Abstract
Study Objectives:
The beneficial effect of sleep on memory consolidation is widely accepted in the adult population and has recently been shown in children. However, the few available data almost exclusively refer to school-aged children. Here we explore the effect of a daytime nap on memory consolidation in a sample of preschool children.
Design:
Subjects performed both a figures recognition task and a priming task, in order to differentiate effects on explicit and implicit memory.
Setting:
Nursery school.
Participants:
Twenty-three children (mean age: 52.6 ± 8 mo; 13 males) participated in the study.
Intervention:
After a study phase in which children had to name 40 pictures of objects and animals, each subject either took an actigraphically monitored nap or stayed awake. At retest, children were administered both an implicit and an explicit memory task.
Measurements and Results:
The implicit memory task consisted of naming 40 pictures presented at eight ascending levels of spatial filtering. The explicit memory task consisted of judging 40 pictures as old or new. The number of correct answers at the explicit recognition task was significantly higher in the nap compared to the wake condition, whereas priming effects did not differ between conditions.
Conclusions:
A positive role of sleep in explicit memory consolidation, similar to the one observed in the adult, was detected in our sample of preschool children. In contrast, our data suggest that implicit perceptual learning, involved in priming tasks, does not benefit from sleep.
Citation:
Giganti F, Arzilli C, Conte F, Toselli M, Viggiano MP, Ficca G. The effect of a daytime nap on priming and recognition tasks in preschool children. SLEEP 2014;37(6):1087-1093.
Keywords: sleep, explicit memory, implicit memory, priming, preschool children
INTRODUCTION
Memory is considered as a multicomponent system. According to Tulving,1 four systems of long-term memory— procedural memory, the perceptual representation system (priming effects), semantic and episodic memory—can be observed in humans. Whereas the concept of declarative (or explicit) memory refers to semantic and episodic memory, procedural memory and priming effects are usually labeled as nondeclarative (or implicit) memory.2 As proposed by Voss and Paller,3 implicit memory includes all those neurocognitive processes that support memory “without the concomitant awareness of memory retrieval”. The same stimuli can simultaneously be processed (and then retrieved) in an implicit and explicit manner as shown both in healthy subjects4 and in pathological conditions.5
A well-known approach to assess implicit learning is the study of the priming effect. Priming refers to facilitative changes in the ability to identify, generate, or process an item because of a prior encounter with this item or a similar stimulus.4 The most common type of priming test measures perceptual priming (also called repetition priming or item-specific priming), which is believed to express facilitated or more fluent perceptual processing of the physical features of repeated items.3
As suggested by Rauchs and colleagues,2 priming and procedural skills, both of them implicit forms of memory, may differ in the nature of the stored representation and in its neural substrates. In perceptual priming tasks, stored information usually corresponds to items (i.e., drawings or words) presented only once, whereas in procedural memory tasks the acquisition process takes place over several training sessions. Moreover, priming and procedural memory refer to different memory systems, as suggested by the occurrence of neuropsychological dissociations (e.g., those appearing in subcortical dementias6), and are subserved by different brain areas, as highlighted by neuroimaging studies (subcortical areas such as the striatum for procedural memory7 versus neocortical areas for priming8).
In the adult human, the so-called sleep effect—i.e., the facilitating role of sleep, relative to wakefulness, on consolidation of newly acquired material—has been widely shown for both explicit and implicit memory.9,10 It is acknowledged that sleep's unique properties, in addition to creating the ideal circumstances for memory storage to take place (i.e., by protecting learned material from interference), actively engage consolidation processes through reactivations of memory traces.11 In the past two decades, the debate in sleep memory research has been intensively focused on trying to enlighten which sleep features play the greater role in sustaining memory consolidation. Although we are not going to discuss details with this issue, which is the object of extensive literature and of two systematic reviews of ours,9,10 it is worthwhile to note that many authors, as also commented on by Rauchs et al.,2 have investigated how different types of explicit and implicit memories might benefit from different types of sleep. In the explicit domain, according to the tasks used, consolidation of episodic memory would depend on nonrapid eye movement (NREM) sleep,12 slow wave sleep (SWS),13–15 rapid eye movement (REM) sleep,16,17 or on a combination of SWS and REM sleep,18 whereas semantic learning appears to rely on REM sleep.19 As for implicit memory, perceptual motor skills would mainly be linked to NREM sleep and its neurophysiological features,20,21 whereas REM sleep would be involved in learning more complex cognitive skills (such as those required to solve the Towers of Hanoi Task)22 and in the perceptual representation system,15,23 whereas sensory-perceptual skills would rely more consistently on a combination of SWS and REM sleep.24,25
Much less is known concerning sleep-memory relationships in early development. In 15-mo-old babies, Gomez and colleagues26 found that the learning of an artificial language significantly benefitted from sleep. As for childhood, it has been shown that declarative tasks are enhanced by sleep in the 9–12 y27 and in the 6–8 y age ranges.28 In comparable age ranges, with regard to procedural memory, no postsleep improvement was found for a finger sequence tapping task,28 and a worsening of performance was even shown by Fischer et al.29 using a Serial Reaction Time Task. Research in the preschool age range (4–6 y.) is surprisingly very scarce. The only study, carried out by Wilhelm and colleagues,30 found evidence that sleep dependent improvements in procedural memory may be obtained provided that presleep performance level is enhanced through extended training.
