Abstract
Use of perfluorochemical liquids during intratracheal vector administration enhances recombinant adenovirus and adeno-associated virus (AAV)-mediated lung epithelial gene expression. We hypothesized that inhalation of nebulized perfluorochemical vapor would also enhance epithelial gene expression after subsequent intratracheal vector administration. Freely breathing adult C57BL/6 mice were exposed for selected times to nebulized perflubron or sterile saline in a sealed Plexiglas chamber. Recombinant adenoviral vector was administered by transtracheal puncture at selected times afterward and mice were killed 3 days after vector administration to assess transgene expression. Mice tolerated the nebulized perflubron without obvious ill effects. Vector administration 6 hr after nebulized perflubron exposure resulted in an average 540% increase in gene expression in airway and alveolar epithelium, compared with that with vector alone or saline plus vector control (p<0.05). However, vector administration 1 hr, 1 day, or 3 days after perflubron exposure was not different from either nebulized saline with vector or vector alone and a 60-min exposure to nebulized perflubron is required. In parallel pilot studies in macaques, inhalation of nebulized perflubron enhanced recombinant AAV2/5 vector expression throughout the lung. Serial chest radiographs, bronchoalveolar lavages, and results of complete blood counts and serum biochemistries demonstrated no obvious adverse effects of nebulized perflubron. Further, one macaque receiving nebulized perflubron only was monitored for 1 year with no obvious adverse effects of exposure. These results demonstrate that inhalation of nebulized perflubron, a simple, clinically more feasible technique than intratracheal administration of liquid perflubron, safely enhances lung gene expression.
Beckett and colleagues show that inhalation of a nebulized perfluorochemical (perflubron) vapor 6 hours before transtracheal administration of recombinant adenoviral vector results in a substantial increase in gene expression in mouse airway and alveolar epithelium. Pilot studies in macaques performed in parallel show that nebulized perflubron enhances recombinant adeno-associated vector serotype 5 expression throughout the lung, with no obvious adverse effects.
Introduction
One of the obstacles to successful gene therapy for lung diseases is effective airway-based delivery of vectors to airway and alveolar epithelium. The lung has formidable physical and immunological barriers to prevent infection of respiratory epithelium by viruses and to inactivate and remove other foreign particles in the airways (Bromberg et al., 1998; Look and Brody, 1999; Sueblinvong et al., 2007). Moreover, physical and immunological barriers may be increased in injured or diseased lung, the ostensible target for therapeutic gene transfer (Pilewski, 2002; Weiss, 2002b; Weiss and Pilewski, 2003; Sueblinvong et al., 2007). Various adjunct delivery techniques have been investigated to help overcome some of these barriers (Weiss, 2002b; Weiss and Pilewski, 2003; Sueblinvong et al., 2007). However, these approaches generally involve invasive measures or use substances that may have deleterious effects in lung.
Perfluorochemical (PFC) liquids are inert, nontoxic liquids that had been extensively investigated for use in preclinical models of lung injury and in clinical application of partial liquid ventilation (PLV) for diseased lungs (Leach et al., 1996; Hirschl et al., 2002; Kacmarek et al., 2006). Although liquid ventilation has generally fallen from clinical use, administration of perflubron (LiquiVent; Alliance Pharmaceutical, San Diego, CA) and other PFC liquids in PLV was safe and could improve oxygenation and lung mechanics while also decreasing histopathological injury and inflammation in injured lungs (Hirschl et al., 1995, 2002; Leach et al., 1996; Rotta et al., 1999; Kacmarek et al., 2006). More recently it has been found that inhalation of nebulized perflubron and other PFC compounds comparably enhances oxygenation and improves lung mechanics in preclinical models of lung injury (Bleyl et al., 1999; Kandler et al., 2001, 2004; Ragaller et al., 2001; von der Hardt et al., 2004; Gama de Abreu et al., 2005; Meinhardt et al., 2005). The mechanism of nebulized PFC for these effects is unclear. However, inhalation of nebulized PFC is safe and suggests a more clinically feasible and appealing therapeutic approach than instillation of liquid PFC into the airways.
We and others had previously investigated the use of perflubron and other PFC liquids as vehicles for enhancing vector delivery to the lung airways. Instillation of PFC liquids during intratracheal administration of recombinant viral vectors enhances airway and alveolar epithelial gene expression in normal rodent and primate lungs as well as in injured rodent lungs (Lisby et al., 1997; Weiss et al., 1999a,b, 2000, 2001, 2002a,b). One mechanism of PFC liquid action is to propel vectors more effectively into the lung (Weiss et al., 1999a,b). However, administration of PFC liquid before vector administration also enhances lung gene expression (Weiss et al., 2003). This finding suggests that other actions of PFCs are important for enhancing gene expression in respiratory epithelium. For example, intratracheal administration of PFC liquid results in the transient opening of tight junctions between airway epithelial cells, allowing improved access of vectors to binding sites on basolateral membranes (Weiss et al., 2003). In addition, PFC liquids may further enhance lung gene expression by other, as yet unknown mechanisms.
We therefore reasoned that if the actions of PFCs are not solely dependent on propulsion of vector into the lung airways, inhalation of nebulized PFC would also enhance gene expression in lung epithelium. This hypothesis was tested in normal mice and in normal macaques receiving intratracheal administration of recombinant viral vectors after exposure to nebulized perflubron.
Materials and Methods
Animals
All in vivo studies were subject to approval by the Institutional Animal Care and Use Committees of the University of Vermont (UVM, Burlington, VT) and the Tulane National Primate Research Center (TNPRC, Covington, LA) and conformed to national and institutional IACUC and AAALAC (Association for Assessment and Accreditation of Laboratory Animal Care International) guidelines. Adult male C57BL/6 mice (age, 8–12 weeks) were obtained from Jackson Laboratory (Bar Harbor, ME). Adult pigtail macaques (Macaca nemestrina, 3–5 kg) were maintained according to standard practice at the TNPRC.
Vectors
Recombinant type 2 E1a/E1b-deleted replication-deficient human adenovirus carrying bacterial (Escherichia coli) lacZ driven by the cytomegalovirus (CMV) reporter (Ad2/CMVβgal-4; generously donated by J. St. George, Genzyme, Framingham, MA) has been previously characterized (Armentano et al., 1997) and used in previous studies of gene delivery to rodent lungs (Weiss et al., 1999a,b). Aliquots of vector were stored at −70°C and used after thawing without further modification at the desired dilution. The vector is notated as AdlacZ for this paper. Recombinant pseudotyped AAV2/5 vector encoding human placental alkaline phosphatase, generously provided by G.-P. Gao (University of Pennsylvania, Philadelphia, PA), was stored at −70°C and used after thawing without further modification at the desired dilution.
Administration of nebulized perfluorochemical
Mice
Clinical-grade perflubron (LiquiVent; Alliance Pharmaceutical) was nebulized with a customized nebulizer (Ernest Shott, Alliance Pharmaceuticals). In brief, the liquid was heated to 82°C in the stainless steel nebulizer chamber and nebulized by running compressed oxygen at 8–10 liters/min through the heated liquid. This generated a fog that was passed through inert plastic tubing (Nalgene; inner diameter, 0.5 cm) to a closed Plexiglas box (Fig. 1A). The length of tubing used (42.5 in.) allowed for heat loss, so that the aerosol was approximately 37°C when it reached the Plexiglas box. The dense fog of nebulized perflubron was easily visible in the tubing and the Plexiglas box (Fig. 1A). Mice were allowed to move freely in the Plexiglas box during exposure to nebulized perflubron. The box dimensions (17×9×7.5 in.) allowed for simultaneous exposure of up to 20 mice. The level of fog in the Plexiglas box completely enveloped the mice and provided for continuous inhalational exposure. As the nebulized perflubron condensed when its temperature fell below approximately 30°C, a raised metal grate was placed in the bottom of the box to prevent direct contact of mice with the condensed liquid. Mice were exposed to nebulized perflubron for periods of up to 60 min. Control animals were similarly exposed to sterile saline comparably heated and nebulized.
FIG. 1.
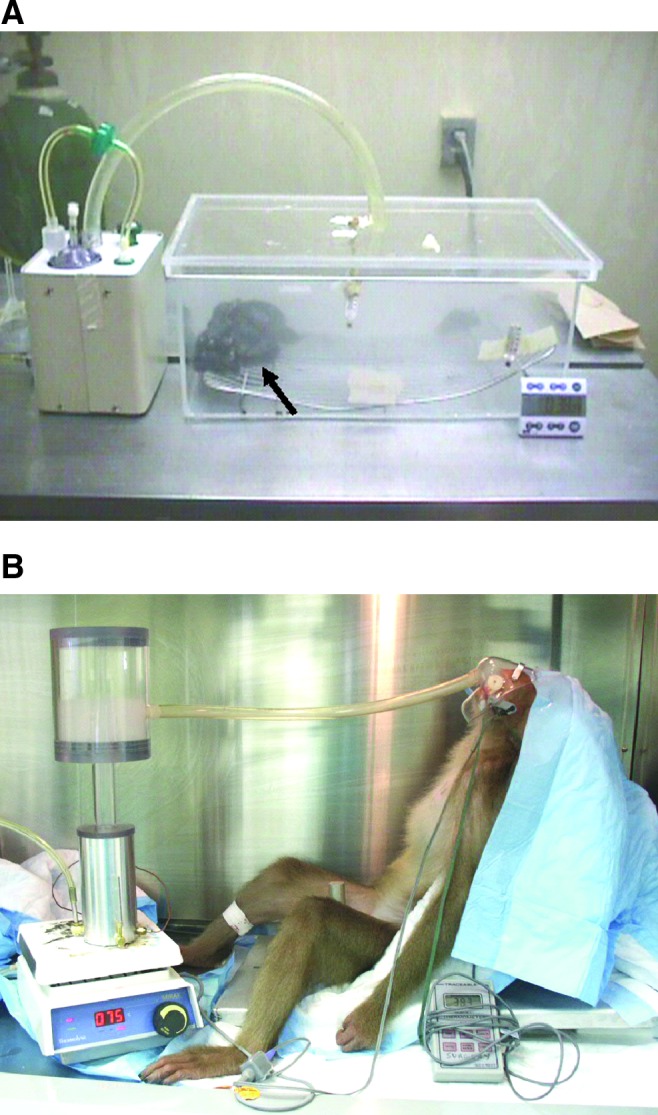
Systems for administration of nebulized perflubron to (A) mice and (B) anesthetized nonhuman primates (pigtail macaques). Perflubron was heated to 82°C and nebulized by blowing oxygen through at 8–10 liters/min. The resulting nebulized material flowed through inert plastic tubing and is visible as a dense fog inside (A) the enclosed Plexiglas box and (B) the nebulization chamber. A metal grate was placed in the chamber to prevent direct contact of the mice with condensed perflubron that pooled on the chamber floor (A). Mice are visible enveloped in the fog, indicated by the arrow in (A). Color images available online at www.liebertpub.com/hgtb
Macaques
Perflubron was similarly heated in a nebulization chamber that resulted in a dense fog produced in an attached clear Plexiglas chamber (chamber courtesy of Ernest Shott, Alliance Pharmaceuticals) (Fig. 1B). Nalgene tubing leading out from the chamber was attached to a facemask, allowing the macaque to directly inhale the nebulized perflubron. The length of tubing used (42.5 in.) allowed for heat loss, so that the aerosol was approximately 37°C when it reached the facemask. The macaques were anesthetized (tiletamine–zolazepam, 3–7 ml/kg) but spontaneously breathing for the duration of the 1-hr inhalation period. Heart rate, blood pressure, respiratory rate, and oxygen saturation were monitored throughout the inhalation period and until the animals recovered from anesthesia. Two macaques were exposed to nebulized perflubron by this approach.
