Abstract
One of the most common oral manifestations of menopause is xerostomia. Oral dryness can profoundly affect quality of life and interfere with basic daily functions, such as chewing, deglutition, and speaking. Although the feeling of oral dryness can be ameliorated after estrogen supplementation, the side effects of estrogen greatly restrict its application. We previously found that N-myc downstream-regulated gene 2 (NDRG2) is involved in estrogen-mediated ion and fluid transport in a cell-based model. In the present study, we used an ovariectomized rat model to mimic xerostomia in menopausal women and constructed two adenovirus vectors bearing NDRG2 to validate their therapeutic potential. Ovariectomized rats exhibited severe sialaden hypofunction, including decreased saliva secretion and ion reabsorption as well as increased water intake. Immunohistochemistry revealed that the expression of NDRG2 and Na+ reabsorption-related Na+/K+-ATPase and epithelial sodium channels (EnaC) decreased in ovariectomized rat salivary glands. We further showed that the localized delivery of NDRG2 improved the dysfunction of Na+ and Cl− reabsorption. In addition, the saliva flow rate and water drinking recovered to normal. This study elucidates the mechanism of estrogen deficiency-mediated xerostomia or sialaden hypofunction and provides a promising strategy for therapeutic intervention.
Introduction
Menopause is accompanied by physical changes in the oral cavity.1 The major oral symptoms of menopause are xerostomia with or without a decrease in absolute saliva volume.2,3 Xerostomia is the subjective feeling of dry mouth and has widespread implications, including oral pain and impaired function of deglutition and speech.4 Despite it is generally thought that menopause-induced xerostomia is estrogen-related3,5,6 and many estrogens are known to regulate saliva composition and secretion,7,8,9,10 the specific mechanism has not been fully elucidated. Previous studies have reported that estrogen supplements can relieve xerostomia in menopausal women,11,12,13 but the side effects of estrogen, such as endometrial proliferation, augment the need for more specific and effective therapies.
The salivary secretion process was described as early as 1954.14 According to the two-stage hypothesis, the NaCl-rich hyperosmotic or isotonic plasma-like primary saliva is initially secreted by salivary acinar cells. Subsequently, as primary saliva travels through the duct system, salivary ducts reabsorb some electrolytes, such as NaCl, modifying the electrolyte composition of the primary saliva. Because the duct cells do not allow any water movement, the final saliva becomes hypotonic.15,16 Throughout this process, ductal reabsorption plays a vital role in modulating the osmotic pressure of saliva, and aberrant reabsorption may contribute to the feeling of dry mouth. Epithelial sodium channels (ENaC) were found to be the primary molecular determinants of Na+ reabsorption in salivary ducts.17,18 Another important factor in the modulation of saliva electrolytes is Na+/K+-ATPase,19,20,21 which is widely present in the cell membrane and consists of three subunits, α, β, and γ. The β subunit of Na+/K+-ATPase facilitates the transport of the α subunit to the plasma membrane, and the α subunit executes the catalytic function.22 Na+/K+-ATPase is mainly localized on the basolateral side of the ductal cell membrane18 and extrudes Na+ into the intercellular space, creating a low intracellular Na+ concentration and providing the driving force of Na+ reabsorption through ENaC on the apical membrane.23 Although ENaC and Na+/K+-ATPase play important roles in the saliva-forming process, their correlation with impaired saliva secretion and reabsorption in xerostomia is largely unknown.
The N-myc downstream-regulated gene 2 (NDRG2) is a member of the NDRG family and was first identified and cloned in our laboratory.24 NDRG2 was found to be a potential sodium transport regulator. In addition to its known functions in stimulating amiloride-sensitive Na+ current in Xenopus laevis oocytes and Fischer rat thyroid cells,25 NDRG2 can also activate Na+/K+-ATPase to promote Na+ transport in human salivary duct cells.26 Interestingly, when we studied the expression profile of NDRG2 by immunohistochemistry, we found that NDRG2 specially expressed in the duct cells of adult mouse sublingual glands, but not in acinar cells.27 Moreover, we recently found that NDRG2 is a novel estrogen target gene and suggested a novel estrogen/NDRG2/Na+/K+-ATPase regulation pathway in cell electrolyte transport.26 Therefore, delivery of NDRG2 into the salivary gland may represent a potential therapeutic option for the relief of estrogen deficiency-xerostomia.
The present study addressed whether and how NDRG2 is involved in sialaden hypofunction caused by estrogen deprivation and evaluated the potential therapeutic effect of adenovirus (Ad)-mediated NDRG2 expression on sialaden hypofunction, including saliva composition and symptoms.
Results
Sialaden hypofunction induced in salivary gland cells by ovariectomy (Ovx)
To examine the in vivo effects of estrogen deprivation in rats, Ovx was performed at the age of 8 weeks. A radioimmunoassay confirmed that β-estradiol was quickly decreased in the sera of Ovx-rats compared with control or sham Ovx groups (Supplementary Figure S1). The saliva electrolyte composition, saliva flow rate, and water intake in different groups were longitudinally measured before and after Ovx. At 10–30 days after Ovx, elevated concentrations of Na+ and Cl−, but no changes in K+ and Ca2+ were detected in the saliva (Figure 1a–d). Ovx-rats also showed significantly decreased saliva flow rates in a time-dependent manner (Figure 1e). In addition, water intake increased from 120 ml/kg•24 hours (basic water intake) to 170 ml/kg•24 hours and 210 ml/kg•24 hours at 20 and 30 days after Ovx, respectively (Figure 1f). Therefore, estrogen deficiency-induced sialaden hypofunction in Ovx-rats can mimic xerostomia in menopausal women.
Figure 1.
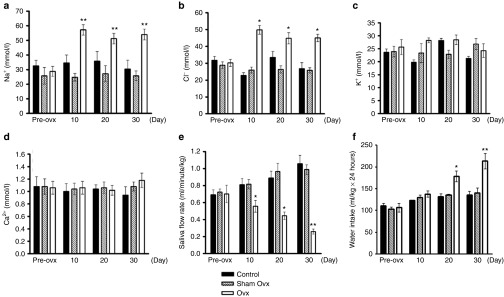
Estrogen deprivation suppresses salivary gland functions in Ovx-rats. Saliva was collected pre-Ovx, or 10, 20, and 30 days after Ovx, from Sprague Dawley rats (n = 8). (a–d) Salivary ion concentrations were measured in the different groups of rats. (a) Na+, (b) Cl−, (c) K+, (d) Ca2+. (e) Pilocarpine-stimulated salivary flow rates were detected in the Ovx and control groups. (f) The effect of estrogen deprivation on rats' water intake. Data are expressed as the mean ± SEM. *P < 0.05; **P < 0.01 versus control.
Estrogen deficiency inhibited the reabsorption of Na+ and Cl− in salivary ductal cells
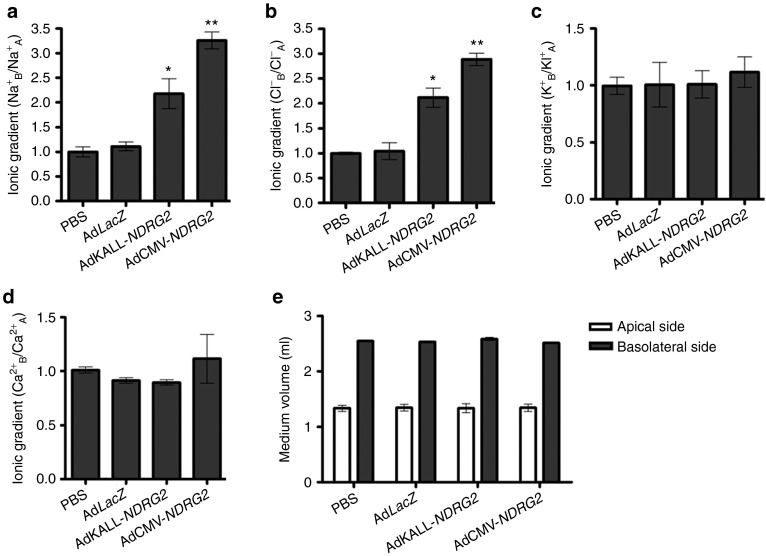
To further explore the mechanism of estrogen deficiency-induced impaired saliva electrolyte composition, we built a monolayer salivary duct cell model with a Transwell-Col plate (Figure 2f and Supplementary Figure S2) as described in the Materials and Methods section. Primary submandibular gland cells were isolated and cultured in Transwell-Col plates. The ion concentration on the apical or basolateral side was analyzed after cells had become confluent. Under basal conditions, salivary duct cells were generated along progressive basolateral to apical Na+ and Cl− gradients, indicating NaCl reabsorption from the apical to the basolateral side. Consistent with the electrolyte composition in the saliva of Ovx-rats, a significant decrease in Na+ reabsorption and a comparable decrease in Cl− reabsorption were detected with the monolayer Ovx-salivary duct cell model (Figure 2a,b). However, estrogen deprivation did not alter K+ and Ca2+ gradients (Figure 2c,d). In addition, medium volumes in both the apical and basolateral compartments showed no differences between the three groups (Figure 2e).
