Abstract
Background:
Diagnosing narcolepsy without cataplexy is often a challenge as the symptoms are nonspecific, current diagnostic tests are limited, and there are no useful biomarkers. In this report, we review the clinical and physiological aspects of narcolepsy without cataplexy, the limitations of available diagnostic procedures, and the differential diagnoses, and we propose an approach for more accurate diagnosis of narcolepsy without cataplexy.
Methods:
A group of clinician-scientists experienced in narcolepsy reviewed the literature and convened to discuss current diagnostic tools, and to map out directions for research that should lead to a better understanding and more accurate diagnosis of narcolepsy without cataplexy.
Recommendations:
To aid in the identification of narcolepsy without cataplexy, we review key indicators of narcolepsy and present a diagnostic algorithm. A detailed clinical history is mainly helpful to rule out other possible causes of chronic sleepiness. The multiple sleep latency test remains the most important measure, and prior sleep deprivation, shift work, or circadian disorders should be excluded by actigraphy or sleep logs. A short REM sleep latency (≤ 15 minutes) on polysomnography can aid in the diagnosis of narcolepsy without cataplexy, although sensitivity is low. Finally, measurement of hypocretin levels can helpful, as levels are low to intermediate in 10% to 30% of narcolepsy without cataplexy patients.
Citation:
Baumann CR, Mignot E, Lammers GJ, Overeem S, Arnulf I, Rye D, Dauvilliers Y, Honda M, Owens JA, Plazzi G, Scammell TE. Challenges in diagnosing narcolepsy without cataplexy: a consensus statement. SLEEP 2014;37(6):1035-1042.
Keywords: narcolepsy, insomnia, multiple sleep latency test, polysomnography
1. INTRODUCTION
Narcolepsy with Cataplexy is usually easy to diagnose as cataplexy is often distinctive and occurs in almost no other conditions. In contrast, Narcolepsy without Cataplexy is often a challenging diagnosis, even for highly experienced clinicians. This uncertainty arises from the nonspecific nature of the symptoms, the limitations of our current diagnostic tests, and the lack of useful biomarkers. The problem is further compounded by the fact that in some patients, narcolepsy may evolve over time; for example, patients in whom daytime sleepiness is the sole initial manifestation may develop cataplexy many years later.
To advance understanding of Narcolepsy without Cataplexy, a group of clinician scientists experienced in narcolepsy convened in June 2012 to discuss these diagnostic challenges. The participants have all published extensively on narcolepsy, and the meeting (but not the writing of this paper) was funded by Jazz Pharmaceuticals. This meeting helped clarify aspects of this disorder and inspired us to write this manuscript.
The goals of this paper are to review briefly what is known about Narcolepsy without Cataplexy, to discuss the usefulness and limitations of current diagnostic tools, and to suggest directions for research that should lead to a better understanding and more accurate diagnosis of Narcolepsy without Cataplexy.
2. BASICS OF NARCOLEPSY WITHOUT CATAPLEXY
2.1. Current diagnostic criteria
In this paper, we use the new terms Narcolepsy type 1 (Na-1) and Narcolepsy type 2 (Na-2) in keeping with the revised nomenclature of the 3rd Edition of the International Classification of Sleep Disorders (ICSD-3). Na-1 includes Narcolepsy with Cataplexy, and Na-2 is simply Narcolepsy without Cataplexy.
In the ICSD-3, Na-1 is defined as excessive daytime sleepiness (EDS) and at least one of the following criteria: (a) cataplexy and a positive MSLT, or (b) hypocretin deficiency. A MSLT is considered positive if the mean sleep latency is ≤ 8 minutes and there are 2 or more sleep onset REM periods (SOREMP). Hypocretin deficiency is defined as cerebrospinal fluid (CSF) hypocretin-1 level < 1/3 of normal or ≤ 110 pg/mL if a Stanford reference sample is used for radioimmunoassay. This new definition is much like that of the ICSD-2 except that low CSF hypocretin plays a more important role in the diagnosis of Na-1, and the counting/analysis of SOREMPs can now include a SOREMP within 15 minutes of sleep onset on the preceding nocturnal polysomnogram (PSG). Usually, the diagnosis of Na-1 is straightforward as definite cataplexy can often be elicited by a careful history and low hypocretin levels are highly specific for narcolepsy.1 A thoughtful approach usually leads to high diagnostic certainty in Na-1 patients.
In contrast, the diagnosis of Na-2 remains challenging. In the ICSD-3, Na-2 is defined by chronic sleepiness plus a positive MSLT as defined above. Of course, the patient must also lack clear cataplexy, and if CSF is tested, hypocretin-1 levels are in the normal range. Thus, the diagnosis of Na-2 critically hinges on clinical findings and the MSLT, yet as discussed below, the MSLT has limitations and a positive MSLT is not specific for narcolepsy.
2.2. Epidemiology
Little is known about the epidemiology of Na-2, but two groups independently examined the population prevalence of a positive MSLT result, and their findings are corroborative. In one sample of 539 subjects, 3.9% had 2 or more SOREMPs, and 2.5% of subjects met MSLT criteria for narcolepsy with mean sleep latencies ≤ 8 minutes plus ≥ 2 SOREMPs.2 These findings suggest that in the general population, SOREMPs are not uncommon, especially in subjects with EDS.
A separate cohort study of 556 subjects found a surprisingly high prevalence of positive MSLTs, especially in men3: 13.1% of men had ≥ 2 SOREMPs, and 4.1% met the criteria for narcolepsy with ≥ 2 SOREMPs, a mean sleep latency ≤ 8 minutes, and subjective sleepiness (Epworth Sleepiness Scale score > 10). These findings were especially common in shift workers. In contrast, 5.6% of women had ≥ 2 SOREMPs, but only 0.4% had positive MSLTs plus subjective sleepiness. It is possible that some of these subjects have Na-2, but none had cataplexy. A more likely explanation is that the MSLT can produce false-positive results, especially in shift workers.
