Abstract
Cancer cells, besides reproducing uncontrollably, lose cohesiveness and orderliness of normal tissue, invade and get detached from the primary tumor to travel and set up colonies elsewhere. Dislodging neoplastically altered cells from a tumor during biopsy or surgical intervention or during simple procedure like needle aspiration is a possibility because they lack cohesiveness, and they attain the capacity to migrate and colonize. Considering the fact that, every tumor cell, is bathed in interstitial fluid, which drains into the lymphatic system and has an individualized arterial blood supply and venous drainage like any other normal cell in our body, inserting a needle or a knife into a tumor, there is a jeopardy of dislodging a loose tumor cell into either the circulation or into the tissue fluid. Tumor cells are easier to dislodge due to lower cell-to-cell adhesion. This theory with the possibility of seeding of tumor cells is supported by several case studies that have shown that after diagnostic biopsy of a tumor, many patients developed cancer at multiple sites and showed the presence of circulating cancer cells in the blood stream on examination. In this review, we evaluate the risk of exposure to seeding of tumor cells by biopsy and aspiration cytology and provide some suggested practices to prevent tumor cell seeding.
Keywords: Aspiration cytology, biopsy, cancer, metastasis, seeding
INTRODUCTION
There are two common methods of obtaining tissue from a tumor or lesion for the microscopic examination and diagnosis. One is biopsy, which is the removal of living tissue by surgical means and the other is aspiration of cells from the tumor with the help of a fine-needle (fine needle aspiration cytology [FNAC]). These procedures are associated with the risk of seeding tumor cells either into the interstitial tissue fluid from where they are carried to lymph nodes, or into the veins draining the tissue from where they enter the vasculature and may travel to lodge into any organ or tissue. There is also a risk of dragging cells along the surgical incision or needle track leading to the possibility of increasing the spread of cancer through biopsy.[1]
Cancer cells, besides reproducing uncontrollably, lose cohesiveness and orderliness of normal tissue, invade and get detached from the primary tumor to travel and set up colonies elsewhere. Dislodging neoplastically altered cells from a tumor during biopsy or surgical intervention or during simple procedure like needle aspiration is a possibility because they lack cohesiveness, and they attain the capacity to migrate and colonize.[1]
Every tumor cell is bathed in interstitial tissue fluid which drains into the lymphatic system and has an individualized arterial blood supply and venous drainage just like any other normal cell in our body. Whenever a needle for FNAC or scalpel for biopsy is inserted, the risk of dislodging a cell is high. The dislodged tumor cells may metastasize either through the blood stream or through the interstitial fluid.[1] Tumor cells are easier to dislodge due to lower cell-to-cell adhesion.[1] This theory with the possibility of seeding of tumor cells is supported by several case studies that have shown that after diagnostic biopsy of a tumor, many patients developed cancer at multiple sites and/or blood stream showed the presence of cancer cells.[2]
This review includes a review of articles from English literature and data from internet sources published between 1983 and 2012. In this review, we evaluate the risk of exposure to seeding of tumor cells by biopsy and aspiration cytology and provide some suggested practices to prevent tumor cell seeding.
LITERATURE REVIEW
FNAC began to establish itself as the procedure in the 1950's and 1960’ which is a technique of obtaining cells and tissue fragments through a needle introduced in to the abnormal tissue. Though, the low risk of complications is an advantage with FNAC, instances of complications have been reported in relation to different sites and organs, such as hemorrhage, septicemia, bile peritonoitis, acute pancreatitis, pneumothorax etc.[3] The most serious complication that evoked the interest of health care workers is the possibility of cancer cells being disseminated along the needle track.
Biopsy, a gold standard procedure in medical and dental fraternity, which involves removal of part of or whole of a lesion for microscopic or other investigative procedures has been seen to cause tumor cell seeding along the surgical margins and even dissemination to distant sites.