Intratracheal administration of recombinant vector and of liquid perflubron
Mice
Direct intratracheal administration of recombinant viral vectors by transtracheal puncture in mice is a well-established and reliable technique in our laboratory (Weiss et al., 1999a,b, 2000, 2001, 2002a,b, 2003). In brief, mice were anesthetized by an intraperitoneal injection of Avertin (tribromoethanol and tertiary amyl alcohol, 1 mg/kg). With the animal in the supine position, a midline vertical incision was made in the neck and the trachea was exposed by blunt dissection of the underlying connective tissue and musculature. The trachea was punctured just below the larynx with a 28.5-gauge insulin syringe (Becton Dickinson, Franklin Lakes, NJ) and 50 μl of recombinant adenoviral vector solution (5×1010 viral particles/mouse, in sterile phosphate-buffered saline [PBS]) was instilled over approximately 1–2 min. Vector administration was immediately followed by administration of liquid perflubron (10 cm3/kg) slowly administered over the next approximately 5 min. The neck incision was sutured with 6.0 silk and the animal was allowed to recover from anesthesia.
Macaques
Bronchoscopic administration of recombinant viral vectors has been previously described (Weiss et al., 2002b). In brief, macaques were anesthetized with tiletamine–zolazepam and a pediatric bronchoscope (Olympus, Tokyo, Japan) was passed through the mouth and into the trachea. In one of the two macaques exposed to nebulized perflubron, bronchoscopy was performed 6 hr after exposure and a microsprayer (PennCentury, Wyndmoor, PA) was inserted through the bronchoscope and positioned in the lower trachea, approximately 1–2 cm above the main carina (Harvey et al., 1999). Using the microsprayer, 2.24×1013 DNase-resistant AAV2/5 particles were instilled. A second, unexposed naive macaque received similar instillation through the microsprayer. A third macaque received 1.12×1013 DNase-resistant AAV2/5 particles directly instilled into the left caudal (lower) lobe through the bronchoscope. A similar amount of virus, followed immediately by 10 ml of liquid perflubron, was instilled through the bronchoscope into the right caudal (lower) lobe of this same animal. All the macaques studied underwent serial thoracic radiography, bronchoalveolar lavage, and blood collection for analyses of complete blood counts and serum chemistries.
Computerized tomographic scanning of mice
Immediately after exposure to nebulized perflubron for 1 hr or intratracheal instillation of liquid perflubron, mice were killed by intraperitoneal injection of sodium pentobarbital. The trachea was exposed by dissection and tied off with suture ligature. The mice were then scanned for 80 min (80 kVp [kilovoltage peak], 450 mA·sec) in an eXplore RS80 MicroCT scanner (GE Healthcare Medical Systems, Piscataway, NJ). Three-dimensional data sets were reconstructed at a pixel resolution of 47 μm and visualized with MicroView software (GE Healthcare Medical Systems).
Bronchoalveolar lavage and lung harvests
Mice
Animals were killed 3 days after instillation of AdlacZ by lethal overdose of pentobarbital (intraperitoneal injection of 150 mg/kg body weight). The chest was opened in situ and the trachea was cannulated with a small-gauge butterfly syringe (23 gauge). The lungs were lavaged three times with 1 ml of 0.6 mM EDTA in PBS (Weiss et al., 1999a,b, 2001, 2002a). The recovered bronchoalveolar lavage (BAL) fluid was stored on ice pending further analyses. The heart–lung block was then removed from the chest. The left lung was tied off with suture, removed, and immediately frozen in a polypropylene cryotube by immersion in liquid nitrogen. The remaining trachea and right lung were fixed at room temperature by infusing PBS–1% glutaraldehyde (pH 7.3) through a 19-gauge needle into the trachea at 30 cmH2O to maintain inflation. The lung was also immersed in the same buffered fixative for a total fixation time of 1 hr.
Macaques
Each anesthetized animal, sitting in an upright position in a chair designed for BAL collection, underwent BAL of the right middle lobe (RML) 2, 14, 28, and 56 days after instillation and fluid was assessed for cell counts, differentials, and total protein. Animals that received vector were killed 56 days after the final lavage by lethal overdose of pentobarbital (100 mg/kg, intravenous). After euthanasia, the chest was opened and the heart–lung block was removed. The trachea was cannulated with a 60-cm3 syringe and a 1:1 mixture of sterile PBS and Tissue-Tek OCT (Sakura Finetek USA, Torrance, CA) was instilled under 30 cmH2O pressure. The heart–lung blocks were simultaneously immersed in the PBS–OCT mixture and placed on ice until the OCT had hardened. Each lung lobe and the trachea were then cut into sequential pieces for subsequent analyses and stored at −70°C. The macaque that was exposed to nebulized perflubron only underwent serial BAL and blood draws over a 1-year period. This animal also underwent thoracoscopic lung biopsy 4 weeks after exposure. Tissue obtained from the biopsy was fixed in 10% formalin for subsequent histological analyses.
Bronchoalveolar lavage fluid analyses
Aliquots of unprocessed BAL fluid from both mice and macaques were assessed with a hemacytometer (Reichert, Buffalo, NY), using trypan blue to determine total nucleated cell count (Weiss et al., 1999a,b, 2001, 2002a,b). BAL fluid was centrifuged at 4°C (1600 rpm for 20 min), and the cell pellet was washed twice with sterile PBS and resuspended in 1 ml of PBS. Hema 3 (Thermo Fisher Scientific, Waltham, MA)-stained Cytospin preparations of resuspended cell pellets were used to assess cell differentials. Differentials were determined in blinded fashion by two investigators, and the average differential count was used. The remaining undiluted BAL fluid supernatant was assessed for total protein content (modified Bradford reaction; Bio-Rad, Hercules, CA) (Bradford, 1976).
Detection of in situ β-galactosidase expression
The fixative was removed by aspiration from the trachea and right lung lobes, the airways were rinsed with PBS, and 0.2% 5-bromo-4-chloro-indolyl-β-d-galactopyranoside (X-Gal; Sigma-Aldrich, St. Louis, MO) in 100 mM Tris (pH 8.0), containing 5 mM ferric ferrocyanide and 2 mM MgCl2, was instilled into the trachea. Lungs were also immersed in X-Gal solution and incubated overnight (18–24 hr) at 37°C (Weiss et al., 1997). X-Gal solution was then removed by aspiration, and the airways were rinsed with PBS and postfixed in 10% buffered formalin. The lung lobes and trachea were then embedded in paraffin, sectioned, mounted on glass slides, and counterstained with nuclear fast red. Lung sections were examined visually and also analyzed for total percentage of lung surface area expressing β-galactosidase (β-Gal). To accomplish this, each entire lung section was preprocessed with Photoshop (Adobe, San Jose, CA) and then examined and processed using ImageJ version 1.3.0h, an imaging program developed by the National Institutes of Health (NIH) (ImageJ program [public domain], NIH; available on the Internet at http://rsb.info.nih.gov/ij/). Photoshop was used to enhance the contrast identification of stained and nonstained lung. Using ImageJ, the image was split into its respective red, green, and blue channels. Using the red channel, ImageJ was used initially to measure the area (pixels) of the image that was considered lung. Contrast thresholding was then applied to distinguish β-Gal-positive areas from lung area. These areas were divided by the total lung area to generate a value representing the percentage of β-Gal-positive lung. At least three sections were assessed for each lung.
Detection of alkaline phosphatase activity
Quantitative alkaline phosphatase activity was assessed in macaque lung homogenates, using a standardized histochemical reaction (Tropix Phospha-Light gene reporter system; Life Technologies, Carlsbad, CA). In situ alkaline phosphatase activity was detected in mounted cryosections of macaque lungs incubated overnight with 2-dicyclohexylphosphino-2′,4′,6′-triisopropylbiphenyl (XPhos) and nitroblue tetrazolium, using standard techniques (Weiss et al., 2000).
