Abstract
A library of potentially bioactive compounds through the novel 1H-indole-methyl-isocyanide and MCRs has been described. A flexible and efficient synthesis affording great complexity and diversity is achieved, with moderate to good yields with no need for protection and deprotection steps.
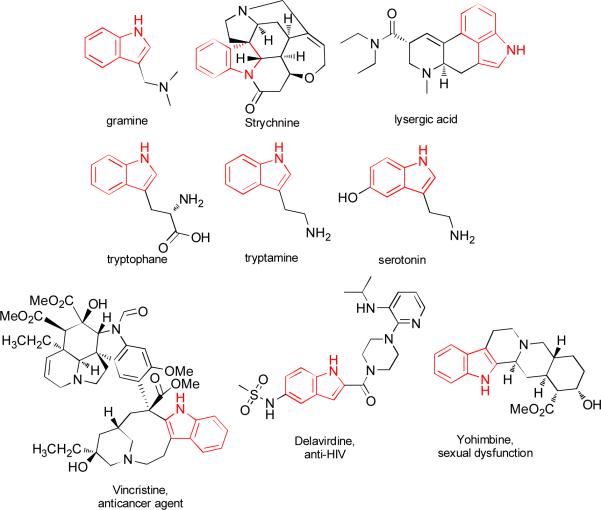
In 1869, Adolf von Baeyer proposed the structure of one of the most important heterocyclic compounds(1): indole. Indole is unambiguously the king of heterocycles: An electron-rich heteroatomic system with enhanced reactivity in electrophilic aromatic substitutions, especially in C-3 position (enamine type). The importance of indole is recognized both by the number of publications (more than 80000 publications in 114 years) and with its presence in pharmaceuticals, agrochemicals, organic electronics, in addition to the vast of natural products alkaloids containing indole moieties. Alkaloids like gramine, strychnine and lysergic acid (LSD), aminoacids and other bioactive compounds like tryptophan, tryptamine and serotonin and pharmaceuticals like vincristine, delavirdine and yohimbine (fig. 1) are just very few examples showing why chemists have this continuous interest in employing the indole core in the compounds.(2-5)
Fig 1.
Selected examples of indole-containing compounds
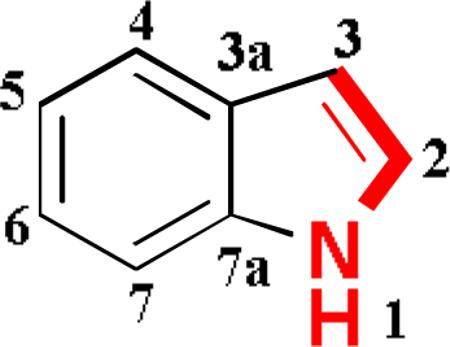
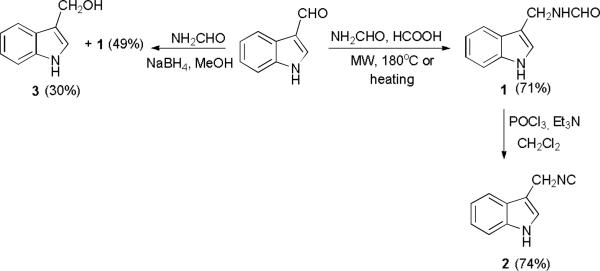
MCRs are valuable tools that give us the opportunity to access complex and diverse scaffolds with potential bioactivity.(6) Despite the importance of Ugi-type reactions in the synthesis of heterocyclic compounds, only scant attention has been paid to the incorporation of indoles as multicomponent reaction partners. Recently the Ugi-4C reactions of indole-carboxaldehydes and their post-Ugi transformations was reported.(7) In continuation of our interest in isocyanide based MCRs, we envisaged using “Mannich-type” isocyanides from electron-rich heterocycles, such as indole, for the construction of scaffolds which otherwise their skeleton would be accessible via lengthy sequential synthesis. In past, we reported Ugi reactions with a tryptophan derived isocyanide and subsequent Pictet-Spengler reactions(8-9) but herein we report the first “gramine-type” isocyanide and its employment in various MCRs giving access to unique drug-like indole scaffolds. To the best of our knowledge 1H-indole-methyl-isocyanide 2 has never been mentioned before in literature and thought to be the most suitable precursor. It could be synthesized from the corresponding formamide 1 by dehydration, whereas the latter could be synthesized from the readily available indole-3-carboxaldehyde via a reductive amination reaction (scheme 1).