In 1974, in a book on cancer, Dr. Philip Rubin of the University of Rochester declared that surgical biopsies may contribute to the spread of cancer in some cases.[4]
John Wayne Cancer Institute of Santa Monica, CA conducted a study on 663 Breast Cancer women out of which half of the women underwent breast biopsies, while in the other half tumors were completely removed without performing biopsy. The result of the study was that compared with the women who had their tumors surgically removed there was 50% more chance of spread of cancer to sentinel node in those who had a needle biopsy.[4,5]
There was an article published in July 2004, by The British Medical Journal and it cautioned against the risks of needle biopsies of the liver due to the serious potential for needle track seeding of the tumor.[3,6]
A report published in March, 2007 in the journal nature described the inflammation associated with the immune system's attack on prostate tumors could be involved in their metastasis.[7] Dr. Micheal Karin found that an inflammatory cytokine called RANK ligand, initiates a chain reaction and activates IKKa a protein kinase, which enters the cancer cell nucleus and reduces the expression of the antimetastatic gene Maspin.[7] This report probably explains the molecular pathogenesis behind seeding of tumor cells, as inflammation definitely sets in when tissues are inserted with a needle or scalpel.
The authors reported a case of needle tract implantation of hepatocellular carcinoma following percutaneous biopsy of the liver. The patient with a small hepatocellular carcinoma diagnosed by needle biopsy was found to have developed a nodule of hepatocellular carcinoma at the site of the previous biopsy, 8 months after the biopsy and the lobectomy.[8]
Lundstedt et al. reported 5 cases of percutaneous tumor seeding recorded after 5,000 fine-needle biopsies of abdominal malignancies at their institution. They suggested that in patients with abdominal malignancies, performing fine-needle biopsy runs the risk of implantation metastases, which may compromise the outcome of radical surgery. They also suggested that it should only be performed when the result of the procedure has a direct impact on the choice of therapy.[9]
Cedrone et al. and Goletti et al., Ishii et al. have reported cases of needle tract seeding after ethanol injection for treatment of hepatocellular carcinoma shedding more light on the neoplastic cell seeding.[10,11,12]
Case reports during the period of 1993-2003 have established the possibility of subcutaneous seeding of hepatocellular carcinoma after percutaneous fine-needle aspiration.[13,14,15,16,17,18,19]
Further, risk of dissemination of cancer cells in to circulation after incisional biopsy of an oral cancer has been confirmed by Kusukawa et al. by means of cytokeratin 19 (CK19) reverse-transcriptase polymerase chain reaction (RT-PCR) and they concluded that this may result in increased risk of metastasis. In contrast, CK19 transcript was not detected either in the excisional biopsy group or in controls.[20]
