Abstract
Background
Corticotropin releasing hormone (CRH) and urocortins (UCNs) bind to corticotropin releasing hormone type 2 receptor (CRH-R2), a Gs protein-coupled receptor that plays an important role in modulation of anxiety and stress responses. The Crhr2 gene maps to a quantitative trait locus (QTL) for alcohol preference on chromosome 4 previously identified in inbred alcohol-preferring (iP) and non-preferring (iNP) F2 rats.
Methods and Results
Crhr2 mRNA expression was determined in male alcohol-naïve iP and iNP rats in several brain regions, and lower levels of Crhr2 mRNA were observed in iP rats compared to iNP rats. To identify genetic variation that may underlie differences detected in Crhr2 expression, DNA sequencing was performed and variations were identified in the promoter region, coding region and 3’-untranslated region between the iP and iNP rats. A 7bp insertion in the Crhr2 promoter of iP rats altered expression in vitro as measured by reporter assays. While CRH-R2 binding affinity did not significantly differ between male iP and iNP receptor variants, CRH-R2 density was lower in the amygdala of iP as compared to iNP rats. Male P rats displayed decreased social interaction and significantly higher corticosterone levels directly following 30 minute restraint when compared to male NP rats.
Conclusions
This study identified Crhr2 as a candidate gene of interest underlying the chromosome 4 QTL for alcohol consumption that was previously identified in the P and NP model. Crhr2 promoter polymorphism reduced mRNA expression in certain brain regions, particularly the amygdala, and lowered the density of CRH-R2 in the amygdala of iP compared to iNP rats. Together, these differences between the animals may contribute to the drinking disparity as well as the anxiety differences of the P and NP animals.
Keywords: Alcoholism, corticotropin releasing hormone type 2 receptor, Selective breeding, Stress, Amygdala
INTRODUCTION
The selectively bred alcohol-preferring (P) and –nonpreferring (NP) rats have been established as an animal model of alcoholism displaying the necessary phenotypic criteria (Cicero, 1979). Utilizing quantitative trait locus (QTL) analysis, a previous study identified a highly significant QTL (LOD = 9.2) for alcohol consumption and alcohol preference on chromosome 4 in a population of iP X iNP F2 rats (Bice et al., 1998; Carr et al., 1998). This QTL was later confirmed utilizing congenic strains that were derived from the iP and iNP lines (Carr et al., 2006). Crhr2 has been identified as a potential candidate gene of interest due to its role in alcohol drinking behavior (Ryabinin et al., 2012) and because it maps to the peak of the chromosome 4 QTL region (83.8 Mbp).
CRF1 and CRF2 receptors are Gs-coupled 7-transmembrane receptors. Numerous studies have shown that binding of corticotropin-releasing hormone (CRF) to the type 1 receptor (CRF1 receptor) plays an important role in the regulation of the adaptive response to stress as well as alcohol drinking (Greetfeld et al., 2009; Heilig and Koob, 2007). However, only limited research has studied the role of CRF2 receptor in the regulation of alcohol drinking in rodents. CRF2 receptor is involved in excessive dependent-like ethanol drinking in rats (Funk and Koob, 2007). Additionally, Crhr2 knockout mice exhibit increased alcohol drinking when utilizing a limited-access procedure (Sharpe et al., 2005), and the administration of a CRF2 receptor agonist reverses the increases in ethanol self-administration during the early stages of ethanol withdrawal in rats (Valdez et al., 2004).
In addition to CRF itself, the CRF family consists of urocortin 1 (coded by UCN I), urocortin 2 (UCN II), and urocortin 3 (UCN III), as well as CRF-binding protein (Ryabinin et al., 2002). These ligands differ in their distribution within the central nervous system (CNS) as well as their receptor pharmacology. For example, CRF binds to CRF1 receptor with high affinity, whereas Urocortin 1 binds with high affinity to both CRF1 receptor and CRF2 receptor (Lovenberg et al., 1995). Unlike CRF and Urocortin 1, Urocortin 2 and Urocortin 3 appear to be selective for CRF2 receptor (Chen et al., 2005; Inoue et al., 2003; Lewis et al., 2001). The infusion of UCN 3, a CRF2 receptor agonist, into the central amygdala has been shown to decrease ethanol self-administration in alcohol-dependent Wistar rats (Funk and Koob, 2007). Both Ucn1 KO mice and Crhr2 KO mice showed a lack of ethanol-induced conditioned placed preference compared to WT littermates indicating that Urocortin 1 acting on CRF2 receptor may be a mechanism for alcohol preference (Giardino et al., 2011). Interestingly, inbred P (iP) rats have significantly lower levels of Urocortin 1 than iNP rats in the centrally-projecting Edinger-Westphal nucleus (EWcp) where Urocortin 1 is primarily expressed (Turek et al., 2005).
The CRF and CRF1 receptor system is involved in activating the HPA axis and regulating emotional and cognitive function after stress (Arborelius et al., 1999; Todorovic et al., 2005). However, the involvement of urocortins and the CRF2 receptor system involvement is just emerging (Chen et al., 2005; Kageyama et al., 2003; Valdez et al., 2004). While CRF1 receptors are distributed throughout the brain, the tissue distribution of the CRF2 receptor is more restricted to specific brain regions (Van Pett et al., 2000). Studies utilizing Crhr2 knockout mice suggest that CRF2 receptor may dampen or modulate responses to stress associated with CRF1 receptor activation (Bale and Vale, 2004). Together, these receptors are thought to maintain the homeostasis of stress responses with CRF1 receptor activation yielding an anxiogenic response and CRF2 receptor activation playing an anxiolytic role (Greetfeld et al., 2009; Valdez et al., 2004).
P rats are more “anxious” than NP rats (Pandey et al., 2005; Stewart et al., 1993), and P rats show a greater stress-induced increase in alcohol drinking than NP rats (Chester et al., 2004b). Both acoustic startle and fear potentiated startle responses are consistently greater in P than NP rats (Chester et al., 2004a; McKinzie et al., 2000). P and NP strains exhibited both neurobiological and neurochemical differences in CRH (Murphy et al., 2002). For example, CRH levels were lower in the amygdala, hypothalamus, prefrontal cortex, and cingulate cortex in P rats compared to NP rats, (Ehlers et al., 1992), particularly in the central nucleus of the amygdala (Hwang et al., 2004).
The purpose of this study was to screen for functional variation in the Crhr2 gene, and then further characterize the variations in the P and NP lines. Quantitative real-time PCR (qRT-PCR) was conducted to measure Crhr2 mRNA expression in iP and iNP rats, and DNA sequence analysis used to screen for polymorphisms in the Crhr2 promoter and transcript between the iP and iNP lines. Luciferase assays and binding studies were performed to determine the functional significance of identified polymorphisms. To examine potential consequences of Crhr2 polymorphism on behavioral and endocrine responses associated with environmental stress, corticosterone levels and social interaction (SI) were assessed before and after 30 minutes of restraint.
MATERIALS AND METHODS
Experimental animal care
The experimental protocol used in this study was reviewed and approved by the Indiana University Institutional Animal Care and Use Committee and was carried out in accordance with the Guide for the Care and Use of Laboratory Animals. All rats were housed under twelve hour light–dark conditions (7:00 am / 7:00 pm) with free access to food and water. To avoid confounding results due to gender, only males were used for this research.