Figure 2.
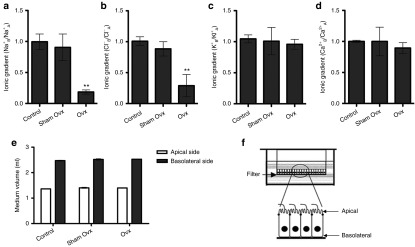
Estrogen deprivation inhibits Na+ and Cl− transport from the apical to basolateral side in submandibular gland ductal cells of Ovx-rats. Primary submandibular gland ductal cells were isolated from control rats, sham Ovx-rats, and Ovx-rats at the 30th day after ovariectomy. Then, cells were cultured in Transwell-Col plates and the ion concentrations were analyzed after cell growth to complete confluency. (a–d) Ion concentrations in the culture medium were measured on both the apical and basolateral sides of the Transwell-Col compartment. Data represent the ion concentration at the basolateral side divided by the ion concentration at the apical side. The value of control cells was designated as 1. (a) Na+, (b) Cl−, (c) K+, (d) Ca2+. (e) Medium volumes in the apical and basolateral compartments were measured. (f) Schematic diagram of primary submandibular gland ductal cells grown on a filter of the top well. Cells were cultured on collagen I-coated Transwell-Col filters to produce the monolayer. Data are expressed as the mean ± SEM of relative changes in individual groups compared with the control. **P < 0.01 versus control.
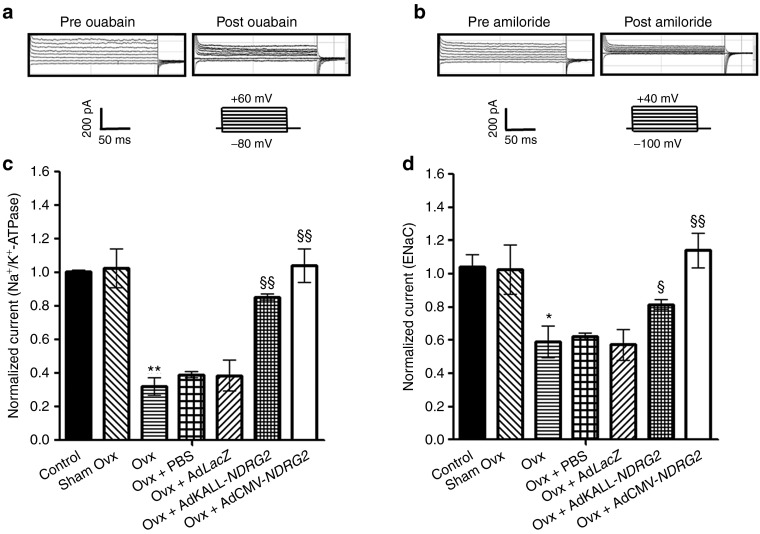
We also examined the effect of estrogen deficiency on Na+-activated currents in salivary duct cells using the whole-cell patch clamp technique. The changes in cell current revealed that estrogen deficiency resulted in significant reduction in both Na+/K+-ATPase-mediated and ENaC-mediated Na+ current in Ovx-salivary duct cells (Figure 7c,d). The above results suggest that estrogen deficiency-induced impaired electrolyte reabsorption correlates with Na+/K+-ATPase and ENaC activity.
Figure 7.
Effects of estrogen deprivation and NDRG2 adenoviral vector administration in rat submandibular gland ductal cells on Na+ currents. Primary submandibular gland ductal cells were isolated from Ovx-rats or NDRG2 adenoviral vector-delivered rats. Currents were measured under whole-cell clamp conditions. (a) The Na+/K+-ATPase-mediated Na+ current was elicited by 200-ms voltage steps from −80 to +60 mV in 20-mV increments. This outward current was blocked by 1 mmol/l ouabain. (b) ENaC-mediated Na+ current was elicited by 200-ms voltage steps from −100 to +40 mV in 20-mV increments. This inward current was blocked by 10 µmol/l amiloride. (c) The average Na+/K+-ATPase-mediated Na+ current (n = 8 cells) and (d) ENaC-mediated Na+ current (n = 8 cells) in different groups. All values were normalized to the value of the control current. Data are expressed as the mean ± SEM of the relative values of each group of cells, and the value of the control cells was designed as 1. *P < 0.05; **P < 0.01 versus control. §P < 0.05; §§P < 0.01 versus Ovx group. PBS, phosphate-buffered saline.
Effect of estrogen deficiency on Na+ transporters expression in salivary duct cells
To determine the potential molecular mechanism underlying the action of dramatic estrogen reduction associated with the dysfunction of rat salivary glands, the expression of Na+/K+-ATPase and ENaC was assessed. We previously proposed a novel estrogen/NDRG2/Na+/K+-ATPase regulation pathway in cell Na+ transport.26 As an estrogen target gene, NDRG2 can activate Na+/K+-ATPase in human salivary gland (HSG) cells, so we also examined NDRG2 expression in estrogen-deficient salivary duct cells.
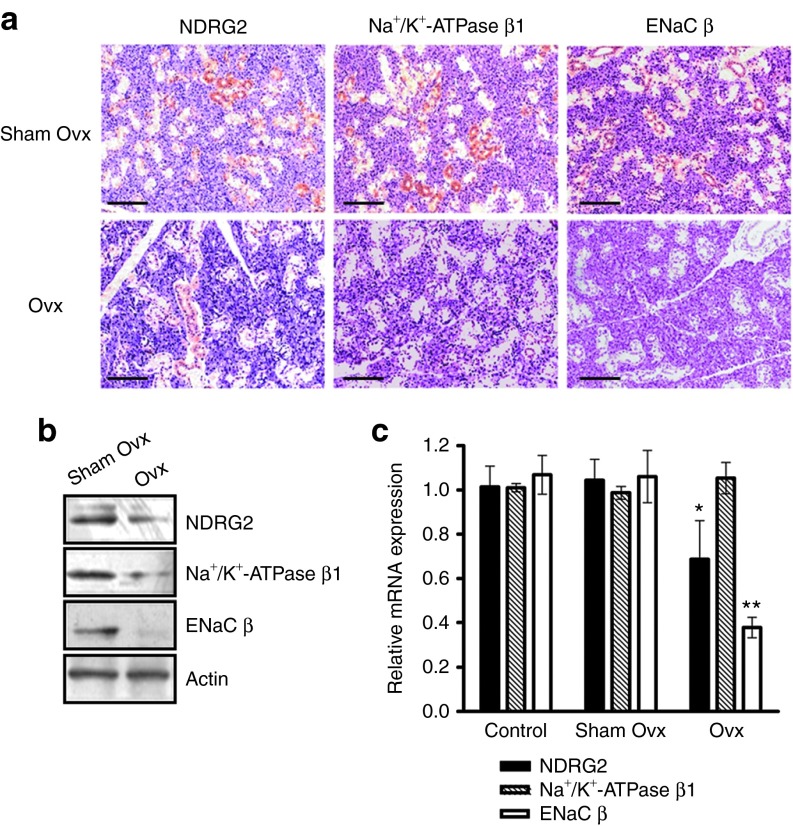
To analyze the protein level and distribution of NDRG2, Na+/K+-ATPase, and ENaC in salivary glands from Ovx-rats, immunohistochemistry analysis was performed. A significant decrease in NDRG2, Na+/K+-ATPase, and ENaC staining was detected in Ovx-ductal cells of the submandibular gland (Figure 3a and Supplementary Figure S3). The result of protein quantitative analysis was consistent with the findings in immunohistochemical analysis (Figure 3b). Interestingly, NDRG2, Na+/K+-ATPase, and ENaC were all predominantly expressed in ductal cells, compared with the weak staining in acinar cells in sham Ovx-rats (Figure 3a). In addition, a similar NDRG2 distribution pattern was observed in the human parotid gland, submandibular gland, and sublingual gland. NDRG2 was predominantly located in the cytoplasm of duct cells, and weak NDRG2 staining was detected in acinar cells in salivary gland epithelia (Supplementary Figure S4).
