Abstract
Context
Increased inflammatory biomarkers predict antidepressant non-response, and inflammatory cytokines can sabotage and circumvent mechanisms of action of conventional antidepressant therapy.
Objective
To determine whether inhibition of the inflammatory cytokine tumor necrosis factor (TNF)-alpha reduces depressive symptoms in patients with treatment resistant depression (TRD) and whether increased baseline plasma inflammatory biomarkers including high sensitivity c-reactive protein (hs-CRP), TNF-alpha and its soluble receptors predict treatment response.
Design
Double-blind, placebo-controlled, randomized clinical trial.
Setting
Outpatient infusion center, Emory University.
Participants
Sixty medically-stable outpatients with major depression on a consistent antidepressant regimen or medication-free (n=23) for ≥4 weeks and moderate treatment resistance as determined by the Massachusetts General Hospital Staging method.
Interventions
Three infusions of the TNF-alpha antagonist infliximab (5mg/kg)(n=30) or placebo (n=30) at baseline and weeks 2 and 6 of a 12-week trial.
Main Outcome Measure
17-item Hamilton Depression Rating Scale (HAM-D-17).
Results
No overall difference in change of HAM-D-17 scores between treatment groups across time was found. However, there was a significant interaction between treatment, time and log baseline hs-CRP (p=0.01), with change in HAM-D-17 scores (Baseline to Week 12) favoring infliximab-treated patients at a baseline hs-CRP>5mg/L and placebo-treated patients at a baseline hs-CRP≤5mg/L. Exploratory analyses focusing on patients with a baseline hs-CRP>5mg/L revealed a treatment response (≥50% reduction in HAM-D-17 at any point during treatment) of 62% (8/13) in the infliximab group versus 33% (3/9) in placebo-treated patients (p=0.19). Baseline concentrations of TNF-alpha and its soluble receptors were significantly higher in infliximab-treated responders versus non-responders (p<0.05), and infliximab-treated responders exhibited significantly greater decreases in hs-CRP from Baseline to Week 12 compared to placebo-treated responders (p<0.01). Drop-outs and adverse events were limited and did not differ between groups.
Conclusions
This proof-of-concept study suggests that TNF-alpha antagonism does not have generalized efficacy in TRD, but may improve depressive symptoms in patients with high baseline inflammatory biomarkers.
Trial Registration
ClinicalTrials.gov Identifier: NCT00463580
Introduction
Despite advances in the treatment of major depression, one-third of depressed patients fail to respond to conventional antidepressant medication.1 One pathophysiologic mechanism hypothesized to contribute to treatment resistance in depression is inflammation. Increased inflammatory biomarkers including inflammatory cytokines, acute phase proteins, chemokines and adhesion molecules have been found to be reliably elevated in depressed patients, and have been associated with decreased likelihood of response to conventional antidepressants.2–4 Moreover, factors linked to a poor antidepressant treatment response, including early life stress, anxiety disorders and neuroticism have been associated with increased inflammation.5–11 Data also indicate that inflammatory cytokines can sabotage and circumvent mechanisms of action of conventional antidepressant medications.2 For example, inflammatory cytokines can increase expression and activity of monoamine transporters, the primary antidepressant target for monoamine reuptake inhibition.12,13 In addition, inflammatory cytokines can reduce monoamine precursors through activation of enzymes such as indoleamine 2,3 dioxygenase, which breaks down tryptophan, the primary amino acid precursor for serotonin, into kynurenine.14 Inflammation can also reduce availability of the enzyme co-factor, tetrahydrobiopterin, which is essential for activities of tryptophan hydroxylase and tyrosine hydroxylase, which are rate limiting enzymes for synthesis of serotonin, norepinephrine and dopamine.15,16 Inflammatory cytokines have also been shown to inhibit neurogenesis through activation of nuclear factor kappa B.17 Neurogenesis is an important component of the salutary effects of conventional antidepressants in several depressive-like behaviors in animal models of depression including anhedonia.18–20 Finally, inflammatory cytokines can reduce expression of glutamate transporters and increase glutamate release from astrocytes, thereby activating pathophysiologic mechanisms (e.g. glutamate excitotoxicity) that are not targets of conventional antidepressant medications.2,15,21,22
Given the association of inflammatory cytokines with treatment resistance, there has been interest in testing whether inhibiting inflammatory cytokines might have therapeutic potential in treatment resistant depression (TRD). One inflammatory cytokine, tumor necrosis factor (TNF)-alpha, may be especially relevant in this regard. TNF-alpha has been reliably shown to be elevated in depressed patients.23 Moreover, increases in TNF-alpha have been associated with depressive symptoms during chronic exposure to interferon (IFN)-alpha.24 In addition, peripheral administration of a TNF-alpha antagonist has been shown to improve depressed mood in patients with psoriasis.25 TNF-alpha antagonism has also been found to resolve major depression in patients with Crohn’s disease,26 and reduce fatigue in patients with advanced cancer.27 Moreover, gene-targeted deletion of TNF-alpha receptors in mice leads to an antidepressant-like phenotype and reduced anxiety-like behavior during immune activation.28,29 Nevertheless, no previous study has tested whether administration of a peripherally active cytokine antagonist to otherwise healthy patients with TRD might reverse depressive symptoms.
Therefore, we endeavored to determine whether repeated intravenous administration of a monoclonal antibody directed at TNF-alpha (infliximab) would improve depressed mood in patients with TRD. Such a targeted biologic therapy was chosen not only to directly test the cytokine-hypothesis of depression,30,31 but also to obviate non-immunologic effects that may potentially confound interpretation of mechanism of action of other readily available medications with anti-inflammatory properties including acetylsalicylic acid, cyclo-oxygenase inhibitors and minocycline, all of which have relevant off-target effects.32–35 Based on the hypothesis that an anti-cytokine strategy might only be effective in patients with high inflammation prior to treatment, we also measured the acute phase protein, high sensitivity c-reactive protein (hs-CRP) as well as TNF-alpha and its soluble receptors I and II (sTNFRI and sTNFRII) at baseline and throughout the study as biomarkers of inflammation. Like TNF-alpha, CRP has been shown to be reliably elevated in depressed patients and has been associated with development of depression as well as antidepressant non-response.36–38 In addition, baseline CRP has been found to be a potent predictor of response in patients treated with infliximab for inflammatory disorders including Crohn’s Disease.39,40 sTNFRI and II also have been associated with depression, anxiety and fatigue in laboratory animals and humans and are believed to reflect TNF-alpha activity.4,28,29,41–43
Methods
Study Overview and Eligibility Criteria
The study was a single site, parallel-group, randomized, double-blind trial of infliximab versus placebo for antidepressant non-responders with major depression according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV), as assessed by Structured Clinical Interview for DSM-IV (SCID).44 Due to the exploratory nature of the study, subjects with Bipolar Disorder, depressed, were also included.
Eligible participants, recruited from television, radio and newspaper advertisements, were males and females between the ages of 25–60 years on a consistent antidepressant regimen or off antidepressant therapy for at least 4 weeks prior to baseline. No changes in antidepressant treatment were allowed during the study. All subjects were required to have moderate treatment resistance as determined by a score ≥2 on the Massachusetts General Hospital Staging (MGH-S) method for treatment resistance in the current episode,45 and exhibit moderate severity of depression as determined at screening by a score ≥14 on the Quick Inventory of Depressive Symptomatology (QIDS)-SR-16.46 Exclusion criteria included any autoimmune disorder (confirmed by laboratory testing); history of tuberculosis (confirmed by chest X-ray, skin and blood testing) or high risk of tuberculosis exposure; hepatitis B or C or human immunodeficiency virus infection (confirmed by laboratory testing); evidence of active fungal infection; history of recurrent viral or bacterial infections; history of cancer excluding basal cell or squamous cell carcinoma of the skin (fully excised with no recurrence); unstable cardiovascular, endocrinologic, hematologic, hepatic, renal, or neurologic disease (determined by physical examination and laboratory testing); history of schizophrenia (determined by SCID); active psychotic symptoms of any type; substance abuse/dependence within the past 6 months (determined by SCID); active suicidal ideation determined by a score ≥3 on item #3 on the Hamilton Depression Rating Scale (HAM-D);47 and/or a score <28 on the Mini-Mental State Exam.48 All subjects provided written informed consent, and all procedures were approved a priori by the Emory University Institutional Review Board. The study was registered at ClinicalTrials.gov (NCT00463580) in April, 2007.
