Abstract
Reovirus, an oncolytic RNA virus exhibiting antiglioma activity, was shown in a previous single institution phase 1 study found that the inoculation of the virus to be well tolerated in patients with recurrent malignant glioma (MG). The goals of multicenter study reported herein were to determine the dose-limiting toxicity, maximum tolerated dose, and target lesion response rate when reovirus was administered in a novel fashion via intratumoral infusion for 72 hours in patients with recurrent malignant glioma. Fifteen adult patients were treated in a dose escalation study ranging from 1 × 108 to 1 × 1010 tissue culture infectious dose 50, tentimes the dose achieved in the previous trial. Neurological, functional examinations, and imaging studies were completed pre- and postinfusion. There was one grade 3 adverse event (convulsions) felt to be possibly related to treatment, but no grade 4 adverse events considered probably or definitely related to treatment. Dose-limiting toxicity were not identified and a maximum tolerated dose was not reached. Evidence of antiglioma activity was seen in some patients. This first report of intratumoral infusion of reovirus in patients with recurrent malignant glioma demonstrated the approach to be safe and well tolerated, warranting further studies.
Introduction
Little progress has been made in the treatment strategies for patients with malignant glioma (MG). Glioblastoma multiforme patients exhibit a median survival of ~12–15 months, with only ~15% surviving to 3 years, even in the temozolomide era.1 In light of these grim survival data, a need exists for novel therapeutic strategies. Oncolytic viruses have been increasingly popular for study as antineoplastic agents.2 By taking advantage of various tumor cell genetic defects, these viruses are able to kill tumor cells while leaving normal cells unharmed. Glioma cells are deficient in many antiviral pathways, making them susceptible to infection with viruses such as reovirus, herpes simplex virus, Newcastle disease virus, adenovirus, and others.3
Reovirus (respiratory enteric orphan) is a double-stranded RNA virus that can be isolated from the respiratory and gastrointestinal tracts of humans. The lack of symptoms associated with reovirus infection in humans has resulted in its designation as an orphan virus. Previous studies indicate that 70–100% of adults carry antireovirus antibodies, indicating previous exposure. Preclinical studies of reovirus have demonstrated its capacity to infect and lyse tumor cells while sparing healthy tissue.4,5,6,7,8,9 An interruption of the normal cellular innate immune response present in glioma cells gives rise to an overexpression of the ras signaling pathway, conferring reovirus with tumor specificity.4 The receptor tyrosine kinase-ras pathway has been shown to be implicated in 88% of MGs.5
Studies of reovirus in animal glioma models show that intratumoral injection of reovirus demonstrated efficacy; safety was confirmed by intracranial injection of reovirus in primates.10,11,12 The first trial of reovirus in humans involved a single intratumoral injection of dose 1 × 1010 tissue culture infectious dose 50 (TCID50), treating 19 patients with various non–central nervous system (CNS) malignancies. No dose-limiting toxicity (DLT) or maximum tolerated dose (MTD) was achieved, while three and two patients experienced partial and stable responses, respectively.
Reovirus was also studied via direct injection of dose 5 × 109 TCID50 into six patients with T2 prostate cancer and found to be well tolerated. Analysis of pathology specimens revealed that five out of six patient specimens had evidence of apoptosis and necrosis within the tumor tissue without damage to adjacent healthy prostatic tissue.13,14 Another phase 1 study of 18 patients used cycles of monthly i.v. infusions of reovirus at 1 × 108 to 3 × 1010 TCID50 over 1 hour in patients with various non-CNS malignancies. To date, no DLTs have been reported.15
A single clinical trial of reovirus has been conducted in patients with recurrent MG.16 This study involved a dose escalation (1 × 107 to 1 × 109 TCID50) of single stereotactic intratumoral injection of reovirus. In this initial study of direct CNS intratumoral inoculation, reovirus was again found to be well tolerated: patients had a median progression-free survival of 4.3 weeks (2.9–39) and a median survival of 21 weeks (6–234+). The tolerability of reovirus at the doses administered in this study of MG as well as other previous studies prompted us to design a clinical trial incorporating a dose escalation of virus, with the objective of reaching higher doses and better distribution of the investigational agent. By placement of catheters with direct infusion of virus into the tumor, the direct intraoperative time needed for the study was minimized. This study represents the first report of oncolytic virus delivery in humans via direct prolonged intratumoral infusion.
Convection-enhanced delivery (CED) is a novel drug-delivery method that relies on sustained, continuous low-pressure infusion via catheter to the exact tissue location. This method is especially promising in brain tissue, as it circumvents the blood–brain barrier and allows enhanced delivery to the site of interest, limiting systemic side effects, and increasing efficacy.17 CED allows particles to distribute over a large area of the brain regardless of intrinsic diffusibility due to pressure-driven delivery.18 This study represents the first attempted use of a CED-type of approach using an oncolytic virus in a clinical trial in North America.
The use of CED for the distribution of viral vectors in the CNS has been explored by various groups in recent years.19,20,21,22,23 Chen et al. first examined adeno-associated virus and adenovirus in a primate model and demonstrated that viral particles that did not bind extensively to the extracellular matrix had the best opportunity for distribution. Attempts to interrupt such binding with co- or preinfusion with agents such as heparin and albumin have met with variable success, indicating the need for further study. Nonetheless, the promise for increasing delivery of viral agents seems clear, particularly along white matter tracks towards infiltrating tumor; further work will need to be done to clarify methods for optimizing delivery of individual agents, including reovirus.
