Abstract
G207, a mutant herpes simplex virus (HSV) type 1, is safe when inoculated into recurrent malignant glioma. We conducted a phase 1 trial of G207 to demonstrate the safety of stereotactic intratumoral administration when given 24 hours prior to a single 5 Gy radiation dose in patients with recurrent malignant glioma. Nine patients with progressive, recurrent malignant glioma despite standard therapy were included. Patients received one dose of G207 stereotactically inoculated into the multiple sites of the enhancing tumor margin and were then treated focally with 5 Gy radiation. Treatment was well tolerated, and no patient developed HSV encephalitis. The median interval between initial diagnosis and G207 inoculation was 18 months (mean: 23 months; range: 11–51 months). Six of the nine patients had stable disease or partial response for at least one time point. Three instances of marked radiographic response to treatment occurred. The median survival time from G207 inoculation until death was 7.5 months (95% confidence interval: 3.0–12.7). In conclusion, this study showed the safety and the potential for clinical response of single-dose oncolytic HSV therapy augmented with radiation in the treatment of malignant glioma patients. Additional studies with oncolytic HSV such as G207 in the treatment of human glioma are recommended.
Introduction
Glioblastoma multiforme (GBM), the most commonly diagnosed, aggressive, and lethal primary brain tumor, is almost uniformly fatal.1 Prognosis for GBM remains bleak in spite of advances in therapeutic radiotherapy, chemotherapy, and surgical techniques. Recent adaptation of a combined regimen of survival, but this remains less than 15 months. Certain patient subsets, particularly those lacking MGMT promoter methylation, remain more resistant to this regimen.2,3
The characteristic, multitherapeutic resistance of glioma cells makes them prime targets for combined treatment modalities and novel therapeutic agents, including virotherapeutic agents. Preclinical and clinical studies suggest that targeted oncolytic viral and oncolytic gene therapy may enhance current GBM treatment options. In previous clinical trials, we have examined the safety and tolerability of a genetically engineered herpes simplex virus type I (HSV-1), G207, as a potential therapeutic agent for GBM.4,5 Direct inoculation of G207 into the tumor does not produce encephalitis; however, it retains the capacity to replicate in and kill multiple classes of malignant neoplasms. G207 contains deletions in both loci of the viral γ134.5 gene, as well as a disabling lacZ insertion in UL39, the gene that encodes for the large subunit of the viral ribonucleotide reductase.6 Two prior studies utilizing stereotactic inoculation of G207 directly into recurrent malignant gliomas have been reported; each demonstrated the safety and tolerability of the agent in this patient population and suggested that antitumor activity was present in some patients as evidenced by long-term survival posttreatment.4,5
Preclinical studies in orthotopic murine models have demonstrated the capacity of adjuvant therapies to improve HSV antitumor efficacy. In particular, HSV treatment followed by radiation treatment has shown improved antitumor effects.7,8,9 These findings supported the development of a clinical trial in patients with malignant glioma to examine the effects of administering these interventions in combination.
The primary objective of this phase 1 study (deemed CT2001) was to determine the safety of direct inoculation of G207 into recurrent malignant gliomas followed by focal irradiation to the tumor volume the following day. To evaluate the potential for efficacy of this approach, secondary end points assessed radiographic and performance response, overall survival, HSV/G207-specific antibody response, and degree of viral shedding posttreatment.
Results
Patients
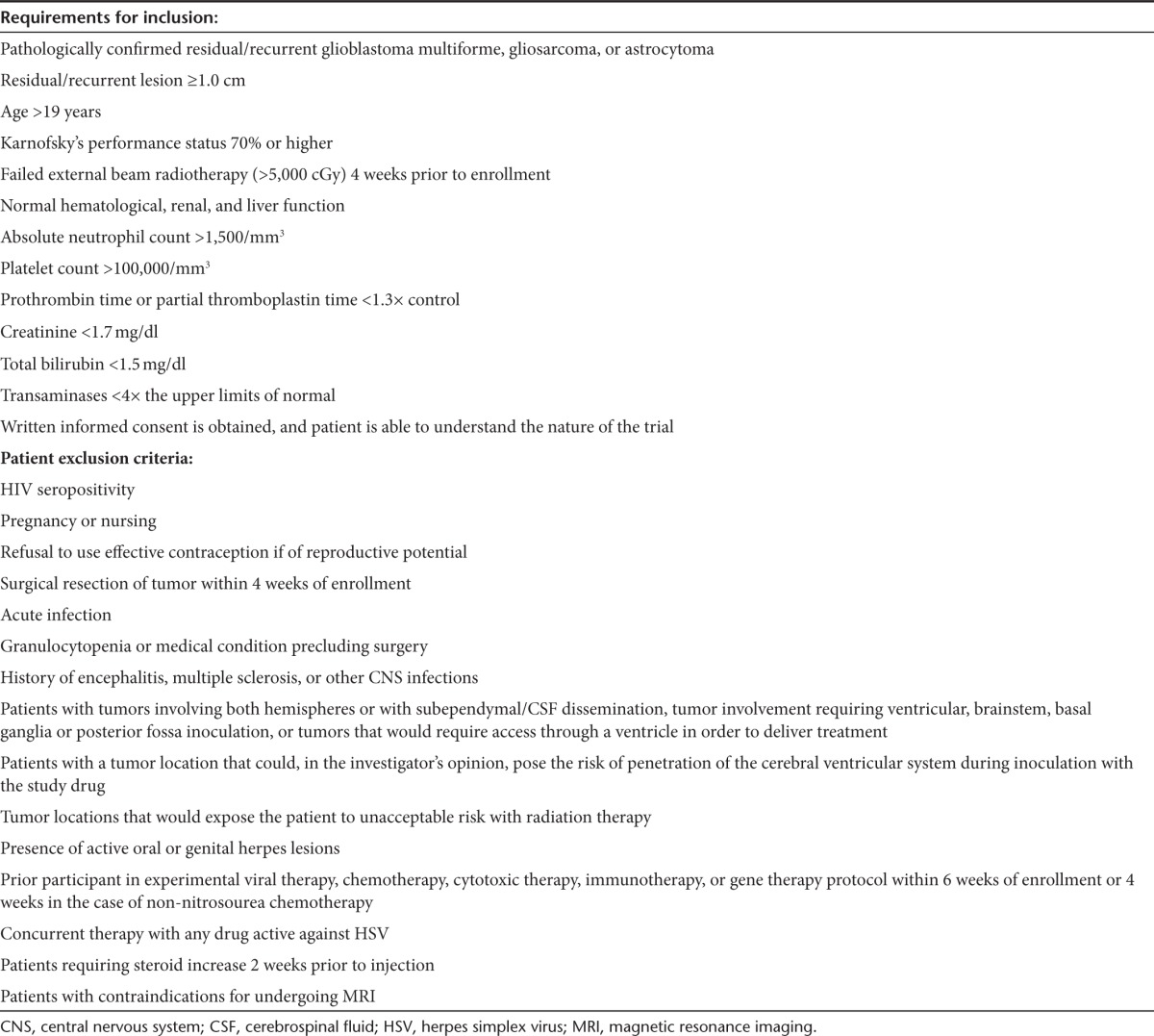
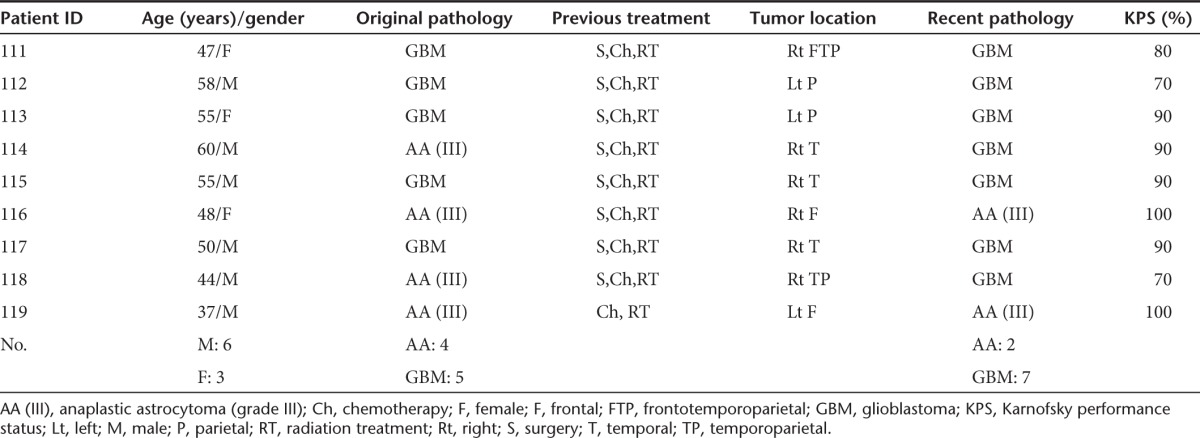
