Overview of Olfactory Epithelium and Vomeronasal Organ Structure and Cell Types
In terrestrial vertebrates, the primary relay neurons of the olfactory system are contained within two specialized sensory epithelia in the head – the main olfactory epithelium (OE), which lines the nasal cavity and is involved in detection of odors, and the vomeronasal sensory epithelium, which lines the vomeronasal organ (VNO; a bean-shaped structure located in the anterior–ventral region of the nasal septum) and mediates detection of nonvolatile pheromones (Figure 1(a)). Cell bodies of olfactory receptor neurons (ORNs) lie within the epithelia of the OE and VNO. ORNs are bipolar primary sensory neurons: they have apical dendrites, with cilia- or microvilli-covered surfaces that extend into the nasal or VNO cavities, and they extend axons that synapse upon neurons within the central nervous system. In the case of the OE, the axons of ORNs form the first cranial nerve and synapse upon cells of the main olfactory bulb (MOB) of the forebrain. The sensory neurons of the VNO extend axons to form the vomeronasal nerve, which synapses upon the accessory olfactory bulb (AOB), a prominent structure in rodents that lies immediately adjacent to the MOB.
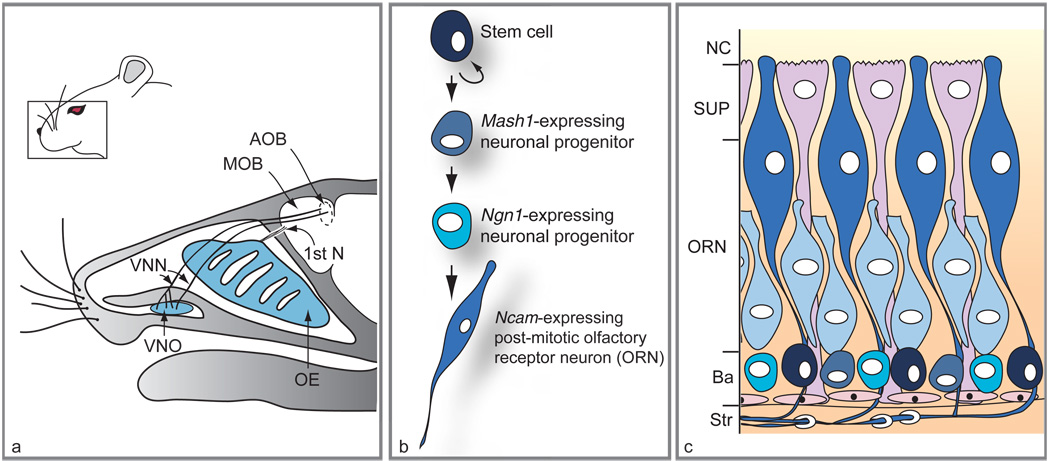
Figure 1.
Location of main olfactory epithelium (OE) and vomeronasal organ (VNO) in mouse head, and lineage and distribution of different cell types within the OE. (a) Sagittal section through an adult head reveals the position of the VNO relative to the OE. While the vomeronasal nerves (VNN) target the accessory olfactory bulb (AOB), axons of olfactory receptor neurons (first cranial nerve; 1st N) target the main olfactory bulb (MOB). (b) Scheme of the neuronal differentiation pathway in the OE. (c) Histological arrangement of cells in mature OE. Neuronal stem cells (black) located at the basal layer (Ba) adjacent to the stroma (Str) give rise to transit-amplifying progenitors expressing the Mash1 gene (round gray-blue cells) followed by Ngn1-expressing precursors (turquoise cells). The immature ORNs (light blue elongated cells) arise from the Ngn1-positive cells and mature into Ncam-expressing neurons (dark blue elongated cells). Supporting cells (SUP) lie in a single layer on the apical surface of the OE, at the nasal cavity (NC), while olfactory ensheathing cells wrap around nerve bundles in the underlying stroma. The VNO (not shown) has a similar histological arrangement, with basal cells at the boundary between epithelium and mesenchyme and neurons populating the bulk of the epithelium. Adapted from Kawauchi S, Beites CL, Crocker CE, et al. (2004) Molecular signals regulating proliferation of stem and progenitor cells in mouse olfactory epithelium. Developmental Neuroscience 26: 166–180, with permission. Copyright 2004 by S. Karger AG, Basel, Switzerland.
Because rodents have a highly developed sense of smell and have been used for many years as model organisms in the study of olfaction, information provided in this article concentrates primarily on studies of OE and VNO in rodents, particularly rats and mice. Studies of primary olfactory pathway development in mouse mutants have shown that the process of neurogenesis in both the OE and the VNO is regulated to a large extent by the same genes, which are expressed by cells at distinct stages in the neuronal lineages of the two epithelia (Figure 1(b)). The OE and VNO are also similar in that, once their structures are established during the final third of gestation, the neural cell types within them are arranged in roughly comparable laminar organizations (Figure 1(c)). Thus, in both epithelia, neural stem cells and committed neuronal progenitors are located in the basal compartment (near the basement membrane, which forms the boundary between sensory epithelium and underlying connective tissue stroma – i.e., the lamina propria). Committed neuronal progenitors give rise to terminally differentiated sensory neurons (ORNs), which comprise the majority of cells within both epithelia. Another major cell type within the two sensory epithelia is the supporting, or sustentacular, cell, best characterized in the main OE. The cell bodies of supporting cells lie in a distinct layer in the apical compartment of the OE and their processes extend to the basal lamina; they appear to be analogous to glial cells of the brain, and studies of mouse mutants suggest that ORNs and supporting cells are both derived from a common neural stem cell in the OE.
Another cell type found associated with the OE is the olfactory ensheathing cell (OEC). OECs, which encircle bundles of ORN axons in the stroma underlying OE, possess characteristics of both Schwann cells and astrocytes. Interestingly, transplanted OECs appear to promote recovery in a variety of nerve lesion models, and it has been proposed that their presence is responsible for the ability of lesioned ORN axons to regenerate and reinnervate the central nervous system. Some experimental embryology studies suggest that OECs may originate from the olfactory placode, and tissue culture studies have also reported that cells resembling OECs arise in cultures made from OE proper. However, no definitive lineage-tracing study has been performed to demonstrate unequivocally that OECs arise from the olfactory placode or OE proper, and at present their origin remains uncertain. Thus, although the provocative possibility exists that OECs are, like ORNs, products of a multipotential OE stem cell, this hypothesis remains to be proved, and OECs are not discussed further in this article.
Neuronal Cell Types of the OE and VNO
Newly generated neurons in the OE and VNO are products of differentiation pathways known to contain at least three distinct proliferating cell types. These cell types have been most extensively characterized in the main OE (Figure 1(b)): (1) neural stem cells divide to give rise to a population of (2) committed neuronal progenitor cells that express the proneural gene mammalian achaete scute homolog 1 (Mash1; also known as Ascl1), and Mash1-expressing progenitor cells are committed to a neuronal fate and undergo amplifying divisions to rise to (3) immediate neuronal precursors (INPs), which express a different proneural gene, neurogenin1 (Ngn1; also known as Nurog1). Studies using retroviral lineage tracing and molecular markers for different cell types indicate that, in the established OE (see later), INPs, Mash1-expressing neuronal progenitors, and neural stem cells are all found in the basal compartment of the epithelium. INPs are, like Mash1-expressing progenitors, transit-amplifying cells, but INPs are committed ultimately to generating daughter cells that undergo terminal differentiation into ORNs. ORNs express many genes generally characteristic of terminally differentiated neurons, as well as genes specific to their sensory function (odorant receptors, cell-type-specific channels, etc.). Two well-known markers for ORNs are the neuronal cell adhesion molecule (NCAM1) and the olfactory marker protein (OMP). Interestingly, in the main OE, the differentiation and maturation of ORNs from stem and progenitor cells observed during embryonic development appear to be maintained during regenerative neurogenesis, which occurs when ORNs in the main OE have been lost due to disease or environmental insult.