Very little is known about the natural history of Na-2. Andlauer and colleagues classified 171 narcolepsy without cataplexy subjects by their CSF hypocretin levels: 24% had low, 8% had intermediate, and 68% had normal levels.4 To avoid suspected confounding factors, subjects with shift work, insufficient sleep, or untreated sleep-disordered breathing were excluded. They found that 33% of the subjects with low hypo-cretin levels at baseline developed cataplexy an average of 14.7 years after sleepiness onset. In those with intermediate levels, 18% developed cataplexy, but among those with normal levels, cataplexy occurred in only one subject. This study demonstrates that hypocretin deficient patients may lack cataplexy when first evaluated and develop it many years later. Whether this is caused by progressive injury to the hypocretin-producing neurons, maladaptive changes in brain function, or another process remains unknown. Perhaps in some individuals hypo-cretin deficiency develops slowly, so that the > 90% hypocretin cell loss observed at autopsy in individuals with cataplexy is only achieved after a long evolution. Supporting this hypothesis, a diagnostic cut off of 200 pg/mL rather than 110 pg/ mL performed slightly better in narcolepsy without cataplexy, although a minority were positive.
2.3. Neuropathology of narcolepsy without cataplexy
Though researchers have learned much about the pathology of Na-1, very little is known about the underlying cause(s) of Na-2. Among patients with narcolepsy, hypocretin levels are low or undetectable in over 90% of those with cataplexy but in only about 10% to 24% of those lacking cataplexy.1,5 In subjects with Na-1, several postmortem studies have consistently demonstrated a severe (> 85%) loss of the hypo-cretin-producing neurons across the hypothalamus.6–11 Thus far, only one complete hypothalamus from a subject with Na-2 has been studied, and this individual had only a 33% loss of the hypocretin neurons.12 Interestingly, the cell loss was restricted to the posterior part of the hypocretin field, suggesting a more focal attack on the hypocretin neurons than in Na-1. The density of hypocretin axons in the anterior hypothalamus was normal in this and a second Na-2 subject, suggesting that with partial injury to the hypocretin neurons, axonal sprouting by the remaining neurons may help compensate. These observations indicate that at least some Na-2 cases may simply be caused by less extensive injury to the hypocretin neurons, but clearly there is a need for additional neuropathological evaluations of many more individuals with Na-2.
In contrast to Na-1, there is no biomarker for Na-2, and its pathophysiology remains elusive. Some researchers debate whether Na-2 exists as a distinct entity or instead lies in the borderland between Na-1 and idiopathic hypersomnia. Other investigators argue that Na-2 exists as a specific disease because patients have chronic sleepiness, persuasive MSLT findings, positive HLA typing, and frequent hypnagogic hallucinations, without sleep inertia or chronic sleep deprivation. On the other hand, the observation that cataplexy can appear years after the onset of sleepiness demonstrates that in some patients, Na-2 can be an early form of Na-1. As the symptoms of Na-2 overlap with other disorders, this controversy will likely persist until useful biomarkers can be identified.
3. LIMITATIONS OF CURRENT DIAGNOSTIC PROCEDURES
3.1. Clinical history
Chronic daytime sleepiness is the key symptom of Na-2; it is often severe and occurs every day. Daytime naps are usually short (< 30 min), restorative, and often include dreaming. Patients score high on the Epworth Sleepiness Scale. In a Japanese series, the mean ESS (14.9 ± 3.5) in 62 patients with Na-2 was similar to the mean ESS (14.6 ± 3.7) of 52 patients with Na-1.13 A similar finding was observed in a French series (ESS: 19 ± 3.3 in 54 patients with Na-1 vs. 17.5 ± 2.7 in 46 patients with Na-2).14 Of course, EDS can be caused by a wide variety of sleep disorders, but ESS scores are usually lower, except for psychiatric disorders.
Hypnagogic or hypnopompic hallucinations with or without sleep paralysis occur in about 28% of Na-2 patients,14 but these symptoms also occur in the general population. For example, a cohort study of 36,533 subjects revealed an overall 7.6% lifetime prevalence of sleep paralysis, with much higher prevalence in students (28.3%).15 Hypnagogic hallucinations were also common (24.8%) in a European cohort of 13,057 subjects, whereas hypnopompic hallucinations were less common (6.6%).16 In 90% of these subjects, however, hypnagogic hallucinations occurred less than once per month.16
Similarly, in a small community sample, all subjects with a lifetime occurrence of sleep paralysis recalled not more than 3 events in total.17 Among patients with narcolepsy, in contrast, the frequency of these events is much higher: 54 patients with Na-1 reported a mean frequency of 10 hallucinations (sometimes with sleep paralysis) per month, and 46 patients with Na-2 had an average of 3 episodes each month.14 Frequent hallucinations and sleep paralysis can also occur in patients with psychiatric disorders.14,15 Thus, hallucinations or paralysis during sleep may be useful for diagnosing Na-2 if they occur frequently in the absence of psychiatric disorders.
A good clinical history is also helpful in identifying alternative diagnoses. For instance, patients who report a sleep need of more than 10 hours per day and sleep inertia are more likely to have idiopathic hypersomnia. Shift-workers or young adults with irregular bedtimes may suffer from insufficient sleep syndrome. Patients with mood disorders may score high on the ESS, but most often have normal or borderline MSLT values. The history might also reveal symptoms suggestive of a neurological or systemic disorder causing sleepiness or tiredness.
Na-2 is likely in patients with severe EDS plus frequent sleep paralysis or hallucinations, but the clinical history is mainly helpful to rule out other possible causes of sleepiness.
3.2. Multiple sleep latency test
The diagnosis of Na-2 depends on the MSLT, but this test has many limitations.18 One large cohort study showed that about 4% of men had ≥ 2 SOREMPs, a mean sleep latency ≤ 8 minutes, and subjective sleepiness.3 Most likely, the majority of these MSLTs were falsely positive due to factors such as shift work, insufficient sleep, sleep apnea, or medications. Recent discontinuation of antidepressants or stimulants that suppress REM sleep may also produce multiple SOREMPs. Unfortunately, there are few controlled studies that document how often such findings confound interpretations and how they might be best minimized.