Results
Patient characteristics
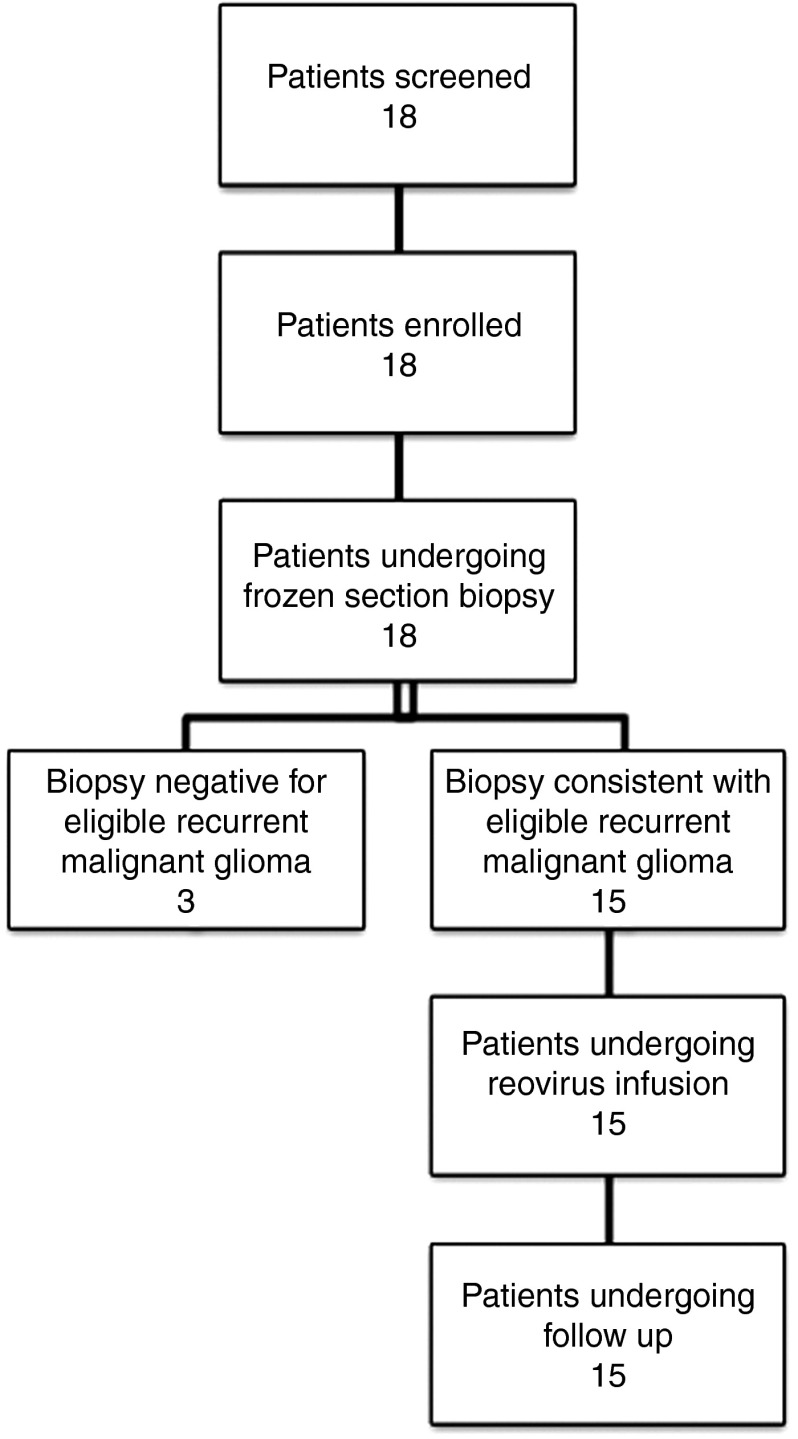
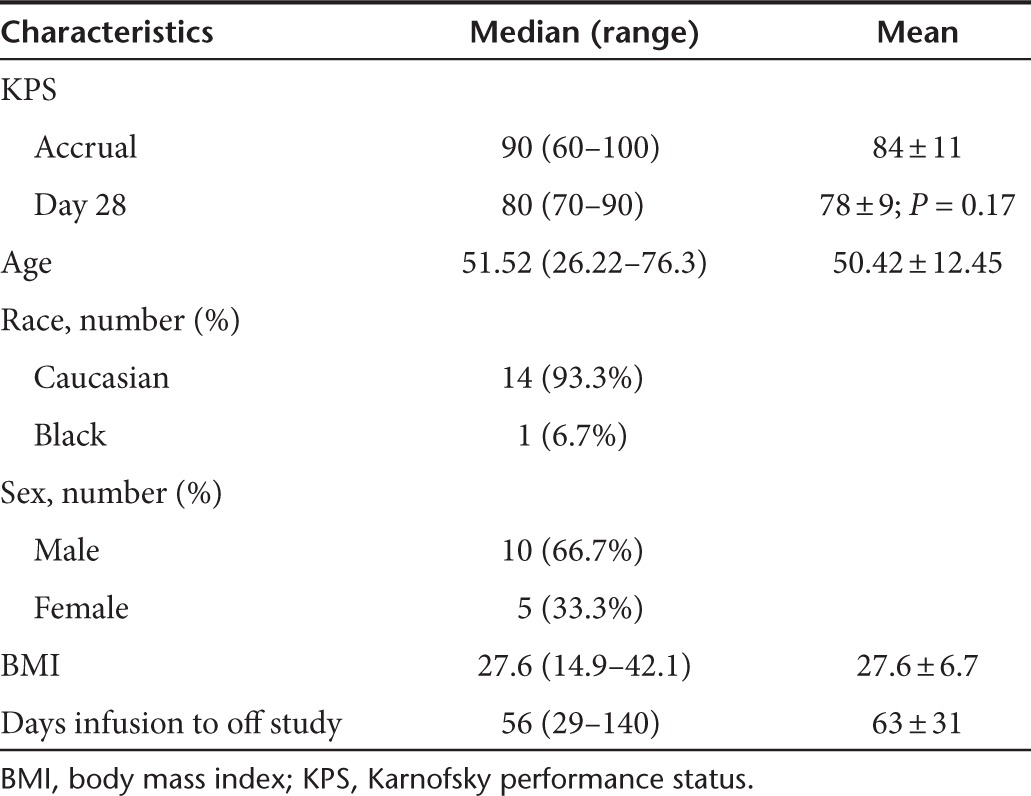
Patients were accrued from March 2006 to January 2010. Eighteen patients were enrolled and 15 were treated (Figure 1). Three patients were enrolled in the 3 × 109 cohort but were not treated. Three patients had pathology consistent with grade 3 tumors (anaplastic astrocytomas) while the remaining was grade 4 (glioblastoma multiforme). The last patient to complete the study ended official participation in April 2010. However, some patients had informal follow-up information available through September 2011. Although complete data on additional study protocols in which the study patients participated are unavailable, patients had a likely range of subsequent treatments. Data is known about some patients at the primary site. One patient went on to receive no additional therapies while another was enrolled in an additional experimental protocol. This range likely represents the study patients for which data is unavailable.
Figure 1.
Patient evaluation. Patients were evaluated for possible inclusion into the trial, enrolled, and treated with REOLYSIN as indicated in the accompanying flow diagram. A total of 18 patients were enrolled, and 15 were treated and received follow-up.
