Abstract
Hfq is an RNA-binding protein that plays an important role in many cellular processes. In this study, we examined the biological effect of the Hfq of Edwardsiella tarda, a severe fish pathogen with a broad host range that includes humans. To facilitate the study, a markerless hfq in-frame deletion wild type, TXhfq, was constructed. Compared to the wild type TX01, TXhfq exhibited (i) retarded planktonic and biofilm growth, (ii) decreased resistance against oxidative stress, (iii) attenuated overall virulence and tissue dissemination and colonization capacity, (iv) impaired ability to replicate in host macrophages and to block host immune response. Introduction of a trans-expressed hfq gene into TXhfq restored the lost virulence of TXhfq. To identify potential Hfq targets, comparative global proteomic analysis was conducted, which revealed that 20 proteins belonging to different functional categories were differentially expressed in TXhfq and TX01. Quantitative real time RT-PCR analysis showed that the mRNA levels of two thirds of the genes of the identified proteins were consistent with the proteomic results. Since TXhfq is dramatically attenuated in virulence, we further examined its potential as a naturally delivered vaccine administered via the immersion route in a flounder model. The results showed that TXhfq induced effective protection against lethal E. tarda challenge. Taken together, our study indicated that Hfq is required for the normal operation of E. tarda in multiple aspects, and that Hfq probably exerts a regulatory effect on a wide range of target genes at both transcription and post-transcription levels.
Introduction
Edwardsiella tarda is a Gram-negative, motile, rod-shaped bacterium belonging to the family of Enterobacteriaceae. It is a serious fish pathogen that causes edwardsiellosis, a systematic disease that affects a wide range of farmed fish species including Japanese eel (Anguilla japonica, Temminck & Schlegel, 1847), Japanese flounder (Paralichthys olivaceus, Temminck & Schlegel 1846), turbot (Scophtalmus maximus, Linnaeus 1758), red sea bream (Pagrus major, Temminck & Schlegel 1843), tilapia (Oreochromis niloticus, Linnaeus 1758), and channel catfish (Ictalurus punctatus, Rafinesque 1818) [1]. Heavy economic losses due to E. tarda-related edwardsiellosis have been reported in aquaculture industries worldwide [2]. In addition to fish, E. tarda is also a human pathogen and known to cause gastroenteritis and extraintestinal infections [3]. Recent studies indicate that E. tarda possesses numerous virulence factors, which participate in different aspects of host infection [2,4]. Currently, owing to the lack of effective vaccines, control of E. tarda in aquaculture depends mainly on the use of antibiotics in most countries including China.
Hfq was originally identified as a host factor required for RNA replication of phage Qβ in Escherichia coli[5], and was classified into the conserved RNA-binding Lsm (like-Sm)/Sm-like protein family found in both eukaryotes and prokaryotes [6]. Accumulating evidences have shown that Hfq is an RNA chaperone involved in posttranscriptional gene regulation via several mechanisms, including interaction with small RNAs (sRNAs) and facilitating their binding to target mRNA, modulating mRNA degradation, and regulating the process of mRNA translation [7]. For pathogenic bacteria such as Brucella abortus, E. coli, Legionella pneumophila, Pseudomonas aeruginosa, Salmonella Typhimurium, Vibrio cholerae, Yersinia enterocolitica, and Klebsiella pneumoniae, Hfq is known to be a virulence factor [8-18]. Inactivation of the hfq gene leads to defect in a variety of biological aspects, notably cellular growth and motility, quorum sensing, stress tolerance, and infectivity [19,20]. However, to date very little study has been documented on the Hfq of fish pathogens, and for E. tarda, the role of Hfq is entirely unknown.
In the present study, we aimed to investigate the biological property of Hfq in E. tarda and, in particular, whether Hfq plays any role in E. tarda infection. For this purpose, we compared the effect of an hfq wild type, TXhfq, to that of the wild type strain. We found that deletion of hfq in E. tarda had pleiotropic effects on bacterial growth, infection, and global protein expression. Additionally, we also observed the potential of TXhfq as a naturally delivered vaccine to elicit protective immunity in host against E. tarda. Our results provide the first insight to the biological function of E. tarda Hfq as well as its applicability in the control of edwardsiellosis in aquaculture.
Materials and methods
Ethics statement
Experiments involving live animals were conducted in accordance with the “Regulations for the Administration of Affairs Concerning Experimental Animals” promulgated by the State Science and Technology Commission of Shandong Province. The study was approved by the ethics committee of Institute of Oceanology, Chinese Academy of Sciences. Efforts were taken to ensure that all research animals received good care and humane treatment as stipulated in the above regulations.
Bacterial strains and growth conditions
Escherichia coli BL21(DE3) was purchased from Tiangen (Beijing, China). E. coli S17-1λpir was purchased from Biomedal (Sevilla, Spain). E. tarda TX01 was isolated from diseased fish [21]. Bacteria were cultured in Luria-Bertani broth (LB) at 37 °C (for E. coli) or 28 °C (for E. tarda). Where indicated, chloramphenicol, polymyxin B, and 2,2’dipyridyl were supplemented at the concentration of 30 μg/mL, 100 μg/mL, and 100 μM respectively. Biofilm growth on polystyrene surface was conducted as reported previously [22].
Fish
Clinically healthy Japanese flounder (Paralichthys olivaceus) (average 11.9 g) were purchased from a local commercial fish farm. The fish were maintained at ~19 °C in aerated seawater that was changed twice daily. The seawater was sand-filtered and activated carbon-absorbed, with pH of 8.1, oxygen > 6 mg/L, and ammonia < 0.1 mg/L. Fish were acclimatized in the laboratory for two weeks before experimental manipulation. Fish were fed daily with commercial dry pellets (purchased from Shandong Sheng-suo Fish Feed Research Center, Shandong, China) at the amount of ~1.2% body weight. Before experiment, fish (6%) were randomly sampled and examined for the presence of bacteria in blood, liver, kidney, and spleen, and no bacteria were detected from the sampled fish. For tissue collection, fish were euthanized with an overdose of MS222 (tricaine methanesulfonate) (Sigma, USA) as described previously [23].
Sequence analysis
hfq was cloned from E. tarda TX01 with primers F1/R1 (Table 1), which were designed based on the known hfq sequence of E. tarda EIB202 and E. tarda FL6-60 (GenBank accession nos. ACY83199.1 and ADM40428.1 respectively). The sequence of Hfq was analyzed using the BLAST program at the National Center for Biotechnology Information (NCBI) and the Expert Protein Analysis System.
Table 1.
Primers used in this study
| Primer | Sequences (5′ → 3′) a |
|---|---|
| F1 |
ATGGCTAAGGGGCAATCT |
| R1 |
TTATTCAGCGTCATCACTGC |
| F2 |
GGATCCGGATTGATCCGGTCGCC (BamHI) |
| R2 |
CTGCCTGCTTGCCCCTTAGCCATTCT |
| F3 |
GGCAAGCAGGCAGCAGCTACCA |
| R3 |
GGATCCCGGTCGGTCTCCAACTG (BamHI) |
| F4 |
GATATCATGGCTAAGGGGCAATC (EcoRV) |
| R4 |
GATATCTTCAGCGTCATCACTGC (EcoRV) |
| HP1-F |
CCCGGGATGCGAGCTGAAACCATTCTG (SmaI) |
| HP1-R |
CCCGGGTTTTAAGCATGATGCGCT (SmaI) |
| HP2-F |
TACGTAATGCTGATAATGCAAATATCAACT (SnaBI) |
| HP2-R |
TACGTACAGGTGCGCGTTAAACCACG (SnaBI) |
| DPFP-F |
GATATCATGTCTCAGCCTCAGAGCG (EcoRV) |
| DPFP-R | GATATCCAGCGTCAGCAAACGCGT (EcoRV) |
aItalicized nucleotides are restriction sites of the enzymes indicated in the brackets at the ends.
hfq knockout
The primers used in this study are listed in Table 1. To construct the hfq knockout wild type TXHfq, in-frame deletion of a 222 bp segment (residues 6 to 79) of hfq was performed by overlap extension PCR as follows: the first overlap PCR was performed with the primer pair F2/R2, the second overlap PCR was performed with the primer pair F3/R3, and the fusion PCR was performed with the primer pair F2/R3. The PCR products were inserted into the suicide plasmid pDM4 [24] at the BglII site, resulting in pDMHfq. S17-1λpir was transformed with pDMHfq, and the transformants were conjugated with TX01 as described previously [21]. The transconjugants were selected on LB agar plates supplemented with 10% sucrose. One of the colonies that were resistant to sucrose and sensitive to chloramphenicol (marker of pDM4) was analyzed by PCR, and the PCR products were subjected to DNA sequencing to confirm in-frame deletion. This strain was named TXHfq.
