Abstract
Cutaneous leishmaniasis (CL) is the most frequent clinical form of tegumentary leishmaniasis and is characterised by a single or a few ulcerated skin lesions that may disseminate into multiple ulcers and papules, which characterise disseminated leishmaniasis (DL). In this study, cells were quantified using immunohistochemistry and haematoxylin and eosin staining (CD4+, CD68+, CD20+, plasma cells and neutrophils) and histopathology was used to determine the level of inflammation in biopsies from patients with early CL, late CL and DL (ulcers and papules). The histopathology showed differences in the epidermis between the papules and ulcers from DL. An analysis of the cells present in the tissues showed similarities between the ulcers from localised CL (LCL) and DL. The papules had fewer CD4+ T cells than the DL ulcers. Although both CD4+ cells and macrophages contribute to inflammation in early CL, macrophages are the primary cell type associated with inflammation intensity in late ulcers. The higher frequency of CD20+ cells and plasma cells in lesions demonstrates the importance of B cells in the pathogenesis of leishmaniasis. The number of neutrophils was the same in all of the analysed groups. A comparison between the ulcers from LCL and DL and the early ulcers and papules shows that few differences between these two clinical forms can be distinguished by observing only the tissue.
Keywords: leishmaniasis, disseminated leishmaniasis, histopathological aspects, inflammation, inflammatory cells
Leishmaniases are diseases caused by protozoa of the genus Leishmania. Human infection occurs through the bite of an infected sandfly and it is estimated that approximately two million new cases of leishmaniasis occur each year; however, only approximately 600,000 are reported (Ashford et al. 1992). In Brazil, cases are reported for all regions and leishmaniasis is especially common in the states of Ceará, Maranhão and Bahia (MS 2007).
Leishmaniasis can manifest as a tegumentary form that includes cutaneous and mucocutaneous leishmaniasis or as a visceral form of the disease. The difference in clinical manifestation depends on the host immune system and the infecting parasite strain (Yoneyama et al. 2007). The most common clinical form of tegumentary leishmaniasis (TL) is cutaneous leishmaniasis (CL). CL is characterised by a single ulcer, which is also referred to as localised CL (LCL) and this ulcer may spontaneously regress or disseminate into the multiple ulcers and papules, which are characteristic of disseminated leishmaniasis (DL), an emerging form of leishmaniasis (Costa et al. 1986, Turetz et al. 2002). Multiple studies of the morphological pathology of leishmaniasis have been performed in attempts to establish a morphological pattern that could be used to differentiate between the lesions (Azulay 1960, Ridley et al. 1980, Magalhães et al. 1986a).
In infected individuals who do not develop lesions or disease, the immune response expresses a T-helper (Th)1 pattern that can be detected in peripheral blood cells (Carvalho et al. 1985). However, the intensity of inflammation can also result in ulcerations in which the healing capacity of these cells is lost, thus favouring the immunopathology of the disease (Gollob et al. 2005).
In LCL, the lesions involve a local inflammatory reaction composed primarily of plasma cells, macrophages and lymphocytes. Experimental studies have evaluated T lymphocytes and their contributions to the lesion resolution process and to resistance to infection (Müller et al. 1994, Milon et al. 1995, Scott & Farrell 1998). In humans, several studies have evaluated the frequency and the pattern of T cells in the peripheral blood and tissues from TL (Uyemura et al. 1993, Carvalho et al. 1994, da-Cruz et al. 1994, Diaz et al. 2002, Antonelli et al. 2005, Campanelli et al. 2006).
One study suggested that the humoral response was not essential for the development of protective immunity in human leishmaniasis (McSorley et al. 1996). However, studies in experimental models have shown that B cell depletion leads to disease exacerbation, suggesting that B cells are necessary for the activity of the T cells that mediate lesion healing; thus, humoral responses may in fact play a role in mediating protective immunity against leishmaniasis (Scott et al. 1986). Comparisons of the immune responses involved in LCL and DL have indicated that the host immunity in LCL is more efficient, with higher production of interferon (IFN)-γ and tumour necrosis factor (Turetz et al. 2002, Guimarães et al. 2005).
The differences in the immune responses between the localised and disseminated forms may provide information regarding protection and resistance against leishmaniasis parasites. The present study used histopathology and the detection of cells in situ to examine the differences between the two clinical forms of leishmaniasis. Correlations between cell populations and the total extent of inflammation may highlight aspects of the tissue response and elucidate leishmaniasis pathogenesis. Thus, the present study aimed to compare the histopathology and cell infiltrates in biopsies from patients with early and late LCL and from patients with DL who presented both ulcers and papules.
SUBJECTS, MATERIALS AND METHODS
Patients characteristics - We evaluated 30 patients divided into the following four groups: 10 patients with skin lesions diagnosed with recent LCL (LCL-E) (within 20 days after the appearance of the first lesion), 10 patients with late LCL (LCL-L) (lesions for more than 30 days) and 10 patients with DL who had both ulcers (DL-U) and papules (DL-P), resulting in two biopsies per patient in this group. Biopsies were performed for diagnosis and selected according to the clinical criteria specified above. The analysed variables were obtained from medical records.
