Abstract
Study Objectives:
Evaluate the relative contributions of weight status and obstructive sleep apnea (OSA) to cardiopulmonary exercise responses in children.
Design:
Prospective, cross-sectional study. Participants underwent anthropometric measurements, overnight polysomnography, spirometry, cardiopulmonary exercise function testing on a cycle ergometer, and cardiac doppler imaging. OSA was defined as ≥ 1 obstructive apnea or hypopnea per hour of sleep (OAHI). The effect of OSA on exercise function was evaluated after the parameters were corrected for body mass index (BMI) z-scores. Similarly, the effect of obesity on exercise function was examined when the variables were adjusted for OAHI.
Setting:
Tertiary pediatric hospital.
Participants:
Healthy weight and obese children, aged 7–12 y.
Interventions:
N/A.
Measurements and Results:
Seventy-one children were studied. In comparison with weight-matched children without OSA, children with OSA had a lower cardiac output, stroke volume index, heart rate, and oxygen consumption (VO2 peak) at peak exercise capacity. After adjusting for BMI z-score, children with OSA had 1.5 L/min (95% confidence interval -2.3 to -0.6 L/min; P = 0.001) lower cardiac output at peak exercise capacity, but minute ventilation and ventilatory responses to exercise were not affected. Obesity was only associated with physical deconditioning. Cardiac dysfunction was associated with the frequency of respiratory-related arousals, the severity of hypoxia, and heart rate during sleep.
Conclusions:
Children with OSA are exercise limited due to a reduced cardiac output and VO2 peak at peak exercise capacity, independent of their weight status. Comorbid OSA can further decrease exercise performance in obese children.
Citation:
Evans CA, Selvadurai H, Baur LA, Waters KA. Effects of obstructive sleep apnea and obesity on exercise function in children. SLEEP 2014;37(6):1103-1110.
Keywords: child, exercise function, heart, obesity, obstructive sleep apnea
INTRODUCTION
Growing evidence suggests that a bidirectional relationship exists between childhood obesity and sleep dysfunction. Childhood obesity is a major risk factor for obstructive sleep apnea (OSA), and the likelihood of an obese child developing OSA is four to five times greater than in a nonobese child.1 Conversely, school-aged children are at risk of developing future obesity if they sleep less than 9 h per night2 or if they have OSA.3
OSA is associated with left ventricular hypertrophy, elevated heart rate (HR), and impaired ventilatory responses to carbon dioxide during sleep. Children with an apnea-hypopnea index (AHI) greater than 10 h-1 are at risk of left ventricular hyper-trophy and this correlates with AHI and the severity of hypoxia during sleep.4 Children with OSA have impaired left ventricular diastolic function,5 but systolic function and left ventricular ejection fraction are maintained.5–7 HR is elevated during wake and sleep, whereby children with an AHI greater than 5 h-1 have a mean HR approximately 5 to 9 bpm higher than nonsnoring or snoring children.8 This increase in HR is likely a consequence of changes in autonomic function.9–11 Children with OSA have significantly elevated sympathetic activity at rest9,12 and blunted sympathetic activity (baroreflex gain) in response to sleep and stress, whereas parasympathetic activity remains unaffected.11,13 The increase in baseline sympathetic activity correlates with AHI and hypoxia.12,14 In regard to lung function, pulmonary function is generally unaffected by OSA,15,16 but children with OSA have impaired ventilatory responses to increased carbon dioxide during sleep17 but not during quiet wakefulness.18
Cardiopulmonary exercise testing (CPET) can be used to assess physical conditioning, and in adults, it can also be used as a prognostic marker.19 Two variables used to assess future risk of cardiovascular disease and mortality in adults are oxygen consumption and the ventilatory response to carbon dioxide at peak exercise capacity (i.e. VO2peak and VE/VCO2, respectively).20–22 In healthy individuals, VO2peak is approximately 30 to 50 mL/kg/min and VE/VCO2 at peak exercise capacity is approximately 25, but a VO2peak less than 14 to 15 mL/kg/min and a VE/VCO2 greater than 33 have been shown to be markers of a poor prognosis and future risk of mortality in adults.19–22 In children, little is known about the longitudinal effect of a low VO2peak or high VE/VCO2.23 One study examined exercise function in children with OSA and found that children with OSA achieve peak exercise capacity at a lower workload, HR, and a lower VO2peak relative to age and sex.24 Although estimates of obese children having diagnosed OSA are anywhere from 19–78%25–29 the outcomes from this study were not adjusted for weight status, nor did they evaluate cardiac output.24
Previous studies have found obese children achieve peak exercise earlier and at a lower workload than healthy weight children.30,31 This poor exercise function in obese children is thought to be a consequence of physical deconditioning and tiring earlier because of carrying a heavier body mass, but no studies have documented the prevalence of OSA in their sample groups or determined the effect of OSA on exercise function in children.30–35
The first aim of this study was to assess cardiopulmonary exercise function in children with OSA. Because obesity is a risk factor for OSA, we than compared the relative contributions of obesity versus OSA to cardiopulmonary function. We hypothesized that children with OSA, irrespective of weight status, are exercise restricted because of an impaired cardiovascular response to stress.