ORNs of the main OE and VNO express different classes of odorant receptors (the catalytic receptors that transduce odor stimuli into cytoplasmic signals), reflecting their different functions: detection of ‘common’ odors in the main OE, and pheromone detection in the VNO. Odorant receptors in both sensory epithelia are seven-transmembrane, G-protein-coupled receptors. In the main OE, each ORN appears to express only one odorant receptor gene (of over 1000 estimated receptor genes), and those ORNs expressing the same odorant receptor converge their axons homotypically onto selected glomeruli of the MOB. Thus, a specific set of ORNs, with receptors that have been activated by a specific odorant, will stimulate postsynaptic neurons (mitral and tufted cells) within a specific set of MOB glomeruli, which then transduce this information to higher-order olfactory structures in the brain. In the VNO, two families of receptors, the V1Rs and V2Rs, serve as pheromone receptors, and expression of different receptors from these two families appears to divide VNO sensory neurons into two distinct populations: neurons within the apical zone of the VNO neuroepithelium express V1Rs, and their axons project to the anterior portion of the AOB, while neurons in the basal zone of the VNO neuroepithelium express V2Rs and project to the posterior portion of the AOB.
Two Phases of Olfactory Neurogenesis
Both anatomical studies and, more recently, studies of mouse mutants indicate that olfactory neurogenesis can be divided into two phases. The first is an early, morphogenetic phase, in which the neuronal lineage is established and the basic structures of the primary olfactory pathway (OE and VNO) are set up (primary neurogenesis); the second phase is established neurogenesis, during which the characteristic morphology of the nasal cavity and mature pattern of distribution of different cell types within the epithelia emerge, and the signaling systems that control expansion and regenerative neurogenesis come to predominate in the regulation of neuronal cell number. These phases of neurogenesis are depicted in Figure 2.
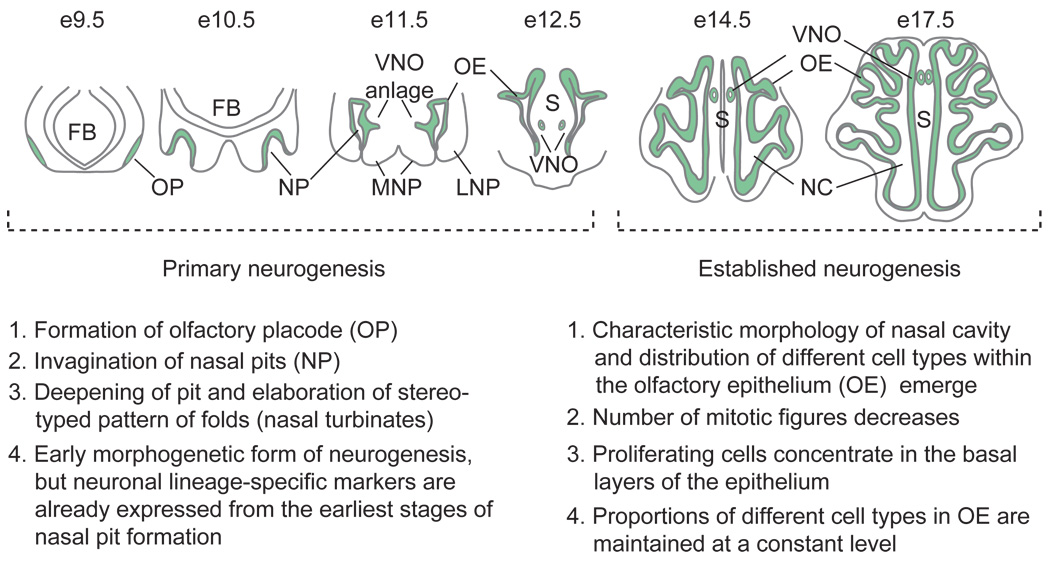
Figure 2.
Schematic model and description of primary and established neurogenesis during mouse olfactory epithelium development. Coronal sections through heads at days 9.5–12.5 of gestation (e9.5–e12.5) are depicted for the phase of primary neurogenesis, while horizontal sections at e14.5 and e17.5 (immediately before birth) are portrayed for the phase of established neurogenesis. FB, forebrain; MNP, medial nasal process; VNO, vomeronasal organ; LNP, lateral nasal process; S, nasal septum; NC, nasal cavity. Adapted from Kawauchi S, Shou J, Santos R, et al. (2005) Fgf8 expression defines a morphogenetic center required for olfactory neurogenesis and nasal cavity development in the mouse. Development 132(23): 5211–5223.
During primary neurogenesis, the OE forms from the olfactory placodes, thickenings of embryonic ectoderm that first appear as two oval epithelial patches in the anterolateral region of the head around day 9 of gestation (e9) in the mouse. As development proceeds, the olfactory placodes invaginate to form the nasal pits, which by e10.5 are already lined by neuroepithelium that contains cells at every stage of the neurogenic pathway. By e11.5, the nasal pits continue to deepen and fold, and it is during this period that the VNO becomes recognizable as a thickening in the epithelium of the medial wall of the nasal pit. At e12.5, the end of primary neurogenesis, developing nasal turbinates – the elaborate foldings of which will permit a greatly expanded OE surface area – begin to be recognizable in the main olfactory cavity. It is during this period that VNO morphogenesis takes place, as the ventral medial fold of developing neuroepithelium invaginates and pinches off from the main nasal cavity, forming a tube that is closed posteriorly, and anteriorly communicates via its own duct with the developing oral cavity, nasal cavity, or sometimes both.
In the OE that lines the developing nasal pit as early as e10, the three mitotic neuronal cell types (neural stem cells, Mash1-expressing progenitors and Ngn1-expressing INPs), as well as Ncam-expressing ORNs, are all present. These cell types are initially localized in concentric patterns, with the least differentiated cells lying closest to the rim and the most differentiated cells (ORNs) at the center (Figure 3(a)). Expression of Sox2, which encodes a marker for OE neural stem cells during established neurogenesis, defines the entire neuroepithelial domain of the developing nasal pit during primary neurogenesis. However, a small group of Sox2-expressing cells, which lie closest to the rim of the pit, also express fibroblast growth factor 8 (Fgf8). Fgf8 encodes a signaling molecule known to be required for OE neurogenesis, nasal cavity formation, and development of the VNO(see later); the cells in the rim of the olfactory pit that co-express Sox2 and Fgf8 are thought to be primordial neural stem cells responsible for initiating primary olfactory neurogenesis (by analogy to the primordial germ cells that ultimately give rise to gametes) (Figure 3(b)).