The effects of sleep on priming are also poorly investigated and the few studies are limited to the adult. Plihal and Born15 used a word stem completion task to assess the effects of partial sleep deprivation on a priming task. The authors found that subjects who had slept during the second half of the night produced, in the test phase, significantly more items belonging to the initial list than those who had slept during the first half, proposing a role of REM sleep for the consolidation of implicit information. Likewise, using the same partial sleep deprivation paradigm, Wagner and colleagues17 found that priming effects in recognition of unknown faces were stronger when the retention interval was dominated by REM sleep (late night sleep). Nevertheless, in a more recent study, Rauchs and colleagues31 did not confirm a beneficial effect of sleep on perceptual priming. In this study, priming effects seemed instead affected by circadian factors (i.e., they were stronger during the day than at night).
We believe that priming could be particularly interesting to study in childhood because (1) the tasks used to show priming effects do not require awareness in the learning and retrieval phase; and (2) the development of implicit memory precedes that of explicit memory. Notable dissociations in the developmental trajectories of the priming system compared to the explicit memory system were already remarked by Tulving and Schacter.4 Converging evidence indicates that cognitive processes that mediate perceptual priming are operative by 4 y of age and do not undergo significant developmental changes over the school years, whereas the intentional component of recognition performance does improve with increasing age.32–34 Despite the relevance of the implicit memory system in the early years, to our knowledge, there has not been any research carried out so far in children on the relationships between sleep and priming.
Given the paucity of data on sleep and memory in the preschool age, the aims of this study are to explore the effect of sleep on memory consolidation, through a daytime nap model, in a sample of 3- to 5-y-old children, through both a figures recognition task and an identification (priming) task. This will allow us to differentiate between effects on explicit and implicit memory.
METHODS
Subjects
Twenty-three children (mean age: 52.6 ± 8 mo; age range: 38–70 mo, 13 males) participated in the study. Inclusion criteria were: (1) the absence of any sleep disturbance; (2) no intake of medications acting on the central nervous system, with particular regard to tranquilizers, antiepileptic drugs, antihistamines; (3) the absence of any type of cognitive impairment because of genetic syndromes or other pathologies, as reported by parents and/or teachers, as well as an IQ higher than 90 on the Italian versions of Wechsler Preschool and Primary Scale of Intelligence (WPPSI) (Orsini A, Picone L. WPPSI. Contributo alla taratura Italiana [WPPSI. Contribution to Italian standardization]. Organizzazioni Speciali, Florence, Italy, 1996).
Memory Tasks
Children were individually tested in a dimly lit and quiet room, sitting in front of a computer screen at a distance of 57 cm. For each subject, stimuli consisted overall of 160 black-and-white photographs of real-life objects and animals, taken from a standardized pool with equal perceptual complexity and familiarity,35 divided into two sets (A and B) of 80 figures (40 animals and 40 objects), each one used for a single experimental condition, wake (W) versus sleep (S). In order to control for the difficulty of stimuli sets, the association between stimuli set (A and B) and condition (W and S) was also balanced.
In the study phase, children were asked to name 40 pictures (20 animals and 20 objects) presented in random order on the LCD screen of a laptop. Each figure was presented with canonical orientation for 1000 ms, with a variable interstimuli interval (ISI) depending on the time required by the child to give his answer. All stimuli were centered on a white background and subtended a visual angle of between 5° and 8°. In the test phase, a priming and a recognition task were performed in counterbalanced order within subjects (sleep and wake conditions) and between subjects. Furthermore, to control for the difficulty of items, two additional sets were created (A2 and B2), whereas stimuli presented as new in the set A and B became old and vice-versa. Stimuli sets (A, B, A2, B2) were presented in counter-balanced order in both memory tasks.
Identification Task (Priming)
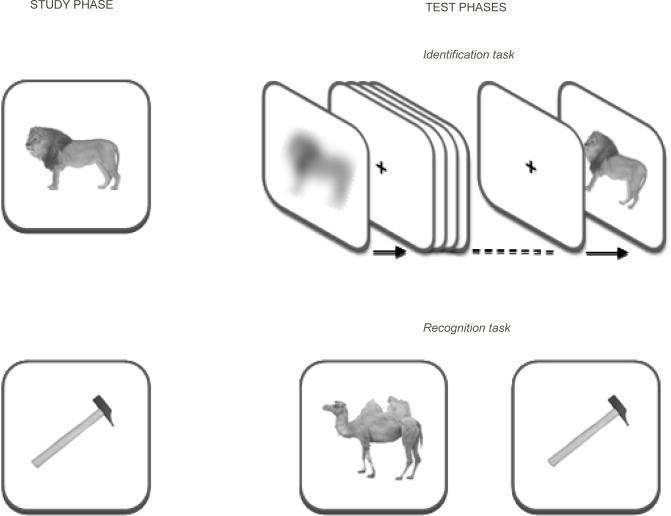
In the priming task, 40 pictures (10 animals and 10 objects presented in the study phase plus 10 new animals and 10 new objects) were subjected to seven different levels of spatial filtering, following a coarse-to-fine order that gradually integrated spatial information, as shown in Figure 1.