The trial was a single-site, open-label protocol conducted at the University of Alabama at Birmingham (UAB, Birmingham, AL) over a 3-year period and enrolled nine patients whose tumor had progressed despite surgery (stereotactic biopsy or resection), chemotherapy, and radiation therapy for malignant glioma. All nine patients were treated, and none were lost to follow-up. The median age was 50.2 years (mean: 50.4 years; range: 37–60 years; Table 1). In two of the four patients, anaplastic astrocytoma had transformed to GBM at the time of stereotactic biopsy, just prior to G207 inoculation. Thus, at the time of treatment, seven of the nine patients enrolled had a confirmed diagnosis of GBM. The median interval between initial diagnosis and G207 inoculation was 18 months (mean: 23 months; range, 11–51 months). No patients received any additional antitumor treatment (besides the one-time G207 inoculation and one-time fraction of radiation) until progression. As typical in this population, patients were on variable doses of corticosteroids leading up to and following treatment. A summary of steroid doses is provided as Supplementary Table S1.
Table 1. Patient inclusion/exclusion criteria.
Treatment course
We report the combined results of the CT 2001 trial, which comprise a 12-month study that included screening, treatment visit (5 days), and follow-up (1 year). The dose utilized, 1.0 × 109 plaque-forming units (pfu), was selected as 0.5 log lower than the highest dose shown to be safe in a prior study without radiation.5 All patients were followed until death.
Toxicity
National Cancer Institute Common Terminology Criteria for Adverse Events 3.0 and protocol severity scales were used to distinguish between occurrences of adverse events (AEs) in response to treatment versus underlying disease.10 In total, 108 AEs were reported (Supplementary Table S2). The most common were headache, 10 (representing 9.3% of the total AEs reported); seizures, 6 (5.6%); hemiparesis, 4 (3.7%); and fever, 5 (4.6%). Most events were 1-mild (n = 50), 2-moderate (n = 34), or 3-severe (n = 24) in severity. Some AEs were severe but not serious, and 21 met one of the “serious” criteria. All patients experienced serious AEs, the majority of which were determined to be due to their underlying disease, a common finding in patients with recurrent malignant glioma in other similar studies. Three serious AEs (seizures after administration) were considered possibly related to G207 administration, and one, pyrexia due to improper administration technique (see patient 114, below), as probably related to the study agent. No patients discontinued study participation because of AEs presumably related to G207 administration or because of clinically significant laboratory abnormalities. G207 was not detected in the samples tested for the presence of HSV (saliva and serum). None of the patients showed magnetic resonance imaging (MRI), laboratory, or pathologic evidence of inflammatory changes or other findings to suggest encephalitis. Three patients merit further discussion:
Patient 114 had a generalized seizure 1 dpi with a temperature to 105 °F; this resolved quickly with no sequelae. Discharge was delayed 1 day. A neuronavigation software idiosyncrasy allowed a trajectory in which the needle delivering the virus apparently traversed the ventricular system. This AE was attributed to inadvertent ventricular contamination with virus.
Patient 115 presented 17 dpi with cerebrospinal fluid leak from the incision, but no signs of meningitis were found. The wound was resutured, and the patient was discharged. At scheduled visit 2 (28 dpi), increasing lethargy and edema that involved the arms, trunk, and face, along with frequent staring spells, were reported. Cerebrospinal fluid was positive for Staphylococcus epidermidis; polymerase chain reaction (PCR) on the cerebrospinal fluid for G207 was negative. Antibiotics were initiated, and the patient was discharged 2 days later on antibiotics. Ongoing posttreatment cerebrospinal fluid leak and meningitis sequelae consisted of lethargy and weakness.
Patient 118 had mild worsening of his left hemiparesis from 4/5 to 3/5 following his procedure, along with a progressive left neglect. On 5 dpi, shortness of breath developed that was attributed to a new onset supraventricular tachycardia after ruling out pulmonary embolism. The arrhythmia effects resolved; however, the left sided weakness persisted.
