Abstract
Retrograde viral transport (i.e., muscle to motoneuron) enables targeted gene delivery to specific motor pools. Recombinant adeno-associated virus serotype 9 (AAV9) robustly infects motoneurons, but the retrograde transport capabilities of AAV9 have not been systematically evaluated. Accordingly, we evaluated the retrograde transduction efficiency of AAV9 after direct tongue injection in 129SVE mice as well as a mouse model that displays neuromuscular pathology (Gaa−/−). Hypoglossal (XII) motoneurons were histologically evaluated 8 weeks after tongue injection with AAV9 encoding green fluorescent protein (GFP) with expression driven by the chicken β-actin promoter (1×1011 vector genomes). On average, GFP expression was detected in 234±43 XII motoneurons 8 weeks after AAV9-GFP tongue injection. In contrast, tongue injection with a highly efficient retrograde anatomical tracer (cholera toxin β subunit, CT-β) resulted in infection of 818±88 XII motoneurons per mouse. The retrograde transduction efficiency of AAV9 was similar between the 129SVE mice and those with neuromuscular disease (Gaa−/−). Routine hematoxylin and eosin staining and cluster of differentiation (CD) immunostaining for T cells (CD3) indicated no persistent inflammation within the tongue or XII nucleus after AAV9 injection. Additional experiments indicated no adverse effects of AAV9 on the pattern of breathing. We conclude that AAV9 can retrogradely infect a significant portion of a given motoneuron pool in normal and dystrophic mice, and that its transduction efficiency is approximately 30% of what can be achieved with CT-β.
ElMallah and colleagues evaluate the transduction efficiency of AAV9 after direct intramuscular tongue injection in both normal and dystrophic mice. They show that AAV9 can retrogradely infect a significant portion of a given motoneuron pool in normal and dystrophic mice and that its transduction efficiency is approximately 30% of what can be achieved with a highly efficient retrograde anatomical tracer called cholera toxin β subunit (CT-β).
Introduction
Muscle injection of recombinant adeno-associated virus (AAV) followed by retrograde axonal viral transport provides a means to target gene delivery to specific motoneuron pools. After the initial report by Kaspar and colleagues (2003), several studies have confirmed motoneuron transduction after intramuscular AAV injection (Kaspar et al., 2003; Pirozzi et al., 2006; Hollis et al., 2008; Towne et al., 2010; Zheng et al., 2010). The relative transduction efficacy, however, is typically low. For example, Hollis and colleagues found that self-complementary (sc) AAV serotype 1 (scAAV1, 1.05×109 viral particles [VP]) transduced approximately 4% of the extensor carpi motoneuron pool after muscle injection, whereas scAAV6 (1.05×109 VP) transduced less than 1% of the pool after a similar procedure (Hollis et al., 2008). In contrast, Towne and colleagues found that gastrocnemius injection of AAV6 (1.3×1012 VP) in primates resulted in transduction of approximately 15% of the motoneuron pool (Towne et al., 2010). Thus, the efficacy of retrograde viral transport appears variable across AAV serotypes.
AAV9 is a serotype that effectively crosses the blood–brain barrier (Manfredsson et al., 2009) and can robustly infect motoneurons (Snyder et al., 2011). Intrathecal delivery of scAAV9-GFP (2.5×109 vector genomes [VG]) transduced approximately 700 motoneurons in adult mice, but this was not restricted to a given motor pool (Snyder et al., 2011). Intravenously administered scAAV9-GFP (doses ranging from 4×1011 to 4×1012 VP) was reported to transduce motoneurons in neonatal but not adult mice (Foust et al., 2009). However, another study found that intravenous delivery of scAAV9-GFP caused transduction of approximately 15% of motoneurons in both adult mouse (2×1012 VG) and cat (1.2×1012 VG) cervical spinal cords (Duque et al., 2009), with no efficient transduction achieved with single-stranded (ss) AAV9-GFP. Thus, AAV9 vectors are capable of gene delivery to motoneurons after intrathecal or intravenous delivery. To our knowledge, however, the present work represents the first systematic evaluation of retrograde transduction efficacy of AAV9 after intramuscular injection.
Our primary purpose was to compare and contrast the relative efficiency of ssAAV9 retrograde motoneuron transduction. The ssAAV9 vector has approximately double the transgene capacity compared with scAAV9 (McCarty et al., 2001; Hollis et al., 2008). This larger capacity offers distinct advantages for treating neuromuscular disorders. Accordingly, we compared the transduction efficiency of ssAAV9 with what could be achieved using a standard retrograde neuranatomical tracer (cholera toxin β subunit, CT-β). We and others have shown that CT-β will infect a majority of the associated motoneuron pool after direct intramuscular injection (Lane et al., 2008; Qiu et al., 2010; Lee et al., 2011) The efficiency of retrograde AAV9 transport was tested using the hypoglossal (XII) motor system.
There are a wide range of neuromuscular impairments that could potentially benefit from direct gene transfer to XII motoneurons. Obstructive sleep apnea, for example, is associated with reduced neural drive to the tongue during sleep (Horner, 2009). Gene therapies that enhance XII motoneuron excitability and/or output during sleep could mitigate apneic events. In addition, single-gene defect muscular dystrophies that are accompanied by motoneuron pathology (Byrne et al., 2011a) would potentially benefit from parallel transduction of muscle and motoneurons after a single AAV injection. However, retrograde viral transport may be influenced by aspects of both muscle and neural pathology. Accordingly, our secondary purpose was to determine whether the retrograde transduction efficiency of motoneurons with AAV9 would be blunted in a mouse model with both muscular dystrophy and neuronal pathology. Accordingly, AAV9 retrograde transduction was evaluated in a murine model of Pompe disease (the Gaa−/− mouse) (Raben et al., 1998), in which pronounced muscle wasting (Mah et al., 2005) and motoneuron pathology have been reported (DeRuisseau et al., 2009; Lee et al., 2011).
The results of this investigation indicate that ssAA9 can effectively transduce approximately 230 XII motoneurons after a single intramuscular injection in both normal and dystrophic mice. Accordingly, the AAV9 vector is a promising candidate for targeted gene delivery to motoneurons in neuromuscular disorders.
Materials and Methods
Animals
All experimental procedures were approved by the Institutional Animal Care and Use Committee at the University of Florida (Gainesville, FL). 129SVE mice (n=20; age, 4 weeks±3 days) and Gaa−/− mice (n=4; age, 8 weeks±5 days) were used. The Gaa−/− mice were previously described (Raben et al., 1998; DeRuisseau et al., 2009), and were originally obtained from Taconic (Hudson, NY).