Molecular characterization of iP versus iNP rats
Dissection of brain regions
The entire brain was removed from alcohol-naïve adult male iP (n=5) and iNP (n=4) rats and dissected using the coordinates of Paxinos and Watson (Paxinos, 1998) to isolate seven brain regions including the (1) nucleus accumbens, (2) frontal cortex, (3) amygdala, (4) septum, (5) hippocampus, (6) caudate-putamen and (7) hypothalamus. These brain regions were selected due to their involvement in the regulation of the reinforcing properties of alcohol, the motor and memory impairing effects of alcohol, and the adaptation to stress (McBride and Li, 1998). The detailed methods of the dissections were previously published (Bice et al., 2008; Liang et al., 2004). All equipment used to dissect the tissues was treated with RNase Zap (Ambion, Austin, TX) to prevent RNA degradation.
mRNA expression analysis
The microdissected tissues were snap frozen on dry ice and stored at -80°C until RNA isolation. RNA was isolated according to the RNeasy Midi manufacturer’s protocol (Qiagen, Valencia, CA). The isolated RNA was then treated with DNase I. Using the ABI PRISM 7700 (Life Technology, Foster City, CA), the relative mRNA expression levels of Crhr2 were determined in the dissected brain regions. Vector NTI (Invitrogen, Carlsbad, CA) was utilized to design primer (LC551 and LC552, Table 1). For the qRT-PCR assay, cDNA was generated from the RNA each sample obtained from iP and iNP rats using 50 ng of total RNA template, 0.2 μM forward and reverse primers, and SYBR Green PCR Master Mix (Life Technology). Amplification was performed in triplicate for 40 cycles in two separate experiments resulting in six values for each sample. The specificity of the PCR product was confirmed using gel electrophoresis.
Table 1.
List of primers
|
Crhr2 sequencing primers
|
||
| Transcript sequencing | ||
| LC530 | CCCTCATCTCCGTGA | |
| LC531 | GTGAGTAGTTGATCCTTGAG | |
| LC532 | GCATCAAGTACAACACGACC | |
| LC533 | TGGAGTACGTCATGACGATG | |
| LC534 | TCACCAACTTCTTCTGGATG | |
| LC535 | CTGAAAGGACTGCAGGAAAG | |
| LC536 | GTCACAGATTGTGTTCATCTAC | |
| lc537 | CCTGGTCTTTCAGAGCCTC | |
| LC537 | GAGGCTCTGAAAGACCAGG | |
| Promoter sequencing | ||
| LC730-rCrhr2PrSF | AGCCCTGCTTAAGCCAG | |
| LC731-rCrhr2PrSR | TATCCTGGGCACCAGATAGAGTGAGAG | |
| LC732-rCrhr2PrF | TCATGCTCAGACCTAAGACTGCCG | |
| LC733-rCrhr2PrR | GCCTGCTCGACCATGACCAATAG | |
| qRT-PCR primers
| ||
| lc551-crhr2-f | CTGCAGTCCTTTCAGGGTTTCTTTG | |
| lc552-crhr2-r | CAACGGTGCCACCGCTTTCT | |
| lc555-cyc-f | AAAGACACCAATGGCTCCCAGTTC | |
| lc556-cyc-r | GTACCACATCCATGCCTTCCAGAAC | |
The relative values of expression were determined using the standard curve method, and normalized to the Ct values of “housekeeping” gene cyclophillin B, (LC555 and LC556, Table 1). No differences were detected in the average cyclophilin B Ct values when comparing iP versus iNP in each respective brain region, indicating that cyclophilin B represents an appropriate control for normalization. Repeated measurement ANOVA was used and statistical significance was set at p<0.05.
DNA sequence analysis
Genomic DNA isolated from male iP and iNP rats was used to sequence the promoter regions using four primers (LC730 to LC733, Table 1). Primers were designed based on the Crhr2 1.5 kb promoter region sequence accessed from the Ensembl Genome Browser (accession # ENSRNOG00000011145). Next, total RNA was isolated from male iP (n=4) and iNP (n=4) rats, pooled, and reverse transcribed to generate cDNA (RETROscript kit; Ambion). Based on the rat Crhr2 cDNA sequence (NM_022714; U16253), four primer pairs were designed: LC530 to LC537 (Table 1). These primers were utilized to amplify four overlapping DNA fragments that encompassed the entire rat Crhr2 cDNA sequence. The PCR products were purified (GenElute PCR Cleanup Kit, Sigma, St. Louis, MO) and subsequently sequenced (Thermo Sequenase Cycle Sequencing Kit, USB, Cleveland, OH).
Transient transfection and dual-luciferase activity assays
The reporter plasmids utilized in this study were constructed as previously described (Liang et al., 2003). Briefly, the promoter regions of the iP and iNP animals were generated and spanned from -1396 to -214 (Ref Seq NM_022714). These DNA fragments were fused upstream of the luciferase gene in the pGL3-basic vector to test the promoter polymorphism function. In addition, 3’-UTR fragments were generated from the iP and iNP lines from +1452 to +1626 relative to the translational start site (+1) to measure the effects of polymorphism on 3’UTR function. For transient transfections, human neuroblastoma SK-N-SH cells were cultured and transfected as previously described (Liang et al., 2003). Using Tfx-50 reagent (Promega, Madison, WI), 0.5 μg of the pGL-3 luciferase plasmids were co-transfected along with 4 ng of CMV Renilla luciferase vector (pRL-CMV) per well. The pRL-CMV was utilized as an internal control for transfection efficiency. The resulting cell extracts were assayed for firefly and Renilla luciferase activities in a TD-20/20 Luminometer using the Dual-Luciferase Reporter Assay System. The reporter assays were performed five times in triplicate using plasmids that were independently purified at least twice.
CRF2 receptor binding studies
Characterization of CRF2 receptor binding affinity in membrane homogenates
The conditions that were utilized for receptor binding assays were performed as previously described (Grigoriadis et al., 1996; Rominger et al., 1998). Frozen brain tissues dissected from the frontal cortex of iP (n=8) and iNP (n=8) rats were homogenized in assay buffer (50 mM Tris HCL, 2 mM EGTA, 10 mM MgCL2, pH 7.2). Typically, 50 mg of brain wet tissue was homogenized in a 1 ml assay buffer, and then incubated for 30 minutes in a 37°C water bath to degrade endogenous CRH. The assay buffer was enriched with 0.1% BSA, 0.1% zinc-free Bacitracin and 100 KU/ml aprotinin, and all displacers and radioactive ligand solutions were diluted in enriched buffer. Each assay was initiated by adding 50 μl of membrane suspension (~250 μg protein) to 150 μl of enriched buffer containing [125I]-Tyr0-sauvagine (PerkinElmer, Inc., Boston, MA) and 50 μl of the displacer Astressin 2B, a selective CRF2 receptor antagonist (Tocris Bioscience, Ellisville, MI) or a non-specific binding peptide. The final concentration of radioactive ligand was 0.175 nM and non-specific binding was determined in the presence of 1 μM of CRH (American Peptide Company, Sunnyvale, CA). Astressin 2B was then used to determine inhibition profiles at the following final concentrations: 0.1, 0.3, 1.0, 3.0, 10.0, 30.0, 100, or 1000 nM. Binding assays were performed in duplicate. Tubes were incubated at ambient temperature for 2 hrs and were added to 0.5 ml of ice-cold enriched assay buffer containing 0.01 % Triton X-100 (wash buffer) followed by centrifuging at 4°C for 5 minutes at 12,000 × g. Aspiration of buffer followed by washing and aspiration. Separation of bound from free radioligand occurred during the centrifugation, and the bottom 0.75 cm of each tube containing the pellet was cut with a microtube cutter into 12 × 75 mm test tubes for counting in a gamma counter (ICN, Huntsville, AL) at 80% efficiency. Graph Pad Prism Version 4 software was used to generate the competition profile of Astressin 2B.