Study Procedures
Subjects were enrolled at Emory University between December, 2008 and March, 2011. In order to achieve similar representation of baseline inflammatory status in each group, group assignment was stratified based on a hs-CRP of ≥ or <2mg/L determined at screening. A hs-CRP of 2mg/L was chosen because it is the central value in the “medium” relative risk category of inflammation (1–3mg/L) recommended by the American Heart Association and the Centers for Disease Control.49 Group assignment was also stratified by sex. Following screening for inclusion and exclusion criteria, all subjects reported to the infusion center in the Emory Division of Digestive Diseases on 3 separate occasions (baseline, 2 weeks and 6 weeks) to receive an infusion of either infliximab (5mg/kg) or placebo through an indwelling catheter over 120 minutes. The baseline visit was scheduled no later than one month after screening. The dosing protocol and scheduling of infliximab infusions were matched to the standard induction regimen for treatment of inflammatory bowel disease.50 Independent pharmacists dispensed infliximab or placebo in a 250ml saline bag according to a computer-generated randomization list, blocked in units of 4 provided by a study statistician. The placebo was matched to infliximab on the basis of color and consistency when dissolved in saline. Infliximab and placebo were provided free-of-charge by Centocor OrthoBiotech Services (Horsham, PA). Assessments of clinical [HAM-D-17 and Clinical Global Impression Severity (CGI-S)]51 and inflammatory status (hs-CRP, TNF-alpha, sTNFRI and II) were conducted at baseline and Weeks 1,2,3,4,6,8,10, and 12. For any subject who exhibited evidence of infection, infusions were delayed until symptoms resolved and/or appropriate treatment (e.g. antibiotics) was initiated. During the trial, HAM-D-17 assessments on Week 3 were discontinued due to subject/staff burden (11 subjects had Week 3 assessments). HAM-D-17 assessments on Week 10 were discontinued for the same reason but were resumed for a total of 32 subjects. Patients were not allowed to take non-steroidal or steroidal anti-inflammatory medications during the study except for 81mg of aspirin if medically indicated (n=3). Medications for hypertension, diabetes, hypothyroidism, allergies, infections and other medical conditions were allowed as dictated by the patients’ treating physicians. All study staff were blinded to treatment assignment until the entire trial was completed.
Outcomes
As indicated in the registered trial (ClinicalTrials.gov Identifier: NCT00463580), the primary end point was change in depression severity as measured by the 17-item HAM-D (HAM-D-17). Additional endpoints included treatment response which was defined as a ≥50% reduction in HAM-D-17 at any point during the study, and remission, which was considered a HAM-D-17 ≤7 at end of treatment (Week 12). Secondary endpoints included CGI-S. Analyses were also conducted on interactive effects of baseline inflammatory status as assessed by plasma hs-CRP with change in depression severity over time as well as change in inflammatory status from Baseline to Week 12. TNF-alpha and its soluble receptors were similarly considered in exploratory analyses.
Laboratory Assessments
To limit impact of circadian rhythms and stress on inflammatory measures, blood was obtained in EDTA-coated tubes through an indwelling catheter between 8–10am after at least 30 minutes of rest. Blood samples were immediately centrifuged at 1,000Xg for 15 minutes at 4°C, and plasma was removed and stored at −80°C until assay. CRP was measured by the immunoturbidometric method using the Beckman AU 480 chemistry analyzer and the Ultra WR CRP reagent kit (Sekisui Diagnostics, Framingham, MA). Inter- and intra-assay coefficients of variation were reliably less than 3%. TNF-alpha and sTNFRI and II were assessed as previously described in duplicate using sandwich ELISA (R & D Systems, Minneapolis, MN).24 Mean inter-and intra-assay coefficients of variation were reliably 10% or less.
Sample Size
Power calculations were based on standard deviations of HAM-D scores in patients with TRD derived from published literature.52 Given a standard deviation of 7 and the other parameters adopted (60 subjects, 80% power and a two-sided alpha level of 0.05), the trial was powered to detect differences in change scores between groups of 5 points on the HAM-D-17.
Statistical analysis
T tests and chi-square analyses were used to compare sociodemographic and clinical variables between groups as well as number/percent of subjects who achieved treatment response/remission or experienced an adverse event. Non-parametric tests were used in cases where data were not normally distributed. An intent-to-treat analysis using mixed-effects model for repeated measures (MMRM) was used to analyze change from baseline of HAM-D-17 scores as a function of treatment, time and their interaction. To evaluate impact of baseline inflammatory status on treatment assignment and response, baseline hs-CRP (log transformed linear and quadratic terms) was considered in the model. Quadratic terms were employed because each treatment group exhibited a unique convex (upside down U-shaped) trend of primary end points with increasing levels of baseline hs-CRP. Study week was entered into MMRM models as a categorical variable for purposes of illustrating group least square means over time (see Figures 1,4 and 7). For determining significance and effect sizes for treatment group differences by time, study week was analyzed as a continuous variable. An autoregressive covariance structure (AR-1) best fit the data, and thus was used for final MMRM analyses. TNF-alpha and its soluble receptors were also tested in similar fashion to hs-CRP in exploratory analyses. Fixed effect parameters were tested with the Wald test (t test). The independent effect of age, sex, body mass index (BMI), MGH-S score, diagnosis of bipolar disorder, concomitant psychotropic medications, and comorbid medical conditions also were examined in MMRM analyses to evaluate possible interactions with treatment and time. In addition, MMRM analyses were applied to examine effect of treatment on longitudinal assessments of hs-CRP. Where indicated, t tests were employed in exploratory analyses of differences between inflammatory biomarkers at baseline in responders versus non-responders and in changes from Baseline to Week 12. Statistical significance was based on a two-sided alpha of 0.05. No interim analysis was conducted.
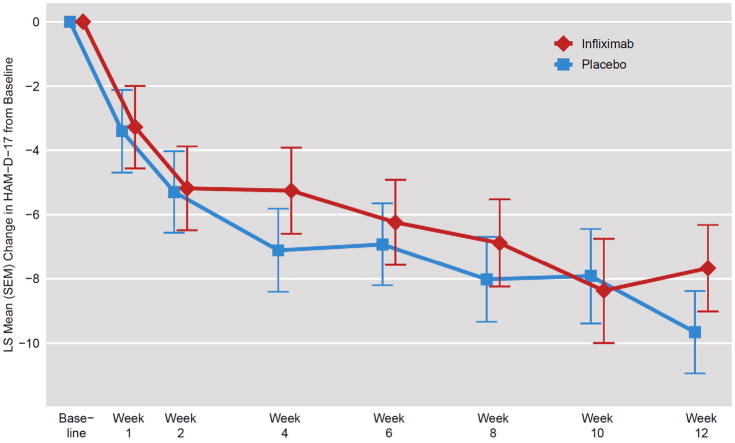
Figure 1. Change in HAM-D-17 Scores in TRD Patients Randomly Assigned to Infliximab or Placebo.
Using a double-blind, placebo-controlled, randomized design, the change in the 17-item Hamilton Depression Rating Scale (HAM-D-17) was examined in an intent-to-treat MMRM analysis of 60 patients with treatment resistant depression (TRD) administered 3 infusions of the TNF-alpha antagonist infliximab (n=30) or placebo (n=30) at baseline and 2 and 6 weeks of a 12 week trial. There was no main effect of treatment assignment or a treatment by time interaction. However, there was a significant effect of time with both groups exhibiting significant decreases in HAM-D-17 scores across treatment weeks (p=0.01). Depicted is LS Mean (SEM) Change in HAM-D-17 from Baseline to the indicated week using an unstructured covariance matrix with time as a categorical variable.
Figure 4. Change in HAM-D-17 Scores from Baseline to Week 12 in Infliximab- or Placebo-Treated TRD Patients with a Baseline CRP>5 mg/L versus ≤5mg/L.
An intent-to-treat MMRM analysis of patients with treatment resistant depression (TRD) administered the TNF-alpha antagonist infliximab or placebo was separately conducted in patients with a baseline high sensitivity c-reactive protein (hs-CRP)>5mg/L (n=22)(Panel A) and those with a baseline hs-CRP ≤5mg/L (n=48)(Panel B). In general, opposite effects of infliximab were found depending on baseline hs-CRP. Subjects with baseline hs-CRP>5 mg/L tended to do better than placebo whereas the opposite effect was seen in subjects with a baseline hs-CRP≤5mg/L. Although there was a main effect of time on change in HAM-D-17 scores in patients with a baseline hs-CRP>5mg/L, there were no effects of treatment assignment or a treatment by time interaction. In addition, no main effects of treatment assignment, time or their interaction were found in subjects with a baseline hs-CRP ≤5mg/L. Depicted is least squares (LS) Mean [standard error of the mean (SEM)] Change in HAM-D-17 from Baseline to the indicated week using an unstructured covariance matrix with time as a categorical variable.
