Abstract
Aims:
The aim of this study is to evaluate the expression of human papilloma virus (HPV) in oral squamous cell carcinoma (OSCC) and to correlate the association of HPV in histological grades of OSCC using p16 (p16INK4a) immunohistochemistry (IHC).
Subjects and Methods:
This study consists of 30 histological diagnosed cases of OSCC (10-well-differentiated oral squamous cell carcinoma [WDOSCC], 10-moderately differentiated oral squamous cell carcinoma [MDOSCC] and 10-poorly differentiated oral squamous cell carcinoma [PDOSCC]). The sections were subjected to IHC procedure using p16. Two parameters in immunohistochemical p16 expression were evaluated by 3 observers based on the criteria by Galgano M. Tetal (2010) (a) percentage of p16 positive cases (b) pattern of p16 staining in various grades of OSCC.
Statistical Analysis Used:
Kappa test.
Results:
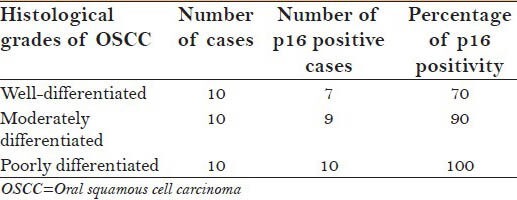
Totally, 30 samples of 0SCC, p16 positivity was noted in 26/30 (86.66%). Of 26 positive cases, p16 staining was positive in 7/10 (70%) of WDOSCC, 9/10 (90%) in MDOSCC and, 10/10 (100%) PDOSCC. Incidentally, we also found single dispersed cell staining in WDOSCC, patchy staining in MDOSCC and more diffuse staining pattern predominant in PDOSCC.
Conclusions:
Our study revealed an association between HPV and OSCC. Diffuse staining pattern was noted in PDOSCC, which in turn depicts the increase viral overload, which might have an influence on its aggressive behavior.
Keywords: Human papilloma virus, oral squamous cell carcinoma, p16
INTRODUCTION
Cancer is one of the most common causes, which results in morbidity and mortality today, further, more than 10 million new cases and more than 6 million deaths occur each year worldwide.[1] In India, oral squamous cell carcinoma (OSCC) is the most common malignancy accounting up to 40-50% of all malignancies. Although tobacco and alcohol are the main etiologic factors in three-fourth of these cancers, the etiological factor in 1/4th of the cases remains unnoticed. There is growing evidence that human papilloma virus (HPV) may act as a cocarcinogen along with tobacco which eventually results in oral cancers.[2]
Human papilloma virus are small, circular double stranded deoxyribonucleic acid (DNA) viruses that belong to the papillomaviridae family.[3] Over 130 HPV types are known; these are classified as low or high-risk, based on their association with cervical carcinoma. HPV-16 and 18 are the most commonly detected high-risk types. HPV transforms infected epithelial cells and causes defects in genes controlling apoptosis, cell cycle, and DNA repair, thereby promoting tumorigenesis.[4]
The protein p16 is a cellular protein involved in cell cycle regulation. In normal cells, p16 protein is expressed in very low levels and is almost undetectable by IHC. Due to the transforming activity of E7 oncogene, p16 is strongly expressed in tumor cells affected by HPV and may be easily detected by IHC.[5] Hence, p16 positivity correlates strongly with HPV positivity.[6] However, literature search revealed meager data exclusively correlating HPV and histological grades of OSCC.
Hence, this study is focused on evaluating the expression of HPV in OSCC and to correlate the association of HPV in histological grades of OSCC using p16.
