Abstract
During the influenza pandemic of 2009, the A(H1N1)pdm09, A/H3N2 seasonal and influenza B viruses were observed to be co-circulating with other respiratory viruses. To observe the epidemiological pattern of the influenza virus between May 2009-August 2011, 467 nasopharyngeal aspirates were collected from children less than five years of age in the city of Salvador. In addition, data on weather conditions were obtained. Indirect immunofluorescence, real-time transcription reverse polymerase chain reaction (RT-PCR), and sequencing assays were performed for influenza virus detection. Of all 467 samples, 34 (7%) specimens were positive for influenza A and of these, viral characterisation identified Flu A/H3N2 in 25/34 (74%) and A(H1N1)pdm09 in 9/34 (26%). Influenza B accounted for a small proportion (0.8%) and the other respiratory viruses for 27.2% (127/467). No deaths were registered and no pattern of seasonality or expected climatic conditions could be established. These observations are important for predicting the evolution of epidemics and in implementing future anti-pandemic measures.
Keywords: influenza, A(H1N1)pdm09, children, epidemiology, seasonality, climate
Influenza is a great threat to public health because it can lead to a broad array of respiratory illnesses that cause significant morbidity and mortality in children. The virus is transmitted through respiratory airborne droplets and/or indirect contact transmission (Tellier 2006). Influenza activity has marked patterns of circulation in the Northern Hemisphere, circulating in winter months from November-April, while in the Southern Hemisphere, circulation begins in May.
The Brazilian seasonal climates are nearly the reverse of the seasons in Europe and the United States of America. The main latitude and longitude of Brazil is 16º South and 55º West. Although 90% of the country is within the tropical zone, the climate of Brazil varies considerably from the mostly tropical north to the temperate zones below the Tropic of Capricorn (23º27’S latitude), which crosses the country at the latitude of the city of São Paulo.
The transmission of influenza and its relationship between weather variability and seasonality have been considered by several researchers and suggested to be strongly associated with climatic aspects. A colder temperature and low RH are believed to induce the widespread and rapid dissemination that have long been associated with viral seasonality in temperate regions. However, in tropical and subtropical countries, the dynamics of seasonal influenza A occur in a pattern that is poorly understood and in some areas, the activity peak may vary throughout the year (Viboud et al. 2006, Alonso et al. 2007, Brankston et al. 2007, Shaman & Kohn 2009, Chowell et al. 2010). This behaviour has also been associated with solar radiation and vitamin D levels. Because influenza infection is most common in winter in temperate regions when solar ultraviolet-B (UVB) doses are believed to be low, the study of the possible effects of UVB radiation on sunlight and vitamin D in innate immunity is rapidly growing (Sagripanti & Lytle 2007, Adams & Hewison 2008). In fact, the sustained circulation of these respiratory viruses and the resulting deaths are mainly dependent on host immunity and overall susceptibility to the viral strain. Virological and epidemiological characteristics of influenza viruses depend on the nature of their genomes, which are known to promote constant evolution that results in antigenic changes and therefore, a susceptible population. Eventually, new strains can emerge following a sudden antigenic shift when a reassortment event occurs, as observed in 2009, when the world experienced the emergence of the pandemic influenza A virus strain A(H1N1)pdm09 (CDC 2009, WHO 2011, WHO/GISN 2011).
We aimed to observe the behaviour of influenza A in relation to epidemiological aspects during the pandemic and post-pandemic in outpatients younger than five years old. In addition, we observed the circulation of influenza virus and its relationship with climatic conditions in a tropical city of northeastern Brazil.
SUBJECTS, MATERIALS AND METHODS
Subject data and specimen collection - Four hundred and sixty-seven nasopharyngeal aspirate samples collected between May 2009-August 2011 from selected patients with respiratory clinical manifestations were analysed. Reports were requested for all cases of children younger than five years of age who presented influenza-like illness (ILI). Three main criteria were requested (fever ≥ 36.5ºC, cough and rhinorrhoea) and at least one other criterion for inclusion of ILI, such as sore throat, headache or myalgia was considered (WHO 2009a, MS 2012). A standardised report form was used that included questions about one or more pre-existing conditions, such as asthma, immunosuppression and chronic metabolic, lung or heart disease (WHO 2009a). The data in the medical records were collected at the moment of consultation in a paediatric teaching hospital of the Federal University of Bahia (UFBA), Prof Hosannah de Oliveira Paediatric Centre, Prof Edgard Santos University Hospital Complex in the city of Salvador, state of Bahia (BA).