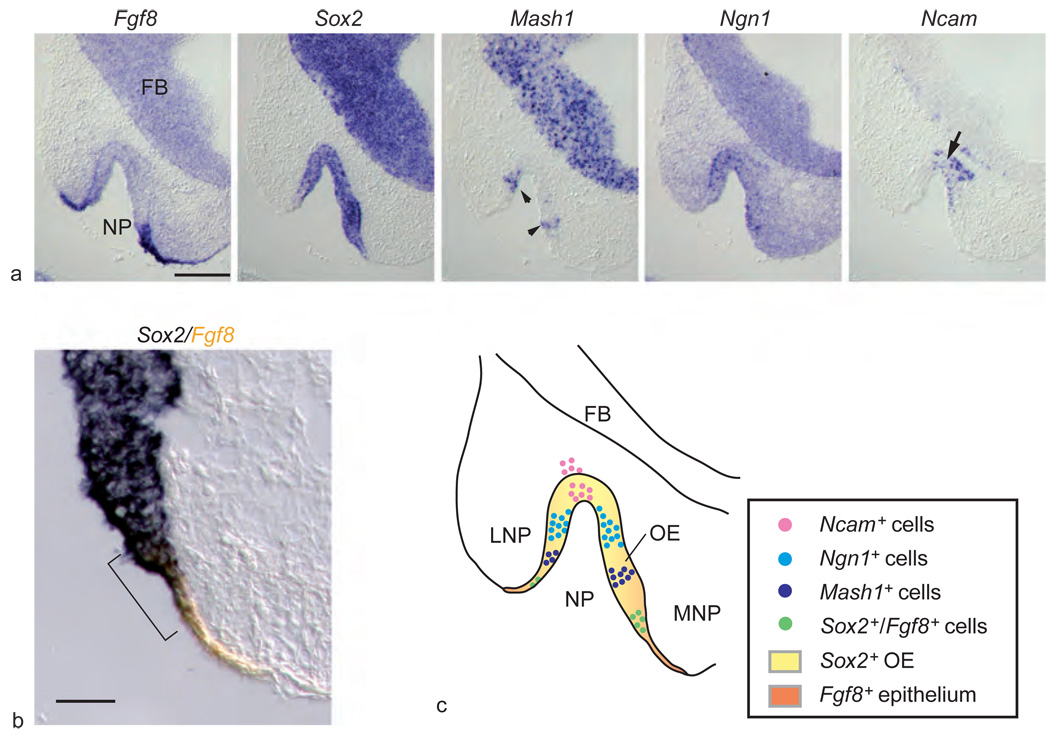
Figure 3.
Expression of Fgf8 and neuronal cell markers in developing olfactory epithelium (OE). (a) Five successive images show in situ hybridization for Fgf8 and OE neuronal lineage markers in invaginating nasal pit (NP) at day 10.5 of gestation (FB, forebrain). While Sox2 is expressed throughout the neuroepithelium, Fgf8 is localized to the borders of the invaginating pit. Mash1 (arrowheads) expression is located next to the Fgf8-expressing cells at the inner rim of the nasal pit, while Ncam-expressing neurons (arrow) are located at the center of the pit. (b) Double-label in situ hybridization for Fgf8 (orange) and Sox2 (blue) demonstrates overlap of the two markers in a small rim of surface ectoderm and adjacent invaginating neuroepithelium (bracket). (c) Model of peripheral-to-central process of neuronal differentiation in developing OE and origin of Sox2-expressing neural stem cells from Fgf8-expressing ectoderm (LNP, lateral nasal process; MNP, medial nasal process). Scale bar = 200 µm (a), 50 µm (b). Adapted from Kawauchi S, Shou J, Santos R, et al. (2005) Fgf8 expression defines a morphogenetic center required for olfactory neurogenesis and nasal cavity development in the mouse. Development 132(23): 5211–5223.
Around e14.5 in the mouse, the overall number of mitotic figures in the OE starts to decrease in the epithelium, and different cell types of the OE begin to take on the laminar positions that they will ultimately maintain throughout life. This is the onset of the established phase of neurogenesis, when the mature structure of the nasal cavity also emerges: turbinates and definitive septum form, the sensory epithelium becomes localized to the posterior and dorsal nasal cavity, and the more anterior nasal cavity comes to be lined with respiratory epithelium. Within the OE proper, supporting (sustentacular) cells emerge as a distinct cell population at this time, and their cell bodies begin to form the apical cell layer of the neuroepithelium. Ncam-expressing ORNs now lie in the middle compartment of the epithelium, and Mash1-and Ngn1-expressing neuronal progenitors predominate in the basal compartment of both OE and VNO (Figure 4). With the emergence of this organized pattern begins a new phase of neurogenesis Scale bar = 200 µm (a), 50 µm (b) that is dedicated to maintaining and regulating ORN number. From this time on, the proportions of different cell types are maintained at a fairly constant level within the OE, and the locations of populations expressing different cell-type-specific genes within the epithelium remain essentially the same throughout life.
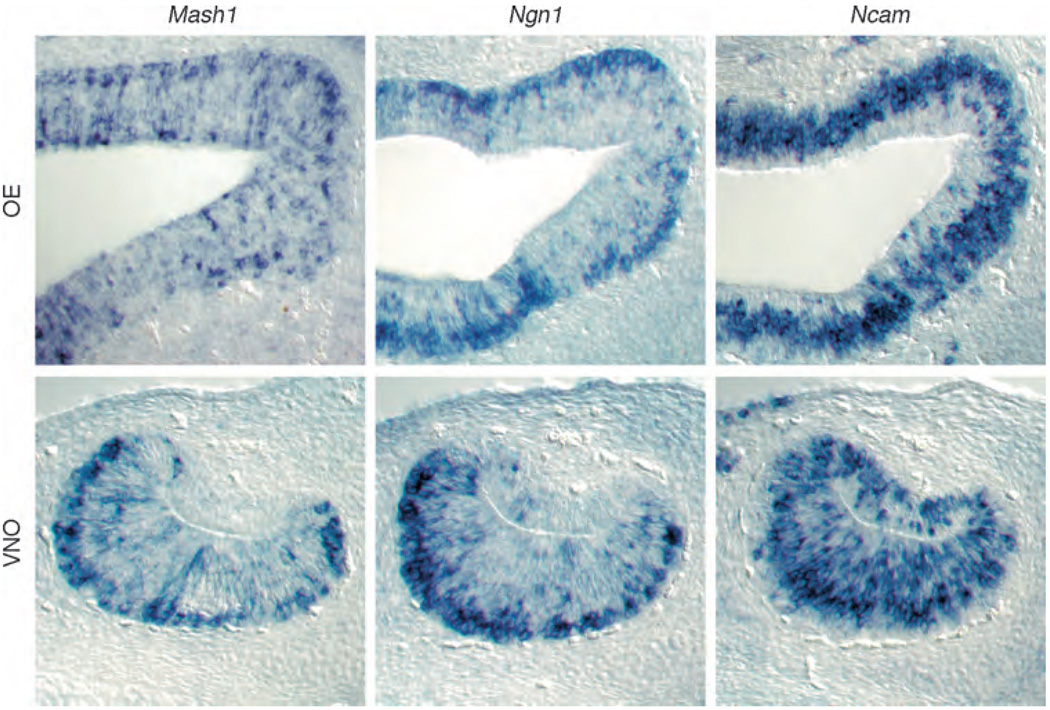
Figure 4.
