Abstract
Treatment of cultures with toll-like receptor (TLR) ligands or cytokines has become a popular approach to investigate astrocyte neuroinflammatory responses and to simulate the neural environment in various CNS disorders. However, despite much effort, the mechanism of astrocyte activation such as their responses to the TLR ligands and IL-1 remain highly debated. We compared highly pure primary mouse and human astrocyte cultures in their ability to produce proinflammatory mediators (termed “A1”) and immunoregulatory mediators (termed “A2”) in response to LPS, poly IC and IL-1 stimulation. In human astrocytes, IL-1 induced both A1 and A2 responses, poly IC induced mostly A2, and LPS induced neither. In mouse astrocytes, LPS induced mostly an A1 predominant response, poly IC induced both A1 and A2, and IL-1 neither. In addition, mouse astrocytes produce abundant IL-1 protein while human astrocytes did not, despite robust IL-1 mRNA expression. Of the TLR4 receptor complex proteins, human astrocytes expressed TLR4 and MD2 but not CD14, while mouse astrocytes expressed all three. Mouse astrocyte CD14 (cell-associated and soluble) was potently upregulated by LPS. Silencing TLR4 or CD14 by siRNA suppressed LPS responses in mouse astrocytes. In vivo, astrocytes in LPS-injected mouse brains also expressed CD14. Our results show striking differences between human and mouse astrocytes in the use of TLR/IL-1R and subsequent downstream signaling and immune activation. IL-1 translational block in human astrocytes may be a built-in mechanism to prevent autocrine and paracrine cell activation and neuroinflammation. These results have important implications for translational research of human CNS diseases.
Keywords: brain, TLR, innate immunity, poly IC, cytokines, species, inflammation, evolution
INTRODUCTION
Astrocyte cultures derived from neonatal rodent or human fetal brains are readily available systems that have aided our understanding of astrocyte responses to various insults. Treatment of cultures with toll-like receptor (TLR) ligands or cytokines has become a popular approach to investigate astrocyte neuroinflammatory responses and to simulate the neural environment in various CNS disorders. Glial innate immune activation is a mechanism that is elicited in virtually all CNS conditions whereby astrocytes and microglia play intricate roles in modulating neuronal function and fate. One of the highly debated issues in glial biology is the context in which astrocytes participate in the innate immune responses. In particular, astrocyte responses to TLR ligands such as LPS have been debated for well over two decades. While there are early studies reporting LPS activation of primary cultures of rodent astrocytes (Chung et al, 1991; Lieberman et al, 1989), these studies were often suspected of having “contaminating” microglial cells as the source of LPS responses (Giulian et al, 1986; Holm et al, 2012; Saura, 2007). A number of approaches have been taken to eliminate microglia in these cultures including the use of L-leucine methyl ester with or without anti-mitotic agents (Giulian et al, 1986; Hamby et al, 2006; Uliasz et al, 2012), liposomal clodronate (Kumamaru et al, 2012), transgenic expression of a myeloid-specific suicide gene (Holm et al, 2012), or repeated passage of the mixed culture (Du et al, 2010; Lee et al, 1992). A study comparing four major mouse astrocyte culture protocols reported that the subculture procedure yielded significantly higher purity than primary or shaken cultures (Du et al, 2010). The role of “contaminating” microglia issue notwithstanding, LPS response of (rodent) astrocytes has been reported by numerous groups using various astrocyte culture protocols (Brahmachari et al, 2006; Galea et al, 1996; Gorina et al, 2011; Hamby et al, 2006; Hamby et al, 2012; Krasowska-Zoladek et al, 2007; Lange et al, 2012; Ma et al, 2013; Pang et al, 2001). Most studies reported induction of cytokines, chemokines and other inflammatory mediators by LPS.
Our laboratories have long been engaged in the study of human astrocyte immune responses and we have found that human astrocytes are unresponsive to LPS but highly sensitive to IL-1 (with or without IFNγ). The amounts/types of inflammatory genes induced by IL-1 in human astrocytes resemble those of LPS-activated human microglia (John et al, 2003; Lee et al, 1993b; Liu et al, 1996; Tarassishin et al, 2011a), suggesting that astrocytes are capable of mounting potent immune responses but to different stimuli. Evidence supports that adult human astrocytes also respond robustly to IL-1 (Krause et al, 2011; Zhao et al, 1998). However, it is unclear what the effective activating stimuli for rodent astrocytes are, as stated above. The differences between human and rodent astrocytes in morphology, Ca2+ propagation, and their role in neural processing have been demonstrated (Han et al, 2013; Oberheim et al, 2009), and recent studies have also demonstrated enhanced learning in chimeric mice engrafted with human glial progenitor cells through TNFα action (Han et al, 2013).
Given that much of our current knowledge base in neuroscience and glial biology is built on mice (in vivo and in vitro), the goal of this study was to determine whether significant species differences exist in astrocyte innate immune responses, by performing side-by-side comparisons of primary human and mouse cultures. The results show that mouse but not human astrocytes respond robustly to LPS and that they also respond remarkably differently to IL-1 and poly IC, indicating that a fundamental shift in the astrocyte innate immune sensing mechanism has occurred during evolution. These results have important implications for the translational research of human CNS diseases.
MATERIALS AND METHODS
Preparation of mouse astrocyte cultures and BV2 cells
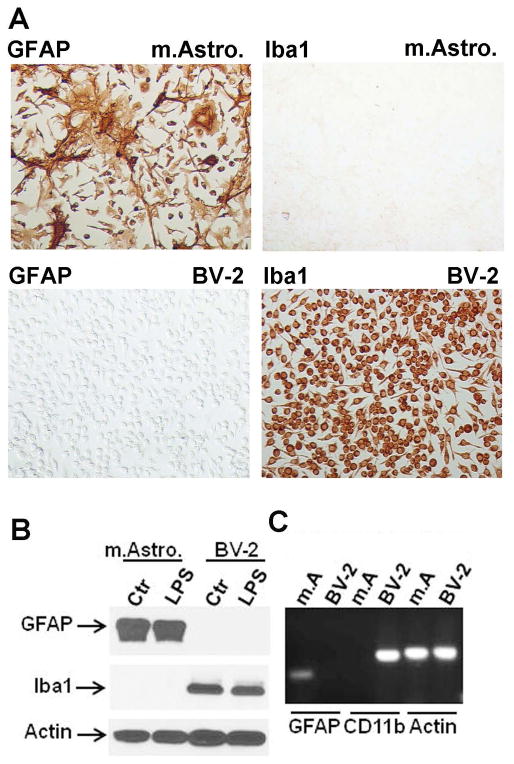
Primary mouse astrocyte cultures were prepared by dissociating brains of newborn C57BL6 mice (1–2 days postnatal) by triturating in Hank’s Balanced Salt Solution without calcium or magnesium (Cellgro). Cell preparations were passed through a 70 μM mylon mesh (BD Biosciences), pelleted, washed, and then seeded in DMEM containing 10% FCS and antibiotics (Invitogen)(complete media) in 10 cm tissue culture dishes (BD Biosciences). Cultures were passaged ~ every 2 weeks into new 10 cm dishes at least 3 times (= G3) to achieve highly pure astrocyte cultures (Du et al, 2010; Lee et al, 1992). Culture purity was determined by GFAP (astrocytes) and Iba-1 (microglia) immunostains (see Figure 1), as well as Western blot and RT-PCR analyses. BV2 cells (Blasi et al, 1990; Henn et al, 2009) were obtained from Dr Linda Van Eldik (University of Kentucky, Lexington, KY), maintained in complete media and propagated twice a week.
Figure 1. Purity of mouse astrocyte culture.
(A) Characterization of the mouse astrocyte cultures used to investigate their immune responses. Newborn pup - derived mixed CNS cell cultures were serially passaged at least three times until non-astrocytic cells are almost absent. The cultures are immunostained for GFAP and Iba-1 and show that Iba-1+ microglia are absent. Control cultures consisted of BV2 mouse microglial cell lines which are negative for GFAP but positive for Iba-1. (B) Western blot analysis of the mouse astrocyte cultures for GFAP and Iba-1 confirming the purity of the culture. β-actin was used as the protein loading control. There was no change in the amount of GFAP or Iba-1 expression after LPS stimulation. BV2 cells were used as controls. (C) RT-PCR analysis of mouse astrocyte (m.A.) and BV-2 cultures for GFAP, CD11b and β-actin, confirming the absence of CD11b mRNA expression in astrocyte cultures.
Preparation of human fetal astrocyte and microglial cultures
Human fetal astrocytes cultures were prepared as previously described (Lee et al, 1992) and according to the protocols approved by the Albert Einstein College of Medicine Institutional Review Board. Briefly, brain tissues of abortuses were dissociated by mincing and trituration and incubated in 0.05% Trypsin-EDTA for 45 min at 37°C. This was followed by filtering through 270 μM and 130 μM pore nylon meshes. Cells were seeded in complete media and cultured until monolayer was formed (~ 2 weeks). Thereafter, monolayers were passaged ~ every 2 weeks at least 3 times (= G3) to enrich for astrocytes. Microglial cultures were prepared by pooling the medium of monolayer cultures at 2–3 weeks in vitro, as previously described (Lee et al, 1992; Liu et al, 1996).
Reagents and cell treatment
Mouse and human IL-1α, IL-1β and IFNγ were purchased from PeproTech (Rocky Hill, NJ) and used at 10 ng/ml unless stated otherwise. Lipopolysaccharides (LPS), both the smooth strain (S-form) from Escherichia coli 0111:B4 and the rough strain (R-form) from Escherichia coli EH100 (Ra mutant), as well as and poly IC (PIC) were purchased from Sigma-Aldrich (St. Louis, MO). The smooth form of LPS was used for experiments, unless stated otherwise. For standard cell stimulation, LPS was used at 100 ng/ml and poly IC at 10 μg/ml. All culture treatments were done in DMEM containing 0.5% FCS (low serum medium). Cells/culture supernatants were collected at 6 h and 22 h after cell stimulation for real-time PCR, western blot and ELISA, and at 1 day (D) - 3 D for Griess reaction, unless stated otherwise.
Real-time RT-PCR
Cells were collected in TRIzol and total RNA were prepared by chloroform extraction and isopropanol precipitation according to manufacturer’s recommendations (Invitrogen). cDNA was synthesized with superscript III Reverse Transcriptase (Invitrogen). Real-time RT-PCR was performed using a SYBR Green PCR mix and conducted with the ABI Prism 7900HT (Applied Biosystems). β-Actin, GAPDH or PBDA were used as housekeeping genes. The relative amount of target gene expression was calculated as 2ΔCt where ΔCt is the difference between Ct of housekeeping gene and Ct of target gene. Fold changes were determined by dividing the 2ΔCt of test sample by 2ΔCt of control sample. The primers were designed using Primer-BLAST or Primer 3 program. All primer sequences (both murine and human) are listed in Table 1S as supplemental material.