Effect of nebulized perflubron on tight junctions between airway epithelial cells
Mice were exposed to nebulized perflubron for 1 hr. At specified times afterward (immediately, 1 hr, 6 hr, 1 day, and 3 days, n=3–5 for each experimental condition) mice were killed by lethal overdose of pentobarbital. Lungs were removed by dissection and fixed at room temperature by infusing 2.5% glutaraldehyde–4% lanthanum hydroxide–0.1 M sodium cacodylate buffer (pH 7.4) through a butterfly syringe (23 gauge) into the trachea at 30 cmH2O to maintain inflation (Johnson et al., 1998; Weiss et al., 2003). Lungs were also immersed in the same buffered fixative for a total fixation time of 1 hr. The lungs were then rinsed for 16 hr in 4% lanthanum hydroxide–0.1 M sodium cacodylate buffer (pH 7.4) and subsequently fixed for 1 hr in 4% lanthanum hydroxide–2% osmium tetroxide in 0.1 M sodium cacodylate buffer. After dehydration in a graded series of ethanol and propylene oxide, lungs were mounted, sectioned (70- to 90-nm sections stained with saturated aqueous uranyl acetate and lead tartrate), and evaluated by electron microscopy with a JEM 1010 electron microscope (JEOL, Tokyo, Japan) at 80 K. Six to 8 sections with a minimum of 100–150 intercellular junctions between large airway epithelial cells and between alveolar epithelial cells were evaluated for the presence of lanthanum staining in each lung (Johnson et al., 1998; Weiss et al., 2003).
Statistical analyses
Analysis of variance (ANOVA) was used to determine differences among groups. Controls (naive, adenovirus only, and saline nebulization only) were analyzed by one-way ANOVA. Differences between saline-treated and perflubron-treated animals administered vector at various time points after nebulization were analyzed by two-way ANOVA (drug vs. time) to determine differences between saline- and perflubron-treated animals (Drug) and differences relative to timing of vector administration (Time). If a significant group effect was found, the Fisher least significant difference (LSD) test or Tamhane T2 post hoc test was used to identify differences among groups. In addition, analysis of percent positive β-Gal-expressing area included nonparametric (Mann–Whitney) tests as an adjunct analysis to confirm significance between groups where a strong trend (p<0.08) with unequal variance between groups was observed. Statistics were performed with SPSS software (SPSS, Chicago, IL) in consultation with the UVM Statistical Consulting Clinic. Differences between mean values were considered statistically significant at p<0.05 (Zar, 1974).
Results
Systems for administration of nebulized perflubron
Figure 1 depicts the apparatuses for nebulizing and administering perflubron or saline to mice (Fig 1A) and to macaques (Fig 1B). The fog generated by the nebulizer completely enveloped the mice and provided for continuous inhalational exposure. Mice behaved normally during the exposure. On initial removal from the chamber, the mice were also coated with condensed perflubron. However, this rapidly evaporated over 5–10 min and no obvious adverse effects were observed. Mice subsequently behaved normally including grooming and feeding. For administration to the macaques, the animals were anesthetized and nebulized perflubron was delivered through a facemask. There was some pooling of the condensed perflubron in the oral cavity of the macaques and some was swallowed. However, once recovered from anesthesia, macaques exhibited normal behavior including feeding and grooming.
Radiographic evaluations after nebulized perflubron administration
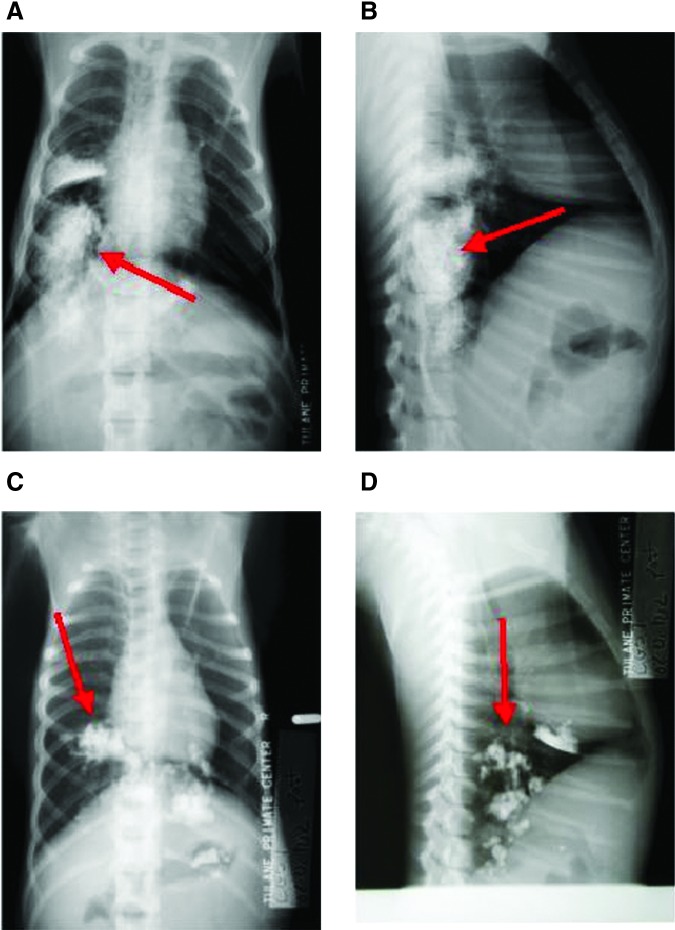
Mice exposed to nebulized perflubron for 1 hr and then immediately killed had no discernible perflubron visible in the lungs by micro-computerized tomography (CT) scanning (Fig. 2A). Small amounts of perflubron were found in the stomach and intestines, likely reflecting nebulized perflubron that had condensed on the animals' fur and was ingested during grooming. In contrast, intratracheal administration of liquid perflubron resulted in diffuse dispersion throughout the lungs as previously demonstrated (Fig. 2B) (Weiss et al., 1999a). As CT scans were not feasible for the macaques, thoracic radiographs were taken immediately after nebulized perflubron exposure and demonstrated scattered areas of condensed liquid perflubron pooling in dependent regions of the lung and also in the stomach (Fig. 3). This likely represented random pooling of inhaled perflubron in dependent lung regions and also swallowing of nebulized perflubron that had condensed in the oropharyngeal cavity. Follow-up thoracic radiographs demonstrated clearance of the perflubron within 1–2 days from both the lungs and the gastrointestinal tract.
FIG. 2.
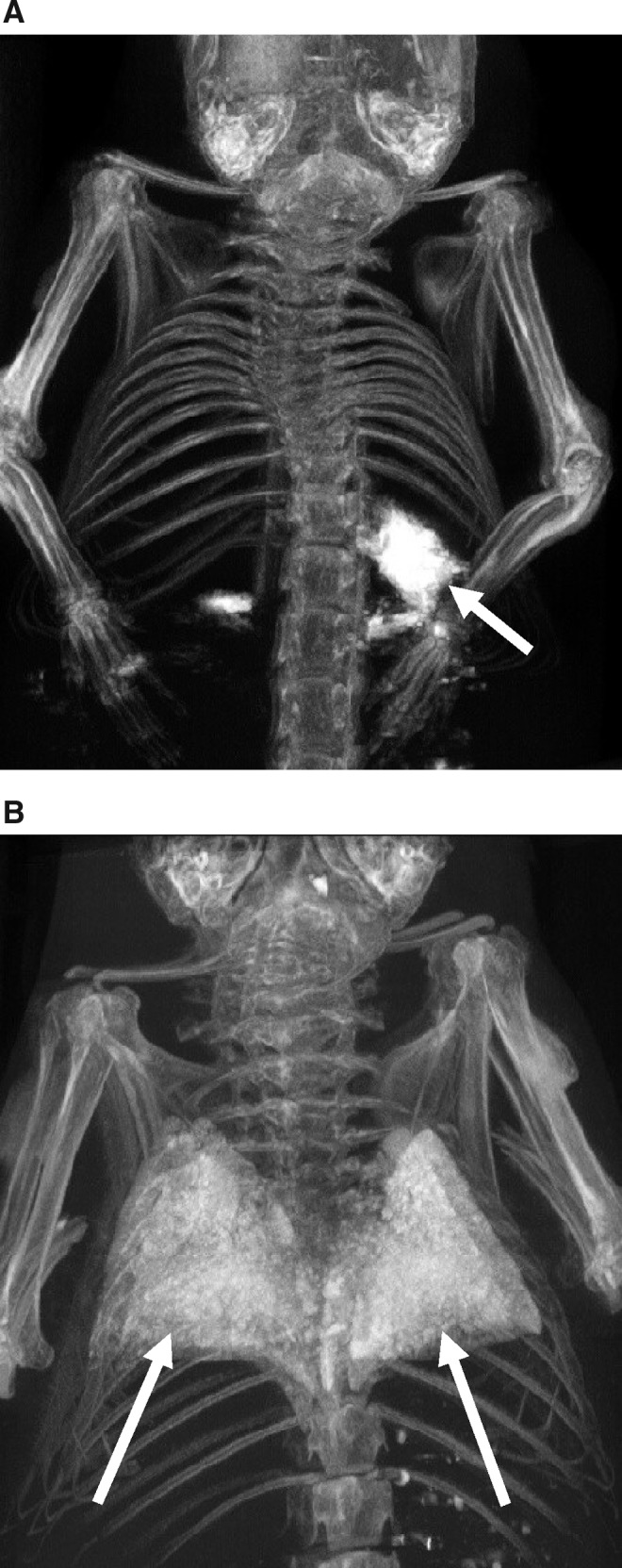
Computerized tomographic scans of mice exposed to nebulized or intratracheal liquid perflubron. Maximal-intensity projection images demonstrate regions of greatest X-ray attenuation, which in these specimens correspond to bone and to perflubron. The “double-vision/ghosting” artifact is due to specimen settling during the 80-min scan. (A) Nebulized perflubron (arrow highlights perflubron in the stomach). (B) For comparison, intratracheal instillation of liquid perflubron is depicted (arrows highlight perflubron in the lungs). Representative scans from one of three similarly treated mice for each condition are shown.
FIG. 3.