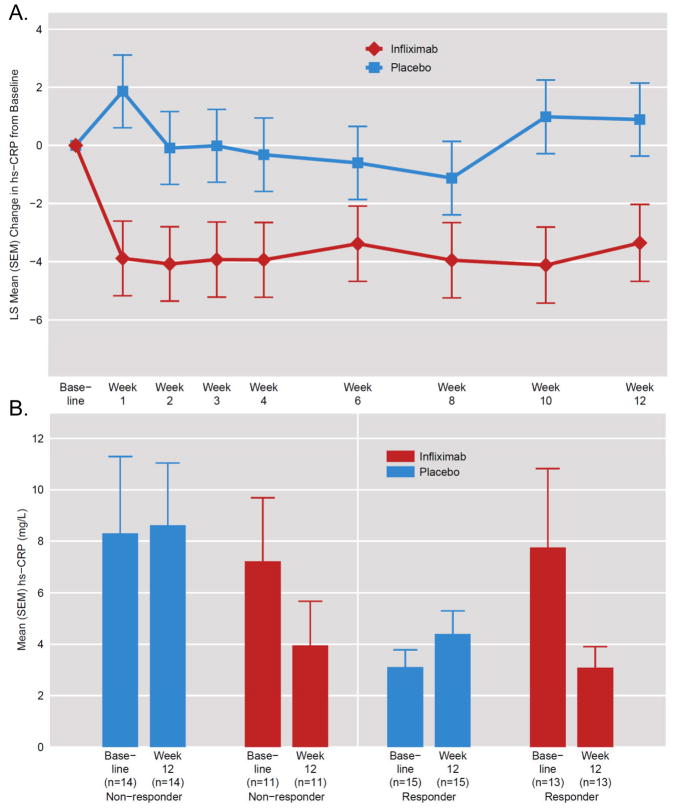
Figure 7. Change in Plasma hs-CRP Concentrations Across Treatment and as a Function of Treatment Response in Infliximab- versus Placebo-Treated TRD Patients.
Plasma high sensitivity c-reactive protein (hs-CRP) was measured across the study in all patients with treatment resistant depression (TRD). Infliximab treatment had a significant main effect on plasma hs-CRP (p<0.05), leading to significant decreases in hs-CRP compared to placebo at all time points. Depicted is least squares (LS) Mean [standard error of the mean (SEM)] Change in hs-CRP from Baseline to the indicated week using an unstructured covariance matrix with time as a categorical variable (Panel A). Comparing placebo responders versus non-responders, placebo responders had lower hs-CRP. Infliximab responders exhibited higher baseline and lower Week 12 hs-CRP than infliximab non-responders, but these results also did not reach statistical significance. There was a main effect of treatment on change from Baseline to Week 12 in hs-CRP (p<0.05). Moreover, infliximab responders exhibited a significantly greater decrease in hs-CRP from Baseline to Week 12 than placebo responders (p<0.01) (Panel B).
Results
Baseline sociodemographic and clinical data for the 60 patients randomly assigned to infliximab or placebo are depicted in Table 1. No statistically significant differences between groups in any of the variables listed were observed. It should be noted that both groups exhibited mean hs-CRP values above 5mg/L, and 45% (27/60) had a hs-CRP>3mg/L. Of the 60 patients randomized, 27 subjects in the infliximab group and 28 subjects in the placebo group completed all 3 infusions. Three subjects in the infliximab group received only 1 infusion and dropped out (2 due to scheduling conflicts and 1 lost to follow-up). Two subjects in the placebo group received only 2 infusions (both due to medical/psychiatric complications) and one of these subjects discontinued participation at that time. Two subjects in the infliximab group discontinued in the follow-up phase (1 due to scheduling conflicts and 1 lost to follow-up). In total, 90% of the subjects completed all 12 weeks of the trial [97% placebo (29/30) and 83% infliximab (25/30), p=0.19] (eFigure 1, Consort Diagram, Online-Only Material).
Table 1.
Sociodemographic and Clinical Characteristics of the Study Sample
| Characteristic | Infliximab (n=30) | Placebo (n=30) |
|---|---|---|
| Age (yrs.) – mean (SD) | 42.5 (8.2) | 44.3 (9.4) |
| Sex (female) – no. (%) | 20 (66%) | 20 (66%) |
| Ethnic Origin - no. (%) | ||
| Caucasian | 23 (77%) | 23 (77%) |
| Black | 6 (20%) | 5 (17%) |
| Other | 1 (3%) | 2 (6%) |
| Education (Highest Degree) – no. (%) | ||
| Graduate Degree | 8 (27%) | 7 (23%) |
| College Graduate | 13 (43%) | 13 (43%) |
| Partial College | 8 (27%) | 9 (30%) |
| High School Graduate | 1 (3%) | 1 (3%) |
| Unemployed (looking for work or disabled) – no. (%) | 12 (41%)+ | 12 (40%) |
| Age of Onset of MDD (yrs.) – mean (SD) | 19.1 (8.3) | 18.9 (10.7) |
| Lifetime Episodes of MDD – no. (SD) | 8.7 (24.8) | 7.8 (24.8) |
| Duration of Current MDD Episode (mos.) – mean (SD) | 184.4 (148.8) | 238.7 (165.25) |
| Antidepressant Trials in Current Episode – no. (SD) | 4.6 (3.2) | 3.7 (2.1) |
| MGH-S score – mean (SD) | 7.73 (6.6) | 6.1 (3.5) |
| Family History of MDD – no. (%) | 27 (90%) | 23 (82%)+ |
| Mood-Relevant Psychotropic Medication – no. (%) | 16 (53%) | 21 (70%) |
| Co-Morbid Medical Illness – no. (%) | 13 (43%) | 19 (63%) |
| Bipolar Disorder – no. (%) | 3 (10%) | 6 (20%) |
| BMI (kg/m2) – mean (SD) | 31.2 (6.9) | 32.7 (8.0) |
| Baseline hs-CRP (mg/L) – mean (SD) | 6.34 (8.9) | 5.45 (8.2) |
| Baseline HAM-D 17 – mean (SD) | 24.1 (4.0) | 23.6 (3.8) |
| Baseline CGI – mean (SD) | 4.8 (0.59) | 4.8 (0.81) |
yrs-years; SD-standard deviation; no.-number; MDD-major depressive disorder; mos.-months; MGH-S-Massachusetts General Hospital staging method; BMI-body mass index; hs-CRP-high sensitivity c-reactive protein; HAM-D 17-17-item Hamilton Depression Rating Scale; CGI-Clinical Global Impression severity scale; +-data on one subject was missing
No differences in change in HAM-D-17 scores over time were found between treatment groups (t=0.10, df=58, p=.92), nor was there a significant interaction between treatment and time (t=0.86, df=305, p=0.39) (Figure 1). However, there was a significant effect of time, with HAM-D-17 scores significantly decreasing from baseline to end of treatment (t=2.53, df=305, p=0.01) in both infliximab- and placebo-treated groups (p<0.05). Of note, no significant interactive effects of the above noted sociodemographic or clinical covariates were observed with treatment group and time. Interestingly, when baseline hs-CRP (log linear and quadratic terms) was entered into the model, there was a significant interaction among treatment, time and log hs-CRP (t=2.65, df=302, p=0.01). To identify the critical threshold for the influence of baseline hs-CRP, we examined change in HAM-D-17 from Baseline to Week 12 (Infliximab-Placebo) in relation to log hs-CRP. A baseline hs-CRP>5mg/L was found to be the point at which infliximab-treated patients began to exhibit a greater decrease in HAM-D-17 scores than placebo-treated subjects (Figure 2). Similar results were found for CGI-S (eFigure 2, Online-Only Material). Figure 3 illustrates the difference between infliximab versus placebo based on analysis of subgroups of patients with successively higher baseline hs-CRP concentrations (>1,>3, and >5mg/L), with infliximab being superior to placebo by >3 points in subjects with a baseline hs-CRP>5mg/L.