Reverse transcription (RT)-PCR: Semiquantitative RT-PCR analysis was performed as described (Brahmachari et al, 2006). Briefly, 1 μg of total RNA was reverse transcribed using oligo(dT)12–18 as primer and SuperScript III reverse transcriptase (Life Technologies) in a 20-μl reaction mixture. The resulting cDNA was amplified using platinum PCR Supermix (Life Technologies) and the following primers. CD11b, sense: 5′-CAGATCAACAATGTGACCGTATGGG-3′, antisense: 5′-CATCATGTCCTTGTACTGCCGCTTG-3′; GFAP, sense: 5′-GGCGCTCAATGCTGGCTTCA-3; antisense: 5′-TCTGCCTCCAGCCTCAGGTT-3′; β-actin, sense: 5′-ATATCGCTGCGCTGGTCGTC-3′, antisense: 5′-AGGATGGCGTGAGGGAGAGC-3′. Amplified products were electrophoresed on a 1.5% agarose gel and visualized by ethidium bromide staining.
Western blotting
Cells were lyzed in PBS buffer containing 0.5% Triton X-100, 0.5% Tween 20 and a cocktail of protease inhibitors (Roche Diagnostics) for 30 min at 4°C with constant rotation. The lysates were cleared by centrifugation at 12,000 rpm for 20 min. The samples (40–50 μg total protein) were run in 10% SDS-polyacrylamide or 4–20% grandient mini-protean precast gel and the proteins were transferred to PVDF membrane (Bio-Rad). Membranes were blocked with 5% milk/PBS then incubated with primary antibodies (see below) for 18 h at 4°C with constant rotation and then with secondary antibodies (anti-goat at 1:5,000: Rockland Immunochemicals; anti-rat at 1:10,000: Pierce/Thermo Scientific; anti-mouse at 1:500: Pierce/Thermo Scientific; or anti-rabbit at 1:500: Pierce/Thermo Scientific) for 1 h at RT. The signals were detected using West Pico or Femto Chemiluminescent Reagents (Pierce/Thermo Scientific) or ProtoGlow ECL (National Diagnosctics). Densitometry was performed with Image J NIH software using β-actin as the loading control.
Antibodies
Primary antibodies for western blot were as follows: anti-MD2 (rabbit polyclonal, Abcam, 1:500), anti-TLR4 (rabbit polyclonal, Santa Cruz, 1:200), anti-GFAP (rat monoclonal, Invitrogen/Life Technologies, 1:1000), anti-Iba-1 (mouse monoclonal, Abcam, 1:1000), anti-iNOS (rabbit polyclonal, Novus Biologicals, 1:500), anti-human CD14 that is known to cross-react with mouse CD14 (rabbit polyclonal, Abcam, 1:200), anti-human CD14 (rabbit polyclonal, Thermo Scientific, 1:100), anti-human CD14 (rabbit polyclonal, Sigma, catalogue #HPA001887 at 1:250), anti-mouse CD14 (rat monoclonal, BD Biosciences, catalogue #553738 at 1:250), and anti-β-actin (mouse monoclonal, Cell Signaling, 1:500).
ELISA
ELISA for mouse and human cytokines was performed using the R&D System DuoSet antibody kits as previously described (Tarassishin et al, 2011a; Tarassishin et al, 2011b). Murine IFNβ ELISA was performed using a kit from PBL Interferon Source (Piscataway, NJ) with a sensitivity range of 15.6 – 1000 pg/ml. Detection of soluble CD14 (sCD14) was done using commercial ELISA kits from R&D Systems. The human sCD14 ELISA had the detection range of 62.5–4,000 pg/ml. The mouse sCD14 ELISA had the dection range of 1.56–100 ng/ml.
Griess reaction
Nitrite production was measured in triplicates using the Griess reaction by mixing 100 μl of astrocyte culture supernatants with 100 μl of Griess reagent with the known amounts of sodium nitrite as standards, as previously described (Lee et al, 1993a). OD was measured at 540 nm using a plate reader MRX Revelation (Dynex Technologies).
Immunocytochemistry (tissue culture)
Monolayers were fixed in ice-cold methanol and immunostained as previously described (Liu et al, 1996). Primay antibodies were: anti-GFAP (rat, 1:100, Invitrogen), anti-Iba-1 (rabbit, 1:500, Wako), anti-mouse IL-1α (goat, 1:100, R&D Systems), and anti-mouse CD14 (rat, 1:100, BD Biosciences). The detection was performed using anti-rat, anti-rabbit, anti-mouse ImmPress reagents (Vector Laboratories) following the manufacturer’s instructions. Double labeling was performed with sequential applications of the goat anti-IL-1α (1:100) antibody and anti-goat ImmPress reagent labelled with HRP, then rat anti-GFAP (1:100) antibody and anti-rat IgG conjugated with alkaline phosphatase (AP) at 1:100 (Invitrogen/Life Technologies). Incubation with the primary antibodies was for 1 h at RT and then overnight at 4°C, and the secondary antibodies was for 1 h at RT. Substates used were diaminobenzidine (DAB: DAKO) for HRP and BCIP/nitroblue tetrazolium (NBT: Sigma-Aldrich) for AP.
CD14 immunohistochemistry (IHC) and immunofluorescence (mouse brain)
Formalin-fixed paraffin-enbedded (FFPE) sections of mouse brains that are intracerebrally injected (i.c.) with LPS or PBS (Suh et al, 2010; Sun et al, 2008) were utilized for this study. Immunhistochemistry and immunofluorescence were performed as previously described (Cosenza-Nashat et al, 2006; Cosenza-Nashat et al, 2011), using antigen retreival and ImmPress secondary antibody methods. Briefly, deparaffinized sections were subjected to antigen retrieval in citrate buffer, pH 6.1 (Target Retrieval Solution: DAKO, Carpinteria, CA) at 95 °C for 10 min. The sections were incubated with rat anti-mouse CD14 (1:100) overnight at 4 °C followed by 2 h at RT. Sections were then incubated with a polymer-based secondary antibody (ImmPRESS) following the manufacturer’s instructions. Color was develped using DAB (brown). For double-labeling, CD14 staining was first completed followed by GFAP staining using the AP-labeled secondary antibody and BCIP/NBT, as described above. Double immunofluorescence stain was performed by combining the primary antibodies into a cocktail and incubating the sections in the primary solution (same concentrations as above) overnight at 4 °C and then 2 h at RT. This was followed by incubation with a cocktail of alexa fluor® 488 goat anti-rabbit IgG (H+L) and alexa fluor® 568 goat anti-rat IgG (H+L) both at 1:1000 for 2 h at RT. Slides were washed and mounted using Vectashield HardSet Mounting Medium containing 4′6-diamidino-2-phenylindole (DAPI) nuclear counterstain (Vector Laboratories, Ltd.).
TLR4 and CD14 knockdown
Mouse astrocytes were transfected with SmartPool TLR4 siRNA or control siRNA (Dharmacon/Thermo Scientific) using the transfection reagent TransfectIT-TKO (Mirus Bio LLC), following the manufacturer’s instructions. The mouse CD14 27mer siRNA duplex was purchased from OriGene Technologies, Inc., and was transfected using the SiPORT NeoFX Transfection reagent (Life Technologies/Invitrogen) according to the manufacturer’s instructions. Cells were first incubated with the siRNA (10 nM) for 48 h, then treated with LPS or medium alone for 24 h. The knockdown efficiency was determined by western blotting for TLR4 and CD14.
Statistics
The data shown are representative of 2–5 independent experiments with similar results. Triplicate samples were run for all ELISAs for each individual experiment and the results are shown as mean ± SD. Statistics were performed using one-way ANOVA with post-hoc analysis or Student’s t-test as stated. P values of <0.05 were considered significant. All statistics were run using the GraphPad Prism 5.0 software.
RESULTS
Purity of the mouse astrocyte cultures
Mouse astrocyte cultures prepared with multiple passages (at least three times = G3) consisted of virtually pure GFAP+ cells, lacking Iba-1+ or CD11b+ microglia as demonstrated by immunostain (Figure 1A), western blot (Figure 1B) or RT-PCR (Figure 1C). BV2 cells were used as positive and negative controls for Iba-1/CD11b and GFAP, respectively. The amount of GFAP immunoreactivity in individual astrocytes varied from weak to very strong (Figure 1A). In four separate cultures examined, Iba-1 stain failed to demonstrate positive cells in (G3) astrocyte cultures (not shown). These results demonstrate that the astrocyte cultures we obtained by repeated passage and used in this study are highly pure with no contamination by microglial cells.
Purity of the human astrocyte cultures
Using the same protocol, we have obtained highly pure human astrocyte cultures with < 1% microglia contamination (Lee et al, 1992). Lack of significant microglial contamination is shown repeatedly by the demonstration that while human microglia robustly respond to LPS, human astrocyte cultures do not (Lee et al, 1993b; Lee et al, 1993a; Lee et al, 1993c)(data not shown and this study).
