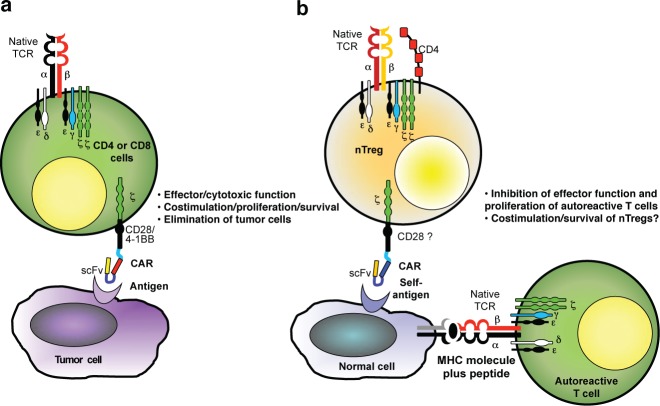
The concept of chimeric antigen receptor (CAR) was pioneered in 1989 by Eshhar and colleagues with the specific goal of providing an alternative means by which T lymphocytes can engage antigens expressed by target cells.1 Until recently, the focus of CAR–T cell research has been on achieving effector function in T lymphocytes that can target cancer cells and destroy them. In this issue of Molecular Therapy, however, Eshhar's group illuminates another face of CAR-based technology.2 They engrafted CARs in naturally occurring regulatory T cells (nTregs) to produce “loss of function” of an unwanted T-cell response that causes inflammation, thereby ameliorating an ongoing autoimmune disorder (Figure 1).
Figure 1.
CAR function in (a) effector T cells to eliminate tumor cells and (b) nTregs to control autoimmune diseases. Costimulation in CAR-redirected nTregs remains to be defined. CAR, chimeric antigen receptor; MHC, major histocompatibility complex; nTregs, native T-regulatory cells; scFv, single-chain variable fragment; TCR, T-cell receptor.
CARs are chimeric proteins with two main components: the extracellular moiety usually derived from a monoclonal antibody and an intracellular signaling moiety usually derived from the ζ-chain of the CD3–T cell receptor (TCR) complex. By expressing these proteins in T lymphocytes, Eshhar and colleagues in their first discovery overcame two major obstacles limiting the clinical translation of adoptive T-cell therapies.1 Expression of CARs by polyclonal T cells allows rapid production of antigen-specific effector T cells without having to expand this population from a small number of cell precursors. Moreover, and in contrast to the native αβTCR of T cells, CAR-based antigen recognition does not require antigen processing by the cell for presentation in association with major histocompatibility complex (MHC), and so can be used for all patients, irrespective of their MHC phenotype, even if the targeted tumor cells downregulate their antigen-processing machinery or expression of MHC.
CAR technology was described more than two decades ago, but successful clinical implementation has been much more recent. Although the gradual evolution of effective techniques for producing viral vectors and for transducing and expanding human T lymphocytes certainly contributed to the clinical development of CAR-based T-cell therapies, the key event was the adaptation of the concept of T-cell costimulation to CAR molecules.3,4 By incorporating a third component within CARs—the intracytoplasmic domains derived from early and late costimulatory molecules—investigators showed that these elements produced sufficient in vivo survival and/or expansion of CAR-redirected T cells for the effective control of tumor growth in humans.5,6
Although effector T cells that target malignant tissues are of clinical value, an unwanted effector immune response that damages normal tissues, as in autoinflammatory diseases, can be highly destructive. Eshhar and colleagues sought to adapt the CAR–T cell concept so that it could inhibit ongoing, but unwanted, immune responses. Using murine autoimmune colitis as a model, they found that adoptive transfer of nTregs engineered to express a CAR that targets the carcinoembryonic antigen (CEA) can block the inflammatory disease. This model is relevant to human inflammatory colitis (e.g., Crohn's disease), because CEA is expressed in human inflamed intestinal tissues.7,8 In addition, colitis was observed as a side effect in a clinical trial designed to target CEA expressed by gastrointestinal tumors using T cells redirected with a high-affinity CEA-specific αβTCR, indicating that there is sufficient antigen expression and processing in the intestine to trigger T-cell activation.9
Clinical translation of CAR-modified nTregs may be feasible; several approaches have been developed to select and expand functional nTregs ex vivo that are sufficiently robust and compliant with good manufacturing practice for clinical use,10,11 and several clinical studies are investigating their ability to suppress unwanted immune reactivity.12 If these nTregs could be more specific to antigens expressed by inflamed tissues, then these cells would become activated (and hence fully inhibitory) at the site of autoimmune inflammation, and in humans, as in mice, exert effective and selective immune suppression. Importantly, Eshhar and colleagues point out that even if we do not know the antigens that are the true targets for autoimmune effector cells in human diseases, nTregs can be activated at the site of inflammation if the CAR they express can recognize a known tissue-restricted self-antigen, such as CEA, present at the site of disease.