Chest X-rays of macaques exposed to nebulized or bronchoscopically administered perflubron. Representative posteroanterior (PA; A and C) and lateral (B and D) chest X-rays from two macaques, taken immediately after 1 hr of nebulized perflubron inhalation, demonstrate random pooling of inhaled perflubron in dependent lung regions and also swallowing of nebulized perflubron that had condensed in the oropharyngeal cavity (red arrows). Color images available online at www.liebertpub.com/hgtb
Effect of nebulized perflubron on adenovirus-mediated in situ gene expression in mice
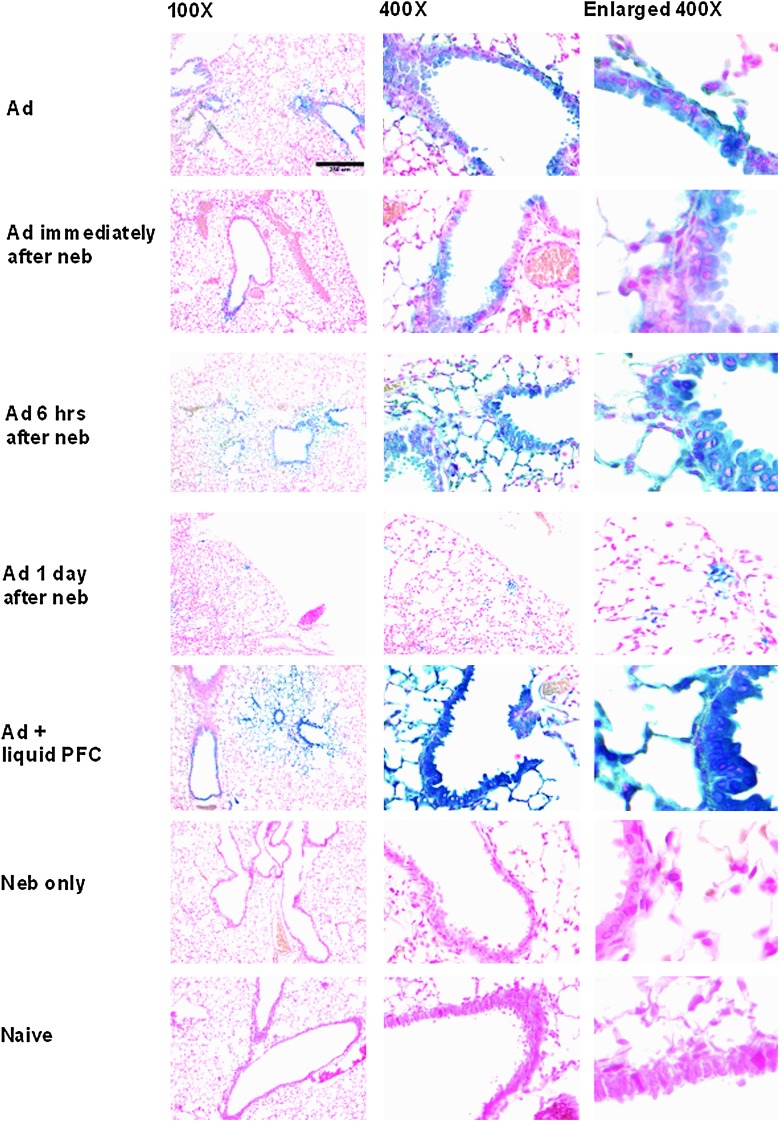
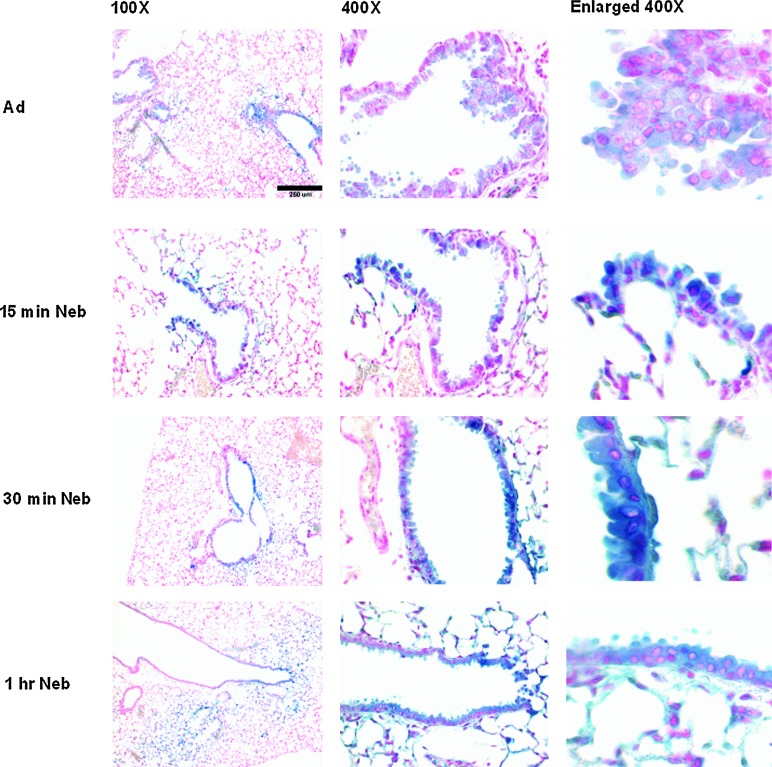
After exposure of mice for 1 hr to nebulized perflubron, administration of AdlacZ immediately afterward did not significantly enhance gene expression compared with that observed in mice receiving vector alone (Fig. 4). In contrast, significant enhancement of gene expression was observed in mice receiving nebulized perflubron when vector was administered 6 hr after aerosol exposure (Fig. 4). Gene expression in mice receiving vector 6 hr after nebulized perflubron was increased compared with that observed in mice receiving AdlacZ only and was comparable to previously observed levels after simultaneous administration of vector with liquid perflubron (Fig. 4) (Weiss et al., 1999a,b, 2001, 2002a). No enhancement was observed if vector was administered 1 day (Fig. 4) or 3 days (data not shown) after nebulized perflubron exposure. Expression was enhanced in both airway and alveolar epithelial cells (corresponding high-power views are depicted in Fig. 4, middle and right-hand columns). Comparably, inhalation of nebulized saline for 1 hr followed by vector administration immediately, 6 hr, 1 day, or 3 days later did not enhance in situ gene expression over that observed after administration of vector alone (data not shown). An exposure of at least 60 min to nebulized perflubron was required to enhance gene expression after subsequent vector administration; no significant effect on in situ gene expression was seen with a 15- or 30-min exposure (Fig. 5).
FIG. 4.
Inhalation of nebulized perflubron enhances AdlacZ-mediated lung gene expression in mice. Shown are representative low-magnification (×100) overviews, corresponding high-magnification detailed views (×400), and further enlargements of selected areas in the×400 magnifications of lungs from mice receiving either intratracheal instillation of AdlacZ alone (Ad), AdlacZ instillation immediately after a 60-min exposure to nebulized perflubron (Ad immediately after neb), AdlacZ instillation 6 hr after a 60-min exposure to nebulized perflubron (Ad 6 hr after neb), AdlacZ instillation 1 day after a 60-min exposure to nebulized perflubron (Ad 1 day after neb), intratracheal instillation of AdlacZ with liquid perflubron (Ad + liquid PFC), a 60-min exposure to nebulized perflubron only (neb only), or naive control (Naive). n=4–11 for each experimental condition. Color images available online at www.liebertpub.com/hgtb
FIG. 5.