Autoradiagraphic determination of CRF2 receptor binding density
Sections from selected brain regions were prepared from each iP (n=10) and iNP rat (n=10) that were sacrificed at 12-13 weeks of age using decapitation. The brains were rapidly removed and flash frozen in a pre-cooled isopentane solution on dry ice and stored at -70°C until sectioned. Autoradiography was performed as previously described (Sanchez et al., 1999). Briefly, tissue sections were pre-incubated twice for 10 minutes in 50 mM Tris-HCl (pH 7.4) containing 10 mM MgCl2 and 2 mM EGTA. The sections were then incubated for 2 hours at room temperature with 200 pM [125I]-Tyr0-sauvagine in the above solution with 0.1% bovine serum albumin (BSA), aprotinin (0.04 TIU/ml), and 0.1 mM bacitracin. Two sets of adjacent sections were used for the detection of CRF1 receptor and CRF2 receptor, respectively. Specific CRF1 receptor binding was determined using 200 pM [125I]-Tyr0-sauvagine in the presence of 1uM Astressin 2B, and specific CRF2 receptor binding was determined in the presence of 1 uM Stressin I (Tocris Bioscience, Ellisville, MI), a specific CRF1 receptor agonist. Non-specific binding was determined with [125I]-Tyr0-sauvagine in the presence of 1 uM unlabeled sauvagine due to its high affinity for both CRF1 receptor and CRF2 receptor (Grigoriadis et al., 1996; Primus et al., 1997). Following a 90 minute incubation at room temperature, all sections were washed twice in PBS (pH 7.4) that contained 1% BSA and in deionized water at 4°C. Brain slices were air dried, placed in cassettes with [125I]-Microscale slides (American Radiolabeled Chemicals, Inc., St. Louis, MO), and were exposed to BAS-TR2025 imaging plates overnight. The imaging plates were scanned using a BAS5000 system. Digital images were generated from the Phosphor imager, and were analyzed using Multi Gauge software (V2.3) where the brain regions of interest were defined by anatomical landmarks as described in the atlas of Paxinos (Paxinos, 1998). Microscale standards were used as quantitative reference points for analysis.
Characterization of the stress response in selectively bred P and NP rats
The selectively bred P and NP lines (n=8 per group) were used for the stress response study because the iNP rats display a severe bleeding problem due to the absence of platelet aggregation making implantation of arterial catheters very difficult (Smith et al., 1996). Notably, the coding and promoter sequence differences between iP and iNP also exist in the selectively bred P and NP rats. Age-matched, adult Wistar rats (Harlan, Indianapolis, IN) that had no previous contact with the “test” rats were used as partner rats for the social behavior tests.
Stress response protocol
A restraint stress response protocol was conducted to measure the effect(s) of 30 minute restraint on plasma corticosterone levels and social interaction. Six time points (30 minute intervals) were measured during two sessions. During the first session (no restraint), blood samples were drawn for measurement of plasma corticosterone at baseline 1 (T0), baseline 2 (T1), 30 (T2), 60 (T3), 90 (T4) and 120 (T5) minutes after exposure to a novel male rat during the social interaction test. During the second session (30 minute restraint), blood samples were drawn for measurement of plasma corticosterone at baseline (T6), 30 (T7), 60 (T8), 90 (T9), 120 (T10) and 150 (T11) minutes after 30 minutes of restraint (see Figure 6). The social interaction test was conducted immediately following the second blood draw during both sessions (at 30 minutes), and the P and NP rats were restrained for 30 minutes immediately following the first blood draw (T6) during the second testing session. All experiments were conducted in the morning during the light phase of the light/dark cycle between 8:00 AM and 11:30 AM.
Figure 6. Differences in corticosterone levels between P and NP rat lines.
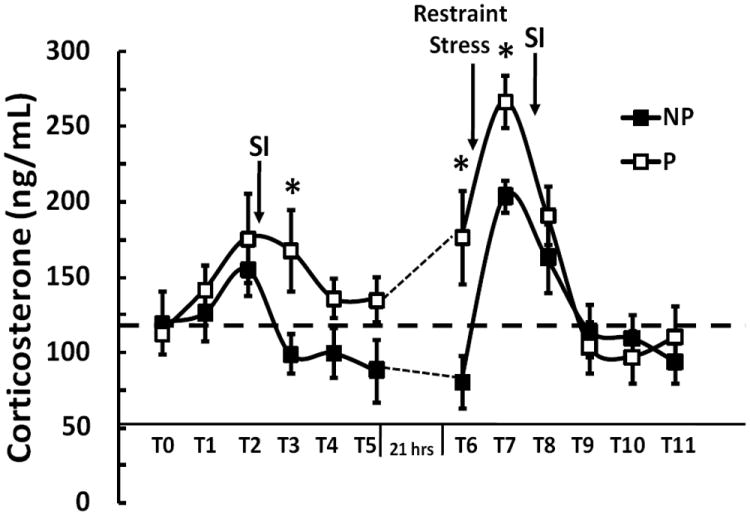
Corticosterone levels were determined at each time point (T0 – T11) with 30 minutes between the time intervals. The time at T0 and T6 was 8:00 AM. The SI test was performed immediately following the 2nd blood draw each day (T1 and T7). Restraint stress was for 30 minutes from T6 – T7. Following 30 minute restraint stress, a significant time by genotype interaction was observed from T7 to T11 (p=0.035). Asterisk denotes significant differences between P and NP rats at specific time points (*p<0.05).
Plasma corticosterone measurements
At approximately fourteen weeks of age (350-450 g), P and NP rats were individually housed. Arterial catheters were then inserted in anesthetized rats into the femoral artery, filled with heparinized saline (25 U/ml), and routed subcutaneously to the dorsal midline of the neck where they were fixed using a leather jacket. Arterial catheters were flushed daily with heparinized saline to prevent clotting. Blood samples were drawn via the arterial catheter. Samples were immediately placed on dry ice in tubes. Plasma was then isolated from each sample by centrifugation and stored at -80°C. Plasma corticosterone levels were assayed using the ImmuChem Double Antibody Corticosterone 125I RIA kit (MP Biomedicals, Orangeburg, NY), and the inter- and intra-assay coefficients of variation were less than 7% and 10%, respectively. To test time and strain interactions, we first utilized the data points from T1 to T5 with T0 as baseline, and then used the data points from T7 to T11 with T6 as baseline. Plasma corticosterone level data was analyzed using a repeated measure ANOVA, and post-hoc analysis was performed at each specific time point. Statistical significance was set at p<0.05.
Social interaction test
The social interaction (SI) test was performed to characterize the social response to stress. This test of anxiety has been repeatedly shown to be highly sensitive to the effects of CRH or other neuropeptides in the CNS (Gehlert et al., 2005; Rainnie et al., 2004). Before conducting the SI tests, the P and NP rats were singly housed for one week and handled daily. Prior to the SI test, the rats were placed into the SI apparatus for a 5 minute habituation session and were exposed to the test room for at least 30 minutes. A modified SI test was used to measure SI. In this test, the experimental P and NP rats were placed in an open field (0.9 m long × 0.9 m wide with walls 0.3 m high) with a novel male Wistar rat. During the five minute test, the total amount of time the experimental rats (i.e., P or NP) initiated interaction with the partner Wistar rat was recorded as described previously (Shekhar and Keim, 1997). The primary behaviors that are measured in this test are social approach behaviors (nose-pokes, grooming, mutual exploration, etc.) that are specifically initiated by the experimental animal towards the partner animal during the 5 minute observation period. All tests were videotaped and independently scored at a later time by two individuals who were unaware of the animals’ genotype using cumulative stopwatches. Between each session, the apparatus was cleaned with 70% ethanol. A repeated ANOVA was performed to test genotype and time interaction. Statistical significance was set at p<0.05.