Progression and survival
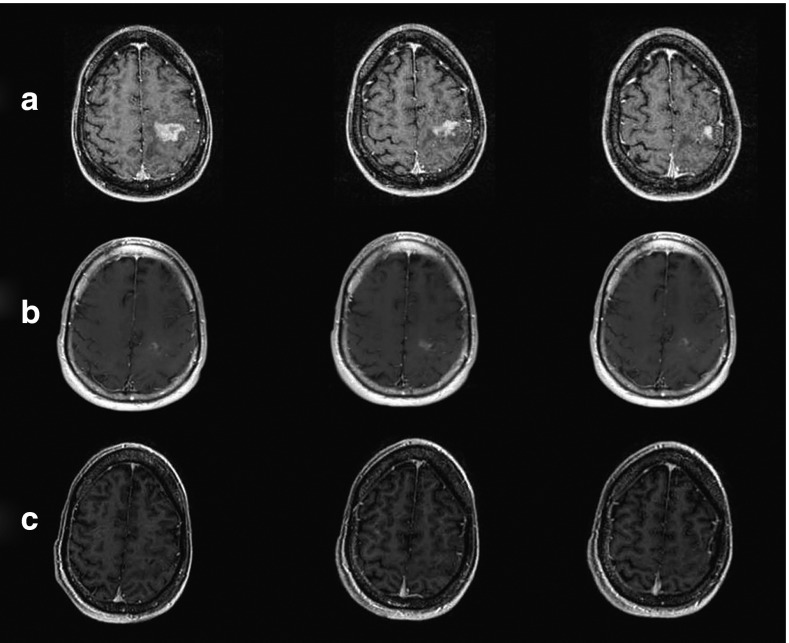
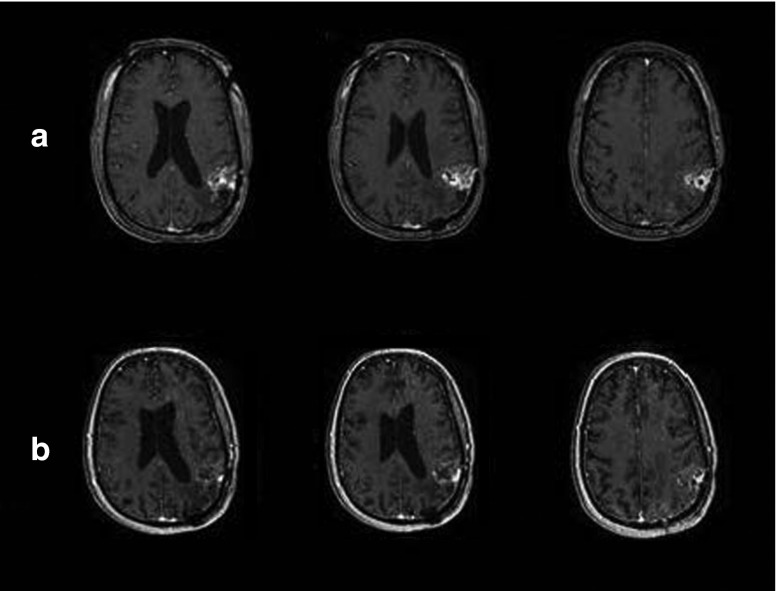
While no conclusions about efficacy can be drawn from this phase 1 study, secondary end points of response including progression-free survival and overall survival were recorded. Macdonald response criteria were used to evaluate the radiographic response to treatment.11 For patient 119, a marked response occurred, consistent with a partial response (PR) seen at 1, 3, and 6 months (Figure 1). Six of the nine patients had stable disease or PR for a least one time point. Notably, some patients had significant responses to treatment that did not strictly meet criteria for PR due to the presence of a minimally enhancing residual rim (Figure 2).
Figure 1.
Patient 119: pre-G207 and at 3 and 6 months post–G207 inoculation showing a partial response. (a) Pre–G207 inoculation, (b) 3 months post–G207 inoculation, and (c) 6 months post–G207 inoculation.
Figure 2.
Patient 113: pre– and post–G207 inoculation, showing a clear response to viral treatment. (a) Pre–G207 inoculation and (b) 1-month post–G207 inoculation.
The Kaplan–Meier estimate of median survival time from initial diagnosis to G207 inoculation was 18.7 months (95% confidence interval: 13–35 months). The median progression-free survival time was ~2.5 months (95% confidence interval: 1–5.75). The estimated median survival time from G207 inoculation until death was 7.5 months (95% confidence interval: 3.0–12.7). The influence of time between the end of G207 inoculation and beginning of radiation treatment on overall survival was analyzed with Cox modeling (range: 18 hours 12 minutes to 25 hours 38 minutes with a median of 20 hours 55 minutes). The corresponding regression coefficient of 0.09 (P = 0.4967) and hazard ratio of 1.096 did not support a difference in response with earlier radiation. Details on response to treatment are included in Supplementary Table S3.
Neurological status
Median Karnofsky performance status (KPS) at screening was 90 (range: 70–100) (Table 2). Shortly after inoculation (days 1–4), KPS of some patients decreased but recovered thereafter by day 28. At 1-month follow-up, five patients had stable KPS (±10 points), and in two patients, KPS increased by 20 points. Patient 119 had an increase in KPS from 70 on day 1 to 90 at 1, 3, and 6 months, respectively. This corresponded with the PR seen at the same time intervals.
Table 2. Demographic data and primary clinical information at screening.
Laboratory evaluation and immune status
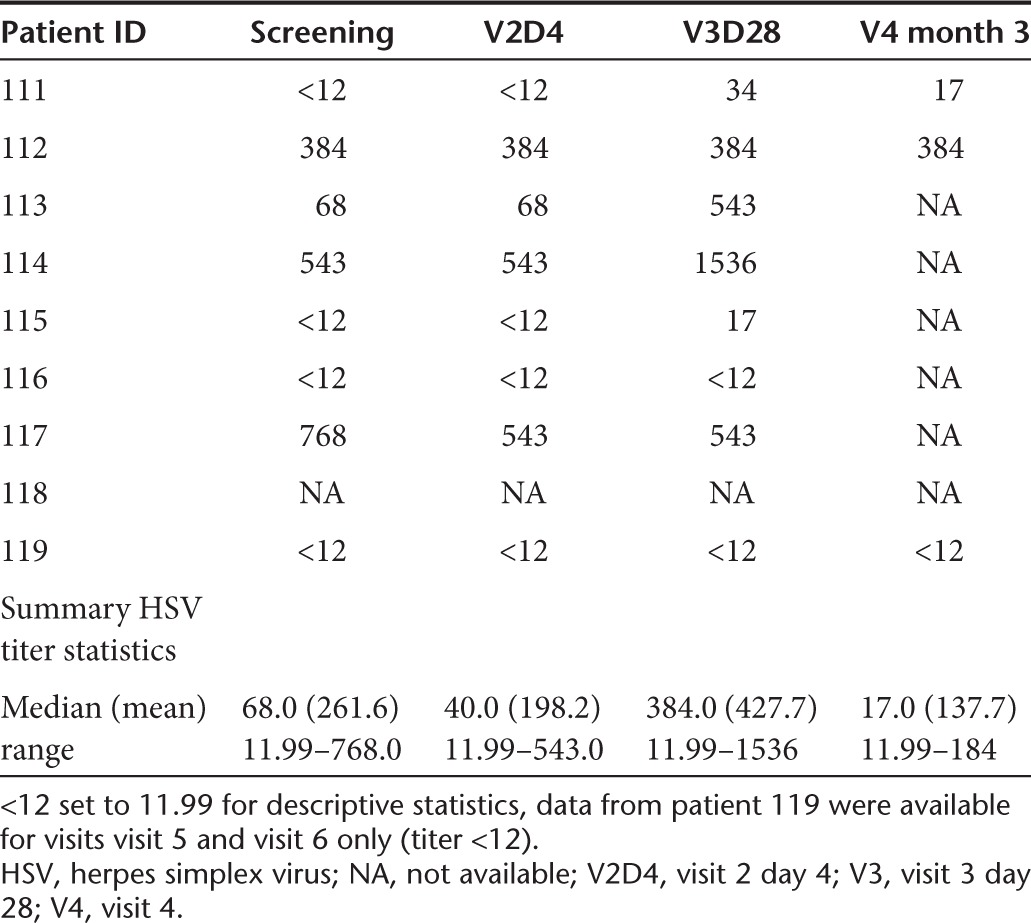
The CD4 and CD8 counts are reported in Supplementary Table S4. At screening, three patients were seropositive for HSV-1; all were negative for HSV-2. On visit 3 (day 28), one patient (patient 113) seroconverted for HSV-1 only (Supplementary Table S5). Quantitative titers of antibodies to HSV were also performed (Table 2). Patients 111, 115, 116, and 119 had low antibody titers to HSV (<40) at screening and maintained the low titers (≤17) until the end of the trial. Patient 113 had nearly an eightfold increase in antibody titers on day 28. Patient 114 had high antibody titer to HSV at screening and then had a further threefold increase by the end of 1 month. Patient 117 was an exception since the high anti-HSV titers at screening actually decreased by day 4 and were stable at 1-month posttreatment.