Clinical samples and laboratory investigation - All specimens were first tested with a commercial Indirect Immunofluorescence (IFI) kit (Respiratory Panel 1 Viral Screening and Identification kit, Light Diagnostics™ Respiratory Viral Screen IFA, included in kit #3105 EMD Millipore Corporation, Billerica, MA, USA). The assay screened for influenza A and B and respiratory syncytial virus (RSV), adenovirus (Adv), parainfluenza virus (PV) types I, II and III. In 2009, during the pandemic, all samples that were collected (100%) were tested by IFI, real-time transcription reverse polymerase chain reaction (rRT-PCR) and/or RT-PCR. In 2010 and 2011, two-thirds of the negative samples for influenza and other viruses were tested by rRT-PCR and/or conventional RT-PCR, considering clinical criteria that could detect any influenza virus (A/H3N2, A/H1N1, A(H1N1)pdm09 or influenza B present in the sample).
The rRT-PCR was performed in accordance with protocols established by WHO/CDC (2009) for the detection and characterisation of swine influenza A (swInfA), including a panel of oligonucleotide primers and dual-labelled hydrolysis (Taqman ® ). This assay was utilised to test respiratory specimens taken from suspected influenza A-infected patients. The influenza A primer and probe sets were used for the universal detection of type A influenza viruses. A primer and probe set for all swInfA viruses and a primer and probe set to match haemagglutinin (HA) genes (swH1) were used. The ABI Fast Real-time PCR System 7500 thermocycler and its corresponding software (Applied Biosystems, Foster City, CA, USA) were used. The subtype A/(H1N1pdm09) was confirmed by conventional multiplex RT-PCR using different primer sets that targeted six fragments of neuraminidase and five of HA genes. The primers were also used for seasonal influenza A/H3N2, influenza B and the former seasonal A/H1N1 virus. Direct sequencing was performed for the purified products using the ABI Prism Big Dye Terminator in accordance with the World Health Organization protocols (WHO 2009d).
Distinct climatic conditions - Meteorological data, such as the maximum and minimum temperature, air relative humidity (RH) and rainfall, were obtained during the study period from the Meteorological National Institute of Brazil, which is an official governmental institute (inmet.gov.br). Sunlight incidence was also obtained from climatetemp.info.
City geographic, climate characteristics and population dates - Salvador, latitude and longitude 12º54’S 38º20’W, has an average temperature of 25.1ºC (77ºF). The city receives on average of 1,866 mm (73.5 in) of precipitation annually or 156 mm (6.1 in) each month. The driest month is January, with an average of 77 mm (3.0 in) of rain and the wettest month is May, with an average of 293 mm (11.5 in) of rain. The mean RH for an average year is 80.2% and this region experiences 2,694 sunshine hours annually (climatetemp.info). According to the 2010 Brazilian Institute of Geography and Statistics Census (IBGE) (censo2010.ibge.gov.br/dados), 2,480,790 individuals reside in Salvador, which makes it the third-most populous city in Brazil, with a large concentration of people living in the urban area and suburbs.
Ethics approval - This study was approved by the Committee on Ethics of Maternity Climério de Oliveira, Faculty of Medicine, UFBA (159/2007). Informed consent was obtained from the parents or guardians of all children enrolled in the study and guidelines for human clinical sample ethics were strictly observed.
RESULTS
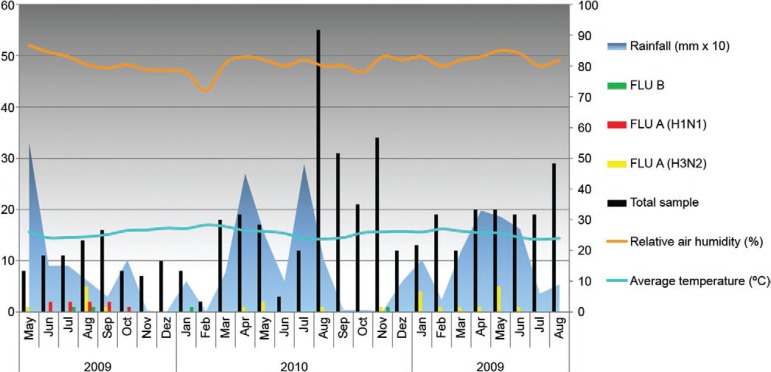
Of the 467 clinical samples collected between May 2009-August 2011, 34 (7%) were typed as influenza A and four were positive for influenza B (0.8%). Regarding influenza A subtyping, Flu A/H3N2 and influenza A(H1N1)pdm09 were identified in 25/34 (74%) and nine/34 (26%) of the samples, respectively. All pandemic influenza samples were detected from June-October 2009, most of which were equally distributed until September (Fig. 1). When analysing only the 85 samples that were collected in 2009, nine (10.5%) were confirmed to be H1N1pdm09-positive. Of the ILI cases that were confirmed to be influenza A(H1N1)pdm09, four/nine (44.4%) occurred in children aged less than or equal to one year, two/nine (22.2%) in children from one-two years and three/nine (33.3%) in children two-five years old. Dual influenza A infection A(H1N1)pdm09 and A/H3N2 viruses was detected in a four-year-old child, while influenza A(H1N1)pdm09/RSV co-infection was found in a child less than one year. The seasonal A/H3N2 virus was detected throughout the study years, with annual rates of 8.2%, 2.1% and 8.6% in 2009, 2010 and 2011, respectively, and affected all age ranges (Table I).