Rallis et al. in 2008 published their observation in 60 hamsters, which showed metastases following biopsy of oral carcinoma, which was reduced by an intratumoral administration of bleomycin prebiopsy.[21]
Liebens et al. reviewed (between 1900 and 2008) the clinical significance of epithelial cell displacement after core needle biopsy in breast carcinoma patients, and associated risk factors (delay between biopsy and surgery, needle passes, duration of the procedure, tumor size, histological type, tumor grade, margins, type of surgery, and of adjuvant treatment). In their study Malignant epithelial cell displacement on surgical specimens occurred in 22% of the patients. A short interval between core needle biopsy and surgical excision increased risk of detecting displaced cells.[22]
Supriya et al. have reported in 2008 the first case of tumor seeding after FNAC of a benign parotid tumor.[23]
Falleti et al. in 2010 reported a case of cutaneous needle track seeding of mesothelioma after thoracentesis was performed using a 22-gauge needle.[24]
Guralp and Kushner reviewed articles on dissemination of endometrial cancer cells during procedures such as hysteroscopy, saline infusion sonography and laparoscopy and said that the majority of studies suggest that they increase the risk of spill of tumor cells. They also suggested that there are too few in vivo and in vitro studies to comment definitively on the viability of the disseminated endometrial cancer cells. The limited data available, however, questions the ability of disseminated endometrial cancer cells to maintain and grow.[25]
Another group of researchers worked on risk of tumor incisional recurrence in patients receiving surgery and post-operative radiation therapy for locally advanced sinonasal malignancies. In their study, Medical records for 70 patients diagnosed with non-metastatic Stage II to Stage IV sinonasal malignancies were retrospectively reviewed and suggested that actuarial risk of incisional recurrence for the entire group at 1 year was 3%.[26]
Conners and Rilling have reported a case of tumor seeding in to pleural space following percutaneous cryoablation of hepatocellular carcinoma.[10,27]
Kuo et al. reported a rare case of metastasis at the colostomy site after rectal cancer surgery probably occurred owing to ablative cancer cell reflux and seeding from the obstruction during decompressive colostomy rather than local, lymphatic or hematogenous spread.[28]
There is histological evidence of seeding of tumor cells from primary neoplastic site into adjacent breast tissue following biopsy. However, as the interval between biopsy and surgery lengthens then the incidence of seeding declines, which suggests that displaced tumor cells are not viable.[29]
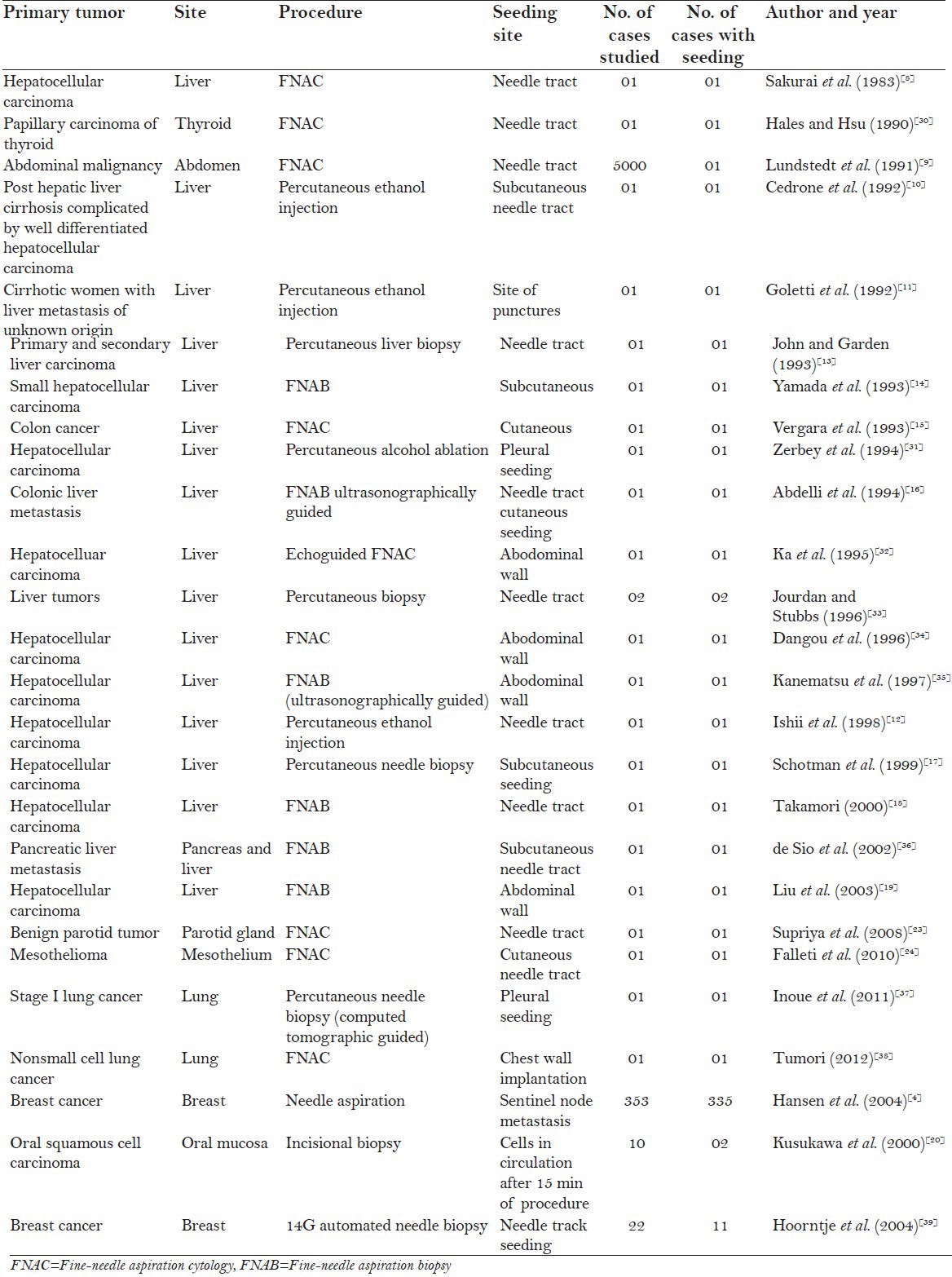
Here is compilation of the results of various studies [Table 1] between 1983 and 2012.