Mouse and human astrocytes respond differently to the TLR ligands and IL-1
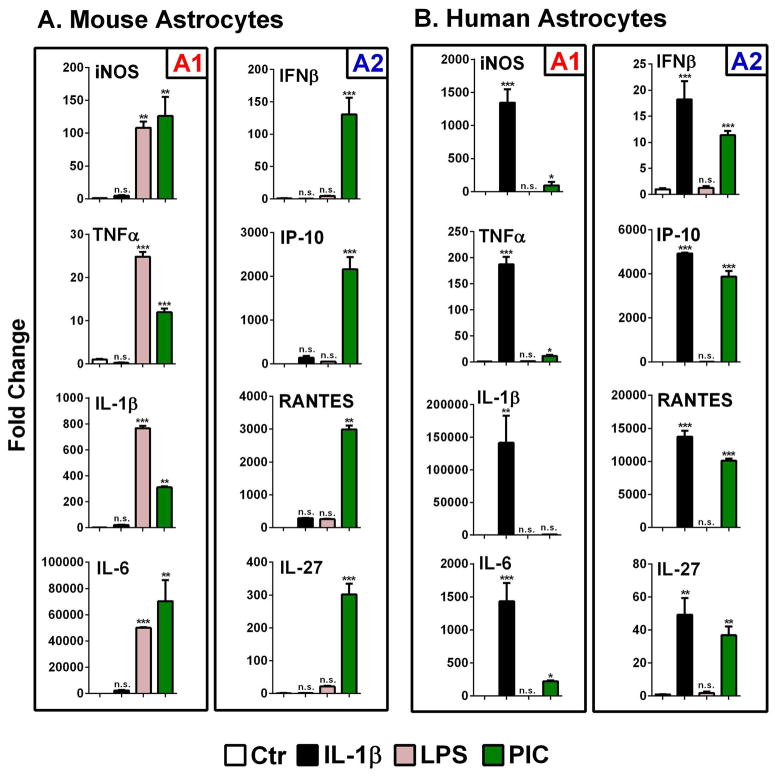
We compared highly pure primary murine and human astrocyte cultures in their responses to LPS (TLR4 ligand), poly IC (TLR3 ligand) and the cytokine (IL-1β) as the three main immune triggers (Figure 2). The target genes are grouped into NF-κB-dependent proinflammatory genes (IL-1β, TNFα, IL-6 and iNOS, termed “A1” response) and IRF3-dependent immunoregulatory genes (IFNβ, IP-10/CXCL10, RANTES/CCL5, and IL-27, termed “A2” response) (Grandvaux et al, 2002; Molle et al, 2010) following the M1 and M2 scheme demonstrated for macrophages and microglia (Downer et al, 2011; Grandvaux et al, 2002; Marsh et al, 2009). The proposal for A1 and A2 designations for astrocyte activation phenotypes and their dependence on IRF3 have been previously published (Tarassishin et al, 2011a; Tarassishin et al, 2011b).
Figure 2. Q-PCR analysis of astrocyte gene expression.
(A) Mouse astrocytes and (B) human astrocyte cultures were stimulated with LPS (100 ng/ml), poly IC (PIC, 10 μg/ml) or cytokines (recombinant murine or human IL-1β at 10 ng/ml) for 22 h, then real-time PCR was performed for select cytokines, chemokines and iNOS using mouse and human-specific primers, as described in the Materials and Methods. Values (mean ± SD, n = 3) are normalized to those in control, unstimulated cultures (Ctr = 1) (ns = not significant, *p<0.05, **p<0.01, ***p <0.001 vs. Ctr). Data are grouped into NF-κB-dependent proinflammatory genes (“A1”, iNOS, TNFα, IL-1β and IL-6) and IRF3-dependent immunoregulatory genes (“A2”, IFNβ, IP-10, RANTES and IL-27). See text for detailed interpretation of the results. Data are representative of multiple (2–5) independent experiments with similar results.
Representative results are shown in Figure 2. In mouse astrocytes (Figure 2A), inflammatory cytokine genes were induced by LPS or poly IC but not by IL-1. Interestingly, in mouse astrocytes, LPS induced predominantly A1 response genes, while poly IC induced both A1 and A2 response genes. In contrast to mouse astrocytes, human astrocytes (Figure 2B) responded robustly to IL-1 but not to LPS. IL-1 activated both A1 and A2 genes but poly IC activated mostly A2 genes. These results show that mouse and human astrocytes respond differently to the same TLR/IL-1R ligands, as depicted in Figure 10 (see below).
Figure 10. Summary and hypothesis.
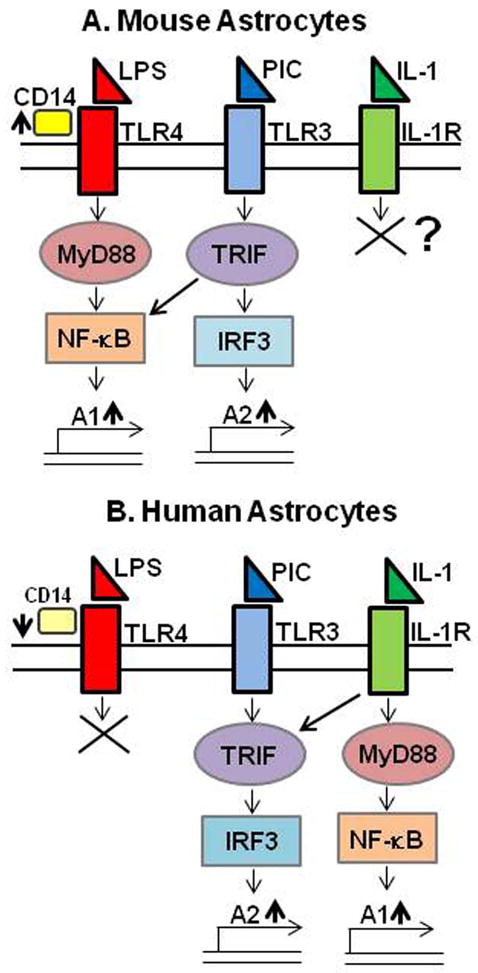
(A) Mouse astrocytes and (B) human astrocytes appear to have distinct intracellular signaling mechanims in response to IL-1 or TLR ligands. LPS activated mouse astrocytes only. While human astrocytes responded robustly to IL-1, mouse astrocytes lacked similar responses to IL-1. In addition, mouse and human astrocytes also showed significant differences in their responses to poly IC. Poly IC induced high levels of TNFα and iNOS (“A1” genes) in mouse astrocytes, but not in human astrocytes. Remarkably, LPS induced little or no immunoregulatory genes (IFNβ, IP-10, RANTES and IL-27: “A2” genes) in mouse astrocytes, many of which are IRF3-dependent. Instead, these genes were robustly induced by poly IC, as in human astrocytes. See Figure 2 and also text for details.
Different stimuli are involved in mouse vs. human astrocyte TNFα induction
We next investigated the details of astrocyte proinflammatory cytokine protein expression using TNFα ELISA as the readout. Mouse or human astrocyte cultures were stimulated with different concentrations of IL-1β (± IFNγ) or with LPS or poly IC and the amount of secreted TNFα was determined by ELISA. The results are shown in Figure 3. As expected, human astrocyte TNFα was induced by IL-1 alone in a dose-dependent manner (10 pg/ml to 100 ng/ml) but LPS or poly IC had no effect. Conversely, murine astrocyte TNFα was induced by LPS or poly IC but not by IL-1 regardless whether IFNγ was present or not. These results confirm the Q-PCR data in Figure 2. Note that the amounts of TNFα protein induced in mouse astrocyte cultures were higher than those in human astrocyte culture. These results show that TNFα induction in mouse and human astrocytes are induced by activation of TLR3/4 and IL-1R, respectively.
Figure 3. TNFα is induced by different stimuli in murine and human astrocyte cultures.
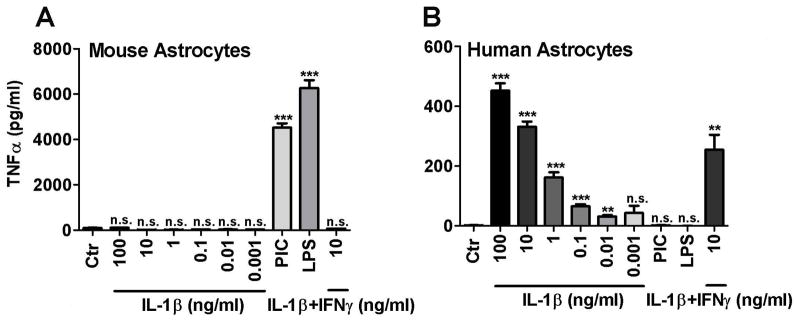
Cultures of mouse (A) and human (B) astrocytes were stimulated with different concentrations of recombinant human or murine IL-1β (± human or murine IFNγ at 10 ng/ml), LPS (100 ng/ml) or poly IC (PIC, 10 μg/ml) for 24 h and the amounts of released TNFα in the culture supernatants were determined by ELISA. Data shown are mean ± SD from triplicate cultures. ** p <0.01, *** p <0.001 and n.s. = not significant vs. Ctr. The results show that while human astrocyte TNFα was induced by IL-1 in a dose-dependent manner, LPS or poly IC had no effect. Conversely, murine astrocyte TNFα was induced by LPS or poly IC, but not by IL-1 (with or without IFNγ). Data are representative of three independent experiments.
Role of IL-1, IFNγ and TLR ligands in mouse astrocyte iNOS induction
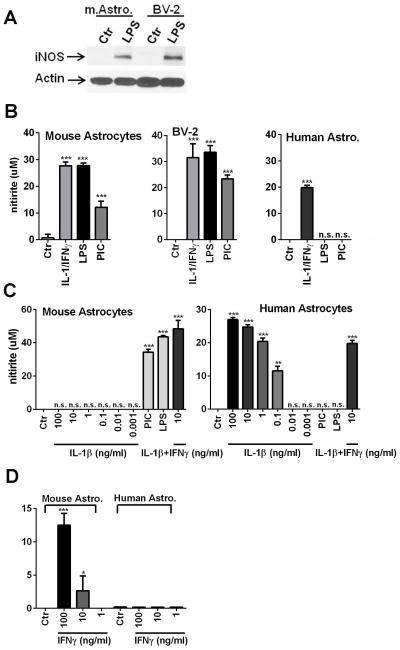
Since the mouse and human iNOS gene promoters display multiple transcription factor-binding elements including NF-κB, AP-1 and IFNγ-activated site (GAS), we compared iNOS/nitrite production in mouse astrocyte, mouse microglia (BV2) and human astrocyte cultures activated with different stimuli. Results are shown in Figure 4. Immunoblotting with an anti-iNOS antibody showed a single protein band of ~ 130 kDa in LPS-stimulated mouse astrocytes and BV2 cells (Figure 4A). iNOS activity was determined by measurement of nitrite in day 1 (1D) and day 3 (3D) culture supernatants by the Griess reaction. The amount of nitrite was higher in 3D than 1D cultures in all experiments (not shown). While iNOS was induced only by IL-1/IFNγ in human astrocytes, mouse astrocyte and BV2 iNOS was induced by all three stimuli with comparable potency (Figure 4B). Furthermore, IL-1β alone (1 pg/ml to 100 ng/ml) without IFNγ had no effect on mouse astrocyte iNOS induction (Godoy et al, 2012), in contrast to human astrocytes (Figure 4C). Unexpectedly, mouse astrocyte iNOS was also inducible by IFNγ alone, similar to the reports in murine macrophages (see Discussion) (Figure 4D). These results together demonstrate that IL-1 signaling in mouse astrocytes is ineffective, relative to human astrocytes.
Figure 4. iNOS expression by mouse astrocytes.