Fig. 1. : virological and climatic data in the city of Salvador, state of Bahia, Brazil, from May 2009-August 2011. Bars represent the influenza (Flu) virus detected. The X-axis shows the years and respective months and the left Y-axis shows the absolute number of positive specimens for influenza viruses (black bars). The right side Y-axis shows the average temperature (ºC), relative air humidity (%) and rainfall (mm x 10). In 2009, the total annual rain volume was 1,176 mm, in 2010, 1,780 mm, and 2011, 1,470 mm.
TABLE I. Prevalence of influenza A, including the pandemic virus and influenza B, in children under five years-old with influenza-like illness attending at a paediatric teaching hospital during 2009-2011 in the city of Salvador, state of Bahia, Brazil .
| Year | Influenza type | 0-1 year n (%) | 1-2 years n (%) | 2-5 years n (%) | Total n (%) |
|---|---|---|---|---|---|
| 2009 | A/H1N1pdm09 | 4 (16.7) | 2 (6.3) | 3 (10.3) | 9 (10.6) |
| A/H3N2 | 1 (4.2) | 4 (12.5) | 2 (6.9) | 7 (8.2) | |
| B | 2 (8.3) | 0 (-) | 0 (-) | 2 (2.4) | |
| Influenza negative | 17 (70.8) | 26 (81.3) | 24 (82.8) | 67 (78.8) | |
|
| |||||
| Total | 24 (100) | 32 (100) | 29 (100) | 85 (100) | |
|
|
|||||
| 2010 | A/H1N1pdm09 | 0 (-) | 0 (-) | 0 (-) | 0 (-) |
| A/H3N2 | 2 (1.8) | 0 (-) | 3 (4.6) | 5 (2.2) | |
| B | 0 (-) | 2 (3.5) | 0 (-) | 2 (0.9) | |
| Influenza negative | 108 (98.2) | 55 (96.5) | 62 (95.4) | 225 (9.07) | |
|
| |||||
| Total | 110 (100) | 57 (100) | 65 (100) | 232 (100) | |
|
|
|||||
| 2011 | A/H1N1pdm09 | 0 (-) | 0 (-) | 0 (-) | 0 (-) |
| A/H3N2 | 4 (5.3) | 4 (10) | 5 (14.3) | 13 (8.7) | |
| B | 0 (-) | 0 (-) | 0 (-) | 0 (-) | |
| Influenza negative | 71 (94.7) | 36 (90) | 30 (85.7) | 137 (91.3) | |
|
| |||||
| Total | 75 (100) | 40 (100) | 35 (100) | 150 (100) | |
the percentage of detection of viruses was stratified by range age.
Regarding influenza B, two/four positive samples were found in 2009 and one of these was in a child who was less than or equal to one year old, which corresponds to 8.3% in this age range and the other two specimens were detected in 2010, both in children who were less than or equal to five years old. The prevalence of influenza A in individuals of all age groups of the studied population is described in Table I. Most study patients had no chronic health conditions that resulted in risk factors or subsequent complications from influenza.
Although some patients two/nine (22.2%) who were infected with the pandemic virus and aged four and 10 months developed mild acute bronchiolitis, they did not require hospitalisation. A child of four months had a coinfection of the H1N1pdm09/RSV viruses. In addition, another child of eight months, who had radiological evidence of pneumonia, was hospitalised and released three days later. Other respiratory viruses were also identified by IFI within the study period in 27% (127/467) of patients with ILI, which circulated together with the pandemic virus. The distribution was as follows: in 2009, 26/85 (30.5%), in 2010, 57/232 (24.5%) and in 2011, 44/150 (29.3%) samples were positive for a number of respiratory viruses. RSV was the most prevalent and accounted for 55% (70/127) of those tested (for a detailed distribution, see Table II). RSV circulated in Salvador at low levels in 2009; however, a considerable increase was noted in 2010 between March-April and between February-April 2011, with an increase in circulation from May-July and a decrease in August. Adv circulation was also detected, as well as PV3, but at low levels compared with RSV (Fig. 2).