Figure 1.
Schematic illustration of study and test phases. Study phase: images of animals and tools were presented in their complete form (a lion as an example). Test phase: in the identification task (i.e., priming task), subjects were asked to name each picture, presented in an ascending sequence of nine levels of filtering (see text for details); in the recognition task, old and new images were displayed asking subjects whether they had been presented in the study phase or not.
The low-pass filtering process was achieved using a Gaussian digital filter applied to the bidimensional array representing the original image scanned at a resolution of 300 dpi.36 Each picture was presented in an ascending sequence of eight frames starting from the most blurred and adding new ranges of high spatial frequencies up to its complete version (Figure 1). Each filtered image was shown for a duration of 700 ms. Children were asked to name each picture, at each level of filtering, and feedback on identification accuracy (‘‘right’’ or ‘‘wrong’’) was given. If children were not able to name the picture, they were not prompted to guess, but encouraged to describe the functions (for objects) and characteristics (for animals).
Recognition Task (Explicit Memory)
In the recognition task, 40 pictures (10 animals and 10 objects presented in the study phase plus 10 new animals and 10 new objects) were presented. Each picture was presented in its complete version for 500 ms. Children were asked to judge whether each picture was old or new (Figure 1).
Sleep Assessment
Daytime naps were recorded at school by means of actigraphy, using Actiwatch-Plus actimeters (Cambridge Neurotechnology Ltd, Pampisford, Cambridge, UK).
Through the analysis of activity levels (Sleep Analysis Software, Copyright Cambridge Neurotechnology Ltd, Version 3.24), we were able to determine the following sleep measures: bedtime, rise time, sleep latency (SL), total sleep time (TST), duration of wake after sleep onset (WASO), and sleep efficiency (SE).
The parents were interviewed about children's habitual sleep schedules in order to control that the nighttime sleep episodes preceding the days of experimental sessions did not differ from each other or from habitual sleep in duration or quality.
Vigilance Assessment
To rule out that children's performances were affected by a vigilance trough because of the lack of the habitual nap in the wake condition, or by sleep inertia effects, a reaction time test was performed in both conditions (wake, W and sleep, S) both before the study (RTtask1) and before the test phase (RTtask2). Stimuli for the reaction time task consisted of 20 ghosts appearing on the laptop screen and subjects were instructed to press the keyboard space bar as soon as possible at the ghost's appearance.
Design and Procedure
The study was carried out during wintertime in a public Nursery School located in Florence, whose staff had expressed its availability and where all children were accustomed to take a nap during the afternoon in the context of their daily school routine.
The headmaster of the selected school was first contacted through a formal letter, introducing the research and the professionals involved. All the procedures, instruments, and aims of the study were explained in a meeting, extended to teachers and parents' representatives. A final meeting served to illustrate the study to all the children's parents and to collect their informed consent. The study was approved by the Ethical Committee of the Department of Psychology, University of Naples II.
The parents were interviewed about their children's habitual sleep schedules in order to control that the night sleep preceding the days of the experimental sessions did not differ from habitual sleep in duration or quality. All experimental sessions started at approximately 12:00, when subjects began to wear the actimeter. After administration of the reaction time task, the study phase started at about 12:30.
Each subject underwent two conditions, (S and W) separated by an interval of at least 1 w. In S, subjects went to bed immediately after the study phase and were allowed to spontaneously wake up from the nap until max 15:30, when the teacher was instructed to wake them up. The test phase was carried out 10 min after final awakening, to allow dissipation of sleep inertia. In W, after the study phase children had to stay awake in the retention interval (playing and/or drawing with the experimenter). The W condition always followed S, so that children could undergo the test phase after a time interval corresponding to the one obtained in the sleep condition (sleep duration + 10 min).
Data Analysis
Statistical analysis was performed using the Statistical Package for Social Sciences (SPSS, version 16.0; SPSS Inc, Chicago, IL, USA).
Reaction times were submitted to repeated-measures analysis of variance (ANOVA) with two within-subjects factors: time (two levels: before study phase and before test phase) and condition (two levels: S and W).
The priming effect was tested separately for S and W. In each condition, the percentages of correct identification for each filtering level were calculated and compared by means of repeated-measures ANOVA with two within-subjects variables: priming (two levels: old versus new) and levels of filtering (eight levels). In order to assess the presence of a sleep effect on priming, the percentages of correct identifications for each filtering level for old items were compared between S and W through repeated-measures ANOVA with two within-subjects variables: condition (two levels: S versus W) and levels of filtering (eight levels).