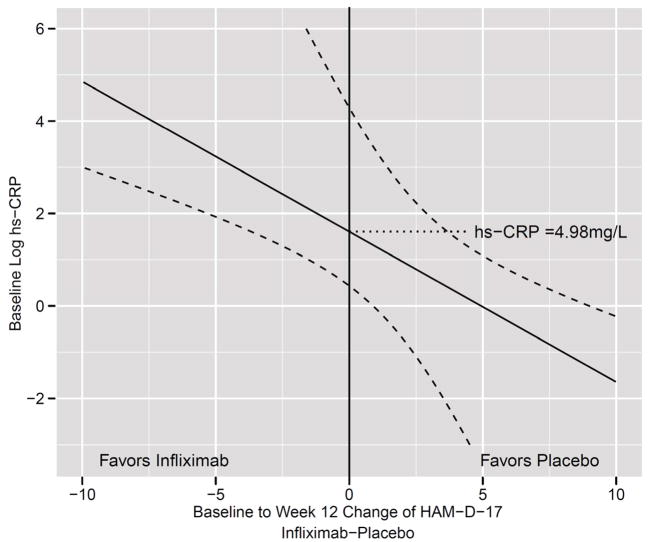
Figure 2. Relationship between Change in HAM-D-17 Score from Baseline to Week 12 (Infliximab-Placebo) and Log Baseline Plasma hs-CRP in Patients with TRD Treated with Infliximab or Placebo.
Based on an interaction between treatment, time and baseline log high sensitivity c-reactive protein (hs-CRP)(p=0.01), the relationship between baseline log plasma hs-CRP and the change in the 17-item Hamilton Depression Rating Scale (HAM-D-17) score from Baseline to Week 12 (Infliximab-Placebo) was examined in 60 patients with treatment resistant depression (TRD) administered 3 infusions of either the TNF-alpha antagonist infliximab (n=30) or placebo (n=30) at baseline and 2 and 6 weeks of a 12 week trial. At plasma concentrations of hs-CRP greater than 4.98 mg/L, the change in HAM-D-17 favored infliximab- versus placebo-treated patients, whereas at plasma concentrations below 4.98mg/L the change in HAM-D-17 favored placebo.
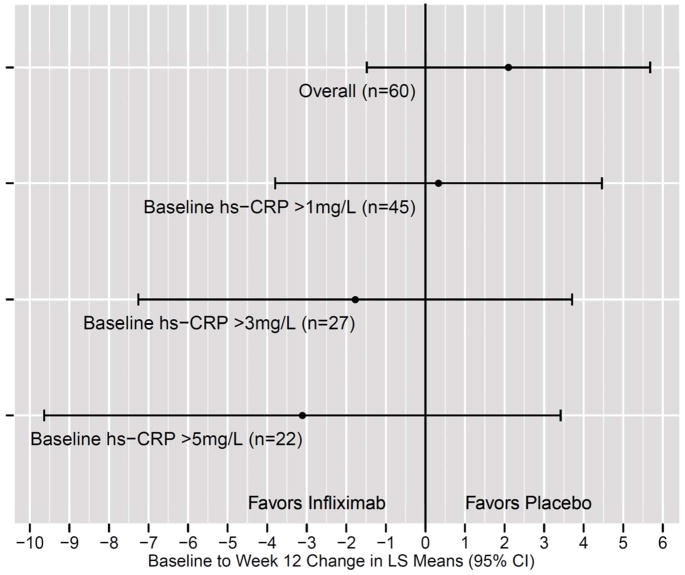
Figure 3. Change in HAM-D-17 Score from Baseline to Week 12 (Infliximab-Placebo) in TRD Patients Subgrouped By Baseline Plasma hs-CRP.
To examine the impact of varying levels of baseline plasma high sensitivity c-reactive protein (hs-CRP) on least squares (LS) mean change [± 95% Confidence Interval (CI)] in the 17-item Hamilton Depression Rating Scale (HAM-D-17) score from Baseline to 12 Week (Infliximab-Placebo), the sample of patients with treatment resistant depression (TRD) were subgrouped into all subjects (Overall), all subjects with a baseline hs-CRP >1mg/L (n=45), all subjects with a baseline hs-CRP >3mg/L (n=27) or all subjects with a baseline hs-CRP >5mg/L (n=22). When only considering subjects with a hs-CRP >5mg/L, a 3.1 difference in the HAM-D-17 favoring infliximab was found between infliximab- versus placebo-treated patients.
Based on these results, exploratory analyses were focused on TRD patients with a baseline hs-CRP > or =5mg/L to maximize potential differences between groups. Of note, no significant differences in sociodemographic or clinical variables were found between infliximab-and placebo-treated TRD subjects with a baseline hs-CRP>5mg/L including distribution of bipolar depressed subjects which was 2/13 (15%) for the infliximab group and 1/9 (11%) for the placebo group (p=0.77)(eTable 1, Online-Only Material). In subjects with a baseline hs-CRP=5mg/L, placebo-treated patients were more likely to have a co-morbid medical illness and were more likely to be on concurrent psychotropic medications for their mood disorder than infliximab-treated subjects (p=0.022 and p=0.026, respectively). Including these variables in the MMRM models discussed below did not substantially change the treatment-by-time interaction.
Although the treatment-by-time interactions were not statistically significant in exploratory analyses of these relatively small groups, separate MMRM analyses highlighted the opposite effect of infliximab versus placebo for groups with baseline hs-CRP>5mg/l (n=22) compared to ≤5 mg/L (n=38) (Figure 4). Infliximab was superior to placebo in improving HAM-D-17 scores for subjects with baseline hs-CRP >5mg/L (Panel A), but placebo was superior within the CRP ≤5 mg/L group (Panel B). Consistent with these findings, the standardized effect size difference at 12 weeks (from MMRM) was moderate to large, in opposite directions: +0.41 favoring infliximab in the >5mg/L hs-CRP group, compared to −0.82 favoring placebo in the ≤5mg/L hs-CRP group.
To explore which specific symptoms were improving in infliximab- versus placebo-treated subjects with hs-CRP>5 mg/L, median change in individual items on the HAM-D-17 from baseline to end of treatment was examined. Symptoms more responsive in infliximab-treated patients versus placebo included anhedonia, psychomotor retardation, depressed mood, psychic anxiety and suicidal ideation (Figure 5). Examination of symptoms that improved in subjects with a baseline hs-CRP≤5mg/L also provided evidence of reduced severity of anhedonia, psychomotor retardation and psychic anxiety in infliximab-treated subjects (eFigure 2, Online-Only Material). In contradistinction, placebo-treated patients with a baseline hs-CRP≤5mg/L, showed reduced severity in HAMD-17 items of hypochondriasis, somatic general, and feelings of guilt (eFigure 3, Online-Only Material).
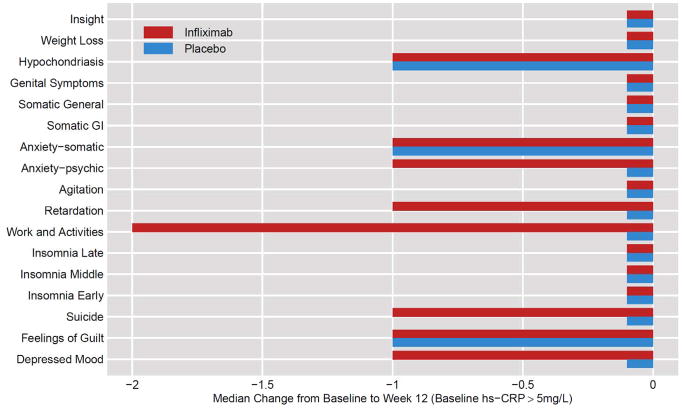
Figure 5. Median Change in Individual HAM-D-17 Items from Baseline to Week 12 in Infiximab- versus Placebo-Treated TRD Patients with Baseline hs-CRP>5mg/L.
To determine which symptoms were differentially improved as a function of infliximab treatment, the median change in the 17 items of the Hamilton Depression Rating Scale (HAM-D-17) was plotted. Symptoms of Anxiety-psychic, Retardation, Work and Activities, Suicide and Depressed Mood exhibited greater improvement in infliximab- versus placebo-treated patients with treatment resistant depression (TRD) from Baseline to Week 12 in patients with a baseline plasma high sensitivity c-reactive protein (hs-CRP)>5mg/L.
