myeloma. J Clin Oncol. 2009;27(34):5713–9. doi: 10.1200/JCO.2009.22.2679. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19786667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma MH, Yang HH, Parker K, Manyak S, Friedman JM, Altamirano C, et al. The proteasome inhibitor PS-341 markedly enhances sensitivity of multiple myeloma tumor cells to chemotherapeutic agents. Clin Cancer Res. 2003;9(3):1136–44. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12631619. [PubMed] [Google Scholar]
- 13.Mitsiades N, Mitsiades CS, Richardson PG, Poulaki V, Tai YT, Chauhan D, et al. The proteasome inhibitor PS-341 potentiates sensitivity of multiple myeloma cells to conventional chemotherapeutic agents: therapeutic applications. Blood. 2003;101(6):2377–80. doi: 10.1182/blood-2002-06-1768. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12424198. [DOI] [PubMed] [Google Scholar]
- 14.San Miguel JF, Schlag R, Khuageva NK, Dimopoulos MA, Shpilberg O, Kropff M, et al. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med. 2008;359(9):906–17. doi: 10.1056/NEJMoa0801479. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18753647. [DOI] [PubMed] [Google Scholar]
- 15.Roussel M, Moreau P, Huynh A, Mary JY, Danho C, Caillot D, et al. Bortezomib and high-dose melphalan as conditioning regimen before autologous stem cell transplantation in patients with de novo multiple myeloma: a phase 2 study of the Intergroupe Francophone du Myelome (IFM) Blood. 115(1):32–7. doi: 10.1182/blood-2009-06-229658. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19884643. [DOI] [PubMed] [Google Scholar]
- 16.Qazilbash MH, Saliba RM, Nieto Y, Parikh G, Pelosini M, Khan FB, et al. Arsenic trioxide with ascorbic acid and high-dose melphalan: results of a phase II randomized trial. Biol Blood Marrow Transplant. 2008;14(12):1401–7. doi: 10.1016/j.bbmt.2008.09.019. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19041063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Durie BG, Harousseau JL, Miguel JS, Blade J, Barlogie B, Anderson K, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20(9):1467–73. doi: 10.1038/sj.leu.2404284. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16855634. [DOI] [PubMed] [Google Scholar]
- 18.Badros AZ. In the age of novel therapies, what defines high-risk multiple myeloma? J Natl Compr Canc Netw. 8(Suppl 1):S28–34. doi: 10.6004/jnccn.2010.0114. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=20141672. [DOI] [PubMed] [Google Scholar]
- 19.Snedecor GWC, Cochran WG. Statistical Methods. 7. Iowa State University Press; Ames, Iowa: 1980. [Google Scholar]
- 20.Fischer R. On the interpretation of x2 from contingency tables, and the calculation of P. Journal of the Royal Statistical Society. 1922;85:87–94. [Google Scholar]
- 21.Randles R, Wolfe DA. Introduction to the Theory of Nonparametric Statistics. New York: John Wiley & Sons; 1979. [Google Scholar]
- 22.Kaplan EL, Meier P. Non-parametric estimation from incomplete observation. J Am Stat Assoc. 1958;53:457–81. [Google Scholar]
- 23.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50(3):163–70. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=5910392. [PubMed] [Google Scholar]
- 24.Ibrahim JG, Chen MH, Sinha D. Bayesian Survival Analysis. New York: Springer; 2001. [Google Scholar]
- 25.Schwarz GE. Estimating the dimension of a model. Annals of Statistics. 1978;6:461–64. [Google Scholar]
- 26.Munshi NC, Anderson KC, Bergsagel PL, Shaughnessy J, Palumbo A, Durie B, et al. Consensus recommendations for risk stratification in multiple myeloma: report of the International Myeloma Workshop Consensus Panel 2. Blood. 117(18):4696–700. doi: 10.1182/blood-2010-10-300970. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=21292777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thomas A, O’Hara B, Ligges U, Sturtz S. R News. 2006. Making BUGS Open; pp. 12–17. [Google Scholar]
- 28.Dimopoulos MA, Weber D, Kantarjian H, Delasalle KB, Alexanian R. HyperCVAD for VAD-resistant multiple myeloma. Am J Hematol. 1996;52(2):77–81. doi: 10.1002/(SICI)1096-8652(199606)52:2<77::AID-AJH2>3.0.CO;2-2. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=8638645. [DOI] [PubMed] [Google Scholar]
- 29.Lonial S, Kaufman J, Tighiouart M, Nooka A, Langston AA, Heffner LT, et al. A phase I/II trial combining high-dose melphalan and autologous transplant with bortezomib for multiple myeloma: a dose- and schedule-finding study. Clin Cancer Res. 16(20):5079–86. doi: 10.1158/1078-0432.CCR-10-1662. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=20739431. [DOI] [PubMed] [Google Scholar]
- 30.Qazilbash MH, Saliba R, De Lima M, Hosing C, Couriel D, Aleman A, et al. Second autologous or allogeneic transplantation after the failure of first autograft in patients with multiple myeloma. Cancer. 2006;106(5):1084–9. doi: 10.1002/cncr.21700. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16456814. [DOI] [PubMed] [Google Scholar]
- 31.Harvey RD, Nettles J, Wang B, Sun SY, Lonial S, et al. Commentary on Perrone. vitamin C: not for breakfast anymore…if you have myeloma. Leukemia. 2009;23(11):1939–40. doi: 10.1038/leu.2009.128. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19904281. [DOI] [PubMed] [Google Scholar]
- 32.Perrone G, Hideshima T, Ikeda H, Okawa Y, Calabrese E, Gorgun G, et al. Ascorbic acid inhibits antitumor activity of bortezomib in vivo. Leukemia. 2009;23(9):1679–86. doi: 10.1038/leu.2009.83. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19369963. [DOI] [PubMed] [Google Scholar]