Eleven patients were discontinued from the study prior to scheduled follow-up secondary to progressive disease. Average time from infusion to discontinuation of the study in these patients was 63 ± 31 days. No patients were lost to follow-up. No protocol violations were recorded during the study. Demographic data for the patients enrolled can be found in Table 1.
Table 1. Patient demographics.
Viral titers and shedding
Pretreatment and posttreatment serum, saliva, urine, and feces were negative for viral genome in all but two patients based upon RT-PCR screening. Viral RNA was not detected in the feces of any patient. Patient four (3 × 108 dose level) had a positive signal in saliva on day 1 of treatment and urine on day 3. Patient 13 (1 × 1010 dose level) had a positive signal in the serum at baseline and day 35 and also in the urine on day 7. These results are consistent with those seen in many other studies where detection of viral genome has also been infrequent. Of note, previous studies from other laboratories have also reported positive signals in baseline specimens. Because reovirus is ubiquitous, such positive signals in serum at baseline may be due to either community-acquired exposure/infection or environmental contamination.
Outcome: time to progression and survival
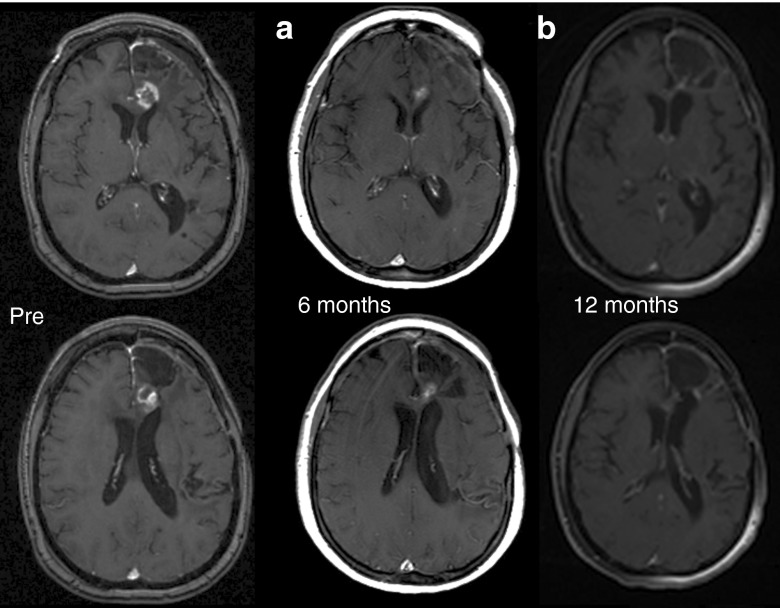
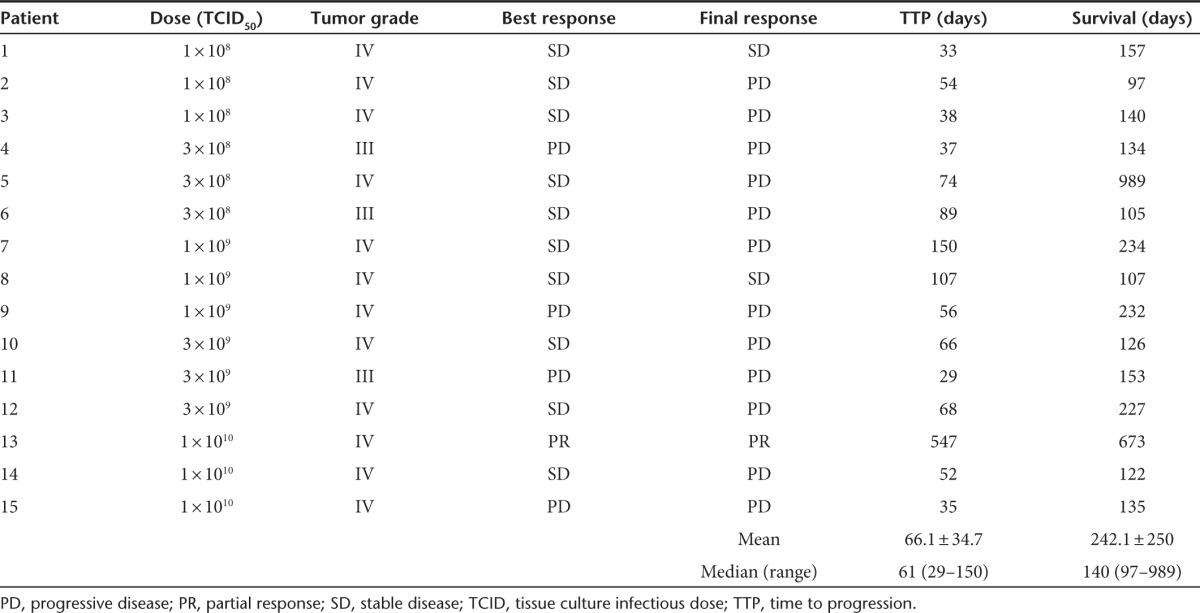
Ten patients had stable disease as their best response during the study period, one had a partial response, and four had progressive disease. Median survival was 140 days (range: 97–989). At the time of study discontinuation, 12 patients had tumor progression, 2 had stable disease, and 1 had a partial response. Median time to progression was 61 days (range: 29–150 days). DLTs were not identified and an MTD was not reached. Although subjects were followed for 24 weeks in the study, further follow-up was available on several patients. All patients were deceased at the time of statistical analysis and manuscript preparation. Of note, patient 5 survived nearly 3 years postreovirus treatment in the 3 × 108cohort while patient 13 survived nearly 2 years in the 1 × 1010cohort (see Figure 2). Full description of patient outcomes can be found in Table 2.
Figure 2.
Pre, 6- and 12-month posttreatment MRI scans. Shown are contrast-enhanced MRI scans from patient 13 with a recurrent glioblastoma multiforme who underwent a 72-hour infusion with 1 × 1010 TCID50 reovirus (highest dose tested). (a) MRI conducted at 6 months postinfusion, at formal end of trial, shows partial response compared to pre-entry MRI. (b) MRI conducted at 12 months postinfusion, on routine follow-up, and without any further antineoplastic therapy, shows a complete response compared to pre-entry MRI. MRI, magnetic resonance imaging; TCID50, tissue culture infectious dose50
Table 2. Patient outcomes.
