Abstract
Study Objectives:
The relationship between sleep and immune function is not well understood at a functional or molecular level. We therefore used a genetic approach in Drosophila to manipulate sleep and evaluated effects on the ability of flies to fight bacterial infection.
Setting:
Laboratory.
Participants:
Drosophila melanogaster.
Methods and Results:
We used a genetic approach to transiently alter neuronal excitability in the mushroom body, a region in the central brain that is known to regulate sleep. Flies with increased sleep for up to two days prior to a bacterial infection showed increased resistance to the infection and improved survival. These flies also had increased expression levels of a subset of anti-microbial peptide mRNA prior to infection, as well as increased NFκB activity during infection as indicated by in vivo luciferase reporter activity. In contrast, flies that experienced reduced sleep for up to two days prior to infection had no effect on survival or on NFκB activity during infection. However, flies with reduced sleep showed an altered defense mechanism, such that resistance to infection was increased, but at the expense of reduced tolerance. This effect was dependent on environmental condition.
Conclusions:
Increasing sleep enhanced activity of an NFκB transcription factor, increased resistance to infection, and strongly promoted survival. Together, these findings support the hypothesis that sleep is beneficial to the host by maintaining a robust immune system.
Citation:
Kuo TH, Williams JA. Increased sleep promotes survival during a bacterial infection in Drosophila. SLEEP 2014;37(6):1077-1086.
Keywords: ion channels, NFκB, innate immunity, bacterial infection, Drosophila
INTRODUCTION
Sleep is an evolutionarily conserved process that is necessary for survival.1 This notion is supported by observations that continuous sleep deprivation leads to death in both rodents and fruit flies.2,3 Although the mechanism by which sleep promotes survival is unknown, one proposal is that mortality as a result of total sleep deprivation is attributed to adverse effects on immune function.4 Excess sleep and fatigue are commonly experienced during infectious illness in humans. Increased sleep associated with infection has also been documented in a wide range of species, including Drosophila.5 It has therefore been proposed that sleep is an adaptive response to infection and has a role in fighting infection.6 Chronic sleep loss is also associated with increased risks to health, such as obesity, diabetes, and cardiovascular disease,7–9 all of which involve an inflammatory process.10–12 A correlation between poor sleep quality and a weakened immune response is further supported by the observation that infectious illness was more common in shift workers who experienced greater levels of fatigue and lower quality sleep than daytime workers.13 Together, these findings suggest that getting a sufficient amount of daily sleep is important for the maintenance of a robust immune system.
It is well established that sleep in Drosophila has features that are similar to that in mammals.14,15 Several genome-wide studies have shown that many immune related genes increase expression with sleep deprivation,16–18 indicating a link between the immune and sleep homeostatic systems. More recently, we demonstrated that infection and injury transiently increased sleep in flies.5 Similar to mammals, the amount of sleep that increased with infection or injury was time-of-day dependent and required expression of an NFκB transcription factor, Relish, which is central to the innate immune response in Drosophila. Flies are therefore a suitable and powerful model to test the hypothesis that sleep supports immune function.
To evaluate a role of sleep in immune function, we used a drug-inducible Gal4-UAS expression system to alter neuronal excitability in mushroom bodies (MB), which is a region of the brain that has a well-established role in controlling sleep.19–22 MB-switch is a mifepristone (RU486) dependent Gal4 driver that enables the expression of upstream activating sequence (UAS)-linked target genes in MB.23 By using this modified Gal4-UAS system, MB-switch>UAS-Kir2.1 flies over-express potassium ion channels in mushroom body when fed with RU486, which consequently hyperpolarizes neurons, decreases synaptic transmission,24 and increases sleep.19 Conversely, RU486-treated MB-switch>UAS-NaChBac flies over-express sodium ion channels in the mushroom body, which increases neuronal excitability25 and decreases sleep.19 Using this approach, we assessed sleep and immune response parameters in MB-switch-UAS flies that were treated with either RU486 or an equivalent amount of vehicle for 3-4 days prior to infection and for the duration of each experiment. Flies with increased sleep showed an improved ability to fight infection with S. marcescens and with S. pneumoniae relative to controls. Flies with reduced sleep showed minor changes in host defense that were influenced by environmental condition. These findings indicate a beneficial role of sleep in immune function.
MATERIALS AND METHODS
Fly Stocks
Flies were grown on standard dextrose-cornmeal media.
MB-switch, UAS-Kir2.1, UAS-NaChBac/cyo, and UAS-mc* flies are in a WRR+ background19 and were provided by Drs. William Joiner and Amita Sehgal, University of Pennsylvania. κB-luc transgenic flies were described previously.5
Behavioral Assays
All experiments were performed in females kept in constant light (LL) at 25°C unless otherwise indicated. Sleep was measured by monitoring locomotor activity in flies using the Trikinetics Drosophila Activity Monitoring System (DAM2; Waltham, MA). Flies 1-4 days of age were loaded individually into glass tubes containing sucrose and agar medium. For induction of the mifepristone (RU486; Sigma) dependent MB-switch-Gal4 driver,23 adult flies were loaded into tubes with 500 μM RU486, or equivalent control vehicle (80% ethanol) dilution in 2% sucrose and 2% agar medium as described previously.5 Sleep in this assay is defined as an activity count of zero for ≥ 5 consecutive minutes.26 Sleep parameters were analyzed using custom Matlab-based software (Insomniac2, written by Dr. Lesley Ashmore, or an updated version, Insomniac3, written in MSVC6, by Thomas Coradetti). Post-infection sleep was measured and reported only in flies that survived beyond the analysis period of 24 h. The number of replicate experiments reported for sleep parameters correspond to those reported in Table S1 (supplemental material), except where otherwise indicated.
One-way ANOVA followed by Tukey post hoc comparison was used to evaluate changes in sleep after infection within each group (either vehicle- or RU486-treated flies). Student t-test was used to compare overall sleep and other behavioral parameters between vehicle- and RU486-treated groups in either 24 h or 12 h increments as indicated.
Measurement of the Immune Response
All methods used for behavioral assays and for measuring immune function are described in detail elsewhere.27 Briefly, S. marcescens (ATCC, #8100) were grown overnight in LB medium and diluted to an OD600 of 0.1 in phosphate buffered saline (PBS) and 1% food coloring (Brilliant Blue FCF). S. pneumoniae (strain D39; P210, gift of Dr. Michael Sebert) were grown in anaerobic conditions in BHI medium, and diluted in PBS/food coloring to an OD600 of 0.05–0.1. Three to 4 days after flies were loaded into activity monitors, they were removed, CO2 anesthetized, and infected by injection with diluted bacteria using glass capillary needles. Flies were subjected to aseptic injury by injection with equivalent dilutions of LB medium and food coloring in PBS. Survival rate was determined by using activity data derived from the Trikinetics system. Custom software (Drosonex, gift of Thomas Coradetti) was used to measure survival in hours following infection or injury. Flies were considered dead when all activity counts reached zero for the remainder of the experiment. For flies maintained in LD conditions, survival was monitored in groups of flies. One- to 4-day-old female flies were placed into vials containing 500 μM RU486 or equivalent vehicle control and entrained at 12: 12 h light: dark (LD) cycles. Three days later, flies were infected with S. marcescens at 6 h into the dark phase (Zeitgeber Time 18). Survival was determined daily by manually counting living flies remaining in the vials. Twenty flies were loaded in vials at Day 0. Two vials per experiment were used for each group.
Two to 5 replicate experiments were conducted for each genotype in either LL or LD as indicated. The survival data across all replicates were pooled and subjected to statistical analysis using the Kaplan-Meier estimator and the log rank test. To justify pooling of data, we analyzed the effect of independent experiments on overall results as follows. Individual experiments were treated as covariates and were subjected to a Cox proportional hazard regression analysis. Results are reported in Table S1, and were compared to an equivalent analysis without the covariate adjustment (pooled data). The results showed that there was no effect of the covariates on the overall P-value. We therefore pooled data and performed a more conservative analysis using the Kaplan-Meier estimator and log rank test.