Scheme 1.
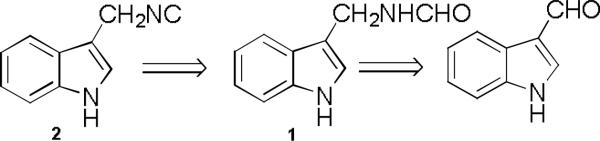
Retrosynthetic analysis of 1H-indole-methyl-isocyanide 2
The reductive amination of indole-3-carboxaldehyde with formamide(10) led to the desired formylated compound 1 in 49% yield, affording also the indole benzyl alcohol 3 in a 30% isolated yield (scheme 2). After quite some experimentation, we “revived” an old but nevertheless very useful reaction: The Leuckart-Wallach reaction.(11) This, rather abandoned in modern synthetic chemistry, reaction is a one-step reductive amination but suffers from some serious practical problems such as prolonged reaction times, high temperatures and compatibility issues with substituted benzaldehydes. Nevertheless, decreasing the concentration of the indole-3-carboxaldehyde with certain specific molar ratios of the formamide and formic acid using microwave assistance (accelerated Leuckart-Wallach), gave us access to the important intermediate 1 in 71% yield reducing also the reaction time to 3 minutes (scheme 2). Typical dehydrating conditions with POCl3 and Et3N afforded the novel 1H-indole-methyl-isocyanide.
Scheme 2.
The Leuckart-Wallach reaction and synthesis of the isocyanide 2
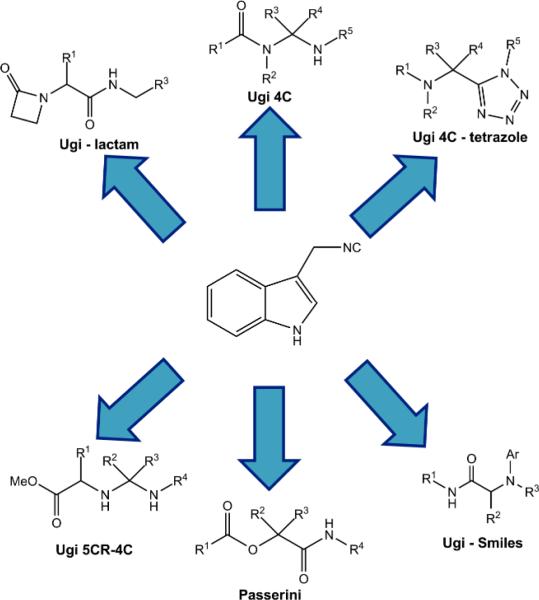
Next, the isocyanide 2 was successfully employed in various multi-component reactions, based on the rapid and easy access to biologically relevant compounds that can give, such as the Ugi reaction-4 component,(6a) Ugi-tetrazole,(6b) Ugi-β-lactam,(6c) Ugi-Smiles,(6d) Ugi-4 component-5 centers(6e) and Passerini-3 component(6f) (fig. 2). Various aldehydes, amines and acids were used in order to illustrate the diversity of these drug-like compounds, which could be obtained assembling the indole moiety.
Fig 2.
Multi-Component Reactions of the novel isocyanide
Both Ugi-4C and Ugi-tetrazole reactions gave very good results using electron withdrawing and donating groups on the amine and aldehyde components (table 1). Scaffolds like 4 illustrate the flexibility and complexity that in a one-step can be achieved, whereas compounds 5 combine two of the most bioactive rings such as the indole and tetrazole ring. Ugi-Smiles reactions (6a-c) gave lower yields but still demonstrate the versatility of the reaction.
Table 1.