Leishmaniasis was diagnosed in subjects depending on clinical criteria, positivity for Montenegro intradermal skin test, parasite culture, polymerase chain reaction and histopathology. Grocott staining was used for fungal differential diagnosis and immunohistochemistry (IHC) using a polyclonal anti-Leishmania antibody was performed for confirmation when necessary.
Biopsy: procedure, processing and analysis - Skin fragments obtained from the borders of ulcers or papules were performed using a 4-mm “punch” while the patient was under local anaesthesia. The fragments were fixed in 10% formalin for 48 h and processed at the histotechnology laboratory at Oswaldo Cruz Foundation (Fiocruz). After paraffin embedding, the tissues were cut into 5-μm sections for staining with haematoxylin and eosin (H&E) and for IHC.
Immunohistochemical staining - The tissue sections obtained from paraffin were deparaffinised and rehydrated. IHC reactions were performed after blocking peroxidase activity with 3% hydrogen peroxide for 5 min. The slides were incubated at 25ºC with anti-CD4 (M3354, Spring, USA), anti-CD68 (M071801-5, DAKO, USA) or anti-CD20 (Mob 004, DBS) antibodies at dilutions of 1:500, 1:50 and 1:50, respectively. A Peroxidase Mouse & Rabbit Kit/HRP (DBS KP500, USA) was used to perform the reaction according to the manufacturer’s recommendations. All of the slides were counter-stained with Harris haematoxylin, dehydrated and mounted with Canadian balsam and glass coverslips.
Cellular composition analysis - A Nikon ACT-1 with v.2.70 software was used to capture 15 random images from 15 different fields of each tissue sample at 400X magnification. The quantification of CD4+, CD20+ and CD68+ cells was performed manually using ImageJ software (National Institutes of Health). Neutrophils and plasma cells were directly quantified from fragments stained with H&E following the same methodology. The numbers of cells were reported as absolute values (number of cells/15 fields).
Inflammation quantification - Images of tissue fragments stained by H&E were captured as described. The analysis was performed on the total area of the tissue fragment and the extent of inflammation (μm2) was determined using ImageJ software. The percentage of inflammation/fragment total area was determined.
Histopathological analysis - Histopathological analysis was performed by two observers on all of the fragments stained with H&E. The morphological characteristics of each biopsy were evaluated for their presence (+) or absence (0) and reported in a worksheet. Changes were observed in the epidermis (fibrin crust, hyperkeratosis, acanthosis, hydropic degeneration, spongiosis and exocytosis) and in the dermis (oedema, granulation tissue, granulomas, giant cells and lytic necrosis).
Ethical considerations - The study was approved by the Human Ethical Committee of the Gonçalo Moniz Research Center under Fiocruz protocol 321-2009.
Statistical analysis - Statistical analyses were performed using GraphPad Prism 5.0. Spearman correlation analysis, Kruskal-Wallis and Pearson correlations and One-Way ANOVA were performed as non-parametric and parametric tests, respectively. The results were considered statistically significant when p < 0.05. A chi-square test was performed to compare the frequencies between variables in independent samples.
RESULTS
Twenty patients with LCL were placed in the following subgroups according to the duration of their lesions: LCL-L, for those with lesions with more than 30 days and LCL-E, when diagnosed with an ulcer for less than 20 days. The other 10 patients had DL and all of these patients had simultaneous ulcerated primary lesions (DL-U) plus secondary lesions due to dissemination (DL-P), thereby forming a fourth group composed of the same patients. The clinical characteristics of the patients are described in Table I.
TABLE I. Clinical characteristics of patients with cutaneous leishmaniasis (CL).
| Characteristics | LCL-L (n = 10) | LCL-E (n = 10) | DL-U (n = 10) | DL-P (n = 10) |
|---|---|---|---|---|
| Male:female | 07:03 | 07:03 | 07:03 | 07:03 |
| Age (mean years ± SD) | 25 ± 11 | 23 ± 9 | 45 ± 17 | 45 ± 17 |
| Lesion duration (mean days ± SD) | 34 ± 10 | 17 ± 2 | 43 ± 20 | 30 ± 25 |
| Number of lesions (mean days ± SD) | 1 | 1 | 3 ± 4 | 29 ± 60 |
| Lesion size (mean mm2 ± SD) | 337 ± 240 | 58 ± 63 | 185 ± 136 | NM |
| MST (mean mm2 ± SD) | 190 ± 125 | 322 ± 256 | 164.6 ± 136 | 164.6 ± 136 |
DL: disseminated leishmaniasis; DL-P: DL with papules; DL-U: DL with ulcers; LCL: localised CL; LCL-E: recent LCL; LCL-L: late LCL; MST: Montenegro skin test; NM: not measured; SD: standard deviation.
The epidermis and dermis were analysed separately. The analysed histopathological-characteristic frequencies and the comparisons between the groups are described in Table II.