Figure 3.
Estrogen deprivation is associated with Na+ transporters in the submandibular gland of Ovx-rats. Submandibular glands were removed from Ovx-rats at the 30th day after ovariectomy. (a) Immunohistochemistry analysis was used to detect NDRG2, Na+/K+-ATPase β1, and ENaC β protein expression and changes in the distribution between the sham Ovx and Ovx groups. Scale bar = 200 μm. (b) Protein from the different groups were extracted and subjected to immunoblotting analysis. β-actin was used as a loading control. (c) NDRG2, Na+/K+-ATPase β1, and ENaC β mRNA levels were quantified by real-time PCR. The β-actin transcript was chosen as the internal reference. Data are expressed as the mean ± SEM of relative changes in the individual groups compared with the control. *P < 0.05; **P < 0.01 versus control.
The transcription levels of NDRG2, Na+/K+-ATPase, and ENaC were also analyzed in the rats' submandibular glands. Compared with the control and sham Ovx groups, significantly lower levels of NDRG2 and ENaC β mRNA levels were detected in Ovx-rats, but no difference in Na+/K+-ATPase β transcription was observed (Figure 3c). These results suggest that estrogen deprivation decreases the amounts of the Na+/K+-ATPase protein but does not affect the levels of Na+/K+-ATPase mRNA level in the salivary gland tissues in vivo. This result is consistent with our previous findings in NDRG2-silenced HSG cells that Na+/K+-ATPase protein, but not RNA, was dramatically reduced.26 In our previous study, we found that NDRG2 was involved in the estrogen-mediated regulation of Na+/K+-ATPase. This estrogen/NDRG2/Na+/K+-ATPase regulation pathway in cell electrolyte transport may provide a reasonable explanation for estrogen deficiency-induced sialaden hypofunction. More importantly, the above findings suggest that NDRG2 could be a potential therapeutic intervention in sialaden hypofunction.
Furthermore, we detected the expression of two other saliva secretion-related molecules aquaporin 5 (AQP5)28 and transmembrane proteins with unknown function 16 (Tmem16A)29 in this estrogen deprivation animal model. A significant decrease of AQP5 expression was found in the Ovx-submandibular gland; however, the expression of Tmem16A was not affected by estrogen deprivation (Supplementary Figure S5a,b). Intriguingly, NDRG226 and AQP530 are both estrogen target genes.
Ad-NDRG2 retrograde ductal administration mediated efficient gene delivery in the rat submandibular gland
Two replication-deficient adenoviruses, AdCMV-NDRG2 and AdKALL-NDRG2, were evaluated with respect to the improvement of sialaden hypofunction (Figure 4a). In these vectors, the expression of NDRG2 was driven by the CMV or the human tissue kallikrein (KALL) promoter, respectively. The CMV promoter can induce global expression, while the KALL promoter can induce ductal cell-specific expression of target genes.31 Retrograde ductal injection of AdCMV-NDRG2 or AdKALL-NDRG2 into the submandibular glands of Ovx-rats (Post-Ovx 37 days) resulted in the widespread or ductal cell-specific expression of NDRG2 (Postinjection 3 days) (Figure 4b,c). The result of protein quantitative analysis of NDRG2 was consistent with the findings in immunohistochemical analysis (Figure 4d). We also detected the transcription and protein levels of Na+/K+-ATPase β and ENaC β in NDRG2 adenovirus-injected submandibular glands (Figure 4d and Supplementary Figure S6). The result of transcription level analysis of EnaC β is consistent with its protein expression patterns in two NDRG2 administrations. But the mRNA levels of Na+/K+-ATPase β 1 were not affected with the exogenous NDRG2. This result is consistent with our previous findings in NDRG2-silenced HSG cells that Na+/K+-ATPase β1 protein, but not mRNA, was dramatically reduced.26 We further found ectopic NDRG2 expression led to a decrease in Na+/K+-ATPase β 1 ubiquitination in the submandibular glands of Ovx-rats by coimmunoprecipitation assays (Supplementary Figure S7). This supports our hypothesis that NDRG2 could stabilize Na+/K+-ATPase β 1 protien via inhibiting the degradation of Na+/K+-ATPase β 1 in vivo. In addition, no significant changes of the expression of saliva secretion-related molecules AQP5 or Tmem16A were found in AdKALL-NDRG2 or AdCMV-NDRG2 treatment group (Supplementary Figure S5c,d).
Figure 4.
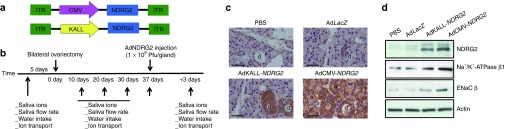
Effect of adenoviral vector delivery on NDRG2 expression and localization on salivary glands from Ovx-rats. (a) Two adenoviruses with different promoters were constructed for NDRG2 gene delivery. (b) The timeline of the procedures conducted in this study. (c) Submandibular glands were removed after 3 days following vector administration, and immunohistochemistry analysis was performed to investigate the expression and distribution of NDRG2. Scale bar = 50 μm. (d) Protein from the different groups were extracted and subjected to immunoblotting analysis. β-actin was used as a loading control. PBS, phosphate-buffered saline.
Adenovirus-mediated submandibular gland delivery of NDRG2 improved sialaden hypofunction in Ovx-rats
To test whether the introduction of exogenous NDRG2 could improve the estrogen deprivation-mediated sialaden hypofunction in Ovx-rats, we injected adenovirus vectors into the targeted submandibular glands the seventh day following the 30-day time point (Figure 4b). Three days after the administration of 109 plaque-forming units (Pfu) of either control (AdLacZ) or experimental (AdCMV-NDRG2 or AdKALL-NDRG2) vectors, we observed a significant decrease in salivary Na+ and Cl− concentrations (Figure 5a,b), with no changes in K+ and Ca2+ concentrations (Figure 5c,d), in glands treated with AdCMV-NDRG2. However, in the AdKALL-NDRG2 treatment group, slightly decreased saliva Na+ and Cl− concentrations (Figure 5a,b), with no changes in K+ and Ca2+ concentrations (Figure 5c,d), were detected compared with those in the AdCMV-NDRG2 group. AdCMV-NDRG2 administration was also found to efficiently increase the saliva flow rate in Ovx-rats, but no improved saliva output was found in the AdKALL-NDRG2 administration group (Figure 5e). Moreover, the amount of water consumption was reduced in both AdCMV-NDRG2 and AdKALL-NDRG2 treatment groups. These results demonstrate that both AdCMV-NDRG2 and AdKALL-NDRG2 can improve estrogen deficiency-induced sialaden hypofunction, although differences in the therapeutic effects of the two treatments were evident.
Figure 5.