Table 1.
Compilation of the various studies and case reports showing tumor cell seeding
DISCUSSION
Tumor seeding, whereby malignant cells are deposited along the tract of a biopsy needle, can have fatal consequences. More than 90% of cancer-associated mortality may be attributed to metastasis. Once cancer cells in a tumor attain metastatic potential it is a great challenge to treatment as it is difficult for one to discern the extent of systemic involvement by the tumor cells even though the primary tumors can be removed by surgical resection, chemotherapy or radiotherapy. Once in the circulation these metastatic seeds or the circulating tumor cells (CTCs) bring about dissemination to anatomically distant organs from a primary tumor.[40] Fortunately, tumor seeding is a rare occurrence, yet the issue invariably receives a high profile and is often regarded as a major contraindication to certain biopsy procedures. Although its existence is in no doubt, realistic insight into its likelihood across the spectrum of biopsy procedures and multiple anatomical sites is required to permit accurate patient counseling and risk stratification.[41]
We analyzed the data from Table 1 and have drawn inference based on the compiled data and made an attempt to provide suggested practices to reduce the risk of tumor cell seeding.
Data in the table leads us to infer two key findings;
Risks are specific to some tumors: [Figures 1 and 2] Breast cancers followed by liver malignancies with seeding complication have been reported more in literature may be relating to more risk with these tumor. In our review, 94% of breast cancers and 4% liver malignancies showed risk of seeding of tumor cells following biopsy or FNAC.
-
Risks are localized to procedures:
- Excisional biopsy associated with less seeding risk than Incisional biopsy: a procedure in which a tumor mass is removed in toto should carry little risk of spread as in Excisional biopsy with wide margins. The main risks of serious spread will apply with incisional biopsies, where a small portion of the large tumor mass is incised to carry out investigation on the biopsy tissue to arrive at a proper diagnosis before carrying out a definitive treatment.[4,42]
- Procedures in which cancer itself is penetrated.
- Improper handling of the tissue while making biopsy
- Core needle shows more seeding risk when compared to fine-needle (FNAC) use.[42]
- Repeated penetrations during needle procedure associated with increased seeding risk: Many a times to obtain sufficient amount of sample during needle biopsy for diagnosis the tumor may need to be penetrated several times. This repeated puncturing and manipulation inside the tumor mass with needle may seed tumor cells into the needle track and also may spill the cancerous cells directly in to the circulation.[2]
Figure 1.
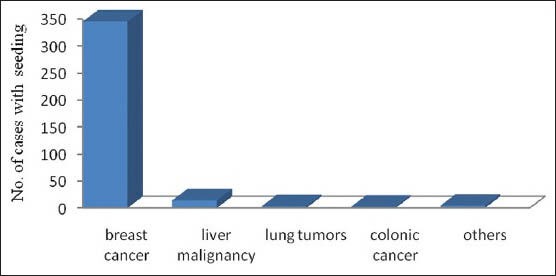
Bar chart showing risk of tumor cell seeding specific to type of tumor
Figure 2.