AAV vectors
The tongue was injected with an ssAAV vector encoding green fluorescent protein (GFP) with expression driven by the chicken β-actin promoter (AAV9-CBA-GFP, 1×1011 VG/ml). All vectors were generated and titered at the University of Florida Powell Gene Therapy Center Vector Core Laboratory, using previously published methods (Zolotukhin et al., 2002). Vectors were purified by iodixanol gradient centrifugation and anion-exchange chromatography as described previously (Zolotukhin et al., 2002). Final formulations of AAV9-CBA-GFP vectors were in lactated Ringer solution.
In vivo delivery
All animal studies were performed in accordance with the guidelines of the University of Florida Institutional Animal Care and Use Committee. Mice were anesthetized with 3% isoflurane in oxygen administered via a nose cone. Ten (5 females, 5 males) 4-week-old 129SVE mice and four (1 female, 3 males) 8-week-old Gaa−/− mice were administered AAV9-CBA-GFP diluted in lactated Ringer solution (20 μl) directly to the left side of the base of the tongue lateral to the lingual frenulum. The needle was held at a 45-degree angle and inserted to a depth of 2 mm. PE50 tubing was placed over the needle to ensure that the depth of injection was uniform in each mouse. Six 4-week-old 129SVE mice were administered the same volume of lactated Ringer solution. These sham-injected mice were used as a control group for studies of ventilation and XII efferent nerve discharge (see below). Four (2 females, 2 males) 4-week-old 129SVE mice received one injection of 5 μl of CT-β (0.1%) diluted in 15 μl of sterile water to the same area. CT-β is a monosynaptic tracer (Yates et al., 1999) that we have previously used to label XII motoneurons in mice (Lee et al., 2011). There were no cases of early mortality and all animals survived until the time of evaluation (2 months postinjection).
Genomic DNA extraction and real-time polymerase chain reaction
PCR was used to measure AAV genome copies in the tongue and medulla of 129SVE mice (n=4). Eight weeks after AAV9-CBA-GFP lingual injection, tissues were harvested in a manner that prevented cross-contamination, snap frozen in liquid nitrogen, and stored at −80°C until genomic DNA (gDNA) was extracted. The medulla was harvested en bloc whereas the entire tongue was harvested and divided into anterior, middle, and posterior tongue. gDNA was isolated with a DNeasy blood and tissue kit (Qiagen, Valencia, CA) according to the manufacturer's instructions. gDNA concentrations were determined with an Eppendorf biophotometer (Eppendorf, Hamburg, Germany). AAV genome copies in the gDNA were quantified by real-time PCR, using an ABI 7900 HT sequence detection system (Applied Biosystems/Life Technologies, Carlsbad, CA) according to the manufacturer's instructions, and results were analyzed with SDS 2.3 software. Briefly, primers and probe were designed to the simian virus 40 (SV40) poly(A) region of the AAV vector as previously described (Mah et al., 2010). A standard curve was created, using plasmid DNA containing the same SV40 poly(A) target sequence. PCRs contained a total volume of 100 μl and were run under the following conditions: 50°C for 2 min, 95°C for 10 min, and 45 cycles of 95°C for 15 sec and 60°C for 1 min.
DNA samples were assayed in triplicate. To assess PCR inhibition, the third replicate was spiked with plasmid DNA at a ratio of 100 copies/μg gDNA. If this replicate was greater than 40 copies/μg gDNA then the results were considered acceptable. If a sample contained greater than or equal to 100 copies/μg gDNA it was considered positive for vector genomes. If a sample contained fewer than 100 copies/μg gDNA it was considered negative for vector genomes. If less than 1 μg of gDNA was analyzed the vector copy number reported was normalized per microgram of gDNA and the plasmid spike-in was reduced to maintain the ratio of 100 copies/μg gDNA. Data were reported as AAV genome copies per microgram of total genomic DNA±SD.
Brainstem histology
Mice were initially anesthetized with isoflurane and then given an intraperitoneal urethane injection (1.5 g/kg). Once an adequate plane of anesthesia was established mice were killed via systemic perfusion with 4% paraformaldehyde. Tissues were harvested 72 hr after CT-β injection (Lane et al., 2008) and 8 weeks after AAV9-CBA-GFP injection. The postinjection time interval for CT-β was selected on the basis of our prior reports (Lane et al., 2008; Lee et al., 2011). The brainstem and spinal cord tissues were extracted and postfixed by immersion in 2% paraformaldehyde (n=12) and stored at 4°C for sectioning. Transverse vibrating-blade microtome (Vibratome) sections (40 μm) were made through the brainstem and sections were placed in 2% paraformaldehyde in 96-well plates until ready for processing. CT-β-labeled neurons were detected as previously described (Lane et al., 2008). The sections were incubated overnight with primary antibodies against CT-β (polyclonal goat antiserum to purified CT-β, choleragenoid isolated from Vibrio cholerae type Inaba 569B) (product no. 703, lot no. 7032A5, diluted 1:10,000; List Biological Laboratories, Campbell, CA). This antiserum labels a single immunoprecipitation band against purified CT-β (0.5 mg/ml) at a dilution of 1:16 on Western blot (manufacturer's technical information). The next day, tissue was washed in phosphate-buffered saline (PBS), incubated with a biotinylated secondary antibody (VECTASTAIN ABC kit; Vector Laboratories, Burlingame, CA), and treated with 3,3′-diaminobenzidine (DAB) for bright-field microscopy. GFP immunohistochemistry, followed by secondary detection with a VECTASTAIN ABC kit and DAB, was performed on tissue from the AAV9-CBA-GFP-injected mice. Tissue was incubated overnight with primary antibody against GFP, diluted 1:20,000 (chicken anti-GFP; Aves Laboratories, Tigard, OR). The next day the tissue was washed in PBS, incubated with a biotinylated anti-chicken IgG secondary antibody (diluted 1:200; Vector Laboratories), and treated with a VECTASTAIN ABC kit and DAB for bright-field microscopy. In four of the animals (two sham- and two vector-injected mice) the medullae were postfixed in 4% paraformaldehyde for 24 hr and then transferred to 70% ethanol until processing. Paraffin serial sections (5 μm) were stained with hematoxylin and eosin (H&E) and by immunohistochemistry. Inflammatory cell immunophenotype was determined with monoclonal antibodies that recognize CD3 (Flotte et al., 2011).