Adverse events
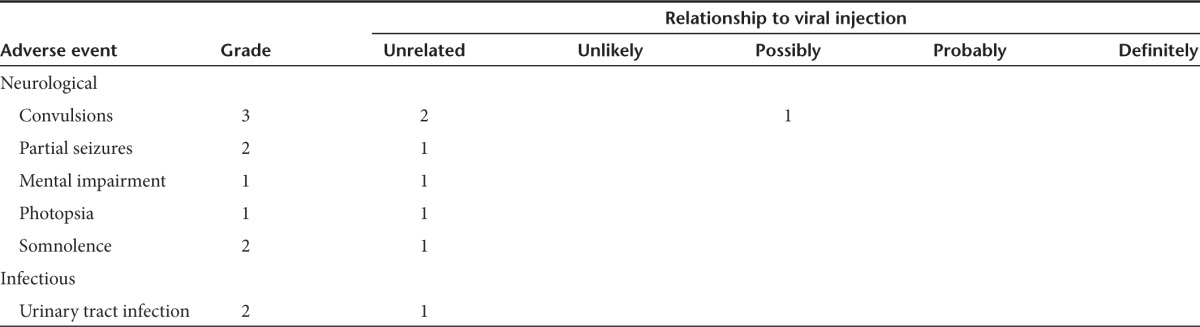
No deaths were considered to be due to the infusion of reovirus, nor were any severe adverse events (SAEs) identified. Five patients were found to have a total of nine SAEs (see Table 3). SAEs were found throughout all dose levels. The most common AEs were convulsions/seizures found in three of the five patients with SAEs. No SAE was designated as probably or definitely related to either reovirus or infusion. Tables 3 and 4 contain a complete listing of SAEs. The AEs encountered in this study are similar to those found previously in a glioma reovirus infusion study such as seizure and mental impairment, and the maximum dose in this study was higher than that of the previous trial (1 × 1010 compared to 1 × 109).16
Table 3. Significant adverse events.
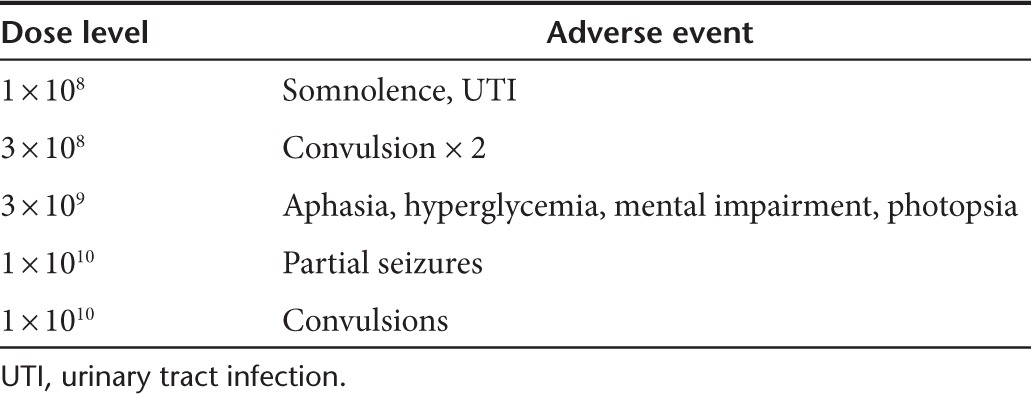
Table 4. Patients with serious adverse events.
Discussion
The aim of this phase 1 investigation was to evaluate the safety and tolerability of reovirus in MG patients. In this investigation of 15 patients enrolled at five dose levels, no DLT or MTD was reached, demonstrating that reovirus is both safe and well tolerated. The primary significant AEs involved seizures and convulsions, a common occurrence among all patients with intracranial mass lesions. These findings were not felt to be related to reovirus by the site investigators. However, these events were concentrated in two patients treated at the highest dose level, and thus future studies of reovirus at this dose level should include an analysis of the potential relationship of such events to reovirus administration.
All significant AEs were clustered at a single accrual site. While this site was also responsible for the largest number of patients (10/15), this raises the possibility of overreporting at this site, as well as underreporting at the other two accrual locations. However, the complete list of AEs, including those labeled unrelated to the study agent did not include any serious or life-threatening events. No event was identified as definitely related to reovirus infusion, supporting its safety profile.
This study represents the first attempted use of CED of virus in brain tumor patients. While studies of other viruses have demonstrated that increased volume of distribution and even convection are possible with viruses larger than reovirus (diameter: 70–80 nm), limitations to convection may be produced by viral size, surface proteins, and the local tumor microenvironment.19,20,21,22,23,24 Without tagged virus imaging studies (not performed due to financial constraints), convection of reovirus is not proven by this study. Nonetheless, previous studies with other viruses, along with the images in Figure 3, support the possibility that an increased volume of distribution of reovirus was produced by the delivery method employed in this study over simple inoculation alone. Other advantages of the approach include increased total volume and thus dose of virus deliverable, imaging confirmation of location of the virus delivery site prior to dosing, decreased operating room (OR) time due to lack of viral delivery in the OR, and confirmation of tumor recurrence by permanent section prior to dosing of virus.
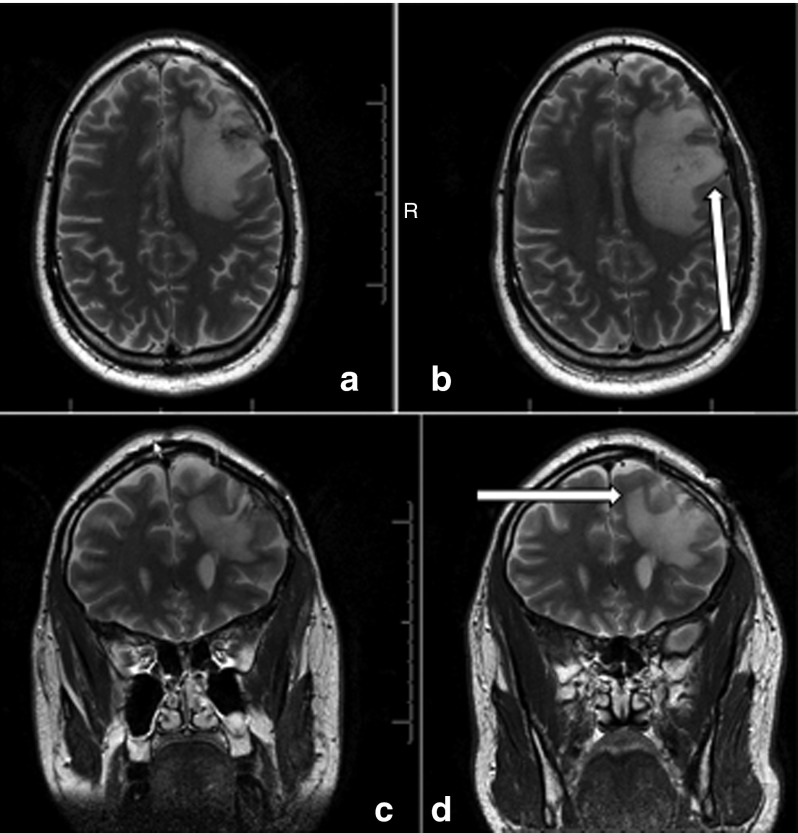
Figure 3.