Detection of HSV in peripheral samples
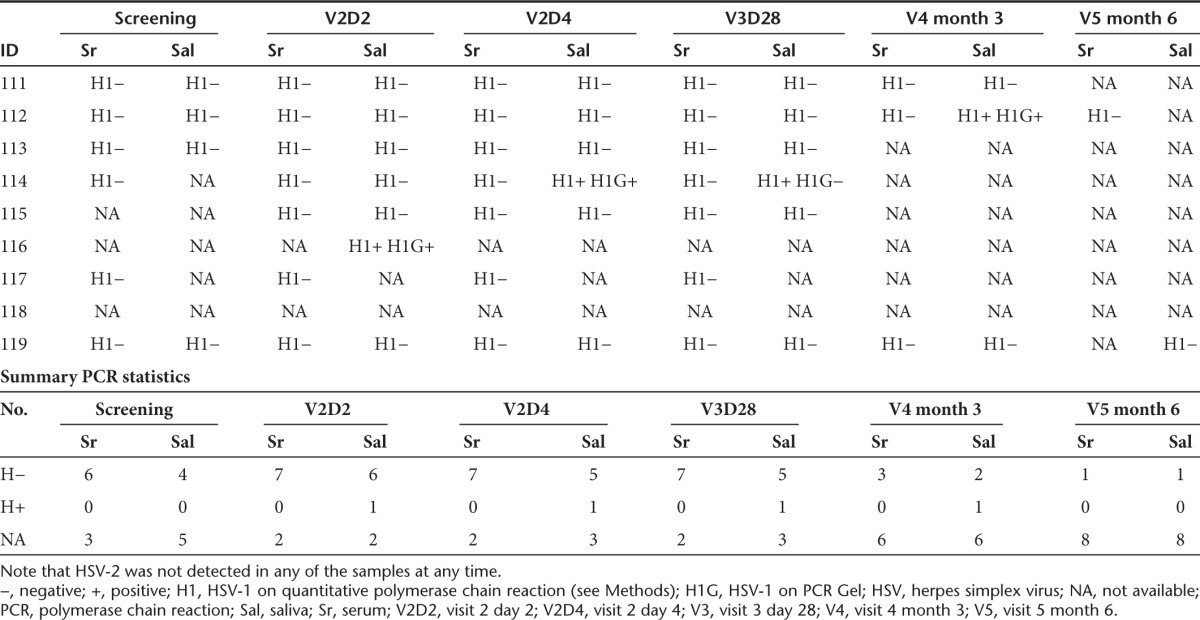
HSV quantitative PCR in serum remained negative in all study subjects throughout the study but was positive in saliva samples in three study subjects at various time points. Patient 114 had HSV-1 isolated from saliva on visit 2 day 4 (PCR) and visit 3 with a consecutive measurable HSV immunoglobulin G antibody titer rise. Patient 112 had HSV-1 isolated from saliva on visit 4 (PCR), and patient 116 had HSV-1 isolated from his saliva on visit 2 day 2 (PCR), both without any change of HSV immunoglobulin G titer (Table 3). The remaining three evaluable patients did not have HSV-1 isolated from their saliva at any time point: quantitative PCR testing for G207 was negative in each of these samples.
Table 3. HSV titers.
Compassionate use
Two patients, patient 113 and 119, underwent retreatment under individualized compassionate use protocols. Both patients demonstrated significant radiographic responses to their initial treatment with G207 under the original protocol and requested retreatment at progression after their first treatment. The same protocol was used for radiation administration for the compassionate use protocol as was used with the original protocol, with each patient receiving an additional 5 Gy radiation on the day following G207 administration. Details regarding the time of retreatment and ultimate survival are presented in Supplementary Table S6.
Toxicity following compassionate use
Patient 113 developed transient increase dysphasia, fatigue, nausea, and ataxia postretreatment, which resolved the following day. Patient 119 developed worsening seizure control and worsening hemiparesis postretreatment. He underwent stereotactic biopsy 2 months postretreatment and was found to have progression to GBM. Microscopic examination revealed minimal inflammation, but no evidence of encephalitis and immunoperoxidase stains for HSV demonstrated the presence of a few positive cells only. Seizures were thus attributed to tumor progression. After treatment for a deep venous and pulmonary artery thrombosis, and resolution of his seizures, patient was discharged to a rehabilitation hospital with ongoing hemiparesis.