Cell-type-specific markers for committed neuronal progenitors and differentiated olfactory receptor neurons in the established phase of neurogenesis in main olfactory epithelium (OE) and vomeronasal organ (VNO). In situ hybridization is shown for mRNAs encoding Mash1, Ngn1, and Ncam in the OE and VNO of mice at day 14.5 of gestation, when the pattern of neurogenesis becomes established in the two sensory epithelia. Even at this stage in prenatal development, the characteristic laminar patterns of cells are apparent, and the relative proportions of cells at different stages of differentiation are similar to what is seen in the postnatal epithelia. (Top panel) Mash1 and Ngn1 messages are all expressed in basal areas of the OE, while some apical cells express Mash1 but not Ngn1. Ncam, an olfactory receptor neuron marker, is expressed throughout the OE but is absent from the supporting cell layer that lines the nasal cavity. (Bottom panel) Mash1 and Ngn1 are similarly located in the VNO, at the boundary between basal epithelium and underlying connective tissue. Ncam-positive cells populate the majority of the sensory epithelium, from the concave side of the lumen to the basal layer. Adapted from Murray RC, Navi D, Fesenko J, et al. (2003) Widespread defects in the primary olfactory pathway caused by loss of Mash1 function. Journal of Neuroscience 23(5): 1769–1780, with permission. Copyright 2003 by the Society for Neuroscience.
During the established phase of neurogenesis, an asymmetry develops in the VNO epithelial lining, such that its lateral wall is lined with nonsensory epithelium, whereas the medial wall is lined with a thicker sensory neuroepithelium. This is analogous to the asymmetry that develops in the main nasal cavity, wherein nonsensory, respiratory epithelium predominates in the lining of the anterior and ventral regions of the cavity, whereas the thicker sensory neuroepithelium containing ORNs tends to be localized to more posterior and dorsal regions, covering the turbinates and the posterior two-thirds of the nasal septum.
Intrinsic Factors: Transcription Factors Regulating Mitotic Cell Populations
Neurogenesis and nerve cell renewal take place throughout life in both the OE and the VNO. This capacity for ongoing neurogenesis is coupled with the ability to regenerate the sensory neuron compartment quickly, at least in the main OE. When ORN number is reduced dramatically by surgical or chemical ablation, progenitor cells in the basal compartment of the OE rapidly upregulate proliferation and produce new differentiated ORNs to replenish the damaged epithelium. These observations imply that stem and neuronal progenitor cells, as well as the microenvironment in which they reside, produce signals that stimulate proliferation and differentiation. Moreover, it must also be the case that OE neuronal stem and progenitor cells express intrinsic factors that endow them with the capacity to respond to these signals.
Work from a number of groups has demonstrated that as cells progress through the OE neuronal lineage, they successively express transcription factors that are characteristic of, and required for, differentiation of stem cells into committed neuronal progenitors and, ultimately, ORNs. Thus, expression of these cell-intrinsic factors both provides unique molecular ‘signatures’ for neuronal progenitor cells at specific developmental stages and determines the ultimate fates of these cells. This feature makes the OE an ideal system for studies of the regulatory roles that such transcription factors play in neurogenesis, and also provides the molecular markers needed to decode effects of extrinsic factors, such as signaling molecules, on the regulation of neurogenesis. Indeed, studies of mice with mutations in genes encoding regulatory transcription factors have proved to be extremely informative in understanding the roles that such factors play in regulating ORN and VNO development.
Although no definitive stem cell marker has been found for OE and VNO stem cells, a likely candidate is the transcription factor, Sox2. Sox2, a transcription factor of the SoxB1-type SRY transcription factor family, is thought to be a general neuronal stem cell marker: It is expressed throughout the neural primordium in rodents, is an important regulator of embryonic development, and has been shown to direct neural progenitor identity. Moreover, in a number of neural tissues, Sox2-expressing cells have been shown to be capable of both self-renewal and differentiation, suggesting that Sox2 gene expression is a trait shared by stem cells in many neural systems. During primary neurogenesis in the OE, expression of Sox2 mRNA defines the neuroepithelial domain of the invaginating olfactory pit, and co-expression of Sox2 and Fgf8 has recently been reported to define a population of primordial neural stem cells that will give rise to all subsequent neural stem and progenitor cell types of the OE (Figure 3). Indeed, in mice in which the Fgf8 gene is inactivated in anterior neural structures, this Sox2–Fgf8 co-expressing cell population undergoes apoptosis, leading to a failure in subsequent OE neurogenesis, nasal cavity formation, and morphogenesis of the VNO (see later). Thus, although no stem cell marker has been identified for the VNO, the observation that the VNO fails to develop at all in Fgf8 conditional mutant mice strongly suggests that the Sox2–Fgf8 co-expressing primordial neural stem cells, observed to play a critical role in the early stages of primary neurogenesis in the OE, give rise to the neurogenic population of the VNO as well.
During primary neurogenesis at e10–e11, cells expressing the proneural gene, Mash1, are found in close apposition to Sox2–Fgf8 co-expressing primordial neural stem cells near the rim of the invaginating olfactory pit (Figure 3). A day or so later in development (e12.5), Mash1 mRNA can be detected in cells found in the apical, middle, and basal compartments of the OE, coincident with the location of mitotic figures at this age. As the OE matures and enters the phase of established neurogenesis, Mash1-expressing cells come to be located primarily in the basal compartment of the OE, suggesting that the action of Mash1 is required early in the ORN lineage. Mash1 has been shown in genetic studies to be required for ORN development, and in studies in vitro, in OE cultures, and in vivo, in surgical models of induced neurogenesis, Mash1 has been shown to be expressed by early-stage transit-amplifying progenitors of the ORN lineage (Figure 1(b)). Indeed, in mice with targeted inactivation of the Mash1 gene, Ngn1-expressing INPs, as well as ORNs, fail to develop, indicating that Mash1 acts upstream of Ngn1 to direct neuronal differentiation in the OE.
Recent studies indicate that, in the absence of Mash1 function, the OE reverts to a state in which it maintains high levels of both proliferation and apoptosis. Proliferating cells express Sox2, the Mash1 3′-untranslated region (3′-UTR; which is still present in the targeted mutant), and Steel, a marker of supporting cells. This has led to the hypothesis that these proliferating cells in the OE of Mash1−/− mice are ‘frozen’ at an early stage of differentiation, and the fact that these cells co-express markers of both supporting cells (Steel) and early neuronal progenitors (Mash1 3′-UTR) suggests that they would be capable, if they did not undergo apoptosis (the precipitating cause of neurogenic failure in the Mash1 mutant), of giving rise to both ORNs and supporting cells. These observations provide indirect evidence that the neural stem cell of the OE is a bipotential stem cell, capable of giving rise to both glial and neuronal cell types. Interestingly, neurogenesis also fails in the VNO of Mash1−/− mice, and in the same manner as in main OE: Ngn1 expression fails to occur and neurons fail to form, while the Mash1 3′-UTR is expressed in abundant proliferating cells that undergo high levels of apoptosis. Thus, the developmental hierarchy of gene expression in both the main OE and the VNO appear to be fundamentally similar.
As they progress through the ORN lineage, Mash1-expressing progenitors lose expression of Mash1 and upregulate expression of a different proneural gene, Ngn1. Ngn1 expression defines the immediate neuronal precursor, which has been shown by tissue culture and genetic studies to be committed to ORN differentiation after one to two rounds of division. Evidence for the function of Ngn1 as a neural determination gene first came from studies in Xenopus. In Xenopus, misexpression of an Ngn1 homolog can convert nonneurogenic ectodermal cells to neurons. In Ngn1 mutant OE, most ORNs fail to develop and differentiate (at least by the end of primary neurogenesis at e12.5), suggesting that mammalian Ngn1 plays a role similar to that of its Xenopus counterpart. Moreover, although Ngn1 expression is severely reduced in the OE of Mash1 mutant mice, Mash1 expression is not significantly affected in the OE of Ngn1-null mice, indicating that Mash1 and Ngn1 expression are essential at different stages of differentiation and that Mash1 acts upstream of Ngn1 in the ORN lineage.