Tongue histology
After perfusion the tongue was removed, postfixed in 4% paraformaldehyde for 24 hr, and then transferred to 70% ethanol until processing. Paraffin serial sections (5 μm) were stained with H&E. Inflammatory cell immunophenotype was also assessed with monoclonal antibodies that recognize CD3. CD3 is a protein complex important in transducing the signal that initiates the T cell activation and differentiation pathway. The presence of CD3 indicates activation of a T cell inflammatory response (Recio et al., 2007; Flotte et al., 2011). In a subset of mice the tongue was processed differently to enable visualization of GFP immunofluorescence. In those animals, the tongue was frozen in optimal cutting temperature (O.C.T.) compound (Sakura Finetek USA, Torrance, CA) and liquid nitrogen. Using a cryostat, each tongue was then sectioned at 8 μm and directly examined by fluorescence microscopy.
Microscopy and quantitative analyses
Bright-field and standard fluorescence photographs were taken with a Zeiss AxioPhot microscope and an AxioCam HRc digital camera (Carl Zeiss Microimaging, Jena, Germany) linked to a PC. By using bright-field microscopy, CT-β- and GFP-positive XII motoneurons with visible nuclei were counted at×10 magnification in consecutive transverse sections of the medulla from each animal. Only cells with a visible nucleus were counted, and an Abercrombie correction was applied to raw counts: total motoneurons counted×[T/(T+h)], where T represents section thickness and h is the diameter of the nuclei (Guillery, 2002; Lane et al., 2008; Qiu et al., 2010).
Ventilation
These studies were undertaken to determine whether AAV9-CBA-GFP injection into the tongue, and subsequent viral infection and GFP expression in motoneurons, had any negative impacts on the respiratory system. Thus, ventilation was quantified by whole-body plethysmography in unrestrained, unanesthetized mice as previously described (DeRuisseau et al., 2009). Mice were placed inside a 3.5×5.75 inch Plexiglas chamber that was calibrated with known airflow and pressure signals before data collection. Data were collected at 10-sec intervals and the Drorbaugh and Fenn equation (Drorbaugh and Fenn, 1955) was used to calculate respiratory volumes including tidal volume and minute ventilation. During both a 30-min acclimation period and subsequent 30- to 60-min baseline period, mice were exposed to normoxic air (21% O2, 79% N2). At the conclusion of the baseline period, the mice were exposed to a brief respiratory challenge, which consisted of a 10-min hypercapnic exposure (7% CO2, balance O2). Experiments were conducted with 129SVE mice that had received tongue injection of AAV9-GFP (n=4) or were sham-injected (n=4). Data were collected 8 weeks postinjection.
Statistical analysis
Statistical comparisons of the number of labeled neurons and body weight between experimental groups were made by unpaired t test. Comparison of ventilation parameters was made by two-way repeated measures analysis of variance. Data were considered to be statistically different when p<0.05.
Results
CT-β labeling
Initial experiments verified the extent of XII motoneuron labeling after tongue injection with the standard retrograde tracer CT-β (Guo et al., 1996; Smith et al., 2005; Qiu et al., 2010). The XII nucleus was observed caudal and lateral to the medullary central canal as previously reported in mice (Millecamps et al., 2001; Lee et al., 2011). On average (n=4), 818±88 XII motoneurons per animal were immunopositive for CT-β. Labeling was restricted to the immediate region of the hypoglossal nucleus, which is consistent with prior reports that CT-β does not cross neuronal synapses (Yates et al., 1999). It has been estimated that the XII nucleus in the adult mouse contains 974 motoneurons (Sturrock, 1991). Accordingly, CT-β was able to infect approximately 84% of XII motoneurons after a single injection into the base of the tongue.
Retrograde transport of AAV9 in 129SVE and Gaa−/− mice
Fluorescence microscopy confirmed the expected GFP expression in lingual myofibers 8 weeks after tongue injection with AAV9-CBA-GFP (Fig. 1). GFP expression was then immunohistochemically examined in the brainstem of the same animals (Fig. 2). On average, 234±43 GFP-positive XII motoneurons could be detected per animal. This was significantly less than the total number of XII motoneurons that were labeled after CT-β injections (p<0.01). Thus, retrograde transport of AAV9 to XII motoneurons appears to be approximately 30% as effective as CT-β. There was no evidence of transsynaptic transport of AAV9, as GFP expression was limited to the immediate region of the XII motor nucleus and could not be detected in neighboring glial cells or interneurons. Similarly, GFP expression could not be detected by immunohistochemistry in rostral brainstem regions or the spinal cord (data not shown).
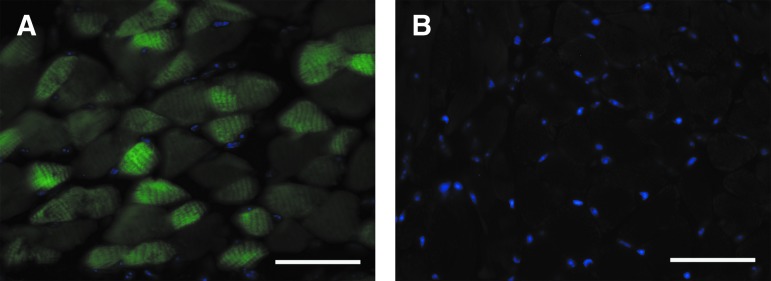
FIG. 1.
Tongue myofibers show GFP fluorescence after intramuscular injection with AAV9-CBA-GFP. Transverse histological sections were taken through the base of the tongue and then examined by fluorescence microscopy. (A) GFP expression in tongue myofibers 8 weeks after injection with AAV9-CBA-GFP. (B) Absence of fluorescence in myofibers from a sham-injected mouse. The tissues were also stained with 4′,6-diamidino-2-phenylindole (DAPI; blue). Scale bars: 50 μm. Color images available online at www.liebertpub.com/hgtb
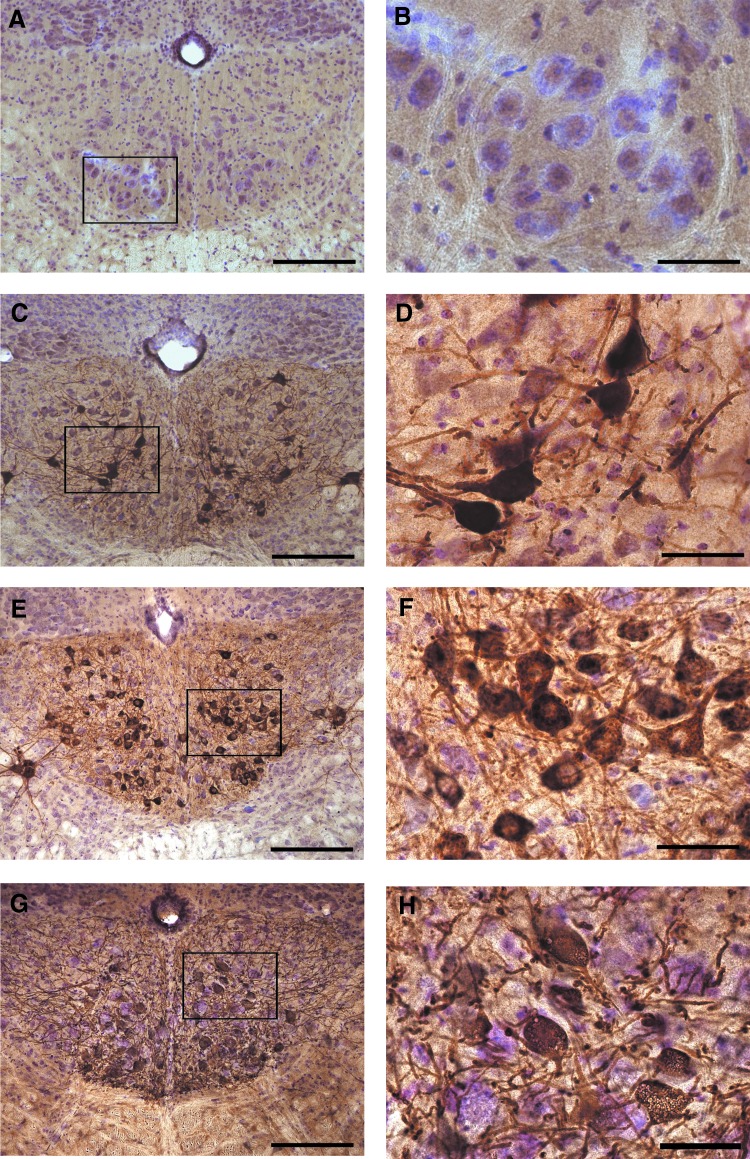
FIG. 2.