Concerning explicit memory, we have preliminarily calculated the percentages of correctly identified old stimuli (hits) versus the percentage of the unrecognized ones (misses), as well as the percentage of correctly rejected new stimuli (correct rejections) versus the percentage of those wrongly judged as already presented (false alarms). Afterward, for both S and W, measures of old/new discrimination (Pr) and response bias (Br) were computed, according to Snodgrass and Corwin,37 as follows: Pr = [p(hit)-p(false alarm)]; Br = [p(false alarm)/1-Pr]. Measures of Pr provide an unbiased estimate of the accuracy in the response to old and new items, with higher values corresponding to more accurate recognition memory. Br indicates the overall tendency to respond “old” or “new”, regardless of accuracy.
All dependent variables were compared between S and W through t tests for paired samples. Significance was set at P ≤ 0.05.
RESULTS
Sleep Measures
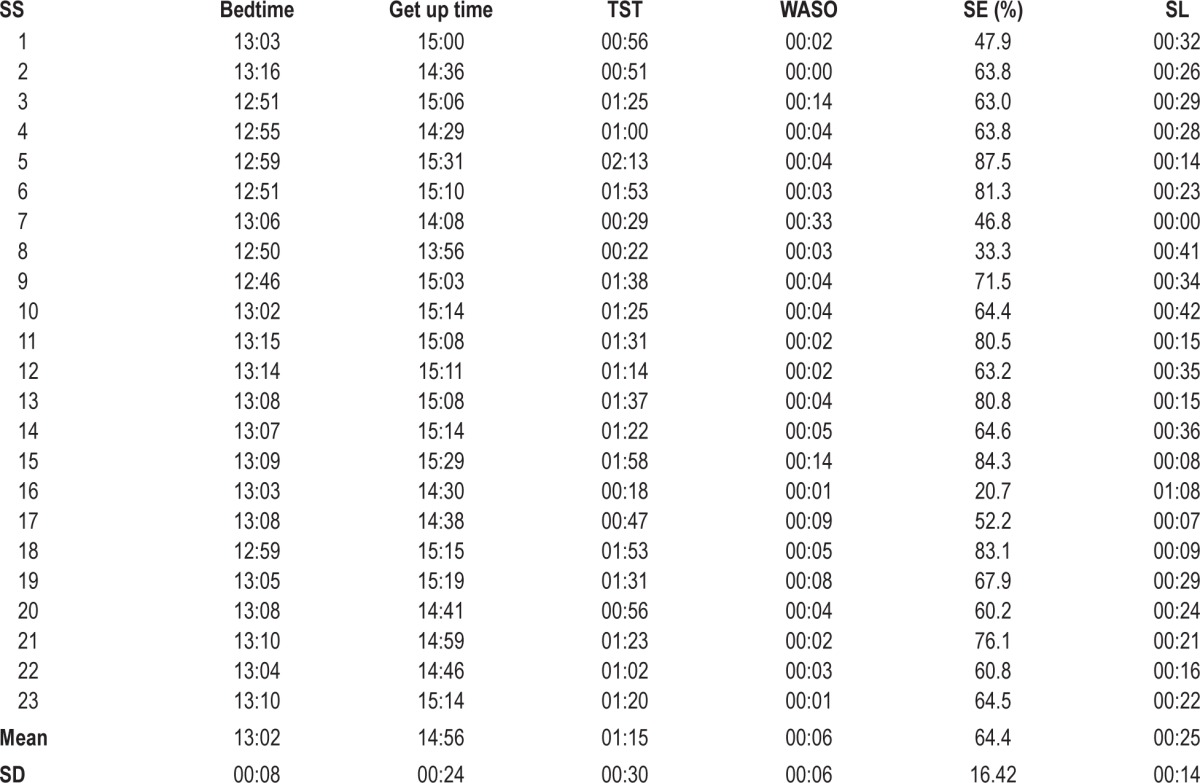
Results from sleep recordings are summarized in Table 1. By means of the interviews carried out with the children's parents, it was ascertained that no subject showed different sleep patterns between conditions or compared to the habitual ones during the nights preceding each experimental session.
Table 1.
Sleep Measures (means ± SD)
Vigilance
At the RTtask1, mean reaction time was 500.66 ± 86.7 ms in the S condition and 492.31 ± 87.5 ms in the W condition; at the RTtask2 mean reaction time was 513.03 ± 104.4 ms in the S condition and 534.96 ± 99.8 ms in W. ANOVA revealed a significant effect of time (F1,22 = 7.49, P = 0.01), whereas condition and the interaction time × condition were not significant (respectively: F1,22 = 0.19, ns; F1,22 = 1.25, not significant [ns]).