Pre- and postinfusion MRIs. Shown are T2-weighted MRI preinfusion axial (a) and coronal (c) and 1-day postcompletion of infusion (postcatheter removal) axial (b) and coronal (d) from patient 13 (1 × 1010). Increased T2 signal is seen associated with cerebral edema produced by the underlying tumor preinfusion (see arrow). Postinfusion, there is a generalized increase in the T2 signal consistent with, but not proof of, convection of the agent. Due to the limitations of this study design and costs, direct labeling of the virus to confirm convection was not done. Such an increase in T2 signal could also be produced by inflammation related to local viral infection of tumor cells, but the uniform nature of the increased volume of T2 hyperintensity would still support an increased distribution of reovirus over simple inoculation. MRI, magnetic resonance imaging.
Despite the limitations of this small, phase 1 study, this method was well tolerated among participants. Although the study was not aimed or designed to determine efficacy, several patients enrolled in the study experienced prolonged survival, a promising finding for both reovirus and the approach.
The patients experiencing prolonged survival were scattered among different dose-ranges, suggesting a possible factor outside of exposure or area under the curve as the driving factor. Factors intrinsic to tumor type—genotype, immune factors, previous treatment modalities as well as viral titer and dosing function to optimize or synergize the antineoplastic effects of reovirus. Future studies should evaluate these factors and their capacity to affect survival. In addition, an MTD was not achieved, and the dosing scheme may represent a subtherapeutic regimen. Higher TCID50 doses could lead to more robust tumor response.
Limitations
The primary limitations of the study include the lack of posttreatment histological data and availability of planned tumor viral titers on enrollees. These limitations were necessitated by the intracranial nature of these tumors and the lack of ability to sample tumors posttreatment without performing an intracranial procedure. In addition, due to the lack of available funding, no concurrent study was performed to confirm an increase in viral distribution via the infusion approach used. The study was small and of a phase 1 nature, so no stratification by tumor subtype was appropriate. Although small, the study followed the standard 3 × 3 dose escalation design.
Future directions
Multiple phase 2 studies are under way involving reovirus combined with chemotherapeutic agents in malignancies such as squamous cell lung, ovarian, head and neck, pancreatic, and non–small cell lung cancers. In addition, phase 2 studies are underway of reovirus in metatastic neoplasms, especially in advanced melanoma and sarcoma. A phase 2 study of reovirus infusion in MG patients is planned as well. Reovirus serves as a versatile pathogen with applications spanning a wide variety of malignancies. With safety and tolerability proven in multiple phase 1 studies, reovirus warrants phase 2 investigations to evaluate efficacy, specifically in the treatment-poor area of MG.
Materials and Methods
Eligibility criteria. Eligible patients were defined as 18 years or older with either first, second, or third occurrence of a supratentorial tumor with a histologic diagnosis consistent with glioblastoma multiforme, gliosarcoma, anaplastic astrocytoma, anaplastic mixed glioma, or anaplastic oligodendroglioma. Histological diagnosis was confirmed by the neuropathologist at the participating medical center prior to trial enrollment. Recurrence was documented by either a biopsy less than 4 weeks prior to reovirus infusion or by frozen section during the same procedure as catheter placement but prior to reovirusinfusion. Tumors were required to be greater than or equal to 1 × 1 cm and accessible for stereotactic delivery without risk of injection into the ventricles, basal ganglia, foramen magnum, or posterior fossa. Patients must have been fully recovered from any AEs related to previous therapies as defined by return to grade 1 toxicity or lower (defined by the Common Terminology Criteria for Adverse Events).25 All patients were required to have been treated by surgical resection and external beam radiation of at least 5,000 cGy at the time of initial diagnosis, and all radiation was to be completed 6 weeks or more prior to reovirus administration. Patients were also required to be 4 weeks removed from any intracranial surgery, excluding needle biopsy, as well as chemotherapeutic medications (6 weeks in the case of nitrosureas). Enrollees were required to have a life expectancy of >8 weeks and a Karnofsky Performance Status of at least 60. Women of child-bearing age underwent a negative urine pregnancy screen, and all enrollees were required to use barrier methods of contraception during the study period. Patients were required to be on a stable steroid dose for 2 weeks prior to baseline/admission magnetic resonance imaging (MRI) scan, not to exceed 24 mg/day of dexamethasone. Patients were also required to prove general health prior to study entry through benchmark results on the following laboratory and diagnostic studies: absolute neutrophil count ≥ 1.5 × 109/l, hemoglobin ≥ 10g/dl, platelets ≥ 100 × 109/l; alanine transaminase, total bilirubin, and creatinine ≤ 1.5× upper limit normal, prothrombin time within normal limits and electrocardiogram with no evidence of active cardiac disease. All patients underwent infusion within 2 weeks of enrollment, and were required to live in a geographically-accessible area to one of the study sites. Three study sites were chosen: The University of Alabama of Birmingham Medical Center, Cedars Sinai Medical Center, and The Ohio State Medical Center, Columbus, OH. The Institutional Review Boards at the respective study sites approved the study, and all patients signed a consent form indicating their understanding of the investigational nature of the study.
Patients were excluded on the basis of severe medical or psychiatric comorbidities, >1 enhancing lesion on MRI, evidence of satellite lesions, or leptomeningeal enhancement. Patients with a history of multiple sclerosis, chronic CNS disease, encephalitis, active CNS infection, meningeal gliomatosis, gliomatosis cerebri, or inclusion in another viral or intratumoral protocol were excluded. Patients receiving therapy with Gliadel wafers were eligible as long as implantation predated reovirus infusion by at least 6 months. Patients were also excluded on the basis of bleeding disorders, acquired or hereditary immune disorders, a history of hepatitis or tuberculosis, treatment with systemic antivirals within 6 months, previous brachytherapy or radiotherapy of the brain, previous or concurrent malignancy, or more than three recurrences.