TABLE II. Distribution of respiratory viruses in children under five years old with influenza-like illness attending at a paediatric teaching hospital during 2009-2011 in the city of Salvador, state of Bahia, Brazil .
| Year | 2009 | 2010 | 2011 | Total |
|---|---|---|---|---|
| Samples analysed | 85 n (%) | 232 n (%) | 150 n (%) | 467 n (%) |
| RSV | 8 (30.8) | 30 (52.6) | 32 (72.7) | 70 (15) |
| Adeno | 8 (30.8) | 15 (26.3) | 9 (20.5) | 32 (6.9) |
| PF1 | 2 (7.7) | 1 (1.8) | 2 (4.5) | 5 (1.1) |
| PF2 | 0 (0) | 0 (0) | 1 (2.3) | 1 (0.2) |
| PF3 | 8 (30.8) | 11 (19.3) | 0 (0) | 19 (4.1) |
| Total positive results | 26 (-) | 57 (-) | 44 (-) | 127 (27.2) |
| Annual positive results | - (30.59) | - (24.57) | - (29.33) | - (-) |
Fig. 2. : virological and climatic data in the city of Salvador, state of Bahia, Brazil, from May 2009-August 2011. Bars represent the respiratory virus detected. The X-axis shows the years and respective month. The left Y-axis shows the absolute number of positive specimens for respiratory viruses (black bars). The right side Y-axis shows the average temperature (ºC), relative air humidity (%) and rainfall (mm X 10). In 2009, the total annual rain volume was 1,176 mm, in 2010, 1,780 mm, and in 2011, 1,470 mm. RSV: respiratory syncytial virus.
During the study period in 2009, the average precipitation was 150 mm monthly; the highest index was in May, with 550 mm. June and July had 150 mm of precipitation and in August, the levels fell to 100 mm. Only one seasonal virus A/H3N2 was detected in May of 2009 and most of the cases were detected only in August (100 mm). The first influenza pandemic case detected in this study population was in June of 2009 (150 mm). In 2010, we had a few seasonal influenza cases that were distributed throughout April (450 mm) and May (250 mm) and one case occurred in August (170 mm) and November (30 mm). The average monthly rainfall was 180 mm. In 2011, the seasonal virus A/H3N2 circulated from January (rainfall 150 mm) to June (250 mm), but no pandemic influenza cases could be detected in these last years of study (Fig. 1). The average temperature was 25.6ºC and the air RH was approximately 81.5%. Similar climatic conditions were observed in 2010 and 2011, with few variations in rainfall (Fig. 2).
DISCUSSION
The present study describes the occurrence of cases of influenza during the period from May of 2009-August 2011. The epidemiology of influenza in Salvador changed in the years following the pandemic and few cases were reported. From 2010-August 2011, no pandemic virus was detected in the studied age groups. Data from Epidemiological Surveillance Board, Bahia State Department of Health (SUVISA/DIVEP 2012) also revealed a considerable decline in the detection of influenza A(H1N1)pdm09 in children less than or equal to five years in Salvador; only one case was reported in February 2010 and three were reported in 2011 (J Dias, unpublished observations). According to data in Brazil, an increase of RSV with influenza A in 2010 and 2011 was reported, with a peak in circulation between February-June (MS 2012). During the pandemic period, other respiratory viruses were also found circulating in São Paulo, in southeastern Brazil, with a predominance of human metapneumovirus (Watanabe et al. 2011).
The seasonal H3N2 virus had the highest rate among children aged one-two years in 2009 and variations in the attack rate among the age groups affected by influenza A seasonal virus in the subsequent years of the study occurred. Because the highest attack rate of the influenza pandemic virus occurred in very young children (0-1 year of age), children are considered a high-risk group for influenza infection, particularly those less than or equal to two years old and regarded as the most critical source of community-wide influenza transmission (Neuzil et al. 2002, WHO 2009c , MS 2012).
A consensus exists that A(H1N1)pdm09 has disproportionately affected children and young adults and spared elderly individuals greater than 65 years old. Although the pandemic A/(H1N1)pdm09 virus is supposed to have a greater attack rate in young adults, the major incidence was in children less than or equal to five years old (Iskander et al. 2007, WHO 2009c). The age distribution of cases of pandemic influenza peaks in children in Brazil was similar to that observed in other countries (Oliveira et al. 2009).