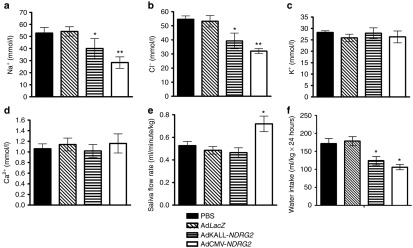
NDRG2 gene delivery improves estrogen deficiency-induced sialaden hypofunction. Three days after adenoviruses were delivered into Ovx-rats' submandibular glands, saliva was collected from the control (phosphate-buffered saline (PBS) or LacZ) or therapeutic (AdCMV-NDRG2 or AdKALL-NDRG2) groups (n = 8). (a–d) Salivary ion concentration was measured from the saliva of the different groups. (a) Na+, (b) Cl−, (c) K+, (d) Ca2+. (e) The pilocarpine-stimulated saliva flow rate was detected in each respective treatment group. (f) The effect of NDRG2 delivery on Ovx-rats' water intake. Data are expressed as the mean ± SEM. *P < 0.05; **P < 0.01 versus the PBS group.
The sialaden hypofunction in Ovx-rats could also be rescued by β-estradiol replacement (Supplementary Figure S8). We observed a decrease in saliva Na+ and Cl− concentrations, with no changes in K+ and Ca2+ concentrations after 50 µg β-estradiol replacement, which was similar to AdKALL-NDRG2 treatment group. These results further confirm that the sialaden hypofunction in Ovx-rats is caused by estrogen deprivation.
Ad-NDRG2 administrations corrected the dysfunction of Na+ and Cl− reabsorption in submandibular duct cells of Ovx-rats
To investigate the effects of exogenous NDRG2 on the function of Ovx-salivary duct cells, we isolated the primary ductal cells from submandibular glands of the phosphate-buffered saline (PBS), AdLacZ, AdKALL-NDRG2, and AdCMV-NDRG2 treatment groups the third day after injection. Cells were cultured in Transwell-Col plates, and the ion concentration on the apical or basolateral side was analyzed after cells had grown to complete confluency. A significant increase in Na+ reabsorption and comparably elevated Cl− reabsorption were detected in both the AdKALL-NDRG2 and AdCMV-NDRG2 groups compared with the PBS or AdLacZ group (Figure 6a,b). Consistent with the findings in NDRG2 adenovirus vector-mediated saliva K+ and Ca2+ compositions (Figure 5c,d), no significant changes in the K+ and Ca2+ concentration ratio were detected in the Transwell-Col system (Figure 6c,d). It is noteworthy to mention that AdCMV-NDRG2 more strongly rescued Na+ and Cl− reabsorption compared to AdKALL-NDRG2. In addition, the medium volumes in both the apical and basolateral compartments in Transwell-Col plates of monolayer salivary duct cells were not significantly different between the control and therapeutic groups (Figure 6e).
Figure 6.
NDRG2 adenovirus injection induces Na+ and Cl− transport from the apical to the basolateral side in submandibular gland ductal cells of Ovx-rats. Primary submandibular gland ductal cells were isolated from phosphate-buffered saline (PBS), AdLacZ, AdKALL-NDRG2, or AdCMV-NDRG2 groups 3 days after adenovirus delivery. Cells were cultured in Transwell-Col plates, and the ion concentration was analyzed after cell growth to complete confluency. The data represent the ion concentration at the basolateral side divided by the ion concentration at the apical side. The value of the control cells was designated as 1. (a) Na+, (b) Cl−, (c) K+, (d) Ca2+. (e) Medium volumes in the apical and basolateral compartments were measured in the four different treatment groups. Data are expressed as the mean ± SEM of relative changes in the individual groups compared with the PBS treatment group. *P < 0.05; **P < 0.01 versus the PBS group.
Ad-NDRG2 injection rescued Na+ currents in the submandibular gland ductal cells of Ovx-rats
To further demonstrate the observed functional interaction between NDRG2 and Na+ transport, we evaluated the Na+/K+-ATPase- and ENaC-mediated Na+ currents after Ad-NDRG2 administration in Ovx-submandibular duct cells. Using the whole-cell patch-clamp technique, activities of the basolateral Na+/K+-ATPase- and apical ENaC-mediated Na+ currents were measured in submandibular duct cells as the currents inhibited by ouabain (1 mmol/l) and amiloride (10 µmol/l), respectively. Basal Na+/K+-ATPase- and ENaC-mediated Na+ currents were recorded as shown in Figures 7a,b. We injected Ovx-rats with PBS, AdLacZ, AdKALL-NDRG2, or AdCMV-NDRG2. Primary ductal cells were isolated from submandibular glands and Na+ currents were measured on ductal cells after 3 days of adenovirus treatment. Rats injected with AdCMV-NDRG2 exhibited a complete recovery with respect to Na+/K+-ATPase- and ENaC-mediated Na+ currents (Figure 7c,d). In AdKALL-NDRG2-administered rats, an 80% greater recovery of Na+/K+-ATPase- and ENaC-mediated Na+ currents was found compared with the recovery resulting from PBS and AdLacZ treatment (Figure 7c,d). The above findings are consistent with previous studies on HSG cells and oocytes that showed that NDRG2 silencing could reduce Na+/K+-ATPase- or ENaC-mediated Na+ current.25,26
Discussion
In this study, we mimicked the development of xerostomia in menopausal women with Ovx-rats and found that NDRG2 was associated with the estrogen deficiency-induced inhibition of saliva ion reabsorption. Then, we demonstrated that adenovirus-mediated delivery of NDRG2 into submandibular glands effectively increased the reabsorption of saliva Na+ and Cl− and improved the saliva flow rate and water intake.
Oral dryness or xerostomia is a subjective symptom and is prevalent in more than one-third of postmenopausal women.2 The mechanisms responsible for the development of xerostomia during the menopausal stage are still unclear, although salivary glands are sensitive to changes in female sex steroid blood levels.3 The decrease in estrogen levels during menopause is thought to affect the oral epithelial maturation process, leading to thin and atrophic epithelium, salivary glands included.32 Salivary glands contain sex hormone receptors, and the estrogen receptor β is the predominant estrogen receptor subtype in human salivary glands.33 In the present study, estradiol replacement can rescue the sialaden hypofunction in Ovx-rats, which confirm that sialaden hypofunction in Ovx-rats is caused by estrogen deprivation. Estrogen deficiency is significantly associated with decreased NDRG2 expression in the rat salivary glands. This result is consistent with our previous study that 17 β-estradiol binds with estrogen receptor β to upregulate NDRG2 expression via transcriptional activation.26 In addition, the transcription level of NDRG2 was downregulated in the submandibular gland tissues of postmenopausal women compared to premenopausal women (Supplementary Figure S9).
In our previous study, an estrogen/NDRG2/Na+/K+-ATPase regulation pathway was illustrated by a series of cell-based experiments.26 Similar to the results of cell-based experiments, NDRG2 was found to participate in the regulation of estrogen-mediated Na+/K+-ATPase protein stability, but not at the transcription level in the present in vivo experiments. We also found the decrease of NDRG2 transcription is only 25% in Ovx-submandibular glands, but there was a dramatic decrease in Na+/K+-ATPase or ENaC protein level. We hypothesize that there may be other estrogen target molecules contribute to the decrease in Na+/K+-ATPase or ENaC protein level, similar to NDRG2. The present animal-based study and our previous cell-based study have demonstrated that NDRG2 as a new estrogen target gene is involved in the modulation of Na+/K+-ATPase and ENaC. In addition, NDRG2 was found to be primarily expressed in ductal cells, compared to very weak staining in acinar cells of both rat and human salivary glands. A similar distribution of NDRG2, Na+/K+-ATPase, and ENaC suggests that saliva Na+ reabsorption is possibly regulated by NDRG2 in salivary ductal cells. Thus, NDRG2 replacement may improve sialaden hypofunction in Ovx-rats.