Progression and survival following compassionate use
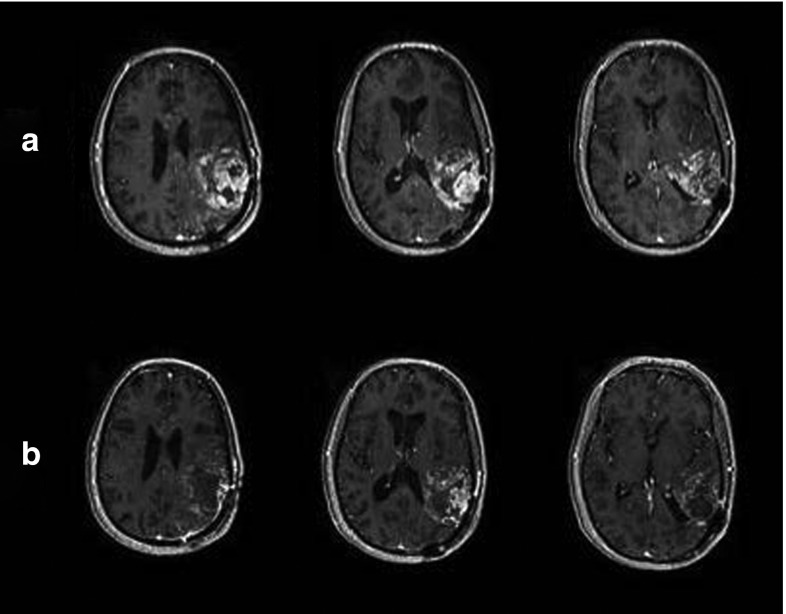
Patient 113 demonstrated a reduction in contrast-enhancing tumor area at day 27 postretreatment (best response: stable disease by strict criteria). MRI findings were impressive for increase in necrosis and decreased mass effect (Figure 3). While imaging at ~9 weeks posttreatment met criteria for stable disease, the patient was started on Gleevec and hydroxyurea by her neuro-oncologist. She succumbed to her disease 5.5 months post–G207 retreatment.
Figure 3.
Patient 113: pre– and post–G207 reinoculation, showing a clear response to viral treatment. (a) Pre G207 reinoculation and (b) 1-month post–G207 reinoculation.
Patient 119 demonstrated a minimal decrease in tumor size in response to retreatment (best response: stable disease). He succumbed to his tumor 8.2 months after retreatment (for details, see Supplementary Table S6). Brain autopsy was undertaken after consent was obtained. Gross findings showed diffuse, bilateral involvement of the brain by tumor. Microscopic analysis confirmed diffuse invasion of the brain with malignant astrocytes, necrosis, and endothelial proliferation. No evidence of viral inclusion bodies, microglial or other inflammatory response or chronic demyelination was seen, supporting an absence of encephalitis or other G207-related toxicity.
Discussion
We report the results of the first clinical trial of intratumoral administration of G207, a conditionally replicating HSV, followed by focal radiation therapy 24 hours postinjection. While wild-type HSV is quite neurovirulent, deletions in the genes for ICP34.5 and one of the ribonucleotide subunits are present in G207, essentially eliminating neurovirulence while allowing the virus to maintain its antitumor effects.12,13,14 The use of engineered HSV-1 with an intact thymidine kinase gene (UL23), including G207, for tumor therapy has the added advantage of the availability of efficacious antiviral therapy for life-threatening disease if needed (e.g., acyclovir).
The rationale for the strategy of our study was based on the observation that treatment with γ134.5 deleted HSV-1, (including G207, and its parent virus, R3616) has been shown to be enhanced by ionizing radiation when administered after viral inoculation. Preclinical studies have demonstrated that treatment with oncolytic HSV-1, when followed within 4–120 hours by administration of a clinically relevant fraction of radiation (5–25 Gy total dose, administered in one to three fractions), increased the efficacy of the oncolytic HSV antitumor effects, increased viral replication and distribution, and improved survival in orthotopic models.7,8,9 The γ134.5 deletion that renders specificity of the oncolytic HSV to tumor cells also results in a defect in the late γ gene expression. Administration of ionizing radiation after viral inoculation may overcome this defect through increased activation of the p38 pathway, resulting in increased viral replication and intratumoral production of new virus.15 Additional evidence suggests that IR-induced expression of cellular homologues for the viral UL39 and γ134.5 genes, both nonfunctional in G207, may act to augment intratumoral G207 replication.15 Interestingly, while preclinical studies of G207 administered with radiation in other histologies have also demonstrated increased efficacy, a single study in prostate cancer did not demonstrate this effect.16,17,18,19
No patient in our study developed MRI, laboratory, or clinical evidence of encephalitis. One previously treated patient had an exacerbation of his prior seizure disorder with an associated hemiparesis after retreatment and radiation. A biopsy indicated that this patient's tumor had progressed from its previously documented status as a grade III astrocytoma to a grade IV astrocytoma, and it was felt that this transition was likely responsible for these findings. We thus consider G207 plus focal radiation safe at the respective doses administered in this study.
One patient had PR at 1-month posttreatment. Five additional patients had SD by MRI 1-month postinoculation, and five of the nine patients had stable (±10 points) Karnofsky scores at that time point; at 3 months, three patients had stable KPS, and in patient 119, it had increased by 20 points. A single patient with a large tumor and a KPS of 70 at screening showed further decrease in KPS and was taken off the trial at 1 month due to tumor progression. Importantly, 5 Gy is considered a subtherapeutic dose of radiation in malignant glioma, and while a treatment effect from 5 Gy cannot be ruled out from our study, this would be extremely unlikely based on decades of clinical experience with this tumor and its known resistance to radiotherapy. Thus, the effect seen is most likely due to either augmentation of G207 effect or some unknown interaction between G207 and radiation. Ultimately, all study patients died from tumor progression. None of the deaths were attributed to G207 administration into the tumor or adjacent brain tissue.
Unexpectedly, only three of the nine (33%) patients were seropositive for HSV-1 (none were positive for HSV-2 exposure) at screening, which is lower than expected for individuals in this age range where rates of 75–85% would have been anticipated, as well as seen in the preclinical study of G207 in Aotus.20,21,22 While a larger, prospective trial will be necessary to determine whether pretreatment exposure status is a biomarker for treatment response, preclinical studies and our previous clinical trials do not support this.23 The combined rate of HSV-1 seropositivity in our two previously published studies of G207 was 17/25 (68%).4,5 Despite the immunocompromised state of patients in this current study, HSV-1 seroconversion occurred in one patient. Previous studies had shown a seroconversion of 1/5 (original phase 1) and 3/3 (two-dose phase 1B), whereas preclinical studies in Aotus showed uniform elevation of serum anti-HSV antibodies.4,5,24 Presumably, the highly immunocompromised nature of the recurrent glioma patients—produced by treatment with steroids, prior chemotherapy and radiation, as well as the tumor itself—impacts the ability of the patient to form an antibody response. It is interesting to consider whether the single fraction of radiation administered in this study could have impacted antibody formation as well, and warrants further study in preclinical models.
Interestingly, two of the most significant radiographic responses seen in this trial occurred in patients who were HSV-1 seronegative at enrollment. In prior G207 studies in GBM, the best responses were often seen in patients seropositive at enrollment. One of these patients, patient 113, had HSV-1 antibody present by quantitative analysis only prior to treatment and seroboosted during the study. This patient went on to have a second, impressive radiographic response under a compassionate use protocol, a response that occurred after having seroconverted. Results presented here are thus consistent with prior findings, suggesting that antibody status to HSV-1 before initiation of treatment may not be significant.