Patients were able to withdraw from the study at any time, for whatever reason, including for progressive disease requiring additional treatment. All statistical analyses were completed under the intention to treat principle.
Treatment plan. Purified reovirus (REOLYSIN) along with dilution instructions were provided by Oncolytics Biotech (Calgary, Alberta, Canada) to each of the three study sites involved in this multicenter trial. Following standard stereotactic frame placement and MRI, patients underwent stereotactic placement of one to four intralesional catheters into areas of MRI enhancement of a single tumor (patients underwent stereotactic biopsy immediately prior to catheter implantation if no biopsy demonstrating recurrent tumor had been obtained within 4 weeks of entry). For the initial six patients, two catheters were placed per patient. However, intermittent clogging of one of these catheters occurred in the majority of these patients for at least a portion of the infusion. While patients did, all receive the complete volume of infusate and total dose of reovirus anticipated, the distribution of the virus was likely impacted negatively. To overcome this potential issue, the protocol was then amended to give the treating neurosurgeon the option to increase the number of catheters placed up to four total. CED catheters (Phoenix Biomedical, Ontario, Canada CD435) were passed with stylet in place using standard stereotactic technique, tunneled subcutaneously, and secured. Luer-locks were placed, and 0.2 ml of sterile saline was used to flush each catheter. Care was taken to use different catheter passes for each to limit viral reflux. Catheters were placed at least 2 cm deep to the cortical surface, and 1 cm from ventricles, sulci, and previous resection cavities. Patients were required to be on therapeutic doses of antiepileptic drugs at the time of stereotactic catheter implantation, and further treatment with antiepileptic drugs was undertaken at the discretion of the treating neurosurgeon, as was perioperative antibiotic administration. Following computed tomography confirmation of catheter location (see Figure 4) and confirmatory diagnosis of recurrent tumor by pathology, patients underwent 72-hour infusion of reovirus via syringe pump (Sims-Deltec CADD-Micro infusion pump, St Paul, Minnesota, model 5900) at a rate of 400 µl/hour divided evenly among the number of catheters for a total of 9.6 µl/24 hours. The rate of infusion chosen was based upon previous studies and was an intermediate rate, with a comparable total volume of infusate.26,27 In the event that a postoperative computed tomography revealed improper position of a catheter, the full dose was distributed between the remaining, appropriately placed catheters. Although reovirus is stable at room temperature, syringes on each pump were changed every 12 hours to protect against titer loss over time.
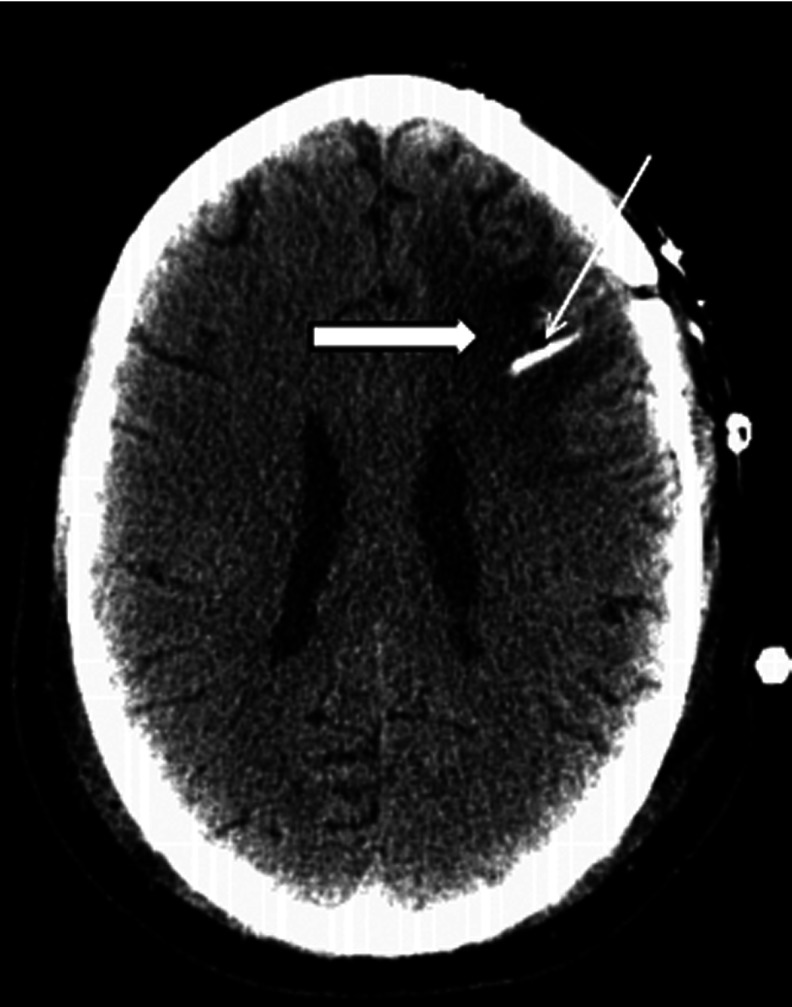
Figure 4.
Catheter placement. Representative computed tomography showing a typical catheter placement. White arrow indicates catheter (white). Block arrow with black outline shows tumor, dark gray. Only a single catheter is visualized on this axial view as catheters were intentionally placed at opposite or at least distant poles of tumor to maximize virus distribution. Catheter placement was made by the neurosurgeon such that the virus would be delivered to the enhancing rim of the tumor as seen on postgadolinium magnetic resonance imaging.
Patients were enrolled sequentially in cohorts starting at the following doses: 1 × 108 TCID50, 3 × 108 TCID50, 1 × 109 TCID50, 3 × 109 TCID50, and 1 × 1010 TCID50.28 Three patients were recruited for each cohort in a standard dose-escalation scheme. The first patient in each cohort was observed for 14 days before accrual of subsequent patients at that dose level. The last enrollee in each cohort was observed for 28 days before patients were accrued for the next cohort.