METHODS
This was a cross-sectional, prospective study approved by the Human Research Ethics Committee. The primary caregiver(s) gave written informed consent and the children gave verbal consent. Data collection was performed during a single visit.
Between February 2009 and June 2011, children age 7 to 12 y were recruited from the Sleep Clinic or Weight Management Service at the Children's Hospital at Westmead NSW Australia, and from the community. Community-based children were a convenience sample of children known to the investigators. Children were included if they snored at least “a little of the time” according to the OSA-18 survey,36 were clinically evaluated to be healthy weight or obese, and were at least 135 cm tall in order to successfully reach the bicycle's pedals and perform the CPET. Using the United States Centers for Disease Control and Prevention (US CDC) guidelines, children were defined as being of healthy weight if their body mass index (BMI) percentile was between the 5th and 85th centiles for age and sex, and obese if their BMI percentile was ≥ 95th for age and sex.37 Age- and weight-matched, nonsnoring children were also recruited for comparison. To clarify the effect of obesity on exercise function, and for time management purposes, children were excluded from the study if they were overweight (i.e. had a BMI between the 85th and 95th percentile). Children were also excluded if they were developmentally delayed, had an underlying syndrome, received a diagnosis of a sleep disorder other than OSA, or were already established on treatment.
Anthropometry
Height, weight, and waist circumference were measured. Standing height was measured to the nearest 0.1 cm and weight was measured to the closest 0.01 kg. BMI was calculated as weight/height2 (kg/m2), and BMI percentiles and z-scores were determined using the US CDC reference values.37 Waist circumference was measured to the nearest 0.1 cm at the smallest point between the lowest rib and iliac crest. To assess for central obesity, waist-to-height ratio (WHtR) was calculated as waist/height (cm/cm), and central obesity was defined as WHtR > 0.5.38
Polysomnography
Overnight polysomnography was performed at the David Read Sleep Unit, at The Children's Hospital at Westmead NSW Australia, in accordance with the 1997 American Thoracic Society (ATS) guidelines39 using the Sandman Elite® Version 9.2 system (Embla Systems, Broomfield, CO, USA). Data collection commenced between 19:30 and 21:00, and ended at 06:00.