Malignant glioma was chosen for the study due to its resistance to traditional therapeutics and because it usually results in death. Approximately 90% of gliomas recur locally, within 2 cm of their resection margins, and systemic spread is exceedingly rare.25,26 As a result, these tumors are potentially excellent targets for experimental therapy with a conditionally replicating virus such as G207 HSV in that it replicates in and destroys tumor cells, while sparing normal nervous tissue. Although lower in our study population, ~75–85% of the adult population at large reports having been exposed to the HSV-1 as demonstrated by seropositive antibody status.20,21,22
The lack of suitable response criteria for assessing therapeutic response in studies using oncolytic viruses and other similar vectors remains an issue as demonstrated by the results of this trial. Patients with impressive posttreatment radiographic effects do not necessarily meet the criteria for significant reduction in maximal volume of enhancing area or even nonenhancing area, as required by the MacDonald and RANO criteria.27,28 The possibility of an inflammatory response capable of producing both enhancement and peritumoral T2 changes that are indistinguishable by MRI from tumor except by the passage of time has been seen in other clinical trials of oncolytic viruses, as noted by F. Lang and E. Galanis (personal communication). As these changes are better characterized, the possibility of developing postoncolytic virus pseudoprogression criteria may need to be entertained.
This is the third published clinical trial using G207 in patients with recurrent glioma. The first study utilized a dose-escalation scheme and showed that G207 could be safely inoculated into recurrent malignant gliomas at doses up to 3 × 109 pfu.5 The second trial utilized a two-dose regimen with an interval resection of the glioblastoma.4 The safety of this approach was also confirmed. In addition, examination of treated tumor tissue following resection demonstrated evidence supporting G207 replication in the tumor after injection and a postinoculation immune response to treatment. Three additional trials using a different HSV-1 mutant, HSV1716, in glioma patients have been successfully conducted in the United Kingdom.29,30,31
All of these studies suggest the following: (i) HSV-1 mutants deleted for the γ134.5 gene are safe for injection into patients with malignant glioma at the doses examined and (2) some of these patients had longer survival times than would be normally expected. This current investigation is the first clinical trial designed to use an HSV-1 mutant in combination with adjuvant radiotherapy, in order to determine the safety of such an approach.
This study demonstrates that a single dose of G207, at 1 × 109 pfu, can be infused into the enhancing portions of the tumor, followed by the administration of 5 Gy of radiation within 24 hours, without producing undue toxicity. While the use of a phase 1 study design, using a limited number of patients with no controls, prevents any conclusions being made about the efficacy of this treatment approach, the decreases in tumor size seen on posttreatment MRIs in some patients, as well as an increase in survival in some patients, are encouraging. The addition of radiation did not make the treatment approach any less safe, with similar AEs seen in this group of patients as in the initial G207 study without radiation, and suggested the potential for augmentation of response over treatment with virus alone. Two patients were treated a second time with G207, ~7 and 10 months after their initial treatments, and did not develop undue immunologic effects, even with a period of several months between treatments, supporting the potential for efficacious multiple treatments with oncolytic HSV. Previous criticisms of oncolytic virus therapy have suggested that the development of a memory immune response might interfere with treatment effects and/or produce toxicity; this was not seen in our study. Further studies with G207 or other genetically engineered HSV in the treatment of human glioma are warranted; consideration of adjuvant treatment with radiation, virotherapy, and other therapies designed to augment the efficacy of treatment, as well as the possibility of multiple viral treatments, should be considered as part of the design of such trials.
Materials and Methods
All patients were informed of the investigational nature of the study and were provided informed consent as required by the Institutional Review Board of the UAB (NCT00157703).This protocol and its amendments were approved by the UAB Institutional Review Board and reviewed by the Recombinant DNA Advisory Committee of the National Institutes of Health and the Food and Drug Administration. Patients meeting screening inclusion/exclusion criteria presented in Table 4 were included.
Table 4. PCR analysis.
Study treatment
Virus. HSV G207 (IND BB-7393) was provided by MediGene AG (Martinsried, Germany) from lots that had been produced cGMP under contract by BioReliance (Rockville, MD), shipped frozen (−80 °C) to the UAB Hospital Research Pharmacy, and processed on the day of surgery for injection of 1 × 109 pfu as we have previously described in detail.5 These lots of cGMP grade G207 HSV have been used in previously reported trials4,5
Biopsy and stereotactic inoculation. On day 0, the patients underwent Cosman-Roberts-Wells stereotactic head frame application under local anesthesia, followed by a contrast-enhanced computed tomography or MRI scan and target localization. A stereotactic biopsy was performed. Following confirmation of recurrent tumor by frozen section, 200 µl of G207 was stereotactically inoculated into five different areas of enhancement of the tumor rim over 2 minutes to avoid possible reflux. Trajectory of the needle placement was undertaken to avoid ventricular entry.
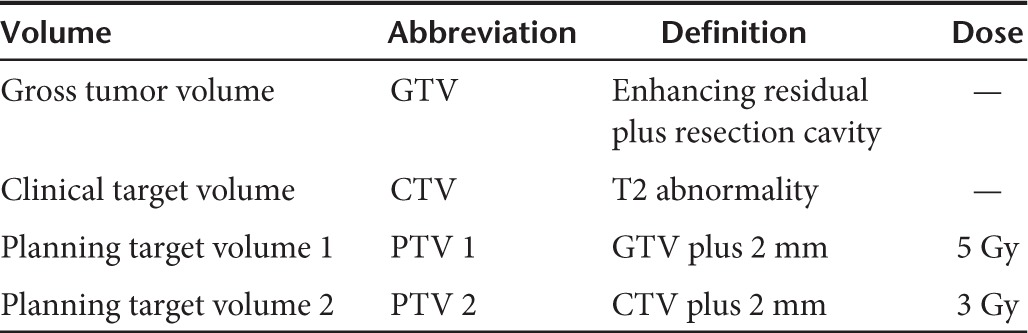
Radiation. Radiation was administered to all nine patients using a 100 SAD linear accelerator with at least 6 MV beam energy at 1 dpi. The time difference between end of G207 inoculation and beginning of radiation treatment ranged from 18 hours 12 minutes to 25 hours 38 minutes with a median of 20 hours 55 minutes. Computed tomography scans were collected at slice thickness of at least 3 mm. The target volumes and prescription doses for radiation treatment planning are shown in Table 5 and followed ICRU-62 conventions.
Table 5. Radiation treatment plan parameters.
