Abstract
Study Objectives:
To study whether positive multiple sleep latency tests (MSLTs, mean sleep latency [MSL] ≤ 8 minutes, ≥ 2 sleep onset REM sleep periods [SOREMPs]) and/or nocturnal SOREMP (REM sleep latency ≤ 15 minutes during nocturnal polysomonography [NPSG]) are stable traits and can reflect incipient narcolepsy.
Design and Setting:
Cross-sectional and longitudinal investigation of the Wisconsin Sleep Cohort Study.
Participants:
Adults (44% females, 30-81 years) underwent NPSG (n = 4,866 in 1,518 subjects), and clinical MSLT (n = 1,135), with 823 having a repeat NPSG-MSLT at 4-year intervals, totaling 1725 NPSG with MSLT studies. Data were analyzed using linear mixed-effects models, and the stability of positive MSLTs was explored using κ statistics.
Measurements and Results:
Prevalence of a nocturnal SOREMP on a NPSG, of ≥ 2 SOREMPs on the MSLT, of MSL ≤ 8 minutes on the MSLT, and of a positive MSLT (MSL ≤ 8 minutes plus ≥ 2 SOREMPs) were 0.35%, 7.0%, 22%, and 3.4%, respectively. Correlates of a positive MSLT were shift work (OR = 7.8, P = 0.0001) and short sleep (OR = 1.51/h, P = 0.04). Test-retest for these parameters was poor, with κ < 0.2 (n.s.) after excluding shift workers and short sleepers. Excluding shift-work, short sleep, and subjects with negative MSLTs, we found one undiagnosed subject with possible cataplexy (≥ 1/month) and a NPSG SOREMPs; one subject previously diagnosed with narcolepsy without cataplexy with 2 NPSG SOREMPs and a positive MSLT, and two subjects with 2 independently positive MSLTs (66% human leukocyte antigen [HLA] positive). The proportions for narcolepsy with and without cataplexy were 0.07% (95% CI: 0.02-0.37%) and 0.20% (95% CI: 0.07-0.58%), respectively.
Conclusions:
The diagnostic value of multiple sleep latency tests is strongly altered by shift work and to a lesser extent by chronic sleep deprivation. The prevalence of narcolepsy without cataplexy may be 3-fold higher than that of narcolepsy-cataplexy.
Citation:
Goldbart A, Peppard P, Finn L, Ruoff CM, Barnet J, Young T, Mignot E. Narcolepsy and predictors of positive MSLTs in the Wisconsin Sleep Cohort. SLEEP 2014;37(6):1043-1051.
Keywords: cataplexy, HLA, MSLT, narcolepsy, polysomnography, REM sleep
INTRODUCTION
Much progress has been made in our understanding of narcolepsy with cataplexy, a disorder now called narcolepsy type 1 in the most recent International Classification of Sleep Disorders.1 The disorder is strongly associated with human leukocyte antigen (HLA) DQB1*06:02 (90% versus 25% in the general population)2 and almost always caused by an autoimmune-based loss of hypocretin (orexin) cells, an etiology that can be documented by the measure of low hypocretin-1 levels in the cerebrospinal fluid (CSF).3–5 In these patients, diagnosis can be made based on the presence of definite cataplexy,6 and should be confirmed by the multiple sleep latency test (MSLT), with the observation of a mean sleep latency (MSL) ≤ 8.0 minutes and ≥ 2 sleep onset REM periods (SOREMP) (referred to as a “positive MSLT” below).7
In cases with cataplexy and hypocretin deficiency, sensitivity for a positive MSLT is 85% to 93% and specificity 96%.5,7–9 Recent studies have also shown that a SOREMP during nocturnal polysomnography (NPSG) is also a very specific finding, although only 50% sensitive.9 Population-based prevalence studies for narcolepsy-cataplexy have been performed in many countries and in Europe, Asia, and North America, indicating a figure of 0.02% to 0.06%, and both sexes are similarly affected.10–12
Since the emergence of the MSLT as a diagnostic tool to diagnose narcolepsy,13–17 patients are increasingly diagnosed on the basis of sleepiness and a positive MSLT alone without cataplexy. Problematically, however, the first studies reporting on the MSLT as a diagnostic test only included narcolepsycataplexy cases and very few young healthy subjects (< 30) as controls,14,16,17 thus not addressing specificity. As first noted by Aldrich et al.,8 the lack of knowledge regarding specificity is problematic, as many more subjects have sleepiness due to other pathologies; thus even a small rate of false positive can lead to widespread misdiagnosis. In 1997, Aldrich noted a high prevalence of ≥ 2 SOREMPs (9.2%) in patients evaluated at a sleep clinic, a minority of which (8.2%) were later diagnosed as narcoleptic.8 Most strikingly, 7% of subjects with SDB and sleep disorders other than narcolepsy (1,913 subjects) also had ≥ 2 SOREMPs. Another study by Chervin and Aldrich also found that in patients with a final diagnosis of sleep disordered breathing (SDB), ≥ 2 SOREMPs occurred in 4.7% of cases and were best predicted by male sex and decreased lowest oxygen saturation, but not AHI.18 Diagnostic validity for overall positive MSLT (rather than for ≥ 2 SOREMPs) is harder to assess in these older studies, however. Indeed, diagnostic criteria for a positive MSLT was MSL ≤ 5 rather than MSL ≤ 8 minutes plus ≥ 2 SOREMPs—MSL criteria that were found to be too strict, with sensitivity at 86% rather than 95%, but similar specificity (∼95%),19 including for cases for hypocretin deficiency.5 In 2005, the criteria were revised to MSL ≤ 8 min and ≥ 2 SOREMPs.7
More recently, studies have shown that not only 4% to 6% of patients with sleep apnea have multiple SOREMPs but similar numbers of subjects in population-based samples as well, with only about half of these subjects complaining of excessive daytime sleepiness.20,21 These samples included both younger and older subjects. It is, therefore, possible that a large majority of narcolepsy without cataplexy patients are in fact false positives on a background of sleepiness caused by, e.g., sleep apnea and recruitment bias favoring the fact that sleepier subjects will be the most likely to consult. In favor of this, a recent study pointed out that test-retest of a positive MSLT in narcolepsy patients without cataplexy is very poor, with only 5 of 15 cases demonstrating a positive MSLT upon repeat testing.22 Further undermining the MSLT as a diagnostic test for narcolepsy, positive MSLTs are prevalent in other neurological conditions such as Parkinson disease, Prader-Willi syndrome, autosomal dominant cerebellar ataxia deafness and narcolepsy, and myotonic dystophy.23–26
At the practical level, sleep clinicians use the MSLT to diagnose narcolepsy but must also take care to exclude factors that could create false positives, notably sleep deprivation and sleep apnea, as causes of differential diagnosis. In large case series of narcolepsy without typical cataplexy diagnosed by experts, 15% to 25% of cases have low CSF hypocretin and 40% to 60% are HLA positive, suggesting that a small portion of narcolepsy without cataplexy cases have a cause similar to type 1 narcolepsy.5,27 The etiology of cases without cataplexy is unknown and, in many cases, could involve false positives.