To determine bacterial load, groups of up to 10 infected flies were homogenized in 400 μL LB medium immediately or 24 h post-inoculation, serially diluted and spread onto LB plates. Plates were incubated at 37°C, and the number of colony forming units (cfu) per fly was determined the next day. Statistical comparisons between experimental conditions were performed across cfu/fly values for each group, which ranged from 4-10 groups total across 3-5 experimental replicates. All statistical analyses were performed using public-accessed software (PAST; http://folk.uio.no/ohammer/past/).28
Luciferase Reporter Assay
One- to 4-day-old female flies carrying the κB-luc reporter maintained in LL for 3-4 days were loaded into vials containing 500 μM RU486, or an equivalent dilution of vehicle (to 4% ethanol) in 2% sucrose and 2% agar medium and maintained for an additional 2 days. Flies were then transferred individually to a 96-well plate containing 2 mM luciferin (Gold Biotechnology Inc.), the substrate of luciferase and 500 μM RU486, or equivalent dilution of vehicle, in 2% sucrose and 1% agar medium. The next day, flies were subjected to infection. Luciferase activity in living flies was measured with a Topcount luminescence counter (Perkin Elmer). For each experiment, 28 flies per condition were loaded into the plates. Average values are reported from living flies, as determined by visual inspection, at each time point across 3-5 replicate experiments for each genotype.
Luminescence data (in arbitrary units) were converted into natural log values. One-way ANOVA followed by Tukey post hoc comparison was performed within each of the vehicle- and RU486-treated groups to evaluate the pattern of induction of reporter activity following infection. Post hoc comparisons were made to a baseline value measured immediately prior to infection. Student t-test was used to compare values between the vehicle and RU486 groups at each time point. A Bonferroni correction for the number of comparisons was applied to the P-value to avoid false positives.
Quantitative PCR
MB-switch>UAS-Kir2.1 or MB-switch>UAS-NaChBac flies that were fed either RU486 or vehicle were collected and immediately frozen at −80°C before or 18 h post-infection with S. marcescens. A time of 18 h was chosen because it corresponded to peak NFκB activity during infection with S. marcescens. Total RNA was extracted from groups of 20 whole flies using RNA isolation reagent (Biotecx Laboratories, TX) according to the manufacturer's instructions. The following steps were then performed: 10 μg total RNA were treated with DNase (TURBO DNA-free Kit; Applied Biosystems); 6 μL of DNase treated samples were used to synthesize cDNA with oligo-dT (AffinityScript QPCR cDNA synthesis kit; Agilent); cDNA samples were diluted 1:5 to perform SYBR green based QPCR (Brilliant II SYBR Green QPCR Master Mix; Agilent) using 7500 Real Time PCR System (Applied Biosystems). Standard cDNA generated from non-treated MB-switch>UASNaChBac flies were used to quantify the relative expression of each gene. rp49 is used as an internal control of total cDNA used. Primers used for QPCR are described in Table S2 (supplemental material).
RESULTS
Enhanced Sleep Improves Immune Function
Both sleep14 and the immune response29,30 are tightly linked to the circadian clock. To ensure that effects of sleep on immune response parameters were independent of a circadian influence, all experiments (unless otherwise indicated) were conducted in constant light (LL). LL in Drosophila degrades core clock proteins and renders flies arrhythmic.31
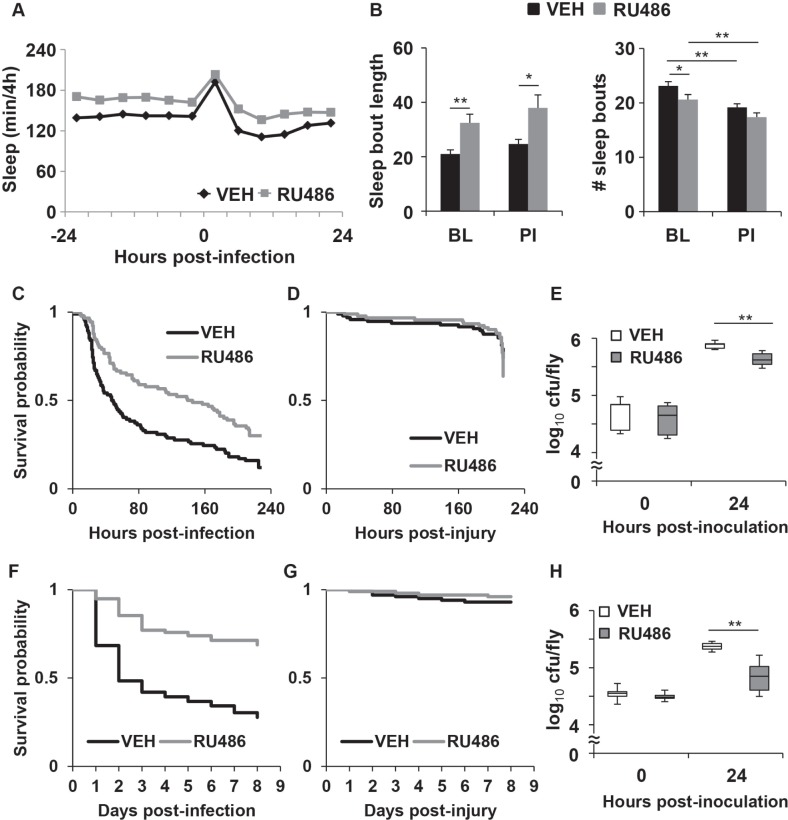
Consistent with previous findings,19 RU486-treated MB-switch>UAS-Kir2.1 flies maintained in LL had more sleep than vehicle-treated control for up to 2 baseline days (48 h) prior to inoculation (VEH: 14.4 ± 0.4 h and RU486: 16.8 ± 0.4 h per 24 h; P < 0.00002, Student t-test). Figure 1A shows sleep per 4 h on the days before and after infection with S. marcescens. RU486-treated flies continued to sleep more than the vehicle-treated group following infection. The increase in sleep in RU486-treated flies was attributed to a significant increase in sleep bout length (Figure 1B left panel). The number of sleep bouts was reduced prior to infection (Figure 1B right panel), which suggests that despite the absence of the circadian clock, RU486-treated flies were experiencing more consolidated periods of sleep relative to vehicle-treated flies. After infection with S. marcescens, both vehicle and RU486-treated groups transiently increased sleep as compared to their own baseline (P < 0.0001, one-way ANOVA), which is measured from a 4 h period immediately before the inoculation. The increase in sleep lasted for 4 h post infection in both RU486- and vehicle-treated groups (P < 0.0005, Tukey post hoc; Figure 1A). However, the RU486-treated flies continued to have more sleep and longer sleep bouts than the vehicle control group after the infection (Figure 1A and 1B).
Figure 1.
MB-switch>UAS-Kir2.1 flies treated with RU486 have increased sleep and improved immune function during infection. (A) Increased sleep is induced by RU486 in MB-switch>UAS-Kir2.1 flies in constant light (LL); mean ± SEM total time sleeping in minutes per 4 h is plotted relative to time of infection with S. marcescens. VEH = vehicle control; (B) Mean ± SEM sleep bout duration in minutes (left panel) and number of sleep bouts (right panel) is plotted for the 12 h period prior to infection (BL: baseline) and 12 h post infection (PI); * P < 0.05 and ** P < 0.01, t-test; n = 74 VEH; n = 82 RU486 treated flies. (C,D) Kaplan-Meier plots of flies surviving (C) infection in LL; P < 0.0003, log rank test; n = 94 VEH, n = 90 RU486 treated flies, and (D) aseptic injury; P > 0.2, log rank test; n = 96 VEH, n = 91 RU486 treated flies. (E) Box-and-whisker plots of cfu/fly are plotted relative to the time of infection in LL; ** P < 0.01 unpaired t-test; n = 5-6 groups for each condition across 3 independent experiments. The bottom, middle and top of the box represent the 25th, 50th (median), and 75th percentile, respectively. Error bars represent standard deviation. (F,G) Kaplan-Meier plots of survival of flies maintained in light: dark conditions (LD) during (F) infection, P < 0.00001, log rank test; n = 155 VEH, n = 157 RU486 treated flies, and (G) aseptic injury, P > 0.3, log rank test; n = 100 for both RU486 and VEH groups. (H) Box-and-whisker plots are as described in (E), except flies were maintained in LD and infected at 6 h into the dark phase n = 6-8 groups for each condition across 3-4 independent experiments.