SUBJECTS AND METHODS
Study design
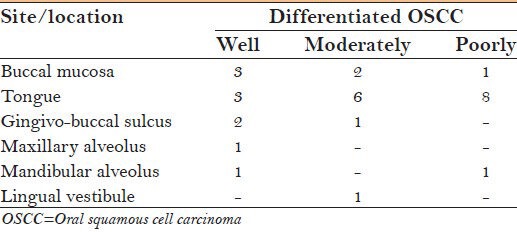
A total of 30 formalin fixed paraffin embedded biopsied samples, histopathologically diagnosed as OSCC were retrieved from the Department of Archival Collection, M.S. Ramaiah Dental College and Hospital, Bangalore [Table 1]. Serial sections of 4 μm were taken, one section was subjected to H and E to ascertain the histological grades (Modified Broder's grading system),[7] while the consecutive sections were subjected to p16 IHC analysis. Of the 30 cases, 10 cases were well differentiated oral squamous cell carcinoma (WDOSCC), 10 cases were moderately differentiated oral squamous cell carcinoma (MDOSCC) and the rest 10 cases were poorly differentiated oral squamous cell carcinoma (PDOSCC).
Table 1.
Different grades of OSCC and their sites/location
Procedure for p16 immunohistochemistry (IHC) - is elucidated in a Flowchart 1 Commercially available mouse monoclonal antibody for p16INK4a protein (Biogenex, USA) was used.
Flow Chart 1.
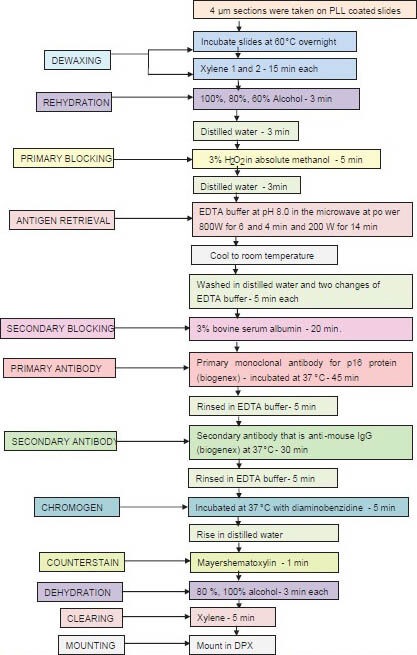
Representing the steps in p16 immunohistochemical staining. PLL = Poly L lysine, EDTA = Ethylenediaminetetraacetic acid, H2O2 = Hydrogen peroxide, IgG = Immunoglobulin G, DPX = Dibutylphathalate xylene
Positive control slides of cervical carcinoma [Figure 1a] harboring HPV were taken to ascertain the validity of IHC kit and the accuracy of the technique. For negative control, the primary antibodies were omitted. The presence of brown precipitate at the site of cytoplasm, nucleus or both were indicative of p16 positive immunoreactivity regardless of staining intensity.[8] The criteria given by[9] opted to evaluate the p16 staining is delineated in Table 2.
Figure 1.
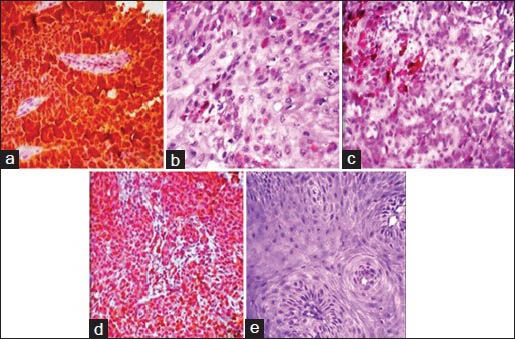
p16 immunohistochemistry staining in histological grades of oral squamous cell carcinoma with positive control. (a) Positive control slide of cervical cancer, (b) dispersed single cell staining in well-differentiated oral squamous cell carcinoma, (c) Patchy staining in moderately differentiated oral squamous cell carcinoma, (d) diffuse staining in poorly differentiated oral squamous cell carcinoma, (e) No staining. Kappa statistics of 0.900, revealed high agreement between the observers
Table 2.
Criteria for evaluating p16 staining
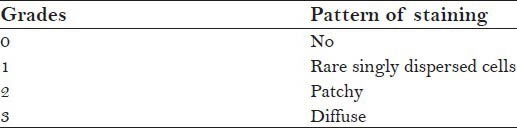
The slides were examined by three pathologists using light microscope and the entire procedure was blinded to minimize the inter observer bias and the values were ascertained using kappa statistics.