Conversely, the MSLT may be falsely negative in a significant portion of subjects clinically suspected to have narcolepsy. In a recent study, Andlauer found that the MSLT was falsely negative in 7% of Na-1 patients.19 In contrast, the false negative rate in Na-2 is unknown since the MSLT results are part of the definition.20 Sometimes the MSLT may be negative due to poor sleep from environmental factors, anxiety, older age, or medications that interfere with sleep.
These weaknesses result in low test-retest reliability of the MSLT. Among 18 individuals with Na-2 who had two MSLTs, fewer than half showed positive MSLT results on both studies.19 In addition, a recent study of 36 patients with Na-2 and other CNS hypersomnias showed that after a second MSLT, differences in mean sleep latencies and number of SOREMPs spurred a change in diagnosis in 53% of patients.21 Without a reliable biomarker for Na-2, it is currently difficult to establish the false positive and false negative rates for the MSLT in identifying Na-2. Repeating the MSLT to reveal consistent results is likely useful.
Some of the variability in MSLT results may arise from methodological problems. The AASM has published clear guidelines for performing the MSLT,22 but nevertheless, specific protocols often differ between sleep labs (e.g., the quantity of the prior night's sleep, the timing of the first nap in relation to habitual sleep times and duration, performing 4 vs. 5 naps, optimal nap durations, rigor in ensuring wakefulness between nap opportunities, methods to score SOREMPs). Scoring of SOREMPs can be a challenge due to the occurrence of saccadic eye movements or atonia in NREM sleep, and comparison to REM sleep on the PSG is often helpful. In addition, chronic insufficient sleep is common, yet most labs do not use actigraphy or even sleep logs prior to MSLT. In the same line, it is unclear whether the minimum of 6 hours sleep during the preceding nocturnal polysomnography is sufficient for all adults or whether this constitutes physiologically significant acute sleep deprivation.22 Also, medications such as antidepressants or other psychotropic drugs may significantly affect REM sleep for weeks or months after discontinuation, but management of these medications is not uniformly defined for MSLT protocols.
Age and sex also influence the MSLT. With advancing age, the number of SOREMPs decreases and the mean sleep latency increases in Na-1.23 For instance, mean sleep latency is lower in adolescents with narcolepsy (3.6 min) compared to narcolepsy patients above 65 years (5.2 min), and the mean number of SOREMPs decreases from 3.7 to 2.8. In the Wisconsin cohort study, positive MSLT results were much more common in men, though the reasons for this are unknown.3
To provide some insights into MSLT parameters in patients reporting sleepiness, we selected two recent studies performing MSLT with the AASM guidelines. These studies also performed 2 weeks of actigraphy or sleep logs plus nocturnal PSG prior to MSLT to rule out confounding disorders (Table 1).24,25 These studies show that SOREMPs are very common in narcolepsy, but they also occur with insufficient sleep and a variety of neurological disorders. In addition, these studies suggest that the sequence of sleep stages on MSLT may be diagnostically helpful, as SOREMPs commonly follow N1 sleep in narcolepsy but not in other disorders (in which REM sleep commonly follows N2 or N3). These rapid transitions into REM sleep may reflect the REM sleep dysregulation of narcolepsy, and if supported by additional research, a more refined definition of SOREMPs might improve their diagnostic usefulness.
Table 1.
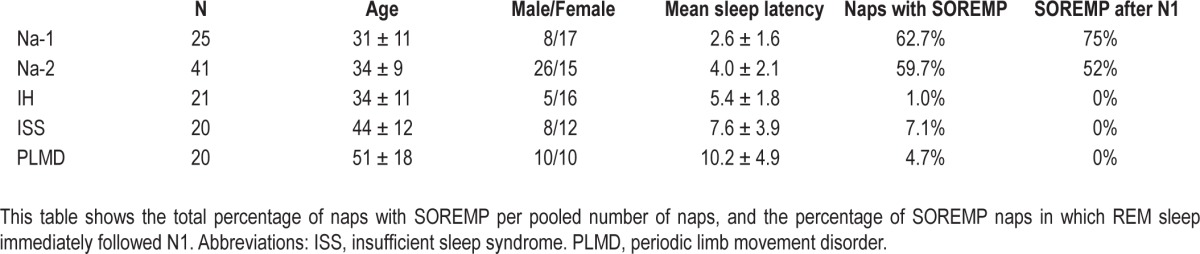
Though it has several limitations, the MSLT remains the most important test for the diagnosis of Na-2. It should be performed according to the AASM guidelines, and prior sleep deprivation or circadian disorders should be excluded using 2 weeks actigraphy or if unavailable, using sleep logs. In this line, patients should be allowed their habitual sleep timing and duration during the preceding PSG. Analyzing whether SOREMPs follow N1 on the MSLT might improve diagnostic specificity, but studies in larger samples are needed to confirm this.
3.3. Nocturnal polysomnography
In the evaluation of possible narcolepsy, the PSG is mainly helpful to rule out other potential causes of sleepiness and to evaluate sleep quality and amount, but a rapid entry into REM sleep can support the diagnosis of narcolepsy. In ICSD-3, REM sleep within 15 minutes of sleep onset on the preceding nocturnal PSG may be included toward the count of ≥ 2 SOREMP on the MSLT. This change in the definition of SOREMPs is based on the finding that a REM latency ≤ 15 minutes on PSG occurs in 51% of patients with Na-1 and has a high diagnostic specificity.19 In addition, REM sleep latencies ≤ 15 minutes during the PSG occur in 64% of Na-2 patients with low hypocretin levels and in 23% of Na-2 patients with normal hypocretin levels.4 Such short REM sleep latencies on PSG were very rare (< 1%) in the Wisconsin cohort,3 suggesting that short REM latency may be closely associated with hypocretin deficiency. It would be helpful to further examine the diagnostic usefulness of a PSG SOREMP in additional, large, ethnically and geographically diverse groups of sleep-satiated normal subjects and patients with sleep disorders. In the future, a PSG SOREMP might make the MSLT unnecessary in some patients, ultimately reducing the labor and cost of diagnosis.4
Sleep fragmentation is also common in Na-2, and future studies should also examine the diagnostic usefulness of low sleep efficiency and increased arousal indices.