To ensure that the increased sleep in the MB-switch>UASKir2.1 flies was independent of a nonspecific effect of RU486, we measured sleep in parental lines, MB-switch and UAS-Kir2.1 flies. RU486 had no effect on sleep in MB-switch flies (Figure S1A, supplemental material). Surprisingly, RU486-treated UAS-Kir2.1 flies in LL showed a significant reduction in baseline sleep as compared to the vehicle-treated groups (VEH: 17.0 ± 0.3 h and RU486: 15.5 ± 0.4 h per 24 h; P < 0.005, t-test; Figure S1D). However, the reduced sleep in the RU486-treated flies did not persist after the infection, as the amount of sleep was no different from the vehicle treated group (VEH: 15.6 ± 0.5 h and RU486: 14.3 ± 0.5 h per 24 h; P > 0.07, t-test; Figure S1D). Together, these data indicate that the increase in sleep observed in the MB-switch>UAS-Kir2.1 flies is indeed due to an RU486-dependent expression of potassium ion channels in the mushroom body.
MB-switch>UAS-Kir2.1 flies were infected with S. marcescens and then monitored for survival. RU486-treated flies had a significantly enhanced survival rate as compared to the vehicle control group (Figure 1C). Both RU486- and vehicle-treated MB-switch>UAS-Kir2.1 flies had nearly 100% survival for up to 9 days following aseptic injury (Figure 1D), indicating that injury did not contribute to the survival rates in the infected groups. RU486- and vehicle-treated parental lines, MB-switch and UAS-Kir2.1, also succumbed to infection with S. marcescens at equal rates (Figures S1B and S1E). Together, these findings indicate that flies that experienced enhanced sleep had a better survival outcome than the control group.
We next measured whether increased sleep influenced the ability of flies to clear the infection. The outcome of host defense comes from the balance of two immune response parameters, resistance and tolerance.32 Resistance is the ability of the host to limit the load of infectious pathogens, while tolerance is the ability of host to limit the damage in response to pathogens.32 We evaluated resistance by determining the number of colony forming units (cfu) remaining in flies after inoculation. Flies were infected with S. marcescens and harvested either immediately or 24 h after infection. Both RU486- and vehicle-treated MB-switch>UAS-Kir2.1 flies had the same number of cfu per fly immediately after infection, indicating the same bacterial load in both groups; 24 h post-inoculation, RU486-treated MB-switch>UASKir2.1 flies had significantly reduced cfu/fly as compared to the vehicle-treated group (Figure 1E). No difference was observed between RU486- and vehicle- treated MB-switch and UAS-Kir2.1 parental lines (Figures S1C and S1F, respectively). These findings suggest that flies that experienced more sleep had greater resistance to the infection.
To ensure that the change in immune response during infection is not attributed to locomotor behavior, waking activity rates were evaluated in RU486- and vehicle-treated flies. RU486- treated MB-switch>UAS-Kir2.1 flies showed a reduced activity rate compared to the vehicle-treated group (Figure S2A, supplemental material). However, a previous study reported that the influence of the MB on sleep did not always correlate with waking activity, and that this latter parameter was sensitive to environmental lighting conditions.20 We therefore conducted a set of experiments in a 12h: 12h light: dark cycle (LD). RU486- treated MB-switch>UAS-Kir2.1 flies slept more (P < 0.001, t-test, n = 46 flies each vehicle and RU486 group; not shown) and showed no change in waking activity rate in LD compared to the vehicle-treated group (Figure S2A). MB-switch>UAS-Kir2.1 flies that were maintained in groups were infected at 6 h into the dark phase in LD. The effect of RU486 on survival and bacterial resistance in these flies during infection were consistent with those observed in flies maintained individually in LL (Figures 1F and 1H). Nearly all flies in both groups survived with aseptic injury (Figure 1G). Thus the positive effect of increased sleep on immune parameters is unlikely to be attributed to a change in waking activity rate.
We next examined whether increased sleep affected survival during infection with another strain of bacteria, Streptococcus pneumoniae. S. pneumoniae is a Gram-positive strain of bacteria that requires the Toll signalling pathway for fighting infection.33,34 Most flies with this type of infection succumbed within 2-3 days after inoculation. However, similar to S. marcescens, RU486 treatment of MB-switch>UAS-Kir2.1 flies significantly improved survival during infection as compared to the vehicle treated group (P < 0.00001, log rank test, Figure 3A and Table S1).
Figure 3.
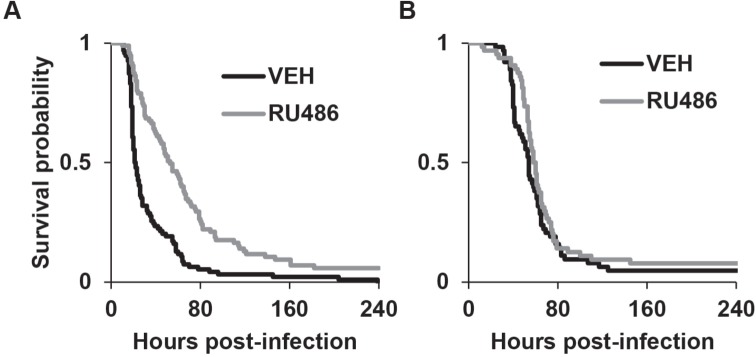
RU486-treated MB-switch>UAS-Kir2.1 flies have improved survival during infection with S. pneumoniae. Kaplan-Meier plots of flies surviving an infection with S. pneumoniae in (A) MB-switch>UAS-Kir2.1 flies, P < 0.00001 log rank test (n = 94 VEH and 95 RU486 treated flies); and (B) MB-switch>UAS-NaChBac flies, P > 0.22 log rank test (n = 64 for both VEH and RU486 treated flies).
Reduced Sleep Does Not Affect Survival during Infection
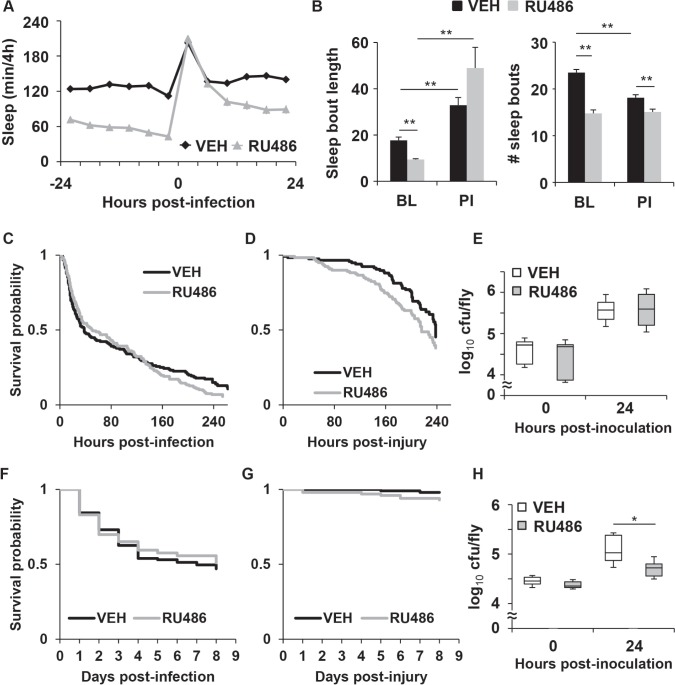
Similar to previous findings,19 RU486-treated MB-switch>UAS-NaChBac flies maintained in LL had decreased sleep as compared to vehicle-treated controls for up to 2 baseline days (48 h) before inoculation with S. marcescens (VEH: 12.8 ± 0.4 h and RU486: 8.1 ± 0.4 h per 24 h; P < 0.00001, t-test). The reduced sleep in the RU486-treated flies was at the expense of both sleep bout length and number of bouts as measured 12 h prior to infection (Figure 2B; “BL”). Both RU486- and vehicle-treated flies increased sleep for up to 24 h after infection as compared to their own baseline (P < 0.0001, ANOVA; P < 0.05, Tukey post hoc; Figure 2A). This increase in sleep is likely attributed to an increase in sleep bout length relative to baseline, which was seen in both groups (Figure 2B). Nonetheless, the RU486-treated flies continued to sleep less than vehicle-treated control (Figure 2A). RU486 had no effect on sleep in UAS-NaChBac parent control flies as compared to vehicle treated siblings either before (VEH: 12.8 ± 1.2 h and RU486: 14.3 ± 0.8 h per 24 h, P > 0.2) or after infection (VEH: 13.3 ± 1.2 h and RU486: 15.5 ± 0.8 h per 24 h, P > 0.1, t-test; Figure S1G).