TABLE II. Frequency of histopathological aspects observed in the 40 cutaneous leishmaniasis (CL) and disseminated leishmaniasis (DL) biopsies.
| Epidermis | Dermis | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Fibrin crust | Hyperkeratosis | Acanthosis | Hydropic degeneration | Spongiosis | Exocytosis | Oedema | Granulation tissue | Granuloma | Giant cells | Lytic necrosis | ||
| LCL-L | 30 | 80 | 100 | 80 | 80 | 50 | 90 | 90 | 80 | 20 | 50 | |
| LCL-E | 30 | 90 | 100 | 80 | 50 | 60 | 100 | 90 | 30 | 20 | 10 | |
| DL-U | 0 | 90 | 10 | 70 | 80 | 50 | 10 | 10 | 20 | 20 | 50 | |
| DL-P | 40 | 70 | 70 | 50 | 50 | 50 | 10 | 10 | 30 | 30 | 50 | |
| p | ||||||||||||
| LCL-L x LCL-E | NS | NS | NS | NS | 0.0001 | NS | NS | NS | 0.0001 | NS | 0.0001 | |
| LCL-L x DL-U | 0.0001 | NS | NS | NS | NS | NS | 0.0015 | 0.0015 | 0.0001 | NS | NS | |
| DL-U x DL-P | 0.0001 | 0.0007 | 0.0001 | 0.0059 | 0.0001 | NS | NS | NS | NS | NS | NS | |
DL-P: DL with papules; DL-U: DL with ulcers; LCL: localised CL; LCL-E: recent LCL; LCL-L: late LCL; NS: not significant.
Biopsies from the LCL-E and LCL-L patients presented similar morphological characteristics in the epidermis and dermis. In the LCL-E group, a fibrin crust was observed in 30% of the cases, hyperkeratosis in 90%, acanthosis in 100%, hydropic degeneration in 80% and exocytosis in 60%. In the dermis, granulation tissue was present in 90% of the cases and giant cells were present in 20% of the cases. However, unlike early ulcers, late lesions had more spongiosis (80%), granulomas (90%) and lytic necrosis (50%) (Table II).
The histopathological characteristics of the epidermis were much different between the secondary lesions and ulcers in DL. The ulcers presented higher frequencies of hyperkeratosis (90%), hydropic degeneration (70%) and spongiosis (80%) and lacked a fibrin crust. The papules presented a fibrin crust in 40% of the cases and presented acanthosis in 70% of the cases. Exocytosis was equal between the ulcers and papules (50%). These differences were not observed in the dermis (Table II).
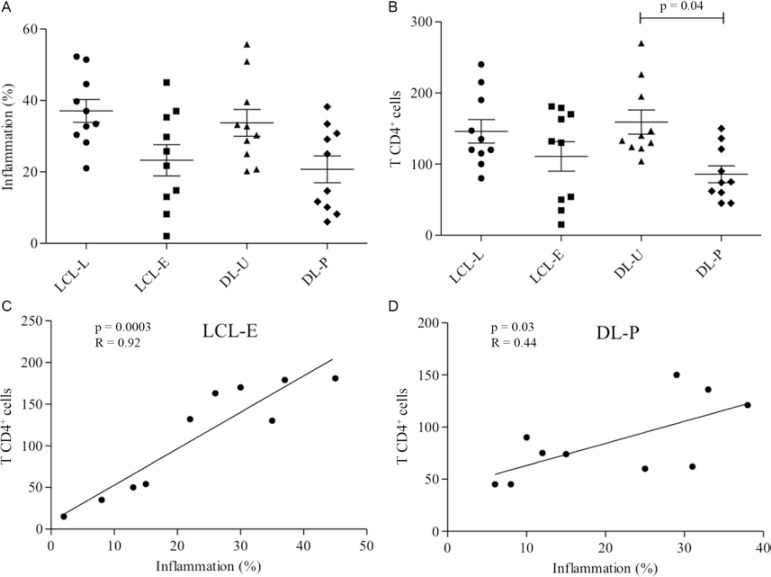
A higher percentage of inflammation was observed in the LCL-L group (37 ± 10) compared with recent lesions (23 ± 13) (Fig. 1A). We evaluated the time and extent of the lesion area and found that the lesions increased with disease progression only in the more advanced ulcers (over 30 days) (data not shown).
Fig. 1A. : inflammation percentage in lesions from localised cutaneous leishmaniasis (LCL) and disseminated leishmaniasis (DL). Mean ± standard deviation (SD) are indicated; B: number of CD4+ T lymphocytes in lesions from LCL and DL. Mean ± SD are indicated; C: linear correlations of Spearman (p = 0.003; R = 0.92) between inflammation (%) and CD4+ T lymphocytes in recent (LCL-E) group; D: linear correlations of Spearman between inflammation (%) and CD4+ T lymphocytes in DL with papule (DL-P) group (p = 0.03; R = 0.44) (10 patients per group). Significance levels are indicated. DL-U: DL with ulcers; LCL-L: late LCL.
The primary and secondary lesions of patients with DL had various degrees of inflammation. The ulcerated lesions (DL-U) had a higher inflammatory infiltrate (35 ± 12) compared with the papular lesions (DL-P) (20 ± 12) (Fig. 1A). Although the ulcerated lesions of this group were present for a similar time as the LCL-L lesions (over 30 days), there was no correlation with lesion area, as observed in the other group (data not shown). The area of the papules was not measured.