Survey on reactions Ugi-4C, Ugi-tetrazole and Ugi-Smiles
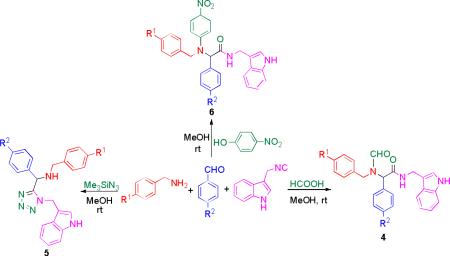
| ||||
|---|---|---|---|---|
| Entry | R1 | R2 | Acid component | Product (% yield) |
| 1 | Cl | Br | formic acid | 4a (85) |
| 2 | H | H | formic acid | 4b (82) |
| 3 | OMe | CN | formic acid | 4c (74) |
| 5 | Cl | Br | TMS-azide | 5a (88) |
| 6 | H | H | TMS-azide | 5b (80) |
| 7 | 3-Cl-C6H4 | F | TMS-azide | 5c (71) |
| 8 | Cl | Br | p-nitro-phenol | 6a (35) |
| 9 | H | H | p-nitro-phenol | 6b (41) |
| 10 | OMe | CN | p-nitro-phenol | 6c (45) |
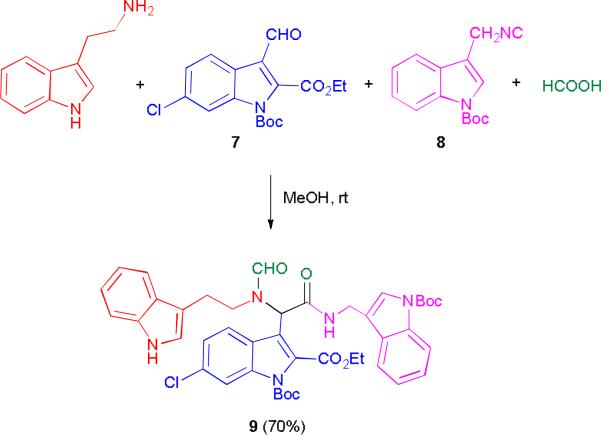
In addition, an Ugi-4C reaction was performed with three different indole moieties(12), giving access to a tris-indole alkaloid-like structure which would otherwise have been very difficult to synthesize in a linear approach (scheme 3). Thus unprotected tryptamine, protected indole aldehyde 7, the protected indole isocyanide 8 (for synthesis see supporting information) and formic acid combined to give the tris-indole compound 9 in 70% yield.
Scheme 3.
Synthesis of a tris-indole alkaloid-like compound
Furthermore, amino acids were thought to be useful for assembling with the new isocyanide. To investigate that, Ugi 4C-5 center and Ugi-lactam reactions were performed with β-amino acids (10a-c) and α-amino acids (11a-c) respectively giving good yields. For compounds (11a-b) diastereoselectivities of 1:2 were observed. Both the complexity that these kinds of reactions ensure and the formation of bioactive rings such as a lactam are of great importance (table 2). Finally, a Passerini reaction (12a-c), performed with different substituents on the aldehyde and acid moiety, giving access to more scaffolds (table 2).
Table 2.
Survey on reactions Ugi 4C-5CR, Ugi-lactam and Passerini
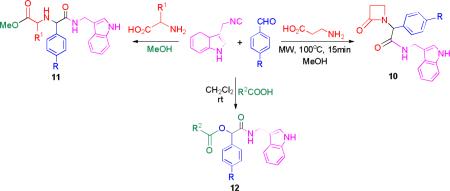
| ||||
|---|---|---|---|---|
| Entry | R1 | R | R2 | Product (% yield) |
| 1 | - | Cl | - | 10a (59) |
| 2 | - | H | - | 10b (45) |
| 3 | - | Me | - | 10c (39) |
| 4 | Me | Br | - | 11a (70) |
| 5 | Me | H | - | 11b (55) |
| 6 | H | CN | - | 11c (71) |
| 7 | - | Br | Me | 12a (67) |
| 8 | - | H | Me | 12b (55) |
| 9 | - | CN | 2-Cl-C6H4 | 12c (69) |
Conclusions
A novel indole isocyanide was synthesized, through the accelerated Leuckart-Wallach reaction, which was subsequently employed in the most important multi-component reactions leading to possible bioactive compounds. The new derivatives expand the indole chemical space, combining both the necessary complexity and diversity. The examples presented herein illustrate the generality and the broad scope of using this isocyanide in MCR chemistry. The 3-aminomethyindole fragment is abundant in multiple natural products and thus it can be predicted that MCRs involving our new 3-isocyanomethylindole will find application in natural product synthesis.