RESULTS
In total 30 samples of OSCC were evaluated. The results revealed p16 positivity in 26 cases (86.66%) of 30 [Graph 1].
Graph 1.
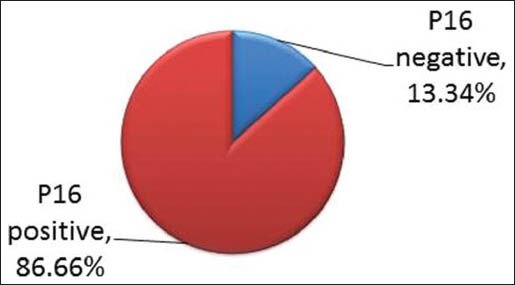
Portrayingp16 positivity in 86.66% of oral squamous cell carcinoma cases
The number of p16 positive cases in different histological grades of OSCC is collated in Table 3.
Table 3.
Demonstrating p16 positive cases in histological grades of OSCC
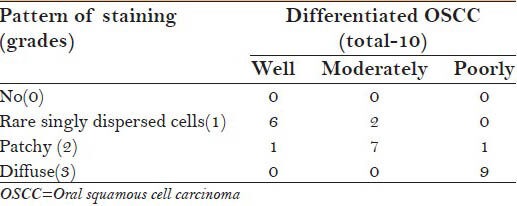
Different grades of OSCC showed difference in grades/patterns of staining. WDOSCC predominantly showed singly dispersed cell staining, while it was predominantly patchy and diffuse in moderately and PDOSCC, respectively [Table 4, Graph 2 and Figure 1].
Table 4.
Different grades of p16 staining in different grades of OSCC
Graph 2.
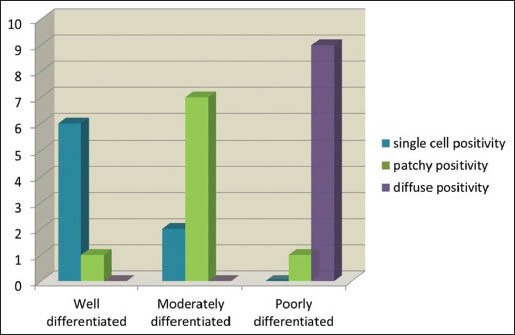
More diffuse p16 staining in poorly differentiated oral squamous cell carcinoma, predominant patchy staining in moderately differentiated oral squamous cell carcinoma and singly dispersed cell staining in well-differentiated oral squamous cell carcinoma
DISCUSSION
Associating HPV and head and neck cancer was first done in 1983 by Syrjänen et al.,[10] and then was supported by several other authors on the basis of the following evidence: (i) The epitheliotropic nature of HPV (ii) the widely confirmed oncogenic potential, especially in cervical squamous cell carcinoma and (iii) the morphological similarities between oropharyngeal and genital epithelia.[11]
Most HPV research has primarily focused on cervical cancer, as >99% of cervical cancers harbor HPV.[3] During the recent years, evidence of HPV infection emerged as a major prognostic and predictive marker in head and neck squamous cell cancer (HNSCC).[6] In oral cavity, among 12 types of HPV found, HPV 16 and 18 are the most common types followed by 6 and 11. Prevalence of HPV in OSCC is now considered to be as high as 50%.[2]
Moreover, the number of people at risk for infections of such oncogenic viruses and subsequent risk of neoplasia is well into the millions throughout the world.[3] Recent data from case-control and meta-analytic studies show that HPV would be indeed, an independent risk factor for the development of oropharyngeal and oral carcinomas.[12]
HPV associated OSCC are more common in younger age groups due to physical contact and oral sex.[13] It is well-established that the genital HPV infections is a sexually transmitted disease, associated with early sexual debut and multiple sexual partners. The mode of transmission of HPV to the mucosa is less understood and less defined at this stage, theories have proposed multiple pathways for HPV transmission, including perinatal transmission, auto-infection from oral-genital contact by hand and sexual transmission by oral-genital contact.[14] It has been postulated that abrasions caused due to this continuous exposure might make mucosal surface more susceptible to HPV facilitating its entry into the basal cells.[13]
There has been wide variation in HPV positivity rates in cancers at different sites in the head and neck region. Approximately, 25-75% of oropharyngeal cancers have tested HPV positive, with rates in tonsillar cancer being the highest, followed by cancers of the tongue and of the buccal mucosa.[2] The probable reason could be that HPV being inhabitant of normal crypt epithelia and Waldeyer's ring, an antigen presenting site, may act as the reservoir for HPV.