There remain significant obstacles to the clinical implementation of CAR-modified nTregs. There are, of course substantial differences between mouse and human nTregs. The selection of CD4+CD25+ T cells in mice allows a significant enrichment of nTregs. In humans, the selection of this scanty T-cell subset does not completely eliminate effector T cells that can express similar phenotypic markers. The addition of rapamycin or its analogs during the ex vivo culture of selected human nTregs cells helps to selectively expand nTregs but does not completely eliminate effector T cells.11,13,14 These residual effector T cells may be of particular concern in an autoimmune disorder because if they are engineered to express a CAR that targets a tissue-restricted self-antigen to the site of autoimmune disease, the consequence may be exacerbation of the disease rather than the intended amelioration. The optimal design of CARs for nTregs also remains unclear. Eshhar and colleagues used a second-generation CAR that encodes the CD28 costimulatory endodomain known to promote costimulation and persistence of CAR effector T cells in cancer patients.15 The current study by Eshhar's group was not designed to evaluate the long-term persistence of CAR-modified nTregs. However, because of the usually chronic nature of human autoimmune diseases, including inflammatory bowel diseases, long-term persistence of CAR-redirected nTregs would be desirable. It is unknown whether CD28 costimulation through a CAR will be sufficient for the required long-term persistence of modified nTregs.16 Indeed, it is unknown whether constitutive costimulation through a CAR will ultimately divert human nTreg cells from their inhibitory function.
Eshhar and colleagues first described CAR-modified effector T cells 25 years ago, and the road to their successful clinical development has turned out to be both long and winding. But we can hope the experience gained on that first journey will enable this new application of CARs to reach its destination more directly and with greater speed.
References
- Gross G, Waks T., and, Eshhar Z. Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc Natl Acad Sci USA . 1989;86:10024–10028. doi: 10.1073/pnas.86.24.10024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blat D, Zigmond E, Alteber Z, Waks T., and, Eshhar Z. Suppression of murine colitis and its associated cancer by carcinoembryonic antigen-specific regulatory T cells. Mol Ther. 2014;22:1018–1028. doi: 10.1038/mt.2014.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finney HM, Lawson AD, Bebbington CR., and, Weir AN. Chimeric receptors providing both primary and costimulatory signaling in T cells from a single gene product. J Immunol . 1998;161:2791–2797. [PubMed] [Google Scholar]
- Maher J, Brentjens RJ, Gunset G, Riviere I., and, Sadelain M. Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRzeta/CD28 receptor. Nat Biotechnol. 2002;20:70–75.. doi: 10.1038/nbt0102-70. [DOI] [PubMed] [Google Scholar]
- Kalos M, Levine BL, Porter DL, Katz S, Grupp SA, Bagg A.et al. (2011T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia Sci Transl Med 395ra73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brentjens RJ, Davila ML, Riviere I, Park J, Wang X, Cowell LG.et al. (2013CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia Sci Transl Med 5177ra38.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammarstrom S. The carcinoembryonic antigen (CEA) family: structures, suggested functions and expression in normal and malignant tissues. Semin Cancer Biol. 1999;9:67–81.. doi: 10.1006/scbi.1998.0119. [DOI] [PubMed] [Google Scholar]
- Smithson JE, Warren BF, Young S, Pigott R., and, Jewell DP. Heterogeneous expression of carcinoembryonic antigen in the normal colon and upregulation in active ulcerative colitis. J Pathol. 1996;180:146–151.. doi: 10.1002/(SICI)1096-9896(199610)180:2<146::AID-PATH643>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Parkhurst MR, Yang JC, Langan RC, Dudley ME, Nathan DA, Feldman SA.et al. (2011T cells targeting carcinoembryonic antigen can mediate regression of metastatic colorectal cancer but induce severe transient colitis Mol Ther 19620–626.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hippen KL, Merkel SC, Schirm DK, Sieben CM, Sumstad D, Kadidlo DM.et al. (2011Massive ex vivo expansion of human natural regulatory T cells (T(regs)) with minimal loss of in vivo functional activity Sci Transl Med 383ra41.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty R, Mahendravada A, Perna SK, Rooney CM, Heslop HE, Vera JF.et al. (2013Robust and cost effective expansion of human regulatory T cells highly functional in a xenograft model of graft-versus-host disease Haematologica 98533–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roncarolo MG., and, Battaglia M. Regulatory T-cell immunotherapy for tolerance to self antigens and alloantigens in humans. Nat Rev Immunol. 2007;7:585–598. doi: 10.1038/nri2138. [DOI] [PubMed] [Google Scholar]
- Battaglia M, Stabilini A, Migliavacca B, Horejs-Hoeck J, Kaupper T, Roncarolo MG. Rapamycin promotes expansion of functional CD4+CD25+FOXP3+ regulatory T cells of both healthy subjects and type 1 diabetic patients. J Immunol. 2006;177:8338–8347. doi: 10.4049/jimmunol.177.12.8338. [DOI] [PubMed] [Google Scholar]
- Tresoldi E, Dell'albani I, Stabilini A, Jofra T, Valle A, Gagliani N.et al. (2011Stability of human rapamycin-expanded CD4+CD25+ T regulatory cells Haematologica 961357–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savoldo B, Ramos CA, Liu E, Mims MP, Keating MJ, Carrum G.et al. (2011CD28 costimulation improves expansion and persistence of chimeric antigen receptor–modified T cells in lymphoma patients J Clin Invest 1211822–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golovina TN, Mikheeva T, Suhoski MM, Aqui NA, Tai VC, Shan X.et al. (2008CD28 costimulation is essential for human T regulatory expansion and function J Immunol 1812855–2868. [DOI] [PMC free article] [PubMed] [Google Scholar]