Posttreatment evaluation. Patient assessments were made, including physical examination, HSV antibody titer, saliva and blood HSV cultures as well as MRI at screening and at 1 and 4 dpi followed by repeat assessments at 1, 3, 6, 9, and 12 months postinoculation. Neurological examination included Karnofsky grading and neurological assessment. Follow-up MRIs were classified as complete response, PR, stable disease, or progressive disease; MacDonald criteria were used to determine progression. Patients who showed signs of clinical or MRI disease progression were declared treatment failures.
Statistical analyses. All statistical analyses were conducted using SAS Ver. 9.1.3 (SAS Institute, Cary, NC). For time-to-event data (e.g., survival time from initial diagnosis to G207 inoculation or time from G207 inoculation until death), the Kaplan–Meier method was used to estimate the median time to event with corresponding 95% confidence intervals rounded to the nearest week. To evaluate the influence of time between the end of G207 inoculation and beginning of radiation treatment on overall survival, a Cox proportional hazards model was performed estimating the corresponding regression coefficient and hazard ratio (significance level: 5%).
HSV culture and PCR. One milliliter of serum and saliva were used for virus isolation and PCR assays. Of this, 0.2 ml was inoculated into duplicate wells of 24-well tissue culture plates containing Vero and A549 cells. Plates were incubated in a CO2 incubator (37 °C, 95% humidity) and examined by inverted microscope daily for cytopathic efficacy for 7 days. Prior experience indicated that Vero cells are able to detect 10 pfu of G207. Positive cultures were passaged in Vero cells, and positive supernates were analyzed by PCR to distinguish between G207 and wild-type HSV. Cells from the initial positive cultures were serotyped using a MicroTrak HSV-1/HSV-2 Culture Identification/typing Test (Trinity Biotech, Bray, Ireland). Viral DNA was extracted from sera using the QIAmp DNA Blood Mini Kit (Qiagen, Germantown, MD). Initial screening of specimens was performed by quantitative PCR using two primer sets that amplify glycoprotein B and DNA polymerase genes of HSV. Laboratory studies were performed in Germany at Medigene AG, Martinsried and at Becker, Olgemoller and Colleagues, Munich. Safety was assessed throughout by evaluating toxicities (rated using the classification developed by the National Institutes of Health),10 laboratory testing, and neurologic status. Efficacy was evaluated by time to progression of disease (clinical and imaging) and immunohistochemistry to monitor viral replication and infection.
Hematology laboratory studies. All screening and monitoring hematology studies were performed at UAB Hospital Laboratories. Blood was collected and transferred according to their specifications and standard institutional procedures. Slides were stained using these standard protocols with immunostains for HSV-1 and HSV-2 (Cell Marque, Rocklin, CA).
SUPPLEMENTARY MATERIAL Table S1. G207 steroid status of subjects; IV, intravenous; po, oral, NA, Not available. Table S2. Seriousness as determined by CTCAE grading scale; SEV, Severity with 1-mild, 2-moderate, and 3-severe. Table S3. Progression-free and overall survival times Table S4. CD 4 and CD 8 counts. Table S5. HSV serostatus. Table S6. Compassionate use event/endpoint times
Acknowledgments
The authors thank James Robinson Hackney (Department of Neuropathology, University of Alabama at Birmingham (UAB)) for his helpful assistance with immunohistochemical methodology and Suzanne Byan-Parker (Comprehensive Cancer Center, UAB) and Wendi Moore (Department of Surgery, UAB) for their assistance in the preparation of this manuscript. The authors declare no competing financial interests.
This research was supported in part by grant awards PO1 CA71933 (R.J.W.) and P50 CA097247 (G.Y.G. from the United States Public Health Service, National Institutes of Health, National Cancer Institute, and MediGene AG, Munich, Germany. This clinical trial is registered on http://www.clinicaltrials.gov as NCT00157703.
Supplementary Material
G207 steroid status of subjects; IV, intravenous; po, oral, NA, Not available.
Seriousness as determined by CTCAE grading scale; SEV, Severity with 1-mild, 2-moderate, and 3-severe.
Progression-free and overall survival times
CD 4 and CD 8 counts.
HSV serostatus.
Compassionate use event/endpoint times
References
- Dolecek TA, Propp JM, Stroup NE, Kruchko C. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2005-2009. Neuro-oncology. 2012;14 suppl. 5:v1–49. doi: 10.1093/neuonc/nos218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352:997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- Markert JM, Liechty PG, Wang W, Gaston S, Braz E, Karrasch M, et al. Phase Ib trial of mutant herpes simplex virus G207 inoculated pre-and post-tumor resection for recurrent GBM. Mol Ther. 2009;17:199–207. doi: 10.1038/mt.2008.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markert JM, Medlock MD, Rabkin SD, Gillespie GY, Todo T, Hunter WD, et al. Conditionally replicating herpes simplex virus mutant, G207 for the treatment of malignant glioma: results of a phase I trial. Gene Ther. 2000;7:867–874. doi: 10.1038/sj.gt.3301205. [DOI] [PubMed] [Google Scholar]
- Mineta T, Rabkin SD, Yazaki T, Hunter WD, Martuza RL. Attenuated multi-mutated herpes simplex virus-1 for the treatment of malignant gliomas. Nat Med. 1995;1:938–943. doi: 10.1038/nm0995-938. [DOI] [PubMed] [Google Scholar]
- Advani SJ, Sibley GS, Song PY, Hallahan DE, Kataoka Y, Roizman B, et al. Enhancement of replication of genetically engineered herpes simplex viruses by ionizing radiation: a new paradigm for destruction of therapeutically intractable tumors. Gene Ther. 1998;5:160–165. doi: 10.1038/sj.gt.3300546. [DOI] [PubMed] [Google Scholar]
- Bradley JD, Kataoka Y, Advani S, Chung SM, Arani RB, Gillespie GY, et al. Ionizing radiation improves survival in mice bearing intracranial high-grade gliomas injected with genetically modified herpes simplex virus. Clin Cancer Res. 1999;5:1517–1522. [PubMed] [Google Scholar]
- Markert JM, Gillespie GY, Weichselbaum RR, Roizman B, Whitley RJ. Genetically engineered HSV in the treatment of glioma: a review. Rev Med Virol. 2000;10:17–30. doi: 10.1002/(sici)1099-1654(200001/02)10:1<17::aid-rmv258>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- National Institutes of Health. Common Terminology Criteria for Adverse Events, Version 3.0. 2003.