To understand the pathogenesis of HPV, [Figure 2] a basic understanding of normal cell cycle is important. In normal cell cycle, the hypophosphorylated retinoblastoma (RB) in complex with E2F transcription factors prevents the progress of cell cycle from G1 to S. When RB is phosphorylated by cyclin D - cyclin-dependent kinase 4 (CDK4), it releases E2F. The latter then induces target genes such as cyclin E, which stimulates DNA replication and progression through the cell cycle. The gene product p16 normally binds to CDK 4, inhibiting their association with cyclin D. The inhibition of the complex cyclin D - CDK4 prevents phosphorylation of RB leading to inhibition of cell cycle progression through G1- to S-phase.
Figure 2.
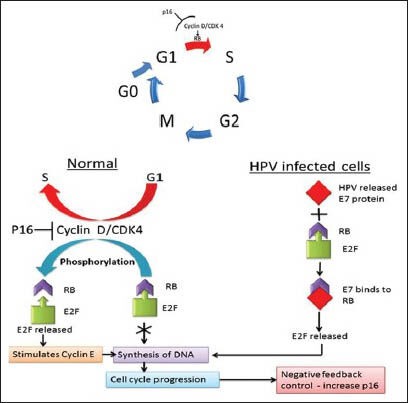
The mechanism of normal cell cycle & pathogenesis of HPV in tumorigenesis. A-Normal Cell cycle, B - Role of RB in regulating G1 -S checkpoint, C-Pathogenesis of HPV GI to S - Red arrow indicates the HPV targeting the cells passing through G1 to S phase. Abbreviations- G - Gap phase, S- synthesis phase, M - mitosis phase, RB -Retinoblastoma, p16 - p16INK4a CDK- Cyclin dependent kinase, HPV - Human Papilloma Virus, DNA-Deoxyribonucleic acid, E7- Early genes, E2F- Transcription factor
The proposed mechanism of action of HPV is by integration of HPV into host genome and up regulates expression of E6 and E7 oncogenes. The interaction of E7 with RB gene results in release of the transcription factor E2F from the RB-E2F complex. As RB-E2F normally inhibits transcription of the p16 gene, expression of HPV E7 results in excessive and deregulated transcription and translation of p16.
The E6 protein induces the loss of G1 checkpoint activation very early due to the degradation of p53. As a result, these infected cells are also resistant to p53 induced growth arrest and apoptosis, making them immortal.[15] The mechanism of normal cell cycle and pathogenesis of HPV in tumorigenesis is represented in schematic way.
Usually, HPV gets detected by IHC, in-situ hybridization (ISH) and polymerase chain reaction (PCR) methods.[16] Studies comparing these three methods revealed that all OSCCs cases, which confirmed HPV positivity by PCR/ISH were also p16 positive by IHC.[16] Detection of p16 by IHC showed very high level of sensitivity but less specificity. p16 is a known surrogate biomarker of HPV-induced carcinomas. Thereby, HPV detection was done using p16 IHC.