RESULTS
Crhr2 mRNA levels are lower in discrete brain regions of alcohol-naïve iP rats compared to iNP rats
To assess the endogenous Crhr2 mRNA expression between iP and iNP rats, discrete brain regions dissected from alcohol-naïve iP and iNP rats were analyzed using qRT-PCR. Crhr2 mRNA expression was observed at significantly lower levels in the hypothalamus (p=0.0329), amygdala (p=0.0001), and caudate putamen (p=0.0157) in the iP rats compared to iNP rats (Fig.1). No significant difference was observed in the nucleus accumbens (p=0.8911), frontal cortex (p=0.327), hippocampus (p=0.1108), or septum (p=0.5057).
Figure 1. Crhr2 mRNA expression differences.
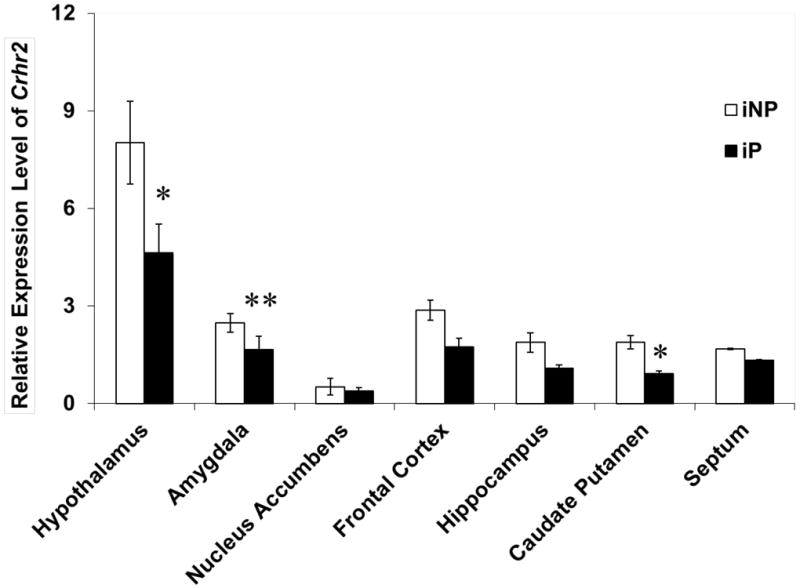
qRT-PCR was utilized to compare the relative levels of Crhr2 expression between iP and iNP in various brain regions. The graph depicts the mean ± SEM of the results from two independent experiments performed in triplicate. Significant differences in regional Crhr2 were determined using ANOVA. Asterisks denote a significant difference in mRNA expression (*p<0.05, ** p=0.0001).
Polymorphism in the Crhr2 promoter, coding region, and 3’UTR between the iP and iNP rats
When 1.5 kb of promoter region was sequenced, a 7bp repeat insertion was identified in the iP sequence compared to the iNP sequence (Fig. 2). In addition, the Crhr2 cDNA, which included 215 bp of the 5’-UTR, the coding region, and 276 bp of the 3’UTR was sequenced in each strain, and a single nucleotide polymorphism (SNP) was observed in the coding region (Fig. 2). For iP rats, DNA sequencing detected a guanine (G) at nt +492, while the iNP sequence had an adenine (A) at this position. The iNP sequence at this position is identical to the published DNA sequence for rat (NM_022714). This coding alteration translates into an isoleucine to valine (Ile93Val) substitution in the extra cellular N-terminal domain (ECD1) of CRF2 receptor. Therefore, this polymorphism could potentially alter ligand binding affinity. In the 3’UTR, DNA sequencing revealed a deletion of a G at nt 1559 in iP compared to iNP and the published DNA sequences (NM_022714) (Fig. 2). These same polymorphisms are also present and homozygous in the P and NP rat lines.
Figure 2. Crhr2 DNA polymorphism in iP rats as compared to iNP rats.
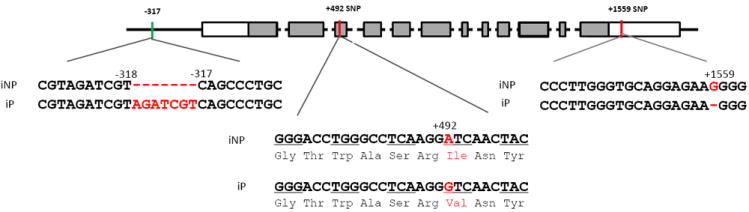
A schematic gene structure delineating the polymorphisms identified in the promoter region, coding region, and 3’UTR region of rat Crhr2. The magnified panel depicts the DNA sequence differences detected in Crhr2 in iP relative to iNP rats including a 7bp insertion in the promoter, a coding mutation at +492 (iNPA/iPG), and an +1559 G deletion in the 3’UTR. The coding mutation identified in iP rats results in an Ile/Val amino acid substitution.
Crhr2 promoter polymorphism in iP rats decreased luciferase reporter gene expression
The functional relevance of the 7 bp insertion in the iP Crhr2 promoter was determined using luciferase assays (Fig. 3A). The transiently transfected pGL3-P luciferase reporter construct expressed lower luciferase activity than the pGL3-NP construct (Fig. 3A). The potential difference in transcription factor binding due to the 7 bp insertion was determined using Genomatix software (Fig. 3B). Three transcription factors were predicted including C2H2 Zinc finger transcription factors (C2H2 ZF), cAMP Response Element Binding Protein (CREB) and Pax3 (the paired box gene 3) (Fig. 3B). Transiently transfected luciferase reporter constructs that contained either the iP or iNP 3’UTR polymorphic Crhr2 fragments did not produce statistically significant differences in luciferase expression (data not shown).
Figure 3. Relative Luciferase activity associated with Crhr2 promoter polymorphism.
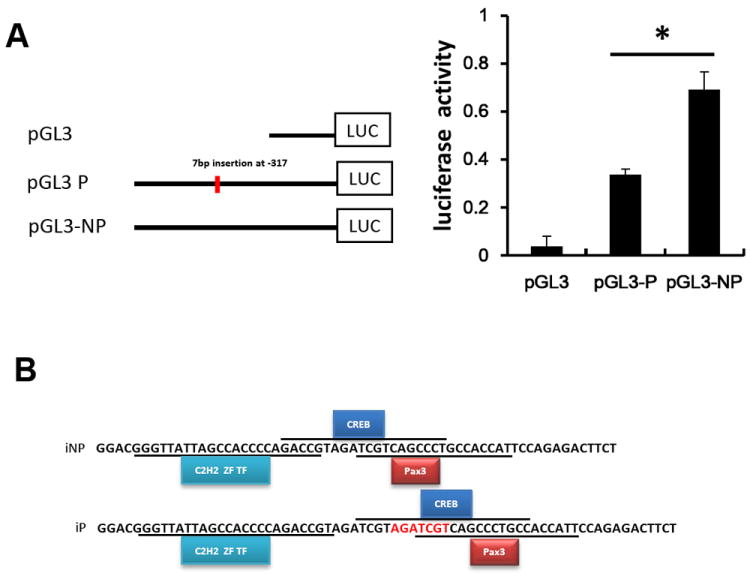
(A) Three pGL-3 promoter plasmids (pGL3-P, pGL3-NP and pGL3 basic) were transfected into SK-N-SH cells to determine whether the Crhr2 promoter polymorphism affects luciferase gene expression. The relative expression of the luciferase gene from each construct is depicted as the mean ± SEM. ANOVA was used to detect significant differences in mean values within and between multiple constructs (p<0.05). (B) Using Genomatix software, the predicted transcription factor binding sites included CREB, C2H2, and Pax3.