We observed that most children less than or equal to five years old who had ILI were not infected with the pandemic virus. In Perth, Australia, a quarter of preschool children and approximately 40% of school-aged children and older teenagers had serological evidence of pandemic influenza infection during the winter of 2009; the authors suggest that high levels of mild or asymptomatic infection must have occurred in the population (Dowse et al. 2011). The clinical syndrome caused by A(H1N1)pdm09 ranged from febrile illness to severe pneumonia; however, the majority of clinical presentations were a influenza-like illness with fever and cough that was occasionally accompanied by dyspnoea, sore throat and runny nose (WC-WHO 2010)
The most common clinical symptom reported by the majority of infected patients with confirmed A(H1N1)pdm09 in our study was fever (100%), followed by cough and/or rhinorrhoea. Among the patients studied, two children had bronchiolitis and dyspnoea and one was co-infected with influenza A/H3N2 and RSV. Another case presented mild pneumonia, which required hospitalisation, but no deaths occurred in the population. The death and hospitalisation rates varied in all countries (WHO 2009a). Morbidity and mortality resulting from seasonal influenza and other respiratory viruses have long been described in the country (Straliotto et al. 2002, Bellei et al. 2007, Vidal et al. 2008).
In Brazil, the mortality rate by influenza A(H1N1)pdm09 was 0.47/100,000 inhabitants and varied according to region. In 2009, 2,060 deaths occurred nationwide. This number decreased to 113 deaths in 2010 and 21 deaths in 2011. The highest absolute number of deaths was observed in the southeastern region in 2009, with 992 deaths and in the South Region, which had 789 deaths. (Shout et al. 2009, MS 2012). The Northeast Region had the lowest rate, with 62 individuals in 2009. In BA, 7.9% of the deaths reported were associated with influenza A(H1N1)pdm09 (SUVISA/DIVEP 2012). In terms of mortality, our findings differ from reports from the southeastern and southern regions, where the highest absolute number of severe acute respiratory infection and mortality associated with the pandemic virus were concentrated. Consideration of these aspects could be of great importance to public health for surveillance and investigations in regions with different patterns of circulation, morbidity and mortality (MS 2012). Mortality rates were also high in other South American countries. In Argentina, which borders the Southern region of Brazil, Libster et al. (2010) reported a higher mortality rate by the pandemic virus at approximately 1.1/100,000 inhabitants; most of the affected population were children.
In temperate climates of the Northern Hemisphere, influenza epidemics occur annually, with the peak activity in cold months, followed by a substantial decrease during warmer periods. In the Southern Hemisphere, the winter occurs between June-September, which is when the transmission dynamics of influenza virus varies between different countries (Viboud et al. 2006, WHO 2009b).
In Brazil, influenza tends to spread throughout the year and in the southern and southeastern regions, it generally occurs during winter, from June-August. In other regions, such as the northern and northeastern, influenza circulation varies between the seasons and its occurrence increases largely in periods associated with rain (MS 2012). The Northeast Region is the driest part of the country, while the South Region and most of the Atlantic coast north to Salvador have similar amounts of rain to most of the Central-West Region, with 1,500-2,000 mm of rain per year and an average variation of rainfall ranging from 168.0-879.0 (mm/month), without distinct periods of a dry season. Winter usually begins in June and extends until August and is characterised by being rainy, with temperatures averaging 22ºC. In May, the precipitation reaches an average of 330 mm and generally decreases to 110 mm in January (countrystudies.us/brazil/23.htm, weather-and-climate.com/average-monthly-Rainfall-Temperature-Sunshine). Because seasonal influenza virus A H3N2 and influenza A pandemic virus circulation did not follow a pattern related to rainfall in the years of this study, no association between the rainy season and virus circulation can be suggested. In contrast, Moura et al. (2009), who investigated the circulation of influenza virus by a temporal approach in the city of Fortaleza, which is also located in northeastern Brazil, observed that influenza virus circulated at low levels throughout the years, with a seasonal increase during the rainy season.