Salivary glands may provide a convenient site for the transfer of genes that encode proteins for local or systemic use.34,35 Here, two adenovirus-mediated NDRG2 vectors were administrated to submandibular glands by retrograde ductal injection in Ovx-rats. Exogenous NDRG2 protein was distributed in a broad or ductal cell-specific manner under CMV promoter- or KALL promoter-constituted adenovirus treatment. The relative duct cell specificity induced by the KALL promoter is consistent with previous in vitro and in vivo studies.31,36
In AdCMV-NDRG2 treatment group, under the action of a strong promoter, NDRG2 was expressed in ductal cells, as well as in acinar cells. It seems likely that Na+ was pumped out of acinar cells via NDRG2-activated Na+/K+-ATPase and subsequently secreted into ducts. Water molecules were passively secreted via aquaporins (AQPs) in acinar cells, which forming the hyperosmotic or isotonic primary saliva. Then, the primary saliva flows through ductal cells, and under the regulation of NDRG2, Na+/K+-ATPase and ENaC synergistically act to increase Na+ reabsorption. Cl− tends to be transported with Na+ to maintain charge balance,14,18,37 and a comparable decrease in Cl− concentration in saliva was detected. Thus, the ion concentrations of the final saliva decreased, and the overall effects were that more saliva was produced and more ions were reabsorbed.
Various strategies have been employed to improve xerostomia. Current medication is confined to hormone replacement therapy,11,13 pilocarpine, anethol trithione, cevimeline, amifostine, or citric acid, which have limited effects and possible side effects.38,39 Gene therapy was also introduced in attempts to study and improve xerostomia. AQP treatment showed improved salivary secretion in radiation-induced xerostomia without significant general adverse effects in rat or miniature pig models.34,40 However, the saliva ion composition cannot be corrected by AQP administration, despite the saliva flow rate was improved with AQP gene therapy. In the present study, the retrograde ductal injection of Ad-NDRG2 into submandibular glands enabled the adverse events posed by the systemic administration of adenovirus to be avoided. AdCMV-NDRG2 treatment exerted greater efficiency in improving all indicators of estrogen deficiency-induced sialaden hypofunction, compared with AdKALL-NDRG2 treatment. However, NDRG2 is weakly expressed in the acinar cells of salivary gland in physiological condition, so AdCMV-NDRG2 treatment was not a strict rescue. The duct cell-specific promoter KALL may lead to a kind of physiological distribution of NDRG2, though AdKALL-NDRG2 yielded a moderate improvement in salivary ion composition and did not affect saliva flow rate compared to AdCMV-NDRG2.
A large number of xerostomia patients actually do not suffer from absolute decreases in saliva but from the changes in viscidity or ionic osmotic pressure of saliva.41,42 Patients usually complain about the feeling of dry month and state that their saliva becomes very viscous, while drinking more water makes little difference. Although premenopausal women have higher salivary flow rate than menopausal women, there is no difference in saliva flow rate between women using hormone replacement therapy and nonusers.43 A significant decrease of AQP5 expression was also detected in Ovx-submandibular glands. Interesting, NDRG2 and AQP5 have been reported to be both estrogen target genes.26,30 We speculate that NDRG2 and AQP5 may play important roles in estrogen deficiency-induced xerostomia by aberrantly modulating Na+ reabsorption and saliva flow, respectively. This hypothesis suggest that AdKALL-NDRG2 treatment can only rescue saliva Na+ reabsorption in Ovx-rats but cannot recover the saliva flow rate is not because of the moderate KALL promoter per se, but because other molecules may be involved in estrogen deficiency-induced xerostomia. Future research will be needed to clarify the relationship between the absolute saliva flow rate and xerostomia.
Consistent with in vivo data, an increase in Na+ or Cl- reabsorption was also detected in Ovx-ductal cells with NDRG2 administration in Transwell-Col system, although the medium volume was not changed. Based on this monolayer salivary duct cell model, we also demonstrated that NDRG2 treatment was associated with a significant increase in Na+/K+-ATPase- and ENaC-mediated Na+ current, which is consistent with the results of Wielputz et al.25 in oocytes and our previous study in HSG cells.26
Our previous26 and present data suggest that NDRG2 is involved in the estrogen regulation of Na+ reabsorption in salivary ductal epithelial cells, and Figure 8 presents a possible mechanistic model for NDRG2. As demonstrated in Figure 8b, in the presence of estrogen, estrogen binds to ER β, forming a transcription factor, which activates NDRG2 transcription. The NDRG2 protein binds and stabilizes the β 1 subunit of Na+/K+-ATPase in cytosol by inhibiting the ubiquitin-proteasome pathway-mediated degradation. The holoenzyme of Na+/K+-ATPase accumulates and translocates into the basolateral side of the cell plasma membrane, where Na+ is pumped out and across which an electrochemical gradient of Na+ is established. ENaC localized on the apical membranes senses this Na+ electrochemical gradient and facilitates the reabsorption of Na+ in primary saliva. Figure 8c shows that NDRG2 transcripts at low levels after estrogen deprivation. Without the protection of NDRG2, Na+/K+-ATPase β 1 is ubiquitinated and targeted for rapid destruction by the proteasome in cytosol. Na+/K+-ATPase and ENaC mediate Na+ transport on the basolateral and apical sides of the plasma membrane, respectively, at low levels in epithelial cells. The reabsorption of Na+ from primary saliva is inhibited. This mechanistic model not only explains how estrogen deficiency induces the inhibition of salivary ion reabsorption, but also enables the analysis of the function of NDRG2 gene therapy in estrogen deficiency-induced xerostomia or sialaden hypofunction.
Figure 8.
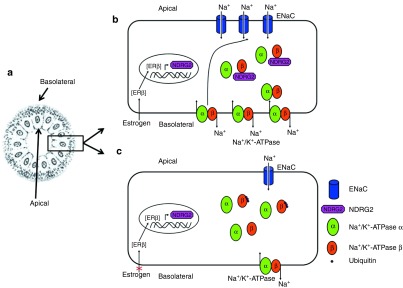
Model for the mechanism of NDRG2 participation in estrogen-mediated Na+ reabsorption in salivary gland ductal cells. (a) The schematic of a section of the striated duct. (b) When estrogen is present, it binds to ERβ, following transfer into cell nuclei, where it activates NDRG2 transcription. The NDRG2 protein binds and stabilizes the Na+/K+-ATPase β1 protein by inhibiting its ubiquitination and degradation in the cytoplasm. A greater accumulation of Na+/K+-ATPase promotes Na+ pumping across the basolateral plasma membrane, leading to a low Na+ concentration in the cell's cytosolic compartment, which stimulates the sensors of cytosolic Na+ and activates the ENaC in the apical plasma membrane, facilitating the reabsorption of Na+ in primary saliva into the ductal cells. (c) In the absence of estrogen, the NDRG2 transcription was reduced. Without the protection from NDRG2, Na+/K+-ATPase β1 was ubiquitinated and degraded quickly in the cytosol. The Na+/K+-ATPase- and ENaC-mediated Na+ transport on the basolateral and apical sides of the plasma membrane in ductal cells, respectively, was decreased.
In summary, this is the first report to show that the exogenous delivery of NDRG2 can improve the saliva flow rate and ion composition in an Ovx-rat model of sialaden hypofunction. This study may enable the development of a potential therapeutic option for the relief of estrogen deficiency-induced xerostomia in postmenopausal women.
Materials and Methods
Subjects. Female Sprague Dawley rats, 8 weeks of age and weighing 200–250 g, were obtained from the Fourth Military Medical University Animal Research Center (Xi'an, China) and individually housed in a specific pathogen-free facility with temperature control and a regular 12-hour light/dark cycle. They were allowed free access to standard chow and tap water ad libitum. All procedures complied with the standards for care and use of animal subjects according to the Guide for the Care and Use of Laboratory Animals (Chinese Association for Laboratory Animal Sciences). The experimental protocols were reviewed and approved by the Ethics Committee of the Fourth Military Medical University.
A total of 10 subjects with tongue cancer (premenopausal women 5, postmenopausal women 5) were recruited from our outpatient and inpatient services at Stomatology School of the Fourth Military Medical University. Their submandibular gland tissues were collected during the surgical procedures of neck dissection. The normal submandibular glands were diagnosed by histopathological examination. The Declaration of Helsinki protocols were followed and all patients gave their written informed consent. The experimental protocols were approved by the Ethics Committee of the Fourth Military Medical University.