Figure 2.
MB-switch>UAS-NaChBac flies treated with RU486 have reduced sleep, but no effect on survival during infection. (A) Reduction in sleep is induced by RU486 in MB-switch>UAS-NaChBac flies in LL. Mean ± SEM total time sleeping in minutes per 4 h is plotted relative to time of infection. (B) Mean ± SEM sleep bout duration in minutes (left panel) and number of sleep bouts (right panel) is plotted for the 12 h period before (BL) and after (PI) infection; ** P < 0.01 t-test; n = 99 VEH, n = 110 RU486 treated flies. (C,D) Kaplan-Meier plots of flies surviving (C) infection in LL; P > 0.4, log rank test, n = 156 VEH, n = 158 RU486 treated flies, and (D) aseptic injury; P > 0.06, log rank test, n = 117 VEH, n = 119 RU486 treated flies. (E) Box-and-whisker plots of cfu/fly are plotted relative to the time of infection in LL, * P < 0.05, unpaired t-test, n = 8 groups each across 4 independent experiments. (F,G) Kaplan-Meier plots of survival of flies maintained in LD during (F) infection; P > 0.7, log rank test; n = 115 VEH and n = 106 RU486 treated flies, and (G) aseptic injury; P > 0.08, log rank test; n = 100 for both RU486 and VEH treated flies. (H) Box-and-whisker plot of cfu/fly relative to the time of infection at 6 h into the dark phase of an LD cycle; * P < 0.05, unpaired t-test, n = 6-10 groups for each condition across 3-5 independent experiments.
RU486-treated MB-switch>UAS-NaChBac flies showed no change in immune function as compared to the vehicle-treated controls as both groups succumbed to infection at equal rates (Figure 2C) and showed no difference in resistance to infection (Figure 2E). Both groups of flies also survived with aseptic injury at rates that were statistically equivalent (Figure 2D). RU486- treated UAS-NaChBac parental control flies also showed no effects on immune function as compared to vehicle treated siblings (Figures S1H and S1I).
Waking activity rate was also evaluated in MB-switch>UASNaChBac flies. RU486-treated MB-switch>UAS-NaChBac flies showed no change in activity rate as compared to the vehicle-treated group (Figure S2B). However, in LD, while RU486-treated MB-switch>UAS-NaChBac flies had less sleep (P < 0.0001, t-test, n = 48, 49 for vehicle and RU486 treated groups, respectively; not shown), they also had a reduced waking activity rate as compared to the vehicle-treated group (Figure S2B). Thus similar to the MB-switch>UAS-Kir2.1 flies, waking activity rates in the MB-switch>UAS-NaChBac flies are also sensitive to environmental condition, but do not correlate with effects on sleep. These flies were then subjected to infection with S. marcescens at 6 h into the dark phase in LD. Interestingly, the RU486-treated flies had no change in survival as compared to the corresponding vehicle-treated group, but showed an improvement in bacterial clearance (Figures 2F and 2H). Because these flies became as sick as controls (as indicated by an equal rate of survival) with a lower number of bacterial cells, they are considered to have reduced tolerance.35 Thus, the increase in resistance to infection with S. marcescens in flies with reduced sleep is balanced by a reduction in tolerance to result in no change in survival outcome relative to controls. No difference in survival was observed when flies received aseptic injury (Figure 2G). These findings suggest that prolonged reduced sleep in flies alters host defense, but in a manner that is dependent on environmental condition. In this case, these flies had increased resistance to infection, but at the expense of reduced tolerance.
Reduction of sleep using a genetic approach may not necessarily affect resilience to sleep loss. That is, some mutants that have less sleep do not show recovery sleep (or sleep rebound) in response to sleep deprivation,36,37 or are not sensitive to adverse consequences of sleep loss, such as those seen with learning.38 To ensure that flies with reduced sleep were not insensitive to the effects of sleep loss, we used the MB-switch Gal4 driver to over-express a catalytic subunit of cAMP-dependent protein kinase (PKA; UAS-mc*). RU486-treated MB-switch>UAS-mc* flies were previously reported to not only have reduced sleep, but to also exhibit a period of recovery sleep upon withdrawal from RU486.19 This finding suggests that RU486-treated flies were indeed accumulating a sleep deficit rather than a reduced need for sleep. In LL, RU486-treated MB-switch>UAS-mc* flies had decreased sleep compared to the vehicle-treated group prior to infection (VEH: 14.1 ± 0.6 h and RU486: 7.9 ± 0.7 h per 24 h; P < 0.00001, t-test; Figure S3A, supplemental material). Similar to post-infection sleep in MB-switch>UAS-NaChBac flies, both RU486- and vehicle-treated flies increased sleep for up to 24 h after infection compared to their own baseline (P < 0.0001, ANOVA; P < 0.05, Tukey post hoc). However, the RU486-treated group continued to sleep less than the vehicle-treated control from 8 to 24 h post-infection (Figure S3A). Survival during infection was not significantly affected in the RU486-treated flies compared to the vehicle-treated control (Figure S3B). Most flies in both groups survived > 7 days after aseptic injury (Figure S3C). RU486 also had no effect on sleep before (VEH: 13.7 ± 0.6 h and RU486: 12.1 ± 0.7 h per 24 h, P > 0.09, t-test) or after infection (VEH: 17.1 ± 0.5 h and RU486: 18.2 ± 0.5 h per 24 h, P > 0.12, t-test) in the UAS-mc* parent control flies (Figure S3D). Finally, there was no difference in survival in response to infection between the VEH- and RU486-treated UAS-mc* parent control flies (Figure S3E). Together, these findings indicate that reduced sleep either by increased neuronal excitability or by enhanced PKA activity does not affect survival during infection with S. marcescens.
To rule out the possibility that the lack of effect of reduced sleep was specific to the type of infection, RU486 and vehicle treated MB-switch>UAS-NaChBac flies were infected with S. pneumoniae. Similar to the findings with S. marcescens, RU486-treated flies (with reduced sleep) succumbed to infection with S. pneumoniae at a rate that was equal to that in vehicle-treated controls (Figure 3B and Table S1).