Predictors of a positive MSLT in the general population have not been well documented. In two different population-based samples, prevalence of a positive MSLT was 2.5% and 3.6% with male predominance.20,21 In the Wisconsin Sleep Cohort, we found a strong effect of shift work, and possible effects of medications and short sleep on MSLT SOREMPs.21 In this study we increased sample size of MSLT testing in the cohort and evaluated test-retest properties of the test in this population based sample of older adults. Furthermore, based on a recent study demonstrating that SOREMPs on NPSG are very specific and ∼50% sensitive for narcolepsy/hypocretin deficiency,9 we also explored the property of this trait in the cohort. Finally, this information was used to identify potential narcolepsy patients.
METHODS
Nocturnal Polysomnography Sample
The population-based Wisconsin Sleep Cohort Study is an ongoing longitudinal study of sleep patterns in the general population. Employed adults aged 30-60 years in south central Wisconsin were mailed a survey on sleep habits, health, and demographics in 1989. Mailed surveys were repeated at 5-year intervals. A stratified random sample of survey respondents was recruited for an NPSG at baseline, with ∼1,500 participants having completed at least one NPSG to date. Exclusion criteria included pregnancy, unstable cardiopulmonary disease, airway cancers, and recent upper respiratory tract surgery. The baseline response rate was 51%. Follow-up NPSG studies have been conducted at 4-year intervals.28–30
The overnight sleep studies were conducted at the University of Wisconsin Clinical Research Unit in rooms resembling typical bedrooms every 4 years. As part of these visits, medication history is documented (most notably intake of psychotropic compounds such as antidepressants), body mass index (BMI, kg/m2) is measured, blood pressure recorded, and health-history, lifestyle and sleep questionnaires administered. The questionnaire includes items detailing habitual sleep time, symptoms of snoring, insomnia, daytime sleepiness (including the Epworth Sleepiness Scale, ESS), sleep paralysis, hypnagogic hallucinations, automatic behavior, and cataplexy-like symptoms. Frequency measures are based on a semi-quantitative 5-point scale that includes “never,” “rarely, only a few times ever,” “sometimes, at least once per month, but less than once per week,” or “often, at least once per week.” It also details whether or not the volunteer is retired or working, and—if working—what type of shift is involved (e.g., rotating shift, stable night shift, stable daytime shift). For analytical purpose, shift work is defined as stable night shift or rotating shifts at the time of the corresponding sleep test. The presence of cataplexy was explored using the following question: Have you ever had episodes of muscle weakness in your legs or buckling of your knees…?, with various emotions listed. Subjects were considered to have cataplexy-like symptoms when they reported positively to this question with “when you laugh” or “when you tell or hear a joke.” Frequency of the symptom had to be sometimes (at least once per month, but less than once per week) or often—at least once per week for either emotion.
A 16-channel polysomnographic recording system (Tele-factor Heritage digital polysomnography systems, Grass Instruments, Quincy, MA, USA) was used to assess sleep states, respiratory, leg movements and cardiac variables. Sleep was studied using EEG, electrooculography, and chin EMG. Leg movements were recorded using leg EMG leads. Oxyhemoglobin saturation was continuously recorded by pulse oximetry (model 3900, Datex-Ohmeda, Louisville, CO, USA). PTAF-2 Nasal pressure transducer (Pro-Tech, Mukilteo, WA, USA) and Dymedix PVDF (polyvinylidene fluoride film) detect oral and nasal airflow. Respiratory inductance plethysmography (Respitrace, Ambulatory Monitoring, Ardsley, NY) records rib cage and abdominal excursions.
Sleep stages and respiratory events were assessed by trained sleep technicians. Sleep variables obtained included total sleep time (TST), defined as the total hours of polysomnographically defined sleep, sleep efficiency, defined as TST divided by time from lights out until arising in the morning, and sleep onset, defined as the interval between light off and the first 3 consecutive epochs of stage 1 sleep or one epoch of stage 2, 3, 4, or REM. REM sleep latency was defined as the interval between sleep onset and the first epoch of REM sleep.
Each 30-s interval of the polysomnographic record was inspected visually for episodes of abnormal breathing. Cessation of airflow for ≥ 10 s was defined as an episode of apnea. A discernible reduction in the sum amplitude of the rib cage plus the abdominal excursions on respiratory inductance plethysmography ≥ 10 s and that was associated with reduction in the oxyhemoglobin saturation ≥ 4% was defined as an episode of hypopnea (Medicare criteria, or AASM-recommended definition of hypopnea). The apnea-hypopnea index (AHI), defined as the average number of episodes of apnea and hypopnea per hour of objectively measured sleep, was the key summary measurement of the occurrence of SDB. Commonly used cutoffs for SDB severity, such as AHI ≥ 5, 15, or 30, were also used. The lowest oxygen saturation during sleep was computed. The number of leg movement per hour of sleep (PLM index) was also recorded.