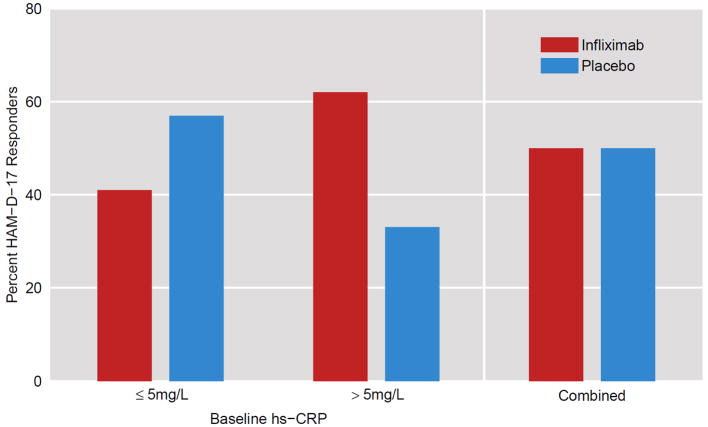
Treatment response rates did not differ between groups in the entire sample and were 50% (X2=0.00, p=1.0). Of note, however, in subjects with a baseline hs-CRP>5 mg/L, the treatment response was 62% (8/13) in the infliximab group and 33% (3/9) in the placebo group (X2=1.69, df=1, p=0.19) (Figure 6). Based on these percentages, the number of patients needed to be treated with infliximab in order to have one more responder (NNT) in subjects with hs-CRP>5 was 3.45 (as opposed to 8–10 for conventional antidepressants)53. In contrast, for subjects with hs-CRP≤5 mg/L, treatment response was 41% (7/17) for the infliximab group and 57% (12/21) for the placebo group (X2=0.96, p=0.33). In this case, the disadvantage to infliximab (number needed to harm – NNH) was calculated as 6.25. In the sample as a whole, remission rates favored placebo at 26% versus 9% for infliximab, although the difference did not reach significance (X2=1.7, p=0.19).
Figure 6. Percent Treatment Responders in Infliximab- Versus Placebo-Treated TRD Patients with a Baseline hs-CRP≤5mg/L or >5mg/L.
Percent Treatment Responders as defined by a ≥50% drop in the 17-item Hamilton Depression Rating Scale (HAM-D-17) at any time during treatment was compared in infliximab- versus placebo-treated patients with treatment resistant depression (TRD) and a baseline plasma high sensitivity c-reactive protein (hs-CRP)≤5mg/L versus >5mg/L as well as the groups combined. Infliximab-treated patients exhibited a higher response rate than placebo when hs-CRP was >5mg/L but a lower response than placebo when hs-CRP was ≤5mg/L. These results did not reach statistical significance (p=0.19). No difference was found between the groups combined (p=1.0).
Regarding impact of infliximab and placebo on hs-CRP concentrations across treatment, there was a significant effect of treatment (t=2.25, df=57, p=0.03), but no effect of time (t=0.10, df=433, p=0.92) or treatment by time interaction (t=0.27, df=433, p=0.78) (Figure 7). Infliximab was found to lead to a significant reduction in hs-CRP compared to placebo at all visits beyond baseline (p<0.05). Placebo non-responders exhibited somewhat higher baseline hs-CRP concentrations than placebo responders (7.8 mg/L SD 11.0 versus 3.1 mg/L SD 2.6, p=0.12), whereas infliximab responders exhibited similar plasma hs-CRP compared to infliximab non-responders (6.9 mg/L SD 7.4 versus 5.8 SD 10.5, p=0.76). Of note, in treatment responders, the mean change in hs-CRP from Baseline to Week 12 in infliximab-treated patients (n=13) was significantly greater than that in placebo-treated subjects (n=15)(−4.7 SD 11.6 mg/L versus 1.3 SD 1.7 mg/L, p<0.01).
Exploratory analyses of TNF-alpha and its receptors revealed no significant interactions between treatment, time and the log of any of the TNF markers. However, all three TNF markers were significantly higher in infliximab responders versus infliximab non-responders (all p<0.05)(eFigure 4, Online-Only Material). No differences in baseline TNF markers were found between placebo responders and non-responders (p>0.36).
Adverse events reported in more than 5% of the total subject population are indicated in Table 2. Except for an increased number of subjects with elevated urinary leukocyte esterase in the placebo group, no statistically significant differences between groups were found. No serious adverse events were reported for either group.
Table 2.
Adverse Events
| Adverse Event – no. (%) | Infliximab (n=30) | Placebo (n=30) |
|---|---|---|
| Headaches | 20 (67%)) | 18 (60%) |
| Coughing | 5 (17%) | 5 (17%) |
| Sore Throat | 4 (13%) | 6 (20%) |
| Insomnia | 5 (17%) | 4 (13%) |
| Diarrhea | 3 (10%) | 6 (20%) |
| Upper Respiratory Infection | 4 (13%) | 2 (7%) |
| Nasal Congestion | 4 (13%) | 2 (7%) |
| Myalgia | 4 (13%) | 1 (3%) |
| Panic Attacks | 2 (7%) | 3 (10%) |
| Rash | 4 (13%) | 1 (3%) |
| Fever | 1 (3%) | 1 (3%) |
| Increased Urinary Leukocyte Esterase | 0 (0%) | 10 (33%)* |
| Sinus Congestion | 4 (13%) | 0 (0%) |
| Yeast Infection | 3 (10%) | 1 (3%) |
| Increased Urinary WBC | 0 (0%) | 3 (10%) |
p<0.005
Comment
Although well-tolerated, infliximab did not show overall superiority to placebo on depressive symptom outcome, suggesting that blockade of peripheral TNF-alpha is not effective as a generalized strategy for TRD. However, an association was observed between baseline concentrations of the inflammatory biomarker hs-CRP and subsequent response to infliximab, such that an increasing advantage for infliximab on depression-related outcome became apparent as baseline values of hs-CRP increased. For subjects with pretreatment values hs-CRP>5mg/L, randomization to infliximab resulted in a 3.1 point greater reduction in HAM-D-17 scores from Baseline to Week 12 compared to placebo. This between-group difference in the study’s primary outcome measure is similar in magnitude to differences typically seen between antidepressant medications and placebo in randomized studies and is considered a clinically meaningful difference according to the National Institute for Health and Clinical Excellence.54
In subjects with baseline hs-CRP>5mg/L, treatment with infliximab benefitted a wide range of depressive symptoms including depressed mood, psychomotor retardation and performance of work and other activities (anhedonia/fatigue) as well as psychic anxiety and suicidal ideation. This pattern of symptom change is intriguing given recent studies showing that these symptoms correspond to brain neurocircuits that are targeted by inflammatory cytokines. For example, peripheral cytokine administration or induction of inflammatory cytokines using typhoid vaccination or endotoxin have been shown to induce psychomotor slowing and anhedonia in association with reduced neural activity in the basal ganglia, an area of central importance to psychomotor speed and hedonic tone.55–57 In addition, administration of inflammatory stimuli have been found to activate subgenual and dorsal areas of the anterior cingulate cortex (ACC), brain regions implicated in anxiety disorders and depression.58–61 Finally, reduction in suicidal ideation may represent a potential advantage of cytokine blockade that warrants further exploration, and is consistent with data showing increased density of microglia and astrocytes in the ACC of suicide victims.62,63
The search for biomarkers that predict antidepressant response has proven remarkably difficult, and the literature is littered with conflicting findings regarding an array of putative genetic and physiological predictor variables. Nevertheless, the specificity of association between TNF-alpha and CRP and infliximab’s mechanism of action may have decreased the “signal-to-noise” ratio sufficiently to allow TNF-alpha and its soluble receptors as well as CRP to function as predictor variables in a way not possible for biomarkers less directly linked to the mechanism of action of more complex pharmacologic or somatic interventions.
Although the results did not reach statistical significance, elevated concentrations of hs-CRP prior to treatment were associated with a reduced response to placebo. These data are consistent with previous findings associating increased peripheral inflammatory biomarkers with resistance to antidepressants.2 The mechanisms by which inflammation might impair the placebo response are unknown. However, the therapeutic relationship has been suggested as a core component of the placebo response,64 and social isolation is a core behavior induced by cytokines.65 These findings raise the possibility that chronic inflammatory activity may impair an individual’s ability to access social perceptions and emotions that are essential for placebo response. Given that ~37% of response to antidepressants results from placebo effects,66 these data may provide a novel explanation for the association between increased inflammation and antidepressant non-responsiveness.
An unexpected observation was that placebo outperformed infliximab in subjects with low levels of baseline inflammation. These results suggest that some minimal level of peripheral inflammatory activity may be required to exhibit an antidepressant response. It is becoming increasingly apparent that low, physiologic levels of cytokine activity are essential for a number of brain processes associated with protection from depression, including neuroplasticity and neurogenesis.67 Moreover, recent data indicate that behavioral effects of serotonergic antidepressants are dependent upon TNF-alpha and IFN-gamma-mediated upregulation of CNS p11, a biochemical marker of antidepressant response.68 In keeping with this finding, studies in animals and observational data in humans suggest that nonsteroidal anti-inflammatory drugs may interfere with behavioral effects of serotonergic antidepressants through their disruption of antidepressant-induced cytokine effects on p11.68 Taken together, these data suggest that caution may be warranted in use of anti-inflammatory strategies in patients with depression without evidence of increased inflammation, and provide a counterbalance to prevailing views of inflammatory processes as primarily depressogenic.