The Ile93Val substitution does not affect CRF2 receptor binding affinity
To determine whether the polymorphism identified in the coding region at 492 bp influenced receptor binding affinity, a competitive homogenate binding experiment was performed using Astressin 2B, a selective CRF2 receptor antagonist (Fig. 4). The IC50 for a series of assays using frontal cortex membrane homogenate was 0.77 nM and 0.71 nM for iP and iNP rats, respectively. Therefore, this SNP does not appear to result in altered receptor binding affinity between the two strains.
Figure 4. CRF2 receptor binding in alcohol-naïve iP and iNP rats.
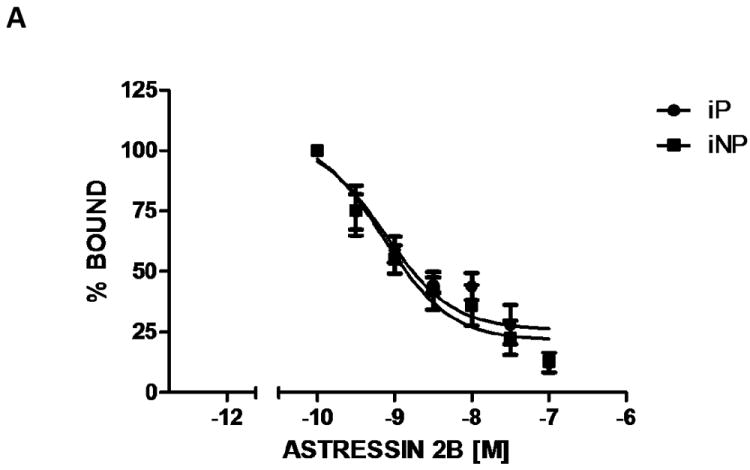
Characterization of CRF2 receptor binding was determined by [125I]-Tyr0-sauvagine affinity in frontal cortex homogenates from naïve iP and iNP rats. The graph depicts the competition profile in the presence of increasing concentrations of Astressin 2B. Each data point represents the mean ± SEM of eight separate experiments performed in duplicate.
The polymorphism in the Crhr2 promoter is associated with altered binding density
To determine whether CRF2 or CRF1 receptor binding differences exist between the iP and iNP strains, in situ (Fig 5, A and B), autoradiography binding was performed using Astressin 2B (a selective CRF2 receptor antagonist) or Stressin I (a specific CRF1 receptor agonist) as displacers. No statistically significant difference was observed in CRF1 receptor binding (Fig. 5C). However, a statistically significant decrease in CRF2 receptor binding was observed in the amygdala of iP relative to iNP rats (Fig. 5D). Non-specific binding was demonstrated in Fig. 5E. Quantification of binding density in the amygdala showed lower density of CRF2 receptor in iP relative to iNP (p<0.01, Fig. 5). No statistically significant binding differences were detected between iP and iNP in the frontal cortex or nucleus accumbens (Fig. 5, bottom panel).
Figure 5. CRF2 receptor density in alcohol-naïve iP and iNP rats.
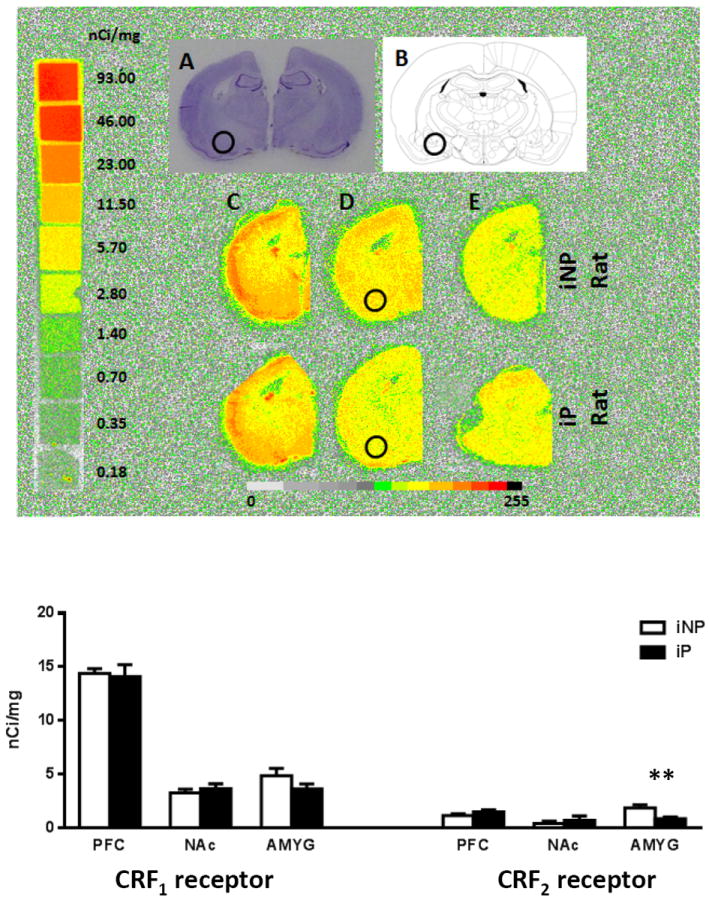
(A) Cresyl violet stained sections indicate that the amygdala sections shown are from the anterior portion of the amygdala. (B) The schematic diagram illustrates the corresponding brain section and localizes the region of interest with a circle. (C) Representative autoradiogram of total [125I]-Tyr0-sauvagine binding which includes CRF1 receptor and CRF2 receptor binding. (D) Representative section showing specific CRF2 receptor binding determined by [125I]-Tyr0-sauvagine binding in the presence of 1 μM of Stressin 1 (a specific CRF1 receptor agonist). (E) Nonspecific binding determined by [125I]-Tyr0-sauvagine binding in the presence of 1 μM of unlabeled sauvagine. The bar graph depicts the density of CRF2 receptor binding in the amygdala (AMYG), pre-frontal cortex (PFC), and nucleus accumbens (NAc). The asterisk indicates a significant difference between the groups (**p<0.01). No significant difference was found in the CRF1 receptor density (bar graph not included).
Differences in plasma corticosterone levels and social interaction following 30 minute restraint stress
In order to characterize the potential effects of Crhr2 polymorphism on HPA activation in the P and NP strains, corticosterone levels were measured before and after 30 minute restraint stress. No statistically significant differences were observed in basal corticosterone levels (T0-T1) between P and NP rats. Repeated measure ANOVA was used to test for any strain and time interactions of corticosterone levels, and no statistically significant interaction was observed from T1 to T5 (P=0.06, Fig. 6). However, a statistically significant time by genotype interaction was observed following 30 minutes of restraint stress from T7 to T11 (p=0.035, Fig. 6). Data was analyzed by subtracting baseline at T6. Increases in corticosterone were also observed preceding the application of restraint stress in P relative to NP rats. Post-hoc analysis at each time point indicated statistically significantly higher corticosterone levels at T3, T6, and T7 in P compared to NP rats.
In order to determine the behavioral effects of Crhr2 polymorphism following environmental stress in the P and NP strains, social interaction (SI) was measured before and after 30 minute restraint stress. While no statistically significant differences were observed in SI between P and NP rats at basal levels (p=0.34), both the P and NP rats displayed decreases in SI following 30 minute restraint (Fig. 7). The P rats showed a modest, but statistically significant decrease in SI following 30 minute restraint compared to NP rats (p=0.008; Fig. 7). ANOVA analyses indicated significant SI differences before and after 30 minute restraint in both the P and NP rats (p<0.0001), and no statistically significant interaction was observed between genotypes (p=0.65).
Figure 7. Social interaction time differences between P and NP rat lines.