Increased Sleep Enhances NFκB-Dependent Activity
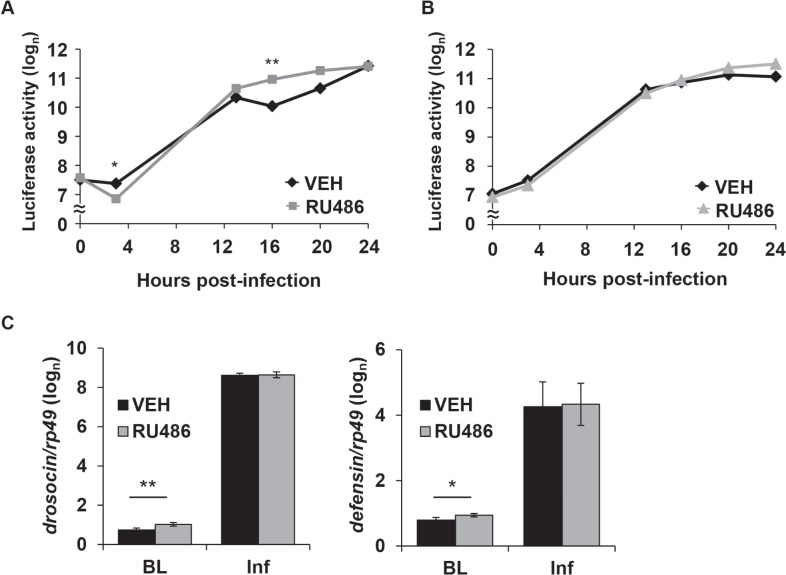
We next determined a molecular mechanism by which sleep influences survival. First, we examined activation of NFκB-dependent transcription in transgenic flies carrying a κB-luciferase reporter (κB-luc).5 This reporter contains a consensus NFκB binding sequence derived from the cecropin promoter and is sensitive to Relish activity in vivo. κB-luc reporter activity dramatically increased after infection with S. marcescens within both the RU486- and vehicle-treated κB-luc;;MB-switch>UAS-Kir2.1 groups. Specifically, κB-luc reporter activity was greater from 12-24 h post-inoculation compared to a baseline time point (P < 0.00001, one-way ANOVA with Tukey post hoc for both vehicle and RU486 treated groups). However, the pattern of induction of κB-luc reporter activity was different between the RU486 and vehicle-treated groups. At 4 h post-infection, reporter activity was significantly lower in the RU486-treated flies, but increased to significantly higher levels at 16 h post infection as compared to the vehicle-treated group (Figure 4A). A higher level of κB-luc reporter activity was also detected in the RU486 group at 20 h post-infection (P < 0.009, t-test), but this fell short of significance with a Bonferroni correction. A similar pattern of κB-luc reporter induction was seen after infection in both RU486- and vehicle-treated κB-luc;;MB-switch>UAS-NaChBac flies, such that reporter activity significantly increased in both groups from 12 to 24 h after infection relative to baseline (P < 0.00002, one-way ANOVA, Tukey post hoc). However, no significant differences were observed between RU486 and vehicle-treated groups (Figure 4B). To rule out nonspecific effects of RU486, κB-luc reporter activity was measured in the parental line, κB-luc;;MB-switch, during infection with S. marcescens. NFκB activity increased in both RU486- and vehicle- treated κB-luc;;MB-switch flies compared to their corresponding baseline (P < 0.00002, one-way ANOVA, Tukey post hoc), and no difference in κB-luc reporter activity was observed between RU486- and vehicle-treated flies up to 24 h post infection (Figure S4, supplemental material). These findings suggest that increasing sleep enhances NFκB transcriptional activity during a later stage of infection, at 16 h post-inoculation.
Figure 4.
Increased sleep increases NFκB activity and expression of AMP mRNA. Mean ± SEM luciferase reporter activity (natural log values, arbitrary units) is plotted against time relative to infection with S. marcescens in (A) κB-luc;;MB-switch/UAS-Kir2.1 flies, and (B) κB-luc;;UAS-NaChBac;MB-switch flies treated with RU486 or vehicle. The reading at hour 0 was performed immediately before inoculation. * P < 0.005 and ** P < 0.0001, t-test (Bonferroni corrected). Values from dead flies were discarded from analysis at each time point. N = 112 flies at time ‘0’ for both VEH and RU486; n = 74 VEH, n = 100 RU486 at time ‘24’ in (A); n = 111 at ‘0’ for both VEH and RU486; n = 94 VEH, n = 81 RU486 at time ‘24’ in (B). (C) Mean ± SEM (log(n) transformed) relative mRNA expression levels of drosocin (left panel) and defensin genes (right panel) in RU486 and VEH treated MB-switch>UAS-Kir2.1 flies immediately before infection (BL; baseline) or 18 h after infection (Inf). mRNA expression was normalized to the rp49 gene as an internal control. ** P < 0.005; * P < 0.05, paired t-test, N = 3.
We next determined changes in mRNA expression of anti-microbial peptides (AMPs) associated with infection in MB-switch>UAS-Kir2.1 and MB-switch>UAS-NaChBac flies using quantitative polymerase chain reaction (Q-PCR). AMPs are crucial to host defense in both insects and mammals, including humans.39 In flies, AMPs are targeted by NFκB transcription factors and are primarily secreted from fat bodies during an immune response.40 They are also necessary for fighting infection,41 although the mechanism by which they target bacteria is unknown. Flies were harvested immediately prior to or 18 h post-infection. In all groups of flies, all AMPs examined showed significant induction of mRNA expression 18 h after infection with S. marcescens (Figure 4C and Table S3, supplemental material). However, in uninfected flies, expression of drosocin and defensin mRNA was significantly increased in RU486-treated MB-switch>UAS-Kir2.1 flies compared to the vehicle control group (Figure 4C). Although no differences in induction of expression of AMP mRNA were seen during infection between RU486- and vehicle-treated flies, these findings indicate that flies experiencing more sleep had increased levels of a subset of AMP mRNA at baseline.
DISCUSSION
The mushroom body is a structure in the brain that is important for integrating sensory information42 as well as in controlling sleep19–22 in flies. Remarkably, manipulation of neuronal excitability in this region was not only effective in altering sleep, but also immune function during a bacterial infection. Previous work has also demonstrated that sensory neurons are involved in mounting an immune response in C. elegans,43,44 which indicates that neuronal influence of the immune response is conserved across species. We show here that decreasing neuronal excitability in MB consequently increased sleep, NFκB-dependent activity, increased resistance to infection and strongly promoted survival. Extra sleep (sleep “banking”) prior to a restricted sleep regimen in human subjects was reported to improve recovery time on a psychomotor vigilance task.45 The results in this current study strongly indicate that extra sleep may also lead to a better survival outcome with infection.
A limitation of the current study is that expression of each of the UAS- transgenes was driven by the RU486-dependent MB-switch-Gal4. Our rationale for using this reagent was that in addition to being a previously established method for manipulating sleep,19 it also allowed us to restrict expression of UAS transgenes to adult flies. The advantage to this approach is that effects on neuronal physiology and sleep are independent of potential abnormalities that may emerge from expression during early stages development that may occur with the use of alternate Gal4 drivers. Nonetheless, using alternate genetic or pharmacological approaches to enhance or reduce sleep in future studies will be important for further elucidating a mechanism by which sleep benefits host defense.
Increased sleep induced by over-expression of potassium ion channels in MB resulted in an increase in a subset of AMP gene expression prior to infection. Sleep has been reported to facilitate or enhance protein synthesis,46,47 which may account for the enhanced levels of AMP mRNA at baseline. A previous study showed that early enhancement of a subset of AMPs during infection in flies was associated with an enhanced survival outcome.29 In this case, an elevated level of AMP expression prior to infection may have contributed to increased resistance seen in flies with more sleep. However, we also observed a small but significant reduction in κB-luc reporter activity during early stages of infection (Figure 4A). One consequence of elevated protein synthesis is activation of the unfolded protein response (UPR). Given the massive increase in NFκB transcriptional activity and increased protein synthesis (such as AMPs) that is necessary for fighting infection, induction of the UPR would be expected during an early stage of infection, as reported in other species.48 The UPR leads to a cascade of signaling events that inhibits protein translation.49 It is therefore possible that a strongly adaptive UPR was effective at transiently reducing NFκB-dependent reporter expression in these flies during the first 4 h of infection. Whether this or the enhanced κB-luc reporter activity that occurs later during the infection contributes to the beneficial effect of enhanced sleep is unknown. It is possible that both events are important factors that underlie improved survival. Surprisingly, despite the increase in κB-luc reporter activity associated with enhanced sleep, we did not detect significant differences in the induction of AMP mRNA expression between RU486- and vehicle-treated groups (Table S3 and Figure 4C). However, NFκB transcription factors have numerous gene targets in flies,50,51 and not all targets are expected to be equally affected during infection.52 The NFκB target genes that may regulate the effect of sleep on immune function may therefore include an alternate subset of AMPs or other components that are involved in fighting infection.