Data analysis was performed in accordance with the 2007 American Academy of Sleep Medicine guidelines.40 Sleep stages and arousals were determined according to central and occipital electroencephalogram (EEG), a left and right electrooculogram (EOG) and submental electromyogram (EMG) signals. Nasal airflow was measured using nasal cannula. Nasal and oral airflow was also monitored using a thermistor, respiratory effort was monitored using diaphragmatic and abdominal surface electrode EMG and chest and abdominal plethysmography, and ventilation was monitored using oximetry (SpO2) and transcutaneous carbon dioxide (TcCO2) monitoring. Respiratory events were scored if they were at least two respiratory cycles long, and significant oxygen desaturations were defined as ≥ 3% desaturation from baseline. An obstructive apneahypopnea index (OAHI) ≥ 1 h-1 was used as a diagnosis for OSA.41
To assess sleepiness among children with and without OSA, the modified Epworth Sleepiness Scale (ESS) questionnaire was completed by the child at the time of the polysomnography.42 The results were analyzed according to set criteria.43
Spirometry
Spirometry was performed according to ATS guidelines44 against predicted values for race, age, sex, and height45 using the Medgraphics CPX/D breath-by-breath exchange system (Medgraphics Corporation, St Paul, MN, USA) and Breeze-Suite Version 6.4.1 software program (Medgraphics Corporation), or Vmax Encore 229 equipment (CareFusion, San Diego, CA, USA) and Vmax Series Version 21-1A software (CareFusion). Results are displayed as absolute (L) and as percent predicted values for race, age, sex, and height.45
Doppler
Cardiac output (L/min), cardiac output index (QI; L/min/m2), stroke volume (SV; mL), stroke volume index (SVI; mL/m2), and HR (bpm) were recorded at rest and at peak exercise, in an upright position using an Ultrasonic Cardiac Output Monitor (USCOM) Doppler, Version 1.8.0.0 (USCOM, Sydney, NSW, Australia).46 Cardiac index and SVI were calculated by adjusting cardiac output and stroke volume for body surface area.47
Cardiopulmonary Exercise Testing
Prior to the CPET, the caregiver(s) reported the number of hours of physical/sporting activity the child did each week. CPET was performed and analyzed in accordance with ATS and American College of Chest Physicians (ACCP) guidelines19 using a cycle ergometer (Excalibur Sport cycle ergometer, the Medgraphics CPX/D Breath-by-Breath Exchange system and BreezeSuite Version 6.4.1 software program, Medgraphics Corporation). Children cycled at 60 revolutions per min (rpm) against increasing resistance (10 watts/min). Tests were terminated when (1) the child felt physically exhausted and could not maintain a speed of 60 rpm despite encouragement, or (2) if the child reached their predicted maximal HR (i.e., maximal HR = 220 – age). The test was deemed satisfactory if the respiratory quotient (RQ) was greater than 1.00; however, the child was encouraged to continue with the test as long as possible (i.e. RQ ≥ 1.05) to ensure true peak exercise capacity was achieved. Exercise data from any child considered not to achieve peak exercise capacity was excluded from the analysis. Physical exhaustion was recorded using the Borg scale,48 a subjective scale validated in children.49,50 Output was averaged at 30-sec intervals and compared with reference values.51
Statistical Analysis
Statistical analysis was performed using SPSS version 19 (SPSS, Chicago, IL, USA). Categorical variables are described as frequencies and compared between groups using the chi-square test. Continuous variables were checked for normality and corrected if required.52 Descriptive data are reported as mean (standard deviation [SD]) and compared using independent t-tests.
Analysis of covariance was used to evaluate effects of weight status by adjusting for OAHI, and to evaluate effects of OSA by adjusting for BMI z-score with results presented as estimated mean (standard error [SE]), mean difference, and 95% confidence intervals (CIs).
Pearson correlation coefficient analysis was used to assess correlations between sleep, and anthropometric and cardiopulmonary variables. Stepwise multiple regression analysis was performed to develop models that predicted cardiopulmonary function while accounting for any interactions amongst variables. P < 0.05 was considered significant.