One of the largest studies on influenza seasonality, circulation and mortality in Brazil was performed by Alonso et al. (2007), who observed a seasonal influenza-travelling wave in a southward direction that originated from equatorial and low-population regions in March-April and moved to a temperate and highly populous region over a three-month period. Weather fluctuations and seasonal-to-interannual climate variability influences many infectious diseases. There is an increasing number of published studies exploring the effects of weather and climate on influenza (Viboud et al. 2004, Simonsen et al. 2011). Rain and cold weather have also been associated with seasonal influenza transmission because they promote crowding inside, which is a trend that could lead to increased risk of contact transmission. In the northern hemisphere, the influenza pandemic exhibited very different timing with regard to the seasons by spreading at low levels throughout the summer months. An abrupt increase in influenza activity occurred early in September and the peak of the fall wave of the pandemic was observed in mid-October, which was much earlier than that normally observed with epidemic strains. The pandemic virus circulated during the summer and early autumn months, likely due to a relative lack of pre-existing immunity in the population (Alonso et al. 2007, Mello et al. 2009, Amato-Gauci et al. 2011, Steel et al. 2011).
The emergence of pandemic influenza has resulted in questions about solar radiation and vitamin D influencing temporal and spatial influenza circulation. Respiratory virus circulation has been associated with UVB radiation. The hypothesis that influenza pandemics are linked to solar cycle variation, sunlight incidence and control of vitamin D levels in humans is growing (Sagripanti & Lytle 2007, Adams & Hewison 2008). In the northern and southern hemispheres, where widespread infections typically occur during the winter, factors such as ozone, clouds and aerosols are closely related to variations in surface UV radiation incidence. Without sufficient sunlight, the skin does not produce vitamin D, which is a deficiency that is common in winter. In southern Brazil, temperature variations between summer and winter and daylight length variation can provide a better characterisation of the season. The highest absolute number of cases and deaths by pandemic influenza were concentrated in the southeast and southern Brazil. Studies in these regions have revealed a high prevalence of hypovitaminosis D in different age groups and both sexes of young resident physicians (mean 26.4 years) of a general hospital in the city of Porto Alegre and in healthy students aged 16-20 years in São Paulo (Peters et al. 2009). Despite these considerations, it is not possible to suggest that the low impact of the pandemic in the Northeast Region, particularly in Salvador, compared with other Regions of the country and other countries could be related to sunlight because many other factors may be involved that explain the low rate of cases by the pandemic virus.
In conclusion, in Salvador, all patients had less severe infection than those previously reported in other studies from Brazil. These data from Salvador suggest that the severity of A(H1N1)pdm09 in children less than or equal to five years may have been diminished. The complications that were observed in patients infected with the pandemic virus were similar to those from previous epidemics by seasonal influenza virus. Although this study was limited to one paediatric hospital, additional surveillance data from other northwestern regions are needed to further study influenza dynamics in this tropical region. Our data may contribute to a better insight of the epidemiology of influenza and may contribute to an understanding of patterns of seasonality that consequently controls influenza in Brazil, especially in the northeastern region.
ACKNOWLEDGEMENTS
To colleagues and collaborators from Laboratory of Influenza and Respiratory Viruses, IOC, Fiocruz, to Priscila Born and Dr Juarez Dias, from DIVEP/SESAB, for technical assistance, and to Sharon Carney, for her helpful English review.
Funding Statement
Financial support: FAPESB (159/2007)
Footnotes
Financial support: FAPESB (159/2007)
Fellowship from CNPq, COM-HUPES, UFBA.
REFERENCES