Priming Effect
In S, the analysis revealed a significant main effect of priming (F1,22 = 33.93, P < 0.001), a significant effect of levels of filtering (F7,154 = 16.81, P < 0.001), and a significant interaction between the factors (F4,87 = 4.03, P = 0.005). As shown in Figure 2A, a greater number of old items were correctly identified compared to new ones. Post hoc analysis revealed a significant difference at level of filtering 1 (t = 2.6, df = 22, P = 0.01), 2 (t = 4.67, df = 22, P < 0.001), 3 (t = 2.65, df = 22, P = 0.01) and 7 (t = 2.51, df = 22, P = 0.01).
Figure 2.
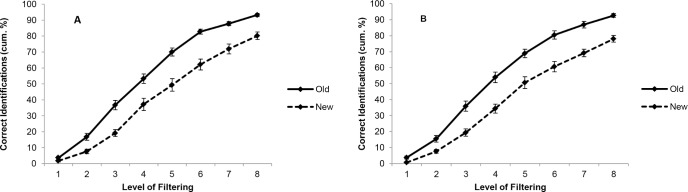
Cumulative percentages (mean ± standard error) of correct identification obtained in the priming task at each level of filtering, in both the S (A) and the W (B) conditions.
In W, the ANOVA displayed a significant main effect of priming (F1,22 = 44.80, P < 0.001), a significant effect of levels of filtering (F7,154 = 19.66, P < 0.001), and a significant interaction between the factors (F7,154 = 2.83, P = 0.023). As shown in Figure 2B, a greater number of old items were correctly identified compared to new ones. Post hoc analysis revealed a significant difference at level of filtering 1 (t = 3.48, df = 22, P = 0.002), 2 (t = 2.71, df = 22, P = 0.01) and 3 (t = 3.10, df = 22, P = 0.005).
Sleep Effect on Implicit Memory
The analysis revealed a significant main effect of levels of filtering (F7,154 = 22.26, P < 0.001), whereas the effect of condition (F1,22 = 0.21, ns) and the interaction between factors (F4,94 = 0.34, ns) were not significant.
Sleep Effect on Explicit Memory
As shown in Figure 3, Pr at test phase turned out to be significantly higher when the retention interval was spent in S than in W (t = 2.89, df = 22, P = 0.008). Instead, no significant differences between S and W were observed in Br (t = 1.12, df = 22, ns).
Figure 3.
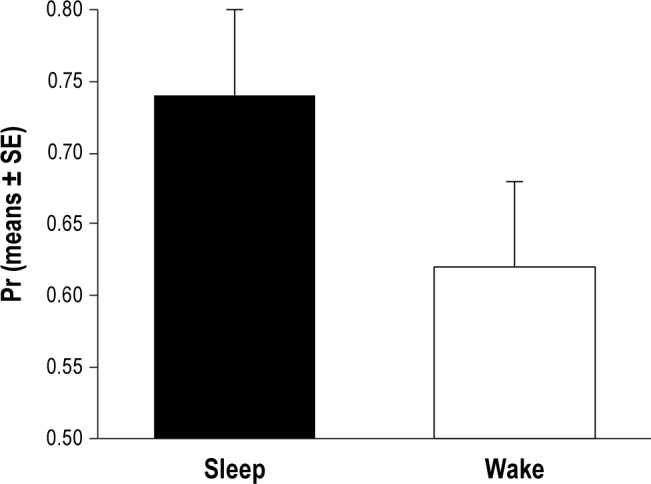
Performance accuracy at the explicit memory task, in the sleep and wake conditions, expressed as Pr values (proportion of hits minus proportion of false alarms).
Furthermore, children achieved a significantly higher percentage of hits (and a lower percentage of misses) when the retention interval was spent in sleep rather than in wake (S: 87.4% hits, 12.6% misses; W = 77.4% hits, 22.6% misses; t = 2.37, df = 22, P = 0.02). Instead, no significant differences between S and W were observed with regard to the percentage of false alarms and correct rejections (S: 86.3% correct rejections, 13.7% false alarms; W: 84.8% correct rejections, 15.2% false alarms; t = 0.41, df = 22, ns).
Through Pearson correlation analysis, we verified that hits did not significantly correlate with reaction times, either in S (RTtask1: r = 0.19, ns; RTtask2: r = 0.24, ns) or in W (RTtask1: r = 0.25, ns; RTtask2: r = 0.33, ns), thus ruling out the possibility that memory performance was affected by sleepiness levels.
DISCUSSION
In our study, we decided to investigate the sleep effect on learning processes addressing both explicit (recognition task) and implicit (priming task) memory, in a sample of preschool children (3-5 y old), thus focusing on an age range that had been almost totally neglected in the past.