Complementation of hfq mutation
The plasmid pJTHfq, which expresses hfq constitutively, was created as follows. hfq was amplified by PCR with primers F4/R4; the PCR product was ligated with the T-A cloning vector pBS-T (Tiangen, Beijing, China), and the recombinant plasmid was digested with SmaI. The fragment containing hfq was retrieved and inserted into plasmid pBT3 [25] at the EcoRV site, resulting in pBT3Hfq. pBT3Hfq was digested with SwaI, and the fragment carrying hfq was inserted into plasmid pJT [26] at the SwaI site, resulting in pJTHfq. S17-1λpir was transformed with pJT, and the transformants were conjugated with TXHfq. The transconjugants were selected on LB agar plates supplement with tetracycline (resistance marker of pJT) and polymyxin B. One of the transformants was named TXHfqC.
H2O2 survival analysis
H2O2 survival analysis was performed as reported previously [27].
Virulence analysis
The median lethal dose (LD50) of TX01, TXHfq, and TXHfqC was determined as described previously [23]. For tissue dissemination and colonization analysis, TXHfq and TX01 were cultured in LB medium at 28 °C for different times until OD600 of 0.8. The cells were washed with PBS and resuspended in seawater to 1 × 108 CFU/mL. Flounder were immersed in seawater containing TXHfq or TX01 or PBS (control) for 2 h. The fish were then moved to 58-liter tanks containing fresh seawater and reared normally as described above in the section of “Fish”. At 1, 2, 3, 4, 5, and 7 days post-infection (dpi), blood, kidney, liver, and spleen were taken aseptically from the fish (five/time point). The tissues were homogenized in a glass homogenizer containing PBS (100 μL/mg tissue). The homogenates and blood were diluted serially and plated in triplicate on LB agar plates. The plates were incubated at 28 °C for 48 h, and the colonies that appeared on the plates were enumerated. The genetic identity of the colonies was verified by PCR with specific primers [27] and sequence analysis of selected PCR products. The experiment was conducted in three replicates at the same time, and the mean values were given in the results.
Bacterial replication in macrophages
Flounder head kidney (HK) macrophages were prepared as described previously [28]. The macrophages were cultured in L-15 medium (Thermo Scientific HyClone, Beijing, China) in 96-well culture plates (~105 cells/well). TXHfq, TX01, and TXHfqC suspensions in PBS were prepared as described above and added to macrophages (106 CFU/well). The cells were incubated at 25 °C for 0.5 h. After incubation, the cells were washed with PBS for three times and added with fresh L-15 containing 100 U/mL penicillin and streptomycin (Thermo Scientific HyClone, Beijing, China), followed by incubation at 25 °C for 1.5 h to kill extracellular bacteria. The plates were then washed three times with PBS and incubated at 28 °C for 1 h, 2 h, 4 h, and 8 h. After incubation, the plates were washed with PBS, and the cells were lysed with 100 μL 1% Triton X-100. The cell lysate was serially diluted and plated in triplicate on LB agar plates. The plates were incubated at 28 °C for 48 h, and the colonies that emerged on the plates were counted. The identities of the colonies were verified as described above.
Reactive oxygen species (ROS) production
ROS production was determined as follows. Flounder HK macrophages in a 96-well microplate (~105 cells/well) were incubated with TXhfq, TX01, or TXhfqC (106 CFU/well) for 1 h or 2 h. The plate was washed with PBS for three times. One hundred microliters of 1 mg/mL nitroblue tetrazolium (Sangon, Shanghai, China) in L-15 was added to the cells. After incubation at 25 °C for 2 h, the reaction was stopped by adding 100% methanol. The plate was washed with 70% methanol, and the reduced formazan was solubilized in 100 μL of 2 M KOH and 120 μL of dimethyl sulfoxide. The plate was read at 630 nm with a microplate reader.
Nitric oxide (NO) assay
NO production was determined as follows. Flounder HK macrophages in a 96-well microplate (~105 cells/well) were incubated with TXhfq, TX01, or TXhfqC (106 CFU/well) for 1 h or 2 h. The supernatants were removed to a separate 96-well plate (50 μL/well), followed by adding into each well 100 μL of 1% sulphanilamide and 100 μL of 0.1% N-naphthylethylene-diamine (Sigma, St. Louis, MO, USA). The plate was read at 540 nm, and the molar concentration of nitrite was determined from standard curve generated using known concentrations of sodium nitrate.
Two-dimensional gel electrophoresis (2-DE)
TX01 and TXhfq were cultured in LB medium at 28 °C and collected at OD600 0.8 by centrifugation at 4000 g for 15 min at 4 °C. The cells were washed with PBS for three times and resuspended in extraction solution (7 M urea, 2 M thiourea, 4% CHAPS, 40 mM DTT, 2% IPG buffer). The cells were disrupted by intermittent sonic oscillation for a total of 15 min on ice with intervals of 30 s. Unbroken cells and cellular debris were removed by centrifugation at 20 000 g for 60 min. The proteins in the supernatant were purified with 2D-Clean-Up Kit (GE Healthcare, Piscataway, NJ, USA) and resuspended in IEF sample loading solution (7 M urea, 2 M thiourea, 2% CHAPS, 40 mM DTT, 0.5% IPG buffer, 0.002% bromophenol blue). Protein concentration was determined using the BCA Protein Assay Kit (Sangon Biotech, Shanghai, China). Two-DE was performed as reported previously [29]. The gel images were acquired using ImageScanner III (GE healthcare, Piscataway, NJ, USA) and analyzed with ImageMaster 2D Platinum 6.0 (GE healthcare, Piscataway, NJ, USA). Triplicate runs were made for each sample to ensure gel reproducibility. For comparative analysis, the percentage intensity volume (%vol) of each spot was used for comparison of matched spots between TXhfq and TX01. To reduce potential errors, a ratio of ≥ 2 (or ≤ 0.5) and analysis of variance (ANOVA) < 0.05 were taken as a threshold for differential expression.
In-gel enzymatic digestion and matrix-assisted laser desorption/ionization time of flight (MALDI-TOF) mass spectrometry analysis
The differentially expressed protein spots were picked from the gels and washed once with 500 μL water and three times with 500 μL 25 mM ammonium bicarbonate in 50% acetonitrile for 60 min. The gel spots were dehydrated by addition of 500 μL acetonitrile, and the samples were then incubated in 200 μL 10 mM DTT at 56 °C for 60 min to reduce disulfide bonds. Alkylation of cysteines was performed by adding 200 μL 55 mM iodoacetamide, and the samples were incubated at room temperature for 45 min in the dark. The samples were washed with PBS and dehydrated with 500 μL acetonitrile. The samples were incubated in trypsin solution (10 μg/mL in PBS) for 30 min on ice. After incubation, the remaining trypsin solution was removed, and 25 μL of PBS was added to the samples. Proteolysis was performed at 37 °C overnight and stopped by adding 5% formic acid. MALDI-TOF mass spectrometry (MS) analysis was performed with ultrafleXtreme (Bruker, Germany) as follows. One microliter peptide solution was dripped onto the Anchorchip target plate and allowed it to dry at room temperature. Matrix solution (CHCA) was added to the plate, and the plate was loaded into the spectrometer. The mass range was from 500 to 3500 Da, and the scan resolution was 50 000. After the scan, five most abundant MS peaks were selected for MS/MS scan. Protein identification was as described previously [29].
Quantitative real-time reverse transcription-PCR (qRT-PCR)
TXhfq and TX01 were cultured in in LB medium to an OD600 of 0.8. Total RNA was extracted with EZNA Total RNA Kit (Omega Bio-tek, Doraville, GA, USA). The RNA was treated with RNase-free DNaseI (TaKaRa, Dalian, China). One microgram of RNA was used for cDNA synthesis with the Superscript II reverse transcriptase (Invitrogen, Carlsbad, CA, USA). qRT-PCR was carried out in an Eppendorf Mastercycler (Eppendorf, Hamburg, Germany) using SYBR ExScript qRT-PCR Kit (Takara, Dalian, China) as described previously [30]. Melting curve analysis of amplification products was performed at the end of each PCR to confirm that only one PCR product was amplified and detected. The expression level of the target genes was analyzed using comparative threshold cycle method (2-ΔΔCT) with 16 s rRNA as an internal control. The data are given in terms of mRNA levels relative to that of 16 s rRNA and expressed as means plus or minus standard errors of the means (SE).