Surgical procedure. The rats were subjected to surgical Ovx through bilateral dorsal incisions under nembutal anesthesia. Subsequently, blood samples were collected longitudinally to measure the concentration of serum estradiol using a radioimmunoassay, according to the manufacturer's instructions (JiuDing Bio-products, Tianjin, China).
Collection of saliva and saliva ion concentration analysis. Saliva was collected following stimulation with 5 mg/kg of pilocarpine as described previously44 using a negative pressure-based vacuum setting (Supplementary Figure S10). The ion concentration in the saliva from individual rats was measured with a Hitachi 7600 chemical autoanalyzer (Hitachi, Tokyo, Japan).
Measurement of water intake. Rats were transferred separately in experimental cages measuring 32 × 25 × 19 cm from home cage the day before water intake test. The animals were allowed free access to standard chow and distilled water ad libitum. On the test day, each rat was given a drinking bottle with 200 ml distilled water, and then we calculated the remaining amount of drinking water after 24 hours. The difference between the amounts of distilled water was the rat's daily water intake. Food was not available during testing.
Ad-NDRG2 vector construction. The two vectors used in this study were human type 5 recombinant adenoviruses. Both vectors employed (AdCMV-NDRG2 and AdKALL-NDRG2) were first-generation constructs that lacked the E1 and E3 regions. AdCMV-NDRG2 was under the control of the CMV promoter, and AdKALL-NDRG2 was under the control of the KALL promoter, a salivary duct cell-specific promoter.31 The vectors were propagated in 293 cells and purified, and titers were determined as previously described.45
Ad-NDRG2 vector injection. Ovariectomized rats were anesthetized and treated intramuscularly with 1 mg of dexamethasone to prevent adenovirus-induced inflammation, and they were then injected with 109 pfu/gland of AdCMV-NDRG2, AdKALL-NDRG2, or AdLacZ in 100 μl of PBS or PBS alone into their submandibular glands by retrograde ductal instillation (Supplementary Figure S11).46 The water consumption, salivary flow rate, and ion concentration in the saliva from individual rats were measured 3 days after adenovirus vector administration.
Isolation and culture of rat primary salivary ductal cells. Four submandibular glands were removed from rats and finely minced in 400 ml of digestion solution containing collagenase (Worthington Biochemical Corporation, Freehold, NJ) and hyaluronidase (Sigma, St Louis, MO) essentially as described elsewhere.47 The minced tissues were transferred to 5 ml of digestion solution in a 50-ml tube and incubated at 37 °C with continuous gassing (5% CO2, 95% O2) and agitation (90 cycles/min) for 20 minutes. Thereafter, the minced, enzyme-digested tissues were dispersed by pipetting 10 times with a 5-ml plastic pipette and centrifuged at 4,800 g for 1 minute. This supernatant was discarded, and the pellet was resuspended in 5 ml of the above digestion solution. The procedure was repeated once more. Then, the resulting cell pellet was resuspended in 15 ml of a solution containing Ham's F12/Dulbecco's modified Eagle medium, albumin (1%), and calcium (29.4 mg/ml); passed through a nylon filter (230-mm diameter); centrifuged; and resuspended in 6 ml of PBS. The cell suspension was then transferred onto a Percoll gradient (Sigma) and centrifuged at 10 rpm for 15 minutes, 2,000 rpm for 5 minutes, and 10,000 rpm for 30 minutes. After these centrifugation steps, two bands were readily apparent. The upper and lower bands contained ductal and acinar cells, respectively.47 The upper bands were collected, washed with PBS, plated onto type 1 collagen (Sigma)-coated culture plates, and cultured in F12/Dulbecco's modified Eagle medium (1:1; Life Technologies, Paisley, UK) supplemented with 10% fetal bovine serum (Hyclone, Logan, UT), 10 ng/ml human recombinant epidermal growth factor, 5 µg/ml insulin, 5 µg/ml human transferrin, and 10 ng/ml cholera toxin at 37 °C in 5% CO2 (all medium supplements were from Sigma).
Ion transport measurement in the Transwell-Col compartment. Primary salivary ductal cells were cultured in F12/Dulbecco's modified Eagle medium with 10% fetal bovine serum on the collagen I-coated filters in the apical chamber of six-well Transwell-coculture chambers.48 After growth to confluency, cells were exposed to 1.6 and 2.5 ml of fresh minimal medium in the apical and basolateral chambers, respectively. Twenty-four hours later, the medium in the apical and basolateral chambers was separately harvested. The ion concentration in the medium was determined with a Hitachi 7600 chemical auto-analyzer (Hitachi).
Immunohistochemistry assay. The expression of NDRG2 in rats and human salivary tissues was characterized by immunohistochemistry using an NDRG2-specific antibody.27,49 Briefly, the salivary tissue sections (4 μm) were dewaxed in xylene, dehydrated in descending concentrations of ethanol, immersed in 0.3% H2O2-methanol for 30 minutes, washed with PBS, and probed with mouse monoclonal anti-NDRG2 antibody (1:100) or isotype control at 4 °C overnight. After washing, the sections were incubated with biotinylated goat anti-mouse IgG (1:300; Boster Biological Technology, Wuhan, China) at room temperature for 2 hours. The immunostaining was visualized with the streptavidin/peroxidase complex and diaminobenzidine, and the sections were then counterstained with hematoxylin.
Real-time polymerase chain reaction (PCR). Total RNA was extracted from individual samples using Trizol (Invitrogen Life Technologies, Carlsbad, CA) and reversely transcribed into cDNA using the RevertAid First-Strand cDNA Synthesis kit (Fermentas, Lithuania), according to the manufacturer's instructions. The levels of mRNA transcripts were determined by quantitative real-time PCR using the cDNA as the template, specific primers, and the standard SYBR Green RT-PCR kit (Takara, Japan), according to the manufacturer's instructions. The PCR reactions (25 μl/tube, in triplicate) were performed first at 95 °C for 10 seconds for denaturation and were then subjected to 35 cycles of 95 °C for 5 seconds and 60 °C for 34 seconds using the specific primers (Supplementary Table S1). The relative levels of target gene mRNA transcripts were normalized to the control β-actin.
Whole-cell patch clamp recordings. Na+-K+-ATPase-Primary salivary ductal cells were voltage clamped using the whole-cell patch technique. Patch electrodes were made from borosilicate glass capillaries (Clark Electromedical Instruments, Reading, UK) and were firepolished (DMG Universal Puller, Zeitz-Instrumente VentreibsGmbH, Germany). The electrodes had a resistance of 3–5 MΩ when filled with the standard pipette solution. Current signals were recorded using an Axopatch 1-C single electrode voltage-clamp amplifier (Axon Instruments, CA) controlled by a microcomputer running pClamp software (v.6, Axon Instruments). A gigaohm seal was rapidly established between the electrode and the cell surface, and the resistance was monitored by a repetitive +5 mV pulse (five is duration) from a holding potential of 0 mV. Access resistance on gaining access to the inside of the cell was <6 MΩ. The pipette and extracellular solutions were designed to inhibit all voltage-gated channels and the Na+/Ca2+ exchanger. The standard pipette solution was made of deionized water, and contained (in mmol/l, Sigma chemicals) NaCl 15, MgCl2 1, CsCl 8, HEPES 10, EGTA 5, MgATP 5, creatine phosphate 5, CsCH3O3S 90, NaCH3O3S 35, and pH 7.2. The standard extracellular solution contained (in mmol/l) NaCl 140, KCl 5, MgCl2 1, NiCl2 2, BaCl2 1, glucose 10, HEPES 10, and pH 7.4 or without KCl and correction for osmolarity for the K+-free extracellular solution. Under these conditions, the Na+/K+-ATPase current can be defined as that inhibited by the removal of extracellular K+.