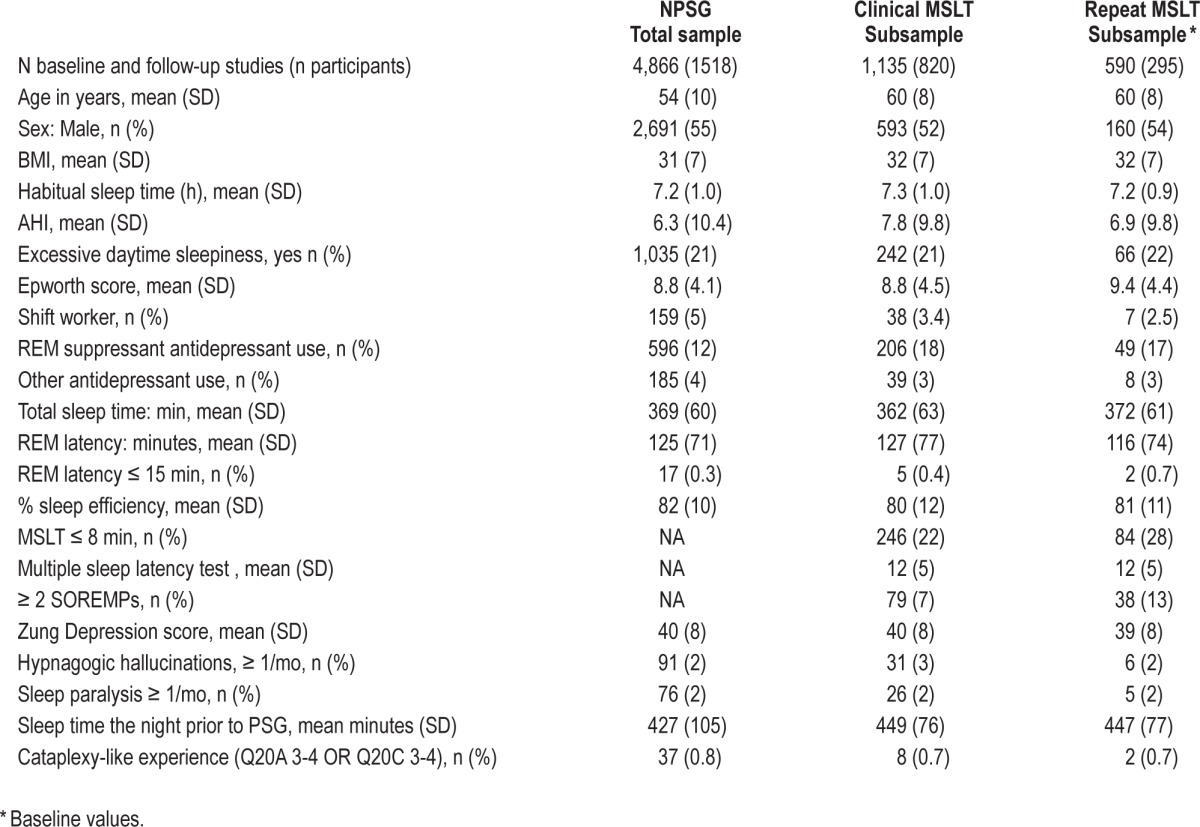
Table 1 describes the entire nocturnal sleep (NPSG) sample used in this study. The sample includes 4,866 sleep studies, performed in up to 4 visits 4 years apart in 1,518 subjects, 45% females, ages 30-81 years (mean age around 60 at the time of the study). Subjects were often obese (mean BMI = 31-32) with 3% to 5% shift workers and a large portion (∼15% to 20%) taking antidepressants. A significant proportion of subjects had sleep apnea, with a mean AHI of 6.2 events/h, with hypopnea defined by the presence of 4% desaturation.
Table 1.
Demographic and sleep testing (NPSG, MSLT) characteristics of the study cohort
MSLT Sample
Beginning in 2001 and ending in 2012, when contacted to schedule their next NPSG appointment, all subjects were asked to complete a clinical MSLT if agreeable. As in the original protocol of Carskadon et al.,31 and subsequent American Academy of Sleep Medicine task-force-approved modifications,32 naps were scheduled at 2-h intervals starting 2 h after initial morning awakening. Bedtime and wake time were decided by the patient, although patients were awakened at 08:00 if still sleeping. If no sleep occurred in 20 min, the nap trial ended and sleep latency recorded as 20 minutes. If sleep occurred within 20 min, onset was defined as time from lights out to the first epoch of sleep (including stage 1). In order to assess for the presence of REM sleep, the test continued ≥ 15 min after sleep onset. If present, latency to REM sleep as noted, thus a SOREMP is defined as REM sleep latency (REML) ≤ 15 min in these naps. Subjects were also asked to complete a daily log for a week prior to the MSLT, so that hours of sleep the nights prior to testing are recorded. Reliability of clinical MSLT scoring in the cohort was demonstrated in a prior study. For further details, please see Mignot et al.21
In addition to repeating the MSLT at a 4-year interval when possible in all comers, we also prioritized subjects having had at least one SOREMP in a prior clinical MSLT for repeats and subjects who never underwent an MSLT. This prioritization only occurred the last year of the study. The MSLT sample includes 1,725 studies in 823 subjects (Table 1). Two hundred ninety-five subjects repeated NPSG-MSLT studies at 4-year interval. As noted, mean age of the MSLT subsample was older, as the inclusion of clinical MSLT protocols started several years after inception of the Wisconsin Sleep Cohort Study. Other parameters were essentially comparable, although the repeat MSLT sample was intentionally enriched in subjects with multiple SOREMPs on initial MSLT (Table 1). The MSLT protocols were closed in 2012.
Other Sleep Cohort Data
The sleep cohort has available a large variety of additional clinical, biochemical, and survey data that we used to enhance our narcolepsy-related investigations.25–27 For example, in addition to clinical MSLTs, experimental MSLTs were also collected in 1990-2003 for a subset of subjects.33 In experimental MSLT protocols, a 4-nap MSLT is conducted 7-14 days following nocturnal polysomnography. The naps are given at 2-h intervals beginning at 09:00 or 09:30, and each nap is interrupted after sleep onset as described in Carskadon et al.31; thus SOREMPs cannot be recorded (these MSLTs are thus not used in these analysis, but as one more descriptive feature to describe possible narcolepsy patients identified). The 4 sleep latencies are then averaged for one measure of sleep latency. We also have depression (Zung) and anxiety ratings,34,35 and other records of work habits and Epworth Sleepiness Scale (ESS) values that are typically repetitively asked at each NPSG visit, for a multiple number of protocols where potential subjects may have participated in over a 20-year period.28–30
Statistical Analysis
In a first analysis, we explored potential associations of cataplexy-like symptoms and various MSLT and NPSG findings either in isolation (nocturnal SOREMP, MSLT: ≥ 2 SOREMPs, MSL ≤ 8) or as a group (“positive MSLT”). Data were analyzed using linear mixed-effects models or generalized estimating equation logistic regression models, accounting for multiple NPSG and MSLT studies per participant. These analyses were also controlled for relevant covariates that had been described in the literature or found in our prior study.21 These included habitual sleep, shift work (rotating or night shift), Horne Ostberg (circadian preference) scores, total sleep time during the NPSG, DQB1*06:02 genotype, apnea hypopnea index (AHI), REM sleep AHI, treatment with REM sleep-suppressing antidepressants or other antidepressants, and amount of sleep on diary the night prior to the NPSG-MSLT.
Stability of positive MSLT findings or cataplexy reports (with various definitions) was next explored using κ statistics and after removing the most relevant covariates identified in the cross-sectional analysis (sex, age, shift work, subjects with short habitual sleep or short sleep the night prior to the MSLT, AHI).