Our results show a significant sleep dependent facilitation of explicit memory, as children in our sample correctly recognized a higher number of the images presented before the retention interval when this was spent in S relative to W. Interestingly, this piece of evidence replicates what is shown in a number of nap studies on adults for both declarative and procedural memory,38 and suggests that short daytime sleep episodes can play a meaningful role even in early development. If this is the case, this beneficial effect of daytime sleep for certain kinds of learning processes may obviously have an applicative fallout when conceiving school timetables and schedules. It goes without saying that support to the link between the sleep episode and the explicit memory enhancement would be provided by the finding of significant correlations between memory performance and sleep parameters. In the current study, actigraphic measures were not sufficient to this aim because they do not include those sleep features believed to play the crucial role in the sleep effect (such as sleep states amount, cycles number and duration, sleep spindles density). Future polysomnographic studies are recommended to further explore this issue.
Conversely, no sleep dependent enhancement was found for children's performance at the priming task. This finding might seem counterintuitive with regard to the centrality of implicit memory in childhood and to the differential trajectories of implicit and explicit memory during early development. According to the relative immaturity of the explicit memory system as compared to the implicit one at this age,4,32,33 we might have expected a greater effect of sleep on implicit memory. However, a few possible explanations of this result should be taken into account.
First, as we propose in a very recent review,10 sleep seems to be more effective for the process of “memory reshaping”— i.e, the formation of connections between related items of knowledge—than for the mere strengthening of rote memories. Because perceptual priming concerns the automatic storage of items as they were perceived in their original form and does not involve specific reelaboration, we can speculate that this particular ability does not require further enhancement through sleep dependent consolidation mechanisms. This hypothesis could explain the apparent disagreement between our finding and that of Gómez and colleagues, the only other research published so far on sleep and implicit memory in early development, showing a positive effect of sleep on this type of memory in infancy.26 In fact, in addition to the different age range selected (15-mo-old babies in Gómez et al.), there is an important difference in the tasks used, although both referable to the general category of “implicit memory”. In Gómez et al.'s study, the children were familiarized with an artificial language, whose learning involved relating words in auditory strings applied to stimuli that were similar but not identical to those from familiarization. Therefore, it cannot be ruled out that, although sleep does not facilitate the elementary memory processes pertaining to the perceptual representation system, it could instead exert a positive role for a task that requires the extraction of abstract relationships among stimuli by reshaping memory traces, as in Gómez et al.26 In this perspective, the differential effect of sleep on explicit and implicit learning emerged in our study is not surprising. In fact, even though the explicit task we used does not overtly put in evidence a reorganization of knowledge, we cannot exclude that the memory process activated by sleep, that which allowed performance enhancement, was based on the integration of the learned images in preexisting knowledge networks, thus on a reconstructive memory process. This would also represent a plausible explanation of why the facilitating role of sleep is exerted, as shown by the separate analysis of hits and correct rejections, on the correct identification of old stimuli (i.e., what can be integrated in networks of preexisting knowledge) rather than on the ability to detect new stimuli.
Furthermore, as shown by studies performed in patients with neurological diseases,5 the neural circuits of implicit learning, and specifically those of visual perceptual memory, are per se more stable and resistant than those underlying other types of memory. Thus, the functional improvement that sleep exerts on different types of memories might not be useful or necessary for this memory system, which would maintain its effectiveness independently from the individual's behavioral state.
Therefore, it should be noted that evidence of a dissociation of the sleep effect on memory, depending on the tasks used and the memory system involved, is not surprising. It is largely accepted that different types of learning processes are served by distinct neural correlates.3 In some studies,3 these have been explored using event-related brain potentials (ERPs) and functional magnetic resonance imaging (fMRI). A common finding from these studies is that distinct neurocognitive events are responsible for implicit and explicit memory. In light of our results, we can hypothesize that neural substrates underlying explicit memory take advantage from sleep to a greater extent than neural substrates underlying implicit memory.
We must acknowledge, however, that the lack of sleep dependent enhancement on priming observed in our study could possibly be because the task used was not sensitive enough to detect an effect of sleep. Because no normative data have been produced so far in the considered age range, we cannot exclude that children of our sample already performed, in the W condition, at the highest possible level, thus leaving no room for improvements related to the memory enhancing effects of the sleep episode.
Finally, an alternative explanation is that sleep features expressed in daytime naps, which are often quite different from those of night sleep (in terms of reduced amount of SWS and REM sleep, as well as of reduced NREM-REM cycles), could not be fully adequate to achieve a facilitating effect on priming. Also, as previously mentioned, actigraphic measures are not able to discriminate different sleep states within the nap, so we cannot rule out that the reported lack of sleep effects on priming could be because of the scarcity of certain sleep components, such as REM sleep or complete NREM-REM cycles. As a matter of fact, when examining the literature on the sleep effect on priming in adults, we find one negative31 and two positive results.15,23 The latter claim that the sleep effect on the priming task should be attributed to REM sleep.
This limitation in sensitivity of measures, however, is counterbalanced by the strength of our methodological choices in terms of feasibility and of ecological validity. The choice of a nap model and of actigraphic rather than polygraphic recordings allowed us experimental access to a very young population, in a specific context (a nursery school) where daytime naps were already part of the daily school schedule. In that particular environment, we deemed the use of actigraphy more suitable, as a noninvasive recording method, than standard polysomnography.