Because infliximab is too large to cross the blood brain barrier (BBB) and enter the CNS at appreciable levels, our findings suggest that direct CNS action may not be necessary for a pharmacologic agent to possess antidepressant activity. If confirmed in subsequent studies, these findings suggest that body-based approaches to treatment of depression may be developed that would avoid the high rate of somatic and behavioral side effects observed with current antidepressants as a result of their indiscriminant action upon receptors throughout the CNS. Relevant in this regard is the relatively low rate of side effects observed in subjects who received infliximab. Nevertheless, it should be noted that the response in subjects with high inflammation may also be the result of increased permeability of infliximab through the BBB. Indeed, high peripheral inflammation has been associated with reduced BBB integrity.69
Several limitations of the current study warrant comment. The study was underpowered to adequately test the possibility that infliximab would only show superiority to placebo in subjects with elevated baseline hs-CRP. Moreover, the low power may have increased the likelihood of false positives including the interaction between treatment, time and log hs-CRP. In addition, the focus of the analyses on subjects with baseline hs-CRP >5mg/L maximized the apparent efficacy of infliximab versus placebo, although it should be noted that baseline inflammatory status was proposed as a potential moderator of treatment response in the statistical analysis plan for this study. Nevertheless, tuture studies focused on subjects with hs-CRP>5 mg/L [which represented 37% (22/60) of our sample] and/or elevated markers of TNF-alpha activation seem warranted. It should also be noted that no guidelines currently exist for either the dose or schedule of administration for infliximab or other cytokine antagonists for depression. Indeed, the finding that patients with lower levels of baseline inflammation actually did worse on infliximab might suggest that the medication was over-dosed for TRD, a condition associated with far lower levels of inflammatory biomarkers than typically seen in inflammatory diseases such as ulcerative colitis and Crohn’s disease. Finally, the relatively short follow-up period may have obviated the capacity to detect interactive or synergistic effects between conventional antidepressants and TNF-alpha antagonism that may appear later in treatment, after the potential negative effects of cytokines on antidepressant action have been removed.
The high rate of placebo response observed in the current study reduced the power to test the antidepressant efficacy of infliximab. This high placebo response was unexpected, given the far lower rates of placebo response typically reported for TRD. Nevertheless, given the long duration of the current MDD episode (mean duration over 15 years for each group) and the fact that some participants were without any medication or other treatment, the sample may have represented a highly motivated and thereby responsive sample for study participation. Although a limitation, the high placebo response serves as a cautionary note for interpretation of studies with highly invasive treatments in highly motivated patients that are not accompanied by adequate placebo control.
A final limitation is that no direct measures of the effect of either infliximab or placebo on the CNS were obtained. Nevertheless, studies in laboratory animals and humans suggest that, when activated, peripheral cytokines access the CNS and produce changes in the brain similar to major depression.70 Moreover, data suggest that antagonism of peripheral cytokines can have activity in the brain. For example, in an animal model of traumatic brain injury, peripheral administration of the TNF-alpha antagonist etanercept reduced CNS levels of proinflammatory cytokines and attenuated inflammatory damage to brain tissue.71 Similarly, in humans with progressive neuro-Behcet’s syndrome, infliximab has been reported to improve behavioral symptoms in concert with reductions in cerebrospinal fluid (CSF) concentrations of IL-6.72 Finally, etanercept was found to reduce percentage and amount of rapid eye movement sleep compared to placebo in abstinent males with a history of alcohol dependence.73
In sum, this proof-of-concept study suggests that TNF-alpha antagonism does not exhibit generalized efficacy in TRD. However, interesting yet complex interrelationships between inflammation and treatment response to both TNF-alpha antagonism and placebo were revealed. While subjects with high inflammation responded preferentially to infliximab, infliximab-treated subjects with low inflammation appeared to do worse than placebo. In contrast, increased inflammation predicted a poor response to placebo. Taken together, the data suggest that there is a subgroup of TRD patients who have increased inflammation and respond to cytokine antagonism, but not to placebo. This data represents an important first step in personalization of antidepressant therapy and provides promise for future development and elaboration of inflammatory biomarkers that identify patients who may be uniquely responsive to immune-targeted therapy.
Supplementary Material
Acknowledgments
Dr. Andrew Miller had full access to all of the data of the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors have had full access to all the data in the study. This work was supported by funds from the National Institute of Mental Health (R21MH0771172) and Centocor Ortho Biotec Services LLC. In addition, the study was supported by PHS Grant UL1 RR025008 from the Clinical and Translational Science Award program and PHS Grant M01 RR0039 from the General Clinical Research Center program, National Institutes of Health, National Center for Research Resources.
References
- 1.Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, Niederehe G, Thase ME, Lavori PW, Lebowitz BD, McGrath PJ, Rosenbaum JF, Sackeim HA, Kupfer DJ, Luther J, Fava M. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163(11):1905–1917. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- 2.Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65(9):732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lanquillon S, Krieg JC, Bening-Abu-Shach U, Vedder H. Cytokine production and treatment response in major depressive disorder. Neuropsychopharmacology. 2000;22(4):370–379. doi: 10.1016/S0893-133X(99)00134-7. [DOI] [PubMed] [Google Scholar]
- 4.Papakostas GI, Shelton RC, Kinrys G, Henry ME, Bakow BR, Lipkin SH, Pi B, Thurmond L, Bilello JA. Assessment of a multi-assay, serum-based biological diagnostic test for major depressive disorder: a Pilot and Replication Study. Mol Psychiatry. 2011 doi: 10.1038/mp.2011.166. [DOI] [PubMed] [Google Scholar]
- 5.Nanni V, Uher R, Danese A. Childhood Maltreatment Predicts Unfavorable Course of Illness and Treatment Outcome in Depression: A Meta-Analysis. Am J Psychiatry. 2011 doi: 10.1176/appi.ajp.2011.11020335. [DOI] [PubMed] [Google Scholar]
- 6.Rush AJ, Wisniewski SR, Warden D, Luther JF, Davis LL, Fava M, Nierenberg AA, Trivedi MH. Selecting among second-step antidepressant medication monotherapies: predictive value of clinical, demographic, or first-step treatment features. Arch Gen Psychiatry. 2008;65(8):870–880. doi: 10.1001/archpsyc.65.8.870. [DOI] [PubMed] [Google Scholar]
- 7.Nathan C. Epidemic inflammation: pondering obesity. Mol Med. 2008;14(7–8):485–492. doi: 10.2119/2008-00038.Nathan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Danese A, Moffitt TE, Pariante CM, Ambler A, Poulton R, Caspi A. Elevated inflammation levels in depressed adults with a history of childhood maltreatment. Arch Gen Psychiatry. 2008;65(4):409–415. doi: 10.1001/archpsyc.65.4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pace TW, Heim CM. A short review on the psychoneuroimmunology of posttraumatic stress disorder: from risk factors to medical comorbidities. Brain Behav Immun. 2011;25(1):6–13. doi: 10.1016/j.bbi.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 10.Bouhuys AL, Flentge F, Oldehinkel AJ, van den Berg MD. Potential psychosocial mechanisms linking depression to immune function in elderly subjects. Psychiatry Res. 2004;127(3):237–245. doi: 10.1016/j.psychres.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 11.Katon W, Unutzer J, Russo J. Major depression: the importance of clinical characteristics and treatment response to prognosis. Depress Anxiety. 2010;27(1):19–26. doi: 10.1002/da.20613. [DOI] [PubMed] [Google Scholar]