Antibody preparation and Western blot
To obtain antibodies against hypothetical protein 1 (HP1), hypothetical protein 2 (HP2), and the dyp-type peroxidase family protein (DPFP), His-tagged recombinant proteins of HP1, HP2, and DPFP were prepared. For this purpose, the plasmids pHP1, pHP2, and pDPFP, which express HP1, HP2, and DPFP respectively, were constructed as follows. The coding sequences of HP1, HP2, and DPFP were amplified by PCR with primer pairs HP1-F/HP1-R, HP2-F/HP2-R, and DPFP-F/DPFP-R, respectively (Table 1). The PCR products were ligated with the pEASY-E2 (TransGen, Beijing, China), resulting in pHP1, pHP2, and pDPFP. For protein preparation, Escherichia coli BL21(DE3) (Tiangen, Beijing, China) was transformed separately with the plasmids. The transformants were cultured in LB medium at 37 °C to mid-log phase, and expression of recombinant proteins was induced by adding isopropyl-β-D -thiogalactopyranoside to a final concentration of 0.4 mM. Growth was continued at 37 °C for 5 h, and recombinant proteins were purified using Ni-NTA agarose (QIAGEN, Valencia, CA, USA) as recommended by the manufacturer. The purified proteins were dialyzed for 24 h against PBS, and protein concentrations were determined using BCA Protein Assay Kit (Sangon Biotech, Shanghai, China). Rat antibodies against the recombinant proteins were prepared as described previously [31]. For Western blot, equal amounts of proteins from TXhfq and TX01 prepared above were resolved in 12% SDS-PAGE and transferred onto nitrocellulose membranes (Amersham, Cambridge, UK). Immunoblot was performed as reported previously [32] using rat polyclonal antibodies against recombinant HP1, HP2, and DPFP prepared above. Densitometry was performed using the Sensiansy gel analysis system (Shanghai Peiqing Science & Technology. Co., Ltd, China).
Vaccination
TXhfq was cultured in LB medium as described above and resuspended in seawater to 108 CFU/mL. Japanese flounder were divided into two groups (150 fish/group) named A and B. Group A was immersed in TXhfq bath for 1 h; group B (control) was similarly immersed in seawater containing PBS. After immersion treatment, fish were placed into new 660-liter tanks and washed with fresh seawater to remove any bacteria that had been carried over from the immersion seawater. The fish were then reared under normal conditions as described above in the section of “Fish”. At one month post-vaccination, 50 fish from each group were taken and challenged via intraperitoneal injection with TX01. Similarly, at two months post-vaccination, 50 fish from each group were taken and challenged with TX01. Mortality was monitored over a period of 20 days after challenge. Three dying fish were randomly selected for the examination of bacterial recovery from liver, blood, and spleen as described above. Relative percent of survival (RPS) was calculated according to the following formula: RPS = {1 – (% mortality in vaccinated fish/% mortality in control fish)} × 100. The vaccination experiment was performed in duplicate at the same time.
Enzyme-linked immunosorbent assay (ELISA)
Sera were taken from vaccinated fish and control fish at one- and two-month post-vaccination and diluted serially in two-fold in PBS. Serum antibody against rEta1 was determined by ELISA analysis as described previously [33]. The assay was conducted with sera from five fish (each as an individual sample), and the mean values were given in the results.
Statistical analysis
Statistical analyses were performed with the SPSS 18.0 package (SPSS Inc., Chicago, IL, USA). Chi-square test with Yates’ correction was used for mortality analysis, and analysis of variance (ANOVA) was used for all other analyses. Except where otherwise indicated, all in vitro experiments were performed at a single time in three replicates, and the results are shown as means plus or minus standard errors of the means (SE). For in vivo experiments, the number of replicate was indicated in the respective methods. In all cases, significance was defined as P < 0.05.
Results
Construction of an E. tarda Δhfq wild type
Hfq of E. tarda is composed of 102 residues and shares 100% and 83.5% overall sequence identities with the Hfq of Edwardsiella ictaluri and E. coli respectively. To examine its functional importance, the hfq gene of E. tarda TX01, a highly pathogenic strain, was knocked out by markerless in-frame deletion of a region encoding amino acid residues 6 to 76. The resulting wild type was named TXhfq.
Mutation of hfq has multiple effects
(i.) Effect on growth and survival under different conditions
Growth analysis showed that when cultured in LB medium, TXhfq exhibited a slower generation time than TX01 at the logarithmic phase but reached similar cell densities as TX01 at the stationary phase (Figure 1). When cultured in LB medium supplemented with the iron chelator 2,2’dipyridyl, the growths of both TX01 and TXhfq were retarded; however, compared to TX01, TXhfq exhibited a much slower growth rate and a much lower maximum cell density at the stationary phase. Examination of biofilm growth on polystyrene surface indicated that TXhfq produced significantly less (3.1 fold) biofilm than TX01 (see Additional file 1). In the presence of H2O2, which damages bacterial cells via its oxidizing effect, the survival rate of TXhfq (2.8% ± 1.1) was significantly lower than that of TX01 (21.8% ± 2.2).
Figure 1.

Growth analysis of TX01 and TXhfq. TX01 and TXhfq were cultured in LB medium supplemented with or without 2,2′dipyridyl (DP), and cell density was measured at different time points by determining absorbance at OD600. Data are presented as means ± SE (N = 3).
(ii.) Effect on overall bacterial virulence
Comparative LD50 analysis showed that TXhfq exhibited a LD50 (1.31 × 108 CFU/fish) that is more than 600 fold higher that of TX01 (1.9 × 105 CFU/fish). When flounder were infected with the same dose of TX01 and TXhfq via immersion, TX01 recoveries from the blood, kidney, liver, and spleen of the infected fish increased from 1, 2, and 3 dpi, and mortality began to occur at 4 dpi (Figure 2A). In contrast, TXhfq recoveries from blood, kidney, liver, and spleen decreased with time and became undetectable after 7 dpi (Figure 2B).
Figure 2.
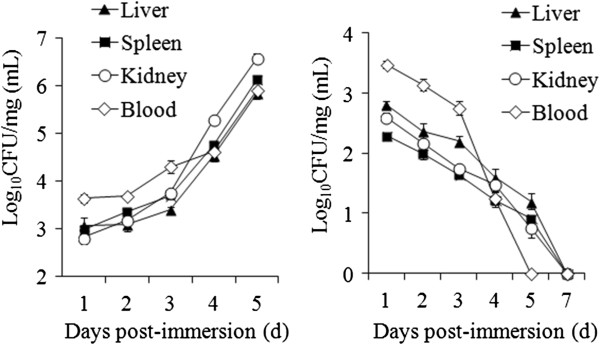
Invasion of TX01 (A) and TXhfq (B) into fish tissues following infection. Flounder were infected via immersion with the same dose of TX01 or TXhfq. Bacterial recovery from the liver, spleen, kidney, and blood of the infected fish was determined at different time points (no data on the group of TX01-infected fish at 7 dpi, because no fish survived to this point). The results are the means of three replicates and shown as means ± SE. CFU: colony forming unit. The Y-axis represents the CFU number per mg of tissue (or per mL of blood) expressed in logarithmic form.
(iii.) Effect on resistance against the immune response of host macrophages
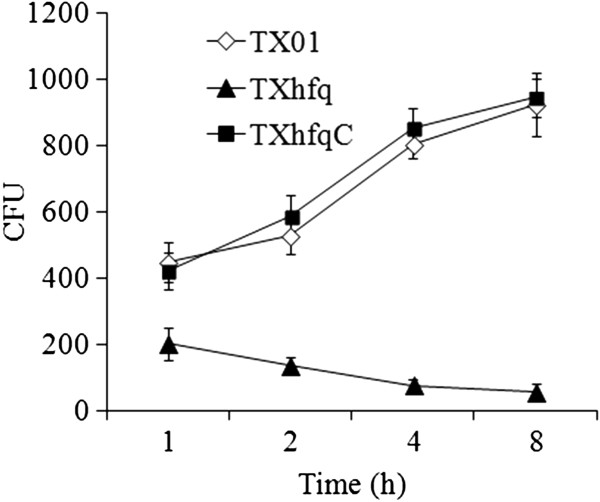
To examine whether hfq mutation affected the ability of E. tarda to block activation of host phagocytes, flounder HK macrophages were infected with TXhfq or TX01, and cellular productions of ROS and NO were determined. The results showed that both ROS and NO levels in TXhfq-infected cells were significantly higher than those in TX01-infected cells (Figure 3). Intracellular bacterial recovery analysis showed that after invasion into HK macrophages, TX01 multiplied inside the cells and increased in number as the time progressed, whereas the intracellular number of TXhfq declined with time (Figure 4).
Figure 3.
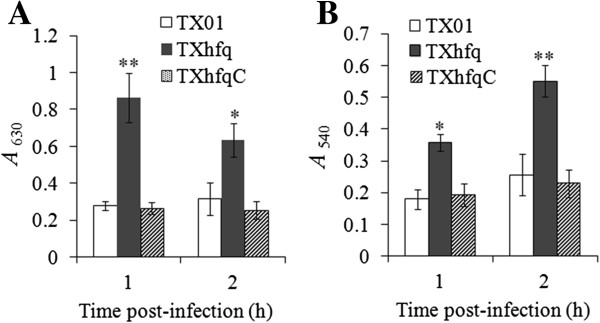
Effect of TXhfq, TX01, and TXhfqC on the immune response of macrophages. Flounder head kidney macrophages were infected with TXhfq, TX01, or TXhfqC, and reactive oxygen species (A) and nitric oxide (B) productions in the cells were determined at 1 h and 2 h post-infection. Data are presented as means ± E (N = 3). *P < 0.05, **P < 0.01.