In conclusion, a daytime nap in preschool children appears able to improve the consolidation of explicit memories, confirming the existence of a nap effect on explicit learning also at that age. Instead, priming does not benefit from sleep in 3- to 5-y-old children, but further studies are needed to replicate these results with a night-sleep paradigm. Also, it has to be kept in mind that our results on priming are not automatically generalizable to all implicit tasks, and that much research is still necessary to obtain a clear and comprehensive picture of the role of sleep on the implicit domain in children.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank Dr. Deborah Warton for her accurate language editing of the text.
REFERENCE
- 1.Tulving E. Organization of memory: Quo vadis? In: Gazzaniga MS, editor. The cognitive neuroscience. Cambridge, MA: MIT Press; 1995. pp. 839–47. [Google Scholar]
- 2.Rauchs G, Desgranges B, Foret J, Eustache F. The relationships between memory systems and sleep stages. J Sleep Res. 2005;14:123–40. doi: 10.1111/j.1365-2869.2005.00450.x. [DOI] [PubMed] [Google Scholar]
- 3.Voss JL, Paller KA. Brain substrates of implicit and explicit memory: the importance of concurrently acquired neural signals of both memory types. Neuropsychologia. 2008;46:3021–9. doi: 10.1016/j.neuropsychologia.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tulving E, Schacter DL. Priming and human memory systems. Science. 1990;247:301–6. doi: 10.1126/science.2296719. [DOI] [PubMed] [Google Scholar]
- 5.Viggiano MP, Gori G, Zaccara G, Righi S, Vannucci M, Giovannelli F. Category- specific visual identification of filtered objects in Alzheimer's disease. Arch Gerontol Geriatr. 2007;44:125–39. doi: 10.1016/j.archger.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 6.Butters N, Heindel WC, Solomon DP. Dissociation of implicit memory in dementia. Neurological implications. Bull Psychonomic Soc. 1990;28:359–66. [Google Scholar]
- 7.Hikosaka O, Miyashita K, Miyachi S, Sakaı¨K Lu X. Differential roles of the frontal cortex, basal ganglia, and cerebellum in visuomotor sequence learning. Neurobiol Learn Mem. 1998;70:137–49. doi: 10.1006/nlme.1998.3844. [DOI] [PubMed] [Google Scholar]
- 8.Buckner RL, Petersen SE, Ojemann JG, Miezin FM, Squire LR, Raichle ME. Functional anatomical studies of explicit and implicit memory retrieval tasks. J Neurosci. 1995;15:12–29. doi: 10.1523/JNEUROSCI.15-01-00012.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ficca G, Salzarulo P. What in sleep is for memory. Sleep Med. 2004;5:225–30. doi: 10.1016/j.sleep.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 10.Conte F, Ficca G. Caveats on psychological models of sleep and memory: a compass in an overgrown scenario. Sleep Med Rev. 2013;17:105–21. doi: 10.1016/j.smrv.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 11.Ellenbogen JM, Payne JD, Stickgold R. The role of sleep in declarative memory consolidation: passive, permissive, active or none? Curr Opin Neurobiol. 2006;16:716–22. doi: 10.1016/j.conb.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 12.Gais S, Mölle M, Helms K, Born J. Learning-dependent increases in sleep spindle density. J Neurosci. 2002;22:6830–4. doi: 10.1523/JNEUROSCI.22-15-06830.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yaroush R, Sullivan MJ, Ekstrand BR. Effect of sleep on memory. II. Differential effect of the first and second half of the night. J Exp Psychol. 1971;88:361–6. doi: 10.1037/h0030914. [DOI] [PubMed] [Google Scholar]
- 14.Plihal W, Born J. Effects of early and late nocturnal sleep on declarative and procedural memory. J Cogn Neurosci. 1997;9:534–47. doi: 10.1162/jocn.1997.9.4.534. [DOI] [PubMed] [Google Scholar]
- 15.Plihal W, Born J. Effects of early and late nocturnal sleep on priming and spatial memory. Psychophysiology. 1999;36:571–82. [PubMed] [Google Scholar]
- 16.Empson JA, Clarke PR. Rapid eye movements and remembering. Nature. 1970;277:287–8. doi: 10.1038/227287a0. [DOI] [PubMed] [Google Scholar]
- 17.Wagner U, Gais S, Born J. Emotional memory formation is enhanced across sleep intervals with high amounts of rapid eye movement sleep. Learn Mem. 2001;8:112–9. doi: 10.1101/lm.36801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rauchs G, Bertran F, Guillery-Girard B, et al. Consolidation of strictly episodic memories mainly requires rapid eye movement sleep. Sleep. 2004;27:395–401. doi: 10.1093/sleep/27.3.395. [DOI] [PubMed] [Google Scholar]