A short REM sleep latency (≤ 15 minutes) on the PSG can aid in the diagnosis of Na-2, although sensitivity is low. Polysomnography is also important to rule out other causes of sleepiness.
3.4. Sleep logs and actigraphy
Sleep logs, actigraphy, and other ancillary tools can strengthen the diagnosis of Na-2 by providing long-term information about the timing and amounts of sleep. Shift work is associated with multiple SOREMPs and short sleep latencies,3 and 15% of chronically sleep deprived subjects have short mean sleep latencies and SOREMPs.26 Sleep logs are an inexpensive method to determine if a subject has obtained adequate sleep, but they may not be reliable, as some patients do not provide complete data and many overestimate their amount of sleep. Actigraphy is much more objective than sleep logs, and 1 to 2 weeks of actigraphy prior to MSLT is strongly recommended. Unfortunately, there is debate about the best methods, and actigraphy is not clinically reimbursed in many countries. Still, these tools should be used whenever possible to provide evidence of a concurrent circadian disorder and to demonstrate that patients have obtained an adequate amount of sleep prior to their sleep studies.
Actigraphy or at least sleep logs should be performed prior to sleep studies to rule out chronic sleep deprivation, abnormal phase of the circadian cycle, or shift work which might produce MSLT findings resembling Na-2.
3.5. Hypocretin level determination in cerebrospinal fluid
CSF hypocretin levels are low (< 110 pg/mL, when using Stanford reference samples) in 87% to 96% of patients with Na-1, and as this test has high specificity and sensitivity in this population, it can be used for diagnosis according to the ICSD-3.1,5,27 However, in patients with suspected Na-2, there has been less interest in measuring hypocretin, as levels are usually normal. In a recent study of 171 narcolepsy without cataplexy patients, Andlauer and colleagues categorized hypocretin levels as low (< 110 pg/mL), intermediate (110-200 pg/mL), or normal (> 200 pg/mL).4 They found that 24% of these individuals had low levels, 8% had intermediate levels, and 68% had normal levels. Using receiver operator curve analysis, they determined that a hypocretin level below 200 pg/mL predicted Na-2 with a specificity of 99%, but because reduced hypo-cretin is uncommon in this population, the sensitivity was only 33%. Further, the sample was highly selected, so positivity for this test is likely much lower in randomly selected narcolepsy without cataplexy patients in most sleep clinics.
The Andlauer study suggests that CSF hypocretin is most likely to be < 200 pg/mL in patients who are HLA DQB1*06:02 positive, developed narcolepsy at a young age, or are African American.4 Patients with reduced hypocretin were also more likely to have a short REM latency (< 15 min) on the PSG and more SOREMPs on the MSLT.
In HLA-positive patients with chronic sleepiness but no cataplexy and with positive MSLT results, hypocretin levels can help confirm the diagnosis of narcolepsy and distinguish between Na-1 and Na-2. Low CSF hypocretin-1 in the absence of cataplexy may be more common in African Americans.
3.6. Genetic testing
Human leukocyte antigen (HLA) DQB1*06:02 is found in 85% to 95% of Na-1 patients.28 The risk to develop narcolepsy is 7 to 25-fold higher in subjects heterozygous for this genotype, and homozygosity for HLA-DQB1*06:02 increases the risk an additional 2 to 4-fold.29 However, only about 40% to 50% of Na-2 patients carry DQB1*06:02.4,30 As 12% to 38% of the general population is DQB1*06:02 positive, testing for this allele is not very helpful for diagnosing Na-2.28
Other genes have been linked to Na-1, and some such as the T cell receptor α polymorphism have been examined in a variety of populations.31 Whether these genes are useful for the diagnosis of either Na-1 or Na-2 remains to be established.
HLA DQB1*06:02 typing is not highly specific for Na-2 and is not generally recommended. However, in patients with other evidence for Na-2, the presence of DQB1*06:02 may slightly strengthen diagnostic certainty.
4. DIFFERENTIATING NARCOLEPSY WITHOUT CATAPLEXY FROM OTHER SLEEP DISORDERS
Ruling out other sleep disorders is a major aspect of diagnosing Na-2. As the symptoms and lab findings of Na-2 can be nonspecific, one must thoroughly search for evidence of other disorders. Narcolepsy without cataplexy is a diagnosis of exclusion.
4.1. Idiopathic hypersomnia without long sleep time
Idiopathic hypersomnia (IH) is a likely diagnosis in a sleepy adult who sleeps more than 10 hours each night, but in the absence of a long sleep time, the distinction between IH and Na-2 mainly relies on the absence of SOREMPs on MSLT.13
Additional but not well-validated findings may also help but will not overrule MSLT results. First, nighttime sleep and naps are usually refreshing in narcolepsy patients, whereas many patients with IH suffer from sleep inertia, i.e., a subjective feeling of grogginess upon awakening. In a study of 77 patients with IH, Anderson and colleagues found that sleep inertia is insensitive (55%) but highly specific (97%) for IH.32 Another study in 75 patients, however, showed that sleep inertia is much rarer in IH patients without long sleep time than in those with long sleep time.33 Furthermore, long (> 60 min) and generally unrefreshing naps are 87% sensitive and specific for IH rather than narcolepsy.32
Second, sleep efficiency on the PSG is generally higher (often > 95%) in IH than in narcolepsy. In addition, IH is thought to respond less well to medications, though evidence for this is weak, with a recent study showing similar responses to modafinil in IH and Na-1 patients.34
Three features can help distinguish idiopathic hypersomnia from Na-2: (1) absence of multiple SOREMPs on MSLT, (2) presence of long habitual sleep periods, long naps, and sleep inertia, and (3) high sleep efficiency on PSG.