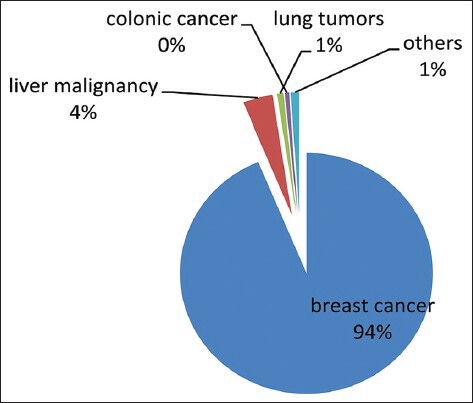
Piechart with percentage of risk of tumor cell seeding specific to type of tumor
Suggested practices to prevent biopsy related tumor cell seeding
For any invasive procedure care needs to be taken during pre-operative, operative and post-operative stages to prevent tumor cell seeding. Hence, we discuss suggested practices given by various authors under these groups.
Pre-operative care
Get a biopsy or surgery on days 18-20 of the menstrual cycle.[1]
Avoid injecting local anesthesia into or closely adjacent to a lesion for biopsy.
Operative care
While considering any surgical procedure for tumors a buffer of normal surrounding tissue should be included. This ensures complete removal as well as reduces the risk of seeding as the knife would not be cutting through the tumor mass. Here, few authors have suggested specifications for buffer for different tumors.
Breast cancer should be removed with a buffer of 1 cm or more whenever possible.[2]
Colon or stomach cancer, at least 5 cm of normal colon or stomach whenever anatomically possible.[2]
Suspected melanoma of the skin, 1 cm of normal skin, and the subcutaneous tissues down to the muscle sheath needs to be removed.[2]
It is important to establish buffer margin for other malignancies with high recurrence rate.
Avoid grasping a lymph node with forceps.[43]
Post-operative care
Strict follow-up of patients.
Suggested practices to prevent aspiration cytology related tumor cell seeding
Pre-operative care
Needle of 22 gauge or less should be used.[3]
Operative care
Multiple insertions to be avoided.[3]
Practice computed tomography (CT) guided ultrasonography directed FNAC.[40]
Coaxial cutting needle technique: Needle introducer remains in position during multiple cutting needle passes protects normal tissue along the tract and may reduce seeding.[44]
Two-step freezing method, by use of percutaneous cryoablation after biopsy but before the removal of the biopsy needle.[45]
Post-operative care
Radiation therapy can be given to kill any tumor cell that may have been dislodged and spread during the surgery.[1]
Periodical CT scans for 3 years after fine-needle aspiration biopsy.[41]
There are few research work which may prove to be promising toward preventing tumor cell seeding:
Identification of novel adhesion molecules and blocking their function can compromise successful seeding and colonization of CTCs in new microenvironment.[30]
Neutralization of CTCs in the circulation.[30]
Evaluation of disseminated cancer cells in to circulation after incisional biopsy using RT-PCR.[46]
CONCLUSION
This study is an attempt to establish seeding risk and bring awareness among patients as well as health care workers. Support from more number of articles and long term follow-up of patients in whom these procedures have been performed may substantiate the results with more authority.
There are very few published data which give us information on the total number of patients undergoing biopsy or the needle procedures in given period of time and among these how many are actually showing tumor cell seeding. Hospitals, health institutions and research workers should work toward providing this data, which in reality will let us know if ‘seeding of tumor cells’ is worth all the attention.
Biopsy and aspiration cytology are the gold standards for the diagnosis of any tumor. They are age old and time tested practices. Cultivating the suggested practices while performing these procedures may make them risk proof.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Biopsy and surgery can spread cancer. Dr. Vincent Gammill Center for the Study of Natural Oncology (CSNO) Solana Beach, California. Available from: www.healingcancernaturally.com/biopsies-surgery-spread-cancer.html .
- 2.Moran P. Cancer treatment watch. Do biopsies or surgical treatment spread cancer? Available from: http://www.cancertreatmentwatch.org/general/biopsiess.html .
- 3.Orell SR, Sterrette GF, Whitaker D. 4th ed. New Delhi: Churchill Livingstone, Published by Elsevier, Reed Elsevier India Private Ltd; 2005. Fine Needle Aspiration Cytology-Text Book; pp. 1–8. [Google Scholar]
- 4.Hansen NM, Ye X, Grube BJ, Giuliano AE. Manipulation of the primary breast tumor and the incidence of sentinel node metastases from invasive breast cancer. Arch Surg. 2004;139:634–9. doi: 10.1001/archsurg.139.6.634. [DOI] [PubMed] [Google Scholar]
- 5.Dangers of cancer spread from a biopsy. [Last Accessed in 2013 Jun 5]. Available from: http://www.karlloren.com/biopsy/p46.htm .