ENaC-Whole-cell macroscopic current recordings of ENaC reconstituted in primary salivary ductal cells were made under voltage-clamp conditions using standard methods. Current through ENaC was the inward, amiloride-sensitive Na+ current with a bath solution of (in mmol/l) NaCl 124, KCl 2.5, CaCl2 2, MgSO4 1, NaHCO3 25, NaH2PO4 1, Glucose 10, and pH 7.2, and the pipette solution of (in mmol/l) CsCl 140, NaCl 5, MgCl2 2, EGTA 1, HEPES 10, MgATP 2, Na3GTP 0.1, and pH 7.4. All currents were filtered at 1 kHz. The cells were clamped to a −60mV holding potential voltage ramps step protocols with potentials ranging from −100 mV up to 40 mV in 20 mV increments to elicit current. Series resistances, on average 3–5 MΩ, were also compensated.
Statistical analyses. All values are expressed as the mean ± SEM. The differences between the different groups were analyzed by analysis of variance and post hoc analysis, and the differences between two groups were analyzed by the Student's t-test using the GraphPad Prism software version 5.0 (GraphPad Software, San Diego, CA). A value of P < 0.05 was considered to be statistically significant.
SUPPLEMENTARY MATERIAL Figure S1. Estradiol level in Ovx-rats. Figure S2. Schematic diagram of primary rat submandibular gland ductal cells grown on a filter in the top well. Figure S3. Estrogen deprivation is associated with Na+ transporters in the submandibular gland of Ovx-rats. Figure S4. Immunohistochemistry analysis of NDRG2 expression in human glands. Figure S5. Effects of estrogen deprivation and NDRG2 adenoviral vector administration on AQP5 or Tmem16A expression. Figure S6. Effects of NDRG2 adenoviral vector administration on NDRG2, Na+/K+-ATPase β1, and ENaC β transcription levels. Figure S7. NDRG2 stabilizes Na+/K+-ATPase β1 protein through inhibiting its ubiquitination. Figure S8. Estradiol replacement rescue estrogen deficiency-induced sialaden hypofunction in Ovx-rats. Figure S9. The transcription level of NDRG2 in the submandibular gland tissues of premenopausal women and postmenopausal women. Figure S10. Saliva collection from rats. Figure S11. Submandibular gland retrograde ductal injection of Ad-NDRG2 vector in a rat using cannulas. Table S1. The sequences of primers.
Acknowledgments
We thank Minggao Zhao and Yanyan Guo for technical help with the patch clamp assays. This work was supported by National Natural Science Foundation of China Grants 81100764, 81230043, 81202139, and 81371446. The authors declare no conflicts of interest.
Supplementary Material
References
- Zachariasen RD. Oral manifestations of menopause. Compendium. 1993;14:1584, 1586–91; quiz 1592. [PubMed] [Google Scholar]
- Nederfors T. Xerostomia and hyposalivation. Adv Dent Res. 2000;14:48–56. doi: 10.1177/08959374000140010701. [DOI] [PubMed] [Google Scholar]
- Agha-Hosseini F, Mirzaii-Dizgah I, Mansourian A, Khayamzadeh M. Relationship of stimulated saliva 17beta-estradiol and oral dryness feeling in menopause. Maturitas. 2009;62:197–9. doi: 10.1016/j.maturitas.2008.10.016. [DOI] [PubMed] [Google Scholar]
- Wolff A, Fox PC, Porter S, Konttinen YT. Established and novel approaches for the management of hyposalivation and xerostomia. Curr Pharm Des. 2012;18:5515–21. doi: 10.2174/138161212803307509. [DOI] [PubMed] [Google Scholar]
- Agha-Hosseini F, Mirzaii-Dizgah I. Unstimulated saliva 17β-estradiol and xerostomia in menopause. Gynecol Endocrinol. 2012;28:199–202. doi: 10.3109/09513590.2011.593668. [DOI] [PubMed] [Google Scholar]
- Singh R, Pallagatti S, Sheikh S, Singh B, Arora G, Aggarwal A. Correlation of serum oestrogen with salivary calcium in post-menopausal women with and without oral dryness feeling. Gerodontology. 2012;29:125–9. doi: 10.1111/j.1741-2358.2011.00580.x. [DOI] [PubMed] [Google Scholar]
- Tenovuo J, Laine M, Söderling E, Irjala K. Evaluation of salivary markers during the menstrual cycle: peroxidase, protein, and electrolytes. Biochem Med. 1981;25:337–45. doi: 10.1016/0006-2944(81)90092-2. [DOI] [PubMed] [Google Scholar]
- Gómez E, Ortiz V, Saint-Martin B, Boeck L, Díaz-Sánchez V, Bourges H. Hormonal regulation of the secretory IgA (sIgA) system: estradiol- and progesterone-induced changes in sIgA in parotid saliva along the menstrual cycle. Am J Reprod Immunol. 1993;29:219–23. doi: 10.1111/j.1600-0897.1993.tb00590.x. [DOI] [PubMed] [Google Scholar]
- Salvolini E, Di Giorgio R, Curatola A, Mazzanti L, Fratto G. Biochemical modifications of human whole saliva induced by pregnancy. Br J Obstet Gynaecol. 1998;105:656–60. doi: 10.1111/j.1471-0528.1998.tb10181.x. [DOI] [PubMed] [Google Scholar]
- Laine M, Pienihäkkinen K. Salivary buffer effect in relation to late pregnancy and postpartum. Acta Odontol Scand. 2000;58:8–10. doi: 10.1080/000163500429361. [DOI] [PubMed] [Google Scholar]
- Yalçin F, Gurgan S, Gurgan T. The effect of menopause, hormone replacement therapy (HRT), alendronate (ALN), and calcium supplements on saliva. J Contemp Dent Pract. 2005;6:10–7. [PubMed] [Google Scholar]
- Sewón L, Laine M, Karjalainen S, Leimola-Virtanen R, Hiidenkari T, Helenius H. The effect of hormone replacement therapy on salivary calcium concentrations in menopausal women. Arch Oral Biol. 2000;45:201–6. doi: 10.1016/s0003-9969(99)00137-5. [DOI] [PubMed] [Google Scholar]
- Eliasson L, Carlén A, Laine M, Birkhed D. Minor gland and whole saliva in postmenopausal women using a low potency oestrogen (oestriol) Arch Oral Biol. 2003;48:511–7. doi: 10.1016/s0003-9969(03)00094-3. [DOI] [PubMed] [Google Scholar]
- THAYSEN JH, THORN NA, SCHWARTZ IL. Excretion of sodium, potassium, chloride and carbon dioxide in human parotid saliva. Am J Physiol. 1954;178:155–9. doi: 10.1152/ajplegacy.1954.178.1.155. [DOI] [PubMed] [Google Scholar]
- Baum BJ. Principles of saliva secretion. Ann N Y Acad Sci. 1993;694:17–23. doi: 10.1111/j.1749-6632.1993.tb18338.x. [DOI] [PubMed] [Google Scholar]
- Turner RJ, Sugiya H. Understanding salivary fluid and protein secretion. Oral Dis. 2002;8:3–11. doi: 10.1034/j.1601-0825.2002.10815.x. [DOI] [PubMed] [Google Scholar]
- Garty H, Palmer LG. Epithelial sodium channels: function, structure, and regulation. Physiol Rev. 1997;77:359–96. doi: 10.1152/physrev.1997.77.2.359. [DOI] [PubMed] [Google Scholar]
- Catalán MA, Nakamoto T, Gonzalez-Begne M, et al. Cftr and ENaC ion channels mediate NaCl absorption in the mouse submandibular gland. J Physiol. 2010;588 Pt 4:713–24. doi: 10.1113/jphysiol.2009.183541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speight PM, Chisholm DM. The relationship between localization of Na+, K+-ATPase and cellular fine structure in the rat parotid gland. Histochem J. 1984;16:721–31. doi: 10.1007/BF01095278. [DOI] [PubMed] [Google Scholar]