4.2. Insufficient sleep syndrome
Chronic sleep deprivation, defined as insufficient sleep syndrome (ISS), is defined by chronic sleepiness, short habitual sleep episodes, and sleep rebound during weekends or holidays. In addition to short mean sleep latencies on MSLT, about one in six ISS subjects have multiple SOREMPs.26,35,36
While multiple SOREMPs alone may be insufficient to discriminate ISS from Na-2, the sequence of sleep stages on MSLT can be helpful. In Na-1, about 75% of SOREMPs immediately follow N1 sleep, and this same pattern occurs with 52% of SOREMPs in Na-2. In contrast, with ISS, nearly all SOREMPs follow N2 sleep, with fewer than 15% after N1 sleep.24,26
Two key findings can help discriminate ISS from Na-1: (1) longer sleep duration on weekends and holidays compared to weekdays, as assessed by sleep logs or actigraphy; and (2) if SOREMPs occur on the MSLT, they usually follow N2 sleep.
4.3. Shift work and phase delay
Shift work commonly leads to circadian phase delay and chronic sleep deprivation that can affect the MSLT. The Wisconsin cohort study (n = 556) revealed a strong correlation between SOREMPs on the MSLT and shift work in men (OR = 29 for multiple SOREMPs).3 Most likely, the neurophysiological basis for this phenomenon is that REM sleep is normally under tight circadian control, and phase changes from shift work permit REM sleep to occur during the daytime.37 With mild phase delay of several hours, one would predict that SOREMPs would be more likely in the morning MSLT naps, but whether the timing of SOREMPs helps distinguish between phase delay and Na-2 has not yet been tested. Still, phase delay and especially shift work must be considered when dealing with sleepy patients with multiple SOREMPs on MSLT.
In addition to a careful history, actigraphy or sleep logs might be the best measures to discriminate shift work from Na-2.
4.4. Secondary narcolepsy
Secondary or symptomatic narcolepsy is rare, but it is easy to recognize and should always be considered, as management differs from that in other forms of narcolepsy.38,39 As a general rule, these individuals have obvious neurological signs related to injury to the hypothalamus or rostral midbrain including visual field cuts, corticospinal deficits, poor memory, and pituitary dysfunction. This hypothalamic injury often injures the hypocretin neurons or their projections, resulting in low CSF hypocretin levels. In addition, nearly all reported patients with secondary narcolepsy sleep more than 10 hours each day.
A wide variety of pathologies can injure the hypothalamus, including strokes, tumors, head trauma, and inflammatory processes such as sarcoidosis or demyelination. These same processes can cause EDS alone, so a positive MSLT is necessary to diagnose secondary Na-2.
To rule out secondary narcolepsy, MRI with and without contrast and fine cuts through the hypothalamus should be performed in any narcolepsy patient with focal neurological deficits. MRI is generally unnecessary in the large majority of adult narcolepsy patients with normal neurological exams.
5. THE DIAGNOSIS OF NA-2 IN CHILDREN
The diagnosis of Na-2 in the pediatric population is equally if not more challenging than it is in adults for several reasons. First, EDS, especially in young children, often presents with symptoms related to cognition, attention, behavior and mood dysfunction that may not be recognized as manifestations of sleepiness,40,41 or with more subtle alterations in sleep patterns such as resumption of daytime napping in a school-aged child. There may be waxing and waning levels of sleepiness throughout the day rather than discrete sleep episodes, and naps may be long and non-refreshing.
In children, the symptoms of cataplexy may also differ from those in adults and thus can potentially be overlooked by clinicians.42 For example, the classic cataplexy presentation in adults of total loss of muscle tone with falls is less common in children,42 while “cataplectic facies” (prolonged episodes of facial muscle weakness with jaw slackness, ptosis, tongue protrusion, and slurred speech) is common in children.42,43 Caregivers may also attribute head nodding to sleepiness rather than loss of head/neck muscle tone due to cataplexy.
Moreover, MSLT testing for Na-2 in children carries more uncertainty, as the test can be more difficult to conduct and to interpret. While normative MSLT values in children younger than 8 years old do not yet exist, it is clear that adult normative sleep latency values should not be used to assess pre-pubertal children,22 and it has been suggested that a mean sleep latency < 10 min should be considered abnormal. Although not a standard, some labs use a 30-minute rather than a 20-minute nap protocol in children, as the normal mean sleep latency in preadolescents is over 20 minutes.44 Children are also more likely to resemble a diagnostic “moving target” as they develop, and in fact, there is some evidence to suggest that serial MSLTs may be required to confirm the diagnosis in some children.45 Other potential logistical challenges include an exaggerated “first-night effect” with difficulty sleeping in the lab during the preceding PSG and larger “normal” sleep needs in children compared to adults.
In pediatric narcolepsy populations, the percent of patients with Na-2 ranges from 0 to 100%, and most studies report 50% or less.46–49 In one study, compared to patients with Na-1, those with Na-2 had a similar age of onset (13.8 vs. 14.3 years), associated symptoms (hallucinations, etc.), and behavioral and academic concerns. The presence of HLA DQB1*06:02 was similar (89% vs. 83%), and MSLT results were not significantly different (sleep latency: 3.7 vs. 4.4 min; SOREMPs: 3.4 vs. 2.8). While these findings seemingly contradict the clinical experience in adults with Na-1 compared to Na-2, the small sample size (N = 30) may have contributed to the apparent lack of between-group differences.
The epidemiology of pediatric narcolepsy occurring in association with an environmental “trigger” may also yield different results. In two recent studies that examined cases of narcolepsy occurring after H1N1 vaccine administration, of which a significant percentage were children, 94%50 and 100%51 of these patients had Na-1, perhaps because the vaccine was a very potent immune system stimulus that triggered extensive hypo-cretin neuron loss.
Another potential confound is differences in the duration of follow-up after initial presentation. It is often assumed that the absence of cataplexy in children with Na-2 represents a temporary condition in the evolution of Na-1. For example, in a case-control study of 150 patients with Na-1, cataplexy first occurred during childhood or adolescence in only 48% of patients, and earlier age of onset of EDS was associated with fewer related symptoms (including cataplexy).52 Clearly, more research is needed to document the epidemiology, clinical presentation, and natural history of Na-2 in the pediatric population.
Unique features of cataplexy in children may present challenges in differentiating Na-1 from Na-2 in this age group. The apparent prevalence of Na-2 in children varies across studies and may be related to age, referral patterns, duration of follow-up, and the presence of environmental “triggers.”