Patients were monitored with neurological examinations and vital sign assessments at 30 minutes, hourly, two-hourly, and every 12 hours thereafter. Patients were hospitalized for at least 90 hours postinitiation of reovirus infusion, and provided with information on infectious precautions at the time of discharge. They were evaluated with imaging studies, laboratory studies, and clinical exams at baseline, prior to discharge, and monthly for 12 weeks in the scheme presented in Table 5. At visits, patients were also monitored for potential toxicities and AEs. With patient permission, enrollees were followed monthly beyond 12 weeks via phone call to the treating physician to ascertain disease status and survival. Contrast-enhanced MRI allowed detailed descriptions of the lesion along with measurement of the maximum perpendicular diameter on axial imaging. Primary endpoints were time-to-progression and survival. Final assessment was done at the time of progression or final follow up, all of which were prior to death. All surplus reovirus was returned to oncolytics for destruction. Appropriate biohazard techniques were utilized intraoperatively as well as postoperatively.
Table 5. Schedule of events.
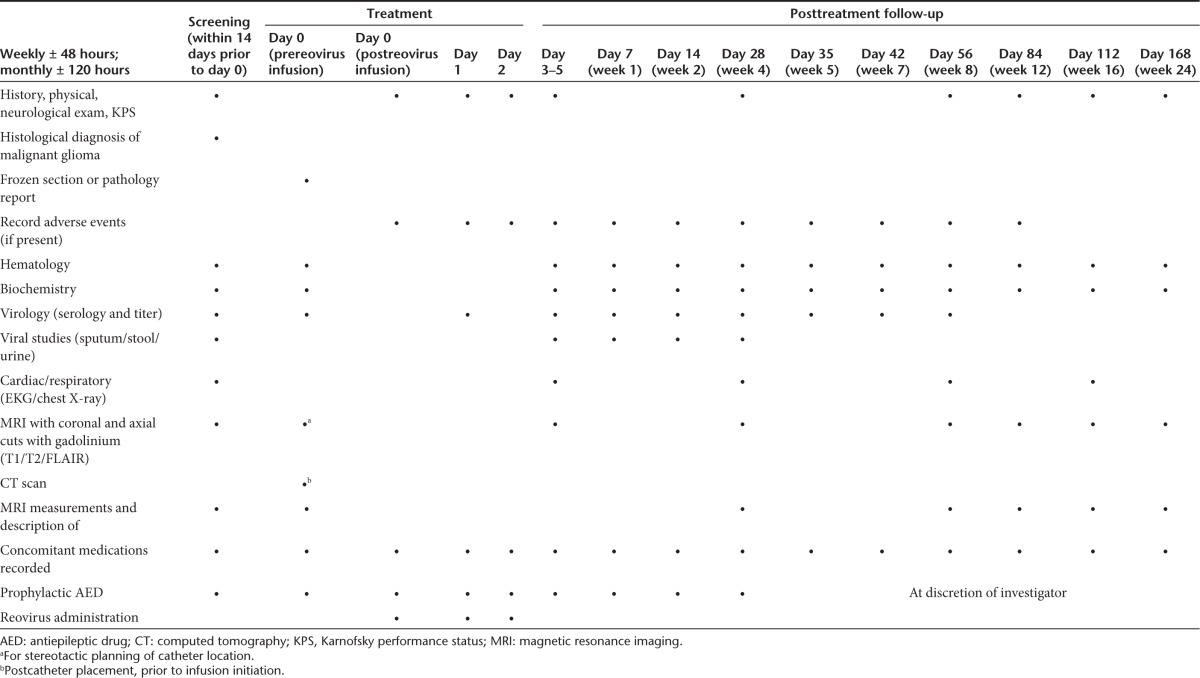
Assessment and efficacy. MRI scan on a stable steroid dose was required for diagnosis of disease response and the MacDonald criteria were applied to assess treatment response.29 Images were centrally reviewed for the determination of disease state. Times from infusion to progression and dates of death were recorded for all patients in order to calculate survival from time of infusion. AEs were reported based on the schedule presented in Table 5 in an attempt to determine an MTD and any DLTs.
Statistical methods. Descriptive statistics were calculated for demographic information as well as outcome and survival data. Analyses were carried out by both the authors and the sponsor.
Acknowledgments
Acknowledgment of Research Support: Oncolytics Biotech; M.C. is CSO of Oncolytics Biotech; J.M.M received funding support, provided by Oncolytics to UAB, for his role as principal investigator; and the clinical trial was sponsored by Oncolytics Biotech Inc. G.M.G. is Senior Vice President of Regulatory Affairs and Chief Safety Officer at Oncolytics. The authors wish to thank Suzanne Byan-Parker (Medical Writer, University of Alabama at Birmingham, Division of Neurosurgery Research Office) for her assistance in the preparation of this manuscript.
References
- Buonerba C, Di Lorenzo G, Marinelli A, Federico P, Palmieri G, Imbimbo M, et al. A comprehensive outlook on intracerebral therapy of malignant gliomas. Crit Rev Oncol Hematol. 2011;80:54–68. doi: 10.1016/j.critrevonc.2010.09.001. [DOI] [PubMed] [Google Scholar]
- Everson RG, Gromeier M, Sampson JH. Viruses in the treatment of malignant glioma. Expert Rev Neurother. 2007;7:321–324. doi: 10.1586/14737175.7.4.321. [DOI] [PubMed] [Google Scholar]
- Parato KA, Senger D, Forsyth PA, Bell JC. Recent progress in the battle between oncolytic viruses and tumours. Nat Rev Cancer. 2005;5:965–976. doi: 10.1038/nrc1750. [DOI] [PubMed] [Google Scholar]
- Strong JE, Coffey MC, Tang D, Sabinin P, Lee PW. The molecular basis of viral oncolysis: usurpation of the Ras signaling pathway by reovirus. EMBO J. 1998;17:3351–3362. doi: 10.1093/emboj/17.12.3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey MC, Strong JE, Forsyth PA, Lee PW. Reovirus therapy of tumors with activated Ras pathway. Science. 1998;282:1332–1334. doi: 10.1126/science.282.5392.1332. [DOI] [PubMed] [Google Scholar]
- Alain T, Kim TS, Lun X, Liacini A, Schiff LA, Senger DL, et al. Proteolytic disassembly is a critical determinant for reovirus oncolysis. Mol Ther. 2007;15:1512–1521. doi: 10.1038/sj.mt.6300207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox ME, Yang W, Senger D, Rewcastle NB, Morris DG, Brasher PM, et al. Reovirus as an oncolytic agent against experimental human malignant gliomas. J Natl Cancer Inst. 2001;93:903–912. doi: 10.1093/jnci/93.12.903. [DOI] [PubMed] [Google Scholar]
- Hirasawa K, Nishikawa SG, Norman KL, Alain T, Kossakowska A, Lee PW. Oncolytic reovirus against ovarian and colon cancer. Cancer Res. 2002;62:1696–1701. [PubMed] [Google Scholar]
- Hirasawa K, Nishikawa SG, Norman KL, Coffey MC, Thompson BG, Yoon CS, et al. Systemic reovirus therapy of metastatic cancer in immune-competent mice. Cancer Res. 2003;63:348–353. [PubMed] [Google Scholar]
- Yang WQ, Senger D, Muzik H, Shi ZQ, Johnson D, Brasher PM, et al. Reovirus prolongs survival and reduces the frequency of spinal and leptomeningeal metastases from medulloblastoma. Cancer Res. 2003;63:3162–3172. [PubMed] [Google Scholar]
- Yang WQ, Senger DL, Lun XQ, Muzik H, Shi ZQ, Dyck RH, et al. Reovirus as an experimental therapeutic for brain and leptomeningeal metastases from breast cancer. Gene Ther. 2004;11:1579–1589. doi: 10.1038/sj.gt.3302319. [DOI] [PubMed] [Google Scholar]