The presence of CD4+ T lymphocytes, although not statistically significant, was higher in the late ulcers than in the recent ulcers (Figs 1B, 2A). A comparison between the number of CD4+ cells and the level of inflammation showed that the amount of CD4+ cells increased with the inflammatory infiltrate in early ulcers (Fig. 1C). In later lesions, no correlation between CD4+ cells and inflammation was observed (data not shown). In DL, CD4+ T lymphocytes were more prevalent in ulcers than in papules (Fig. 1B). Unlike the ulcers, the papules showed a positive correlation between the number of CD4+ lymphocytes and the extent of the inflammatory infiltrate (Fig. 1D).
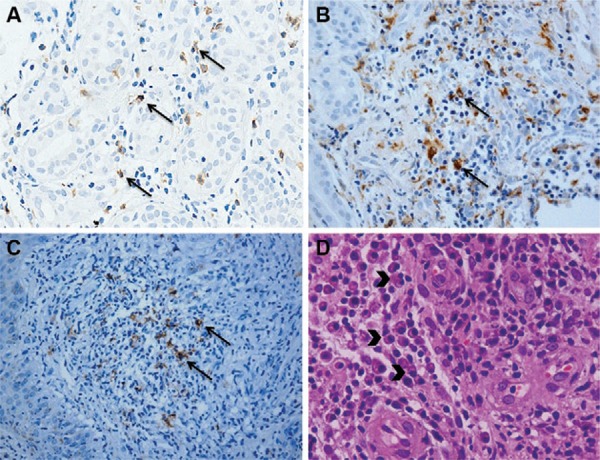
Fig. 2A. : immunostained CD4+ T cells (400X) (arrows); B: immunostained CD68+ cells (400X) (arrows); C: immunostained CD20+ cells (400X) (arrows); D: in haematoxylin and eosin, the presence of plasma cells in the inflammatory infiltrate (arrowheads) (400X).
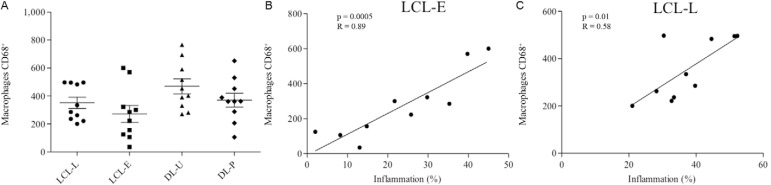
Macrophages were the most prevalent cell type and showed similar frequencies between LCL-L and LCL-E (Figs 2B, 3A). Unlike the CD4+ T lymphocytes, the number of CD68+ macrophages in the inflammatory infiltrate progressively increased as inflammation increased, in both types of lesions (Fig. 3B, C). In DL biopsies, CD68+ cells were slightly higher in the ulcerated lesions than in the papules (Fig. 3A). Unlike what was observed for LCL lesions, the presence of macrophages did correlate with increased inflammatory infiltrate in the DL lesions (data not shown).
Fig. 3A. : number of CD68+ macrophages in lesions from localised cutaneous leishmaniasis (LCL) and disseminated leishmaniasis (DL). Mean ± standard deviation are indicated; B: linear correlations of Spearman between inflammation (%) (p = 0.0005; R = 0.89) and CD68+ macrophages recent LCL (LCL-E) group; C: linear correlations of Spearman (p = 0.01; R = 0.59) between inflammation (%) and CD68+ macrophages in late LCL (LCL-L) group (10 patients per group). DL-P: DL with papules; DL-U: DL with ulcers.
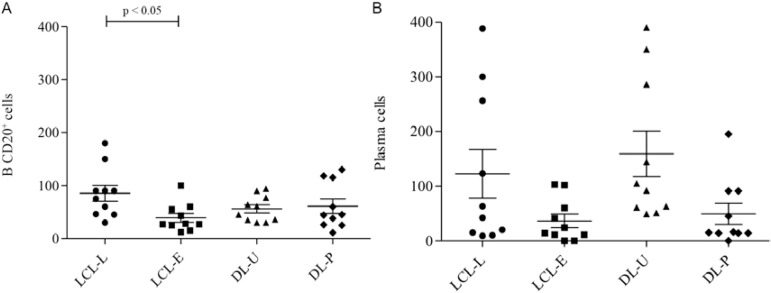
CD20+ B cells and plasma cells (Fig. 2C, D) in the LCL-L and LCL-E groups were similar and appeared more frequently in the late lesions (Fig. 4). In DL, the CD20+ B lymphocytes were present in the same amount in the ulcers and papules. Although not significant, plasma cells tended to be more frequent in DL ulcers (Fig. 4A, B). The number of CD20+ B and plasma cells did not correlate with inflammatory infiltration in any of the four lesions evaluated (data not shown)
Fig. 4A. : number of CD20+ B lymphocytes in lesions from localised cutaneous leishmaniasis (LCL) and disseminated leishmaniasis (DL). Mean ± standard deviation (SD) are indicated; B: number of plasma cells in lesions from LCL and DL. Mean ± SD are indicated (10 patients per group). Significance levels are indicated. DL-P: DL with papules; DL-U: DL with ulcers.