- 12.Zhu CB, Lindler KM, Owens AW, Daws LC, Blakely RD, Hewlett WA. Interleukin-1 Receptor Activation by Systemic Lipopolysaccharide Induces Behavioral Despair Linked to MAPK Regulation of CNS Serotonin Transporters. Neuropsychopharmacology. 2010 doi: 10.1038/npp.2010.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu CB, Blakely RD, Hewlett WA. The proinflammatory cytokines interleukin-1beta and tumor necrosis factor-alpha activate serotonin transporters. Neuropsychopharmacology. 2006;31(10):2121–2131. doi: 10.1038/sj.npp.1301029. [DOI] [PubMed] [Google Scholar]
- 14.Widner B, Laich A, Sperner-Unterweger B, Ledochowski M, Fuchs D. Neopterin production, tryptophan degradation, and mental depression--what is the link? Brain Behav Immun. 2002;16(5):590–595. doi: 10.1016/s0889-1591(02)00006-5. [DOI] [PubMed] [Google Scholar]
- 15.Haroon E, Raison CL, Miller AH. Psychoneuroimmunology meets neuropsychopharmacology: translational implications of the impact of inflammation on behavior. Neuropsychopharmacology. 2012;37(1):137–162. doi: 10.1038/npp.2011.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neurauter G, Schrocksnadel K, Scholl-Burgi S, Sperner-Unterweger B, Schubert C, Ledochowski M, Fuchs D. Chronic immune stimulation correlates with reduced phenylalanine turnover. Curr Drug Metab. 2008;9(7):622–627. doi: 10.2174/138920008785821738. [DOI] [PubMed] [Google Scholar]
- 17.Koo JW, Russo SJ, Ferguson D, Nestler EJ, Duman RS. Nuclear factor-kappaB is a critical mediator of stress-impaired neurogenesis and depressive behavior. Proc Natl Acad Sci U S A. 2010;107(6):2669–2674. doi: 10.1073/pnas.0910658107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goshen I, Kreisel T, Ben-Menachem-Zidon O, Licht T, Weidenfeld J, Ben-Hur T, Yirmiya R. Brain interleukin-1 mediates chronic stress-induced depression in mice via adrenocortical activation and hippocampal neurogenesis suppression. Mol Psychiatry. 2008;13(7):717–728. doi: 10.1038/sj.mp.4002055. [DOI] [PubMed] [Google Scholar]
- 19.Koo JW, Duman RS. IL-1beta is an essential mediator of the antineurogenic and anhedonic effects of stress. Proc Natl Acad Sci U S A. 2008;105(2):751–756. doi: 10.1073/pnas.0708092105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perera TD, Dwork AJ, Keegan KA, Thirumangalakudi L, Lipira CM, Joyce N, Lange C, Higley JD, Rosoklija G, Hen R, Sackeim HA, Coplan JD. Necessity of hippocampal neurogenesis for the therapeutic action of antidepressants in adult nonhuman primates. PLoS One. 2011;6(4):e17600. doi: 10.1371/journal.pone.0017600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tilleux S, Hermans E. Neuroinflammation and regulation of glial glutamate uptake in neurological disorders. J Neurosci Res. 2007;85(10):2059–2070. doi: 10.1002/jnr.21325. [DOI] [PubMed] [Google Scholar]
- 22.Ida T, Hara M, Nakamura Y, Kozaki S, Tsunoda S, Ihara H. Cytokine-induced enhancement of calcium-dependent glutamate release from astrocytes mediated by nitric oxide. Neurosci Lett. 2008;432(3):232–236. doi: 10.1016/j.neulet.2007.12.047. [DOI] [PubMed] [Google Scholar]
- 23.Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, Lanctot KL. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67(5):446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 24.Raison CL, Borisov AS, Woolwine BJ, Massung B, Vogt G, Miller AH. Interferon-alpha effects on diurnal hypothalamic-pituitary-adrenal axis activity: relationship with proinflammatory cytokines and behavior. Mol Psychiatry. 2010;15(5):535–547. doi: 10.1038/mp.2008.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tyring S, Gottlieb A, Papp K, Gordon K, Leonardi C, Wang A, Lalla D, Woolley M, Jahreis A, Zitnik R, Cella D, Krishnan R. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367(9504):29–35. doi: 10.1016/S0140-6736(05)67763-X. [DOI] [PubMed] [Google Scholar]
- 26.Persoons P, Vermeire S, Demyttenaere K, Fischler B, Vandenberghe J, Van Oudenhove L, Pierik M, Hlavaty T, Van Assche G, Noman M, Rutgeerts P. The impact of major depressive disorder on the short- and long-term outcome of Crohn’s disease treatment with infliximab. Aliment Pharmacol Ther. 2005;22(2):101–110. doi: 10.1111/j.1365-2036.2005.02535.x. [DOI] [PubMed] [Google Scholar]
- 27.Monk JP, Phillips G, Waite R, Kuhn J, Schaaf LJ, Otterson GA, Guttridge D, Rhoades C, Shah M, Criswell T, Caligiuri MA, Villalona-Calero MA. Assessment of tumor necrosis factor alpha blockade as an intervention to improve tolerability of dose-intensive chemotherapy in cancer patients. J Clin Oncol. 2006;24(12):1852–1859. doi: 10.1200/JCO.2005.04.2838. [DOI] [PubMed] [Google Scholar]
- 28.Silverman MN, Macdougall MG, Hu F, Pace TW, Raison CL, Miller AH. Endogenous glucocorticoids protect against TNF-alpha-induced increases in anxiety-like behavior in virally infected mice. Mol Psychiatry. 2007;12(4):408–417. doi: 10.1038/sj.mp.4001921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simen BB, Duman CH, Simen AA, Duman RS. TNFalpha signaling in depression and anxiety: behavioral consequences of individual receptor targeting. Biol Psychiatry. 2006;59(9):775–785. doi: 10.1016/j.biopsych.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 30.Maes M. Evidence for an immune response in major depression: a review and hypothesis. Prog Neuropsychopharmacol Biol Psychiatry. 1995;19(1):11–38. doi: 10.1016/0278-5846(94)00101-m. [DOI] [PubMed] [Google Scholar]
- 31.Kent S, Bluthe RM, Kelley KW, Dantzer R. Sickness behavior as a new target for drug development. Trends Pharmacol Sci. 1992;13(1):24–28. doi: 10.1016/0165-6147(92)90012-u. [DOI] [PubMed] [Google Scholar]
- 32.Kircik LH. Doxycycline and minocycline for the management of acne: a review of efficacy and safety with emphasis on clinical implications. J Drugs Dermatol. 2010;9(11):1407–1411. [PubMed] [Google Scholar]
- 33.Langley RE, Burdett S, Tierney JF, Cafferty F, Parmar MK, Venning G. Aspirin and cancer: has aspirin been overlooked as an adjuvant therapy? Br J Cancer. 2011;105(8):1107–1113. doi: 10.1038/bjc.2011.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang YX, Gao JX, Wang XY, Zhang L, Liu CM. Antiproliferative effects of selective cyclooxygenase-2 inhibitor modulated by nimotuzumab in estrogen-dependent breast cancer cells. Tumour Biol. 2012 doi: 10.1007/s13277-012-0324-4. [DOI] [PubMed] [Google Scholar]
- 35.Hu F, Wang X, Pace TW, Wu H, Miller AH. Inhibition of COX-2 by celecoxib enhances glucocorticoid receptor function. Mol Psychiatry. 2005;10(5):426–428. doi: 10.1038/sj.mp.4001644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med. 2009;71(2):171–186. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- 37.Sluzewska A, Sobieska M, Rybakowski JK. Changes in acute-phase proteins during lithium potentiation of antidepressants in refractory depression. Neuropsychobiology. 1997;35(3):123–127. doi: 10.1159/000119332. [DOI] [PubMed] [Google Scholar]
- 38.Gimeno D, Kivimaki M, Brunner EJ, Elovainio M, De Vogli R, Steptoe A, Kumari M, Lowe GD, Rumley A, Marmot MG, Ferrie JE. Associations of C-reactive protein and interleukin-6 with cognitive symptoms of depression: 12-year follow-up of the Whitehall II study. Psychol Med. 2009;39(3):413–423. doi: 10.1017/S0033291708003723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Louis E, Vermeire S, Rutgeerts P, De Vos M, Van Gossum A, Pescatore P, Fiasse R, Pelckmans P, Reynaert H, D’Haens G, Malaise M, Belaiche J. A positive response to infliximab in Crohn disease: association with a higher systemic inflammation before treatment but not with -308 TNF gene polymorphism. Scand J Gastroenterol. 2002;37(7):818–824. [PubMed] [Google Scholar]
- 40.Arends S, Brouwer E, van der Veer E, Groen H, Leijsma MK, Houtman PM, Th AJTL, Kallenberg CG, Spoorenberg A. Baseline predictors of response and discontinuation of tumor necrosis factor-alpha blocking therapy in ankylosing spondylitis: a prospective longitudinal observational cohort study. Arthritis Res Ther. 2011;13(3):R94. doi: 10.1186/ar3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bower JE, Ganz PA, Aziz N, Fahey JL. Fatigue and proinflammatory cytokine activity in breast cancer survivors. Psychosom Med. 2002;64(4):604–611. doi: 10.1097/00006842-200207000-00010. [DOI] [PubMed] [Google Scholar]