The effect of different stimuli on astrocyte iNOS induction was examined by Western blot (A) and the Griess reaction (nitrite: B, D). (A) Western blot analysis shows that LPS induces iNOS in both mouse astrocytes and BV2 cells. (B) Responses of mouse astrocytes, BV2 cells and human astrocytes stimulated with IL-1/IFNγ, LPS or poly IC (PIC) were determined by the Griess reaction. Both mouse astrocyte and BV2 cell iNOS was induced by all three stimuli, while human astrocyte iNOS was induced only by IL-1/IFNγ. (C) Detailed dose analyses showed that IL-1 alone (up to 100 ng/ml) had no effect on mouse astrocyte iNOS induction, while IL-1 (0.1 ng/ml or above) was effective on human astrocyte iNOS. (D) IFNγ alone also induced nitrite in mouse astrocytes, although it was less potent than when IL-1 was present in the stimulating media. Human astrocytes did not respond to IFNγ alone. Mean ± SD from triplicate cultures. *p<0.05, **p <0.01 and ***p<0.001 vs. Ctr. All data are from 3 day samples and are representative of three independent experiments.
Induction of IL-1α protein in LPS-activated mouse astrocytes
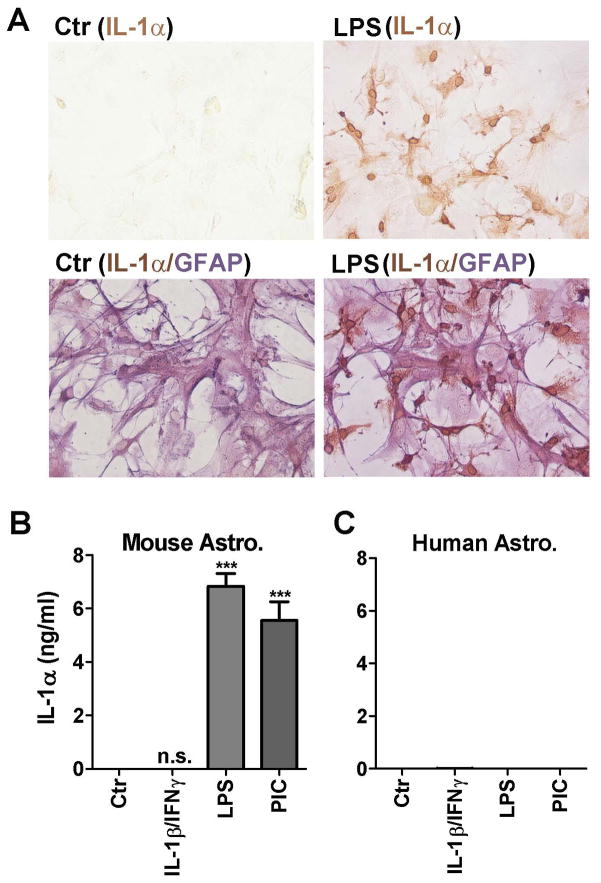
To provide cell-based evidence that mouse astrocytes are responsible for LPS-induced cytokine production, we performed IL-1α immunocytochemistry (Figure 5). As IL-1 proteins are first produced as intracellular proteins (pro-IL-1α and proIL-1β) before release/activation, they provide ideal targets for cell-based immunostaining (Dinarello, 1998; Dinarello, 2010). Figure 5A demonstrates IL-1α positivity in a subset of astrocytes after LPS stimulation. IL-1α immunoreactivity was present in the cytosol and the nuclear membrane. ELISA (cell lysates) in Figure 5B demonstrates that LPS and poly IC but not IL-1/IFNγ induced IL-1α (in nanogram quantities) in mouse astrocyte cultures. These results are similar to those for IL-1β mRNA by Q-PCR (Figure 2).
Figure 5. Induction of IL-1α protein in LPS-activated mouse astrocytes.
(A) Mouse astrocyte cultures were stimulated with LPS or not (Ctr) and subjected to immunostaining for IL-1α (brown, top row) or IL-1α (brown)/GFAP (purple) double labeling (bottom row). IL-1α staining was noted in a subpopulation of LPS-stimulated astrocytes in the nuclear membrane and the cytosol. Double labeling confirms their identity as astrocytes (IL-1α+GFAP+). (B) The amount of cell-associated IL-1α production was determined by ELISA in mouse astrocyte cultures. The results confirm that IL-1 was induced by both LPS and poly IC (PIC) but not by IL-1/IFNγ. (C) Human astrocytes fail to produce IL-1 protein regardless of which stimuli are applied (see text for details).
In human astrocytes, IL-1α protein was not detectable in any of the cultures including those stimulated with IL-1/IFNγ (Figure 5C). Similar results were obtained with IL-1β ELISA (not shown), confirming that human astrocytes fail to produce significant amounts of IL-1 proteins (Liu et al, 1998). This is surprising in light of abundant mRNA induction by IL-1/IFNγ shown by Q-PCR (Figure 2). Together, these results show that while mouse astrocytes express IL-1 proteins in response to LPS or poly IC, in human astrocytes IL-1α and IL-1β expression is blocked at the translational step.
The role of the LPS variant R-LPS in the induction of astrocyte cytokines
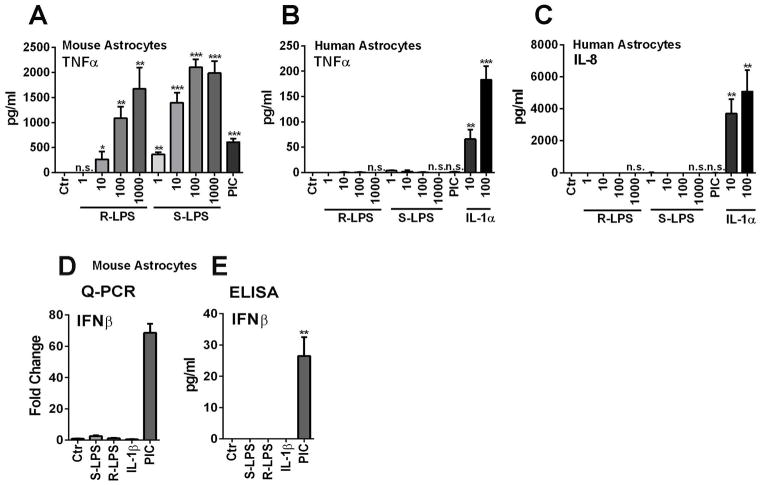
Given that LPS variants have been reported to confer differential abilities to activate macrophages and microglia as well as dendritic cells in various systems (Godowski, 2005; Regen et al, 2011), we wondered whether astrocytes might respond differentially to the rough variant of LPS (R-LPS) as compared to the smooth variant (S-LPS). Mouse and human astrocytes were exposed to R-LPS and S-LPS at 1 ng/ml to 1 μg/ml concentrations and TNFα protein release was measured as the readout (Figure 6A, B). Human astrocytes failed to respond to either form of LPS. The lack of response was also demonstrable by IL-8 ELISA (Figure 6C). In contrast, mouse astrocytes responded to both R-LPS and S-LPS to produce TNFα, with R-LPS being ~ 10-fold less potent than S-LPS (Figure 6A). In contrast to TNFα production, neither form of LPS was able to induce IFNβ production in mouse astrocytes (Figure 6D, E). These results demonstrate that the differential response of human and mouse astrocytes to LPS cannot be attributed to the use of specific LPS variants.
Figure 6. The role of the LPS variant R-LPS in the induction of astrocyte cytokines.
(A, B) Mouse or human astrocytes were stimulated with indicated amounts of rough (R) LPS and their responses were compared with those to smooth (S) LPS (or “LPS”) by TNFα ELISA. The results show that human astrocytes respond to neither form of LPS. Mouse astrocytes responded to both R- and S-LPS, except that R-LPS had the potency of ~ one order of magnitude lower than S-LPS. (C) IL-8 ELISA was performed in human astrocyte samples to confirm that the lack of response to LPS was not limited to TNFα. (D, F) Mouse astrocytes treated as above were examined for IFNβ mRNA and protein expression by Q-PCR and ELISA. The results show that neither variants of LPS nor IL-1 induced IFNβ, while poly IC induced IFNβ. Data are representative of two independent experiments with similar results.
Expression of CD14 and other LPS-receptor and associated proteins in human and mouse astrocytes
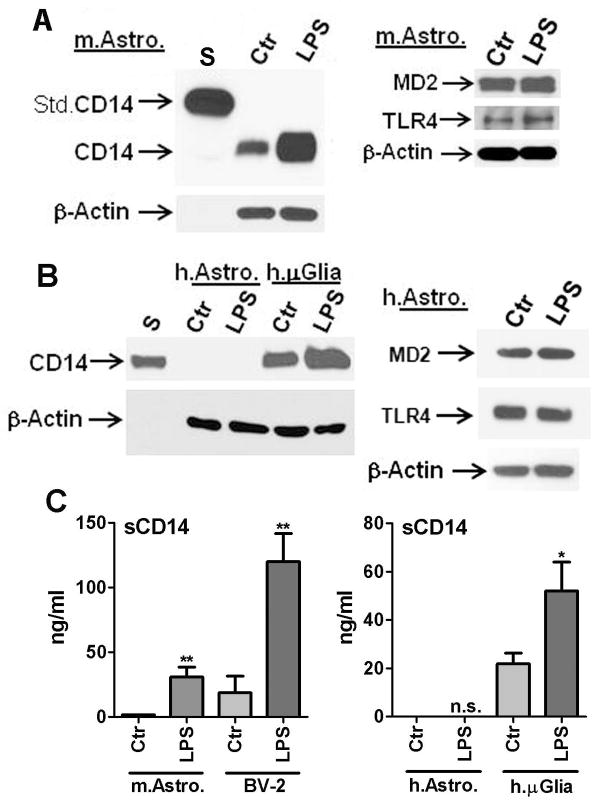
To understand the reasons for the different responses to LPS in mouse and human astrocytes, we examined the level of expression of various molecules involved in the LPS response including CD14, TLR4 and MD2. CD14 is a GPI (glycosyl-phosphatidylinositol)-anchored cell membrane protein that binds to LPS, TLR4 is the recently identified signaling receptor for LPS, and MD2 is the required co-protein necessary for TLR4 signaling. By Western blot analysis, mouse astrocytes expressed all three proteins (Figure 7A). CD14 expression was examined in both mouse and human astrocytes in detail by Western blot analysis using several different commercial antibodies. Known amounts of recombinant mouse CD14-Fc fusion protein and recombinant human CD14 protein were run in parallel as positive controls. A rabbit IgG against human CD14 which also cross-reacts with mouse CD14 (“dual-specific antibody” from Abcam) recognized multiple bands including the one corresponding to the recombinant CD14 at ~ 50 kDa (not shown). A rabbit IgG against human CD14 from Thermo Scientific (formerly Pierce, Rockford IL) also recognized multiple bands (not shown). A rat monoclonal IgG against mouse CD14 (BD Bioscience) demonstrated a single protein band of ~ 50 kDa in mouse samples only. A rabbit polyclonal IgG against human CD14 (Sigma) also demonstrated a single protein band of ~ 50 kDa in human samples only. Therefore, the mouse and human samples were run separately with the known amounts of recombinant CD14 proteins using the last two antibodies. Results representative of at least five independent experiments are shown in Figure 7. In mouse astrocytes, CD14 was readily detectable and the levels were further upregulated following stimulation with LPS. In contrast, human astrocytes lacked CD14 expression in either condition (Figure 7B). Both mouse and human astrocytes expressed TLR4 and MD2 proteins whose level did not change significantly after LPS exposure (Figure 7A, B, and data not shown).