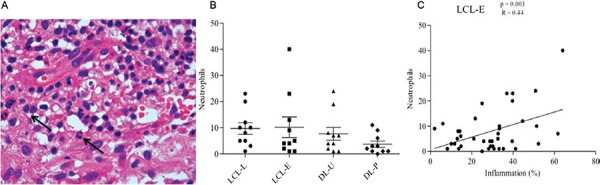
Neutrophils were rarely found in the tissues and appeared in equal numbers in all groups (Fig. 5A, B). However, in the early phase of ulcers, neutrophils appeared to be gradually recruited and correlated with increased inflammatory infiltrate; this did not occur in the older ulcers (Fig. 5C). However, the number of neutrophils did not correlate with lesion duration (data not shown). Neutrophils were also rare in DL (Fig. 5B). Neutrophils appeared to be important during the initial stage of the disease, but their numbers did not correlate with lesion progression or increased infiltration in biopsies (data not shown).
Fig. 5A. : in haematoxylin and eosin, the presence of neutrophils in the inflammatory infiltrate (arrows) (400X); B: number of neutrophils in lesions from localised cutaneous leishmaniasis (LCL) and disseminated leishmaniasis (DL); C: linear correlations of Pearson between inflammation (%) and neutrophils in recent LCL (LCL-E) group (10 patients per group). DL-P: DL with papules; DL-U: DL with ulcers.
DISCUSSION
The analysis of biopsies showed minimal differences in histology between the clinical forms of CL. Studies that have analysed different clinical forms and biopsies before and after treatment of recurrent CL lesions also did not observe any differences (Esterre et al. 1992, Mehregan et al. 1999). The frequency of morphological characteristics in the early and late LCL lesions is consistent with those described by Magalhães et al. (1982) and by Weigle and Saravia (1996). The differences observed at the epidermis in the biopsies from the DL patients were described in the first study on the histology of DL lesions (Carvalho et al. 1994). Therefore, it is not possible to establish an exact time of evolution of a CL lesion using histopathology and we cannot predict whether this lesion may progress or be cured.
The immune response mediated by T cells with a Th1 or Th2 profile in leishmaniasis has been well described based on findings from peripheral blood cells (Reed & Scott 1993, Coutinho et al. 1996, Ribeiro-de-Jesus et al. 1998, Rocha et al. 1999, Campanelli et al. 2006). However, few studies have evaluated the response in situ (Esterre et al. 1992, Palma & Saravia 1997, da-Cruz et al. 2005, Campanelli et al. 2006) compared with the response in DL (Carvalho et al. 1994, Turetz et al. 2002, Machado et al. 2011). Unlike studies based on peripheral blood cells (Esterre et al. 1992, da-Cruz et al. 1994, Campanelli et al. 2006), the analysis of cells in situ made it possible to describe a different immune response pattern (Uyemura et al. 1993, Campanelli et al. 2006).
By comparing the inflammatory infiltrates, we found that the late ulcers and the DL ulcers had higher inflammation than the early ulcers or papules. Thus, as the disease progresses, there is a continuous influx of inflammatory cells into the tissues and many inflammatory cells remain at the site of infection.
The abundance of CD4+ Th1 cells may be responsible for the development of immunity and for the natural healing of lesions (Uyemura et al. 1993). In biopsies of older ulcers from LCL and DL patients, there was a higher frequency of CD4+ T cells than in the early lesions and papules; these data are consistent with previous studies (Palma & Saravia 1997, da-Cruz et al. 2005). The early lesions and papules, unlike late and disseminated ulcers, demonstrated a positive correlation between the CD4+ cells and the amount of inflammatory cell infiltration, suggesting that during the early development of the disease, the recruitment of CD4+ cells occurs gradually. However, no association with the extent of inflammation and these cells was found in the late lesions. This may indicate a change to a lesion-healing phase.
Similar numbers of macrophages were observed in both stages of LCL and in the two clinical forms, but there was a slight tendency for increased numbers of macrophages in DL lesions; this predominance was also observed by Vieira et al. (2002). Macrophages were the cell type that showed the strongest correlation with inflammation in LCL.
Little has been reported about B cells and antibodies in CL patients. Like the study by Vieira et al. (2002), our findings revealed that late LCL lesions had a higher frequency of B lymphocytes than early lesions. Higher production of IFN-γ is associated with increased antibody secretion by B cells, helping to contain the parasites, but IFN-γ is also associated with Leishmania entry into macrophages, a process that results in permanent lesions (Vieira et al. 2002). Increased levels of antibodies in the blood have been associated with dissemination to the mucosa in patients with LCL (Carvalho et al. 1994). Similar numbers of B cells in ulcers and papules may be associated with the dissemination of the parasite. Because of the differences in the immune responses of DL patients, B cells may be more closely related to parasite spreading in these cases. However, in LCL, B cells may contribute more to persistent lesions. A new class of B cells capable of acting as regulatory cells (Bregs) has been studied in chronic and autoimmune diseases (Yang et al. 2009, Carter et al. 2012, Giannoukakis & Trucco 2012). An evaluation of the number of Breg cells in lesions from DL may show that Breg cells also enable the spread of Leishmania.
Plasma cells were frequently observed in the infiltrates of tissue lesions in all of the groups and they were more common than B lymphocytes in the LCL and DL late ulcerative lesions; this observation is consistent with previous studies showing that most of these cells are mature at the lesion site and are responsible for immunoglobulin production in situ (Magalhães et al. 1986, Esterre et al. 1992, Vieira et al. 2002). In contrast to CD4+ T lymphocytes, the recruitment of B lymphocytes and plasma cells did not occur gradually with disease progression in any clinical form. CD4+ T cells may play a role in inflammation, while B and plasma cells are coordinated differently.