It is interesting to note that earlier work demonstrated that sleep deprivation prior to infection also increased mRNA expression of the NFκB Relish and increased resistance to infection18—findings that are consistent with those in our companion paper.53 However, NFκB is necessary for post-infection sleep,5 and benefits of acute sleep deprivation to survival during infection are lost in the absence of two NFκB genes, Relish and Dif.53 These previous findings suggest that NFκB activity during infection increased as a result of acute sleep deprivation, but the ensuing recovery sleep (as well as survival) was prolonged due to this rise in transcriptional activity. The current findings, in contrast, show that increased sleep both prior to and during infection are also associated with elevated NFκB activity. Furthermore, the elevation in NFκB activity was restricted to a specific time frame along the course of infection (at 16 h post-inoculation). Together with the results discussed above, these findings suggest that increased sleep and acute sleep deprivation are likely to enhance NFκB activity during infection through separate mechanisms. Moreover, despite the increased resistance to infection associated with acute sleep deprivation, we found that prolonged sleep reduction may also reduce tolerance to infection, depending on the environmental condition.
Prolonged reduction of sleep in flies produced by over-expression of sodium ion channels or of a catalytic subunit of PKA had little or no effect on immune function in LL. This result was somewhat surprising, given the increased risks to health that have been reported with sleep loss.7–9 However, in both cases, we noted that these flies had elevated sleep relative to the corresponding baseline for at least 24 h after infection (Figure 2A and Figure S3A), which may have compensated for any detrimental effects of sleep loss. As mentioned above, flies with prolonged reduced sleep showed reduced tolerance to infection when they were maintained as groups in LD. The reduced tolerance is indicated by the lack of change in survival outcome despite the increased resistance to infection.32,35 It is possible that prolonged reduced sleep in LD increases resistance to infection through a mechanism that is similar to what was observed with acute sleep deprivation in LD,18 but with no benefit in terms of duration of survival. It is also important to note that these flies were also in a grouped situation. Flies maintained in grouped conditions experience more sleep after they are isolated,54,55 which suggests that flies may accrue a sleep deficit when they are kept in groups. It is therefore possible that the RU486 treated MB-switch>UAS-NaChBac flies in the grouped condition did not have as much post-infection sleep as those in isolation, and were therefore more susceptible to effects of a sleep deficit. Mechanisms that influence tolerance to infection in flies are not well understood, and vary depending on the infecting microorganism.32 Thus the mechanism by which long-term sleep loss reduces tolerance to infection will be an important topic for future study.
In conclusion, increasing sleep by over-expression of ion channels in a restricted region of the brain improved survival during an infection with S. marcescens and with S. pneumoniae. Flies with enhanced sleep had elevated levels of NFκB-dependent reporter activity during infection and showed increased resistance to the infection. Using a similar approach to reduce sleep had surprisingly little effect on immune function. However, flies with reduced sleep also had an altered balance between resistance and tolerance to infection, but in a manner that was influenced by environmental condition. These findings indicate that sleep is indeed beneficial to the host for fighting infection. Chronic inflammation, sleep disturbances, and fatigue are associated with a number of diseases in humans. Given the high degree to which molecular components of innate immune responses are shared between flies and humans, these results also suggest that finding ways of enhancing restorative sleep will have a positive impact on clinical outcome in human disease.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
This work was supported by the National Science Foundation under grant #IOS-1025627. The authors would like to thank Dr. Jung-Eun Lee and Dr. Isaac Edery for providing S. marcescens and primer sequences, Dr. Michael Sebert for providing S. pneumoniae and relevant culture protocols, Thomas Coradetti for survival analysis software, and Brendan Keenan for advice on statistical analyses.
SUPPLEMENTAL MATERIAL
Cox proportional hazard survival regression analyses.
Primers used for QPCR
RU486 has no effect on immune response measurements in MB-switch, UAS-Kir2.1, and UAS-NaChBac parent control flies. Effects of RU486 on sleep, survival, and resistance to infection are shown for (A-C) MB-switch, (D-F) UAS-Kir2.1, and (G-I) UAS-NaChBac parent control flies. (A,D,G) Mean ± SEM total time sleeping in minutes per 4 h is plotted relative to time of infection with S. marcescens in (A) MB-switch; n = 68 VEH and 76 RU486 treated flies, (D) UAS-Kir2.1; n = 77 VEH and 80 RU486 treated flies, and (G) UAS-NaChBac; n = 20 VEH and 30 RU486 treated flies. (B,E,H) Kaplan-Meier plots of flies surviving an infection with S. marcescens in (B) MB-switch flies, P > 0.09 log rank test, n = 127 VEH and 126 RU486 treated flies, (E) UAS-Kir2.1 flies, P > 0.92 log rank test, n = 92 VEH and 93 RU486 treated flies, and (H) UAS-NaChBac flies, P > 0.06 log rank test, n = 92 VEH and 90 RU486 treated flies. (C,F,I) Box-and-whisker plots of cfu/fly are plotted relative to the time of infection with S. marcescens. Error bars represent standard deviation. No significant differences were found between VEH and RU486 treated groups for (C) MB-switch, (F) UAS-Kir2.1, and (I) UAS-NaChBac flies (n = 6 groups for each condition across 3 independent experiments).
Waking activity rates in MB-switch>UAS-Kir2.1 and MB-switch>UAS-NaChBac flies. (A) Mean ± SEM activity per waking minute for a 24 h period is plotted for MB-switch>UAS-Kir2.1 flies maintained in constant light (LL; n = 64 VEH and 62 RU486 treated flies) or in a 12h: 12h light: dark cycle (LD; n = 46 for both VEH and RU486 treated groups); ** P < 0.01; student t-test. (B) Mean ± SEM activity per waking minute for a 24 h period is plotted for MB-switch>UAS-NaChBac flies maintained in LL (n = 32 each of VEH and RU486 treated flies) or in LD (n = 48 VEH and 49 RU486 treated flies), * P < 0.02, student t-test.
RU486-dependent reduction in sleep in MB-switch>UAS-mc* flies has no effect on immune function during bacterial infection. (A) Mean ± SEM total time sleeping in minutes per 4 h is plotted relative to time of infection with S. marcescens in MB-switch>UAS-mc* flies, n = 50 VEH and 55 RU486 treated flies. (B,C) Kaplan-Meier plots of MB-switch>UAS-mc* flies surviving (B) infection, P > 0.14, log rank test, n = 85 VEH and 92 RU486 treated flies, and (C) aseptic injury, P > 0.09, n = 94 each of VEH and RU486 treated flies. (D) Mean ± SEM total time sleeping in minutes per 4 h is plotted relative to time of infection with S. marcescens in UAS-mc* parent control flies, n = 71 VEH and 68 RU486-treated flies. (E) Kaplan-Meier plot of UAS-mc* flies surviving infection with S. marcescens, P > 0.09 log rank test, n = 109 VEH and 111 RU486-treated flies.
Expression of antimicrobial peptide genes in MB-switch>UAS-Kir2.1 and MB-switch>UAS-NaChBac flies with and without infection.
RU486 does not affect κB-luc reporter activity during infection in the MB-switch parent control flies. Mean ± SEM Luciferase activity (natural log, arbitrary units) is plotted against time of infection with S. marcescens in MB-switch Gal4 parent control flies carrying the κB-luc reporter. The reading at hour 0 was performed immediately before infection. RU486 had no effect on κB-luc activity relative to vehicle treated controls. Dead flies were discarded from the analysis at each time point; n = 84 flies total at time ‘0’ for both VEH and RU486 groups; n = 39 for VEH and n = 37 for RU486 group at time ‘24’. N = 3 independent experiments.