Identification of Possible Narcolepsy Patients in the Cohort
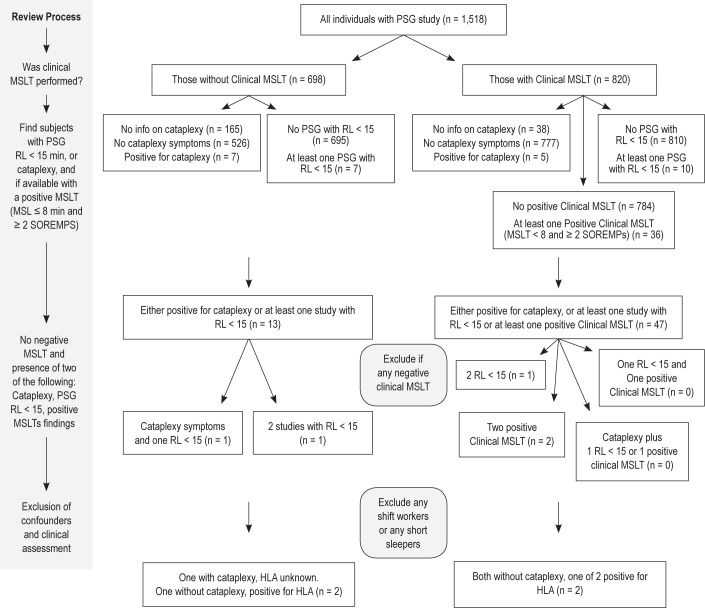
In order to identify potential participants with narcolepsy, we hypothesized that based on our results (poor κ for positive MSLTs) and the literature,22,8 patients with narcolepsy have a stable sleep phenotype demonstrating REM sleep abnormalities when PSG-MSLT tests are repeated. If cataplexy-like symptoms were reported, a positive MSLT (93% sensitive, 96% specificity)36 or a SOREMP at night (45% to 50% sensitive, 99% to 99.5% specific)8,36 was considered sufficient. If cataplexy was not present, 2 independent findings of either a SOREMP at night or a positive MSLT had to be observed without a negative MSLT. The mandated absence of any negative MSLT in these subjects was justified by the high sensitivity of the test, with only 5% to 10% of narcolepsy-cataplexy or hypocretin deficiency cases having a false negative MSLT.5 In the last step, the algorithm excluded subjects that were either shift workers or were chronically sleep deprived (< 6 h). Finally, the identified subjects' files were carefully reviewed and living subjects sent a letter asking for a sleep specialist phone interview to confirm a diagnosis (Figure 1).
Figure 1.
Algorithm used to identify narcolepsy cases in the cohort. The step-by-step review process used is detailed on the left-hand side. Positive MSLT criteria: MSL ≤ 8 min and ≥ 2 SOREMPs. Nocturnal SOREMPs: REML ≤ 15 min. Cataplexy: participants experiencing episodes of muscle weakness in your legs or buckling of your knees… “when you laugh” or “when you tell or hear a joke,” at least once per month.
RESULTS
Prevalence and Predictors of Nocturnal SOREMPs, Cataplexy-Like Symptoms, and Positive MSLT Findings in the Cohort
Prevalence of MSLT findings was similar to our prior study,21 varying by age and sex. Prevalence of multiple SOREMPs was 7%, while 22% of the cohort had an MSL ≤ 8 min. In combination, 3.4% of the cohort had a positive MSLT (Table 2). Prevalence of cataplexy-like symptoms (0.9%) was similar to prior reports.34,35 It was also significantly associated with depression scores (Table 2), as previously reported.35
Table 2.
Predictors of MSLT abnormalities, cataplexy and NPSG SOREMP, estimated by multiple logistic regression
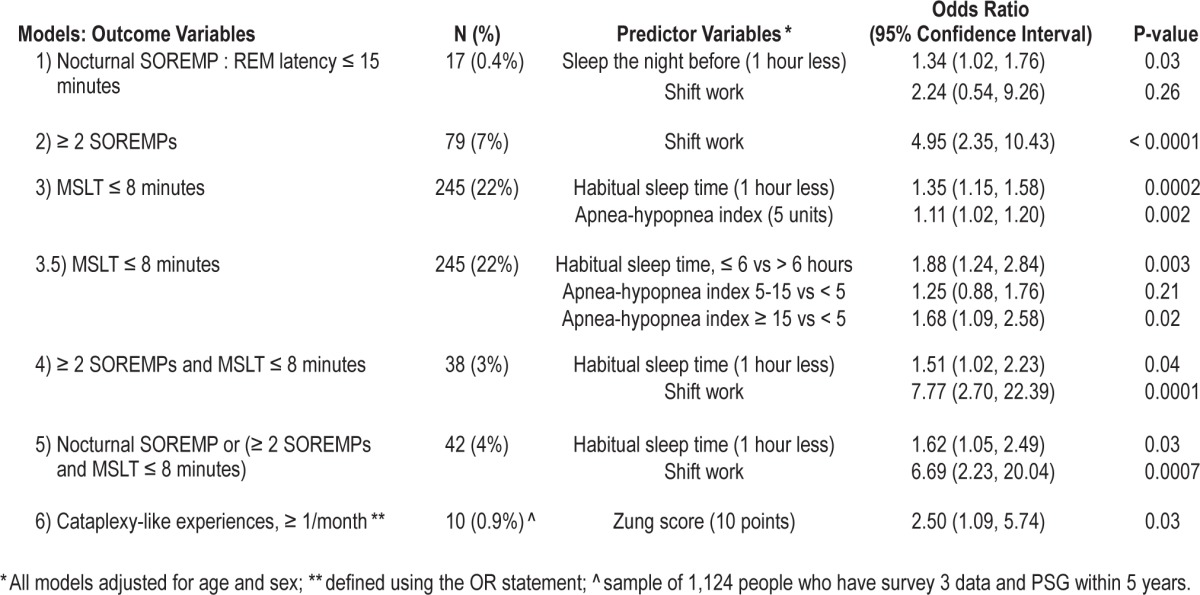
Interestingly, some of the associations reported in the prior study using a smaller sample21 were not confirmed, whereas others became more apparent. As shown in Table 2, nocturnal SOREMPs were a rare finding (only 17 times) in the large sample of 4,866 NPSGs in 1,518 subjects (0.35%), with only sleep the night prior to the NPSG-MSLT showing a modest association. However, the low number of positive nocturnal SOREMPs made it difficult to assess predictors with sufficient power.
As previously reported in a smaller size sample in this cohort,21 a major correlate of multiple SOREMPs either in isolation or in the context of a positive MSLT was shift work, which increased propensity for multiple SOREMPs 4-7 times depending of the combination of parameters examined (Table 2). Male sex also had a highly significant effect,21 and was controlled for. In contrast to our prior study, however, we could not confirm a statistically significant effect of REM sleep suppressing or NREM sleep suppressing antidepressants and mean % oxygen saturation on SOREMPs. As in our prior study, Horne-Ostberg morningness evening scores also did not correlate (data not shown).
Interestingly, sleep latency dichotomized at more or less than 8 minutes was related to AHI and, as previously reported for experimental MSLTs, to markers of sleep deprivation (Table 2).33 When combined with multiple SOREMPs, however, only short habitual sleep had minor effects on the propensity to have a positive MSLT. AHI, studied categorically (≥ 5, ≥ 15, or ≥ 30) or as a continuous variable, overall and during REM sleep only was also not associated with MSLT SOREMPs (data not shown).