6. A PROPOSED APPROACH FOR DIAGNOSING NA-2
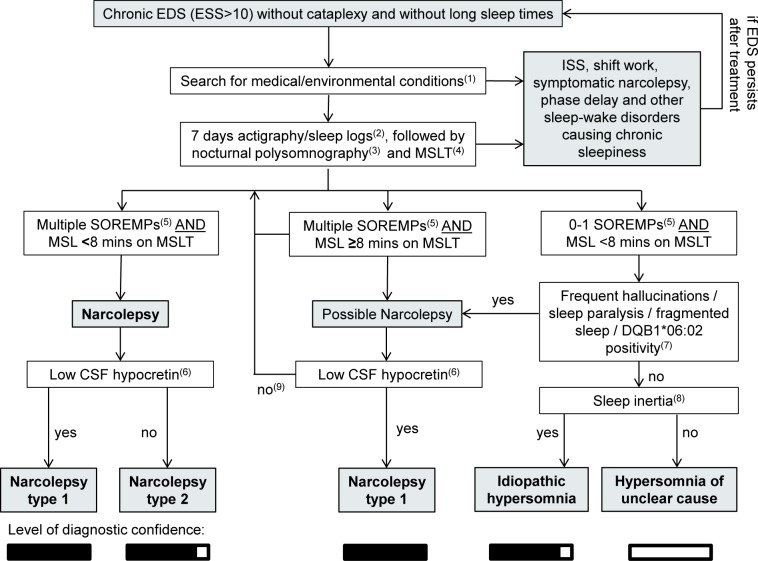
Based on the considerations above and some clinical intuition, we propose an algorithm for the diagnosis of Na-2 (Figure 1).
Figure 1.
Proposed algorithm for the diagnosis of narcolepsy without cataplexy and its differential diagnoses. ISS, insufficient sleep syndrome (chronic sleep deprivation); CSF, cerebrospinal fluid; EDS, excessive daytime sleepiness; ESS, Epworth Sleepiness Scale; MSL, mean sleep latency; MSLT, multiple sleep latency test; SOREMP, sleep onset rapid eye movement sleep. (1) In patients with an atypical history or neurological deficits, other causes of narcolepsy-like findings should be considered, and a brain MRI should be performed. Suggested laboratory parameters include a full iron panel, complete blood count, vitamin B12, and thyroid markers (TSH, T4). Children with Na-2 should receive a more extensive workup for unusual causes of sleepiness (e.g., tumors, metabolic disorders, seizures). (2) Sleep logs or preferably actigraphy over 14 days should be performed before the PSG and MSLT to exclude ISS or shift work. If the habitual sleep schedule is a concern, it may be helpful to schedule the MSLT just after 1-2 weeks' vacation to provide an opportunity for adequate sleep on a regular schedule. (3) During the nocturnal PSG, the patient should be permitted their habitual amount of sleep, which will usually be more than 6 hours of sleep. Long sleepers should be allowed to sleep up to 10 hours. (4) MSLT should be performed according to AASM guidelines, and medications that might alter sleep pressure or REM sleep should be discontinued well in advance. For example, antidepressants should be discontinued at least 3 weeks prior to the sleep studies. (5) According to ICSD-3 criteria, one SOREMP within 15 minutes of sleep onset on the preceding nocturnal PSG can be included in the total SOREMP count. (6) In patients without cataplexy, we recommend measuring CSF hypocretin to distinguish Na-1 from Na-2. (7) A clinical history of frequent hypnagogic/hypnopompic hallucinations, frequent sleep paralysis, fragmented nocturnal sleep, or positive HLA DQB1*06:02 typing may increase the likelihood of Na-2. (8) Sleep inertia, the need for multiple alarm clocks, and long but unrefreshing daytime naps are more indicative of idiopathic hypersomnia. (9) In patients with multiple SOREMPs but normal mean sleep latency or normal hypocretin levels, the MSLT should be repeated, preferably after a period of documented sleep extension.
7. PROPOSED FUTURE DIRECTIONS
We appreciate that diagnosing Na-2 with confidence remains a challenge, and we hope that the opinions and suggestions presented in this paper will make it easier. Looking forward, several lines of research could improve diagnostic certainty. Large prospective studies of patients with EDS would be very helpful—especially studies that include objective measures such as actigraphy, sleep laboratory tests, vigilance tests, and biochemical and genetic markers. Research to improve our current methods is needed such as improving our understanding of the effect of age and other factors that may confound the MSLT; defining the importance of sleep stage sequences (e.g., REM sleep after N1); and optimizing and standardizing the interpretation of actigraphy data. Furthermore, development of more sensitive and easily obtained hypocretin assays would be very helpful. Progress along these lines should make the diagnosis of narcolepsy easier and more reliable, and ultimately, it should provide a much better understanding of narcolepsy in general.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
REFERENCE
- 1.Mignot E, Lammers GJ, Ripley B, et al. The role of cerebrospinal fluid hypocretin measurement in the diagnosis of narcolepsy and other hypersomnias. Arch Neurol. 2002;59:1553–62. doi: 10.1001/archneur.59.10.1553. [DOI] [PubMed] [Google Scholar]
- 2.Singh M, Drake CL, Roth T. The prevalence of multiple sleep-onset REM periods in a population-based sample. Sleep. 2006;29:890–5. doi: 10.1093/sleep/29.7.890. [DOI] [PubMed] [Google Scholar]
- 3.Mignot E, Lin L, Finn L, Lopes C, Pluff K, Sundstrom ML, Young T. Correlates of sleep-onset REM periods during the multiple sleep latency test in community adults. Brain. 2006;129:1609–23. doi: 10.1093/brain/awl079. [DOI] [PubMed] [Google Scholar]
- 4.Andlauer O, Moore H, 4th, Hong SC, et al. Predictors of hypocretin (orexin) deficiency in narcolepsy without cataplexy. Sleep. 2012;35:1247–55. doi: 10.5665/sleep.2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luca G, Haba-Rubio J, Dauvilliers Y, et al. European Narcolepsy Network (EU-NN) Clinical, polysomnographic and genome-wide association analyses of narcolepsy with cataplexy: a European Narcolepsy Network study. J Sleep Res. 2013;22:482–95. doi: 10.1111/jsr.12044. [DOI] [PubMed] [Google Scholar]
- 6.Peyron C, Faraco J, Rogers W, et al. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat Med. 2000;6:991–7. doi: 10.1038/79690. [DOI] [PubMed] [Google Scholar]