- Conteas CN, McDonough AA, Kozlowski TR, Hensley CB, Wood RL, Mircheff AK. Mapping subcellular distribution of Na+-K+-ATPase in rat parotid gland. Am J Physiol. 1986;250 3 Pt 1:C430–41. doi: 10.1152/ajpcell.1986.250.3.C430. [DOI] [PubMed] [Google Scholar]
- Simson JA, Chao J. Subcellular distribution of tissue kallikrein and Na,K-ATPase alpha-subunit in rat parotid striated duct cells. Cell Tissue Res. 1994;275:407–17. doi: 10.1007/BF00318811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HY, O'Doherty GA. Modulators of Na/K-ATPase: a patent review. Expert Opin Ther Pat. 2012;22:587–605. doi: 10.1517/13543776.2012.690033. [DOI] [PubMed] [Google Scholar]
- Cook DI, Dinudom A, Komwatana P, Young JA. Control of Na+ transport in salivary duct epithelial cells by cytosolic Cl- and Na+ Eur J Morphol. 1998;36:67–73. [PubMed] [Google Scholar]
- Deng Y, Yao L, Chau L, et al. N-Myc downstream-regulated gene 2 (NDRG2) inhibits glioblastoma cell proliferation. Int J Cancer. 2003;106:342–7. doi: 10.1002/ijc.11228. [DOI] [PubMed] [Google Scholar]
- Wielputz MO, Lee IH, Dinudom A, et al. (NDRG2) stimulates amiloride-sensitive Na+ currents in Xenopus laevis oocytes and fisher rat thyroid cells. J Biol Chem. 2007;282:28264–73. doi: 10.1074/jbc.M702168200. [DOI] [PubMed] [Google Scholar]
- Li Y, Yang J, Li S, et al. N-myc downstream-regulated gene 2, a novel estrogen-targeted gene, is involved in the regulation of Na+/K+-ATPase. J Biol Chem. 2011;286:32289–99. doi: 10.1074/jbc.M111.247825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu XL, Liu XP, Deng YC, et al. Expression analysis of the NDRG2 gene in mouse embryonic and adult tissues. Cell Tissue Res. 2006;325:67–76. doi: 10.1007/s00441-005-0137-5. [DOI] [PubMed] [Google Scholar]
- Ma T, Song Y, Gillespie A, Carlson EJ, Epstein CJ, Verkman AS. Defective secretion of saliva in transgenic mice lacking aquaporin-5 water channels. J Biol Chem. 1999;274:20071–4. doi: 10.1074/jbc.274.29.20071. [DOI] [PubMed] [Google Scholar]
- Romanenko VG, Catalán MA, Brown DA, et al. Tmem16A encodes the Ca2+-activated Cl- channel in mouse submandibular salivary gland acinar cells. J Biol Chem. 2010;285:12990–3001. doi: 10.1074/jbc.M109.068544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang XX, Xu KH, Ma JY, et al. Reduced migration of Ishikawa cells associated with downregulation of aquaporin-5. Oncol Lett. 2012;4:257–61. doi: 10.3892/ol.2012.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng C, Hoque AT, Braddon VR, Baum BJ, O'Connell BC. Evaluation of salivary gland acinar and ductal cell-specific promoters in vivo with recombinant adenoviral vectors. Hum Gene Ther. 2001;12:2215–23. doi: 10.1089/10430340152710559. [DOI] [PubMed] [Google Scholar]
- Forabosco A, Criscuolo M, Coukos G, et al. Efficacy of hormone replacement therapy in postmenopausal women with oral discomfort. Oral Surg Oral Med Oral Pathol. 1992;73:570–4. doi: 10.1016/0030-4220(92)90100-5. [DOI] [PubMed] [Google Scholar]
- Välimaa H, Savolainen S, Soukka T, et al. Estrogen receptor-beta is the predominant estrogen receptor subtype in human oral epithelium and salivary glands. J Endocrinol. 2004;180:55–62. doi: 10.1677/joe.0.1800055. [DOI] [PubMed] [Google Scholar]
- Delporte C, O'Connell BC, He X, et al. Increased fluid secretion after adenoviral-mediated transfer of the aquaporin-1 cDNA to irradiated rat salivary glands. Proc Natl Acad Sci U S A. 1997;94:3268–73. doi: 10.1073/pnas.94.7.3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Goldsmith CM, Marmary Y, et al. Systemic action of human growth hormone following adenovirus-mediated gene transfer to rat submandibular glands. Gene Ther. 1998;5:537–41. doi: 10.1038/sj.gt.3300622. [DOI] [PubMed] [Google Scholar]
- Zheng C, Baum BJ. Evaluation of viral and mammalian promoters for use in gene delivery to salivary glands. Mol Ther. 2005;12:528–36. doi: 10.1016/j.ymthe.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Delporte C, Steinfeld S. Distribution and roles of aquaporins in salivary glands. Biochim Biophys Acta. 2006;1758:1061–70. doi: 10.1016/j.bbamem.2006.01.022. [DOI] [PubMed] [Google Scholar]
- Visvanathan V, Nix P. Managing the patient presenting with xerostomia: a review. Int J Clin Pract. 2010;64:404–7. doi: 10.1111/j.1742-1241.2009.02132.x. [DOI] [PubMed] [Google Scholar]
- Berk L. Systemic pilocarpine for treatment of xerostomia. Expert Opin Drug Metab Toxicol. 2008;4:1333–40. doi: 10.1517/17425255.4.10.1333. [DOI] [PubMed] [Google Scholar]
- Shan Z, Li J, Zheng C, et al. Increased fluid secretion after adenoviral-mediated transfer of the human aquaporin-1 cDNA to irradiated miniature pig parotid glands. Mol Ther. 2005;11:444–51. doi: 10.1016/j.ymthe.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Närhi TO. Prevalence of subjective feelings of dry mouth in the elderly. J Dent Res. 1994;73:20–5. doi: 10.1177/00220345940730010301. [DOI] [PubMed] [Google Scholar]
- Ben Aryeh H, Gottlieb I, Ish-Shalom S, David A, Szargel H, Laufer D. Oral complaints related to menopause. Maturitas. 1996;24:185–9. doi: 10.1016/s0378-5122(96)82008-1. [DOI] [PubMed] [Google Scholar]
- Streckfus CF, Baur U, Brown LJ, Bacal C, Metter J, Nick T. Effects of estrogen status and aging on salivary flow rates in healthy Caucasian women. Gerontology. 1998;44:32–9. doi: 10.1159/000021980. [DOI] [PubMed] [Google Scholar]
- Benarde MA, Fabian FW, Rosen S, Hoppert CA, Hunt HR. A method for the collection of large quantities of rat saliva. J Dent Res. 1956;35:326–7. doi: 10.1177/00220345560350022801. [DOI] [PubMed] [Google Scholar]
- Rosenfeld MA, Siegfried W, Yoshimura K, et al. Adenovirus-mediated transfer of a recombinant alpha 1-antitrypsin gene to the lung epithelium in vivo. Science. 1991;252:431–4. doi: 10.1126/science.2017680. [DOI] [PubMed] [Google Scholar]
- Adesanya MR, Redman RS, Baum BJ, O'Connell BC. Immediate inflammatory responses to adenovirus-mediated gene transfer in rat salivary glands. Hum Gene Ther. 1996;7:1085–93. doi: 10.1089/hum.1996.7.9-1085. [DOI] [PubMed] [Google Scholar]
- Dehaye JP, Turner RJ. Isolation and characterization of rat submandibular intralobular ducts. Am J Physiol. 1991;261 3 Pt 1:C490–6. doi: 10.1152/ajpcell.1991.261.3.C490. [DOI] [PubMed] [Google Scholar]
- Wakabayashi Y, Chua J, Larkin JM, Lippincott-Schwartz J, Arias IM. Four-dimensional imaging of filter-grown polarized epithelial cells. Histochem Cell Biol. 2007;127:463–72. doi: 10.1007/s00418-007-0274-x. [DOI] [PubMed] [Google Scholar]
- Liu X, Hu X, Zhang J, et al. Preparation and application of monoclonal antibody against hNDRG2. Appl Biochem Biotechnol. 2009;152:306–15. doi: 10.1007/s12010-008-8267-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.