Lack of Stability of MSLT Findings at a 4-Year Interval
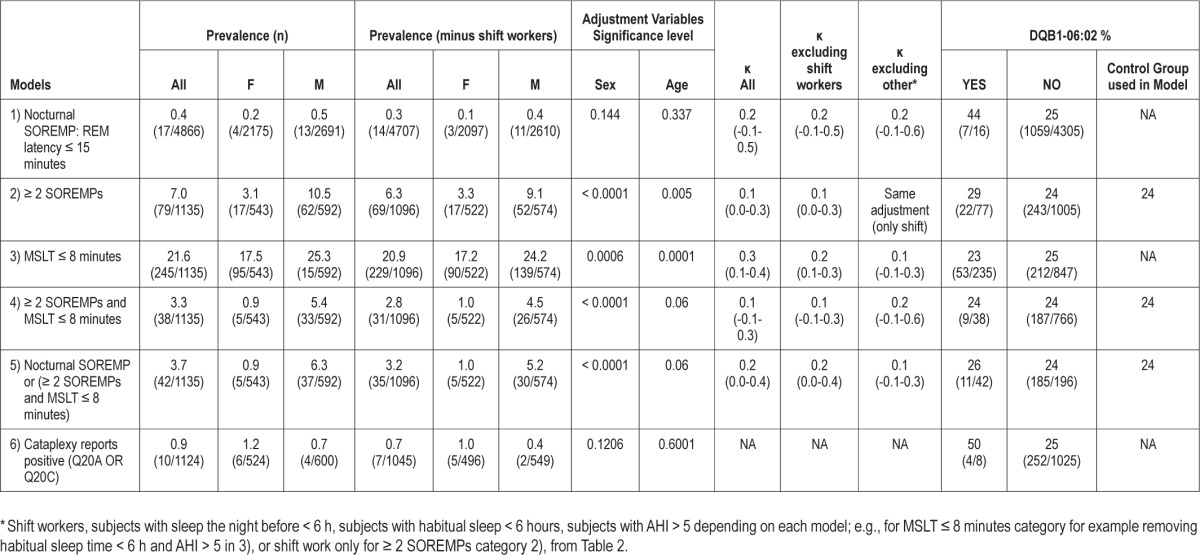
Stability of MSLT findings was examined using κ statistics (Table 3), either in the entire sample, or after removing subjects who had identified covariate characteristics that could alter the results (from Table 2). As can be seen, κ values were very low; for example, reaching 0.1-0.2 for positive MSLT findings in non-shift workers and those sleeping > 6 h (habitual sleep time). DQB1*06:02 positivity was also examined in the various categories and found to be unremarkable.
Table 3.
Prevalence and κ statistics of MSLT abnormalities, NPSG SOREMP, and cataplexy in the cohort
Potential Narcoleptic Patients
To identify potential narcolepsy subjects, we hypothesized that true cases would report cataplexy-like symptoms and/or multiple sleep recording findings consistent with narcolepsy, as those characteristics are rarely reproducible in most cohort participants (Table 3) but are stable in genuine narcolepsy cases with hypocretin deficiency. Using the algorithm depicted in Figure 1, 4 potential narcolepsy cases were identified among non-shift workers and without short habitual sleep.
Patient 1, a male participant with a BMI of 31 kg/m2 (first visit) was evaluated at ages 42 and 46, last had a study in 1995. Mean ESS was 19. Cataplexy-like symptoms were reported weekly with laughing, anger, and joking when first asked at age 51. Significant sleepiness was reported at both visits. Two nocturnal NPSGs were performed at these ages, one displaying a REML of 117 min, the other a REML of 2 min. The participant had sleep apnea treated by CPAP (untreated AHI = 55), and wore CPAP during all studies. An experimental MSLT (SOREMPs not recorded) was performed after the second NPSG with a MSL of 2 min, indicating profound sleepiness. At his last study he was middle-aged and reported good general health, no medication, and 8 h of sleep nightly. Considering that patients with cataplexy display nocturnal SOREMPs about half of the time, the result is consistent with this diagnosis. The participant declined participation to genetic typing and refused the phone interview; thus HLA status is unknown.
Patient 2, a male participant, was evaluated at ages 57, 62, 66, and 70. Mean ESS was 22. BMI was 27 kg/m2 at 57. He reported nocturnal sleep of 8 h nightly. He was diabetic and had hypercholesterolemia, both which were treated. He died at age 70 from cardiovascular complications. Two of his 4 NPSGs had a REML < 15 min (REML 9, 74, 2.5, and 128 min). AHIs were 35/h, 21/h, 13/h and 10/h. The participant had been diagnosed at an independent sleep center with both narcolepsy and sleep apnea. He had 2 experimental MSLTs with very short sleep latencies (1.5 and 2.8 min) and one clinical MSLT at age 70 with a MSL of 2.7 and 3 SOREMPs. He was HLA positive and reported no cataplexy-like symptoms.
Patient 3 was evaluated at ages 40, 44, 48, 52, 56, and 60. He was also male, and his BMI was 27 kg/m2 at age 40. Mean ESS was 14 and reported nocturnal sleep was 8 h. He denied excessive daytime sleepiness. AHI (and REM sleep AHI) was < 5/h in all studies. HLA was negative, and he did not report cataplexy-like symptoms or any other ancillary symptoms He never had a SOREMP in any of the 6 NPSGs (REML 93, 47, 44, 48, 41, and 128 min), had one experimental MSLT with MSL of 4.4 min), had 2 positive clinical MSLTs at age 52 and 56 (5.6 min, 3 SOREMPs; 7.2 min, 2 SOREMPs). The patient replied positively to a letter asking for a clinical interview and was contacted by phone by one of us (EM). Retired, he reported mild significant daytime sleepiness and no unwanted sleep episodes, but said he was able to fall asleep easily whenever he tried. His sleep was regular and normal in length (as reported by questionnaires). He had no cataplexy, sleep paralysis, hypnagogic hallucinations, or snoring. Interestingly, however, he reported always having vivid dreaming (pleasant or unpleasant) when napping and during nocturnal sleep, with frequent remembrance since childhood.
Patient 4 was a man evaluated at ages 52, 56, and 60. BMI was 29 kg/m2 at age 52. Mean ESS was 16, and he reported daytime sleepiness in spite of 7 h of nightly sleep. REM sleep latency was below 15 min in 2 NPSG of 3 NPSGs (4.5, 9, 35 min). AHI was insignificant in all studies (0.2, 0.5 and 0/h). He had 2 experimental MSLTs with short mean sleep latencies of 4 minutes. HLA was positive, and he did not report cataplexy-like symptoms.
Based on these findings, a conservative estimation of narcolepsy frequency in the total sample is 0.0026 (1 out of 380). This estimation is conservative, as not all subjects have had multiple MSLTs or NPSGs, and subjects with short sleep and shift work were excluded. The proportion of subjects with narcolepsy with cataplexy is 0.07% (95% CI: 0.02-0.37%). The proportion of subjects with narcolepsy without cataplexy is 0.20% (95% CI: 0.07-0.58%), although if HLA positive only are considered, it would be 0.13% (95% CI: 0.04%-0.48%).