Medullary sections showing retrograde transduction of XII motoneurons. Transverse sections through the rostral medulla were immunolabeled for the presence of GFP or CT-β and counterstained with cresyl violet. Each area highlighted by a box on the left is shown at higher magnification on the right. (A) and (B) demonstrate the complete lack of GFP-positive cells in a sham-injected mouse. In contrast, (C) and (D) show tissues harvested after tongue injection with AAV9-CBA-GFP. GFP-positive cells are evident throughout the XII motor nuclei. (E) and (F) show the results of retrograde labeling, using CT-β tongue injections. CT-β-positive cells are present throughout the XII nuclei. (G) and (H) show that AAV9-CBA-GFP injection into the tongue of a Gaa−/− mouse also resulted in robust retrograde transduction of XII motoneurons. Scale bars: (A, C, E, and G) 200 μm; (B, D, F, and H) 50 μm. Color images available online at www.liebertpub.com/hgtb
In additional experiments, PCR was used to quantify AAV vector genome copies in samples from both the tongue and brainstem of 129SVE mice after AAV9-CBA-GFP injection. The tongue was divided into anterior, middle, and posterior segments before analyses. A predictably high number of copies (60,533±28,784 VG copies/μg DNA) was detected in the base of the tongue (i.e., the site of injection). The middle portion of the tongue also had a high number of copies (25,460±8160 VG copies/μg DNA) whereas the anterior third had a reduced number (820±273 VG copies/μg DNA). Vector genome copies were also detected in the en bloc medulla after AAV9-CBA-GFP injection (673±306 VG copies/μg DNA) but not in sham-injected controls (0 VG; p<0.001). Because the entire medulla was processed for PCR studies, it is likely that the relative number of AAV9 vector copies (i.e., copies per microgram of tissue) in the XII nucleus was substantially higher. Nevertheless, these data are consistent with the histological results (Fig. 2) and with retrograde AAV9 transport.
Additional AAV9 tongue injections were performed in Gaa−/− mice. The intent was to compare the retrograde transport of AAV9 in healthy animals with that in animals with a known neuromuscular pathology (Raben et al., 1998; DeRuisseau et al., 2009). In Gaa−/− mice, an average of 333±102 GFP-positive XII motoneurons could be detected after tongue injection (Fig. 2). This value was similar to the number of labeled cells in the 129SVE mice (p=0.336).
AAV9-CBA-GFP: Immune response and impact on breathing
Histological evaluation of tongue and brainstem tissues indicated no persistent immune response after AAV9-CBA-GFP injection. Specifically, H&E-stained tissues showed no evidence of inflammatory cells, and CD3 immunostaining did not detect an increase in T lymphocytes (Fig. 3). In addition, tongue muscle fibers showed no signs of fibrosis or necrosis. Myonuclei were of normal diameter, number, and position with no evidence of disorganization of nuclei.
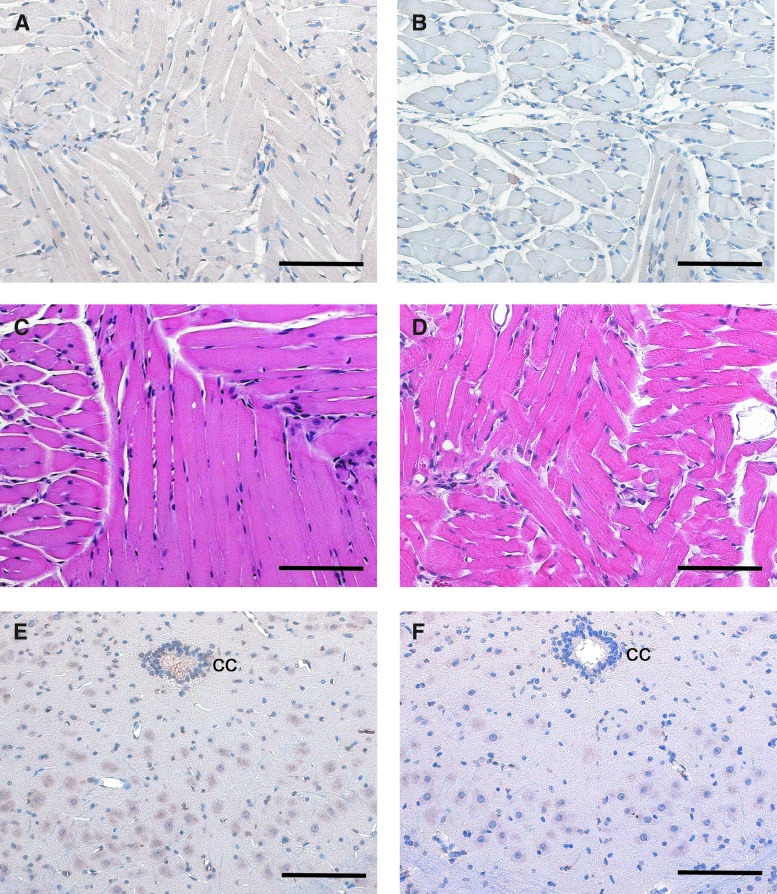
FIG. 3.