- 42.Schroder J, Stuber F, Gallati H, Schade FU, Kremer B. Pattern of soluble TNF receptors I and II in sepsis. Infection. 1995;23(3):143–148. doi: 10.1007/BF01793854. [DOI] [PubMed] [Google Scholar]
- 43.Aderka D, Engelmann H, Maor Y, Brakebusch C, Wallach D. Stabilization of the bioactivity of tumor necrosis factor by its soluble receptors. J Exp Med. 1992;175(2):323–329. doi: 10.1084/jem.175.2.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV. Washington DC: American Psychiatric Press; 1997. [Google Scholar]
- 45.Petersen T, Papakostas GI, Posternak MA, Kant A, Guyker WM, Iosifescu DV, Yeung AS, Nierenberg AA, Fava M. Empirical testing of two models for staging antidepressant treatment resistance. J Clin Psychopharmacol. 2005;25(4):336–341. doi: 10.1097/01.jcp.0000169036.40755.16. [DOI] [PubMed] [Google Scholar]
- 46.Trivedi MH, Rush AJ, Ibrahim HM, Carmody TJ, Biggs MM, Suppes T, Crismon ML, Shores-Wilson K, Toprac MG, Dennehy EB, Witte B, Kashner TM. The Inventory of Depressive Symptomatology, Clinician Rating (IDS-C) and Self-Report (IDS-SR), and the Quick Inventory of Depressive Symptomatology, Clinician Rating (QIDS-C) and Self- Report (QIDS-SR) in public sector patients with mood disorders: a psychometric evaluation. Psychol Med. 2004;34(1):73–82. doi: 10.1017/s0033291703001107. [DOI] [PubMed] [Google Scholar]
- 47.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 49.Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, 3rd, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL, Rifai N, Smith SC, Jr, Taubert K, Tracy RP, Vinicor F. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107(3):499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 50.Rutgeerts P, Feagan BG, Lichtenstein GR, Mayer LF, Schreiber S, Colombel JF, Rachmilewitz D, Wolf DC, Olson A, Bao W, Hanauer SB. Comparison of scheduled and episodic treatment strategies of infliximab in Crohn’s disease. Gastroenterology. 2004;126(2):402–413. doi: 10.1053/j.gastro.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 51.Guy W, editor. Early Clinical Drug Evaluation Program Assessment Manual for Psychopharmacology, Revised (Publication number, 76-338) Rockville, MD: National Institute of Mental Health; 1976. [Google Scholar]
- 52.McGrath PJ, Stewart JW, Fava M, Trivedi MH, Wisniewski SR, Nierenberg AA, Thase ME, Davis L, Biggs MM, Shores-Wilson K, Luther JF, Niederehe G, Warden D, Rush AJ. Tranylcypromine versus venlafaxine plus mirtazapine following three failed antidepressant medication trials for depression: a STAR*D report. Am J Psychiatry. 2006;163(9):1531–1541. doi: 10.1176/ajp.2006.163.9.1531. quiz 1666. [DOI] [PubMed] [Google Scholar]
- 53.Undurraga J, Baldessarini RJ. Randomized, placebo-controlled trials of antidepressants for acute major depression: thirty-year meta-analytic review. Neuropsychopharmacology. 2012;37(4):851–864. doi: 10.1038/npp.2011.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Excellence NIfC. Clinical practice guideline No 23. London: National Institute for Clinical Excellence; 2004. Depression: management of depression in primary and secondary care; p. 670. [Google Scholar]
- 55.Capuron L, Pagnoni G, Drake DF, Woolwine BJ, Spivey JR, Crowe RJ, Votaw JR, Goodman MM, Miller AH. Dopaminergic Mechanisms of Reduced Basal Ganglia Responses to Hedonic Reward during Interferon-alpha Administration. Archives of General Psychiatry. doi: 10.1001/archgenpsychiatry.2011.2094. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Eisenberger NI, Berkman ET, Inagaki TK, Rameson LT, Mashal NM, Irwin MR. Inflammation-induced anhedonia: endotoxin reduces ventral striatum responses to reward. Biol Psychiatry. 2010;68(8):748–754. doi: 10.1016/j.biopsych.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brydon L, Harrison NA, Walker C, Steptoe A, Critchley HD. Peripheral inflammation is associated with altered substantia nigra activity and psychomotor slowing in humans. Biol Psychiatry. 2008;63(11):1022–1029. doi: 10.1016/j.biopsych.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Eisenberger NI, Lieberman MD. Why rejection hurts: a common neural alarm system for physical and social pain. Trends Cogn Sci. 2004;8(7):294–300. doi: 10.1016/j.tics.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 59.Capuron L, Pagnoni G, Demetrashvili M, Woolwine BJ, Nemeroff CB, Berns GS, Miller AH. Anterior cingulate activation and error processing during interferon-alpha treatment. Biol Psychiatry. 2005;58(3):190–196. doi: 10.1016/j.biopsych.2005.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Harrison NA, Brydon L, Walker C, Gray MA, Steptoe A, Dolan RJ, Critchley HD. Neural origins of human sickness in interoceptive responses to inflammation. Biol Psychiatry. 2009;66(5):415–422. doi: 10.1016/j.biopsych.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Harrison NA, Brydon L, Walker C, Gray MA, Steptoe A, Critchley HD. Inflammation causes mood changes through alterations in subgenual cingulate activity and mesolimbic connectivity. Biol Psychiatry. 2009;66(5):407–414. doi: 10.1016/j.biopsych.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Torres-Platas SG, Hercher C, Davoli MA, Maussion G, Labonte B, Turecki G, Mechawar N. Astrocytic hypertrophy in anterior cingulate white matter of depressed suicides. Neuropsychopharmacology. 2011;36(13):2650–2658. doi: 10.1038/npp.2011.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Steiner J, Bielau H, Brisch R, Danos P, Ullrich O, Mawrin C, Bernstein HG, Bogerts B. Immunological aspects in the neurobiology of suicide: elevated microglial density in schizophrenia and depression is associated with suicide. J Psychiatr Res. 2008;42(2):151–157. doi: 10.1016/j.jpsychires.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 64.Krupnick JL, Sotsky SM, Simmens S, Moyer J, Elkin I, Watkins J, Pilkonis PA. The role of the therapeutic alliance in psychotherapy and pharmacotherapy outcome: findings in the National Institute of Mental Health Treatment of Depression Collaborative Research Program. J Consult Clin Psychol. 1996;64(3):532–539. doi: 10.1037//0022-006x.64.3.532. [DOI] [PubMed] [Google Scholar]
- 65.Eisenberger NI, Inagaki TK, Mashal NM, Irwin MR. Inflammation and social experience: an inflammatory challenge induces feelings of social disconnection in addition to depressed mood. Brain Behav Immun. 2010;24(4):558–563. doi: 10.1016/j.bbi.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Undurraga J, Baldessarini RJ. Randomized, Placebo-Controlled Trials of Antidepressants for Acute Major Depression: Thirty-Year Meta-Analytic Review. Neuropsychopharmacology. 2011 doi: 10.1038/npp.2011.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yirmiya R, Goshen I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav Immun. 2011;25(2):181–213. doi: 10.1016/j.bbi.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 68.Warner-Schmidt JL, Vanover KE, Chen EY, Marshall JJ, Greengard P. Antidepressant effects of selective serotonin reuptake inhibitors (SSRIs) are attenuated by antiinflammatory drugs in mice and humans. Proc Natl Acad Sci U S A. 2011;108(22):9262–9267. doi: 10.1073/pnas.1104836108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Banks WA, Erickson MA. The blood-brain barrier and immune function and dysfunction. Neurobiol Dis. 2010;37(1):26–32. doi: 10.1016/j.nbd.2009.07.031. [DOI] [PubMed] [Google Scholar]
- 70.Raison CL, Borisov AS, Majer M, Drake DF, Pagnoni G, Woolwine BJ, Vogt GJ, Massung B, Miller AH. Activation of central nervous system inflammatory pathways by interferon-alpha: relationship to monoamines and depression. Biol Psychiatry. 2009;65(4):296–303. doi: 10.1016/j.biopsych.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chio CC, Lin JW, Chang MW, Wang CC, Kuo JR, Yang CZ, Chang CP. Therapeutic evaluation of etanercept in a model of traumatic brain injury. J Neurochem. 2010;115(4):921–929. doi: 10.1111/j.1471-4159.2010.06969.x. [DOI] [PubMed] [Google Scholar]
- 72.Kikuchi H, Aramaki K, Hirohata S. Effect of infliximab in progressive neuro-Behcet’s syndrome. J Neurol Sci. 2008;272(1–2):99–105. doi: 10.1016/j.jns.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 73.Irwin MR, Olmstead R, Valladares EM, Breen EC, Ehlers CL. Tumor necrosis factor antagonism normalizes rapid eye movement sleep in alcohol dependence. Biol Psychiatry. 2009;66(2):191–195. doi: 10.1016/j.biopsych.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.