A number of other transcription factor genes play roles in regulating neuronal differentiation in the OE, including RunX1 and NeuroD. Runx1 encodes a member of the Runt/Runx family of transcription factors, whereas NeuroD, like Mash1 and Ngn1, encodes a basic helix–loop–helix transcription factor. In the OE, expression of Runx1 and NeuroD is restricted primarily to cells in the basal half of the epithelium. Evidence from developmental genetic studies suggests that NeuroD is expressed at the stage when late, Ngn1-expressing neuronal progenitors are just differentiating into ORNs. Gene expression studies indicate that only a few cells co-express Runx1 and Mash1, whereas virtually all cells expressing NeuroD also express Runx1. Thus, expression data suggest that both Runx1 and NeuroD act at the time when late-stage neuronal progenitors (INPs) are undergoing terminal differentiation into ORNs. OE development in mice with targeted inactivation of the Runx1 gene has only been examined up to the end of the primary phase of neurogenesis, since homozygous nulls die at e12.5. Interestingly, in the OE of e12.5 Runx1−/− embryos, the total number of cells, and the number of Mash1-expressing cells, appear to be unchanged; however, there is a decrease in the number of NeuroD-expressing cells and an increase in cells expressing the early neuronal marker β-III tubulin. Since Runx1 is also known to repress expression of cyclin-dependent kinase inhibitors (which act as ‘brakes’ on mitotic cells in the G1/S transition; see later), these observations have been interpreted as showing a role for Runx1 in regulating NeuroD expression and terminal differentiation of OE neuronal progenitors into postmitotic ORNs. Since NeuroD is expressed in the VNO in a pattern analogous to its expression in OE, by extension it seems possible that Runx1 may function in this tissue as well to regulate sensory neuron differentiation, although this has not yet been investigated.
Control of Neurogenesis by Extrinsic Factors
Developmental transitions in expression of transcriptional regulators, cell proliferation, cell differentiation, and intraepithelial cell location in the OE are directed by the actions of extrinsic signaling molecules. Different signaling molecules appear to predominate during the primary and established phases of neurogenesis, and these factors are produced both within the OE itself and by its underlying mesenchymal stroma (also known as the lamina propria of the mature epithelium). The actions of these secreted signaling molecules in regulating programs of neuronal cell proliferation and differentiation in the OE are classified into two categories: (1) proneurogenic effects, which are positive effects on OE neurogenesis and include stimulation of progenitor cell proliferation and cell survival, and (2) antineurogenic effects, which include suppression of cell proliferation and, directly or indirectly, increases in cell death (apoptosis). Both categories of action are important for OE and VNO morphogenesis and acquisition and maintenance of proper sensory neuron number, and therefore for OE function during development and postnatal life. Over the past several years, studies have revealed that OE neurogenesis is critically dependent on signaling molecules from two different polypeptide growth factor superfamilies, fibroblast growth factors (FGFs) and transforming growth factor-βs (TGF-βs). Each of these superfamilies of signaling molecules includes many members, all of which have specific patterns of expression and differing functions during development and tissue homeostasis. During OE neurogenesis it has been found that different factors from these two superfamilies interact in at least two ways: first, opposing signals converge on neuronal stem and progenitor cells at specific developmental stages in the ORN lineage, regulating proliferation and the stepwise progression of neuronal differentiation. Second, TGF-βs and their secreted antagonists play key roles in feedback loops that regulate the size of progenitor cell pools and the number of ORNs that differentiate from these cell pools. Since these developmental pathways have been worked out almost exclusively in the main OE of rodents, particularly the mouse, the following discussion deals primarily with mouse main OE.
Fibroblast Growth Factors
During OE development, several different FGFs are known to be expressed, and since expression of these FGFs is developmentally regulated, it is thought that the functions of different FGFs predominate at different developmental stages. It has recently been established that FGF8 plays a crucial role in OE development during the early stages of primary neurogenesis. Tissue-specific inactivation of the Fgf8 gene has shown that Fgf8 expression, which is highest in the ectoderm and neuroepithelium that outlines the rim of the invaginating pit at e10–e11 in the mouse, defines a morphogenetic center of function that is crucial for OE neurogenesis, nasal cavity morphogenesis, and development of the VNO. As mentioned previously, a subpopulation of Sox2-expressing neural stem cells, located in the outermost rim of the invaginating OE neuroepithelium, also express Fgf8 (Figure 3); these cells have been termed ‘primordial’ neural stem cells of the OE. In mice with tissue-specific inactivation of Fgf8, these Sox2–Fgf8 co-expressing cells undergo apoptosis (Figure 5(a)). As a result, OE neurogenesis fails, as does nasal cavity formation; indeed, by e14.5, only a small vestige of remaining OE, with just a few neural cells, can be observed – and this is seen only in the least severely affected mutants (Figures 5(b) and 5(c)). These animals also lack any VNO, indicating that FGF8 is a crucial signal for morphogenesis and neurogenesis in the VNO as well as in the main OE. The effects of the neurogenic deficits resulting from absence of FGF8 are long lasting: although animals survive until birth, no OE or VNO ever forms, and only a few, scattered neural cells are observed in the malformed oral–nasal cavity that does develop. These observations indicate that FGF8 is required not only for the survival and expansion of the neural stem cell pool during primary neurogenesis (Figure 5(c)), but also that it is required for maintenance of primary neurogenesis and, as a result, the initiation of the established phase of neurogenesis. Interestingly, Fgf8 expression declines by the end of primary neurogenesis (e12.5), when another closely related FGF, encoded by Fgf18, begins to be expressed within the neuroepithelium of the OE. It has been hypothesized that FGF18 assumes FGF8’s role in maintenance of the stem cell population during the embryonic stages of the established phase of neurogenesis, although this idea has not been tested directly in Fgf18 mutant mice.