Staining for CD3 and hematoxylin and eosin (H&E) staining indicate no persistent inflammatory response to AAV9-CBA-GFP. Histology was assessed 8 weeks after delivery of the vector. (A) and (B) show representative CD3 staining in muscle sections taken from the base of the tongue near the site of AAV9-CBA-GFP injection. No differences were detected between (A) sham-injected and (B) AAV9-CBA-GFP-injected mice. (C) and (D) show H&E-stained tongue myofibers with no indication of an increase in inflammatory cells when comparing (C) sham-injected and (D) AAV9-injected mice. Last, (E) and (F) show CD3 staining in the immediate vicinity of the XII nucleus. No evidence of inflammatory activity was seen in (E) sham-injected and (F) AAV9-injected mice. Scale bars: (A–F) 100 μm. CC, central canal. Color images available online at www.liebertpub.com/hgtb
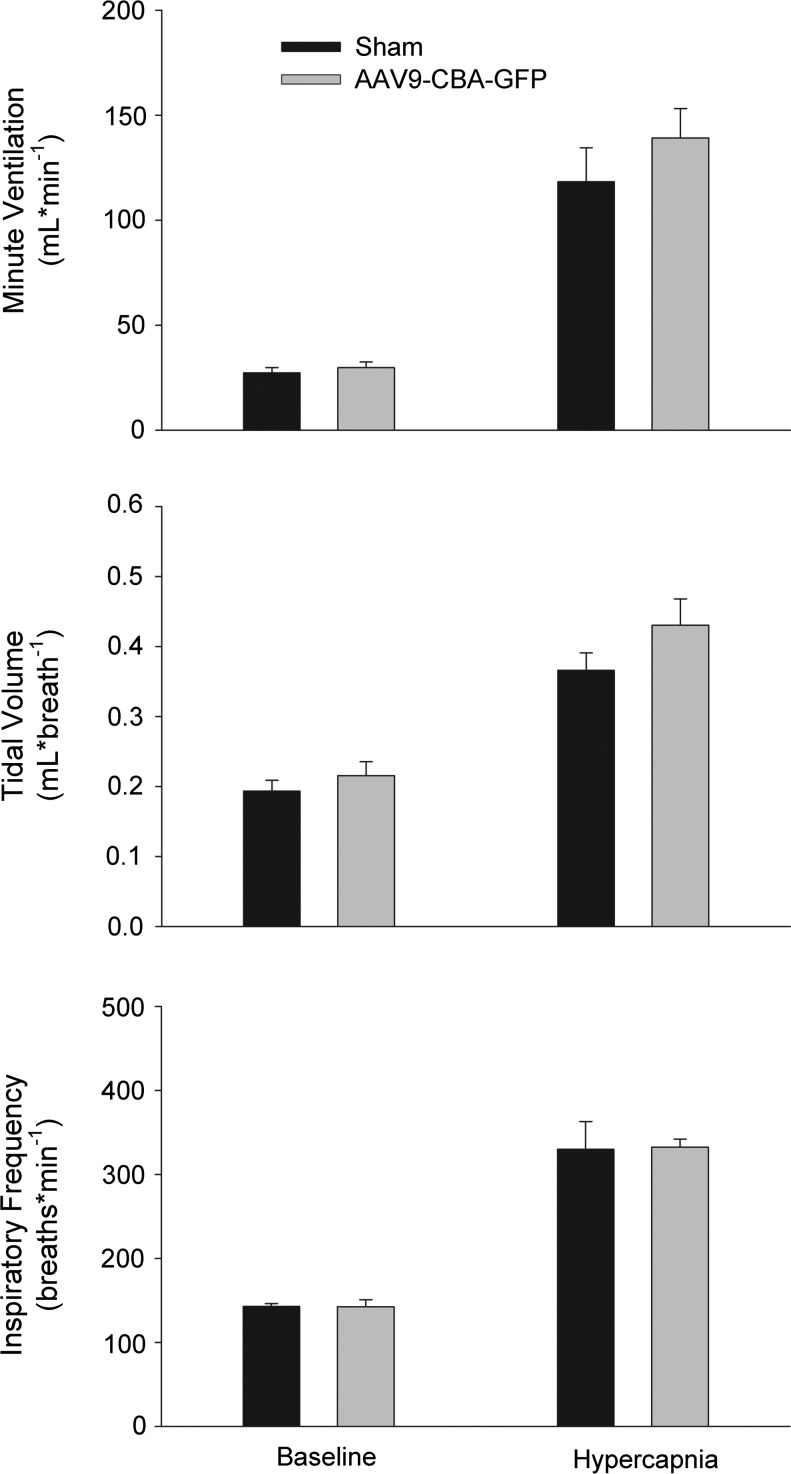
Weight gain after AAV9-CBA-GFP injection was not different compared with sham injection (p=0.66). Whole-body plethysmography (unanesthetized mice) was used to confirm that viral infection and GFP protein expression in tongue myofibers and XII motoneurons had minimal impact on the respiratory control system. At 8 weeks postinjection, respiratory frequency (breaths·min–1, p=0.93), tidal volume (ml·breath–1; p=0.28) and minute ventilation (ml·min–1; p=0.37) were similar between AAV9-CBA-GFP-injected and sham-injected mice (Fig. 4).
FIG. 4.
Ventilation is not altered after AAV9-CBA-GFP tongue injection. Minute ventilation (top), tidal volume (middle), and inspiratory frequency (bottom) were measured 8 weeks after sham or AAV9-CBA-GFP tongue injection in unanesthetized mice, using whole-body plethysmography. The pattern of breathing was similar at baseline (21% O2) and during a hypercapnic respiratory challenge (7% inspired CO2) between the two groups of mice.
Discussion
Our most significant finding is that an AAV9 vector can effectively transduce a significant portion of the XII motor nucleus after direct intramuscular tongue injection. In this regard, the retrograde transport capability of AAV9 is significantly higher than that of previously characterized AAV serotypes. In addition, AAV9 was equally effective at motoneuron transduction after tongue injection in a mouse model of Pompe disease (Raben et al., 1998). Pompe mice show both motoneuron (DeRuisseau et al., 2009; Lee et al., 2011) and muscular pathology (Mah et al., 2007, 2010), but this did not impair the retrograde transport ability of AAV9. The vector is therefore a good candidate for targeted gene delivery to motoneurons in animal models associated with neuromuscular pathology and/or degeneration such as amyotrophic lateral sclerosis (ALS), spinal cord injury, and Pompe disease.