- 7.Thannickal TC, Moore RY, Nienhuis R, et al. Reduced number of hypocretin neurons in human narcolepsy. Neuron. 2000;27:469–74. doi: 10.1016/s0896-6273(00)00058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crocker A, España RA, Papadopoulou M, et al. Concomitant loss of dynorphin, NARP, and orexin in narcolepsy. Neurology. 2005;65:1184–8. doi: 10.1212/01.wnl.0000168173.71940.ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blouin AM, Thannickal TC, Worley PF, Baraban JM, Reti IM, Siegel JM. Narp immunostaining of human hypocretin (orexin) neurons: loss in narcolepsy. Neurology. 2005;65:1189–92. doi: 10.1212/01.wnl.0000175219.01544.c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.John J, Thannickal TC, McGregor R, et al. Greatly increased numbers of histamine cells in human narcolepsy with cataplexy. Ann Neurol. 2013;74:786–93. doi: 10.1002/ana.23968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Valko PO, Gavrilov YV, Yamamoto M, et al. Increase of histaminergic tuberomammillary neurons in narcolepsy. Ann Neurol. 2013;74:794–804. doi: 10.1002/ana.24019. [DOI] [PubMed] [Google Scholar]
- 12.Thannickal TC, Nienhuis R, Siegel JM. Localized loss of hypocretin (orexin) cells in narcolepsy without cataplexy. Sleep. 2009;32:993–8. doi: 10.1093/sleep/32.8.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takei Y, Komada Y, Namba K, et al. Differences in findings of nocturnal polysomnography and multiple sleep latency test between narcolepsy and idiopathic hypersomnia. Clin Neurophysiol. 2012;123:137–41. doi: 10.1016/j.clinph.2011.05.024. [DOI] [PubMed] [Google Scholar]
- 14.Leu-Semenescu S, De Cock VC, Le Masson VD, et al. Hallucinations in narcolepsy with and without cataplexy: contrasts with Parkinson's disease. Sleep Med. 2011;12:497–504. doi: 10.1016/j.sleep.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 15.Sharpless BA, Barber JP. Lifetime prevalence rates of sleep paralysis: a systematic review. Sleep Med Rev. 2011;15:311–5. doi: 10.1016/j.smrv.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ohayon MM. Prevalence of hallucinations and their pathological associations in the general population. Psychiatry Res. 2000;97:153–64. doi: 10.1016/s0165-1781(00)00227-4. [DOI] [PubMed] [Google Scholar]
- 17.Dahmen N, Kasten M, Müller MJ, Mittag K. Frequency and dependence on body posture of hallucinations and sleep paralysis in a community sample. J Sleep Res. 2002;11:179–80. doi: 10.1046/j.1365-2869.2002.00296.x. [DOI] [PubMed] [Google Scholar]
- 18.Johns MW. Sensitivity and specificity of the multiple sleep latency test (MSLT), the maintenance of wakefulness test and the Epworth sleepiness scale: failure of the MSLT as a gold standard. J Sleep Res. 2000;9:5–11. doi: 10.1046/j.1365-2869.2000.00177.x. [DOI] [PubMed] [Google Scholar]
- 19.Andlauer O, Moore H, Jouhier L, et al. Nocturnal REM sleep latency for identifying patients with narcolepsy/hypocretin deficiency. JAMA Neurol. 2013;70:891–902. doi: 10.1001/jamaneurol.2013.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aldrich MS, Chervin RD, Malow BA. Value of the multiple sleep latency test (MSLT) for the diagnosis of narcolepsy. Sleep. 1997;20:620–9. [PubMed] [Google Scholar]
- 21.Trotti LM, Staab BA, Rye DB. Test-retest reliability of the multiple sleep latency test in narcolepsy without cataplexy and idiopathic hypersomnia. J Clin Sleep Med. 2013;9:789–95. doi: 10.5664/jcsm.2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Littner MR, Kushida C, Wise M, et al. Standards of Practice Committee of the American Academy of Sleep Medicine. Practice parameters for clinical use of the multiple sleep latency test and the maintenance of wakefulness test. Sleep. 2005;28:113–21. doi: 10.1093/sleep/28.1.113. [DOI] [PubMed] [Google Scholar]
- 23.Dauvilliers Y, Gosselin A, Paquet J, Touchon J, Billiard M, Montplaisir J. Effect of age on MSLT results in patients with narcolepsy-cataplexy. Neurology. 2004;62:46–50. doi: 10.1212/01.wnl.0000101725.34089.1e. [DOI] [PubMed] [Google Scholar]
- 24.Drakatos P, Suri A, Higgins SE, et al. Sleep stage sequence analysis of sleep onset REM periods in the hypersomnias. J Neurol Neurosurg Psychiatry. 2013;84:223–7. doi: 10.1136/jnnp-2012-303578. [DOI] [PubMed] [Google Scholar]
- 25.Wienecke M, Werth E, Poryazova R, et al. Progressive dopamine and hypocretin deficiencies in Parkinson's disease: is there an impact on sleep and wakefulness? J Sleep Res. 2012;21:710–7. doi: 10.1111/j.1365-2869.2012.01027.x. [DOI] [PubMed] [Google Scholar]
- 26.Marti I, Valko PO, Khatami R, Bassetti CL, Baumann CR. Multiple sleep latency measures in narcolepsy and behaviourally induced insufficient sleep syndrome. Sleep Med. 2009;10:1146–50. doi: 10.1016/j.sleep.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 27.Heier MS, Evsiukova T, Vilming S, Gjerstad MD, Schrader H, Gautvik K. CSF hypocretin-1 levels and clinical profiles in narcolepsy and idiopathic CNS hypersomnia in Norway. Sleep. 2007;30:969–73. doi: 10.1093/sleep/30.8.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mignot E, Lin L, Rogers W, et al. Complex HLA-DR and DQ interactions confer risk of narcolepsy-cataplexy in three ethnic groups. Am J Hum Genet. 2001;68:686–99. doi: 10.1086/318799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pelin Z, Guilleminault C, Risch N, Grumet FC, Mignot E. HLADQB1*06:02 homozygosity increases relative risk for narcolepsy but not disease severity in two ethnic groups. US Modafinil in Narcolepsy Multicenter Study Group. Tissue Antigens. 1998;51:96–100. doi: 10.1111/j.1399-0039.1998.tb02952.x. [DOI] [PubMed] [Google Scholar]