Enhancement of lung gene expression in mice requires a 60-min exposure to nebulized perflubron. Shown are representative low-magnification (×100) overviews, corresponding high-magnification detailed views (×400), and further enlargements of selected areas in the×400 magnifications of lungs from mice receiving intratracheal instillation of AdlacZ alone (Ad), AdlacZ instillation 6 hr after a 15-min exposure to nebulized perflubron (15 min neb), AdlacZ instillation 6 hr after a 30-min exposure to nebulized perflubron (30 min neb), or AdlacZ instillation 6 hr after a 60-min exposure to nebulized perflubron (1 hr neb). n=4–11 for each experimental condition. Color images available online at www.liebertpub.com/hgtb
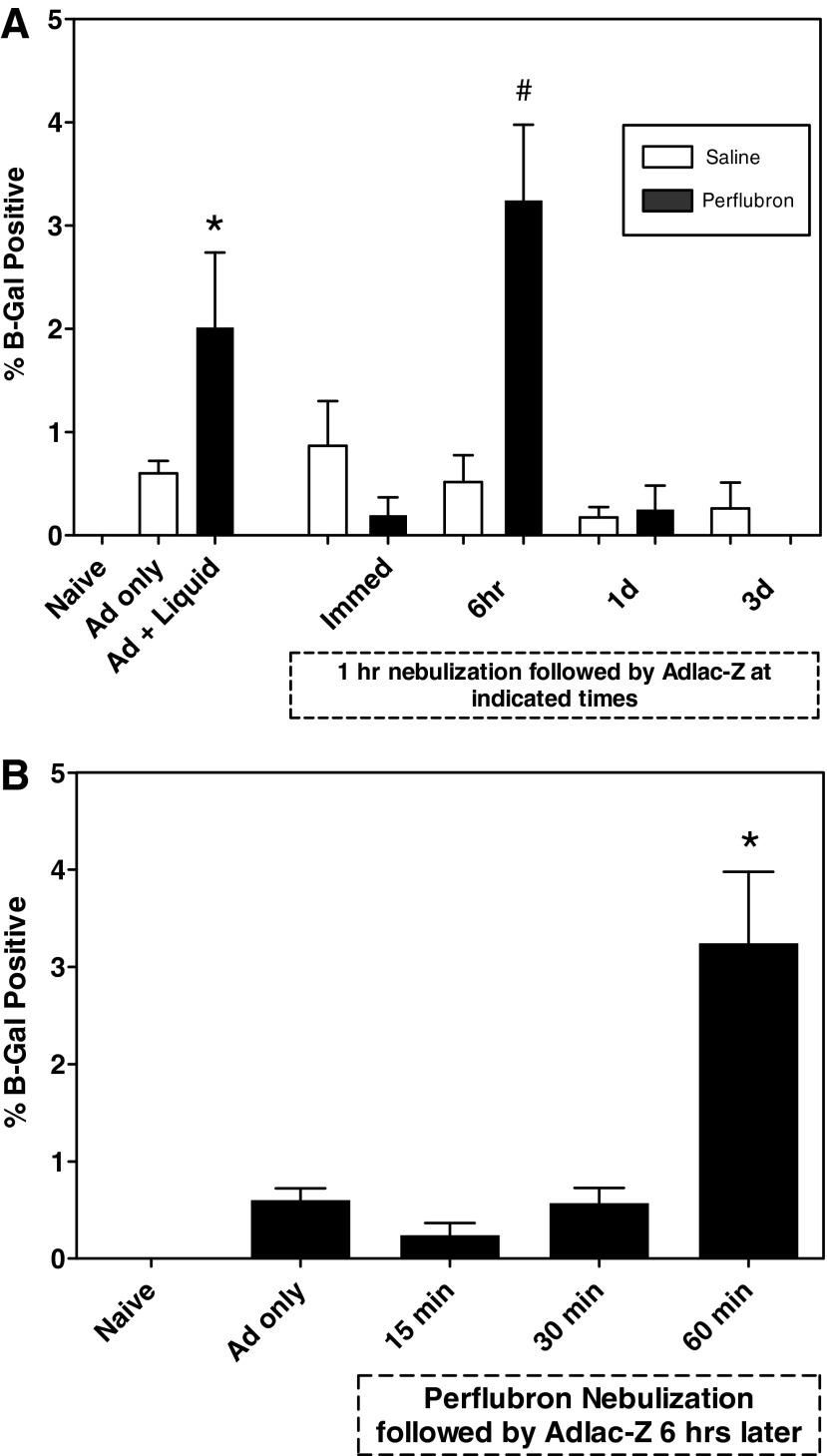
Quantitative measure of the percentage of lung expressing β-Gal activity is depicted in Fig. 6. Intratracheal adenovirus administration plus liquid perflubron resulted in a significant (p<0.05) increase in gene expression compared with naive mice (Fig. 6A), or mice administered vector only. Significant enhancement of gene expression was observed in lungs of mice exposed to nebulized perflubron compared with those exposed to nebulized saline when vector was administered 6 hr after exposure. The timing of vector administration after nebulized perflubron exposure was important, as gene expression was significantly increased in mice receiving AdlacZ 6 hr after perflubron nebulization compared with that observed after AdlacZ administration either immediately after, 1 day, or 3 days after nebulized perflubron exposure (p=0.002). When analyzed for drug effect, AdlacZ administered 6 hr after nebulization exposure (3.25±2.1%) resulted in significantly greater gene expression compared with animals receiving either AdlacZ alone (0.6±0.24%; p=0.004) or nebulized saline followed 6 hr later by AdlacZ (0.52±0.52%; p=0.03). There was no difference between saline and perflubron nebulization exposures when AdlacZ was administered immediately, 1 day, or 3 days after nebulization. Increased gene expression was comparable to that previously observed with simultaneous administration of AdlacZ with liquid perflubron (Weiss et al., 1999a,b, 2001, 2002a).
FIG. 6.
Quantitative estimation of the amount of total lung expression of β-Gal activity. Mounted lung sections from each experimental condition were scanned into Adobe Photoshop and processed with ImageJ software. The percentage of total lung area expressing β-Gal activity is graphically depicted. (A) A 1-hr exposure to nebulized perflubron/saline followed by intratracheal AdlacZ instillation immediately, 6 hr, 1 day, or 3 days later. Control mice were naive or were exposed to AdlacZ only or AdlacZ plus liquid perflubron. *p<0.05 versus Naive or AdlacZ only. #p<0.05 for 6 hr of perflubron versus all other treated groups. (B) Mice were instilled with AdlacZ 6 hr after exposure to 15, 30, or 60 min of nebulized perflubron. *p<0.05 versus AdlacZ only and versus 15 min and 30 min of nebulized perflubron plus AdlacZ. Data are presented as mean±SEM.
AdlacZ administration after exposure to nebulized perflubron for 60 min (3.25±2.1%) resulted in a significant increase in lung gene expression compared with mice receiving AdlacZ alone (0.6±0.24%; p=0.004), or AdlacZ administered 6 hr after either 15 min (0.24±0.28%; p=0.04) or 30 min (0.57±0.36%; p=0.02) of nebulized perflubron exposure (Fig. 6B). There were no significant differences between lung gene expression after administration of AdlacZ alone compared with that observed with AdlacZ administered 6 hr after either a 15- or 30-min exposure to nebulized perflubron.
Effect of nebulized perflubron on inflammatory markers in mouse BAL fluid
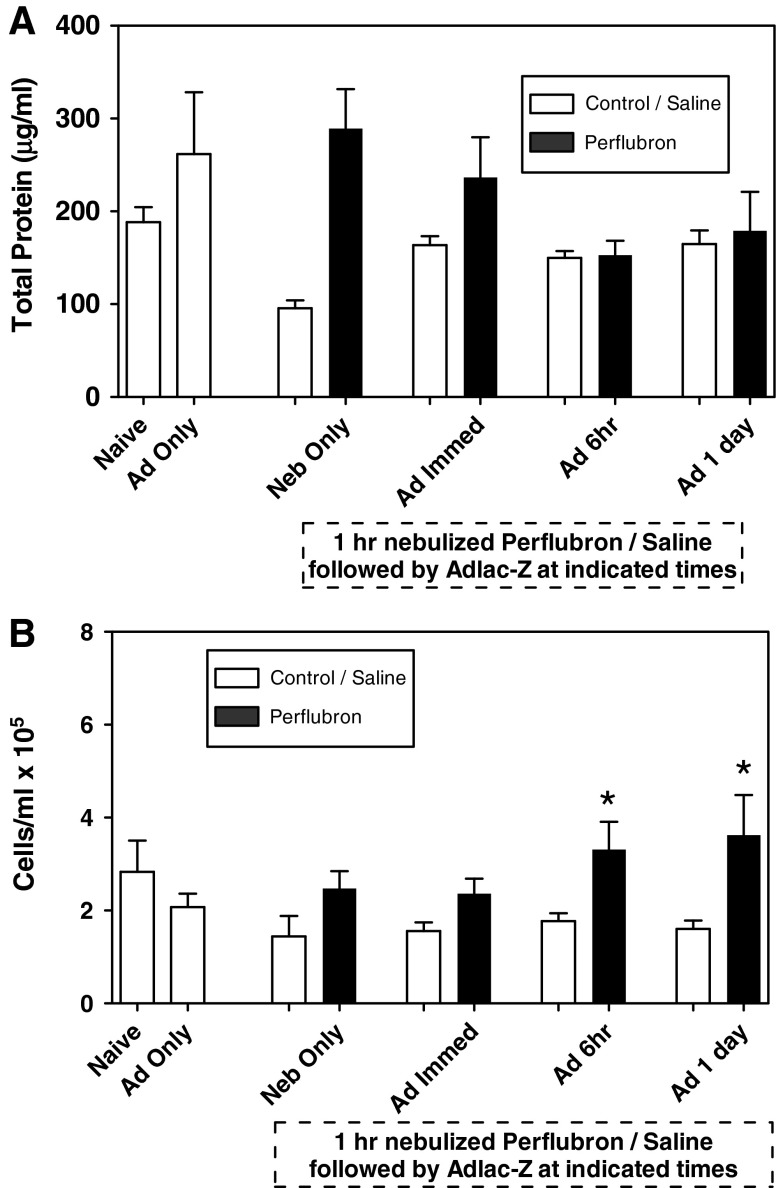
Measurement of BAL fluid total protein (μg/ml) under the various experimental conditions is shown in Fig. 7A. There was no group effect observed in BAL fluid total protein among naive mice or mice receiving AdlacZ alone, nebulized perflubron only, or nebulized saline only. Administration of AdlacZ either alone or immediately, 6 hr, or 1 day after 1 hr of nebulized perflubron exposure resulted in no change in levels of BAL fluid total protein compared with that observed in naive controls. Furthermore, there were no differences in BAL fluid total protein after administration of AdlacZ to perflubron- versus saline-exposed mice at any of the time points measured.
FIG. 7.
Inflammatory markers in mouse lung BAL fluid. Lavage fluid was collected from mice at the time of lung harvest and processed for total protein (A) and total inflammatory cell count (B). *p≤0.05 compared with saline control. Data are presented as mean±SEM.
There was no group effect observed for differences in BAL fluid total cell count among naive mice or mice receiving AdlacZ alone, nebulized perflubron only, or nebulized saline only (Fig. 7B). There was no significant time effect of AdlacZ administration. There was, however, a significant drug effect between perflubron and saline. Total cells were significantly elevated in perflubron- versus saline-treated animals when AdlacZ was administered 6 hr (3.3 [±1.2]×105 vs. 1.8 [±0.4]×105 cells/ml; p=0.03) or 1 day (3.6 [±1.7]×105 vs. 1.6 [±0.4]×105 cells/ml; p=0.04) later, but not if administered either immediately or 3 days after exposure.
The majority (>96%) of the cells seen in BAL fluid from all mice evaluated were macrophages. Administration of AdlacZ alone resulted in no significant change in either lymphocyte or neutrophil content, compared with naive controls, in BAL fluid obtained 3 days after vector administration. However, administration of AdlacZ 6 hr after either nebulized perflubron (2.7±0.7%) or nebulized saline (1.4±1.3%) exposure resulted in a small but significant increase in lymphocytes versus animals administered AdlacZ immediately after nebulized perflubron (0.2±0.4%; p=0.003) or nebulized saline (1.25±0.6%; p=0.003) or 1 day after nebulized perflubron (0.3±0.4%; p=0.001) or nebulized saline (0.8±0.5%; p=0.001) (data not shown). There was no drug effect between perflubron- and saline-treated animals in BAL fluid cell differentials at any of the time points measured.