Commentary on AAV and retrograde transport
AAV2 was the first AAV serotype proposed for extensive use in gene therapy (Hermonat and Muzyczka, 1984; Berns and Giraud, 1996; Flotte et al., 1996, 2003; Rubenstein et al., 1997; Kay et al., 2000; Mueller and Flotte, 2008). Since then, a variety of different AAV serotypes have been described and evaluated (Rutledge et al., 1998; Zolotukhin et al., 2002; Gao et al., 2005; Zincarelli et al., 2008; Mah et al., 2010). Tissue tropism varies across the serotypes (Li et al., 2008; Manfredsson et al., 2009), and this can be exploited for gene therapy applications.
Isolated originally by J. Wilson and colleagues (Gao et al., 2004; Cearley et al., 2008), AAV9 can cross the blood–brain barrier (Manfredsson et al., 2009; Forsayeth and Bankiewicz, 2011) and scAAV9 efficiently transduces neuronal and glial cells after intravenous (Gray et al., 2011) or intrathecal delivery (Federici et al., 2011). However, ssAAV9 does not have a high level of transduction efficiency with intravenous delivery (Gray et al., 2011). In addition, a relatively high dose of AAV9 is required for motoneuron transduction after systemic intravenous delivery in mice (∼1×1013 VG/kg), and this could be an obstacle in translation to clinical use. Overall, an emerging body of evidence supports the use of the AAV9 serotype as perhaps the most ideal vector for gene delivery to the CNS (Foust et al., 2009; Samaranch et al., 2012). Current data indicate that ssAAV9, which has a larger transgene capacity compared with scAAV9, is also an appropriate vector for retrogradely targeting gene delivery to a specific motor pool. Compared with a standard retrograde tracer (CT-β) capable of robust motoneuron infection (Lane et al., 2008; Qiu et al., 2010), we observed that retrograde transport of AAV9 was approximately one-third as efficient. Thus, our AAV9 approach was able to transduce approximately 25% of the XII motor nucleus. This degree of retrograde motoneuron transduction exceeds the relative transduction previously reported for other AAV serotypes, which has ranged from approximately 1 to 15% (Kaspar et al., 2003; Pirozzi et al., 2006; Hollis et al., 2008; Towne et al., 2010).
Pompe disease and AAV9
Pompe disease is an autosomal recessive metabolic myopathy caused by deficiency of the lysosomal enzyme acid α-glucosidase (GAA). This deficiency results in cellular lysosomal and cytoplasmic glycogen accumulation (Byrne et al., 2011a). Myopathy is a hallmark of this disease, but a growing database indicates that motor failure in Pompe disease also involves CNS pathology (Hogan et al., 1969; Martin et al., 1973; Matsui et al., 1983; Teng et al., 2004; Sidman et al., 2008; DeRuisseau et al., 2009; Burrow et al., 2010; Mah et al., 2010). However, the current clinical strategy of intravenous infusion of recombinant GAA (Beck, 2009; Byrne et al., 2011b) will not mitigate CNS glycogen accumulation (DeRuisseau et al., 2009; Lee et al., 2011; Qiu et al., 2012). Experimental observations from both humans (Hogan et al., 1969; DeRuisseau et al., 2009) and animal models (Lee et al., 2011; Qiu et al., 2012) are consistent with the concept that therapy targeting both skeletal muscle and the CNS may be required to fully correct motor deficits in Pompe. A phase 1/2 study administering rAAV2/1-CMV-hGAA to the diaphragm in children with Pompe disease is ongoing (ClinicalTrials.gov identifier, NCT00976352). On the basis of the retrograde transport described here, we suggest that the AAV9 serotype may be a prime candidate for use in future clinical studies to increase retrograde transduction of motor neurons. Tongue injection could be considered in the patient population because there are clinical reports of impaired upper airway function including swallow dysfunction (Jones et al., 2010), speech problems (Muller, 2009), feeding difficulties (Byrne et al., 2011b), and obstructive sleep apnea (Margolis et al., 1994).
Acknowledgments
The authors thank Mr. Roland Federico for assistance with immunohistochemistry experiments. This work was supported by National Institutes of Health grants 1R01HD052682-01A1 (D.D.F.), HL59412 (B.J.B.), 5F32HL095282-02 (D.J.F.), and NIDDK P01 DK58327-03 (B.J.B.). Additional support was provided by an NIH/NCRR Clinical and Translational Science Award to the University of Florida (UL1 RR029890). We would also like to thank Kirsten E. Erger for expert technical assistance.
Author Disclosure Statement
B.J.B., D.D.F., Johns Hopkins University, and the University of Florida could be entitled to patent royalties for inventions described in this paper.
References
- Beck M. Alglucosidase alfa: Long term use in the treatment of patients with Pompe disease. Ther. Clin. Risk Manag. 2009;5:767–772. doi: 10.2147/tcrm.s5776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berns K.I. Giraud C. Biology of adeno-associated virus. Curr. Top. Microbiol. Immunol. 1996;218:1–23. doi: 10.1007/978-3-642-80207-2_1. [DOI] [PubMed] [Google Scholar]
- Burrow T.A. Bailey L.A. Kinnett D.G. Hopkin R.J. Acute progression of neuromuscular findings in infantile Pompe disease. Pediatr. Neurol. 2010;42:455–458. doi: 10.1016/j.pediatrneurol.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Byrne B.J. Falk D.J. Pacak C.A., et al. Pompe disease gene therapy. Hum. Mol. Genet. 2011a;20:R61–R68. doi: 10.1093/hmg/ddr174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne B.J. Kishnani P.S. Case L.E., et al. Pompe disease: Design, methodology, and early findings from the Pompe Registry. Mol. Genet. Metab. 2011b;103:1–11. doi: 10.1016/j.ymgme.2011.02.004. [DOI] [PubMed] [Google Scholar]
- Cearley C.N. Vandenberghe L.H. Parente M.K., et al. Expanded repertoire of AAV vector serotypes mediate unique patterns of transduction in mouse brain. Mol. Ther. 2008;16:1710–1718. doi: 10.1038/mt.2008.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeRuisseau L.R. Fuller D.D. Qiu K., et al. Neural deficits contribute to respiratory insufficiency in Pompe disease. Proc. Natl. Acad. Sci. U.S.A. 2009;106:9419–9424. doi: 10.1073/pnas.0902534106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drorbaugh J.E. Fenn W.O. A barometric method for measuring ventilation in newborn infants. Pediatrics. 1955;16:81–87. [PubMed] [Google Scholar]