- Yang WQ, Lun X, Palmer CA, Wilcox ME, Muzik H, Shi ZQ, et al. Efficacy and safety evaluation of human reovirus type 3 in immunocompetent animals: racine and nonhuman primates. Clin Cancer Res. 2004;10:8561–8576. doi: 10.1158/1078-0432.CCR-04-0940. [DOI] [PubMed] [Google Scholar]
- Yap TA, Vidal L, Pandha H, Spicer J, Digue L, Coffey M, et al. A phase I study of wild-type reovirus, which selectively replicates in cells expressing activated Ras, administered intravenously to patients with advanced cancer. EJC Suppl. 2006;4 [Google Scholar]
- Vidal L, Twigger K, White CL, Ahmed M, Pandha HS, Nutting CM, et al. A phase I trial of intratumoral administration of reovirus type 3 (Reolysin) in combination with radiation in patients with advanced malignancies. Proc Am Assoc Cancer Res. 2006;47 [Google Scholar]
- Gollamudi R, Ghalib MH, Desai KK, Chaudhary I, Wong B, Einstein M, et al. Intravenous administration of Reolysin, a live replication competent RNA virus is safe in patients with advanced solid tumors. Invest New Drugs. 2010;28:641–649. doi: 10.1007/s10637-009-9279-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsyth P, Roldán G, George D, Wallace C, Palmer CA, Morris D, et al. A phase I trial of intratumoral administration of reovirus in patients with histologically confirmed recurrent malignant gliomas. Mol Ther. 2008;16:627–632. doi: 10.1038/sj.mt.6300403. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Saito R, Sugiyama S, Sonoda Y, Kumabe T, Tominaga T. Local convection-enhanced delivery of chemotherapeutic agent transiently opens blood-brain barrier and improves efficacy of systemic chemotherapy in intracranial xenograft tumor model. Cancer Lett. 2011;310:77–83. doi: 10.1016/j.canlet.2011.06.018. [DOI] [PubMed] [Google Scholar]
- Rosenbluth KH, Eschermann JF, Mittermeyer G, Thomson R, Mittermeyer S, Bankiewicz KS. Analysis of a simulation algorithm for direct brain drug delivery. Neuroimage. 2012;59:2423–2429. doi: 10.1016/j.neuroimage.2011.08.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MY, Hoffer A, Morrison PF, Hamilton JF, Hughes J, Schlageter KS, et al. Surface properties, more than size, limiting convective distribution of virus-sized particles and viruses in the central nervous system. J Neurosurg. 2005;103:311–319. doi: 10.3171/jns.2005.103.2.0311. [DOI] [PubMed] [Google Scholar]
- Idema S, Caretti V, Lamfers ML, van Beusechem VW, Noske DP, Vandertop WP, et al. Anatomical differences determine distribution of adenovirus after convection-enhanced delivery to the rat brain. PLoS ONE. 2011;6:e24396. doi: 10.1371/journal.pone.0024396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White E, Bienemann A, Megraw L, Bunnun C, Wyatt M, Taylor H, et al. Distribution properties of lentiviral vectors administered into the striatum by convection-enhanced delivery. Hum Gene Ther. 2012;23:115–127. doi: 10.1089/hum.2010.185. [DOI] [PubMed] [Google Scholar]
- White E, Bienemann A, Sena-Esteves M, Taylor H, Bunnun C, Castrique E, et al. Evaluation and optimization of the administration of recombinant adeno-associated viral vectors (serotypes 2/1, 2/2, 2/rh8, 2/9, and 2/rh10) by convection-enhanced delivery to the striatum. Hum Gene Ther. 2011;22:237–251. doi: 10.1089/hum.2010.129. [DOI] [PubMed] [Google Scholar]
- White E, Bienemann A, Taylor H, Castrique E, Bunnun C, Wyatt M, et al. An evaluation of site-specific immune responses directed against first-generation adenoviral vectors administered by convection-enhanced delivery. J Gene Med. 2011;13:269–282. doi: 10.1002/jgm.1567. [DOI] [PubMed] [Google Scholar]
- Agosto MA, Ivanovic T, Nibert ML. Mammalian reovirus, a nonfusogenic nonenveloped virus, forms size-selective pores in a model membrane. Proc Natl Acad Sci USA. 2006;103:16496–16501. doi: 10.1073/pnas.0605835103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Department of Health and Human Service Common Terminology Criteria for Adverse Events v4.0. 5/28/09.
- Mehta AI, Choi BD, Raghavan R, Brady M, Friedman AH, Bigner DD, et al. Imaging of convection enhanced delivery of toxins in humans. Toxins (Basel) 2011;3:201–206. doi: 10.3390/toxins3030201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson JH, Akabani G, Friedman AH, Bigner D, Kunwar S, Berger MS, et al. Comparison of intratumoral bolus injection and convection-enhanced delivery of radiolabeled antitenascin monoclonal antibodies. Neurosurg Focus. 2006;20:E14. doi: 10.3171/foc.2006.20.4.9. [DOI] [PubMed] [Google Scholar]
- Darling AJ, Boose JA, Spaltro J. Virus assay methods: accuracy and validation. Biologicals. 1998;26:105–110. doi: 10.1006/biol.1998.0134. [DOI] [PubMed] [Google Scholar]
- Wilson CB, Crafts D, Levin V. Brain tumors: criteria of response and definition of recurrence. Natl Cancer Inst Monogr. 1977;46:197–203. [PubMed] [Google Scholar]