DISCUSSION
Multiple SOREMPs during the MSLT have long been considered a pathognomonic finding in narcolepsy,13,14,17 yet more recent studies have found that the prevalence of multiple SOREMPs is higher than suspected in the general population20,21 or in patients with other sleep disorders such as SDB8,18 and Kleine-Levin syndrome,37 reaching very high proportions in subjects with selected neurological disorders.23–26 The current study confirm these findings and demonstrates that shift work and to a lesser extent, insufficient sleep can lead to false positive MSLTs independent of narcolepsy. The study was performed in older adults, however, so that it is possible other correlates are more relevant in children or younger adults.
Shift work is known to be strongly associated with shorter sleep duration, sleepiness, and motor vehicle accident risk. This is consistent with an association with short MSLT sleep latency.38 As REM sleep is strongly regulated by the circadian clock, with REM sleep propensity peaking close to the temperature minimum of the circadian cycle,39,40 it is not surprising that shift workers have SOREMPs during the daytime. Carskadon et al. also reported multiple SOREMPs in 16% of 10th grade adolescents (notably in morning naps) in correlation with delayed sleep phase syndrome and resulting insufficient sleep, suggesting delayed sleep phase may also result in false positive,41 although Horne Ostberg scores did not correlate with SOREMPs in our study of older adults.
The effect of sleep deprivation and insufficient sleep on REM sleep latency has not been fully documented. Clearly, sleep deprivation reduces mean sleep latency in the MSLT, as the test was first used for this purpose in the late 1980s.31 In general, sleep deprivation results in the building of NREM sleep first, so that it does not induce SOREMPs at night in most cases. In rats, however, long-term sleep deprivation can result in SOREMPs,42 and there are reports of subjects with insufficient sleep who had positive MSLTs that improved following extended sleep.43 In addition, a recent study of 20 subjects with insufficient sleep syndrome found 15% positive MSLTs, suggesting increased risk (although non-statistically significant when compared to 20 healthy controls with no positive MSLT tests).44
Interestingly, sleep disordered breathing had only a small effect on MSLT sleep latency, but none on SOREMPs and overall MSLT positivity (Table 2). This finding is in line with studies showing very similar proportions of positive in the general population20,21 and sleep disorders patients.8,18 No relationship between positive MSLTs and REM sleep AHI was also found, but as both AHI and REM AHI were strongly correlated, this is not surprising. Based on these findings, we believe a sleep log or actigraphy for two weeks prior to MSLT should always be performed for a proper interpretation of the MSLT, and that shift workers should return to a normal schedule for several months prior to the MSLT or be excluded. Alternatively, although this is not tested, shifting the timing of the MSLT may be an option, after verification that patients have sufficient sleep between shifts.
In narcolepsy-cataplexy, available data suggest high repeatability of positive MSLTs. As almost all these subjects have hypocretin deficiency,5 this likely extends to all subjects with the biochemical abnormality as well. Aldrich et al.,8 studying 7 subjects, found 5 subjects (71%) had a second repeat positive MSLT using the older MSL < 5 minutes and ≥ 2 SOREMP diagnostic criteria (instead of MSL ≤ 8 min). Aldrich found the positive rate for narcolepsy with cataplexy with the stricter MSL criteria was 83%, suggesting high positive rates in the first and repeat MSLT in true type 1 narcoleptics. Folkerts et al.,17 studying 25 HLA positive cases with cataplexy, found that 24 (96%) had a second positive MSLT when repeated.
In contrast to this, Trotti et al.,22 found that only 33% (5 of 15 patients) without cataplexy with a positive MSLT had a second positive MSLT, suggesting poor repeatability for these cases. In a group of 36 patients with hypersomnia and no cataplexy, a change in diagnosis was made in 53% of patients between the first and second MSLTs, and was accounted for by a difference in the mean sleep latency, or the number of sleep onset REM periods.22 This led these authors to suggest that narcolepsy without cataplexy is indistinguishable from idiopathic hypersomnia, a claim that has been made before on a clinical basis.45 Our κ statistics analysis in population-based subjects reinforces this observation, as only 10% to 20% of subjects with a positive MSLT at baseline repeated positively four years later (Table 3). As the 10% to 20% repeatability is closer to the 33% of the Trotti study,22 it is likely that the majority of patients without cataplexy had a first positive MSLT by chance. Based on these results, we believe that observation of two positive MSLTs in the absence of cataplexy may be useful to confirm a genuine diagnosis of narcolepsy without cataplexy.
Based on these findings, we next assessed if any participants in the Wisconsin Sleep Cohort had a consistent pattern of narcolepsy based on successive sleep tests (MSLT or NSPG) showing positivity. Among 1,518 subjects, four males were identified as positive, one with cataplexy-like symptoms and three without cataplexy. Two of these subjects had sleep apnea, including one subject with cataplexy and one without. The observation of a case with cataplexy-like symptoms within 1,518 subjects (0.066%) is compatible with previously estimated prevalence value for the condition (∼0.03%) in the US population.10–12 It is notable that to our knowledge this participant was undiagnosed and not aware of his condition. Unfortunately, the participant declined the phone interview, so that confirmation of cataplexy by a sleep physician was not possible.
Regarding narcolepsy without cataplexy, our study is the first to assess frequency in a population-based sample of adults with NPSG, although sample size is small and true prevalence not estimable (Figure 1). Further, shift workers and short sleepers were excluded, and not all subjects had enough nocturnal NPSGs or clinical MSLTs to yield two positive tests, thus our calculation likely underestimates prevalence. Nonetheless, a total of three possible narcolepsy cases without cataplexy were found, two carrying HLA DQB1*06:02. One of the two HLA-positive subjects (patient 2) is a near-certain case, as he had an independent narcolepsy diagnosis (by an external sleep physician) and 3 positive sleep test findings (one positive clinical MSLT of one conducted, 2 of 4 NPSGs with a SOREMP at night). The second HLA positive subject (patient 4) is also likely genuine, as he has severe sleepiness (documented by two experimental MSLTs as well) and 2 out of 3 NPSGs conducted had a SOREMP. The HLA negative patient may be the most uncertain case, as he never had SOREMPs during 6 nocturnal NPSGs, an unusual finding considering that ∼50% of NPSG instances are positive in such cases (1.5% probability of having no SOREMPs in 6 studies). Further, he did not complain of significant daytime sleepiness or any ancillary symptoms, although ESS was borderline, and after clinical phone interview, he reported frequent dreaming.