- 6. [Last Accessed in 2013 Jun 5]. Available from: http://www.karlloren.com/biopsy/p31.htm .
- 7.Luo JL, Tan W, Ricono JM, Korchynskyi O, Zhang M, Gonias SL, et al. Nuclear cytokine-activated IKKalpha controls prostate cancer metastasis by repressing Maspin. Nature. 2007;446:690–4. doi: 10.1038/nature05656. [DOI] [PubMed] [Google Scholar]
- 8.Sakurai M, Okamura J, Seki K, Kuroda C. Needle tract implantation of hepatocellular carcinoma after percutaneous liver biopsy. Am J Surg Pathol. 1983;7:191–5. doi: 10.1097/00000478-198303000-00010. [DOI] [PubMed] [Google Scholar]
- 9.Lundstedt C, Stridbeck H, Andersson R, Tranberg KG, Andrén-Sandberg A. Tumor seeding occurring after fine-needle biopsy of abdominal malignancies. Acta Radiol. 1991;32:518–20. [PubMed] [Google Scholar]
- 10.Cedrone A, Rapaccini GL, Pompili M, Grattagliano A, Aliotta A, Trombino C. Neoplastic seeding complicating percutaneous ethanol injection for treatment of hepatocellular carcinoma. Radiology. 1992;183:787–8. doi: 10.1148/radiology.183.3.1316621. [DOI] [PubMed] [Google Scholar]
- 11.Goletti O, De Negri F, Pucciarelli M, Sidoti F, Bertolucci A, Chiarugi M, et al. Subcutaneous seeding after percutaneous ethanol injection of liver metastasis. Radiology. 1992;183:785–6. doi: 10.1148/radiology.183.3.1584934. [DOI] [PubMed] [Google Scholar]
- 12.Ishii H, Okada S, Okusaka T, Yoshimori M, Nakasuka H, Shimada K, et al. Needle tract implantation of hepatocellular carcinoma after percutaneous ethanol injection. Cancer. 1998;82:1638–42. [PubMed] [Google Scholar]
- 13.John TG, Garden OJ. Needle track seeding of primary and secondary liver carcinoma after percutaneous liver biopsy. HPB Surg. 1993;6:199–203. doi: 10.1155/1993/39539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamada N, Shinzawa H, Ukai K, Wakabayashi H, Togashi H, Takahashi T, et al. Subcutaneous seeding of small hepatocellular carcinoma after fine needle aspiration biopsy. J Gastroenterol Hepatol. 1993;8:195–8. doi: 10.1111/j.1440-1746.1993.tb01513.x. [DOI] [PubMed] [Google Scholar]
- 15.Vergara V, Garripoli A, Marucci MM, Bonino F, Capussotti L. Colon cancer seeding after percutaneous fine needle aspiration of liver metastasis. J Hepatol. 1993;18:276–8. doi: 10.1016/s0168-8278(05)80269-7. [DOI] [PubMed] [Google Scholar]
- 16.Abdelli N, Bouche O, Thiefin G, Renard P, Flament JB, Zeitoun P. Subcutaneous seeding on the tract of percutaneous cytologic puncture with a fine needle of a hepatic metastasis from colonic adenocarcinoma. Gastroenterol Clin Biol. 1994;18:652–6. [PubMed] [Google Scholar]
- 17.chotman SN, De Man RA, Stoker J, Zondervan PE, Ijzermans JN. Subcutaneous seeding of hepatocellular carcinoma after percutaneous needle biopsy. Gut. 1999;45:626–7. doi: 10.1136/gut.45.4.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takamori R, Wong LL, Dang C, Wong L. Needle-tract implantation from hepatocellular cancer: Is needle biopsy of the liver always necessary? Liver Transpl. 2000;6:67–72. doi: 10.1002/lt.500060103. [DOI] [PubMed] [Google Scholar]
- 19.Liu C, Frilling A, Dereskewitz C, Broelsch CE. Tumor seeding after fine needle aspiration biopsy and percutaneous radiofrequency thermal ablation of hepatocellular carcinoma. Dig Surg. 2003;20:460–3. [Google Scholar]