- Adams JS, Hewison M. Unexpected actions of vitamin D: new perspectives on the regulation of innate and adaptive immunity. Nat Clin Pract Endocrinol Metab. 2008;4:80–90. doi: 10.1038/ncpendmet0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso WJ, Viboud C, Simonsen L, Hirano EW, Daufenbach LZ, Miller MA. Seasonality of influenza in Brazil: a traveling wave from the Amazon to the subtropics. Am J Epidemiol. 2007;165:1434–1442. doi: 10.1093/aje/kwm012. [DOI] [PubMed] [Google Scholar]
- Amato-Gauci A, Zucs P, Snacken R, Ciancio B, Lopez V, Broberg E, Penttinen P, Nicoll A, European influenza surveillance network (EISN) Surveillance trends of the 2009 influenza A(H1N1) pandemic in Europe. Euro Surveill. 2011;16 doi: 10.2807/ese.16.26.19903-en. [DOI] [PubMed] [Google Scholar]
- Bellei N, Carraro E, Perosa A, Granato C. Patterns of influenza infections among different risk groups in Brazil. Braz J Infect Dis 11. 2007:399–402. doi: 10.1590/s1413-86702007000400005. [DOI] [PubMed] [Google Scholar]
- Brankston G, Guitterman L, Hirji Z, Lemieux C, Gardam M. Transmission of influenza A in human beings. Lancet. 2007;7:257–265. doi: 10.1016/S1473-3099(07)70029-4. [DOI] [PubMed] [Google Scholar]
- CDC - Center for Disease Control and Prevention Outbreak of a swine-origin influenza A (H1N1) virus infection. MMWR. 2009;58:1–3. [PubMed] [Google Scholar]
- Chowell G, Viboud C, Simonsen L, Miller M, Alonso WJ. The reproduction number of seasonal influenza epidemics in Brazil, 1996-2006. Proc Biol Sci. 2010;277:1857–1866. doi: 10.1098/rspb.2009.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowse GK, Smith DW, Kelly H, Barr I, Laurie KL, Jones AR, Keil AD, Effler P. Incidence of pandemic (H1N1) 2009 influenza infection in children and pregnant women during the 2009 influenza season in Western Australia - a seroprevalence study. Med J Aust. 2011;194:68–72. doi: 10.5694/j.1326-5377.2011.tb04170.x. [DOI] [PubMed] [Google Scholar]
- Iskander M, Booy R, Lambert S. The burden of influenza in children. Curr Opin Infect Dis. 2007;20:259–263. doi: 10.1097/QCO.0b013e3280ad4687. [DOI] [PubMed] [Google Scholar]
- Libster R, Bugna J, Coviello S, Hijano DR, Dunaiewsky M, Reynoso N, Cavalieri ML, Guglielmo MC, Areso S, Gilligan T, Santucho F, Cabral G, Gregorio GL, Moreno R, Lutz MI, Panigasi AL, Saligari L, Caballero MT, Almeida RME, Meyer MEG, Neder MD, Davenport MC, Del Valle MP, Santidrian VS, Mosca G, Domínguez MG, Alvarez L, Landa P, Pota A, Boloñati N, Dalamon R, Mercol VIS, Espinoza M, Peuchot JC, Karolinski A, Bruno M, Borsa A, Ferrero F, Bonina A, Ramonet M, Albano LC, Luedicke N, Alterman E, Savy V, Baumeister E, Chappell JD, Edwards KM, Melendi GA, Polack FP. Pediatric hospitalizations associated with 2009 pandemic influenza A (H1N1) in Argentina. N Engl J Med. 2010;362:45–55. doi: 10.1056/NEJMoa0907673. [DOI] [PubMed] [Google Scholar]
- Mello WA, Paiva TM, Ishida MA, Benega MA, dos Santos MC, Viboud C, Miller MA, Alonso WJ. The dilemma of influenza vaccine recommendations when applied to the tropics: the Brazilian case examined under alternative scenarios. PLoS ONE. 2009;4:1–8. doi: 10.1371/journal.pone.0005095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moura FEA, Perdigão ACB, Siqueira MM. Seasonality of influenza in the tropics: a distinct pattern in northeastern Brazil. Am J Trop Med Hyg. 2009;81:180–183. [PubMed] [Google Scholar]
- Ministério da Saúde-Brasil Informe técnico de influenza 2012. 1. Secretaria de Vigilância em Saúde/MS; Brasília: 2012. 15 [Google Scholar]
- Neuzil KM, Hohlbein C, Zhu Y. Illness among schoolchildren during influenza season: effect on school absenteeism, parental absenteeism from work and secondary illness in families. Arch Pediatr Adolesc Med. 2002;156:986–991. doi: 10.1001/archpedi.156.10.986. [DOI] [PubMed] [Google Scholar]
- Oliveira WK, Carmo EH, Penna GO, Kuchenbecker RS, Santos HB, Araujo WN, Malaguti R, Duncan BB, Schmidt MI, surveillance team for the pandemic influenza A(H1N1) 2009 in the Ministry of Health Pandemic H1N1 influenza in Brazil: analysis of the first 34,506 notified cases of influenza-like illness with severe acute respiratory infection (SARI) pii=19362Euro Surveill. 2009;14 doi: 10.2807/ese.14.42.19362-en. [DOI] [PubMed] [Google Scholar]
- Peters BSE, Santos LC, Fisberg M, Wood RJ, Martini LA. Prevalence of vitamin D insufficiency in Brazilian adolescents. Ann Nutr Metab. 2009;54:15–21. doi: 10.1159/000199454. [DOI] [PubMed] [Google Scholar]