Effect of nebulized perflubron on lanthanum staining of intercellular spaces between airway epithelial cells in mouse lungs
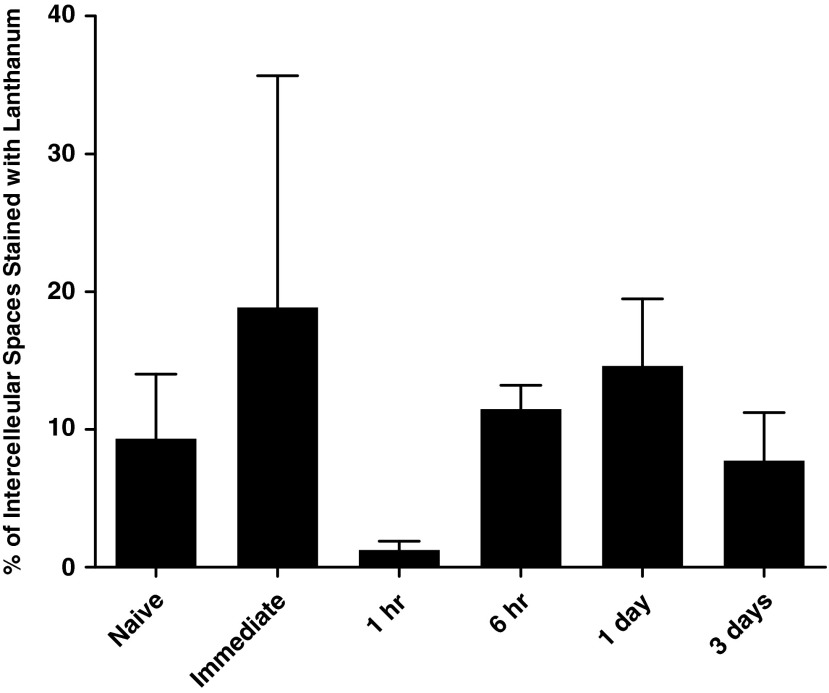
In a separate series of experiments, mice were exposed to nebulized perflubron for 1 hr and then killed immediately, 1 hr, 6 hr, 1 day, or 3 days later. The lungs were subsequently harvested and fixed with lanthanum to assess tight junction permeability (Weiss et al., 2003). No significant increase in lanthanum staining of intercellular spaces was observed at the time points studied (Fig. 8). A nonsignificant trend toward a decrease in number of intercellular spaces stained with lanthanum was observed after 1 hr of nebulized perflubron exposure.
FIG. 8.
Effect of nebulized perflubron on lanthanum permeability of tight junctions between airway epithelial cells. Mice were exposed to nebulized perflubron for 1 hr and killed immediately, 1 hr, 6 hr, 1 day, and 3 days later. Electron micrographs of representative lung sections were assessed for lanthanum staining of intercellular spaces. Each column represents the average for each condition (n=3–5 for each time point). Data are presented as mean±SEM.
Effect of nebulized perflubron on AAV2/A5-mediated lung gene expression in macaques
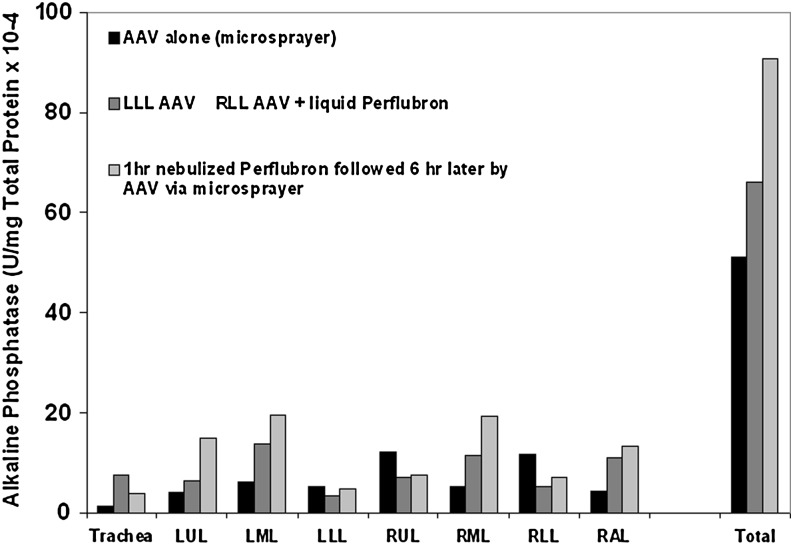
To assess the effects of nebulized perflubron on lung epithelial gene expression in a more relevant preclinical model, two anesthetized adult pigtail macaques inhaled nebulized perflubron for 1 hr. Six hours later, one animal received recombinant AAV2/5 vector (2.24×1013 DNase-resistant particles/ml) encoding human placental alkaline phosphatase delivered to the caudal (lower) trachea through a microsprayer inserted into a pediatric bronchoscope. A third (unexposed) macaque received vector only through the microsprayer whereas a fourth (unexposed) macaque received vector instilled directly through a bronchoscope into the left caudal (lower) lobe. The fourth macaque also received vector directly instilled into the right caudal (lower) lobe followed by 10 ml of liquid perflubron. The three animals receiving vector were killed 2 months later and the lungs were assessed for inflammation and for alkaline phosphatase activity. In the animal exposed to nebulized perflubron, quantitative alkaline phosphatase levels were significantly increased overall and appeared to be more uniformly spread throughout the various lung lobes compared with the other two animals that received vector either alone or chased with liquid perflubron (Fig. 9). Only minimal qualitative alkaline phosphatase expression was observed by immunohistochemistry in any of the animals receiving the vector, and no conclusions could be drawn about distribution or specific cell types expressing the transgene. No significant inflammation was observed on serial thoracic radiographs, blood collections, or in analyses of BAL fluid total cell count or cytokine levels obtained before (days 2, 14, and 28) and at necropsy (day 56) and no evidence of histological tissue inflammation was observed at necropsy in animals receiving vector, with or without either nebulized or liquid perflubron (data not shown). Further, the animal receiving nebulized perflubron only was similarly assessed over a 1-year period by serial chest radiographs, bronchoscopies, and blood collections with no obvious adverse effects of exposure. Although only a small number of animals were assessed, these pilot results demonstrate that inhalation of nebulized perflubron can safely enhance lung gene expression in nonhuman primates.
FIG. 9.
Inhalation of nebulized perflubron enhances AAV2/5-mediated lung gene expression in nonhuman primates. Total quantitative alkaline phosphatase expression is increased in lungs of macaques receiving recombinant vector after inhalation of nebulized perflubron compared with lungs of macaques receiving either vector alone or vector chased by liquid perflubron. Overall distribution of alkaline phosphatase expression appears to be more widespread in lungs of macaques receiving the nebulized perflubron before vector administration. n=1 for each experimental condition. LUL, left upper lobe; LML, left middle lobe; LLL, left caudal (lower) lobe; RUL, right upper lobe; RML, right middle lobe; RLL, right caudal (lower) lobe; RAL, right accessory lobe.
Discussion
Although lung gene therapy has not yet resulted in substantive therapeutic approaches for original target diseases such as cystic fibrosis, several potential therapeutic candidates remain, including lung cancers and genetic lung diseases such as α1-antitrypsin deficiency. Effective vector delivery to lung airways and alveolar spaces remains one of the obstacles to successful lung gene therapy (Bromberg et al., 1998; Look and Brody, 1999; Pilewski, 2002; Weiss, 2002b; Weiss and Pilewski, 2003; Sueblinvong et al., 2007). Intratracheal administration of recombinant adenoviral vectors by catheter or bronchoscopic techniques generally targets only selected regions, usually of proximal airways. Use of a microsprayer device inserted through a bronchoscope does increase vector distribution through the airways, although this remains an invasive technique (Harvey et al., 1999; Cipolla et al., 2000; Beck et al., 2002). Administration of aerosolized vectors, both viral and nonviral, can result in more effective distribution throughout the lungs (Sené et al., 1995; Brown and Chowdhury, 1997; Gautam et al., 2000; Lerondel et al., 2001; Perricone et al., 2001). However, the efficiency of many aerosolization devices remains low, with a significant portion of the vector deposited in the machinery or upper airways. The aerosolization process may also disrupt certain vectors such as liposomes (Brown and Chowdhury, 1997).
Various adjunct techniques have been used to enhance airway-based vector delivery. These include use of agents such as surfactant that physically increase distribution of vector throughout the airways as well as thixotropic agents that decrease mucociliary clearance of vectors (Factor et al., 2001; Seiler et al., 2002). Agents that alter tight junction permeability between airway epithelial cells and other apical membrane properties, such as EGTA, caprate, and detergents, have also been used (Parsons et al., 1998; Coyne et al. 2000; Walters et al., 2000; Wang et al., 2000). Although effective and promising, the clinical feasibility and safety of these agents have not been established except for caprate, which has been used to enhance intestinal absorption (Lennernäs et al., 2003).