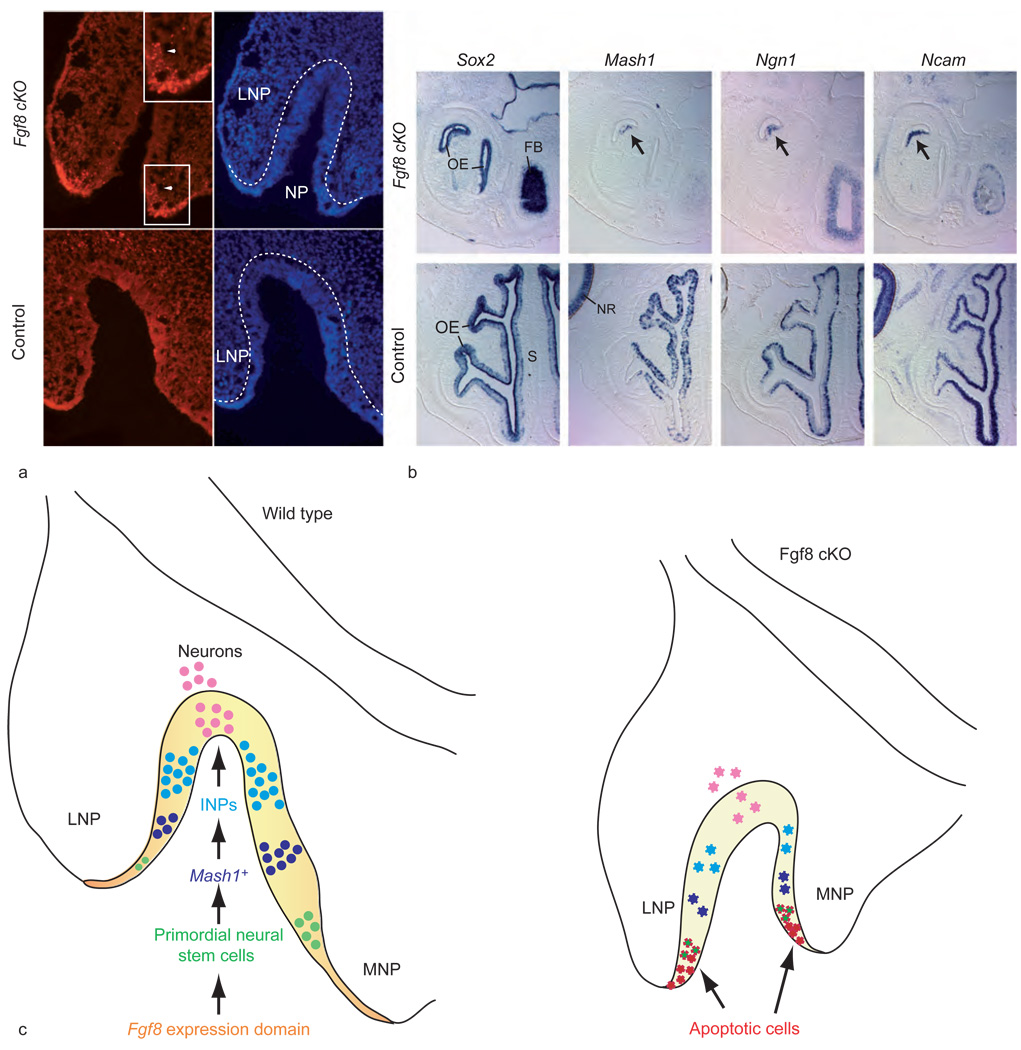
Figure 5.
Fgf8 is required for cell survival in the neurogenic domain. (a) Terminal dUTP nick end labeling (TUNEL) of olfactory epithelium of mutant (cKO; conditional knockout) and control littermates at day 10.5 of gestation shows a high number of apoptotic cells in mutants in ectoderm and olfactory epithelium (white arrowhead; magnified in inset) of invaginating nasal pit (NP). Hoescht panel (blue, right) shows extent of invaginating NP. Broken white line indicates the boundary of the neuroepithelium lining the NP and lateral nasal process (LNP). (b) In situ hybridization on horizontal sections of mutant and control littermates (day 14.5 of gestation) shows the near absence of OE in the mutants and the lack of neuronal markers in the remaining OE (arrow) (FB, forebrain; S, nasal septum; NR, neural retina). (c) Role of Fgf8 in olfactory neurogenesis. Schematic of primary neurogenesis at day 10.5 of gestation in wild-type OE and Fgf8 mutant OE, illustrating the relative positions (MNP, medial nasal process) of the Fgf8 expression domain and different neuronal cell types: Fgf8 expression domain, orange; Sox2-expressing neuroepithelium, yellow; Sox2- and Fgf8-expressing primordial stem cells, green; Mash1-expressing progenitors, blue; immediate neuronal precursors (INPs),turquoise; Ncam-expressing olfactory receptor neurons, pink. In the mutant invaginating pit, cells undergoing apoptosis due to Fgf8 inactivation are shown in red and apoptotic primordial neural stem cells are green with red jagged borders. Diminished populations of other neuronal cell types are shown in their corresponding colors, but with jagged edges. Adapted from Kawauchi S, Shou J, Santos R, et al. (2005) Fgf8 expression defines a morphogenetic center required for olfactory neurogenesis and nasal cavity development in the mouse. Development 132(23): 5211–5223.
Unlike Fgf8, Fgf2 does not appear to be expressed at significant levels in prenatal mouse OE, but in adult mice, it is expressed throughout the neuronal cell layers of the main OE. By 3 weeks of age, FGF2 expression can also be detected in axon bundles that converge below the basal lamina, en route to the olfactory bulb. Like FGF8, FGF2 can also function as a proneurogenic factor: In cultures of OE, addition of FGF2 increases the number of proliferating INPs, by promoting multiple rounds of INP divisions before INP daughter cells undergo terminal differentiation into ORNs; FGF2 has also been shown to be capable of promoting survival and proliferation of putative stem cells in OE cultures. Taken together with the pattern of Fgf2 expression, these observations suggest that FGF2 is likely to be involved in maintaining neuronal stem and progenitor cells, as well as in serving as a mitogen for these cells, in order to regulate ORN number in postnatal OE.
TGF-β Superfamily
Bone morphogenetic proteins
Many studies indicate that the bone morphogenetic proteins (BMPs), a subfamily of TGF-βs structurally related to the Drosophila signaling molecules Dpp and 60A, have important roles in regulating neuronal fate determination and neurogenesis during vertebrate development. For example, endogenous BMP4 promotes acquisition of an epidermal fate, at the expense of neural tissue, in developing ectoderm, and BMP2 and BMP4 have been shown to inhibit proliferation and/or induce apoptosis of neural progenitor cells in several systems. Studies of the OE have shown that BMPs can exert both pro- and antineurogenic effects, depending on the concentration and identity of the BMP in question and the identity of the target cell that is acted upon.
Bmp2, Bmp4, and Bmp7 are all expressed in and/or near OE proper during embryonic development in the mouse, and all three of the secreted proteins (BMP4, BMP2, and BMP7) can inhibit OE neurogenesis in vitro by acting on Mash1-expressing neuronal progenitors. Exposure to any of the three BMPs causes these progenitors to target preexisting Mash1-encoded protein for proteasome-mediated degradation, resulting in apoptosis and termination of the ORN developmental pathway at the Mash1-expressing stage. Interestingly, however, low concentrations of BMP4, but not BMP7, stimulate OE neurogenesis: In this case, the action of BMP4 is to promote survival of newly generated ORNs. Since BMP4 and BMP7 selectively activate different subsets of serine–threonine kinase type I BMP receptors, which are known to be differentially expressed within the OE, it is likely that differential responsiveness of specific OE neuronal stem/progenitor cells to different concentrations of specific BMPs is dictated by the identity of the cell surface receptors that are activated in the various target cell types. The OE is thus proving to be a useful model system for dissecting out the cellular basis of concentration-dependent responses to BMPs, an area of broad interest to both developmental biologists and cancer biologists.