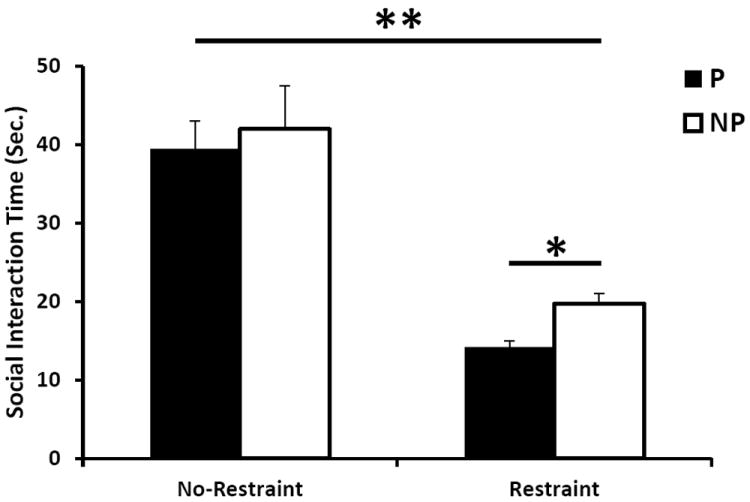
The social interaction test was performed on the selectively bred P and NP rats before and after 30 minute restraint stress. The SI time (seconds) is the total amount of interaction time (sniffing, grooming, etc.) initiated between the P or NP rat and a Wistar rat. Asterisk denotes significant differences between P and NP rats and between restraint and no restraint (*p<0.05, ** p<0.0001).
DISCUSSION
This study was conducted to characterize Crhr2 as a candidate gene of interest underlying the chromosome 4 QTL identified in iP and iNP rats and to examine its potential behavioral and endocrine consequences. First, mRNA studies detected statistically significantly lower Crhr2 transcript levels in three of seven brain regions of the iP strain compared to the iNP strain, and the same trend was observed in two other brain regions (Fig.1). Next, DNA sequence found polymorphism in the coding region (Ile93Val), promoter, and 3’UTR of the Crhr2 gene between the iP and iNP lines. Significant functional differences were associated with the promoter polymorphism in that the iP constructs showed lower transcriptional activity than the iNP. No significant differences were found for the 3’UTR polymorphism. CRF2 receptor binding affinity ex vivo was not altered by the Ile93Val. However, binding density was lower in the amygdala of iP rats compared to iNP rats, consistent with the mRNA expression data. Observed behavioral and physiological effects were associated with this variation in CRF2 receptor in that P rats displayed decreased social interaction and significantly higher corticosterone levels following 30 minute restraint compared to NP rats.
The polymorphism detected at nt +495 (amino acid 93) is located in the extracellular N-terminal domain of the receptor where ligand binding was observed in the CRF1 receptor (Dautzenberg et al., 1999). However, the Ile93Val substitution does not alter ligand binding affinity. In contrast, decreased CRF2 receptor density was detected in the amygdala of iP compared to iNP, which is consistent with lower CRF2 receptor expression in our in vitro reporter data. Our findings suggest that the differences in expression between the two sequence variants are preferentially present within amygdala neurons and likely driven by transcription factors or combinations of such factors.
Using Genomatix transcription factor binding sites analysis software, binding sites were found for C2H2 Zinc fingers, CREB, and Pax3 (Fig. 3D). Notably, the distance between C2H2 ZF transcription factor and CREB binding sites is increased due to the repeated 7bp (AGATCGT) insert in the iP rat, and this increase may potentially account for the difference in expression between the iP and iNP strains.
Evidence suggests that the CRF1 receptor and CRF2 receptor may work in concert to regulate alcohol drinking behavior (Bale et al., 2002; Sommer et al., 2008). Binge-like ethanol intake of C57BL/6J mice is modulated by CRF1 receptor and CRF2 receptor signaling, such that blockade of CRF1 receptor or activation of CRF2 receptor effectively reduces excessive ethanol intake (Lowery et al., 2010). Furthermore, upregulation of Crhr1 and down regulation of Crhr2 mRNA expression was observed in the basolateral amygdala of rats following a history of dependence when compared to alcohol naive animals (Sommer et al., 2008). Interestingly, the present study found that iP rats expressed lower levels of Crhr2, and unpublished data obtained by Dr. Liang also indicated higher levels of Crhr1 in multiple brain regions including the amygdala when compared to iNP rats. Higher Crhr1 mRNA expression in P rats is consistent with innate up-regulation of the Crhr1 transcript in several limbic brain areas of high alcohol preferring msP rats (Hansson et al., 2006). Previous studies found that both brain region injection of CRF can promote drinking and administration of CRF1 receptor antagonists prevented increased drinking in P rats (Gilpin et al., 2008; Knapp et al., 2011). Thus, lower levels of CRF2 receptor and higher levels of CRF1 receptor may play an important role in excessive drinking seen in P rats.
Other studies also suggest that Crhr2 is implicated in the modulation of alcohol consumption, anxiety, and the adaptive response to stress. For instance, Crhr2 knockout mice display increases in anxiety-like behavior (Bale and Vale, 2004), which is consistent with lower expression of Crhr2 and higher in anxiety seen in iP compared to iNP rats (Stewart et al., 2004). Another study found that CRH over-expression was associated with up-regulation of Crhr2 mRNA and down regulation of Crhr1 mRNA (Korosi et al., 2006). Thus, a positive correlation between CRF and Crhr2 mRNA, but a negative relationship between CRF and Crhr1 mRNA were previously observed (Ehlers et al., 1992).
CRF2 receptor system is involved in anxiety and the adaptive response to stress (Joels and Baram, 2009; Ryabinin et al., 2012). Experimental manipulations that lead to increased anxiety are associated with decreased SI (Sajdyk et al., 2006). In our studies, SI was associated with an increase in corticosterone levels in the P and NP animals with the P rats exhibiting higher levels than the NP rats (Fig. 6). In addition, 30 minutes of restraint stress resulted in significantly higher levels of corticosterone in P rats relative to NP rats, and larger decrease in social interaction time in P than NP rats (Fig. 7) which is consistent with previous observation (File and Seth, 2003).
The urocortin system, particularly the centrally-projecting Edinger-Westphal (EWcp) nuclei, could influence alcohol drinking. (EWcp)-Ucn1 neurons have been shown to play a role in EtOH drinking as well as reward (Giardino et al., 2011). iNP rats show significantly higher levels of UCN 1 than iP rats in the EWcp (Turek et al., 2005), and lower Ucn2 mRNA expression levels in than iP in multiple brain regions (Liang, unpublished). We speculate that promoter polymorphism in Crhr2 regulates its gene expression, and may likely lead to alteration of expression of Crhr1, CRH and Urocortins. The difference in alcohol drinking and anxiety is due to changes in expression for some of these genes; however, we speculate that the primary reason for these differences in drinking is due to the difference in CRF2 receptor expression. We understand that other interactions between CRF and NPY systems are possible (Sajdyk et al., 2006).
Together, these data provide novel information regarding how selective breeding for alcohol drinking has contributed to a divergence in multiple systems involved in the regulation of both alcohol consumption and the adaptive response of the CNS to environmental stressors. Ultimately, the CRF2 receptor may provide an interesting target for the treatment of alcoholism related to anxiety.
Acknowledgments
This research was supported by the state high-tech program (863-2012AA022403), the National Institute on Alcohol Abuse and Alcoholism (NIAAA) grants R01AA10707, P60 AA007611, AA07611, and R01 MH065702. JPS was supported by a predoctoral award from the Indiana Clinical & Translational Institute through a CTSA award from NIH, NCATS (TR000162).
Footnotes
The authors declare that there are no conflicts of interest of any kind regarding this work.