Figure 4.
Multiplication of TXhfq, TX01, and TXhfqC in host macrophages. Flounder head kidney macrophages were infected with TXhfq, TX01, or TXhfqC. After removing uninfected bacteria, the cells were incubated at 28 °C for different hours, and intracellular bacterial recovery was determined by plate count. Data are presented as means ± E (N = 3).
Genetic complementation of hfq deletion and its effect on virulence
To examine whether the virulence defect observed with TXhfq was indeed due to hfq deletion, the strain TXhfqC was created, which is a genetic variant of TXhfq that expresses hfq in trans from a plasmid. In contrast to TXhfq, TXhfqC exhibited a LD50 (1.2 × 105 CFU/fish) comparable to that of TX01. Following infection of flounder HK macrophages, TXhfqC-induced productions of ROS and NO were similar in levels to those induced by TX01 infection (Figure 3). Likewise, the intracellular multiplication capacity of TXhfqC was comparable to that of TX01 (Figure 4).
Comparative analysis of the protein expression profiles in TXhfq and TX01
(i.) Two-DE protein maps of TXhfq and TX01
To examine whether there was any difference in the protein profiles of TXhfq and TX01, whole cell proteins of the two strains were subjected to 2-DE analysis. Proteins whose expressions differed by more than 2-fold were further analyzed and listed as putative targets of Hfq regulation. The results showed that 20 protein spots exhibiting apparently differential expressions in TXhfq and TX01 were identified (Figure 5). Of these proteins, eight were significantly upregulated (ratio of TXhfq/TX01 ≥ 2, P ≤ 0.05) and twelve were significantly downregulated (ratio of TX01/TXhfq ≥ 2, P ≤ 0.05).
Figure 5.
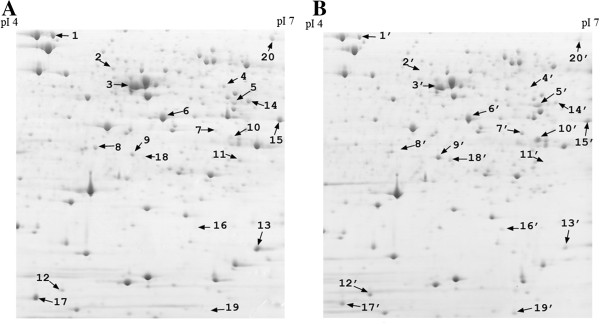
Representative 2-DE maps showing the protein profiles of TX01 and TXhfq. Whole cell proteins prepared from TX01 (A) and TXhfq (B) were subjected to 2-DE analysis. Numbers indicate protein spots with differential expression.
(ii.) Mass spectral identification of differentially expressed proteins
The 20 differentially expressed protein spots were subjected to MALDI-TOF/TOF analysis. Fifteen protein spots were successfully identified and grouped into seven functional categories (Table 2). Three proteins (rpsA, Tuf2, and Tsf) belong to the translation group, five proteins (YgeW, ybaS, cysteine synthase, putative iron-containing alcohol dehydrogenase, and dyp-type peroxidase) belong to the metabolism group, one protein (Mrp) is involved in transport, one protein (FkpA) is a putative chaperone, two proteins (OmpR and YqjD) belong to membrane proteins, and one protein (IscU) plays a role in iron-sulfur cluster assembly. The remaining two proteins are hypothetical proteins with unknown functions.
Table 2.
Summary of the differentially expressed proteins
| Spot no. a | NCBI no. | Protein name | Abrr. | MASCOT score b | Theoretical pI/Mr (kD) | Coverage (%) c | Fold ratio wild type/wild type (mean ± SD) d |
|---|---|---|---|---|---|---|---|
|
Translation | |||||||
| 1e |
gi|269139520 |
rpsA gene product [Edwardsiella tarda EIB202]: 30S ribosomal protein S1 |
RpsA |
351 |
4.61/61.17 |
41.65 |
0.42 ± 0.05 |
| 3 |
gi|18858425 |
tuf2 gene product [Edwardsiella tarda EIB202]: elongation factor Tu |
Tuf2 |
381 |
4.91/43.43 |
54.31 |
0.35 ± 0.09 |
| 6 |
gi|269138094 |
tsf gene product [Edwardsiella tarda EIB202]: translation elongation factor Ts |
Tsf |
223 |
5.3/30.70 |
36.84 |
0.45 ± 0.07 |
|
Metabolism | |||||||
| 4 |
gi|294637737 |
Putative carbamoyltransferase YgeW [Edwardsiella tarda ATCC 23685] |
YgeW |
65.1 |
6.07/44.38 |
17.97 |
0.21 ± 0.12 |
| 7 |
gi|269138485 |
Dyp-type peroxidase family protein [Edwardsiella tarda EIB202] |
|
260 |
5.76/33.27 |
38.46 |
3.62 ± 0.78 |
| 8 |
gi|269140209 |
ybaS gene product [Edwardsiella tarda EIB202]: glutaminase |
YbaS |
362 |
4.83/33.06 |
81.35 |
-∞ |
| 14 |
gi|387866356 |
Putative iron-containing alcohol dehydrogenase [Edwardsiella tarda FL6-60] |
|
218 |
5.73/42.61 |
74 |
0.27 ± 0.06 |
| 15 |
gi|469762796 |
Cysteine synthase [Edwardsiella tarda C07-087] |
|
199 |
6.03/33.99 |
77 |
0.46 ± 0.05 |
|
Transport | |||||||
| 5 |
gi|387867244 |
Mrp (Multiple resistance and pH adaptation) protein [Edwardsiella tarda FL6-60] |
Mrp |
340 |
6.22/39.70 |
26.76 |
2.81 ± 1.11 |
|
Chaperones | |||||||
| 10 |
gi|269140581 |
fkpA gene product [Edwardsiella tarda EIB202]: FKBP-type peptidyl-prolyl cis-trans isomerase |
FkpA |
425 |
8.7/28.76 |
58.39 |
4.24 ± 1.35 |
|
Membrane proteins | |||||||
| 11 |
gi|269140620| |
ompR gene product [Edwardsiella tarda EIB202]: osmolarity response regulator |
OmpR |
127 |
6.31/27.41 |
38.91 |
-∞ |
| 13 |
gi|304557842 |
Uncharacterized membrane protein YqjD [Edwardsiella tarda FL6-60] |
YqjD |
600 |
6.26/10.94 |
82.18 |
0.25 ± 0.04 |
|
Cellular processes | |||||||
| 12 |
gi|269140157 |
iscU gene product [Edwardsiella tarda EIB202]: FeS cluster assembly scaffold |
IscU |
102 |
4.74/13.86 |
55.47 |
2.23 ± 0.62 |
|
Unknown function and hypothetical protein | |||||||
| 2 |
gi|269139286 |
Hypothetical protein 1 [Edwardsiella tarda EIB202] |
|
153 |
5.64/58.02 |
29.04 |
-∞ |
| 9 |
gi|269140100 |
Hypothetical protein 2 [Edwardsiella tarda EIB202] |
|
318 |
5.33/33.70 |
54.90 |
3.21 ± 1.28 |
| 16e |
NA |
NA |
NA |
NA |
NA |
NA |
5.21 ± 1.25 |
| 17 |
NA |
NA |
NA |
NA |
NA |
NA |
0.31 ± 0.05 |
| 18 |
NA |
NA |
NA |
NA |
NA |
NA |
2.94 ± 0.85 |
| 19 |
NA |
NA |
NA |
NA |
NA |
NA |
4.22 ± 1.77 |
| 20 | NA | NA | NA | NA | NA | NA | 0.32 ± 0.06 |
aSpot ID represents the number on the 2-DE gels (Figure 5).
bMOWSE score is -10 log (p), where p is the probability that the observed match is a random event. Based on the NCBInr database using the MASCOT searching program as MS/MS data. Scores greater than 65 are significant (p < 0.05).
cNumber of amino acids spanned by the assigned peptides divided by the protein sequence length.
dMean, the average protein abundance ratio for three paired samples. SD means the standard deviation of protein abundance ratios of one certain spot of three paired samples.
eNot analyzed.