Growth and differentiation factor 11 and feedback inhibition of neurogenesis
Studies in vitro and in vivo have shown that generation of new ORNs by their progenitors is inhibited by a signal produced by OE neuronal cells themselves; this process has been termed feedback inhibition of neurogenesis. Recently, growth and differentiation factor 11 (GDF11), a member of a small subgroup of activin-like TGF-βs that includes the muscle regulatory factor GDF8 (also called myostatin), has been shown to mediate feedback inhibition of INP proliferation during the established phase of OE neurogenesis. Gdf11 is expressed by both committed neuronal progenitors and newly differentiated ORNs in the OE, as are its serine–threonine kinase transmembrane receptors (ALK5 and ActRIIb). Both Gdf11 and the gene that encodes its secreted antagonist, follistatin (FST), are first expressed at significant levels at the onset of the established phase of neurogenesis, and their expression continues through adult life (Gdf11 is expressed only within OE neuroepithelium proper; Fst is expressed in OE stroma and, at lower levels, in OE proper). Recombinant GDF11 inhibits OE neurogenesis in vitro, by reversibly arresting INP divisions; this action is accompanied by induction of the cyclin-dependent kinase inhibitor p27Kip1 in these neuronal progenitors. The antiproliferative effect of GDF11 on INPs is abrogated by addition of FST to OE cultures, but, interestingly, GDF11 actions predominate over the proliferative effect of FGFs on INPs. Gdf11-null mice show increased cell proliferation in the OE, and the number of Ngn1-expressing INPs is increased, as is overall OE thickness, relative to that of wild-type control littermates. These super-numerary INPs go on to give rise to ORNs, as demonstrated by an increase in Ncam-expressing cells in Gdf11-null OE (Figure 6(a)). FST, a secreted protein that prevents effective binding and signaling by GDF11, is able to completely antagonize the antineurogenic effect of GDF11 on INPs in OE cultures. Importantly, the number of INPs and ORNs is dramatically decreased in Fst−/− OE, consistent with what would be expected with removal of a crucial brake on the antineurogenic actions of endogenous GDF11 in the OE neuroepithelium (Figure 6(b)). These observations have led to the proposal of a model in which GDF11 and FST interact to maintain neuron number in the OE by tightly regulating INP divisions and ORN production. Since GDF11 arrests INP proliferation but does not kill INPs, its role in feedback inhibition of OE neurogenesis is likely to be one of maintaining INPs in stasis, such that they are capable of responding rapidly to a decrease in ambient GDF11 levels (as when OE neuronal cells are lost through injury or disease) by rapidly reentering the cell cycle and giving rise to ORNs (Figure 6(c)).
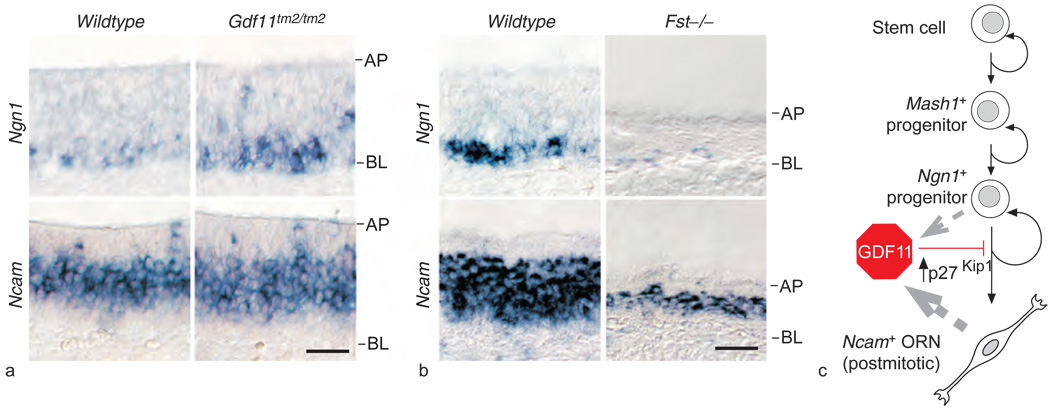
Figure 6.
Disruption of neurogenesis in mice with loss or gain of function of Gdf11. (a) In situ hybridization on horizontal sections (AP, apical layer; BL, basal layer) of olfactory epithelium of Gdf11tm2/tm2 mice reveals an increase in Ngn1-expressing cells and a corresponding increase in Ncam-expressing cells: the Ncam-expressing cell layer is thicker by 20% (9 µm), about the diameter of one olfactory receptor neuron (ORN), in Gdf11tm2/tm2 olfactory epithelium, compared to in wild type. (b) Mice lacking a functional follistatin (Fst) gene show decreased olfactory epithelium neurogenesis. In situ hybridization for Ngn1 and Ncam shows large decreases in expression of both markers as well as aberrantly thin olfactory epithelium in the Fst mutant. (c) Schematic model of growth and differentiation factor 11 (GDF11) action in ORN neurogenesis. GDF11 is produced by both Ngn1+ progenitors and Ncam+ ORNs (gray broken-line arrows). GDF11 reversibly arrests Ngn1+ progenitors through induction of the cycle-dependent kinase inhibitor p27Kip1, thus preventing ORN generation. Reproduced from Wu HH, Ivkovic S, Murray RC, et al. (2003) Autoregulation of neurogenesis by GDF11. Neuron 37: 197–207, with permission from Elsevier.
Summary and Conclusions
In addition to revealing major steps in morphogenesis and neurogenesis of the OE and VNO, studies of the development of these two sensory neuroepithelia have revealed important aspects of the molecular regulation of neurogenesis. Studies of main OE development in particular have led to the concept that neurogenesis in olfactory sensory epithelia proceeds in a linear manner, with expression of specific transcription factors marking the progressive restriction in fate observed as neuronal stem and committed progenitor cells differentiate through the ORN developmental pathway. Both these intrinsic signals, which operate in a cell-autonomous manner, and extrinsic (secreted) signals present in the local microenvironment of developing cells (the stem/progenitor cell niche) play important roles in regulating this process. The factors that predominate during primary olfactory neurogenesis play an expansionary, morphogenetic role. For example, FGF8, which is crucial for increasing the size of the stem cell pool, is of vital importance at this stage in order to establish the ORN lineage in the main OE and to permit formation of the VNO (Figure 7). Once the OE neurogenesis enters its established phase, both FGFs and TGF-βs participate in regulating neuronal cell number. Studies of the actions of TGF-βs and their secreted antagonists in regulating OE neurogenesis have led to the concept of feedback inhibition of neurogenesis, in which feedback circuits consisting of endogenous secreted antineurogenic factors regulate the size of the stem/progenitor cell pool and total neuron number. Ultimately, determining whether similarities in these processes exist between the OE and other regions of the nervous system should provide important knowledge for understanding how neuronal stem/progenitor cell pools are controlled in all neural tissues, and for overcoming roadblocks to recovery in regions of the adult nervous system where – unlike the OE – neuronal regeneration is limited or absent.