References
- Arborelius L, Owens MJ, Plotsky PM, Nemeroff CB. The role of corticotrophin-releasing factor in depression and anxiety disorders. The Journal of endocrinology. 1999;160:1–12. doi: 10.1677/joe.0.1600001. [DOI] [PubMed] [Google Scholar]
- Bale TL, Picetti R, Contarino A, Koob GF, Vale WW, Lee KF. Mice deficient for both corticotropin-releasing factor receptor 1 (CRFR1) and CRFR2 have an impaired stress response and display sexually dichotomous anxiety-like behavior. J Neurosci. 2002;22:193–199. doi: 10.1523/JNEUROSCI.22-01-00193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol. 2004;44:525–557. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- Bice P, Foroud T, Bo R, et al. Genomic screen for QTLs underlying alcohol consumption in the P and NP rat lines. Mamm Genome. 1998;9:949–955. doi: 10.1007/s003359900905. [DOI] [PubMed] [Google Scholar]
- Bice PJ, Liang T, Zhang L, Strother WN, Carr LG. Drd2 expression in the high alcohol-preferring and low alcohol-preferring mice. Mamm Genome. 2008;19:69–76. doi: 10.1007/s00335-007-9089-2. [DOI] [PubMed] [Google Scholar]
- Carr LG, Foroud T, Bice P, et al. A quantitative trait locus for alcohol consumption in selectively bred rat lines. Alcohol Clin Exp Res. 1998;22:884–887. [PubMed] [Google Scholar]
- Carr LG, Habegger K, Spence JP, Liu L, Lumeng L, Foroud T. Development of congenic rat strains for alcohol consumption derived from the alcohol-preferring and nonpreferring rats. Behav Genet. 2006;36:285–290. doi: 10.1007/s10519-005-9021-z. [DOI] [PubMed] [Google Scholar]
- Chen A, Perrin M, Brar B, et al. Mouse corticotropin-releasing factor receptor type 2alpha gene: isolation, distribution, pharmacological characterization and regulation by stress and glucocorticoids. Mol Endocrinol. 2005;19:441–458. doi: 10.1210/me.2004-0300. [DOI] [PubMed] [Google Scholar]
- Chester JA, Blose AM, Froehlich JC. Acoustic startle reactivity during acute alcohol withdrawal in rats that differ in genetic predisposition toward alcohol drinking: effect of stimulus characteristics. Alcohol Clin Exp Res. 2004a;28:677–687. doi: 10.1097/01.alc.0000125345.19665.09. [DOI] [PubMed] [Google Scholar]
- Chester JA, Blose AM, Zweifel M, Froehlich JC. Effects of stress on alcohol consumption in rats selectively bred for high or low alcohol drinking. Alcohol Clin Exp Res. 2004b;28:385–393. doi: 10.1097/01.alc.0000117830.54371.7a. [DOI] [PubMed] [Google Scholar]
- Cicero T. In: A critique of animal analogues of alcoholism, in Biochemistry and Pharmacology of Ethanol. Majchrowicz E, Noble EP, editors. Vol. 2. New York: Plenum Press; 1979. pp. 533–560. [Google Scholar]
- Dautzenberg FM, Kilpatrick GJ, Wille S, Hauger RL. The ligand-selective domains of corticotropin-releasing factor type 1 and type 2 receptor reside in different extracellular domains: generation of chimeric receptors with a novel ligand-selective profile. J Neurochem. 1999;73:821–829. doi: 10.1046/j.1471-4159.1999.0730821.x. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Chaplin RI, Wall TL, et al. Corticotropin releasing factor (CRF): studies in alcohol preferring and non-preferring rats. Psychopharmacology (Berl) 1992;106:359–364. doi: 10.1007/BF02245418. [DOI] [PubMed] [Google Scholar]
- File SE, Seth P. A review of 25 years of the social interaction test. Eur J Pharmacol. 2003;463:35–53. doi: 10.1016/s0014-2999(03)01273-1. [DOI] [PubMed] [Google Scholar]
- Funk CK, Koob GF. A CRF(2) agonist administered into the central nucleus of the amygdala decreases ethanol self-administration in ethanol-dependent rats. Brain Res. 2007;1155:172–178. doi: 10.1016/j.brainres.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehlert DR, Shekhar A, Morin SM, et al. Stress and central Urocortin increase anxiety-like behavior in the social interaction test via the CRF1 receptor. Eur J Pharmacol. 2005;509:145–153. doi: 10.1016/j.ejphar.2004.12.030. [DOI] [PubMed] [Google Scholar]
- Giardino WJ, Cocking DL, Kaur S, Cunningham CL, Ryabinin AE. Urocortin-1 within the centrally-projecting Edinger-Westphal nucleus is critical for ethanol preference. PLoS One. 2011;6:e26997. doi: 10.1371/journal.pone.0026997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Richardson HN, Koob GF. Effects of CRF1-receptor and opioid-receptor antagonists on dependence-induced increases in alcohol drinking by alcohol-preferring (P) rats. Alcohol Clin Exp Res. 2008;32:1535–1542. doi: 10.1111/j.1530-0277.2008.00745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greetfeld M, Schmidt MV, Ganea K, Sterlemann V, Liebl C, Muller MB. A single episode of restraint stress regulates central CRH receptor expression and binding in specific areas of the mouse brain. J Neuroendocrinol. 2009 doi: 10.1111/j.1365-2826.2009.01865.x. [DOI] [PubMed] [Google Scholar]
- Grigoriadis DE, Liu XJ, Vaughn J, et al. 125I-Tyro-sauvagine: a novel high affinity radioligand for the pharmacological and biochemical study of human corticotropin-releasing factor 2 alpha receptors. Mol Pharmacol. 1996;50:679–686. [PubMed] [Google Scholar]
- Hansson AC, Cippitelli A, Sommer WH, et al. Variation at the rat Crhr1 locus and sensitivity to relapse into alcohol seeking induced by environmental stress. Proc Natl Acad Sci U S A. 2006;103:15236–15241. doi: 10.1073/pnas.0604419103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M, Koob GF. A key role for corticotropin-releasing factor in alcohol dependence. Trends Neurosci. 2007;30:399–406. doi: 10.1016/j.tins.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang BH, Stewart R, Zhang JK, Lumeng L, Li TK. Corticotropin-releasing factor gene expression is down-regulated in the central nucleus of the amygdala of alcohol-preferring rats which exhibit high anxiety: a comparison between rat lines selectively bred for high and low alcohol preference. Brain Res. 2004;1026:143–150. doi: 10.1016/j.brainres.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Inoue K, Valdez GR, Reyes TM, et al. Human urocortin II, a selective agonist for the type 2 corticotropin-releasing factor receptor, decreases feeding and drinking in the rat. J Pharmacol Exp Ther. 2003;305:385–393. doi: 10.1124/jpet.102.047712. [DOI] [PubMed] [Google Scholar]
- Joels M, Baram TZ. The neuro-symphony of stress. Nat Rev Neurosci. 2009;10:459–466. doi: 10.1038/nrn2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kageyama K, Li C, Vale WW. Corticotropin-releasing factor receptor type 2 messenger ribonucleic acid in rat pituitary: localization and regulation by immune challenge, restraint stress, and glucocorticoids. Endocrinology. 2003;144:1524–1532. doi: 10.1210/en.2002-221046. [DOI] [PubMed] [Google Scholar]
- Knapp DJ, Overstreet DH, Huang M, et al. Effects of a stressor and corticotrophin releasing factor on ethanol deprivation-induced ethanol intake and anxiety-like behavior in alcohol-preferring P rats. Psychopharmacology (Berl) 2011;218:179–189. doi: 10.1007/s00213-011-2366-5. [DOI] [PubMed] [Google Scholar]