We have extensively investigated the use of another adjunct agent, perfluorochemical (PFC) liquids, for use in airway-based vector delivery. PFC liquids are inert fluorinated hydrocarbon compounds, one of which, perflubron, has been extensively investigated for use in clinical trials of partial liquid ventilation (Leach et al., 1996; Hirschl et al., 2002; Kacmarek et al., 2006). Even though randomized clinical trials of liquid ventilation using perflubron versus conventional gas ventilation did not show significant improvement in survival (and as such, liquid ventilation has largely fallen out of clinical use), a strong safety record for intratracheal administration of perflubron has been established. PFC liquids have been used to enhance drug delivery to the lungs and we and others have demonstrated enhancement of gene expression after administration of recombinant adenovirus and adeno-associated viral vector vectors with PFC liquids (Lisby et al., 1997; Weiss et al., 1999a,b, 2000, 2001, 2002a,b, 2003). One mechanism of PFC liquid action is to more effectively distribute vector particles throughout the airways. Importantly, this has been demonstrated to be an effective approach to airway-based gene delivery in models of lung injury in which increased physical barriers to vector delivery, such as proteinaceous debris and inflammatory fluids, are present in airways and alveolar spaces (Weiss et al., 1999a,b, 2001). This is in large part due to a propulsive effect in which the immiscible PFC liquids are administered immediately after aqueous vector solutions and result in improved penetration and distribution throughout the airways. Some formulations of PFC liquids allow some degree of mixing with aqueous vector solutions, and these suspensions may also improve distribution of transgene expression in lung epithelium (Kazzaz et al., 2011). PFC liquids also have other effects that enhance gene expression. PFC liquids result in a transient opening of tight junctions that peaks approximately 6 hr after PFC administration. This ostensibly allows improved access of recombinant viral vectors to relevant binding sites on basolateral surfaces of differentiated polarized airway epithelial cells (Weiss et al., 2003). PFC liquids also appear to decrease phagocytosis and inactivation of recombinant adenoviral vectors by alveolar macrophages (Bonneau et al., 2000). This could significantly increase the number of vector particles reaching the epithelium, as up to 70% of adenoviral particles instilled into the airways may be phagocytosed and inactivated by macrophages (Worgall et al., 1997).
Although effective in animal studies and conceivable for vector delivery to intubated critically ill patients, instillation of PFC liquid is not easily suited to routine use in otherwise healthy patients (Leach et al., 1996; Hirschl et al., 2002; Weiss, 2002a; Kacmarek et al., 2006). As administration of PFC liquid before vector administration also enhanced gene delivery, through transient opening of tight junctions and possibly other mechanisms as well, we postulated that administration of PFC in nebulized form would also enhance gene expression resulting from subsequent vector administration. If successful, this would provide a more clinically feasible approach to the use of PFC liquids for enhancing lung gene expression. This hypothesis was bolstered by studies demonstrating that administration of PFC liquids in either nebulized or aerosolized form improved lung mechanics and oxygenation in experimental models of lung injury (Bleyl et al., 1999; Ragaller et al., 2001; Kandler et al., 2004; von der Hardt et al., 2004; Gama de Abreu et al., 2005; Meinhardt et al., 2005).
The data presented in this report support this hypothesis. Administration of nebulized perflubron to mice enhanced subsequent recombinant adenovirus-mediated gene expression. In this study, overall gene expression with all the techniques was low because a relatively low dose of vector was used so as to maximize the effects of nebulized perflubron. As such, the clinical relevance of the statistically significant yet small increase in transgene expression with low vector doses may be more clinically relevant with higher vector doses. The procedure is simple and was well tolerated by the mice, with no obvious adverse effects. Recombinant adenoviral vectors are well known to cause acute yet transient increases in lung inflammation. We have previously demonstrated that instillation of PFC liquid does not increase or prolong recombinant adenoviral vector-stimulated lung inflammation assessed 3 days after vector administration (Weiss et al., 2002a,b). Using a similar approach, minimal changes in inflammatory markers in BAL fluid were noted under each of the experimental conditions assessed 3 days after vector administration. Exposure to nebulized perflubron alone did cause an increase in total BAL fluid cell counts, but this was not affected by subsequent vector administration and is similar to transient increases observed with administration of liquid perflubron alone (Weiss et al., 2002a,b). Although only a few macaques were studied, similar enhancement of gene expression as well as no obvious adverse effects of nebulized perflubron were observed. As demonstrated by computerized tomographic imaging of mice and by serial thoracic radiography in macaques, little perflubron accumulated in lungs after nebulized administration. Small amounts accumulated in the gastrointestinal tract without obvious adverse effect. Of note, similar findings were observed in trials of liquid ventilation, and PFC liquids have previously been approved by the U.S. Food and Drug Administration as gastrointestinal (GI) contrast agents (Leach et al., 1996; Hirschl et al., 2002).
The mechanism of nebulized PFC action in enhancing gene expression is unclear. Similar to enhancement of gene expression when liquid perflubron is administered before adenoviral vector, peak enhancement occurred if vector was administered 6 hr after nebulized perflubron administration. This coincides with the timing of maximal increase in tight junction permeability after administration of liquid perflubron (Weiss et al., 2003). However, there was no obvious change in permeability after administration of nebulized perflubron. This suggests that another mechanism of nebulized perflubron action remains to be determined.
In summary, although gene therapy approaches for lung diseases have not yet reached clinical usefulness, several candidate diseases remain potentially amenable. Use of nebulized perflubron during recombinant viral vector administration is a viable and clinically feasible approach to enhancing gene expression in lung epithelium.
Acknowledgments
The authors gratefully acknowledge Wendy Walker, Shari Hunt, and other members of the Animal Care Facility at the Fred Hutchinson Cancer Research Center; James Hall, Kathy Yager, and other members of the Animal Care Facility at the University of Vermont College of Medicine; and Dot Kuebler, Maury Duplantis, Carole Elliott, Peggy Smith, and Shixuan Huang at the Tulane National Primate Research Center. The authors further acknowledge David Hemenway (University of Vermont) and Ernie Schutt and Mark Wedel (Alliance Pharmaceutical) for assistance with perflubron nebulization, John Thompson-Figueroa (University of Vermont) for assistance with computerized tomographic scanning, and Michael Sullivan (University of Vermont) for assistance with imaging analyses. This work was supported by HL03864 (D.J. Weiss, PI), the Cystic Fibrosis Foundation (D.J. Weiss, PI), the American Lung Association (D.J. Weiss, PI), NCRR P20 RR15557 (C. Irvin, PI), Venture Pilot Funding at the TNPRC (D.J. Weiss, PI), and TNPRC base grant 2 P51 RR000164-52 (B.P. Sachs, PI).
Author Disclosure Statement
No competing financial interests exist.
References
- Armentano D. Zabner J. Sacks C., et al. Effect of the E4 region on the persistence of transgene expression from adenovirus vectors. J. Virol. 1997;71:2408–2416. doi: 10.1128/jvi.71.3.2408-2416.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck S.E. Laube B.L. Barberena C.I., et al. Deposition and expression of aerosolized rAAV vectors in the lungs of rhesus macaques. Mol. Ther. 2002;6:546–554. doi: 10.1006/mthe.2002.0698. [DOI] [PubMed] [Google Scholar]
- Bleyl J.U. Ragaller M. Tschö U., et al. Vaporized perfluorocarbon improves oxygenation and pulmonary function in an ovine model of acute respiratory distress syndrome. Anesthesiology. 1999;91:461–469. doi: 10.1097/00000542-199908000-00021. [DOI] [PubMed] [Google Scholar]
- Bonneau L. Shaffer T.H. Lukason M., et al. Ingestion in vivo by alveolar macrophages of perfluorochemical (PFC) liquid correlates with altered pro-inflammatory cytokine release [abstract]. Am. J. Respir. Crit. Care Med. 2000;161:A902. [Google Scholar]
- Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bromberg J.S. Debruyne L.A. Qin L. Interactions between the immune system and gene therapy vectors: Bidirectional regulation of response and expression. Adv. Immunol. 1998;69:353–409. [PubMed] [Google Scholar]
- Brown A.R. Chowdhury S.I. Propellant-driven aerosols of DNA plasmids for gene expression. J. Aerosol Med. 1997;10:129–146. [Google Scholar]
- Cipolla D.C. Gonda I. Shak S., et al. Coarse spray delivery to a localized region of the pulmonary airways for gene therapy. Hum. Gene Ther. 2000;20:361–371. doi: 10.1089/10430340050016085. [DOI] [PubMed] [Google Scholar]
- Coyne C.B. Kelly M.M. Boucher R.C. Johnson L.G. Enhanced epithelial gene transfer by modulation of tight junctions with sodium caprate. Am. J. Respir. Cell Mol. Biol. 2000;23:602–609. doi: 10.1165/ajrcmb.23.5.4164. [DOI] [PubMed] [Google Scholar]
- Factor P. Mendez M. Mutlu G. Dumasius V. Gene transfer to severely injured rat lungs. Mol. Ther. 2001;3:S337–S338. [Google Scholar]