Figure 7. Differential expression of cell-associated and soluble CD14 in mouse and human astrocytes.
(A) Western blot analysis of total cell lysates from mouse astrocyte cultures (Ctr or LPS) demonstrating expression of CD14 protein. Mouse astrocyte CD14 expression is greatly upregulated following LPS stimulation (22 h). The blot was incubated with a rat IgG against anti-mouse CD14 (BD Biosciences) showing a single band of ~50 kDa protein. Positive control (Std.CD14, first lane “S”) was 4 ng of mouse CD14-Fc fusion protein (85–95 kDa, R&D Systems). In addition, mouse astrocytes also expressed MD2 (~ 26 kDa, predicted size 18 kDa) and TLR4 (~ 95 kDa), the amounts of which did not change after LPS stimulation (right panel). (B) Western blot analyses of human astrocyte cultures (± LPS) show lack of CD14 expression. The blot was incubated with a rabbit polyclonal IgG against anti-human CD14 (Sigma) showing a single band of ~50 kDa protein. Positive control was 5 ng of recombinant human CD14 (R&D Systems, first lane “S”), as well as control and LPS-stimulated primary human fetal microglia (last two lanes). MD2 and TLR4 protein expression was also detectable in human astrocytes (right panel). β-actin was used to ensure equal amounts of protein loading. (C) ELISA was performed to determine the amounts of sCD14 in culture supernatants of mouse astrocytes and BV2 cells (left panel) and human astrocytes and microglia (right panel), using two separate commercially available ELISAs (R&D Systems, see Materials and Methods). Data shown are mean ± SD (n = 3). Mouse astrocyte sCD14 is barely detectable but shows significant increase after LPS stimulation. BV2 cells show large amounts of basal production of sCD14 which is increased after LPS stimulation (** p <0.01 vs. Ctr). ELISA for human sCD14 was performed using culture supernatants of human astrocytes and microglial cultures and this shows detectable sCD14 only in microglial cultures. Human microglial sCD14 production is also significantly increased after LPS stimulation (* p<0.05). Results are representative of three independent experiments.
In addition to western blot analysis, we also utilized species-specific ELISAs (R&D Systems) to determine the production of soluble CD14 (sCD14) in these cultures (Figure 7C). BV2 cells and human microglial culture supernatants were used as internal controls. The results show that both mouse microglia (BV2) and human microglia produce large amounts of sCD14 which is significantly upregulated by LPS. Mouse astrocytes produce very low levels of sCD14 (below the detection limit of the ELISA, 1.56 ng/ml), which was also highly upregulated by LPS. sCD14 was not detectable in either the control or LPS-stimulated human astrocyte cultures using ELISAs with lower limit sensitivity of 62.5 pg/ml. These results together demonstrate that mouse but not human astrocytes express significant amounts of CD14.
Role of TLR4 and CD14 in mouse astrocyte LPS response
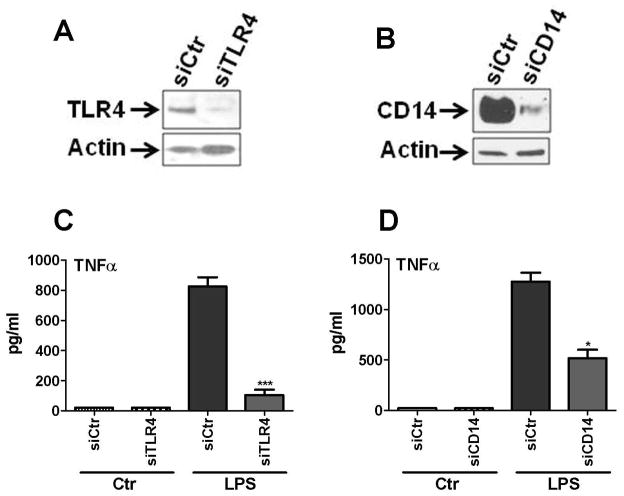
We next examined the role that TLR4 and CD14 play in LPS-induced TNFα production in mouse astrocytes (Figure 8). Astrocytes were treated with siRNA specific to TLR4 or CD14 (or control siRNA, siCtr) as described in Methods. The amount of gene silencing was determined by Western blot (Figure 8A, B). The results show that TLR4-specific siRNA strongly inhibited astrocyte TNFα production compared to control siRNA (Figure 8C). Two independent experiments showed 81% and 91% suppression. CD14 siRNA also significantly inhibited LPS-induced TNFα production (Figure 8D), but to a lesser degree than TLR4 siRNA (65% and 49% inhibition in two independent experiments, see Discussion). These results demonstrate that mouse astrocytes respond to LPS through CD14 and TLR4. The possibility that LPS that we used triggered non-TLR4 response due to impurity is unlikely because of the high degrees of inhibition conferred by CD14 siRNA, as well as the complete lack of response of human astrocytes to LPS (John et al, 2003; Lee et al, 1993b; Lee et al, 1993a; Lee et al, 1993c; Tarassishin and Lee, 2013)(data not shown and this study).
Figure 8. Role of TLR4 and CD14 in LPS-induced mouse astrocyte TNFα production.
Mouse astrocyte cultures were treated with siRNA specific for TLR4 or CD14, or with non-specific control siRNA (siCtr) and then treated with LPS or not (Ctr), as described in the Materials and Methods section. (A, B) Western blot for TLR4 and CD14 demonstrate the efficiency of RNAi. (C, D) The amount of TNFα protein release in each culture was measured by ELISA and this demonstrated that LPS-induced mouse astrocyte TNFα production was significantly inhibited by TLR4 siRNA. Less robust, but significant inhibition was observed with CD14 siRNA. ***p<0.001 and * p <0.05 (vs. siCtr). Results are representative of two independent experiments.
Astrocytes in LPS-injected mouse brains express CD14
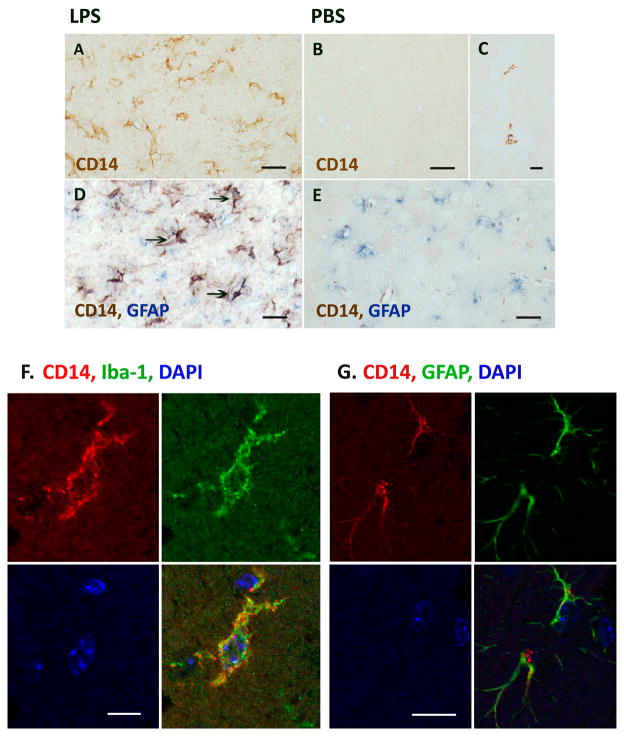
Since the rapid upregulation of CD14 in LPS-stimulated astrocytes was unexpected, we next examined whether astrocytes in vivo can also upregulate CD14 in response to LPS. We obtained brain sections of mice that were injected with LPS or PBS intracerebrally (i.c.) and immunostained them using the rat monoclonal antibody against mouse CD14. Additional controls included uninjected brains, as well as brains of mice injected with LPS intraperitoneally (i.p.). Results show that whereas PBS-injected brains (and all other controls) showed no parenchymal CD14+ staining, robust CD14 immunoreactivity was found in the brains of LPS-i.c. injected mice (Figure 9A, B). Specifically, the deep gray matter including the thalamus and basal ganglia (site of injection) showed diffuse, strong staining, whereas the cortex and hippocampus lacked staining (not shown). Rare non-parenchymal (perivascular or intravascular) CD14+ cells were noted in some brains (Figure 9C). High power magnification demonstrated CD14 staining of cell bodies with processes resembling reactive glial cells (Figure 9A). Double labeling immunohistochemistry for CD14 (brown) and GFAP (astrocytes, blue) demonstrated that some of CD14+ cells were also GFAP+ (Figure 9D, arrows). In control PBS-injected mice, only GFAP staining is noted without parenchymal CD14 stain (Figure 9E). The identity of CD14+ cells was also investigated using double-label immunoflurescence as previously described (Cosenza-Nashat et al, 2011) (Suh et al, 2010) utilizing cell-specific markers, Iba-1 (microglia, green) and GFAP (astrocytes, green). As shown in Figure 9F and G, the identity of CD14+ parenchymal cells was observed to be both microglia and astrocytes. These results confirm that local LPS injection upregulates CD14 protein expression in vivo including diffuse (likely representing sCD14) and cell-associated forms, in both microglia and astrocytes.
Figure 9. Astrocytes in vivo expression CD14 in LPS-injected mouse brains.