Neutrophils were rare compared with the other cells analysed in this study and were present at similar numbers in all types of lesions. Several studies have evaluated neutrophils in leishmaniasis, especially in the early phase of the disease. However, some of the reports are controversial. For example, neutrophils may contribute to protection from the disease, but may also be important for the immunopathology (Müller et al. 2001, Aga et al. 2002, Laskay et al. 2003, von Stebut 2007, Afonso et al. 2008, Daboul 2010). Some studies have suggested that Leishmania can use neutrophils to invade macrophages during the initial attempt of these cells to contain the parasite (Aga et al. 2002, van Zandbergen et al. 2004). However, Daboul (2010) reported that neutrophils are recruited gradually during the initial phase and that their presence at the late stage occurs after the rupture of phagocytes filled with amastigotes, thus restarting an acute reaction in which neutrophils limit the disease progression (Daboul 2010). These data explain the positive correlation between early LCL and inflammatory infiltrate and the absence of a correlation with late lesions.
The tissues of ulcers from both clinical forms are similar and it is not possible to predict disease dissemination. The early ulcers and papules also showed some histopathological similarities. Although these two clinical forms are similar, they represent distinct immunological moments. The analysis of the dynamics of cellular infiltration into CL lesions in its various clinical forms contributes to the characterisation of the local inflammatory response and provides a better understanding of the pathogenesis of the disease.
ACKNOWLEDGEMENTS
To Mr Lago, Ms Neuza and Ms Renda, from Corte de Pedra Health Service, and to technicians of the histotechnology service of CPqGM-Fiocruz.
Funding Statement
Financial support: NIH (AI-30639), INCT, ICIDR
Footnotes
Financial support: NIH (AI-30639), INCT, ICIDR
REFERENCES
- Afonso L, Borges VM, Cruz H, Ribeiro-Gomes FL, dos Reis G, Dutra AN, Clarêncio J, de Oliveira CI, Barral A, Barral-Netto M, Brodskyn CI. Interactions with apoptotic but not with necrotic neutrophils increase parasite burden in human macrophages infected with Leishmania amazonensis. J Leukoc Biol. 2008;84:389–396. doi: 10.1189/jlb.0108018. [DOI] [PubMed] [Google Scholar]
- Aga E, Katschinski DM, van Zandbergen G, Laufs H, Hansen B, Müller K, Solbach W, Laskay T. Inhibition of the spontaneous apoptosis of neutrophil granulocytes by the intracellular parasite Leishmania major. J Immunol. 2002;169:898–905. doi: 10.4049/jimmunol.169.2.898. [DOI] [PubMed] [Google Scholar]
- Antonelli LRV, Dutra WO, Almeida RP, Bacellar O, Carvalho EM, Gollob KJ. Activated inflammatory T cells correlate with lesion size in human cutaneous leishmaniasis. Immunol Lett. 2005;101:226–230. doi: 10.1016/j.imlet.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Ashford RW, Desjeux P, Deraadt P. Estimation of population at risk of infection and number of cases of leishmaniasis. Parasitol Today. 1992;8:104–105. doi: 10.1016/0169-4758(92)90249-2. [DOI] [PubMed] [Google Scholar]
- Azulay RD. Histopatologia da leishmaniose tegumentar. Dermatol Iber Lat Am. 1960;2:7–15. [Google Scholar]
- Campanelli AP, Roselino AM, Cavassani K, Pereira MSF, Mortara R, Brodskyn CI, Goncalves HS, Belkaid Y, Barral-Netto M, Barral A, Silva JS. CD4+CD25+ T cells in skin lesions of patients with cutaneous leishmaniasis exhibit phenotypic and functional characteristics of natural regulatory T cells. J Infect Dis. 2006;193:1313–1322. doi: 10.1086/502980. [DOI] [PubMed] [Google Scholar]
- Carter NA, Rosser EC, Mauri C. Interleukin-10 produced by B cells is crucial for the suppression of Th17/Th1 responses, induction of T regulatory type 1 cells and reduction of collagen-induced arthritis. R32Arthritis Res Ther. 2012;14 doi: 10.1186/ar3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho EM, Barral A, Costa JM, Bittencourt A, Marsden P. Clinical and immunopathological aspects of disseminated cutaneous leishmaniasis. Acta Trop. 1994;56:315–325. doi: 10.1016/0001-706x(94)90103-1. [DOI] [PubMed] [Google Scholar]
- Carvalho EM, Johnson WD, Barreto E, Marsden PD, Costa JL, Reed S, Rocha H. Cell mediated immunity in American cutaneous and mucosal leishmaniasis. J Immunol. 1985;135:4144–4148. [PubMed] [Google Scholar]