- Korosi A, Veening JG, Kozicz T, et al. Distribution and expression of CRF receptor 1 and 2 mRNAs in the CRF over-expressing mouse brain. Brain Res. 2006;1072:46–54. doi: 10.1016/j.brainres.2005.12.034. [DOI] [PubMed] [Google Scholar]
- Lewis K, Li C, Perrin MH, et al. Identification of urocortin III, an additional member of the corticotropin-releasing factor (CRF) family with high affinity for the CRF2 receptor. Proc Natl Acad Sci U S A. 2001;98:7570–7575. doi: 10.1073/pnas.121165198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang T, Habegger K, Spence JP, et al. Glutathione S-transferase 8-8 expression is lower in alcohol-preferring than in alcohol-nonpreferring rats. Alcohol Clin Exp Res. 2004;28:1622–1628. doi: 10.1097/01.alc.0000145686.79141.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang T, Spence J, Liu L, et al. alpha-Synuclein maps to a quantitative trait locus for alcohol preference and is differentially expressed in alcohol-preferring and - nonpreferring rats. Proc Natl Acad Sci U S A. 2003;100:4690–4695. doi: 10.1073/pnas.0737182100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovenberg TW, Chalmers DT, Liu C, De Souza EB. CRF2 alpha and CRF2 beta receptor mRNAs are differentially distributed between the rat central nervous system and peripheral tissues. Endocrinology. 1995;136:4139–4142. doi: 10.1210/endo.136.9.7544278. [DOI] [PubMed] [Google Scholar]
- Lowery EG, Spanos M, Navarro M, Lyons AM, Hodge CW, Thiele TE. CRF-1 antagonist and CRF-2 agonist decrease binge-like ethanol drinking in C57BL/6J mice independent of the HPA axis. Neuropsychopharmacology. 2010;35:1241–1252. doi: 10.1038/npp.2009.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride WJ, Li TK. Animal models of alcoholism: neurobiology of high alcohol-drinking behavior in rodents. Crit Rev Neurobiol. 1998;12:339–369. doi: 10.1615/critrevneurobiol.v12.i4.40. [DOI] [PubMed] [Google Scholar]
- McKinzie DL, Sajdyk TJ, McBride WJ, et al. Acoustic startle and fear-potentiated startle in alcohol-preferring (P) and -nonpreferring (NP) lines of rats. Pharmacol Biochem Behav. 2000;65:691–696. doi: 10.1016/s0091-3057(99)00252-x. [DOI] [PubMed] [Google Scholar]
- Murphy JM, Stewart RB, Bell RL, et al. Phenotypic and genotypic characterization of the Indiana University rat lines selectively bred for high and low alcohol preference. Behav Genet. 2002;32:363–388. doi: 10.1023/a:1020266306135. [DOI] [PubMed] [Google Scholar]
- Pandey SC, Zhang H, Roy A, Xu T. Deficits in amygdaloid cAMP-responsive element-binding protein signaling play a role in genetic predisposition to anxiety and alcoholism. J Clin Invest. 2005;115:2762–2773. doi: 10.1172/JCI24381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos GW. The rat brain in stereotaxic coordinates. San Diego, CA: Academic Press; 1998. [Google Scholar]
- Primus RJ, Yevich E, Baltazar C, Gallager DW. Autoradiographic localization of CRF1 and CRF2 binding sites in adult rat brain. Neuropsychopharmacology. 1997;17:308–316. doi: 10.1016/S0893-133X(97)00071-7. [DOI] [PubMed] [Google Scholar]
- Rainnie DG, Bergeron R, Sajdyk TJ, Patil M, Gehlert DR, Shekhar A. Corticotrophin releasing factor-induced synaptic plasticity in the amygdala translates stress into emotional disorders. J Neurosci. 2004;24:3471–3479. doi: 10.1523/JNEUROSCI.5740-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryabinin AE, Bachtell RK, Heinrichs SC, et al. The corticotropin-releasing factor/urocortin system and alcohol. Alcohol Clin Exp Res. 2002;26:714–722. [PubMed] [Google Scholar]
- Ryabinin AE, Tsoory MM, Kozicz T, et al. Urocortins: CRF’s siblings and their potential role in anxiety, depression and alcohol drinking behavior. Alcohol. 2012;46:349–357. doi: 10.1016/j.alcohol.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajdyk TJ, Fitz SD, Shekhar A. The role of neuropeptide Y in the amygdala on corticotropin-releasing factor receptor-mediated behavioral stress responses in the rat. Stress. 2006;9:21–28. doi: 10.1080/10253890600557315. [DOI] [PubMed] [Google Scholar]
- Sanchez MM, Young LJ, Plotsky PM, Insel TR. Autoradiographic and in situ hybridization localization of corticotropin-releasing factor 1 and 2 receptors in nonhuman primate brain. J Comp Neurol. 1999;408:365–377. [PubMed] [Google Scholar]
- Sharpe AL, Coste SC, Burkhart-Kasch S, Li N, Stenzel-Poore MP, Phillips TJ. Mice deficient in corticotropin-releasing factor receptor type 2 exhibit normal ethanol-associated behaviors. Alcohol Clin Exp Res. 2005;29:1601–1609. doi: 10.1097/01.alc.0000179371.46716.5e. [DOI] [PubMed] [Google Scholar]
- Shekhar A, Keim SR. The circumventricular organs form a potential neural pathway for lactate sensitivity: implications for panic disorder. J Neurosci. 1997;17:9726–9735. doi: 10.1523/JNEUROSCI.17-24-09726.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SV, Lumeng L, Read MS, et al. Characterization of a new hereditary thrombopathy in a closed colony of Wistar rats. J Lab Clin Med. 1996;128:601–611. doi: 10.1016/s0022-2143(96)90133-x. [DOI] [PubMed] [Google Scholar]
- Sommer WH, Rimondini R, Hansson AC, et al. Upregulation of voluntary alcohol intake, behavioral sensitivity to stress, and amygdala crhr1 expression following a history of dependence. Biol Psychiatry. 2008;63:139–145. doi: 10.1016/j.biopsych.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Stewart RB, Badia-Elder NE, Stogsdill TR, et al. Behavioral phenotyping of inbred alcohol-preferring and nonpreferring (iP, iNP) rat strains. Alcohol Clin Exp Res. 2004;28:89A. [Google Scholar]
- Stewart RB, Gatto GJ, Lumeng L, Li TK, Murphy JM. Comparison of alcohol-preferring (P) and nonpreferring (NP) rats on tests of anxiety and for the anxiolytic effects of ethanol. Alcohol. 1993;10:1–10. doi: 10.1016/0741-8329(93)90046-q. [DOI] [PubMed] [Google Scholar]
- Todorovic C, Jahn O, Tezval H, Hippel C, Spiess J. The role of CRF receptors in anxiety and depression: implications of the novel CRF1 agonist cortagine. Neurosci Biobehav Rev. 2005;29:1323–1333. doi: 10.1016/j.neubiorev.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Turek VF, Tsivkovskaia NO, Hyytia P, Harding S, Le AD, Ryabinin AE. Urocortin 1 expression in five pairs of rat lines selectively bred for differences in alcohol drinking. Psychopharmacology (Berl) 2005;181:511–517. doi: 10.1007/s00213-005-0011-x. [DOI] [PubMed] [Google Scholar]
- Valdez GR, Sabino V, Koob GF. Increased anxiety-like behavior and ethanol self-administration in dependent rats: reversal via corticotropin-releasing factor-2 receptor activation. Alcohol Clin Exp Res. 2004;28:865–872. doi: 10.1097/01.alc.0000128222.29875.40. [DOI] [PubMed] [Google Scholar]
- Van Pett K, Viau V, Bittencourt JC, et al. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol. 2000;428:191–212. doi: 10.1002/1096-9861(20001211)428:2<191::aid-cne1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]