CD14 immunohistochemistry of the mouse brains that are injected with LPS (left column) or PBS (right column) 24 h prior to sacrifice. Robust CD14 immunoreactivity is observed in the brains of LPS-injected mice (A) at the site of injection (deep gra matter, thalamus and basal ganglia) whereas PBS injected brains showed no parenchymal CD14+ staining (B). Rare vessel-associated CD14+ cells are also noted (C). Double labeling with GFAP (blue) shows some of the CD14+ cells (brown) are also GFAP+ (D: arrows). Double labeling of PBS-injected mouse brain showing only GFAP stain (E). (F, G) Double label immunofluorescence shows both microglia and astrocytes are CD14+ in intracerebral LPS-injected mice: Immunofluorescence stain for CD14 (red) and cell specific markers, GFAP (astrocytes, green) or Iba-1 (microglia, green), was performed on paraffin-embeded sections of mouse brains injected with LPS, as described in the Materials and Methods section. Shown are examples of astrocytes (GFAP+) and microglia (Iba-1+) that also label for CD14 in these brains. DAPI (blue) was used for nuclear staining. CD14 immunoreactivity appears cytoplasmic, membrane, as well as granular in these cells. Scale bars represent 20 μm in A, B, D and E, 5 μm in C, and 25 μm in G.
Summary and Hypothesis
Based on the data presented in the current work, we propose a simplified scheme that summarizes the differential responses of mouse and human astrocytes to IL-1 or TLR ligands (Figure 10).
DISCUSSION
Several pieces of evidence support the conclusion that mouse astrocytes directly respond to LPS. (1) Mouse astrocyte cultures after repeated trypsinizations were virtually devoid of microglia (Iba-1 negative) but still responded to LPS. (2) Human fetal astrocytes obtained following the same protocol were completely refractory to LPS (i.e., lacked microglia). (3) The magnitude of responses (the amount of cytokine, nitrite or sCD14 production) of mouse astrocytes were in the same order as BV2 cells and reported primary microglia, excluding minor contaminating microglial cells as the source of LPS response.
In addition to the contrasting responses to LPS, our study also revealed another important distinction between mouse and human astrocytes, i.e., their differential responses to IL-1. Given the robust responses of human astrocytes to IL-1 (John et al, 2003; Lee et al, 1993b; Liu et al, 1996; Tarassishin et al, 2011a), the lack of response of mouse astrocytes to IL-1 in our study (such as lack of TNFα and nitrite production) was surprising. When cytokine mixture (IL-1/IFNγ) was used, iNOS was induced in mouse astrocytes in magnitudes similar to LPS stimulation. However, further examination revealed that mouse iNOS was induced by IFNγ alone, but not by IL-1 alone and that IL-1 in the presence of IFNγ acted as a priming agent. This situation is directly opposite of human astrocytes in which IL-1 alone (but not IFNγ alone) induces TNFα or iNOS with IFNγ acting as a priming agent (Hua et al, 2002; Liu et al, 1996).
The ability of IFNγ to induce cytokine genes such as TNFα is an important early response in the activation of macrophages and microglia, both murine and human (O’Connell et al, 2007)(and data not shown). IFNγ also plays a significantly different role in CNS immunity between humans and rodents. For example, IFNγ administration to patients with multiple sclerosis (MS) proved harmful, yet in mice with experimental autoimmune encephalomyelitis (an animal model of MS), IFNγ gene deletion made the disease worse (Steinman, 2008a; Steinman, 2008b). These results together suggest that species-dependent astrocyte immune activation mechanisms may play a significant role in the species-dependent CNS immune responses.
The mechanism underlying the meager IL-1 response in mouse astrocytes is unknown, but previous studies of rat astrocytes have indeed reported LPS ≫ IL-1 potency in cytokine activation (Gottschall et al, 1994), as well as lack of effect (alone) or even downregulation of LPS/IFNγ-induced iNOS by IL-1 (Godoy et al, 2012). Additional requirements such as adhesion to extracellular matrix have been reported for IL-1β action (Summers et al, 2010), as well. These findings together show a significant shift that has occurred in the astrocyte activation mechanisms during evolution. Whereas rodent astrocytes have overlapping mechanisms with myeloid cells, human astrocytes have evolved to utilize distinct mechanisms.
Additional intriguing points raised in our study included that while LPS was effective in inducing proinflammatory (“A1”) genes in mouse astrocytes, it was ineffective in inducing anti-inflammatory or immunoregulatory (“A2”) genes. Furthermore, poly IC, a synthetic double stranded RNA (dsRNA) that activates IRF3 through TLR3 or other cytosolic dsRNA sensors (Hiscott et al, 2006; Tarassishin et al, 2013), induced both A1 and A2 type genes in mouse astrocytes. Our results are corroborated by previous studies of rat astrocytes which showed that both LPS and poly IC induced proinflammatory (“A1”) mediators with similar potency, but only poly IC (and not LPS) induced IFNβ (“A2”) (Krasowska-Zoladek et al, 2007). Another recent study showed that LPS activated MyD88-dependent (“A1”) but not MyD88-independent (“A2”) genes in rodent astrocytes (Gorina et al, 2011).
These results taken together demonstrate differential innate immune activation pathways for human and mouse astrocytes, as summarized in Figure 10. The IL-1R and TLRs have the same intracellular domain called TIR (TLR/IL-1R domain) which recruits MyD88 as the adaptor activating the downstream NF-κB pathway. Activation of the MyD88-independent pathway involves the adaptor TRIF and the transcription factor IRF3. The central dogma in the field is that TLR4 activates both the MyD88 and non-MyD88 (TRIF) pathways and TLR3 activates only the TRIF pathway (Akira et al, 2001; Akira et al, 2006; Kawai et al, 2001). However, in the case of mouse astrocytes, our data suggest that this is not the case.
While investigating the mechanisms underlying all of the stimulus- and species-dependent innate immune activation is clearly beyond the scope of this study, we were most intrigued by the contrasting roles that LPS played in the activation of human and mouse astrocytes. Our initial analysis of the expression of TLR4-associated molecules revealed that while both human and mouse astrocytes expressed TLR4 and MD2, a clear difference existed in their expression of CD14. Employing various detection methods including several anti-CD14 antibodies, we found that CD14 expression is (virtually) absent in human astrocytes but it is readily detectable in mouse astrocytes, especially after LPS stimulation. We also found that the mouse astrocyte LPS response (TNFα production) is dependent on TLR4, and to a lesser degree on CD14, indicating that these receptor components are functional in mouse astrocytes. The differential inhibition also suggests that CD14 may not be absolutely required for the LPS response (see below).
CD14 serves as a high affinity receptor for LPS and is expressed primarily in cells of monocyte and macrophage lineage (Pugin et al, 1993; Wright et al, 1990; Ziegler-Heitbrock and Ulevitch, 1993). In non-myeloid cells, expression of CD14 (by plasmid transfection) has been shown to increase LPS response by as much as 1,000-fold. CD14 exists as a GPI-anchored membrane protein as well as a soluble protein (sCD14) both of which can activate TLR4 signaling. In mouse macrophages, CD14 appears to play a differential role in TLR4 signaling. For example, CD14 is required for MyD88-independent but not MyD88-dependent TLR4 signaling (Godowski, 2005; Jiang et al, 2005), and CD14 is required for the response to S-but not R-chemotype (R-LPS can activate MyD88 in CD14−/− macrophages) (Jiang et al, 2005). In B cells and mast cells (that normally lack CD14 expression), R-LPS can trigger much higher responses than S-LPS (Huber et al, 2006; Minguet et al, 2008). However, the extent to which these findings extend to astrocytes is unclear, since we found that mouse astrocytes responded to both LPS chemotypes (S > R), while human astrocytes responded to neither, indicating that more complex mechanisms are operative.
The significance of tissue culture-derived data can be questioned, as both human and mouse astrocyte cultures are derived from immature brain (fetal and neonatal, respectively) and their purity, while convenient, also poses as a problem of lifting cell-cell inhibition and other contextual influences in vivo. Since robust CD14 expression by mouse astrocytes was unexpected, and since LPS-induced upregulation of CD14 appears to be central to TLR4 signaling in vivo (Fearns et al, 1995; Fearns and Loskutoff, 1997; Xia et al, 2006), we wished to examine whether astrocytes in vivo can express CD14. Using a specific antibody, we demonstrated that CD14 expression (immunoreactivity) is de novo induced following LPS injection to brain. In addition to diffuse immunoreactivity, cell-associated CD14 was also induced in astrocytes as well as in microglia. These observations are in keeping with the well-established fact that LPS injection to mice induces CD14 expression in (systemic) epithelial cells as well as myeloid cells (Fearns et al, 1995; Fearns and Loskutoff, 1997). Astrocyte expression of CD14 mRNA was also found in a recent study of LPS injected mice (Zamanian et al, 2012). In contrast to astrocytic expression of CD14 in mice, CD14 expression in human CNS appears limited to macrophages and microglia, as shown in several clinical situations (Beschorner et al, 2002a; Beschorner et al, 2002b; Cosenza et al, 2002). These results suggest that the differential innate activation mechanisms that we see in cultured mouse (rodent) and human astrocytes may apply to the in vivo situation.
Additional unexpected findings in our study include the contrasting roles that human and murine astrocytes have in the production of IL-1 α/β proteins. Whereas mouse astrocytes produced large amounts of IL-1 when stimulated with LPS (or poly IC), human astrocytes did not produce IL-1 proteins despite high levels of IL-1 mRNA (induced by IL-1). IL-1 is another myeloid-lineage restricted protein, being expressed exclusively by macrophages and microglia in the human CNS (Griffin et al, 1995; Liu et al, 2001; Zhao et al, 2001). Given the powerful role of IL-1 in the activation of human astrocytes, this IL-1 “translational” block may represent a built-in mechanism that protects astrocytes from autocrine/paracrine activation, thereby protecting the brain from perpetuating neuroinflammation.
Our results provide some answers to decades-long debates regarding the mechanism of astrocyte activation. The rather unexpected but striking species-dependent differences that we observe reinforce the notion that astrocytes may contribute to the uniqueness of the human brain. Our study adds significantly to the recent reports which showed morphologic and functional distinctions of human astrocytes in comparison to murine astrocytes (Han et al, 2013) and further shows that the differences may extend to the immunologic responses as well. These studies have significant implications for translating astrocyte data derived from rodents to humans.
Supplementary Material
Acknowledgments
This study was supported in part by NIH RO1 MH55477, Molecular Neuropathology Training Grant T32 NS007098 and Einstein CFAR NIH P30 AI051519. The authors thank Dr Namjong Choi for assisting with the mouse CD14 immunohistochemistry, Ms Christina Polumbo at the Einstein Analytical Imaging Facility for assisting with confocal microscopy, Dr Brad Poulos, Director of the Einstein Human Fetal Tissue Repository for human brain tissue and Dr Linda Van Eldik of University of Kentucky for providing BV2 cells. We are very grateful to Dr Celia F. Brosnan for critical reading of the manuscript.