- 19.Mandai O, Guerrien A, Sockeel P, Dujardin K, Leconte P. REM sleep modifications following a Morse code learning session in humans. Physiol Behav. 1989;46:639–42. doi: 10.1016/0031-9384(89)90344-2. [DOI] [PubMed] [Google Scholar]
- 20.Huber R, Ghilardi MF, Massimini M, Tononi G. Local sleep and learning. Nature. 2004;430:78–81. doi: 10.1038/nature02663. [DOI] [PubMed] [Google Scholar]
- 21.Walker MP, Brakefield T, Morgan A, Hobson JA, Stickgold R. Practice with sleep makes perfect: sleep-dependent motor skill learning. Neuron. 2002;35:5–7. doi: 10.1016/s0896-6273(02)00746-8. [DOI] [PubMed] [Google Scholar]
- 22.Conway J, Smith CT. REM sleep and learning in humans: a sensitivity to specific types of learning tasks. J Sleep Res. 1994;3:48. [Google Scholar]
- 23.Wagner U, Hallschmid M, Verleger R, Born J. Signs of REM sleep dependent enhancement of implicit face memory: a repetition priming study. Biol Psychol. 2003;62:197–210. doi: 10.1016/s0301-0511(02)00125-4. [DOI] [PubMed] [Google Scholar]
- 24.Gais S, Plihal W, Wagner U, Born J. Early sleep triggers memory for early visual discrimination skills. Nat Neurosci. 2000;3:1335–9. doi: 10.1038/81881. [DOI] [PubMed] [Google Scholar]
- 25.Stickgold R, Whidbee D, Schirmer B, Patel V, Hobson JA. Visual discrimination task improvement: a multi-step process occurring during sleep. J Cogn Neurosci. 2000;12:246–54. doi: 10.1162/089892900562075. [DOI] [PubMed] [Google Scholar]
- 26.Gómez RL, Bootzin RR, Nadel L. Naps promote abstraction in language-learning infants. Psychol Sci. 2006;17:670–4. doi: 10.1111/j.1467-9280.2006.01764.x. [DOI] [PubMed] [Google Scholar]
- 27.Backhaus J, Hoeckesfeld R, Born J, Hohagen F, Junghanns K. Immediate as well as delayed post learning sleep but not wakefulness enhances declarative memory consolidation in children. Neurobiol Learn Mem. 2008;89:76–80. doi: 10.1016/j.nlm.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 28.Wilhlem I, Diekelmann S, Born J. Sleep in children improves memory performance on declarative but not procedural tasks. Learn Mem. 2008;15:373–7. doi: 10.1101/lm.803708. [DOI] [PubMed] [Google Scholar]
- 29.Fischer S, Wilhelm I, Born J. Developmental differences in sleep's role for implicit offline learning: comparing children with adults. J Cogn Neurosci. 2007;19:214–27. doi: 10.1162/jocn.2007.19.2.214. [DOI] [PubMed] [Google Scholar]
- 30.Wilhelm I, Metzkow-Mészàros M, Knapp S, Born J. Sleep-dependent motor memory consolidation in children and adults: The pre-sleep level of performance matters. Dev Sci. 2012;15:506–15. doi: 10.1111/j.1467-7687.2012.01146.x. [DOI] [PubMed] [Google Scholar]
- 31.Rauchs G, Lebreton K, Betran F, et al. Effects of partial sleep deprivation on within-format and cross-format priming. Sleep. 2006;29:58–68. [PubMed] [Google Scholar]
- 32.Parkin AJ, Streete S. Implicit and explicit memory in young children and adults. Br J Psychol. 1988;79:361–9. [Google Scholar]
- 33.Russo R, Nichelli P, Gibertoni M, Cornia C. Developmental trends in implicit and explicit memory: a picture completion study. J Exp Child Psychol. 1995;59:566–78. [Google Scholar]
- 34.Hayes BK, Hennessy R. The nature and development of nonverbal implicit memory. J Exp Child Psychol. 1996;63:22–43. doi: 10.1006/jecp.1996.0041. [DOI] [PubMed] [Google Scholar]
- 35.Viggiano MP, Vannucci M, Righi S. A new standardized set of ecological pictures for experimental and clinical research on visual object processing. Cortex. 2004;40:491–509. doi: 10.1016/s0010-9452(08)70142-4. [DOI] [PubMed] [Google Scholar]
- 36.Vannucci M, Viggiano MP, Argenti F. Identification of spatially filtered stimuli as function of the semantic category. Brain Res Cogn Brain Res. 2001;12:475–8. doi: 10.1016/s0926-6410(01)00086-6. [DOI] [PubMed] [Google Scholar]
- 37.Snodgrass JG, Corwin J. Pragmatics of measuring recognition memory: applications to dementia and amnesia. J Exp Psychol Gen. 1988;117:34–50. doi: 10.1037//0096-3445.117.1.34. [DOI] [PubMed] [Google Scholar]
- 38.Ficca G, Axelsson J, Mollicone DJ, Muto V, Vitiello MV. Naps, cognition and performance. Sleep Med Rev. 2010;14:249–58. doi: 10.1016/j.smrv.2009.09.005. [DOI] [PubMed] [Google Scholar]