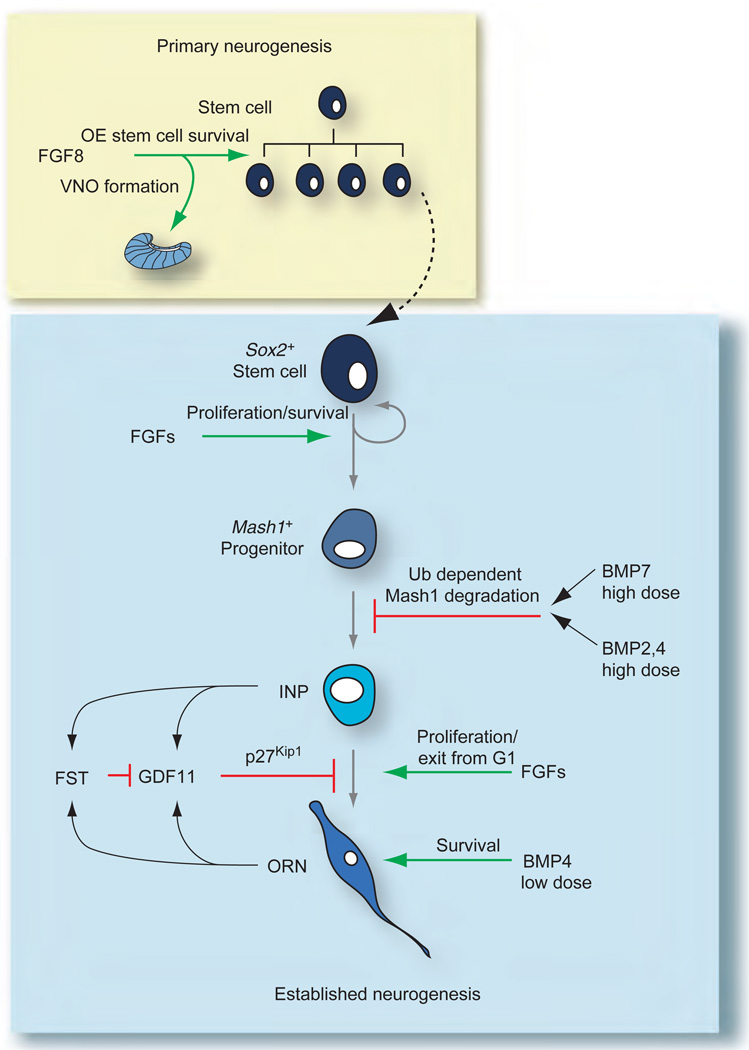
Figure 7.
Summary of molecular signals involved in both primary and established olfactory epithelium (OE) neurogenesis. During primary neurogenesis (yellow box), fibroblast growth factor 8 (FGF8) and other factors are involved in producing the stem cell pool and initially establishing the different progenitors in the olfactory receptor neuron lineage. FGF8 is also necessary for initial vomeronasal organ (VNO) formation. After day 12.5 of gestation, once the lineage is established (blue box), FGFs and transforming growth factor-βs (BMP, bone morphogenetic protein; FST, follistatin; GDF, growth and differentiation factor) converge on different cell types in the OE lineage to achieve and maintain proper neuron number. Green arrows indicate stimulatory interactions; red bars indicate inhibitory interactions. Adapted from Kawauchi S, Beites CL, Crocker CE, et al. (2004) Molecular signals regulating proliferation of stem and progenitor cells in mouse olfactory epithelium. Developmental Neuroscience 26: 166–180, with permission. Copyright 2004 by S. Karger AG, Basel, Switzerland.
Footnotes
See also: Drosophila Apterous Neurons: from Stem Cell to Unique Neuron.
Further Reading
- Beites CL, Kawauchi S, Crocker CE, et al. Identification and molecular regulation of neural stem cells in the olfactory epithelium. Experimental Cell Research. 2005;306:309–316. doi: 10.1016/j.yexcr.2005.03.027. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S, Bronner-Fraser M. Hierarchy of regulatory events in sensory placode development. Current Opinion in Genetics & Development. 2004;14:520–526. doi: 10.1016/j.gde.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Buck L, Axel R. A novel multigene family may encode odorant receptors: A molecular basis for odor recognition. Cell. 1991;65:175–187. doi: 10.1016/0092-8674(91)90418-x. [DOI] [PubMed] [Google Scholar]
- Caggiano M, Kauer JS, Hunter DD. Globose basal cells are neuronal progenitors in the olfactory epithelium: A lineage analysis using a replication-incompetent retrovirus. Neuron. 1994;13:339–352. doi: 10.1016/0896-6273(94)90351-4. [DOI] [PubMed] [Google Scholar]
- Cau E, Gradwohl G, Fode C, et al. Mash1 activates a cascade of bHLH regulators in olfactory neuron progenitors. Development. 1997;124:1611–1621. doi: 10.1242/dev.124.8.1611. [DOI] [PubMed] [Google Scholar]
- Chang H, Brown CW, Matzuk MM. Genetic analysis of the mammalian transforming growth factor-beta superfamily. Endocrine Reviews. 2002;23:787–823. doi: 10.1210/er.2002-0003. [DOI] [PubMed] [Google Scholar]
- Dulac C, Torello AT. Molecular detection of pheromone signals in mammals: From genes to behaviour. Nature Reviews Neuroscience. 2003;4:551–562. doi: 10.1038/nrn1140. [DOI] [PubMed] [Google Scholar]
- Farbman A. Cell Biology of Olfaction. Cambridge, UK: Cambridge University Press; 1992. [Google Scholar]
- Guillemot F, Lo C-C, Johnson JE, et al. Mammalian achaete-scute homolog 1 is required for the early development of olfactory and autonomic neurons. Cell. 1993;75:463–476. doi: 10.1016/0092-8674(93)90381-y. [DOI] [PubMed] [Google Scholar]
- Halpern M, Martinez-Marcos A. Structure and function of the vomeronasal system: An update. Progress in Neurobiology. 2003;70:245–318. doi: 10.1016/s0301-0082(03)00103-5. [DOI] [PubMed] [Google Scholar]
- Itoh N, Ornitz DM. Evolution of the Fgf and Fgfr gene families. Trends in Genetics. 2004;20:563–569. doi: 10.1016/j.tig.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Kawauchi S, Beites CL, Crocker CE, et al. Molecular signals regulating proliferation of stem and progenitor cells in mouse olfactory epithelium. Developmental Neuroscience. 2004;26:166–180. doi: 10.1159/000082135. [DOI] [PubMed] [Google Scholar]
- Kawauchi S, Shou J, Santos R, et al. Fgf8 expression defines a morphogenetic center required for olfactory neurogenesis and nasal cavity development in the mouse. Development. 2005;132(23):5211–5223. doi: 10.1242/dev.02143. [DOI] [PubMed] [Google Scholar]
- Mombaerts P. How smell develops. Nature Neuroscience. 2001;4(supplement):1192–1198. doi: 10.1038/nn751. [DOI] [PubMed] [Google Scholar]
- Murray RC, Calof AL. Neuronal regeneration: Lessons from the olfactory system. Seminars in Cell and Developmental Biology. 1999;10:421–431. doi: 10.1006/scdb.1999.0329. [DOI] [PubMed] [Google Scholar]
- Murray RC, Navi D, Fesenko J, et al. Widespread defects in the primary olfactory pathway caused by loss of Mash1 function. Journal of Neuroscience. 2003;23:1769–1780. doi: 10.1523/JNEUROSCI.23-05-01769.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolay DJ, Doucette JR, Nazarali AJ. Transcriptional regulation of neurogenesis in the olfactory epithelium. Cellular and Molecular Neurobiology. 2006;26:801–819. doi: 10.1007/s10571-006-9058-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pevny L, Placzek M. SOX genes and neural progenitor identity. Current Opinion in Neurobiology. 2005;15:7–13. doi: 10.1016/j.conb.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Vincent AJ, West AK, Chuah MI. Morphological and functional plasticity of olfactory ensheathing cells. Journal of Neurocytology. 2005;34:65–80. doi: 10.1007/s11068-005-5048-6. [DOI] [PubMed] [Google Scholar]
- Wu HH, Ivkovic S, Murray RC, et al. Autoregulation of neurogenesis by GDF11. Neuron. 2003;37:197–207. doi: 10.1016/s0896-6273(02)01172-8. [DOI] [PubMed] [Google Scholar]