Based on these findings, we estimate that 0.13% to 0.20% of the sample has narcolepsy without cataplexy, suggesting a milder form of narcolepsy is more frequent than anticipated. Unfortunately, however, clinical judgment was not available to exclude all possible other diagnostic confounders in one of three narcolepsy without cataplexy cases; thus this estimation remains tentative. As two of three cases without cataplexy were undiagnosed, it is also likely that diagnosed cases of narcolepsy without cataplexy seen at sleep clinic may not be representative of a population sample. Only one study has estimated the prevalence of diagnosed cases of narcolepsy without cataplexy, finding 0.0205%,12 a figure 10 times lower than that found here. Whether or not these subjects have hypocretin-related pathologies or not is impossible to assess, but it is intriguing that 2 out of 3 were HLA positive.
In conclusion, we found that in older adults MSLTs are influenced by shift work and insufficient habitual sleep. Documenting prior sleep history and sleep phase using a sleep log or actigraphy is thus likely of paramount importance. Multiple MSLT positive tests, measuring CSF hypocretin-1,5 or demonstrating autoimmunity toward hypocretin46 may thus be needed to fully confirm a diagnosis of narcolepsy without cataplexy. Using the wealth of data available on the cohort, we could for the first time report on the frequency of MSLT-based narcolepsy without cataplexy, which may be higher than anticipated, affecting 2-3 times more subjects than narcolepsy with cataplexy. Additional studies in children, teenagers and adults would, however, be needed to fully appreciate MSLT confounders and evolution of MSLT findings across the life span.
DISCLOSURE STATEMENT
This was not an industry supported study. The study was supported by NIH-NS23724 grant, NIH-R01HL62252 and NIH-UL1RR025011 grants. Work was performed at the Department of Population Health Sciences, University of Wisconsin-Madison, Madison, WI. Dr. Goldbart was supported by Israel Science foundation 753/11. Dr. Mignot has consulted for and has received research support from Jazz Pharmaceuticals. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank the participants in the Wisconsin Sleep Cohort Study, Erika Hagen and Eileen Leary for protocol assistance, and Ling Lin for DQB1*06:02 typing.
Footnotes
A commentary on this article appears in this issue on page 1027.
REFERENCE
- 1.American Academy of Sleep Medicine. International classification of sleep disorders. 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 2.Mignot E, Lin L, Rogers W, et al. Complex HLA-DR and -DQ interactions confer risk of narcolepsy-cataplexy in three ethnic groups. Am J Hum Genet. 2001;68:686–99. doi: 10.1086/318799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nishino S, Ripley B, Overeem S, Lammers GJ, Mignot E. Hypocretin (orexin) deficiency in human narcolepsy. Lancet. 2000;355:39–40. doi: 10.1016/S0140-6736(99)05582-8. [DOI] [PubMed] [Google Scholar]
- 4.Peyron C, Faraco J, Rogers W, et al. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat Med. 2000;6:991–7. doi: 10.1038/79690. [DOI] [PubMed] [Google Scholar]
- 5.Mignot E, Lammers GJ, Ripley B, et al. The role of cerebrospinal fluid hypocretin measurement in the diagnosis of narcolepsy and other hypersomnias. Arch Neurol. 2002;59:1553–62. doi: 10.1001/archneur.59.10.1553. [DOI] [PubMed] [Google Scholar]
- 6.Anic-Labat S, Guilleminault C, Kraemer HC, Meehan J, Arrigoni J, Mignot E. Validation of a cataplexy questionnaire in 983 sleep-disorders patients. Sleep. 1999;22:77–87. [PubMed] [Google Scholar]
- 7.American Academy of Sleep Medicine. Westchester, IL: American Academy of Sleep Medicine; 2005. International classification of sleep disorders, 2nd ed.: diagnostic and coding manual. [Google Scholar]
- 8.Aldrich MS, Chervin RD, Malow BA. Value of the multiple sleep latency test (MSLT) for the diagnosis of narcolepsy. Sleep. 1997;20:620–9. [PubMed] [Google Scholar]
- 9.Andlauer O, Moore H, Jouhier L, et al. Nocturnal rapid eye movement sleep latency for identifying patients with narcolepsy/hypocretin deficiency. JAMA Neurol. 2013;70:891–902. doi: 10.1001/jamaneurol.2013.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ohayon MM, Priest RG, Zulley J, Smirne S, Paiva T. Prevalence of narcolepsy symptomatology and diagnosis in the European general population. Neurology. 2002;58:1826–33. doi: 10.1212/wnl.58.12.1826. [DOI] [PubMed] [Google Scholar]
- 11.Mignot E. Genetic and familial aspects of narcolepsy. Neurology. 1998;50:S16–22. doi: 10.1212/wnl.50.2_suppl_1.s16. [DOI] [PubMed] [Google Scholar]
- 12.Silber MH, Krahn LE, Olson EJ, Pankratz VS. The epidemiology of narcolepsy in Olmsted County, Minnesota: a population-based study. Sleep. 2002;25:197–202. doi: 10.1093/sleep/25.2.197. [DOI] [PubMed] [Google Scholar]
- 13.Richardson GS, Carskadon MA, Flagg W, Van den Hoed J, Dement WC, Mitler MM. Excessive daytime sleepiness in man: multiple sleep latency measurement in narcoleptic and control subjects. Electroencephalogr Clin Neurophysiol. 1978;45:621–7. doi: 10.1016/0013-4694(78)90162-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mitler MM, Van den Hoed J, Carskadon MA, et al. REM sleep episodes during the Multple Sleep Latency Test in narcoleptic patients. Electroencephalogr Clin Neurophysiol. 1979;46:479–81. doi: 10.1016/0013-4694(79)90149-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mitler MM. The multiple sleep latency test as an evaulation for excessive somnolence. In: Guilleminault C, editor. Sleeping and waking disorders: indications and techniques. Menlo Park, CA: Addison-Wesley; 1982. pp. 145–53. [Google Scholar]
- 16.Amira SA, Johnson TS, Logowitz NB. Diagnosis of narcolepsy using the multiple sleep latency test: analysis of current laboratory criteria. Sleep. 1985;8:325–31. doi: 10.1093/sleep/8.4.325. [DOI] [PubMed] [Google Scholar]
- 17.Folkerts M, Rosenthal L, Roehrs T, et al. The reliability of the diagnostic features in patients with narcolepsy. Biol Psychiatry. 1996;40:208–14. doi: 10.1016/0006-3223(95)00383-5. [DOI] [PubMed] [Google Scholar]