(iii.) Validation of differential expression of selected proteins at mRNA level
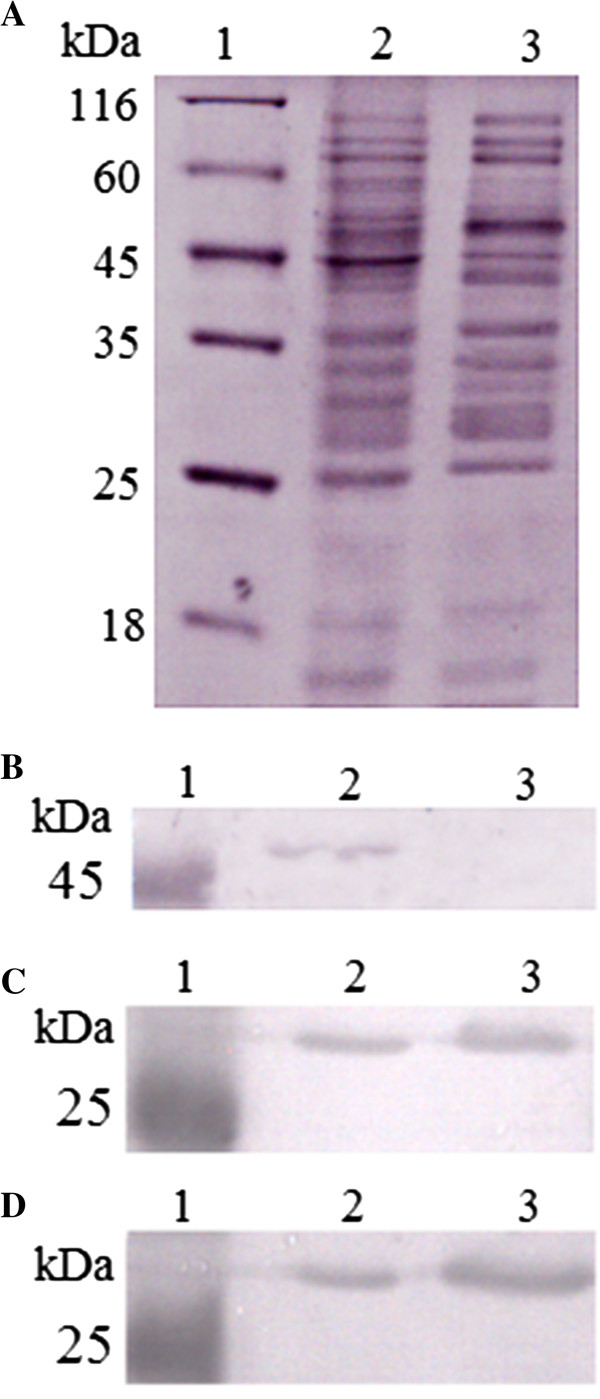
qRT-PCR (which determines the mRNA level) (Table 3) showed that the genes encoding Mrp, Dyp-type peroxidase, hypothetical protein 2, and FkpA were upregulated in TXhfq, while the genes encoding hypothetical protein 1, YgeW, ybaS, OmpR, YqjD and cysteine synthase were downregulated in TXhfq, which is consistent with the 2-DE protein profiles. The expressions of the genes encoding RpsA, Tuf2, Tsf, IscU, and the putative iron-containing alcohol dehydrogenase were comparable in the wild type and wild type strains. Three proteins were selected for further analysis by Western blot. The results showed that hypothetical protein 1, which was downregulated in TXhfq by 2-DE and qRT-PCR analysis, was apparently detected in TX01 but was undetectable in TXhfq, while hypothetical protein 2 and the dyp-type peroxidase family protein, both were upregulated in TXhfq by 2-DE and qRT-PCR, were produced more abundantly in TXhfq than in TX01 (fold difference ~2.8 and 4.1 respectively) (Figure 6).
Table 3.
Summary of mRNA expression in TXhfq (in comparison with that in TX01) as determined by qRT-PCR
| Spo no. | Description | Protein level (by 2-DE) | mRNA level (by qRT-PCR) |
|---|---|---|---|
| 1 |
rpsA gene product [Edwardsiella tarda EIB202]: 30S ribosomal protein S1 |
Down (0.42 ± 0.05) |
Unchanged |
| 2 |
hypothetical protein1 [Edwardsiella tarda EIB202]: |
Down (-∞) |
Down (0.38 ± 0.06) |
| 3 |
tuf2 gene product [Edwardsiella tarda EIB202]: elongation factor Tu |
Down (0.35 ± 0.09) |
Unchanged |
| 4 |
Putative carbamoyltransferase YgeW [Edwardsiella tarda ATCC 23685] |
Down (0.21 ± 0.12) |
Down (0.42 ± 0.09) |
| 5 |
Mrp(Multiple Resistance and pH adaptation) protein [Edwardsiella tarda FL6-60] |
Up (2.81 ± 1.11) |
Up (4.11 ± 0.85) |
| 6 |
tsf gene product [Edwardsiella tarda EIB202]: translation elongation factor Ts |
Down (0.45 ± 0.07) |
Unchanged |
| 7 |
Dyp-type peroxidase family protein [Edwardsiella tarda EIB202] |
Up (3.62 ± 0.78) |
Up (7.72 ± 1.08) |
| 8 |
ybaS gene product [Edwardsiella tarda EIB202]: glutaminase |
Down (-∞) |
Down (0.20 ± 0.07) |
| 9 |
Hypothetical protein [Edwardsiella tarda EIB202]: lysophospholipase |
Up (3.21 ± 1.28) |
Up (5.10 ± 0.84) |
| 10 |
fkpA gene product [Edwardsiella tarda EIB202]: FKBP-type peptidyl-prolyl cis-trans isomerase |
Up (4.24 ± 1.35) |
Up (7.54 ± 1.21) |
| 11 |
ompR gene product [Edwardsiella tarda EIB202]: osmolarity response regulator; cytoplasmic |
Down (-∞) |
Down (0.18 ± 0.04) |
| 12 |
iscU gene product [Edwardsiella tarda EIB202]: FeS cluster assembly scaffold |
Up (2.23 ± 0.62) |
Unchanged |
| 13 |
Uncharacterized membrane protein YqjD [Edwardsiella tarda FL6-60] |
Down (0.25 ± 0.04) |
Down (0.54 ± 0.05) |
| 14 |
Putative iron-containing alcohol dehydrogenase [Edwardsiella tarda FL6-60] |
Down (0.27 ± 0.06) |
Unchanged |
| 15 | Cysteine synthase [Edwardsiella tarda C07-087] | Down (0.46 ± 0.05) | Down (0.33 ± 0.08) |
Figure 6.
Immunoblot analysis of differentially expressed proteins. Equal amounts of proteins prepared from TX01 and TXhfq (lanes 2 and 3 of all panels) were subjected to SDS-PAGE (A). After SDS-PAGE, the proteins were blotted with antibodies against hypothetical protein 1 (B), hypothetical protein 2 (C), or dyp-type peroxidase family protein (D).
The potential of TXhfq as an immersion vaccine
Since TXhfq is dramatically decreased in virulence, we examined its potential as a live attenuated vaccine delivered via the natural route. For this purpose, flounder were immunized with live TXhfq via bath immersion. The fish were challenged with TX01 at one month and two months post-vaccination and monitored for mortality. The results showed that mortality began to occur at 4 days and 3 days post-challenge for TXhfq-vaccinated fish and control fish respectively, and that mortality stopped at 14 days and 12 days post-challenge for TXhfq-vaccinated fish and control fish respectively. The accumulated mortalities of TXhfq-vaccinated fish were 24% and 32% at one month and two months post-vaccination respectively, while the accumulated mortalities of the control fish (mock vaccinated with PBS) were 100% and 92% at one month and two months post-vaccination respectively. Based on these results, the protection rates, in terms of PRS, of TXhfq as an immersion vaccine were 76% and 65% respectively at one month and two months post-vaccination. The two protection rates were statistically comparable. Comparable results were obtained in the duplicate vaccination trial, in which the accumulated mortalities of TXhfq-vaccinated fish were 20% and 30% at one month and two months post-vaccination respectively, while the accumulated mortalities of the control fish were 96% and 98% at one month and two months post-vaccination respectively. Examination of moribund fish indicated that TX01 was the only type of bacterium isolated from the liver, spleen, and blood, suggesting that mortality was caused by the experimental challenge. ELISA analysis showed that specific serum antibodies (titers 26 and 25 respectively) were produced in TXhfq-vaccinated fish at one month and two months post-vaccination.
Discussion
It has been observed that for many bacterial species, mutation of hfq has a profound effect on cell growth, while in other bacteria, such as Haemophilus influenzae and Serratia sp, growth is hardly impaired by hfq deletion [19,34,35]. In this study, we found that compared to the wild type TX01, the Δhfq wild type TXhfq exhibited a slight growth difference when cultured in rich medium. However, TXhfq growth was severely retarded when the bacterium was cultured in iron-depleted medium. This result is in agreement with the reports that Hfq plays a critical role in the regulation of iron homeostasis [36-40]. Biofilm growth is a dynamic process with multiple factors involved [41]. Recent studies showed that the major biofilm regulator CsgD was regulated by Hfq-dependent sRNAs in E. coli and Actinobacillus pleuropneumoniae[42,43], and that in E. coli, Pseudomonas fluorescens, Vibrio alginolyticus, and Stenotrophomonas maltophilia, biofilm development was reduced in the absence of Hfq [20,44-46]. Likewise, in our study we found that TXhfq was impaired in the capacity of biofilm production. These results indicate that in E. tarda, Hfq is required for both planktonic and biofilm growth. Since for bacterial pathogens, biofilm production is often associated with infectivity, these observations suggest that Hfq possibly plays a role under both physiological conditions and during bacterial infection.