- Costa JM, Marsden PD, Llanos-Cuentas EA, Netto EM, Carvalho EM, Barral A, Rosa AC, Cuba CC, Magalhães AV, Barreto AC. Disseminated cutaneous leishmaniasis in a field clinic in Bahia, Brazil: a report of eight cases. J Trop Med Hyg. 1986;89:319–323. [PubMed] [Google Scholar]
- Coutinho SG, Oliveira MP, da-Cruz AM, de Luca PM, Mendonça SC, Bertho AL, Soong L, McMahon-Pratt D. T-cell responsiveness of American cutaneous leishmaniasis patients to purified Leishmania pifanoi amastigote antigens and Leishmania braziliensis promastigote antigens: immunologic patterns associated with cure. Exp Parasitol. 1996;84:144–155. doi: 10.1006/expr.1996.0100. [DOI] [PubMed] [Google Scholar]
- Daboul MW. Role of neutrophils in cutaneous leishmaniasis. East Mediterr Health J. 2010;16:1055–1058. [PubMed] [Google Scholar]
- da-Cruz AM, Bertho AL, Oliveira MP, Neto, Coutinho SG. Flow cytometric analysis of cellular infiltrate from American tegumentary leishmaniasis lesions. Br J Dermatol. 2005;153:537–543. doi: 10.1111/j.1365-2133.2005.06647.x. [DOI] [PubMed] [Google Scholar]
- da-Cruz AM, Conceição-Silva F, Bertho AL, Coutinho SG. Leishmania-reactive CD4+ and CD8+ T cells associated with cure of human cutaneous leishmaniasis. Infect Immun. 1994;62:2614–2618. doi: 10.1128/iai.62.6.2614-2618.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz NL, Zerpa O, Ponce LV, Convit J, Rondon AJ, Tapia FJ. Intermediate or chronic cutaneous leishmaniasis: leukocyte immunophenotypes and cytokine characterisation of the lesion. Exp Dermatol. 2002;11:34–41. doi: 10.1034/j.1600-0625.2002.110104.x. [DOI] [PubMed] [Google Scholar]
- Esterre P, Dedet JP, Frenay C, Chevallier M, Grimaud JA. Cell populations in the lesion of human cutaneous leishmaniasis: a light microscopical, immunohistochemical and ultrastructural study. Virchows Arch A Pathol Anat Histopathol. 1992;421:239–247. doi: 10.1007/BF01611181. [DOI] [PubMed] [Google Scholar]
- Giannoukakis N, Trucco M. A role for tolerogenic dendritic cell-induced B-regulatory cells in type 1 diabetes mellitus. Curr Opin Endocrinol Diabetes Obes. 2012;19:279–287. doi: 10.1097/MED.0b013e328355461b. [DOI] [PubMed] [Google Scholar]
- Gollob KJ, Antonelli LRV, Dutra WO. Insights into CD4+ memory T cells following Leishmania infection. Trends Parasitol. 2005;21:347–350. doi: 10.1016/j.pt.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Guimarães LH, Machado PRL, Lessa HA, Lessa M, D’Oliveira A, Carvalho EM. Aspectos clínicos da leishmaniose tegumentar. Gaz méd Bahia. 2005;75:66–74. [Google Scholar]
- Laskay T, van Zandbergen G, Solbach W. Neutrophil granulocytes-Trojan horses for Leishmania major and other intracellular microbes? Trends Microbiol. 2003;11:210–214. doi: 10.1016/s0966-842x(03)00075-1. [DOI] [PubMed] [Google Scholar]
- Machado PR, Rosa MEA, Costa D, Mignac M, Silva JS, Schriefer A, Teixeira MM, Bacellar O, Carvalho EM. Reappraisal of the immunopathogenesis of disseminated leishmaniasis: in situ and systemic immune response. Trans R Soc Trop Med Hyg. 2011;105:438–444. doi: 10.1016/j.trstmh.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magalhães AV, Chiarini LH, Raick AN. Histopathology of cutaneous leishmaniasis. Rev Inst Med Trop Sao Paulo. 1982;24:268–276. [PubMed] [Google Scholar]
- Magalhães AV, Moraes MAP, Raick AN, Cuentas AL, Costa JML, Cuba CC, Marsden PD. Histopathology of tegumentary leishmaniasis caused by Leishmania braziliensis braziliensis. 2. Tissue humoral response. Rev Inst Med Trop Sao Paulo. 1986;28:293–299. doi: 10.1590/s0036-46651986000500003. [DOI] [PubMed] [Google Scholar]
- McSorley S, Proudfoot L, O’Donnell CA, Liew FY. Immunology of murine leishmaniasis. Clin Dermatol. 1996;14:451–464. doi: 10.1016/0738-081x(96)00037-5. [DOI] [PubMed] [Google Scholar]
- Mehregan DR, Mehregan AH, Mehregan DA. Histologic diagnosis of cutaneous leishmaniasis. Clin Dermatol. 1999;17:297–304. doi: 10.1016/s0738-081x(99)00048-6. [DOI] [PubMed] [Google Scholar]
- Milon G, Giudice GD, Louis JA. Immunobiology of experimental cutaneous leishmaniasis. Parasitol Today. 1995;11:244–247. doi: 10.1016/0169-4758(95)80200-2. [DOI] [PubMed] [Google Scholar]
- MS - Ministério da Saúde Brasil Leishmaniose tegumentar americana. Portal da Saúde. 2007 portal.saude.gov.br/portal/saude/profissional/area.cfm?id_area=1560.