REFERENCE
- 1.Allada R, Siegel JM. Unearthing the phylogenetic roots of sleep. Curr Biol. 2008;18:R670–R9. doi: 10.1016/j.cub.2008.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rechtschaffen A, Gilliland MA, Bergmann BM, Winter JB. Physiological correlates of prolonged sleep deprivation in rats. Science. 1983;221:182–4. doi: 10.1126/science.6857280. [DOI] [PubMed] [Google Scholar]
- 3.Shaw PJ, Tononi G, Greenspan RJ, Robinson DF. Stress response genes protect against lethal effects of sleep deprivation in Drosophila. Nature. 2002;417:287–91. doi: 10.1038/417287a. [DOI] [PubMed] [Google Scholar]
- 4.Everson CA. Sustained sleep deprivation impairs host defense. Am J Physiol. 1993;265:R1148–54. doi: 10.1152/ajpregu.1993.265.5.R1148. [DOI] [PubMed] [Google Scholar]
- 5.Kuo TH, Pike DH, Beizaeipour Z, Williams JA. Sleep triggered by an immune response in Drosophila is regulated by the circadian clock and requires the NFkappaB Relish. BMC Neurosci. 2010;11:17. doi: 10.1186/1471-2202-11-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Imeri L, Opp MR. How (and why) the immune system makes us sleep. Nat Rev Neurosci. 2009;10:199–210. doi: 10.1038/nrn2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patel SR. Reduced sleep as an obesity risk factor. Obes Rev. 2009;10:61–8. doi: 10.1111/j.1467-789X.2009.00664.x. [DOI] [PubMed] [Google Scholar]
- 8.Knutson KL, Cauter EV. Associations between sleep loss and increased risk of obesity and diabetes. Ann N Y Acad Sci. 2008;1129:287–304. doi: 10.1196/annals.1417.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mullington JM, Haack M, Toth M, Serrador JM, Meier-Ewert HK. Cardiovascular, inflammatory, and metabolic consequences of sleep deprivation. Prog Cardiovasc Dis. 2009;51:294–302. doi: 10.1016/j.pcad.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bastard JP, Maachi M, Lagathu C, et al. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw. 2006;17:4–12. [PubMed] [Google Scholar]
- 11.Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol. 2011;11:98–107. doi: 10.1038/nri2925. [DOI] [PubMed] [Google Scholar]
- 12.Motivala SJ. Sleep and inflammation: psychoneuroimmunology in the context of cardiovascular disease. Ann Behav Med. 2011;42:141–52. doi: 10.1007/s12160-011-9280-2. [DOI] [PubMed] [Google Scholar]
- 13.Mohren DC, Jansen NW, Kant IJ, Galama J, van den Brandt PA, Swaen GM. Prevalence of common infections among employees in different work schedules. J Occup Environ Med. 2002;44:1003–11. doi: 10.1097/00043764-200211000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Cirelli C. The genetic and molecular regulation of sleep: from fruit flies to humans. Nat Rev Neurosci. 2009;10:549–60. doi: 10.1038/nrn2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sehgal A, Mignot E. Genetics of sleep and sleep disorders. Cell. 2011;146:194–207. doi: 10.1016/j.cell.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cirelli C, LaVaute TM, Tononi G. Sleep and wakefulness modulate gene expression in Drosophila. J Neurochem. 2005;94:1411–9. doi: 10.1111/j.1471-4159.2005.03291.x. [DOI] [PubMed] [Google Scholar]
- 17.Zimmerman JE, Rizzo W, Shockley KR, et al. Multiple mechanisms limit the duration of wakefulness in Drosophila brain. Physiol Genomics. 2006;27:337–50. doi: 10.1152/physiolgenomics.00030.2006. [DOI] [PubMed] [Google Scholar]
- 18.Williams JA, Sathyanarayanan S, Hendricks JC, Sehgal A. Interaction between sleep and the immune response in Drosophila: a role for the NFkappaB relish. Sleep. 2007;30:389–400. doi: 10.1093/sleep/30.4.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joiner WJ, Crocker A, White BH, Sehgal A. Sleep in Drosophila is regulated by adult mushroom bodies. Nature. 2006;441:757–60. doi: 10.1038/nature04811. [DOI] [PubMed] [Google Scholar]
- 20.Pitman JL, McGill JJ, Keegan KP, Allada R. A dynamic role for the mushroom bodies in promoting sleep in Drosophila. Nature. 2006;441:753–6. doi: 10.1038/nature04739. [DOI] [PubMed] [Google Scholar]
- 21.Ishimoto H, Kitamoto T. The steroid molting hormone Ecdysone regulates sleep in adult Drosophila melanogaster. Genetics. 2010;185:269–81. doi: 10.1534/genetics.110.114587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo F, Yi W, Zhou M, Guo A. Go signaling in mushroom bodies regulates sleep in Drosophila. Sleep. 2011;34:273–81. doi: 10.1093/sleep/34.3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mao Z, Roman G, Zong L, Davis RL. Pharmacogenetic rescue in time and space of the rutabaga memory impairment by using Gene-Switch. Proc Natl Acad Sci U S A. 2004;101:198–203. doi: 10.1073/pnas.0306128101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baines RA, Uhler JP, Thompson A, Sweeney ST, Bate M. Altered electrical properties in Drosophila neurons developing without synaptic transmission. J Neurosci. 2001;21:1523–31. doi: 10.1523/JNEUROSCI.21-05-01523.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nitabach MN, Wu Y, Sheeba V, et al. Electrical hyperexcitation of lateral ventral pacemaker neurons desynchronizes downstream circadian oscillators in the fly circadian circuit and induces multiple behavioral periods. J Neurosci. 2006;26:479–89. doi: 10.1523/JNEUROSCI.3915-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huber R, Hill SL, Holladay C, Biesiadecki M, Tononi G, Cirelli C. Sleep homeostasis in Drosophila melanogaster. Sleep. 2004;27:628–39. doi: 10.1093/sleep/27.4.628. [DOI] [PubMed] [Google Scholar]
- 27.Kuo TH, Handa A, Williams JA. Quantitative measurement of the immune response and sleep in Drosophila. J Vis Exp. 2012:e4355. doi: 10.3791/4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hammer Ø, Harper DAT, Ryan PD. PAST: Paleontological statistics software package for education and data analysis. Palaeontol Electronica. 2001;4:4. [Google Scholar]
- 29.Lee JE, Edery I. Circadian Regulation in the Ability of Drosophila to Combat Pathogenic Infections. Curr Biol. 2008;18:195–9. doi: 10.1016/j.cub.2007.12.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stone EF, Fulton BO, Ayres JS, Pham LN, Ziauddin J, Shirasu-Hiza MM. The circadian clock protein timeless regulates phagocytosis of bacteria in Drosophila. PLoS Pathog. 2012;8:e1002445. doi: 10.1371/journal.ppat.1002445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Devlin PF, Kay SA. Circadian photoperception. Annu Rev Physiol. 2001;63:677–94. doi: 10.1146/annurev.physiol.63.1.677. [DOI] [PubMed] [Google Scholar]
- 32.Schneider DS, Ayres JS. Two ways to survive infection: what resistance and tolerance can teach us about treating infectious diseases. Nat Rev Immunol. 2008;8:889–95. doi: 10.1038/nri2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Filipe SR, Tomasz A, Ligoxygakis P. Requirements of peptidoglycan structure that allow detection by the Drosophila Toll pathway. EMBO Rep. 2005;6:327–33. doi: 10.1038/sj.embor.7400371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pham LN, Dionne MS, Shirasu-Hiza M, Schneider DS. A specific primed immune response in Drosophila is dependent on phagocytes. PLoS Pathog. 2007;3:e26. doi: 10.1371/journal.ppat.0030026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ayres JS, Schneider DS. A signaling protease required for melanization in Drosophila affects resistance and tolerance of infections. PLoS Biol. 2008;6:e305. doi: 10.1371/journal.pbio.0060305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hendricks JC, Lu S, Kume K, Yin JC, Yang Z, Sehgal A. Gender dimorphism in the role of cycle (BMAL1) in rest, rest regulation, and longevity in Drosophila melanogaster. J Biol Rhythms. 2003;18:12–25. doi: 10.1177/0748730402239673. [DOI] [PubMed] [Google Scholar]