- Duque S. Joussemet B. Riviere C., et al. Intravenous administration of self-complementary AAV9 enables transgene delivery to adult motor neurons. Mol. Ther. 2009;17:1187–1196. doi: 10.1038/mt.2009.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federici T. Taub J.S. Baum G.R., et al. Robust spinal motor neuron transduction following intrathecal delivery of AAV9 in pigs. Gene Ther. 2011 doi: 10.1038/gt.2011.130. (in press). [DOI] [PubMed] [Google Scholar]
- Flotte T. Carter B. Conrad C., et al. A phase I study of an adeno-associated virus-CFTR gene vector in adult CF patients with mild lung disease. Hum. Gene Ther. 1996;7:1145–1159. doi: 10.1089/hum.1996.7.9-1145. [DOI] [PubMed] [Google Scholar]
- Flotte T.R. Trapnell B.C. Humphries M., et al. Phase 2 clinical trial of a recombinant adeno-associated viral vector expressing α1-antitrypsin: Interim results. Hum. Gene Ther. 2011;22:1239–1247. doi: 10.1089/hum.2011.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flotte T.R. Zeitlin P.L. Reynolds T.C., et al. Phase I trial of intranasal and endobronchial administration of a recombinant adeno-associated virus serotype 2 (rAAV2)-CFTR vector in adult cystic fibrosis patients: A two-part clinical study. Hum. Gene Ther. 2003;14:1079–1088. doi: 10.1089/104303403322124792. [DOI] [PubMed] [Google Scholar]
- Forsayeth J.R. Bankiewicz K.S. AAV9: Over the fence and into the woods. Mol. Ther. 2011;19:1006–1007. doi: 10.1038/mt.2011.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foust K.D. Nurre E. Montgomery C.L., et al. Intravascular AAV9 preferentially targets neonatal neurons and adult astrocytes. Nat. Biotechnol. 2009;27:59–65. doi: 10.1038/nbt.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao G. Vandenberghe L.H. Alvira M.R., et al. Clades of adeno-associated viruses are widely disseminated in human tissues. J. Virol. 2004;78:6381–6388. doi: 10.1128/JVI.78.12.6381-6388.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao G. Vandenberghe L.H. Wilson J.M. New recombinant serotypes of AAV vectors. Curr. Gene Ther. 2005;5:285–297. doi: 10.2174/1566523054065057. [DOI] [PubMed] [Google Scholar]
- Gray S.J. Matagne V. Bachaboina L., et al. Preclinical differences of intravascular AAV9 delivery to neurons and glia: A comparative study of adult mice and nonhuman primates. Mol. Ther. 2011;19:1058–1069. doi: 10.1038/mt.2011.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillery R.W. On counting and counting errors. J. Comp. Neurol. 2002;447:1–7. doi: 10.1002/cne.10221. [DOI] [PubMed] [Google Scholar]
- Guo Y. Goldberg S.J. McClung J.R. Compartmental organization of styloglossus and hyoglossus motoneurons in the hypoglossal nucleus of the rat. Brain Res. 1996;728:277–280. doi: 10.1016/0006-8993(96)00551-3. [DOI] [PubMed] [Google Scholar]
- Hermonat P.L. Muzyczka N. Use of adeno-associated virus as a mammalian DNA cloning vector: Transduction of neomycin resistance into mammalian tissue culture cells. Proc. Natl. Acad. Sci. U.S.A. 1984;81:6466–6470. doi: 10.1073/pnas.81.20.6466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan G.R. Gutmann L. Schmidt R. Gilbert E. Pompe's disease. Neurology. 1969;19:894–900. doi: 10.1212/wnl.19.9.894. [DOI] [PubMed] [Google Scholar]
- Hollis E.R., II Kadoya K. Hirsch M., et al. Efficient retrograde neuronal transduction utilizing self-complementary AAV1. Mol. Ther. 2008;16:296–301. doi: 10.1038/sj.mt.6300367. [DOI] [PubMed] [Google Scholar]
- Horner R.L. Emerging principles and neural substrates underlying tonic sleep-state-dependent influences on respiratory motor activity. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009;364:2553–2564. doi: 10.1098/rstb.2009.0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones H.N. Muller C.W. Lin M., et al. Oropharyngeal dysphagia in infants and children with infantile Pompe disease. Dysphagia. 2010;25:277–283. doi: 10.1007/s00455-009-9252-x. [DOI] [PubMed] [Google Scholar]
- Kaspar B.K. Lladó J. Sherkat N., et al. Retrograde viral delivery of IGF-1 prolongs survival in a mouse ALS model. Science. 2003;301:839–842. doi: 10.1126/science.1086137. [DOI] [PubMed] [Google Scholar]
- Kay M.A. Manno C.S. Ragni M.V., et al. Evidence for gene transfer and expression of factor IX in haemophilia B patients treated with an AAV vector. Nat. Genet. 2000;24:257–261. doi: 10.1038/73464. [DOI] [PubMed] [Google Scholar]
- Lane M.A. White T.E. Coutts M.A., et al. Cervical prephrenic interneurons in the normal and lesioned spinal cord of the adult rat. J. Comp. Neurol. 2008;511:692–709. doi: 10.1002/cne.21864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K.Z. Qiu K. Sandhu M.S., et al. Hypoglossal neuropathology and respiratory activity in Pompe mice. Front. Physiol. 2011;2:31. doi: 10.3389/fphys.2011.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W. Asokan A. Wu Z., et al. Engineering and selection of shuffled AAV genomes: A new strategy for producing targeted biological nanoparticles. Mol. Ther. 2008;16:1252–1260. doi: 10.1038/mt.2008.100. [DOI] [PubMed] [Google Scholar]
- Mah C. Cresawn K.O. Fraites T.J., Jr., et al. Sustained correction of glycogen storage disease type II using adeno-associated virus serotype 1 vectors. Gene Ther. 2005;12:1405–1409. doi: 10.1038/sj.gt.3302550. [DOI] [PubMed] [Google Scholar]
- Mah C. Pacak C.A. Cresawn K.O., et al. Physiological correction of Pompe disease by systemic delivery of adeno-associated virus serotype 1 vectors. Mol. Ther. 2007;15:501–507. doi: 10.1038/sj.mt.6300100. [DOI] [PubMed] [Google Scholar]