Reports have shown that Hfq is essential to cellular tolerance of various stresses, such as oxidative damage, high salt, and heat shock, in a variety of bacteria including Serratia sp, E. coli, Francisella tularensis, K. pneumoniae, and V. alginolyticus[19,47]. However, in other bacteria such as Listeria monocytogenes, Haemophilus influenzae, and Staphylococcus aureus, Hfq has no effect on resistance to oxidative stress [34,48]. In the case of E. tarda, we found that in the presence of the strong oxidizer H2O2, the survival rate of TXhfq was significantly lower than that of TX01, suggesting that Hfq is required for coping with oxidative stress in E. tarda. Given the fact that production of reactive oxygen species, which induces a state of oxidative stress, is a key defense mechanism in fish as well as mammals against bacterial pathogens, these results indicate a potential involvement of Hfq in the virulence E. tarda.
A large amount of evidences have indicated that Hfq plays a role in pathogenicity. For example, mutation of hfq affects the ability of Brucella melitensis and Salmonella enterica serovar Typhimurium to invade and proliferate inside host cells [49,50]. Similar to these observations, we found that TXhfq exhibited a dramatically increased LD50, and, consistently, the ability of TXhfq to disseminate in flounder tissues and replicate in HK macrophages was significantly impaired. In addition, we found that HK macrophages infected with TXhfq produced much higher levels of ROS and NO than macrophages infected with TX01, suggesting that TXhfq was defective in blocking macrophage activation. In agreement with these results, TXhfq exhibited reduced capacity to survive and replicate inside HK macrophages. These results indicate that Hfq is essential for effective repression of the bactericidal immune response of host cells. Previous studies have shown that E. tarda is an intracellular pathogen that can escape host immune defense and replicate in host phagocytes [51-53]; however, the mechanism behind this phenomenon is unclear. Our results confirm previous observations and suggest a close link between immune evasion and E. tarda virulence. More importantly, our results, together with those of previous reports, point to a possibility that the intracellular replication capacity of E. tarda is not due to the action of a single bacterial factor but more likely to the combined effects of multiple factors.
Hfq is known to regulate gene expression and mRNA stability in post-transcription level, resulting in changes in protein production. Hence, proteomics is an appropriate strategy to detect putative targets of Hfq. Using this approach, Hfq regulons have been identified in Salmonella, Sinorhizobium meliloti, and Neisseria meningitides[39,54-57]. In this study, global proteomic analysis identified 15 proteins differentially expressed between TXhfq and TX01. Of these proteins, YgeW is a carbamoyltransferase that is likely involved in the biosynthesis of antibiotic [58], YbaS is a glutaminase known to contribute to acid resistance [59,60], iron-containing alcohol dehydrogenase and cysteine synthase have been reported to participate in regulation of ethanol utilization and production of antioxidant respectively [61,62], YqjD is an inner membrane protein associated with stationary-phase ribosomes [63], and OmpR is an outer membrane protein that is essential for low pH adaptation and regulates the virulence-associated type VI secretion system [64]. The downregulated expression of these proteins observed in TXhfq may account in part for the defectiveness of TXhfq in growth and in coping with stress conditions. qRT-PCR analysis of the genes encoding the 15 differentially expressed proteins showed that for ten genes, the mRNA levels were consistent with the 2-DE results, while for five genes, the mRNA levels were comparable between TXhfq and TX01. These results suggest that in most cases Hfq regulates the target genes at the transcription level, while in some cases Hfq regulates the target genes at the posttranscriptional level. It will be interesting for future studies to delineate the detail process of Hfq regulation of different types of targets. In addition, since none of the differentially expressed proteins has been studied in E. tarda, works may be carried out in the future to inactivate these proteins (e.g. by gene knock-out) and investigate the potential significance of these proteins in E. tarda survival under different conditions.
Since Hfq wild types usually exhibit vitiated virulence, they are ideal targets for the development of attenuated live vaccines. This idea has been exploited by researchers with different pathogenic species such as S. enterica serovar Typhimurium, Brucella melitensis, and V. alginolyticus[45,65,66]. In our study, we found that flounder immunized with live TXhfq via bath immersion exhibited high levels of survival rates at one- and two-month post-vaccination after lethal E. tarda challenge, suggesting that TXhfq confers effective protection against E. tarda. Given the fact that TXhfq, though highly attenuated in virulence, is still capable of transient infection into flounder via immersion as observed in the tissue dissemination analysis, it is likely that TXhfq mimics natural infection after immersion vaccination and thus induces strong protective immunity in the host. The advantage of TXhfq as a vaccine lies not only in its protectivity but also in its immersion delivery, which for fish is a natural approach. Compared to the commonly used injection method of vaccine delivery, immersion is of low cost and inflicts no stress upon the animals.
In conclusion, we demonstrate in this study that hfq knockout affects multiple aspects of E. tarda, which results in dramatic attenuation of infectivity. Hfq is required for the expression of a wide range of proteins belonging to different functional categories, and the regulatory effects of Hfq likely exert at both transcription and post-transcription levels. In addition, the Δhfq wild type as an immersion vaccine induces effective immunoprotection, a property that may be exploited for the control of E. tarda in aquaculture.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
YHH and YXL performed the experiments and analyzed the data. LS designed the experiment. LS and YHH wrote the paper. All authors read and approved the final manuscript.
Supplementary Material
Biofilm production of TX01 and TXhfq. TX01 and TXhfq were grown in polystyrene plates for 24 h and then assayed for biofilm production. Data are presented as means ± E (N = 3). **, P < 0.01.
Contributor Information
Yong-hua Hu, Email: huyonghua@qdio.ac.cn.
Yong-xin Li, Email: liyongxin116@163.com.
Li Sun, Email: lsun@qdio.ac.cn.
Acknowledgements
This work was supported by the grants from the National Natural Science Foundation of China (31330081, 31302223, and 31025030) and the Taishan Scholar Program of Shandong Province.
References
- Mohanty BR, Sahoo PK. Edwardsiellosis in fish: a brief review. J Biosci. 2007;32:1331–1344. doi: 10.1007/s12038-007-0143-8. [DOI] [PubMed] [Google Scholar]
- Park SB, Aoki T, Jung TS. Pathogenesis of and strategies for preventing Edwardsiella tarda infection in fish. Vet Res. 2012;43:67. doi: 10.1186/1297-9716-43-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson JJ, Nelson CA, Carter JE. Extraintestinal manifestations of Edwardsiella tarda infection: a 10-year retrospective review. J La State Med Soc. 2009;161:103–106. [PubMed] [Google Scholar]
- Leung KY, Siame BA, Tenkink BJ, Noort RJ, Mok YK. Edwardsiella tarda–virulence mechanisms of an emerging gastroenteritis pathogen. Microbes Infect. 2012;14:26–34. doi: 10.1016/j.micinf.2011.08.005. [DOI] [PubMed] [Google Scholar]
- de Fernandez MT F, Eoyang L, August JT, de Fernandez MT F, Eoyang L, August JT. Factor fraction required for the synthesis of bacteriophage QBeta-RNA. Nature. 1968;219:588–590. doi: 10.1038/219588a0. [DOI] [PubMed] [Google Scholar]