- 30.Lin L, Hungs M, Mignot E. Narcolepsy and the HLA region. J Neuroimmunol. 2001;117:9–20. doi: 10.1016/s0165-5728(01)00333-2. [DOI] [PubMed] [Google Scholar]
- 31.Hallmayer J, Faraco J, Lin L, et al. Narcolepsy is strongly associated with the T-cell receptor alpha locus. Nat Genet. 2009;41:708–11. doi: 10.1038/ng.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anderson KN, Pilsworth S, Sharples LD, Smith IE, Shneerson JM. Idiopathic hypersomnia: a study of 77 cases. Sleep. 2007;30:1274–81. doi: 10.1093/sleep/30.10.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vernet C, Arnulf I. Idiopathic hypersomnia with and without long sleep time: a controlled series of 75 patients. Sleep. 2009;32:753–9. doi: 10.1093/sleep/32.6.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lavault S, Dauvilliers Y, Drouot X, et al. Benefit and risk of modafinil in idiopathic hypersomnia vs. narcolepsy with cataplexy. Sleep Med. 2011;12:550–6. doi: 10.1016/j.sleep.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 35.Webb WB, Agnew HW., Jr Sleep: effects of a restricted regime. Science. 1965;150:1745–7. doi: 10.1126/science.150.3704.1745. [DOI] [PubMed] [Google Scholar]
- 36.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 37.Lee ML, Swanson BE, de la Iglesia HO. Circadian timing of REM sleep is coupled to an oscillator within the dorsomedial suprachiasmatic nucleus. Curr Biol. 2009;19:848–52. doi: 10.1016/j.cub.2009.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scammell T. Secondary narcolepsy. In: Culebras A, editor. Sleep disorders and neurologic disease. 2 ed. New York: Informa Healthcare; 2007. pp. 117–34. [Google Scholar]
- 39.Nishino S, Kanbayashi T. Symptomatic narcolepsy, cataplexy and hypersomnia, and their implications in the hypothalamic hypocretin/ orexin system. Sleep Med Rev. 2005;9:269–310. doi: 10.1016/j.smrv.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 40.Dorris L, Scott N, Zuberi S, Gibson N, Espie C. Sleep problems in children with neurological disorders. Dev Neurorehabil. 2008;11:95–114. doi: 10.1080/17518420701860149. [DOI] [PubMed] [Google Scholar]
- 41.Dahl RE, Holttum J, Trubnick L. A clinical picture of child and adolescent narcolepsy. J Am Acad Child Adolesc Psychiatry. 1994;33:834–41. doi: 10.1097/00004583-199407000-00009. [DOI] [PubMed] [Google Scholar]
- 42.Serra L, Montagna P, Mignot E, Lugaresi E, Plazzi G. Cataplexy features in childhood narcolepsy. Mov Disord. 2008;23:858–65. doi: 10.1002/mds.21965. [DOI] [PubMed] [Google Scholar]
- 43.Plazzi G, Pizza F, Palaia V, et al. Complex movement disorders at disease onset in childhood narcolepsy with cataplexy. Brain. 2011;134:3480–92. doi: 10.1093/brain/awr244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gozal D, Wang M, Pope DW., Jr Objective sleepiness measures in pediatric obstructive sleep apnea. Pediatrics. 2001;108:693–7. doi: 10.1542/peds.108.3.693. [DOI] [PubMed] [Google Scholar]
- 45.Kotagal S, Goulding PM. The laboratory assessment of daytime sleepiness in childhood. J Clin Neurophysiol. 1996;13:208–18. doi: 10.1097/00004691-199605000-00004. [DOI] [PubMed] [Google Scholar]
- 46.Viorritto EN, Kureshi SA, Owens JA. Narcolepsy in the pediatric population. Curr Neurol Neurosci Rep. 2012;12:175–81. doi: 10.1007/s11910-011-0246-3. [DOI] [PubMed] [Google Scholar]
- 47.Vendrame M, Havaligi N, Matadeen-Ali C, Adams R, Kothare SV. Narcolepsy in children: a single-center clinical experience. Pediatr Neurol. 2008;38:314–20. doi: 10.1016/j.pediatrneurol.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 48.Nevsimalova S, Jara C, Prihodova I, Kemlink D, Sonka K, Skibova J. Clinical features of childhood narcolepsy. Can cataplexy be foretold? Eur J Paediatr Neurol. 2011;15:320–5. doi: 10.1016/j.ejpn.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 49.Han F, Lin L, Warby SC, et al. Narcolepsy onset is seasonal and increased following the 2009 H1N1 pandemic in China. Ann Neurol. 2011;70:410–7. doi: 10.1002/ana.22587. [DOI] [PubMed] [Google Scholar]
- 50.Partinen M, Saarenpää-Heikkilä O, Ilveskoski I, et al. Increased incidence and clinical picture of childhood narcolepsy following the 2009 H1N1 pandemic vaccination campaign in Finland. PLoS One. 2012;7:e33723. doi: 10.1371/journal.pone.0033723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dauvilliers Y, Montplaisir J, Cochen V, et al. Post-H1N1 narcolepsycataplexy. Sleep. 2010;33:1428–30. doi: 10.1093/sleep/33.11.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ohayon MM, Ferini-Strambi L, Plazzi G, Smirne S, Castronovo V. How age influences the expression of narcolepsy. J Psychosom Res. 2005;59:399–405. doi: 10.1016/j.jpsychores.2005.06.065. [DOI] [PubMed] [Google Scholar]