- Mah C.S. Falk D.J. Germain S.A., et al. Gel-mediated delivery of AAV1 vectors corrects ventilatory function in Pompe mice with established disease. Mol. Ther. 2010;18:502–510. doi: 10.1038/mt.2009.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manfredsson F.P. Rising A.C. Mandel R.J. AAV9: A potential blood–brain barrier buster. Mol. Ther. 2009;17:403–405. doi: 10.1038/mt.2009.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis M.L. Howlett P. Goldberg R., et al. Obstructive sleep apnea syndrome in acid maltase deficiency. Chest. 1994;105:947–949. doi: 10.1378/chest.105.3.947. [DOI] [PubMed] [Google Scholar]
- Martin J.J. de Barsy T. van Hoof F. Palladini G. Pompe's disease: An inborn lysosomal disorder with storage of glycogen. A study of brain and striated muscle. Acta Neuropathol. 1973;23:229–244. doi: 10.1007/BF00687878. [DOI] [PubMed] [Google Scholar]
- Matsui T. Kuroda S. Mizutani M., et al. Generalized glycogen storage disease in Japanese quail (Coturnix coturnix japonica) Vet. Pathol. 1983;20:312–321. doi: 10.1177/030098588302000307. [DOI] [PubMed] [Google Scholar]
- McCarty D.M. Monahan P.E. Samulski R.J. Self-complementary recombinant adeno-associated virus (scAAV) vectors promote efficient transduction independently of DNA synthesis. Gene Ther. 2001;8:1248–1254. doi: 10.1038/sj.gt.3301514. [DOI] [PubMed] [Google Scholar]
- Millecamps S. Nicolle D. Ceballos-Picot I., et al. Synaptic sprouting increases the uptake capacities of motoneurons in amyotrophic lateral sclerosis mice. Proc. Natl. Acad. Sci. U.S.A. 2001;98:7582–7587. doi: 10.1073/pnas.131031098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller C. Flotte T.R. Clinical gene therapy using recombinant adeno-associated virus vectors. Gene Ther. 2008;15:858–863. doi: 10.1038/gt.2008.68. [DOI] [PubMed] [Google Scholar]
- Muller C.W. Jones H.N. O'Grady G., et al. Language and speech function in children with infantile Pompe disease. J. Pediatr. Neurol. 2009;7:147–156. [Google Scholar]
- Pirozzi M. Quattrini A. Andolfi G., et al. Intramuscular viral delivery of paraplegin rescues peripheral axonopathy in a model of hereditary spastic paraplegia. J. Clin. Invest. 2006;116:202–208. doi: 10.1172/JCI26210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu K. Lane M.A. Lee K.Z., et al. The phrenic motor nucleus in the adult mouse. Exp. Neurol. 2010;226:254–258. doi: 10.1016/j.expneurol.2010.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu K. Falk D.J. Reier P.J., et al. Spinal delivery of AAV vector restores enzyme activity and increases ventilation in Pompe mice. Mol. Ther. 2012;20:21–27. doi: 10.1038/mt.2011.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raben N. Nagaraju K. Lee E., et al. Targeted disruption of the acid α-glucosidase gene in mice causes an illness with critical features of both infantile and adult human glycogen storage disease type II. J. Biol. Chem. 1998;273:19086–19092. doi: 10.1074/jbc.273.30.19086. [DOI] [PubMed] [Google Scholar]
- Recio M.J. Moreno-Pelayo M.A. Kiliç S.S., et al. Differential biological role of CD3 chains revealed by human immunodeficiencies. J. Immunol. 2007;178:2556–2564. doi: 10.4049/jimmunol.178.4.2556. [DOI] [PubMed] [Google Scholar]
- Rubenstein R.C. McVeigh U. Flotte T.R., et al. CFTR gene transduction in neonatal rabbits using an adeno-associated virus (AAV) vector. Gene Ther. 1997;4:384–392. doi: 10.1038/sj.gt.3300417. [DOI] [PubMed] [Google Scholar]
- Rutledge E.A. Halbert C.L. Russell D.W. Infectious clones and vectors derived from adeno-associated virus (AAV) serotypes other than AAV type 2. J. Virol. 1998;72:309–319. doi: 10.1128/jvi.72.1.309-319.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaranch L. Salegio E.A. San Sebastian W., et al. AAV9 Transduction in the central nervous system of non-human primates. Hum. Gene Ther. 2012;23:382–389. doi: 10.1089/hum.2011.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidman R.L. Taksir T. Fidler J., et al. Temporal neuropathologic and behavioral phenotype of 6neo/6neo Pompe disease mice. J. Neuropathol. Exp. Neurol. 2008;67:803–818. doi: 10.1097/NEN.0b013e3181815994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J.C. McClung J.R. Goldberg S.J. Postnatal development of hypoglossal motoneurons that innervate the hyoglossus and styloglossus muscles in rat. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 2005;285:628–633. doi: 10.1002/ar.a.20204. [DOI] [PubMed] [Google Scholar]
- Snyder B.R. Gray S.J. Quach E.T., et al. Comparison of adeno-associated viral vector serotypes for spinal cord and motor neuron gene delivery. Hum. Gene Ther. 2011;22:1129–1135. doi: 10.1089/hum.2011.008. [DOI] [PubMed] [Google Scholar]
- Sturrock R.R. Stability of motor neuron and interneuron number in the hypoglossal nucleus of the ageing mouse brain. Anat. Anz. 1991;173:113–116. [PubMed] [Google Scholar]
- Teng Y.T. Su W.J. Hou J.W. Huang S.F. Infantile-onset glycogen storage disease type II (Pompe disease): Report of a case with genetic diagnosis and pathological findings. Chang Gung Med. J. 2004;27:379–384. [PubMed] [Google Scholar]
- Towne C. Schneider B.L. Kieran D., et al. Efficient transduction of non-human primate motor neurons after intramuscular delivery of recombinant AAV serotype 6. Gene Ther. 2010;17:141–146. doi: 10.1038/gt.2009.119. [DOI] [PubMed] [Google Scholar]
- Yates B.J. Smail J.A. Stocker S.D. Card J.P. Transneuronal tracing of neural pathways controlling activity of diaphragm motoneurons in the ferret. Neuroscience. 1999;90:1501–1513. doi: 10.1016/s0306-4522(98)00554-5. [DOI] [PubMed] [Google Scholar]
- Zheng H. Qiao C. Wang C.H., et al. Efficient retrograde transport of adeno-associated virus type 8 to spinal cord and dorsal root ganglion after vector delivery in muscle. Hum. Gene Ther. 2010;21:87–97. doi: 10.1089/hum.2009.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zincarelli C. Soltys S. Rengo G. Rabinowitz J.E. Analysis of AAV serotypes 1–9 mediated gene expression and tropism in mice after systemic injection. Mol. Ther. 2008;16:1073–1080. doi: 10.1038/mt.2008.76. [DOI] [PubMed] [Google Scholar]
- Zolotukhin S. Potter M. Zolotukhin I., et al. Production and purification of serotype 1, 2, and 5 recombinant adeno-associated viral vectors. Methods. 2002;28:158–167. doi: 10.1016/s1046-2023(02)00220-7. [DOI] [PubMed] [Google Scholar]