- 20.Kusukawa J, Suefuji Y, Ryu F, Noguchi R, Iwamoto O, Kameyama T. Dissemination of cancer cells into circulation occurs by incisional biopsy of oral squamous cell carcinoma. J Oral Pathol Med. 2000;29:303–7. doi: 10.1034/j.1600-0714.2000.290703.x. [DOI] [PubMed] [Google Scholar]
- 21.Rallis G, Mourouzis C, Papakosta V, Donta I, Perrea D, Patsouris E, et al. Metastases following biopsy of oral carcinoma in hamsters and the role of local prebiopsy bleomycin. Anticancer Res. 2008;28:2253–7. [PubMed] [Google Scholar]
- 22.Liebens F, Carly B, Cusumano P, Van Beveren M, Beier B, Fastrez M, et al. Breast cancer seeding associated with core needle biopsies: A systematic review. Maturitas. 2009;62:113–23. doi: 10.1016/j.maturitas.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 23.Supriya M, Denholm S, Palmer T. Seeding of tumor cells after fine needle aspiration cytology in benign parotid tumor: A case report and literature review. Laryngoscope. 2008;118:263–5. doi: 10.1097/MLG.0b013e318158f718. [DOI] [PubMed] [Google Scholar]
- 24.Falleti J, Giordano M, Cozzolino I, Vetrani A, De Renzo A, Zeppa P. Cutaneous needle track seeding of mesothelioma diagnosed by fine needle aspiration cytology: A case report. Acta Cytol. 2010;54:811–4. [PubMed] [Google Scholar]
- 25.Guralp O, Kushner DM. Iatrogenic transtubal spill of endometrial cancer: Risk or myth. Arch Gynecol Obstet. 2011;284:1209–21. doi: 10.1007/s00404-011-2031-6. [DOI] [PubMed] [Google Scholar]
- 26.Moore MG, Lin DT, Deschler DG, Wang JJ, Chan AW. Risk of incisional recurrence after midface and anterior skull base surgery in sinonasal malignancies. Skull Base. 2011;21:87–92. doi: 10.1055/s-0030-1266762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Conners D, Rilling W. Pleural tumor seeding following percutaneous cryoablation of hepatocellular carcinoma. Semin Intervent Radiol. 2011;28:258–60. doi: 10.1055/s-0031-1280677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuo YH, Chin CC, Lee KF. Metastasis at the colostomy site: A rare case report. Jpn J Clin Oncol. 2012;42:753–6. doi: 10.1093/jjco/hys081. [DOI] [PubMed] [Google Scholar]
- 29.Loughran CF, Keeling CR. Seeding of tumour cells following breast biopsy: A literature review. Br J Radiol. 2011;84:869–74. doi: 10.1259/bjr/77245199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hales MS, Hsu FS. Needle tract implantation of papillary carcinoma of the thyroid following aspiration biopsy. Acta Cytol. 1990;34:801–4. [PubMed] [Google Scholar]
- 31.Zerbey AL, Mueller PR, Dawson SL, Hoover HC., Jr Pleural seeding from hepatocellular carcinoma: A complication of percutaneous alcohol ablation. Radiology. 1994;193:81–2. doi: 10.1148/radiology.193.1.8090925. [DOI] [PubMed] [Google Scholar]
- 32.Ka MM, Dangou JM, Fall B, Pouye A, Ndiaye MF, Diop TM, et al. Tumor seeding of the abdominal wall after fine needle cytologic puncture of the liver. Apropos of a case. Ann Gastroenterol Hepatol (Paris) 1995;31:221–5. [PubMed] [Google Scholar]
- 33.Jourdan JL, Stubbs RS. Percutaneous biopsy of operable liver lesions: Is it necessary or advisable? N Z Med J. 1996;109:469–70. [PubMed] [Google Scholar]