Supplementary Material
Acknowledgements
This work was supported partially by grants from NIH (1R21GM087617-01A1, 1P41GM094055-01, and 1R01GM09708201).
Notes and references
- 1.a Baeyer A. Justus Liebigs Ann. Chem. 1866;140:295. [Google Scholar]; b Baeyer A, Emmerling A. Ber. Dtsch. Chem. Ges. 1869;2:679. [Google Scholar]
- 2.Bandini M, Eichholzer A. Angew. Chem. Int. Ed. 2009;48:9608. doi: 10.1002/anie.200901843. [DOI] [PubMed] [Google Scholar]
- 3.Douglass TF, Pavan TK. Tetrahedron. 2011;67:7195. [Google Scholar]
- 4.Bandini M. Org. Biomol. Chem. 2013;11:5206. doi: 10.1039/c3ob40735g. [DOI] [PubMed] [Google Scholar]
- 5.Sharma V, Kumar P, Pathaka D. Journal of Heterocyclic Chemistry. 2010;47:491. [Google Scholar]
- 6.a Sheehan SM, Masters JJ, Wiley MR, Young SC, Liebeschuetz JW, Jones SD, Murray CW, Franciskovich JB, Engel DB, Weber WW, Marimuthu J, Kyle JA, Smallwood JK, Farmen MW, Smith GF. Bioorg. Med. Chem. Lett. 2003;13:2255. doi: 10.1016/s0960-894x(03)00462-1. [DOI] [PubMed] [Google Scholar]; b Nixey T, Hulme C. Tetrahedron Lett. 2002;43:6833. [Google Scholar]; c Ribelin TP, Judd AS, Akritopoulou-Zanze I, Henry RF, Cross JL, Whittern DN, Djuric SW. Org. Lett. 2007;9:5119. doi: 10.1021/ol7023373. [DOI] [PubMed] [Google Scholar]; d El Kaim L, Grimaud L, Oble J. Angew. Chem. Int. Ed. 2005;44:7961–7964. doi: 10.1002/anie.200502636. [DOI] [PubMed] [Google Scholar]; e Khoury K, Sinha MK, Nagashima T, Herdtweck E, Dömling A. Angew. Chem. Int. Ed. 2012;51:10280. doi: 10.1002/anie.201205366. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Banfi L, Guanti G, Riva R, Basso A, Calcagno E. Tetrahedron Lett. 2002;43:4067. [Google Scholar]; g Dömling A, Wang W, Wang K. Chem. Rev. 2012;112:3083. doi: 10.1021/cr100233r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.a Zhang L, Zhao F, Zheng M, Zhai Y, Liu H. Chem. Commun. 2013;49:2894. doi: 10.1039/c3cc00111c. [DOI] [PubMed] [Google Scholar]; b Kumar A, Li Z, Sharma SK, Parmar VS, Van der Eycken EV. Chem. Commun. 2013;49:6803. doi: 10.1039/c3cc42704h. [DOI] [PubMed] [Google Scholar]
- 8.Liu H, Dömling A. J. Org. Chem. 2009;74:6895. doi: 10.1021/jo900986z. [DOI] [PubMed] [Google Scholar]
- 9.Wang W, Herdtweck E, Dömling A. Chem. Commun. 2010;46:770. doi: 10.1039/b917660h. [DOI] [PubMed] [Google Scholar]
- 10.Yamada F, Hashizume T, Somei M. Heterocycles. 1998;47:509. [Google Scholar]
- 11.a Loupy A, Monteux D, Petit A, Aizpurua JM, Domínguez E, Palomo C. Tetrahedron Lett. 1996;37:8177. [Google Scholar]; b Lejon T, Helland I. Acta Chemica Scandinavica. 1999;53:76–78. [Google Scholar]; c Bobylev M, Krueger J. US patent 2009, WO2009/018353 A1. [Google Scholar]
- 12.Kumar D, Rawat DS. Opportunity, Challenge and Scope of Natural Products in Medicinal Chemistry. 2011:213. [Google Scholar]
- 13.Hwu JR, Wein YS, Leu YJ. J. Org. Chem. 1996;61:1493. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.