In this study, we observed 86.66% of OSCC cases being p16 positive representing HPV positivity in 87% of cases of OSCC. This was in accordance with Balaram et al., Kojima et al., Ostwald et al., who found 74%, 66%, and 62% HPV positivity, respectively, whereas Koppikar et al., Khovidhunkit et al., Zeuss et al. studies revealed 6%, 2%, and 0%, respectively exhibiting a low prevalence of HPV in OSCC independent of the method used.[17]
It is also observed that HPV infection is more prominent in OSCC cases from India than in patients from other countries; for example, only 23% of Japanese patients, 8-20% of American patients, and 19% of Dutch patients are HPV positive. In India, 33.6% of OSCC patients were HPV positive in Eastern India as compared with 67% in South India and 15% in Western India.[18] This variability may be attributable to: Ethnicity and geography; small number of samples analyzed; possible contamination; detection technique used.[4] Interestingly, we also noted all the cases of PDOSCC were HPV positive, which correlates to the aggressive nature.
Controversy still exits regarding the prognosis of HPV positive OSCC. While few studies reported that detection of HPV was associated with late stage disease and poor prognosis, other studies have shown HPV positive cases having better prognosis and improved survival rate, with favorable to radiation treatment. However, treatment strategies do not currently differ on the basis of HPV status. In the future, determination of HPV status may be used to guide treatment decisions and posttreatment surveillance.[19]
On literature search, there were only a meager number of studies correlating HPV and histological grades of OSCC. To the best of our knowledge, we found none of the studies emphasizing the correlation of HPV in histological grades of OSCC. In this study, we found diffuse staining was more in the PDOSCC, which may correlate increased viral overload and in turn correlates to the aggressive behavior of the tumor and vice versa singly dispersed cells in WDOSCC showed less aggressiveness. This was in accordance with Narges et al., who observed the significant relationship between histological grades and the percentage of positive cells in premalignant and malignant cervical lesions. However, further studies with increased sample size and other markers for correlating the aggressive behavior of the tumor are required which would provide a better understanding of their biological behavior.
The identification of HPV 16 and 18 as high-risk types have resulted in the development of prophylactic vaccines based on their viral capsids. At present, two vaccines for prevention of HPV-related diseases have been developed - Cervarix and Gardasil. The vaccines are now part of the public vaccination program in several countries and are offered to girls from the age of 12 years or prior to sexual debut. The value and cost benefit of vaccinating boys is under discussion in many countries. The efficacy of the vaccines in preventing HPV-related HNSCC is at present unknown.[17] This is equally encouraging and gives us hope that it should be possible to develop such vaccines against head and neck cancers too; which would be especially useful in advanced cases.[1] Public education especially focusing on young adults about of the role of HPV and unsafe sexual practices is of paramount importance in prevention of HPV associated oral cancer.
CONCLUSION
Our study shows that strong association exists between HPV infection and OSCC. The diffuse pattern of p16 positivity in PDOSCC cases needs to be investigated further, which would provide better understanding of the biological behavior of OSCC caused by HPV.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Petersen PE. Oral cancer prevention and control – The approach of the World Health Organization. Oral Oncol. 2009;45:454–60. doi: 10.1016/j.oraloncology.2008.05.023. [DOI] [PubMed] [Google Scholar]
- 2.Chocolatewala NM, Chaturvedi P. Role of human papilloma virus in the oral carcinogenesis: An Indian perspective. J Cancer Res Ther. 2009;5:71–7. doi: 10.4103/0973-1482.52788. [DOI] [PubMed] [Google Scholar]