- 18.Chervin RD, Aldrich MS. Sleep onset REM periods during multiple sleep latency tests in patients evaluated for sleep apnea. Am J Respir Crit Care Med. 2000;161:426–31. doi: 10.1164/ajrccm.161.2.9905071. [DOI] [PubMed] [Google Scholar]
- 19.Moscovitch A, Partinen M, Guilleminault C. The positive diagnosis of narcolepsy and narcolepsy's borderland. Neurology. 1993;43:55–60. doi: 10.1212/wnl.43.1_part_1.55. [DOI] [PubMed] [Google Scholar]
- 20.Singh M, Drake CL, Roth T. The prevalence of multiple sleep-onset REM periods in a population-based sample. Sleep. 2006;29:890–5. doi: 10.1093/sleep/29.7.890. [DOI] [PubMed] [Google Scholar]
- 21.Mignot E, Lin L, Finn L, et al. Correlates of sleep-onset REM periods during the Multiple Sleep Latency Test in community adults. Brain. 2006;129:1609–23. doi: 10.1093/brain/awl079. [DOI] [PubMed] [Google Scholar]
- 22.Trotti LM, Staab BA, Rye DB. Test-retest reliability of the multiple sleep latency test in narcolepsy without cataplexy and idiopathic hypersomnia. J Clin Sleep Med. 2013;9:789–95. doi: 10.5664/jcsm.2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rye DB, Bliwise DL, Dihenia B, Gurecki P. FAST TRACK: daytime sleepiness in Parkinson's disease. J Sleep Res. 2000;9:63–9. doi: 10.1046/j.1365-2869.2000.00201.x. [DOI] [PubMed] [Google Scholar]
- 24.Camfferman D, McEvoy RD, O'Donoghue F, Lushington K. Prader Willi Syndrome and excessive daytime sleepiness. Sleep Med Rev. 2008;12:65–75. doi: 10.1016/j.smrv.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 25.Winkelmann J, Lin L, Schormair B, et al. Mutations in DNMT1 cause autosomal dominant cerebellar ataxia, deafness and narcolepsy. Hum Mol Genet. 2012;21:2205–10. doi: 10.1093/hmg/dds035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dauvilliers YA, Laberge L. Myotonic dystrophy type 1, daytime sleepiness and REM sleep dysregulation. Sleep Med Rev. 2012;16:539–45. doi: 10.1016/j.smrv.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 27.Andlauer O, Moore Ht, Hong SC, et al. Predictors of hypocretin (orexin) deficiency in narcolepsy without cataplexy. Sleep. 2012;35:1247–55F. doi: 10.5665/sleep.2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 29.Taheri S, Lin L, Austin D, Young T, Mignot E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004;1:e62. doi: 10.1371/journal.pmed.0010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Young T. Rationale, design and findings from the Wisconsin Sleep Cohort Study: Toward understanding the total societal burden of sleep disordered breathing. Sleep Med Clin. 2009;4:37–46. doi: 10.1016/j.jsmc.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carskadon MA, Dement WC, Mitler MM, Roth T, Westbrook PR, Keenan S. Guidelines for the multiple sleep latency test (MSLT): a standard measure of sleepiness. Sleep. 1986;9:519–24. doi: 10.1093/sleep/9.4.519. [DOI] [PubMed] [Google Scholar]
- 32.Littner MR, Kushida C, Wise M, et al. Practice parameters for clinical use of the multiple sleep latency test and the maintenance of wakefulness test. Sleep. 2005;28:113–21. doi: 10.1093/sleep/28.1.113. [DOI] [PubMed] [Google Scholar]
- 33.Punjabi NM, Bandeen-Roche K, Young T. Predictors of objective sleep tendency in the general population. Sleep. 2003;26:678–83. doi: 10.1093/sleep/26.6.678. [DOI] [PubMed] [Google Scholar]
- 34.Szklo-Coxe M, Young T, Finn L, Mignot E. Sleep paralysis, hypnagogic hallucinations, cataplexy: Narcolepsy spectrum and aternate etiology. In: Bassetti C, Billiard M, Mignot E, editors. Narcolepsy and hypersomnia. New York: Marcel Dekker/Taylor & Francis Health Sciences; 2007. pp. 133–50. [Google Scholar]
- 35.Szklo-Coxe M, Young T, Finn L, Mignot E. Depression: relationships to sleep paralysis and other sleep disturbances in a community sample. J Sleep Res. 2007;16:297–312. doi: 10.1111/j.1365-2869.2007.00600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Andlauer O, Moore H, Jouhier L, et al. Nocturnal rapid eye movement sleep latency for identifying patients with narcolepsy/hypocretin deficiency. JAMA Neurol. 2013;70:891–902. doi: 10.1001/jamaneurol.2013.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang YS, Lin YH, Guilleminault C. Polysomnography in Kleine-Levin syndrome. Neurology. 2008;70:795–801. doi: 10.1212/01.wnl.0000304133.00875.2b. [DOI] [PubMed] [Google Scholar]
- 38.Ohayon MM, Smolensky MH, Roth T. Consequences of shiftworking on sleep duration, sleepiness, and sleep attacks. Chronobiol Int. 2010;27:575–89. doi: 10.3109/07420521003749956. [DOI] [PubMed] [Google Scholar]
- 39.Czeisler CA, Weitzman E, Moore-Ede MC, Zimmerman JC, Knauer RS. Human sleep: its duration and organization depend on its circadian phase. Science. 1980;210:1264–7. doi: 10.1126/science.7434029. [DOI] [PubMed] [Google Scholar]
- 40.Carskadon MA, Dement WC. Sleep studies on a 90-minute day. Electroencephalography and clinical neurophysiology. 1975;39:145–55. doi: 10.1016/0013-4694(75)90004-8. [DOI] [PubMed] [Google Scholar]
- 41.Carskadon MA, Wolfson AR, Acebo C, Tzischinsky O, Seifer R. Adolescent sleep patterns, circadian timing, and sleepiness at a transition to early school days. Sleep. 1998;21:871–81. doi: 10.1093/sleep/21.8.871. [DOI] [PubMed] [Google Scholar]
- 42.Rechtschaffen A, Bergmann BM, Everson CA, Kushida CA, Gilliland MA. Sleep deprivation in the rat: I. Conceptual issues. Sleep. 1989;12:1–4. doi: 10.1093/sleep/12.1.1. [DOI] [PubMed] [Google Scholar]
- 43.Janjua T, Samp T, Cramer-Bornemann M, Hannon H, Mahowald MW. Clinical caveat: prior sleep deprivation can affect the MSLT for days. Sleep Med. 2003;4:69–72. doi: 10.1016/s1389-9457(02)00065-5. [DOI] [PubMed] [Google Scholar]
- 44.Marti I, Valko PO, Khatami R, Bassetti CL, Baumann CR. Multiple sleep latency measures in narcolepsy and behaviourally induced insufficient sleep syndrome. Sleep Med. 2009;10:1146–50. doi: 10.1016/j.sleep.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 45.Bassetti C, Aldrich MS. Idiopathic hypersomnia. A series of 42 patients. Brain. 1997;120:1423–35. doi: 10.1093/brain/120.8.1423. [DOI] [PubMed] [Google Scholar]
- 46.De la Herrán-Arita AK, Kornum BR, Mahlios J, et al. CD4+ T cell autoimmunity to hypocretin/orexin and cross-reactivity to a 2009 H1N1 influenza A epitope in narcolepsy. Sci Transl Med. 2013;5:216ra176. doi: 10.1126/scitranslmed.3007762. [DOI] [PubMed] [Google Scholar]