- 34.Dangou JM, Ka M, Fall B, Ndiaye MF, Diop TM, Bao O, et al. Tumor seeding of the abdominal wall after fine needle aspiration of a hepatocellular carcinoma. Ann Pathol. 1996;16:227–8. [PubMed] [Google Scholar]
- 35.Kanematsu M, Hoshi H, Takao H, Sugiyama Y. Abdominal wall tumor seeding at sonographically guided needle-core aspiration biopsy of hepatocellular carcinoma. AJR Am J Roentgenol. 1997;169:1198–9. doi: 10.2214/ajr.169.4.9308498. [DOI] [PubMed] [Google Scholar]
- 36.de Sio I, Castellano L, Calandra M, Del Vecchio-Blanco C. Subcutaneous needle-tract seeding after fine needle aspiration biopsy of pancreatic liver metastasis. Eur J Ultrasound. 2002;15:65–8. doi: 10.1016/s0929-8266(02)00008-3. [DOI] [PubMed] [Google Scholar]
- 37.Inoue M, Honda O, Tomiyama N, Minami M, Sawabata N, Kadota Y, et al. Risk of pleural recurrence after computed tomographic-guided percutaneous needle biopsy in stage I lung cancer patients. Ann Thorac Surg. 2011;91:1066–71. doi: 10.1016/j.athoracsur.2010.12.032. [DOI] [PubMed] [Google Scholar]
- 38.Scotti V, Di Cataldo V, Falchini M, Meattini I, Livi L, Ugolini D, et al. Isolated chest wall implantation of non-small cell lung cancer after fine-needle aspiration: A case report and review of the literature. Tumori. 2012;98:126e–9. doi: 10.1700/1190.13213. [DOI] [PubMed] [Google Scholar]
- 39.Hoorntje LE, Schipper ME, Kaya A, Verkooijen HM, Klinkenbijl JG, Borel Rinkes IH. Tumour cell displacement after 14G breast biopsy. Eur J Surg Oncol. 2004;30:520–5. doi: 10.1016/j.ejso.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 40.Li J, King MR. Adhesion receptors as therapeutic targets for circulating tumor cells. Front Oncol. 2012;2:79. doi: 10.3389/fonc.2012.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Robertson EG, Baxter G. Tumour seeding following percutaneous needle biopsy: The real story! Clin Radiol. 2011;66:1007–14. doi: 10.1016/j.crad.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 42.Shinohara S, Yamamoto E, Tanabe M, Maetani T, Kim T. Implantation metastasis of head and neck cancer after fine needle aspiration biopsy. Auris Nasus Laryn×. 2001;28:377–80. doi: 10.1016/s0385-8146(01)00093-1. [DOI] [PubMed] [Google Scholar]
- 43.Fortner JG. Inadvertent spread of cancer at surgery. J Surg Oncol. 1993;53:191–6. doi: 10.1002/jso.2930530313. [DOI] [PubMed] [Google Scholar]
- 44.Maturen KE, Nghiem HV, Marrero JA, Hussain HK, Higgins EG, Fox GA, et al. Lack of tumor seeding of hepatocellular carcinoma after percutaneous needle biopsy using coaxial cutting needle technique. AJR Am J Roentgenol. 2006;187:1184–7. doi: 10.2214/AJR.05.1347. [DOI] [PubMed] [Google Scholar]
- 45.Mu F, Liu SP, Zhou XL, Chen JB, Li HB, Zuo JS, et al. Prevention of needle-tract seeding by two-step freezing after lung cancer biopsy. Pathol Oncol Res. 2013;19:447–50. doi: 10.1007/s12253-012-9601-1. [DOI] [PubMed] [Google Scholar]
- 46.Dyavanagoudar S, Kale A, Bhat K, Hallikerimath S. Reverse transcriptase polymerase chain reaction study to evaluate dissemination of cancer cells into circulation after incision biopsy in oral squamous cell carcinoma. Indian J Dent Res. 2008;19:315–9. doi: 10.4103/0970-9290.44534. [DOI] [PubMed] [Google Scholar]