- 3.Hafed L, Farag H, Shaker O, El-Rouby D. Is human papilloma virus associated with salivary gland neoplasms? An in situ-hybridization study. Arch Oral Biol. 2012;57:1194–9. doi: 10.1016/j.archoralbio.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 4.Machado J, Reis PP, Zhang T, Simpson C, Xu W, Perez-Ordonez B, et al. Low prevalence of human papillomavirus in oral cavity carcinomas. Head Neck Oncol. 2010;2:6. doi: 10.1186/1758-3284-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Izadi-Mood N, Asadi K, Shojaei H, Sarmadi S, Ahmadi SA, Sani S, et al. Potential diagnostic value of p16 expression in premalignant and malignant cervical lesions. J Res Med Sci. 2012;17:428–33. [PMC free article] [PubMed] [Google Scholar]
- 6.Brunner M, Koperek O, Wrba F, Erovic BM, Heiduschka G, Schoppper C, et al. HPV infection and p16 expression in carcinomas of the minor salivary glands. Eur Arch Otorhinolaryngol. 2012;269:2265–9. doi: 10.1007/s00405-011-1894-2. [DOI] [PubMed] [Google Scholar]
- 7.Rajendran R, Sivapathasundharam B. 6th ed. New Delhi, India: Elsevier; 2009. Shafer's Textbook of Oral Pathology. [Google Scholar]
- 8.Bradley KT, Budnick SD, Logani S. Immunohistochemical detection of p16INK4a in dysplastic lesions of the oral cavity. Mod Pathol. 2006;19:1310–6. doi: 10.1038/modpathol.3800649. [DOI] [PubMed] [Google Scholar]
- 9.Galgano MT, Castle PE, Atkins KA, Brix WK, Nassau SR, Stoler MH. Using biomarkers as objective standards in the diagnosis of cervical biopsies. Am J Surg Pathol. 2010;34:1077–87. doi: 10.1097/PAS.0b013e3181e8b2c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Syrjänen K, Syrjänen S, Lamberg M, Pyrhönen S, Nuutinen J. Morphological and immunohistochemical evidence suggesting human papillomavirus (HPV) involvement in oral squamous cell carcinogenesis. Int J Oral Surg. 1983;12:418–24. doi: 10.1016/s0300-9785(83)80033-7. [DOI] [PubMed] [Google Scholar]
- 11.Termine N, Panzarella V, Falaschini S, Russo A, Matranga D, Lo Muzio L, et al. HPV in oral squamous cell carcinoma vs head and neck squamous cell carcinoma biopsies: A meta-analysis (1988-2007) Ann Oncol. 2008;19:1681–90. doi: 10.1093/annonc/mdn372. [DOI] [PubMed] [Google Scholar]
- 12.Martín-Hernán F, Sánchez-Hernández JG, Cano J, Campo J, del Romero J. Oral cancer, HPV infection and evidence of sexual transmission. Med Oral Patol Oral Cir Bucal. 2013;18:e439–44. doi: 10.4317/medoral.18419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jaiswal R, Pandey M. Human papilloma virus in oral carcinogenesis and its routes of transmission. World J Epidemiol Cancer Prev. 2012;1:1–9. [Google Scholar]
- 14.Termine N, Giovannelli L, Rinaldi G, Campisi G. Human papillomavirus infection and head and neck squamous cell carcinoma: Current debates. J Stomatol Invest. 2008;2:27–33. [Google Scholar]
- 15.Thomas M, Pim D, Banks L. The role of the E6-p53 interaction in the molecular pathogenesis of HPV. Oncogene. 1999;18:7690–700. doi: 10.1038/sj.onc.1202953. [DOI] [PubMed] [Google Scholar]
- 16.Pannone G, Rodolico V, Santoro A, Lo Muzio L, Franco R, Botti G, et al. Evaluation of a combined triple method to detect causative HPV in oral and oropharyngeal squamous cell carcinomas: p16 Immunohistochemistry, polymerase chain reaction human papillomavirus-DNA, and in situ hybridization. Infect Agent Cancer. 2012;7:1–14. doi: 10.1186/1750-9378-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lajer CB, von Buchwald C. The role of human papillomavirus in head and neck cancer. APMIS. 2010;118:510–9. doi: 10.1111/j.1600-0463.2010.02624.x. [DOI] [PubMed] [Google Scholar]
- 18.D’Costa J, Saranath D, Dedhia P, Sanghvi V, Mehta AR. Detection of HPV-16 genome in human oral cancers and potentially malignant lesions from India. Oral Oncol. 1998;34:413–20. doi: 10.1016/s1368-8375(98)00028-1. [DOI] [PubMed] [Google Scholar]
- 19.Snow AN, Laudadio J. Human papillomavirus detection in head and neck squamous cell carcinomas. Adv Anat Pathol. 2010;17:394–403. doi: 10.1097/PAP.0b013e3181f895c1. [DOI] [PubMed] [Google Scholar]