- Müller I, Kropf P, Louis JA, Milon G. Expansion of gamma interferon-producing CD8+ T cells following secondary infection of mice immune to Leishmania major. Infect Immun. 1994;62:2575–2581. doi: 10.1128/iai.62.6.2575-2581.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller K, van Zandbergen G, Hansen B, Laufs H, Jahnke N, Solbach W, Laskay T. Chemokines, natural killer cells and granulocytes in the early course of Leishmania major infection in mice. Med Microbiol Immunol. 2001;190:73–76. doi: 10.1007/s004300100084. [DOI] [PubMed] [Google Scholar]
- Palma GI, Saravia NG. In situ characterization of the human host response to Leishmania panamensis. Am J Dermatopathol. 1997;19:585–590. doi: 10.1097/00000372-199712000-00006. [DOI] [PubMed] [Google Scholar]
- Reed SG, Scott P. T-cell and cytokine responses in leishmaniasis. Curr Opin Immunol. 1993;5:524–531. doi: 10.1016/0952-7915(93)90033-o. [DOI] [PubMed] [Google Scholar]
- Ribeiro-de-Jesus A, Almeida RP, Lessa H, Bacellar O, Carvalho EM. Cytokine profile and pathology in human leishmaniasis. Braz J Med Biol Res. 1998;31:143–148. doi: 10.1590/s0100-879x1998000100020. [DOI] [PubMed] [Google Scholar]
- Ridley DS, Marsden PD, Cuba CC, Barreto AC. A histological classification of mucocutaneous leishmaniasis in Brazil and its clinical evaluation. Trans R Soc Trop Med Hyg. 1980;74:508–514. doi: 10.1016/0035-9203(80)90068-1. [DOI] [PubMed] [Google Scholar]
- Rocha PN, Almeida RP, Bacellar O, de Jesus AR, Filho DC, Filho AC, Barral A, Coffman RL, Carvalho EM. Down-regulation of Th1 type of response in early human American cutaneous leishmaniasis. J Infect Dis. 1999;180:1731–1734. doi: 10.1086/315071. [DOI] [PubMed] [Google Scholar]
- Scott P, Farrell JP. Experimental cutaneous leishmaniasis: induction and regulation of T cells following infection of mice with Leishmania major. Chem Immunol. 1998;70:60–80. doi: 10.1159/000058698. [DOI] [PubMed] [Google Scholar]
- Scott P, Natovitz P, Sher A. B lymphocytes are required for the generation of T cells that mediate healing of cutaneous leishmaniasis. J Immunol. 1986;137:1017–1021. [PubMed] [Google Scholar]
- Turetz ML, Machado PR, Ko AI, Alves F, Bittencourt A, Almeida RP, Mobashery N, Johnson WD, Carvalho EM. Disseminated leishmaniasis: a new and emerging form of leishmaniasis observed in northeastern Brazil. J Infect Dis. 2002;186:1829–1834. doi: 10.1086/345772. [DOI] [PubMed] [Google Scholar]
- Uyemura K, Pirmez C, Sieling PA, Kiene K, Paes-Oliveira M, Modlin RL. CD4+ type 1 and CD8+ type 2 T cell subsets in human leishmaniasis have distinct T cell receptor repertoires. J Immunol. 1993;151:7095–7104. [PubMed] [Google Scholar]
- van Zandbergen G, Klinger M, Mueller A, Dannenberg S, Gebert A, Solbach W, Laskay T. Cutting edge: neutrophil granulocyte serves as a vector for Leishmania entry into macrophages. J Immunol. 2004;173:6521–6525. doi: 10.4049/jimmunol.173.11.6521. [DOI] [PubMed] [Google Scholar]
- Vieira MGS, Oliveira F, Arruda S, Bittencourt AL, Barbosa AA, Jr, Barral-Netto M, Barral A. B-cell infiltration and frequency of cytokine producing cells differ between localized and disseminated human cutaneous leishmaniases. Mem Inst Oswaldo Cruz. 2002;97:979–983. doi: 10.1590/s0074-02762002000700009. [DOI] [PubMed] [Google Scholar]
- von Stebut E. Immunology of cutaneous leishmaniasis: the role of mast cells, phagocytes and dendritic cells for protective immunity. Eur J Dermatol. 2007;17:115–122. doi: 10.1684/ejd.2007.0122. [DOI] [PubMed] [Google Scholar]
- Weigle K, Saravia NG. Natural history, clinical evolution and the host-parasite interaction in new world cutaneous leishmaniasis. Clin Dermatol. 1996;14:433–450. doi: 10.1016/0738-081x(96)00036-3. [DOI] [PubMed] [Google Scholar]
- Yang HZ, Li Z, Liu HZ, Mi S, Hu ZW. The identification and advances of regulatory B cells. Sheng Li Ke Xue Jin Zhan. 2009;40:297–302. [PubMed] [Google Scholar]
- Yoneyama KAG, Peder LD, Lonardoni MVC, Silveira TGV. Diagnosis of American cutaneous leishmaniasis by enzyme immunoassay in patients from northern Paraná state, Brazil. Braz J Infect Dis. 2007;11:360–364. doi: 10.1590/s1413-86702007000300012. [DOI] [PubMed] [Google Scholar]