- De Lay N, Schu DJ, Gottesman S. Bacterial small RNA-based negative regulation: Hfq and its accomplices. J Biol Chem. 2013;288:7996–8003. doi: 10.1074/jbc.R112.441386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel J, Luisi BF. Hfq and its constellation of RNA. Nat Rev Microbiol. 2011;9:578–589. doi: 10.1038/nrmicro2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibova I, Skopova K, Masin J, Cerny O, Hot D, Sebo P, Vecerek B. The RNA chaperone Hfq is required for virulence of Bordetella pertussis. Infect Immun. 2013;81:4081–4090. doi: 10.1128/IAI.00345-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bojer MS, Jakobsen H, Struve C, Krogfelt KA, Løbner-Olesen A. Lack of the RNA chaperone Hfq attenuates pathogenicity of several Escherichia coli pathotypes towards Caenorhabditis elegans. Microbes Infect. 2012;14:1034–1309. doi: 10.1016/j.micinf.2012.06.002. [DOI] [PubMed] [Google Scholar]
- Caswell CC, Gaines JM, Roop RM 2nd. The RNA chaperone Hfq independently coordinates expression of the VirB type IV secretion system and the LuxR-type regulator BabR in Brucella abortus 2308. J Bacteriol. 2012;194:3–14. doi: 10.1128/JB.05623-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang MK, Lu MC, Liu LC, Lin CT, Lai YC. Impact of Hfq on global gene expression and virulence in Klebsiella pneumoniae. PLoS One. 2011;6:e22248. doi: 10.1371/journal.pone.0022248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y, Davis BM, Waldor MK. Hfq is essencial for Vibrio cholera virulence and dowregulates sigma expression. Mol Microbiol. 2004;53:345–354. doi: 10.1111/j.1365-2958.2004.04142.x. [DOI] [PubMed] [Google Scholar]
- Meng X, Meng X, Zhu C, Wang H, Wang J, Nie J, Hardwidge PR, Zhu G. The RNA chaperone Hfq regulates expression of fimbrial-related genes and virulence of Salmonella enterica serovar Enteritidis. FEMS Microbiol Lett. 2013;346:90–96. doi: 10.1111/1574-6968.12206. [DOI] [PubMed] [Google Scholar]
- Nakao H, Watanabe H, Nakayama S, Takeda T. yst gene expression in Yersinia enterocolitica is positively regulated by a chromosomal region that is highly homologous to Escherichia coli host factor 1 gene (hfq) Mol Microbiol. 2005;18:859–865. doi: 10.1111/j.1365-2958.1995.18050859.x. [DOI] [PubMed] [Google Scholar]
- Sittka A, Pfeiffer V, Tedin K, Vogel J. The RNA chaperone Hfq is essential for the virulence of Salmonella typhimurium. Mol Microbiol. 2007;63:193–217. doi: 10.1111/j.1365-2958.2006.05489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnleitner E, Hagens S, Rosenau F, Wilhelm S, Habel A, Jäger KE, Bläsi U. Reduced virulence of a hfq wild type of Pseudomonas aeruginosa O1. Microb Pathol. 2003;35:217–228. doi: 10.1016/S0882-4010(03)00149-9. [DOI] [PubMed] [Google Scholar]
- Wilms I, Möller P, Stock AM, Gurski R, Lai EM, Narberhaus F. Hfq influences multiple transport systems and virulence in the plant pathogen Agrobacterium tumefaciens. J Bacteriol. 2012;194:5209–5217. doi: 10.1128/JB.00510-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Q, McNally RR, Sundin GW. Global small RNA chaperone Hfq and regulatory small RNAs are important virulence regulators in Erwinia amylovora. J Bacteriol. 2013;195:1706–1717. doi: 10.1128/JB.02056-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao Y, Vogel J. The role of Hfq in bacterial pathogens. Curr Opin Microbiol. 2010;13:24–33. doi: 10.1016/j.mib.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Roscetto E, Angrisano T, Costa V, Casalino M, Förstner KU, Sharma CM, Di Nocera PP, De Gregorio E. Functional characterization of the RNA chaperone Hfq in the opportunistic human pathogen Stenotrophomonas maltophilia. J Bacteriol. 2012;194:5864–5874. doi: 10.1128/JB.00746-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun K, Wang HL, Zhang M, Xiao ZZ, Sun L. Genetic mechanisms multi-antimicrobial resistance in a pathogenic Edwardsiella tarda strain. Aquaculture. 2009;289:134–139. doi: 10.1016/j.aquaculture.2008.12.021. [DOI] [Google Scholar]
- Hu YH, Liu CS, Hou JH, Sun L. Identification, characterization, and molecular application of a virulence-associated autotransporter from a pathogenic Pseudomonas fluorescens strain. Appl Environ Microbiol. 2009;75:4333–4340. doi: 10.1128/AEM.00159-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HR, Hu YH, Zhang WW, Sun L. Construction of an attenuated Pseudomonas fluorescens strain and evaluation of its potential as a cross-protective vaccine. Vaccine. 2009;27:4047–4055. doi: 10.1016/j.vaccine.2009.04.023. [DOI] [PubMed] [Google Scholar]
- Milton DL, O’Toole R, Horstedt P, Wolf-Watz H. Flagellin A is essential for the virulence of Vibrio anguillarum. J Bacteriol. 1996;178:1310–1319. doi: 10.1128/jb.178.5.1310-1319.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang WW, Sun K, Cheng S, Sun L. Characterization of DegQVh, a serine protease and a protective immunogen from a pathogenic Vibrio harveyi strain. Appl Environ Microbiol. 2008;74:6254–6262. doi: 10.1128/AEM.00109-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu YH, Wang HL, Zhang M, Sun L. Molecular analysis of the copper-responsive CopRSCD of a pathogenic Pseudomonas fluorescens strain. J Microbiol. 2009;47:277–286. doi: 10.1007/s12275-008-0278-9. [DOI] [PubMed] [Google Scholar]
- Zheng WJ, Hu YH, Sun L. The two Dps of Edwardsiella tarda are involved in resistance against oxidative stress and host infection. Fish Shellfish Immunol. 2011;31:985–992. doi: 10.1016/j.fsi.2011.08.018. [DOI] [PubMed] [Google Scholar]
- Liu CS, Sun Y, Hu YH, Sun L. Identification and analysis of the immune effects of CpG motifs that protect Japanese flounder (Paralichthys olivaceus) against bacterial infection. Fish Shellfish Immunol. 2010;29:279–285. doi: 10.1016/j.fsi.2010.04.012. [DOI] [PubMed] [Google Scholar]
- Zhang J, Hu YH, Xiao ZZ, Sun L. Megalocytivirus-induced proteins of turbot (Scophthalmus maximus): identification and antiviral potential. J Proteomics. 2013;91:430–443. doi: 10.1016/j.jprot.2013.07.033. [DOI] [PubMed] [Google Scholar]
- Zheng WJ, Sun L. Evaluation of housekeeping genes as references for quantitative real time RT-PCR analysis of gene expression in Japanese flounder (Paralichthys olivaceus) Fish Shellfish Immunol. 2011;30:638–645. doi: 10.1016/j.fsi.2010.12.014. [DOI] [PubMed] [Google Scholar]
- Liu CS, Sun Y, Zhang M, Sun L. Identification and analysis of a Sciaenops ocellatus ISG15 homologue that is involved in host immune defense against bacterial infection. Fish Shellfish Immunol. 2010;29:167–174. doi: 10.1016/j.fsi.2010.03.012. [DOI] [PubMed] [Google Scholar]
- Sun Y, Zheng WJ, Hu YH, Sun BG, Sun L. Edwardsiella tarda Eta1, an in vivo-induced antigen that is involved in host infection. Infect Immun. 2012;80:2948–2955. doi: 10.1128/IAI.00063-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Liu CS, Sun L. Identification of an Edwardsiella tarda surface antigen and analysis of its immunoprotective potential as a purified recombinant subunit vaccine and a surface-anchored subunit vaccine expressed by a fish commensal strain. Vaccine. 2010;28:6603–6608. doi: 10.1016/j.vaccine.2010.07.050. [DOI] [PubMed] [Google Scholar]
- Hempel RJ, Morton DJ, Seale TW, Whitby PW, Stull TL. The role of the RNA chaperone Hfq in Haemophilus influenzae pathogenesis. BMC Microbiol. 2013;13:134. doi: 10.1186/1471-2180-13-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilf NM, Williamson NR, Ramsay JP, Poulter S, Bandyra KJ, Salmond GP. The RNA chaperone, Hfq, controls two luxR-type regulators and plays a key role in pathogenesis and production of antibiotics in Serratia sp. ATCC 39006. Environ Microbiol. 2011;13:2649–2666. doi: 10.1111/j.1462-2920.2011.02532.x. [DOI] [PubMed] [Google Scholar]
- Bearson BL, Bearson SM, Uthe JJ, Dowd SE, Houghton JO, Lee I, Toscano MJ, Lay DC Jr. Iron regulated genes of Salmonella enterica serovar Typhimurium in response to norepinephrine and the requirement of fepDGC for norepinephrine-enhanced growth. Microbes Infect. 2008;10:807–816. doi: 10.1016/j.micinf.2008.04.011. [DOI] [PubMed] [Google Scholar]
- Mellin JR, McClure R, Lopez D, Green O, Reinhard B, Genco C. Role of Hfq in iron-dependent and -independent gene regulation in Neisseria meningitidis. Microbiology. 2010;56:2316–2326. doi: 10.1099/mic.0.039040-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metruccio MM, Fantappiè L, Serruto D, Muzzi A, Roncarati D, Donati C, Scarlato V, Delany I. The Hfq-dependent small noncoding RNA NrrF directly mediates Fur-dependent positive regulation of succinate dehydrogenase in Neisseria meningitidis. J Bacteriol. 2009;191:1330–1342. doi: 10.1128/JB.00849-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobrero P, Schlüter JP, Lanner U, Schlosser A, Becker A, Valverde C. Quantitative proteomic analysis of the Hfq-regulon in Sinorhizobium meliloti 2011. PLoS One. 2012;7:e48494. doi: 10.1371/journal.pone.0048494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vecerek B, Moll I, Afonyushkin T, Kaberdin V, Blasi U. Interaction of the RNA chaperone Hfq with mRNAs: direct and indirect roles of Hfq in iron metabolism of Escherichia coli. Mol Microbiol. 2003;50:897–909. doi: 10.1046/j.1365-2958.2003.03727.x. [DOI] [PubMed] [Google Scholar]