- Macdonald DR, Cascino TL, Schold SC, Jr, Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8:1277–1280. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- Markert JM, Malick A, Coen DM, Martuza RL. Reduction and elimination of encephalitis in an experimental glioma therapy model with attenuated herpes simplex mutants that retain susceptibility to acyclovir. Neurosurgery. 1993;32:597–603. doi: 10.1227/00006123-199304000-00016. [DOI] [PubMed] [Google Scholar]
- Mineta T, Markert JM, Takamiya Y, Coen DM, Rabkin SD, Martuza RL. CNS tumor therapy by attenuated herpes simplex viruses. Gene Ther. 1994;1 suppl. 1:S78. [PubMed] [Google Scholar]
- Cobbs C, Markert JM. Gene therapy of glioma: a review. Perspect Neurol Surg. 1999;10:1–20. [Google Scholar]
- Advani SJ, Mezhir JJ, Roizman B, Weichselbaum RR. ReVOLT: radiation-enhanced viral oncolytic therapy. Int J Radiat Oncol Biol Phys. 2006;66:637–646. doi: 10.1016/j.ijrobp.2006.06.034. [DOI] [PubMed] [Google Scholar]
- Adusumilli PS, Stiles BM, Chan MK, Chou TC, Wong RJ, Rusch VW, et al. Radiation therapy potentiates effective oncolytic viral therapy in the treatment of lung cancer. Ann Thorac Surg. 2005;80:409–16; discussion 416. doi: 10.1016/j.athoracsur.2005.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen TJ, Katz S, Wittmack EK, Varghese S, Todo T, Rabkin SD, et al. Ionizing radiation does not alter the antitumor activity of herpes simplex virus vector G207 in subcutaneous tumor models of human and murine prostate cancer. Neoplasia. 2001;3:451–456. doi: 10.1038/sj.neo.7900193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanziale SF, Petrowsky H, Joe JK, Roberts GD, Zager JS, Gusani NJ, et al. Ionizing radiation potentiates the antitumor efficacy of oncolytic herpes simplex virus G207 by upregulating ribonucleotide reductase. Surgery. 2002;132:353–359. doi: 10.1067/msy.2002.125715. [DOI] [PubMed] [Google Scholar]
- Kim SH, Wong RJ, Kooby DA, Carew JF, Adusumilli PS, Patel SG, et al. Combination of mutated herpes simplex virus type 1 (G207 virus) with radiation for the treatment of squamous cell carcinoma of the head and neck. Eur J Cancer. 2005;41:313–322. doi: 10.1016/j.ejca.2004.10.018. [DOI] [PubMed] [Google Scholar]
- Bünzli D, Wietlisbach V, Barazzoni F, Sahli R, Meylan PR. Seroepidemiology of Herpes Simplex virus type 1 and 2 in Western and Southern Switzerland in adults aged 25-74 in 1992-93: a population-based study. BMC Infect Dis. 2004;4:10. doi: 10.1186/1471-2334-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashido M, Kawana T, Matsunaga Y, Inouye S. Changes in prevalence of herpes simplex virus type 1 and 2 antibodies from 1973 to 1993 in the rural districts of Japan. Microbiol Immunol. 1999;43:177–180. doi: 10.1111/j.1348-0421.1999.tb02390.x. [DOI] [PubMed] [Google Scholar]
- Rabenau HF, Buxbaum S, Preiser W, Weber B, Doerr HW. Seroprevalence of herpes simplex virus types 1 and type 2 in the Frankfurt am Main area, Germany. Med Microbiol Immunol. 2002;190:153–160. doi: 10.1007/s00430-001-0102-1. [DOI] [PubMed] [Google Scholar]
- Chahlavi A, Rabkin S, Todo T, Sundaresan P, Martuza R. Effect of prior exposure to herpes simplex virus 1 on viral vector-mediated tumor therapy in immunocompetent mice. Gene Ther. 1999;6:1751–1758. doi: 10.1038/sj.gt.3301003. [DOI] [PubMed] [Google Scholar]
- Todo T, Feigenbaum F, Rabkin SD, Lakeman F, Newsome JT, Johnson PA, et al. Viral shedding and biodistribution of G207, a multimutated, conditionally replicating herpes simplex virus type 1, after intracerebral inoculation in aotus. Mol Ther. 2000;2:588–595. doi: 10.1006/mthe.2000.0200. [DOI] [PubMed] [Google Scholar]
- Choucair AK, Levin VA, Gutin PH, Davis RL, Silver P, Edwards MS, et al. Development of multiple lesions during radiation therapy and chemotherapy in patients with gliomas. J Neurosurg. 1986;65:654–658. doi: 10.3171/jns.1986.65.5.0654. [DOI] [PubMed] [Google Scholar]
- Pasquier B, Pasquier D, N'Golet A, Panh MH, Couderc P. Extraneural metastases of astrocytomas and glioblastomas: clinicopathological study of two cases and review of literature. Cancer. 1980;45:112–125. doi: 10.1002/1097-0142(19800101)45:1<112::aid-cncr2820450121>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Agarwal S, Manchanda P, Vogelbaum MA, Ohlfest JR, Elmquist WF. Function of the blood-brain barrier and restriction of drug delivery to invasive glioma cells: findings in an orthotopic rat xenograft model of glioma. Drug Metab Dispos. 2013;41:33–39. doi: 10.1124/dmd.112.048322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelbaum MA, Jost S, Aghi MK, Heimberger AB, Sampson JH, Wen PY, et al. Application of novel response/progression measures for surgically delivered therapies for gliomas: Response Assessment in Neuro-Oncology (RANO) Working Group. Neurosurgery. 2012;70:234–243. doi: 10.1227/NEU.0b013e318223f5a7. [DOI] [PubMed] [Google Scholar]
- Harrow S, Papanastassiou V, Harland J, Mabbs R, Petty R, Fraser M, et al. HSV1716 injection into the brain adjacent to tumour following surgical resection of high-grade glioma: safety data and long-term survival. Gene Ther. 2004;11:1648–1658. doi: 10.1038/sj.gt.3302289. [DOI] [PubMed] [Google Scholar]
- Papanastassiou V, Rampling R, Fraser M, Petty R, Hadley D, Nicoll J, et al. The potential for efficacy of the modified (ICP 34.5(-)) herpes simplex virus HSV1716 following intratumoural injection into human malignant glioma: a proof of principle study. Gene Ther. 2002;9:398–406. doi: 10.1038/sj.gt.3301664. [DOI] [PubMed] [Google Scholar]
- Rampling R, Cruickshank G, Papanastassiou V, Nicoll J, Hadley D, Brennan D, et al. Toxicity evaluation of replication-competent herpes simplex virus (ICP 34.5 null mutant 1716) in patients with recurrent malignant glioma. Gene Ther. 2000;7:859–866. doi: 10.1038/sj.gt.3301184. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
G207 steroid status of subjects; IV, intravenous; po, oral, NA, Not available.
Seriousness as determined by CTCAE grading scale; SEV, Severity with 1-mild, 2-moderate, and 3-severe.
Progression-free and overall survival times
CD 4 and CD 8 counts.
HSV serostatus.
Compassionate use event/endpoint times