- Sagripanti JL, Lytle CD. Inactivation of influenza virus by solar radiation. Photochem Photobiol. 2007;83:1278–1282. doi: 10.1111/j.1751-1097.2007.00177.x. [DOI] [PubMed] [Google Scholar]
- Shaman J, Kohn M. Absolute humidity modulates influenza survival, transmission and seasonality. Proc Natl Acad Sci USA. 2009;106:3243–3248. doi: 10.1073/pnas.0806852106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shout D, Hajjar LA, Galas FRBG, Uip ED, Levin ASS, Caiaffa HH., Filho Epidemiology of human infection with the novel virus influenza A (H1H1) in the Hospital das Clínicas, São Paulo, Brazil - June-September 2009. Clinics. 2009;64:1025–1030. doi: 10.1590/S1807-59322009001000014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsen L, Viboud C, Taylor RJ, Miller MA. Del Giudice G, Rappuoli R. Influenza vaccines for the future, Birkhäuser Advances in Infectious Diseases Series. 2. Springer; London: 2011. The epidemiology of influenza and its control; pp. 27–54. [Google Scholar]
- Steel J, Palese P, Lowen AC. Transmission of a 2009 pandemic influenza virus shows sensitivity to temperature and humidity similar to that of an H3N2 seasonal strain. J Virol. 2011;85:1400–1402. doi: 10.1128/JVI.02186-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straliotto SM, Siqueira MM, Muller RL, Fisher GB, Cunha MLT, Nestor SM. Viral etiology of acute respiratory infections among children in Porto Alegre, RS, Brazil. Rev Soc Bras Med Trop. 2002;35:283–291. doi: 10.1590/s0037-86822002000400002. [DOI] [PubMed] [Google Scholar]
- Superintendência de Vigilância e Proteção da Saúde/Secretaria de Saúde do Estado da Bahia Boletim influenza-Bahia/2012. SUVISA/DIVEP; Salvador: 2012. 2 [Google Scholar]
- Tellier R. Review of aerosol transmission of influenza A virus. Emerg Infect Dis. 2006;12:1657–1662. doi: 10.3201/eid1211.060426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viboud C, Alonso WJ, Simonsen L. Influenza in tropical regions. PLoS Med. 2006;3:468–471. doi: 10.1371/journal.pmed.0030089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viboud C, Pakdaman K, Boele PY, Wilson ML, Meyers MF, Valleron AJ, Flahault A. Association of influenza epidemics with global climate variability. Eur J Epidemiol. 2004;19:1055–1059. doi: 10.1007/s10654-004-2450-9. [DOI] [PubMed] [Google Scholar]
- Vidal LRR, Siqueira MM, Nogueira MB, Raboni SM, Pereira LA, Takahashi GRA, Rotta I, Debur MC, Dalla-Costa LM. The epidemiology and antigenic characterization of influenza viruses isolated in Curitiba, South Brazil. Mem Inst Oswaldo Cruz. 2008;103:180–185. doi: 10.1590/s0074-02762008000200009. [DOI] [PubMed] [Google Scholar]
- Watanabe ASA, Carraro E, Moreira L, Camargo C, Sinohara J, Puerari D, Guatura S, Granato C, Bellei N. Respiratory virus infections among hospitalized patients with suspected influenza A H1N1 2009 virus during the first pandemic wave in Brazil. Braz J Infect Dis. 2011;15:220–224. doi: 10.1016/s1413-8670(11)70179-9. [DOI] [PubMed] [Google Scholar]
- World Health Organization Transmission dynamics and impact of pandemic influenza A (H1N1) 2009 virus. Wkly Epidemiol Rec. 2009a;84:481–484. [PubMed] [Google Scholar]
- World Health Organization The global influenza surveillance network (GISN) Pandemic (H1N1) 2009. 2009b who.int/csr/don/2009_11_27a/en/index.html
- World Health Organization Clinical management of human infection with pandemic (H1N1) 2009: revised guidance. 2009c who.int/csr/resources/publications/swineflu/clinical_management/en/index.html.
- World Health Organization Sequencing primers and protocol. WHO/CDC; Atlanta: 2009d. 2 [Google Scholar]
- WHO - World Health Organization Standardization of terminology of the pandemic A(H1N1)2009 virus. 2011 who.int/influenza/gisrs_laboratory/terminology_ah1n1pdm09/en/index.html. [PubMed]
- World Health Organization/Center for Disease Control and Prevention CDC protocols of real time RT-PCR for swine influenza A(H1N1) WHO/CDC; Atlanta: 2009. 8 [Google Scholar]
- World Health Organization/Global Influenza Surveilance Network Manual for the laboratory diagnosis and virological surveillance of influenza. WHO/WHO Press; Geneva: 2011. 151 [Google Scholar]
- Writing Committee - World Health Organization Clinical aspects of pandemic 2009 influenza A (H1N1) virus infection. N Engl J Med. 2010;362:1708–1719. doi: 10.1056/NEJMra1000449. [DOI] [PubMed] [Google Scholar]