- Salazar JK, Wu Z, Yang W, Freitag NE, Tortorello ML, Wang H, Zhang W. Roles of a novel Crp/Fnr family transcription factor Lmo0753 in soil survival, biofilm production and surface attachment to fresh produce of Listeria monocytogenes. PLoS One. 2013;8:e75736. doi: 10.1371/journal.pone.0075736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mika F, Busse S, Possling A, Berkholz J, Tschowri N, Sommerfeldt N, Pruteanu M, Hengge R. Targeting of csgD by the small regulatory RNA RprA links stationary phase, biofilm formation and cell envelope stress in Escherichia coli. Mol Microbiol. 2012;84:51–65. doi: 10.1111/j.1365-2958.2012.08002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subashchandrabose S, Leveque RM, Kirkwood RN, Kiupel M, Mulks MH. The RNA chaperone Hfq promotes fitness of Actinobacillus pleuropneumoniae during porcine pleuropneumonia. Infect Immun. 2013;81:2952–2961. doi: 10.1128/IAI.00392-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulesus RR, Diaz-Perez K, Slechta ES, Eto DS, Mulvey MA. Impact of the RNA chaperone Hfq on the fitness and virulence potential of uropathogenic Escherichia coli. Infect Immun. 2008;76:3019–3026. doi: 10.1128/IAI.00022-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Wang Q, Liu Q, Cao X, Shi C, Zhang Y. Roles of Hfq in the stress adaptation and virulence in fish pathogen Vibrio alginolyticus and its potential application as a target for live attenuated vaccine. Appl Microbiol Biotechnol. 2011;91:353–364. doi: 10.1007/s00253-011-3286-3. [DOI] [PubMed] [Google Scholar]
- Wu XG, Duan HM, Tian T, Yao N, Zhou HY, Zhang LQ. Effect of the hfq gene on 2,4-diacetylphloroglucinol production and the PcoI/PcoR quorum-sensing system in Pseudomonas fluorescens 2P24. FEMS Microbiol Lett. 2010;309:16–24. doi: 10.1111/j.1574-6968.2010.02009.x. [DOI] [PubMed] [Google Scholar]
- Christiansen JK, Larsen MH, Ingmer H, Sogaard-Andersen L, Kallipolitis BH. The RNA-binding protein Hfq of Listeria monocytogenes: role in stress tolerance and virulence. J Bacteriol. 2004;186:3355–3362. doi: 10.1128/JB.186.11.3355-3362.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn C, Rigoulay C, Bouloc P. No detectable effect of RNA-binding protein Hfq absence in Staphylococcus aureus. BMC Microbiol. 2007;7:10. doi: 10.1186/1471-2180-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui M, Wang T, Xu J, Ke Y, Du X, Yuan X, Wang Z, Gong C, Zhuang Y, Lei S, Su X, Wang X, Huang L, Zhong Z, Peng G, Yuan J, Chen Z, Wang Y. Impact of Hfq on global gene expression and intracellular survival in Brucella melitensis. PLoS One. 2013;8:e71933. doi: 10.1371/journal.pone.0071933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viegas SC, Mil-Homens D, Fialho AM, Arraiano CM. The Virulence of Salmonella enterica Serovar Typhimurium in the insect model galleria mellonella is impaired by mutations in RNase E and RNase III. Appl Environ Microbiol. 2013;79:6124–6133. doi: 10.1128/AEM.02044-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S, Zhang M, Sun L. The iron-cofactored superoxide dismutase of Edwardsiella tarda inhibits macrophage-mediated innate immune response. Fish Shellfish Immunol. 2010;29:972–978. doi: 10.1016/j.fsi.2010.08.004. [DOI] [PubMed] [Google Scholar]
- Ishibe K, Osatomi K, Hara K, Kanai K, Yamaguchi K, Oda T. Comparison of the responses of peritoneal macrophages from Japanese flounder (Paralichthys olivaceus) against high virulent and low virulent strains of Edwardsiella tarda. Fish Shellfish Immunol. 2008;24:243–251. doi: 10.1016/j.fsi.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Rao PSS, Lim TM, Leung KY. Opsonized virulent Edwardsiella tarda strains are able to adhere to and survive and replicate within fish phagocytes but fail to stimulate reactive oxygen intermediates. Infect Immun. 2001;69:5689–5697. doi: 10.1128/IAI.69.9.5689-5697.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansong C, Yoon H, Porwollik S, Mottaz-Brewer H, Petritis BO, Jaitly N, Adkins JN, McClelland M, Heffron F, Smith RD. Global systems-level analysis of Hfq and SmpB deletion wild types in Salmonella: implications for virulence and global protein translation. PLoS One. 2009;4:e4809. doi: 10.1371/journal.pone.0004809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barra-Bily L, Fontenelle C, Jan G, Flechard M, Trautwetter A, Pandey SP, Walker GC, Blanco C. Proteomic alterations explain phenotypic changes in Sinorhizobium meliloti lacking the RNA chaperone Hfq. J Bacteriol. 2010;192:1719–1729. doi: 10.1128/JB.01429-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantappie L, Metruccio MM, Seib KL, Oriente F, Cartocci E, Ferlicca F, Giuliani MM, Scarlato V, Delany I. The RNA chaperone Hfq is involved in the stress response and virulence in Neisseria meningitidis and is a pleiotropic regulator of protein expression. Infect Immun. 2009;77:1842–1853. doi: 10.1128/IAI.01216-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannekoek Y, Huis in’t Veld R, Hopman CT, Langerak AA, Speijer D, van der Ende A. Molecular characterization and identification of proteins regulated by Hfq in Neisseria meningitidis. FEMS Microbiol Lett. 2009;294:216–224. doi: 10.1111/j.1574-6968.2009.01568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong YS, Lee D, Kim W, Jeong JK, Kim CG, Sohng JK, Lee JH, Paik SG, Lee JJ. Inactivation of the carbamoyltransferase gene refines post-polyketide synthase modification steps in the biosynthesis of the antitumor agent geldanamycin. J Am Chem Soc. 2004;126:11142–11143. doi: 10.1021/ja047769m. [DOI] [PubMed] [Google Scholar]
- Brown G, Singer A, Proudfoot M, Skarina T, Kim Y, Chang C, Dementieva I, Kuznetsova E, Gonzalez CF, Joachimiak A, Savchenko A, Yakunin AF. Functional and structural characterization of four glutaminases from Escherichia coli and Bacillus subtilis. Biochemistry. 2008;47:5724–5735. doi: 10.1021/bi800097h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu P, Ma D, Chen Y, Guo Y, Chen GQ, Deng H, Shi Y. L-glutamine provides acid resistance for Escherichia coli through enzymatic release of ammonia. Cell Res. 2013;23:635–644. doi: 10.1038/cr.2013.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hempel N, Görisch H, Mern DS. Gene ercA encoding a putative iron-containing alcohol dehydrogenase is involved in regulation of ethanol utilization in Pseudomonas aeruginosa. J Bacteriol. 2013;195:3925–3932. doi: 10.1128/JB.00531-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Leary SE, Jurgenson CT, Ealick SE, Begley TP. O-phospho-L-serine and the thiocarboxylated sulfur carrier protein CysO-COSH are substrates for CysM, a cysteine synthase from Mycobacterium tuberculosis. Biochemistry. 2008;47:11606–11615. doi: 10.1021/bi8013664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H, Maki Y, Furuike S, Sakai A, Ueta M, Wada A. YqjD is an inner membrane protein associated with stationary-phase ribosomes in Escherichia coli. J Bacteriol. 2012;194:4178–4183. doi: 10.1128/JB.00396-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Wang Y, Song Y, Wang T, Xu S, Peng Z, Lin X, Zhang L, Shen X. A type VI secretion system regulated by OmpR in Yersinia pseudotuberculosis functions to maintain intracellular pH homeostasis. Environ Microbiol. 2013;15:557–569. doi: 10.1111/1462-2920.12005. [DOI] [PubMed] [Google Scholar]
- Allam US, Krishna MG, Lahiri A, Joy O, Chakravortty D. Salmonella enterica serovar Typhimurium lacking hfq gene confers protective immunity against murine typhoid. PLoS One. 2011;6:e16667. doi: 10.1371/journal.pone.0016667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Guo F, Chen C, Li Z, Zhang H, Wang Y, Zhang K, Du G, Li Y, Wang J, Jian T, Wang Z. Brucella melitensis 16MΔhfq attenuation confers protection against wild-type challenge in BALB/c mice. Microbiol Immunol. 2013;57:502–510. doi: 10.1111/1348-0421.12065. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Biofilm production of TX01 and TXhfq. TX01 and TXhfq were grown in polystyrene plates for 24 h and then assayed for biofilm production. Data are presented as means ± E (N = 3). **, P < 0.01.