Reference List
- Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2:675–680. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Beschorner R, Nguyen TD, Gozalan F, Pedal I, Mattern R, Schluesener HJ, Meyermann R, Schwab JM. CD14 expression by activated parenchymal microglia/macrophages and infiltrating monocytes following human traumatic brain injury. Acta Neuropathol. 2002a;103:541–549. doi: 10.1007/s00401-001-0503-7. [DOI] [PubMed] [Google Scholar]
- Beschorner R, Schluesener HJ, Gozalan F, Meyermann R, Schwab JM. Infiltrating CD14+ monocytes and expression of CD14 by activated parenchymal microglia/macrophages contribute to the pool of CD14+ cells in ischemic brain lesions. J Neuroimmunol. 2002b;126:107–115. doi: 10.1016/s0165-5728(02)00046-2. [DOI] [PubMed] [Google Scholar]
- Blasi E, Barluzzi R, Bocchini V, Mazzolla R, Bistoni F. Immortalization of murine microglial cells by a v-raf/v-myc carrying retrovirus. J Neuroimmunol. 1990;27:229–237. doi: 10.1016/0165-5728(90)90073-v. [DOI] [PubMed] [Google Scholar]
- Brahmachari S, Fung YK, Pahan K. Induction of glial fibrillary acidic protein expression in astrocytes by nitric oxide. J Neurosci. 2006;26:4930–4939. doi: 10.1523/JNEUROSCI.5480-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung IY, Norris JG, Benveniste EN. Differential tumor necrosis factor alpha expression by astrocytes from experimental allergic encephalomyelitis-susceptible and -resistant rat strains. J Exp Med. 1991;173:801–811. doi: 10.1084/jem.173.4.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosenza MA, Zhao ML, Si Q, Lee SC. Human brain parenchymal microglia express CD14 and CD45 and are productively infected by HIV-1 in HIV-1 encephalitis. Brain Pathol. 2002;12:442–455. doi: 10.1111/j.1750-3639.2002.tb00461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosenza-Nashat MA, Bauman A, Zhao ML, Morgello S, Suh HS, Lee SC. Cannabinoid receptor expression in HIV encephalitis and HIV-associated neuropathologic comorbidities. Neuropathol Appl Neurobiol. 2011;37:464–483. doi: 10.1111/j.1365-2990.2011.01177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosenza-Nashat MA, Kim MO, Zhao ML, Suh HS, Lee SC. CD45 isoform expression in microglia and inflammatory cells in HIV-1 encephalitis. Brain Pathol. 2006;16:256–265. doi: 10.1111/j.1750-3639.2006.00027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello CA. Interleukin-1, interleukin-1 receptors and interleukin-1 receptor antagonist. Int Rev Immunol. 1998;16:457–499. doi: 10.3109/08830189809043005. [DOI] [PubMed] [Google Scholar]
- Dinarello CA. IL-1: discoveries, controversies and future directions. Eur J Immunol. 2010;40:599–606. doi: 10.1002/eji.201040319. [DOI] [PubMed] [Google Scholar]
- Downer EJ, Clifford E, Gran B, Nel HJ, Fallon PG, Moynagh PN. Identification of the synthetic cannabinoid R(+)WIN55,212-2 as a novel regulator of IFN regulatory factor 3 (IRF3) activation and IFNβ expression: relevance to therapeutic effects in models of multiple sclerosis. J Biol Chem. 2011;286:10316–10328. doi: 10.1074/jbc.M110.188599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du F, Qian ZM, Zhu L, Wu XM, Qian C, Chan R, Ke Y. Purity, cell viability, expression of GFAP and bystin in astrocytes cultured by different procedures. J Cell Biochem. 2010;109:30–37. doi: 10.1002/jcb.22375. [DOI] [PubMed] [Google Scholar]
- Fearns C, Kravchenko VV, Ulevitch RJ, Loskutoff DJ. Murine CD14 gene expression in vivo: extramyeloid synthesis and regulation by lipopolysaccharide. J Exp Med. 1995;181:857–866. doi: 10.1084/jem.181.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearns C, Loskutoff DJ. Role of tumor necrosis factor alpha in induction of murine CD14 gene expression by lipopolysaccharide. Infect Immun. 1997;65:4822–4831. doi: 10.1128/iai.65.11.4822-4831.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea E, Reis DJ, Fox ES, Xu H, Feinstein DL. CD14 mediate endotoxin induction of nitric oxide synthase in cultured brain glial cells. J Neuroimmunol. 1996;64:19–28. doi: 10.1016/0165-5728(95)00143-3. [DOI] [PubMed] [Google Scholar]
- Giulian D, Baker TJ, Shih LC, Lachman LB. Interleukin 1 of the central nervous system is produced by ameboid microglia. J Exp Med. 1986;164:594–604. doi: 10.1084/jem.164.2.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godowski PJ. A smooth operator for LPS responses. Nat Immunol. 2005;6:544–546. doi: 10.1038/ni0605-544. [DOI] [PubMed] [Google Scholar]
- Godoy B, Murgas P, Tichauer J, Von BR. Scavenger receptor class A ligands induce secretion of IL1beta and exert a modulatory effect on the inflammatory activation of astrocytes in culture. J Neuroimmunol. 2012;251:6–13. doi: 10.1016/j.jneuroim.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorina R, Font-Nieves M, Marquez-Kisinousky L, Santalucia T, Planas AM. Astrocyte TLR4 activation induces a proinflammatory environment through the interplay between MyD88-dependent NFkappaB signaling, MAPK, and Jak1/Stat1 pathways. Glia. 2011;59:242–255. doi: 10.1002/glia.21094. [DOI] [PubMed] [Google Scholar]
- Gottschall PE, Tatsuno I, Arimura A. Regulation of interleukin-6 (IL-6) secretion in primary cultured rat astrocytes: synergism of interleukin-1 (IL-1) and pituitary adenylate cyclase activating polypeptide (PACAP) Brain Res. 1994;637:197–203. doi: 10.1016/0006-8993(94)91233-5. [DOI] [PubMed] [Google Scholar]
- Grandvaux N, Servant MJ, tenOever B, Sen GC, Balachandran S, Barber GN, Lin R, Hiscott J. Transcriptional profiling of interferon regulatory factor 3 target genes: direct involvement in the regulation of interferon-stimulated genes. J Virol. 2002;76:5532–5539. doi: 10.1128/JVI.76.11.5532-5539.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin WS, Sheng JG, Roberts GW, Mrak RE. Interleukin-1 expression in different plaque types in Alzheimer’s disease: significance in plaque evolution. J Neuropathol Exp Neurol. 1995;54:276–281. doi: 10.1097/00005072-199503000-00014. [DOI] [PubMed] [Google Scholar]
- Hamby ME, Coppola G, Ao Y, Geschwind DH, Khakh BS, Sofroniew MV. Inflammatory mediators alter the astrocyte transcriptome and calcium signaling elicited by multiple G-protein-coupled receptors. J Neurosci. 2012;32:14489–14510. doi: 10.1523/JNEUROSCI.1256-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamby ME, Uliasz TF, Hewett SJ, Hewett JA. Characterization of an improved procedure for the removal of microglia from confluent monolayers of primary astrocytes. J Neurosci Methods. 2006;150:128–137. doi: 10.1016/j.jneumeth.2005.06.016. [DOI] [PubMed] [Google Scholar]
- Han X, Chen M, Wang F, Windrem M, Wang S, Shanz S, Xu Q, Oberheim NA, Bekar L, Betstadt S, Silva AJ, Takano T, Goldman SA, Nedergaard M. Forebrain engraftment by human glial progenitor cells enhances synaptic plasticity and learning in adult mice. Cell Stem Cell. 2013;12:342–353. doi: 10.1016/j.stem.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henn A, Lund S, Hedtjarn M, Schrattenholz A, Porzgen P, Leist M. The suitability of BV2 cells as alternative model system for primary microglia cultures or for animal experiments examining brain inflammation. ALTEX. 2009;26:83–94. doi: 10.14573/altex.2009.2.83. [DOI] [PubMed] [Google Scholar]
- Hiscott J, Lin R, Nakhaei P, Paz S. MasterCARD: a priceless link to innate immunity. Trends Mol Med. 2006;12:53–56. doi: 10.1016/j.molmed.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Holm TH, Draeby D, Owens T. Microglia are required for astroglial Toll-like receptor 4 response and for optimal TLR2 and TLR3 response. Glia. 2012;60:630–638. doi: 10.1002/glia.22296. [DOI] [PubMed] [Google Scholar]
- Hua LL, Kim MO, Brosnan CF, Lee SC. Modulation of astrocyte inducible nitric oxide synthase and cytokine expression by interferon beta is associated with induction and inhibition of interferon gamma-activated sequence binding activity. J Neurochem. 2002;83:1120–1128. doi: 10.1046/j.1471-4159.2002.01226.x. [DOI] [PubMed] [Google Scholar]
- Huber M, Kalis C, Keck S, Jiang Z, Georgel P, Du X, Shamel L, Sovath S, Mudd S, Beutler B, Galanos C, Freudenberg MA. R-form LPS, the master key to the activation ofTLR4/MD-2-positive cells. Eur J Immunol. 2006;36:701–711. doi: 10.1002/eji.200535593. [DOI] [PubMed] [Google Scholar]
- Jiang Z, Georgel P, Du X, Shamel L, Sovath S, Mudd S, Huber M, Kalis C, Keck S, Galanos C, Freudenberg M, Beutler B. CD14 is required for MyD88-independent LPS signaling. Nat Immunol. 2005;6:565–570. doi: 10.1038/ni1207. [DOI] [PubMed] [Google Scholar]
- John GR, Lee SC, Brosnan CF. Cytokines: powerful regulators of glial cell activation. Neuroscientist. 2003;9:10–22. doi: 10.1177/1073858402239587. [DOI] [PubMed] [Google Scholar]
- Kawai T, Takeuchi O, Fujita T, Inoue J, Muhlradt PF, Sato S, Hoshino K, Akira S. Lipopolysaccharide stimulates the MyD88-independent pathway and results in activation of IFN-regulatory factor 3 and the expression of a subset of lipopolysaccharide-inducible genes. J Immunol. 2001;167:5887–5894. doi: 10.4049/jimmunol.167.10.5887. [DOI] [PubMed] [Google Scholar]
- Krasowska-Zoladek A, Banaszewska M, Kraszpulski M, Konat GW. Kinetics of inflammatory response of astrocytes induced by TLR 3 and TLR4 ligation. J Neurosci Res. 2007;85:205–212. doi: 10.1002/jnr.21088. [DOI] [PubMed] [Google Scholar]