- Gama de Abreu M. Wilmink B. Hubler M. Koch T. Vaporized perfluorohexane attenuates ventilator-induced lung injury in isolated, perfused rabbit lungs. Anesthesiology. 2005;102:597–605. doi: 10.1097/00000542-200503000-00019. [DOI] [PubMed] [Google Scholar]
- Gautam A. Densmore C.L. Xu B. Waldrep J.C. Enhanced gene expression in mouse lung after PEI–DNA aerosol delivery. Mol. Ther. 2000;2:63–70. doi: 10.1006/mthe.2000.0087. [DOI] [PubMed] [Google Scholar]
- Harvey B.G. Leopold P.L. Hackett N.R., et al. Airway epithelial CFTR mRNA expression in cystic fibrosis patients after repetitive administration of a recombinant adenovirus. J. Clin. Invest. 1999;104:1245–1255. doi: 10.1172/JCI7935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschl R.B. Tooley R. Parent A.C., et al. Improvement of gas exchange, pulmonary function, and lung injury with partial liquid ventilation. Chest. 1995;108:500–508. doi: 10.1378/chest.108.2.500. [DOI] [PubMed] [Google Scholar]
- Hirschl R.B. Croce M. Gore D., et al. Prospective, randomized, controlled pilot study of partial liquid ventilation in adult acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2002;165:781–787. doi: 10.1164/ajrccm.165.6.2003052. [DOI] [PubMed] [Google Scholar]
- Johnson L.G. Mewshaw J.P. Ni H., et al. Effect of host modification and age on airway epithelial gene transfer mediated by a murine leukemia virus-derived vector. J. Virol. 1998;72:8861–8872. doi: 10.1128/jvi.72.11.8861-8872.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kacmarek R.M. Wiedemann H.P. Lavin P.T., et al. Partial liquid ventilation in adult patients with acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2006;173:882–889. doi: 10.1164/rccm.200508-1196OC. [DOI] [PubMed] [Google Scholar]
- Kandler M.A. von der Hardt K. Schoof E., et al. Persistent improvement of gas exchange and lung mechanics by aerosolized perfluorocarbon. Am. J. Respir. Crit. Care Med. 2001;164:31–35. doi: 10.1164/ajrccm.164.1.2010049. [DOI] [PubMed] [Google Scholar]
- Kandler M.A. von der Hardt K. Gericke N., et al. Dose response to aerosolized perflubron in a neonatal swine model of lung injury. Pediatr. Res. 2004;56:191–197. doi: 10.1203/01.PDR.0000132667.47744.F4. [DOI] [PubMed] [Google Scholar]
- Kazzaz J.A. Strayer M.S. Wu J., et al. Perfluorochemical liquid–adenovirus suspensions enhance gene delivery to the distal lung. Pulm. Med. 2011;2011:918036. doi: 10.1155/2011/918036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach C.L. Greenspan J.S. Rubenstein S.D., et al. Partial liquid ventilation with perflubron in premature infants with severe respiratory distress syndrome. N. Engl. J. Med. 1996;335:761–767. doi: 10.1056/NEJM199609123351101. [DOI] [PubMed] [Google Scholar]
- Lennernäs H. Gjellan K. Hallgren R. Graffner C. The influence of caprate on rectal absorption of phenoxymethylpenicillin: Experience from an in-vivo perfusion in humans. J. Pharm. Pharmacol. 2003;54:499–508. doi: 10.1211/0022357021778772. [DOI] [PubMed] [Google Scholar]
- Lerondel S. Vecellio None L. Faure L., et al. Gene therapy for cystic fibrosis with aerosolized adenovirus-CFTR: Characterization of the aerosol and scintigraphic determination of lung deposition in baboons. J. Aerosol Med. 2001;14:95–105. doi: 10.1089/08942680152007945. [DOI] [PubMed] [Google Scholar]
- Lisby D.A. Ballard P.L. Fox W.W., et al. Enhanced distribution of adenovirus-mediated gene transfer to lung parenchyma by perfluorochemical liquid. Hum. Gene Ther. 1997;8:919–928. doi: 10.1089/hum.1997.8.8-919. [DOI] [PubMed] [Google Scholar]
- Look D.C. Brody S.L. Engineering viral vectors to subvert the airway defense response. Am. J. Respir. Cell Mol. Biol. 1999;20:1103–1106. doi: 10.1165/ajrcmb.20.6.f150. [DOI] [PubMed] [Google Scholar]
- Meinhardt J.P. Schmittner M. Herrmann P., et al. Comparison of different inhalational perfluorocarbons in a rabbit model of acute lung injury. ASAIO J. 2005;51:85–91. doi: 10.1097/01.mat.0000151923.48654.32. [DOI] [PubMed] [Google Scholar]
- Parsons D.W. Grubb B.R. Johnson L.G. Boucher R.C. Enhanced in vivo airway gene transfer via transient modification of host barrier properties with a surface-active agent. Hum. Gene Ther. 1998;9:2661–2672. doi: 10.1089/hum.1998.9.18-2661. [DOI] [PubMed] [Google Scholar]
- Perricone M.A. Morris J.E. Pavelka K., et al. Aerosol and lobar administration of a recombinant adenovirus to individuals with cystic fibrosis. II. Transfection efficiency in airway epithelium. Hum. Gene Ther. 2001;12:1383–1394. doi: 10.1089/104303401750298544. [DOI] [PubMed] [Google Scholar]
- Pilewski J.M. Gene therapy for airway diseases: Continued progress towards identifying and overcoming barriers to efficiency. Am. J. Respir. Cell Mol. Biol. 2002;27:117–121. doi: 10.1165/ajrcmb.27.2.f244. [DOI] [PubMed] [Google Scholar]
- Ragaller M. Bleyl J. Tschö U., et al. Effects of inhalation of perfluorocarbon aerosol on oxygenation and pulmonary function compared with PGI2 inhalation in a sheep model of oleic acid-induced lung injury. Intensive Care Med. 2001;27:889–897. doi: 10.1007/s001340100921. [DOI] [PubMed] [Google Scholar]
- Rotta A.T. Gunnarsson B. Hernan L.J., et al. Partial liquid ventilation influences pulmonary histopathology in an animal model of acute lung injury. J. Crit. Care. 1999;14:84–92. doi: 10.1016/s0883-9441(99)90019-9. [DOI] [PubMed] [Google Scholar]
- Seiler M.P. Luner P. Moninger T.O., et al. Thixotropic solutions enhance viral-mediated gene transfer to airway epithelia. Am. J. Respir. Cell Mol. Biol. 2002;27:133–140. doi: 10.1165/ajrcmb.27.2.4793. [DOI] [PubMed] [Google Scholar]
- Sené C. Bout A. Imler J.L., et al. Aerosol-mediated delivery of recombinant adenovirus to the airways of nonhuman primates. Hum. Gene Ther. 1995;6:1587–1593. doi: 10.1089/hum.1995.6.12-1587. [DOI] [PubMed] [Google Scholar]
- Sueblinvong V. Suratt B.T. Weiss D.J. Update in gene and stem cell therapies for cystic fibrosis. Clin. Chest Med. 2007;28:361–380. doi: 10.1016/j.ccm.2007.02.004. [DOI] [PubMed] [Google Scholar]
- von der Hardt K. Kandler M.A. Brenn G., et al. Comparison of aerosol therapy with different perfluorocarbons in surfactant-depleted animals. Crit. Care Med. 2004;32:1200–1206. doi: 10.1097/01.ccm.0000124876.31138.f6. [DOI] [PubMed] [Google Scholar]
- Walters R.W. Duan D. Engelhardt J.F. Welsh M.J. Incorporation of adeno-associated virus in a calcium phosphate coprecipitate improves gene transfer to airway epithelia in vitro and in vivo. J. Virol. 2000;74:535–540. doi: 10.1128/jvi.74.1.535-540.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G.J. Zabner J. Deering C., et al. Increasing epithelial junction permeability enhances gene transfer to airway epithelia in vivo. Am. J. Respir. Cell Mol. Biol. 2000;22:129–138. doi: 10.1165/ajrcmb.22.2.3938. [DOI] [PubMed] [Google Scholar]
- Weiss D.J. Gene therapy and gene transfer approaches for acute lung injury. In: Albelda S.M., editor. In Lung Biology in Health and Disease. Vol. 169. Marcel Dekker; New York: 2002a. pp. 378–411. Gene Therapy in Lung Disease. [Google Scholar]
- Weiss D.J. Delivery of gene transfer vectors to lung: Obstacles and the role of adjunct techniques for airway administration. Mol. Ther. 2002b;6:148–152. doi: 10.1006/mthe.2002.0662. [DOI] [PubMed] [Google Scholar]
- Weiss D.J. Pilewski J.M. The status of gene therapy for cystic fibrosis. Semin. Respir. Crit. Care Med. 2003;24:749–764. doi: 10.1055/s-2004-815670. [DOI] [PubMed] [Google Scholar]
- Weiss D.J. Liggitt D. Clark J.G. In situ histochemical detection of β-galactosidase activity in lung: Assessment of X-Gal reagent in distinguishing lacZ gene expression and endogenous β-galactosidase activity. Hum. Gene Ther. 1997;8:1545–1554. doi: 10.1089/hum.1997.8.13-1545. [DOI] [PubMed] [Google Scholar]
- Weiss D.J. Strandjord T.P. Jackson J.C., et al. Perfluorochemical liquid-enhanced adenoviral vector distribution and expression in lungs of spontaneously breathing rodents. Exp. Lung Res. 1999a;25:317–333. doi: 10.1080/019021499270222. [DOI] [PubMed] [Google Scholar]
- Weiss D.J. Strandjord T.P. Liggitt D. Clark J.G. Perflubron enhances adenoviral-mediated gene expression in lungs of transgenic mice with chronic alveolar filling. Hum. Gene Ther. 1999b;10:2287–2293. doi: 10.1089/10430349950016933. [DOI] [PubMed] [Google Scholar]
- Weiss D.J. Bonneau L. Allen J.M., et al. Perfluorochemical liquid enhances adeno-associated virus-mediated transgene expression in lung. Mol. Ther. 2000;2:624–630. doi: 10.1006/mthe.2000.0207. [DOI] [PubMed] [Google Scholar]
- Weiss D.J. Bonneau L. Liggitt D. Use of perfluorochemical liquid allows earlier detection of gene expression and use of less vector in normal lung and enhances gene expression in acutely injured lung. Mol. Ther. 2001;3:734–745. doi: 10.1006/mthe.2001.0321. [DOI] [PubMed] [Google Scholar]
- Weiss D.J. Mutlu G.M. Bonneau L., et al. Comparison of surfactant and perfluorochemical liquid enhanced adenovirus-mediated gene transfer in normal rat lung. Mol. Ther. 2002a;6:43–49. doi: 10.1006/mthe.2002.0632. [DOI] [PubMed] [Google Scholar]
- Weiss D.J. Baskin G.B. Shean M.K., et al. Use of perflubron to enhance lung gene expression: Safety and initial efficacy studies in non-human primates. Mol. Ther. 2002b;5:8–15. doi: 10.1006/mthe.2001.0507. [DOI] [PubMed] [Google Scholar]
- Weiss D.J. Beckett T. Bonneau L., et al. Transient increase in lung epithelial tight junction permeability: An additional mechanism for enhancement of lung transgene expression by perfluorochemical liquids. Mol. Ther. 2003;8:927–935. doi: 10.1016/j.ymthe.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Worgall S. Leopold P.L. Wolff G., et al. Role of alveolar macrophages in rapid elimination of adenovirus vectors administered to the epithelial surface of the respiratory tract. Hum. Gene Ther. 1997;8:1675–1684. doi: 10.1089/hum.1997.8.14-1675. [DOI] [PubMed] [Google Scholar]
- Zar J.H. Biostatistical Analysis. Prentice-Hall; Englewood Cliffs, NJ: 1974. [Google Scholar]