- 37.Koh K, Joiner WJ, Wu MN, Yue Z, Smith CJ, Sehgal A. Identification of SLEEPLESS, a sleep-promoting factor. Science. 2008;321:372–6. doi: 10.1126/science.1155942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Donlea J, Leahy A, Thimgan MS, et al. Foraging alters resilience/ vulnerability to sleep disruption and starvation in Drosophila. Proc Natl Acad Sci U S A. 2012;109:2613–8. doi: 10.1073/pnas.1112623109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hancock RE, Scott MG. The role of antimicrobial peptides in animal defenses. Proc Natl Acad Sci U S A. 2000;97:8856–61. doi: 10.1073/pnas.97.16.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lemaitre B, Hoffmann J. The host defense of Drosophila melanogaster. Annu Rev Immunol. 2007;25:697–743. doi: 10.1146/annurev.immunol.25.022106.141615. [DOI] [PubMed] [Google Scholar]
- 41.Tzou P, Reichhart JM, Lemaitre B. Constitutive expression of a single antimicrobial peptide can restore wild-type resistance to infection in immunodeficient Drosophila mutants. Proc Natl Acad Sci U S A. 2002;99:2152–7. doi: 10.1073/pnas.042411999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ito K, Awano W, Suzuki K, Hiromi Y, Yamamoto D. The Drosophila mushroom body is a quadruple structure of clonal units each of which contains a virtually identical set of neurones and glial cells. Development. 1997;124:761–71. doi: 10.1242/dev.124.4.761. [DOI] [PubMed] [Google Scholar]
- 43.Styer KL, Singh V, Macosko E, Steele SE, Bargmann CI, Aballay A. Innate immunity in Caenorhabditis elegans is regulated by neurons expressing NPR-1/GPCR. Science. 2008;322:460–4. doi: 10.1126/science.1163673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sun J, Singh V, Kajino-Sakamoto R, Aballay A. Neuronal GPCR controls innate immunity by regulating noncanonical unfolded protein response genes. Science. 2011;332:729–32. doi: 10.1126/science.1203411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rupp TL, Wesensten NJ, Bliese PD, Balkin TJ. Banking sleep: realization of benefits during subsequent sleep restriction and recovery. Sleep. 2009;32:311–21. doi: 10.1093/sleep/32.3.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ramm P, Smith CT. Rates of cerebral protein synthesis are linked to slow wave sleep in the rat. Physiol Behav. 1990;48:749–53. doi: 10.1016/0031-9384(90)90220-x. [DOI] [PubMed] [Google Scholar]
- 47.Mackiewicz M, Shockley KR, Romer MA, et al. Macromolecule biosynthesis: a key function of sleep. Physiol Genomics. 2007;31:441–57. doi: 10.1152/physiolgenomics.00275.2006. [DOI] [PubMed] [Google Scholar]
- 48.Richardson CE, Kooistra T, Kim DH. An essential role for XBP-1 in host protection against immune activation in C. elegans. Nature. 2010;463:1092–5. doi: 10.1038/nature08762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–29. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 50.De Gregorio E, Spellman PT, Tzou P, Rubin GM, Lemaitre B. The Toll and Imd pathways are the major regulators of the immune response in Drosophila. EMBO J. 2002;21:2568–79. doi: 10.1093/emboj/21.11.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Busse MS, Arnold CP, Towb P, Katrivesis J, Wasserman SA. A kappaB sequence code for pathway-specific innate immune responses. EMBO J. 2007;26:3826–35. doi: 10.1038/sj.emboj.7601798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lemaitre B, Reichhart J-M, Hoffmann JA. Drosophila host defense: Differential induction of antimicrobial peptide genes after infection by various classes of microorganisms. Proc Natl Acad Sci U S A. 1997;94:14614–9. doi: 10.1073/pnas.94.26.14614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kuo TH, Williams JA. Acute sleep deprivation enhances post-infection sleep and promotes survival during bacterial infection in Drosophila. Sleep. 2014;37:859–69. doi: 10.5665/sleep.3648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ganguly-Fitzgerald I, Donlea J, Shaw PJ. Waking experience affects sleep need in Drosophila. Science. 2006;313:1775–81. doi: 10.1126/science.1130408. [DOI] [PubMed] [Google Scholar]
- 55.Bushey D, Tononi G, Cirelli C. Sleep and synaptic homeostasis: structural evidence in Drosophila. Science. 2011;332:1576–81. doi: 10.1126/science.1202839. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Cox proportional hazard survival regression analyses.
Primers used for QPCR
RU486 has no effect on immune response measurements in MB-switch, UAS-Kir2.1, and UAS-NaChBac parent control flies. Effects of RU486 on sleep, survival, and resistance to infection are shown for (A-C) MB-switch, (D-F) UAS-Kir2.1, and (G-I) UAS-NaChBac parent control flies. (A,D,G) Mean ± SEM total time sleeping in minutes per 4 h is plotted relative to time of infection with S. marcescens in (A) MB-switch; n = 68 VEH and 76 RU486 treated flies, (D) UAS-Kir2.1; n = 77 VEH and 80 RU486 treated flies, and (G) UAS-NaChBac; n = 20 VEH and 30 RU486 treated flies. (B,E,H) Kaplan-Meier plots of flies surviving an infection with S. marcescens in (B) MB-switch flies, P > 0.09 log rank test, n = 127 VEH and 126 RU486 treated flies, (E) UAS-Kir2.1 flies, P > 0.92 log rank test, n = 92 VEH and 93 RU486 treated flies, and (H) UAS-NaChBac flies, P > 0.06 log rank test, n = 92 VEH and 90 RU486 treated flies. (C,F,I) Box-and-whisker plots of cfu/fly are plotted relative to the time of infection with S. marcescens. Error bars represent standard deviation. No significant differences were found between VEH and RU486 treated groups for (C) MB-switch, (F) UAS-Kir2.1, and (I) UAS-NaChBac flies (n = 6 groups for each condition across 3 independent experiments).
Waking activity rates in MB-switch>UAS-Kir2.1 and MB-switch>UAS-NaChBac flies. (A) Mean ± SEM activity per waking minute for a 24 h period is plotted for MB-switch>UAS-Kir2.1 flies maintained in constant light (LL; n = 64 VEH and 62 RU486 treated flies) or in a 12h: 12h light: dark cycle (LD; n = 46 for both VEH and RU486 treated groups); ** P < 0.01; student t-test. (B) Mean ± SEM activity per waking minute for a 24 h period is plotted for MB-switch>UAS-NaChBac flies maintained in LL (n = 32 each of VEH and RU486 treated flies) or in LD (n = 48 VEH and 49 RU486 treated flies), * P < 0.02, student t-test.
RU486-dependent reduction in sleep in MB-switch>UAS-mc* flies has no effect on immune function during bacterial infection. (A) Mean ± SEM total time sleeping in minutes per 4 h is plotted relative to time of infection with S. marcescens in MB-switch>UAS-mc* flies, n = 50 VEH and 55 RU486 treated flies. (B,C) Kaplan-Meier plots of MB-switch>UAS-mc* flies surviving (B) infection, P > 0.14, log rank test, n = 85 VEH and 92 RU486 treated flies, and (C) aseptic injury, P > 0.09, n = 94 each of VEH and RU486 treated flies. (D) Mean ± SEM total time sleeping in minutes per 4 h is plotted relative to time of infection with S. marcescens in UAS-mc* parent control flies, n = 71 VEH and 68 RU486-treated flies. (E) Kaplan-Meier plot of UAS-mc* flies surviving infection with S. marcescens, P > 0.09 log rank test, n = 109 VEH and 111 RU486-treated flies.
Expression of antimicrobial peptide genes in MB-switch>UAS-Kir2.1 and MB-switch>UAS-NaChBac flies with and without infection.
RU486 does not affect κB-luc reporter activity during infection in the MB-switch parent control flies. Mean ± SEM Luciferase activity (natural log, arbitrary units) is plotted against time of infection with S. marcescens in MB-switch Gal4 parent control flies carrying the κB-luc reporter. The reading at hour 0 was performed immediately before infection. RU486 had no effect on κB-luc activity relative to vehicle treated controls. Dead flies were discarded from the analysis at each time point; n = 84 flies total at time ‘0’ for both VEH and RU486 groups; n = 39 for VEH and n = 37 for RU486 group at time ‘24’. N = 3 independent experiments.