- Krause D, Suh HS, Tarassishin L, Cui QL, Durafourt BA, Choi N, Bauman A, Cosenza-Nashat M, Antel JP, Zhao ML, Lee SC. The tryptophan metabolite 3-hydroxyanthranilic Acid plays anti-inflammatory and neuroprotective roles during inflammation role of hemeoxygenase-1. Am J Pathol. 2011;179:1360–1372. doi: 10.1016/j.ajpath.2011.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumamaru H, Saiwai H, Kobayakawa K, Kubota K, Rooijen NV, Inoue K, Iwamoto Y, Okada S. Liposomal clodronate selectively eliminates microglia from primary astrocyte cultures. J Neuroinflammation. 2012;9:116. doi: 10.1186/1742-2094-9-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange SC, Bak LK, Waagepetersen HS, Schousboe A, Norenberg MD. Primary cultures of astrocytes: their value in understanding astrocytes in health and disease. Neurochem Res. 2012;37:2569–2588. doi: 10.1007/s11064-012-0868-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SC, Dickson DW, Liu W, Brosnan CF. Induction of nitric oxide synthase activity in human astrocytes by IL-1β and IFNγ. J Neuroimmunol. 1993a;46:19–24. doi: 10.1016/0165-5728(93)90229-r. [DOI] [PubMed] [Google Scholar]
- Lee SC, Liu W, Brosnan CF, Dickson DW. Characterization of human fetal dissociated CNS cultures with an emphasis on microglia. Lab Invest. 1992;67:465–475. [PubMed] [Google Scholar]
- Lee SC, Liu W, Dickson DW, Brosnan CF, Berman JW. Cytokine production by human fetal microglia and astrocytes: differential induction by LPS and IL-1β. J Immunol. 1993b;150:2659–2667. [PubMed] [Google Scholar]
- Lee SC, Liu W, Roth P, Dickson DW, Berman JW, Brosnan CF. Macrophage colony-stimulating factor in human fetal astrocytes and microglia. Differential regulation by cytokines and lipopolysaccharide, and modulation of class II MHC on microglia. J Immunol. 1993c;150:594–604. [PubMed] [Google Scholar]
- Lieberman AP, Pitha PM, Shin HS, Shin ML. Production of tumor necrosis factor and other cytokines by astrocytes stimulated with lipopolysaccharide or a neurotropic virus. Proc Natl Acad Sci U S A. 1989;86:6348–6352. doi: 10.1073/pnas.86.16.6348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Zhao M-L, Brosnan CF, Lee SC. Expression of type II nitric oxide synthase in primary human astrocytes and microglia: role of IL-1β and IL-1 receptor antagonist. J Immunol. 1996;157:3569–3576. [PubMed] [Google Scholar]
- Liu JS, Amaral TD, Brosnan CF, Lee SC. IFNs are critical regulators of IL-1 receptor antagonist and IL-1 expression in human microglia. J Immunol. 1998;161:1989–1996. [PubMed] [Google Scholar]
- Liu JS, Zhao ML, Brosnan CF, Lee SC. Expression of inducible nitric oxide synthase and nitrotyrosine in multiple sclerosis lesions. Am J Pathol. 2001;158:2057–2066. doi: 10.1016/S0002-9440(10)64677-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma D, Jin S, Li E, Doi Y, Parajuli B, Noda M, Sonobe Y, Mizuno T, Suzumura A. The neurotoxic effect of astrocytes activated with toll-like receptor ligands. J Neuroimmunol. 2013;254:10–18. doi: 10.1016/j.jneuroim.2012.08.010. [DOI] [PubMed] [Google Scholar]
- Marsh B, Stevens SL, Packard AE, Gopalan B, Hunter B, Leung PY, Harrington CA, Stenzel-Poore MP. Systemic lipopolysaccharide protects the brain from ischemic injury by reprogramming the response of the brain to stroke: a critical role for IRF3. J Neurosci. 2009;29:9839–9849. doi: 10.1523/JNEUROSCI.2496-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minguet S, Dopfer EP, Pollmer C, Freudenberg MA, Galanos C, Reth M, Huber M, Schamel WW. Enhanced B-cell activation mediated by TLR4 and BCR crosstalk. Eur J Immunol. 2008;38:2475–2487. doi: 10.1002/eji.200738094. [DOI] [PubMed] [Google Scholar]
- Molle C, Goldman M, Goriely S. Critical role of the IFN-stimulated gene factor 3 complex in TLR-mediated IL-27p28 gene expression revealing a two-step activation process. J Immunol. 2010;184:1784–1792. doi: 10.4049/jimmunol.0902005. [DOI] [PubMed] [Google Scholar]
- O’Connell RM, Taganov KD, Boldin MP, Cheng G, Baltimore D. MicroRNA-155 is induced during the macrophage inflammatory response. Proc Natl Acad Sci U S A. 2007;104:1604–1609. doi: 10.1073/pnas.0610731104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberheim NA, Takano T, Han X, He W, Lin JH, Wang F, Xu Q, Wyatt JD, Pilcher W, Ojemann JG, Ransom BR, Goldman SA, Nedergaard M. Uniquely hominid features of adult human astrocytes. J Neurosci. 2009;29:3276–3287. doi: 10.1523/JNEUROSCI.4707-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang Y, Cai Z, Rhodes PG. Analysis of genes differentially expressed in astrocytes stimulated with lipopolysaccharide using cDNA arrays. Brain Res. 2001;914:15–22. doi: 10.1016/s0006-8993(01)02766-4. [DOI] [PubMed] [Google Scholar]
- Pugin J, Ulevitch RJ, Tobias PS. A critical role for monocytes and CD14 in endotoxin-induced endothelial cell activation. J Exp Med. 1993;178:2193–2200. doi: 10.1084/jem.178.6.2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regen T, van RD, Scheffel J, Kastriti ME, Revelo NH, Prinz M, Bruck W, Hanisch UK. CD14 and TRIF govern distinct responsiveness and responses in mouse microglial TLR4 challenges by structural variants of LPS. Brain Behav Immun. 2011;25:957–970. doi: 10.1016/j.bbi.2010.10.009. [DOI] [PubMed] [Google Scholar]
- Saura J. Microglial cells in astroglial cultures: a cautionary note. J Neuroinflammation. 2007;4:26. doi: 10.1186/1742-2094-4-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman L. A rush to judgment on Th17. J Exp Med. 2008a;205:1517–1522. doi: 10.1084/jem.20072066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman L. Nuanced roles of cytokines in three major human brain disorders. J Clin Invest. 2008b;118:3557–3563. doi: 10.1172/JCI36532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh HS, Cosenza-Nashat M, Choi N, Zhao ML, Li JF, Pollard JW, Jirtle RL, Goldstein H, Lee SC. Insulin-Like Growth Factor 2 Receptor Is an IFNγ-Inducible Microglial Protein that Facilitates Intracellular HIV Replication: Implications for HIV-Induced Neurocognitive Disorders. Am J Pathol. 2010;177:2446–2458. doi: 10.2353/ajpath.2010.100399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers L, Kangwantas K, Nguyen L, Kielty C, Pinteaux E. Adhesion to the extracellular matrix is required for interleukin-1 beta actions leading to reactive phenotype in rat astrocytes. Mol Cell Neurosci. 2010;44:272–281. doi: 10.1016/j.mcn.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Zheng JH, Zhao M, Lee S, Goldstein H. Increased in vivo activation of microglia and astrocytes in the brains of mice transgenic for an infectious R5 human immunodeficiency virus type 1 provirus and for CD4-specific expression of human cyclin T1 in response to stimulation by lipopolysaccharides. J Virol. 2008;82:5562–5572. doi: 10.1128/JVI.02618-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarassishin L, Bauman A, Suh HS, Lee SC. Anti-Viral and Anti-Inflammatory Mechanisms of the Innate Immune Transcription Factor Interferon Regulatory Factor 3: Relevance to Human CNS Diseases. J Neuroimmune Pharmacol. 2013;8:132–144. doi: 10.1007/s11481-012-9360-5. [DOI] [PubMed] [Google Scholar]
- Tarassishin L, Lee SC. Interferon regulatory factor 3 alters glioma inflammatory and invasive properties. J Neurooncol. 2013;113:185–194. doi: 10.1007/s11060-013-1109-3. [DOI] [PubMed] [Google Scholar]
- Tarassishin L, Loudig O, Bauman A, Shafit-Zagardo B, Suh HS, Lee SC. Interferon regulatory factor 3 inhibits astrocyte inflammatory gene expression through suppression of the proinflammatory miR-155 and miR-155*. Glia. 2011a;59:1911–1922. doi: 10.1002/glia.21233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarassishin L, Suh HS, Lee SC. Interferon regulatory factor 3 plays an anti-inflammatory role in microglia by activating the PI3K/Akt pathway. J Neuroinflammation. 2011b;8:187. doi: 10.1186/1742-2094-8-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uliasz TF, Hamby ME, Jackman NA, Hewett JA, Hewett SJ. Generation of primary astrocyte cultures devoid of contaminating microglia. Methods Mol Biol. 2012;814:61–79. doi: 10.1007/978-1-61779-452-0_5. [DOI] [PubMed] [Google Scholar]
- Wright SD, Ramos RA, Tobias PS, Ulevitch RJ, Mathison JC. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;249:1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- Xia Y, Yamagata K, Krukoff TL. Differential expression of the CD14/TLR4 complex and inflammatory signaling molecules following i.c.v. administration of LPS. Brain Res. 2006;1095:85–95. doi: 10.1016/j.brainres.2006.03.112. [DOI] [PubMed] [Google Scholar]
- Zamanian JL, Xu L, Foo LC, Nouri N, Zhou L, Giffard RG, Barres BA. Genomic analysis of reactive astrogliosis. J Neurosci. 2012;32:6391–6410. doi: 10.1523/JNEUROSCI.6221-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao ML, Kim MO, Morgello S, Lee SC. Expression of inducible nitric oxide synthase, interleukin-1 and caspase-1 in HIV-1 encephalitis. J Neuroimmunol. 2001;115:182–191. doi: 10.1016/s0165-5728(00)00463-x. [DOI] [PubMed] [Google Scholar]
- Zhao ML, Liu JS, He D, Dickson DW, Lee SC. Inducible nitric oxide synthase expression is selectively induced in astrocytes isolated from adult human brain. Brain Res. 1998;813:402–405. doi: 10.1016/s0006-8993(98)01023-3. [DOI] [PubMed] [Google Scholar]
- Ziegler-Heitbrock HW, Ulevitch RJ. CD14: cell surface receptor and differentiation marker. Immunol Today. 1993;14:121–125. doi: 10.1016/0167-5699(93)90212-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.