RESULTS
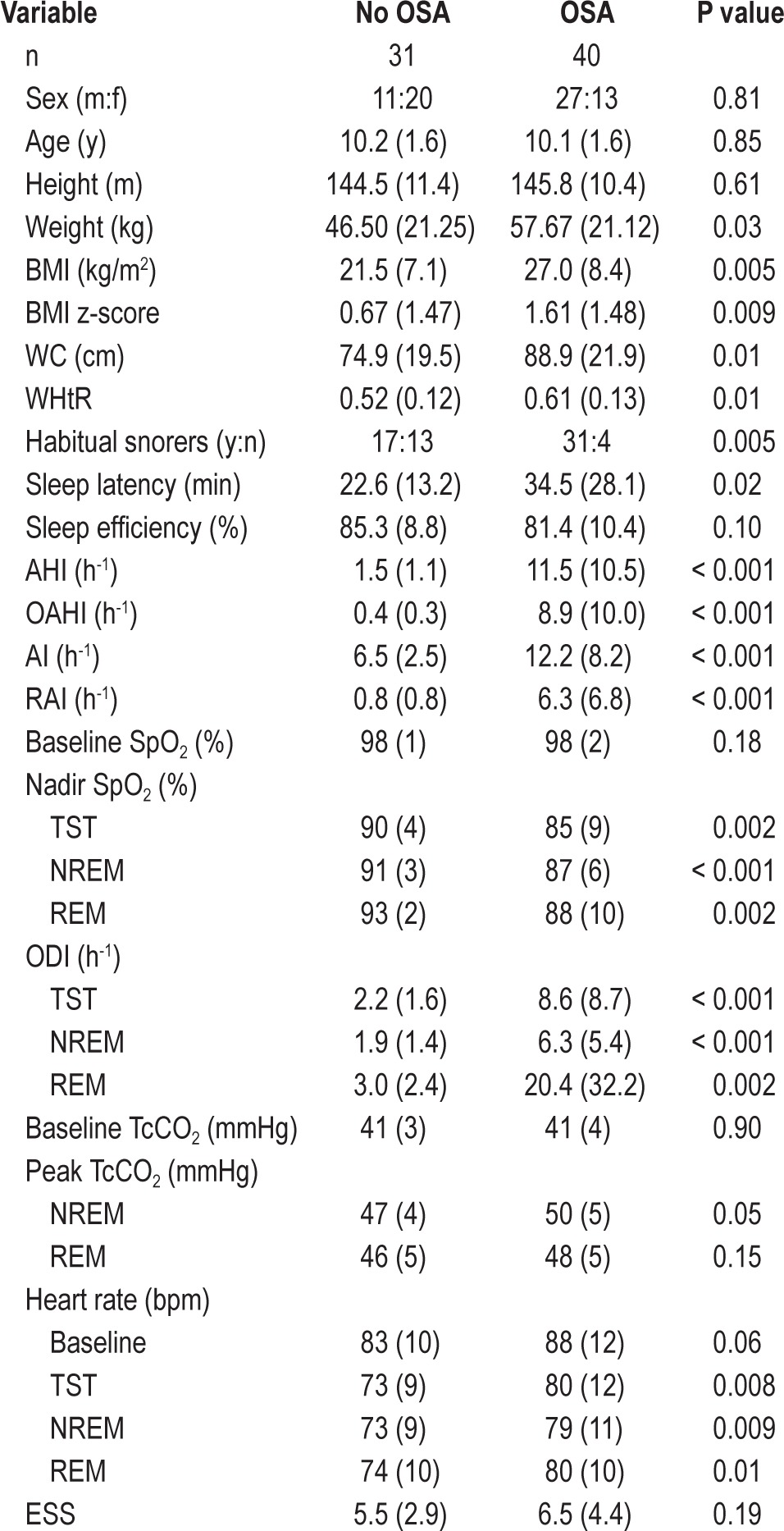
Of 115 children approached to participate in the study, 71 families consented and the children met the inclusion criteria. These 71 children (mean age 10 ± 1.6 y) included 29 (41%) healthy weight and 42 (59%) obese children. Patient demographics and polysomnography results are detailed in Table 1. Forty children had OSA, including 10 healthy weight and 30 obese children. Children with OSA had significantly more arousals, oxygen desaturations, lower nadir SpO2, and higher sleeping HR than children without OSA. There was no difference in the percentage of time spent in each sleep stage (results not shown), or ESS scores between those with and without OSA.
Table 1.
Subject demographics and polysomnography results
Cardiopulmonary Exercise Function in Children With and Without OSA
As shown in Tables 2 and 3, children with OSA had significantly lower RQ, HR, SVI, Q, and VO2peak at peak exercise capacity and a non-significant trend for children with OSA to achieve peak exercise capacity at a lower workload than those without OSA (P = 0.06). Children with OSA had a higher breathing reserve at peak exercise than those without OSA (i.e. VE/maximum voluntary ventilation (MVV) 54% and 45%; P < 0.05).
Table 2.
Cardiopulmonary function for children with and without obstructive sleep apnea
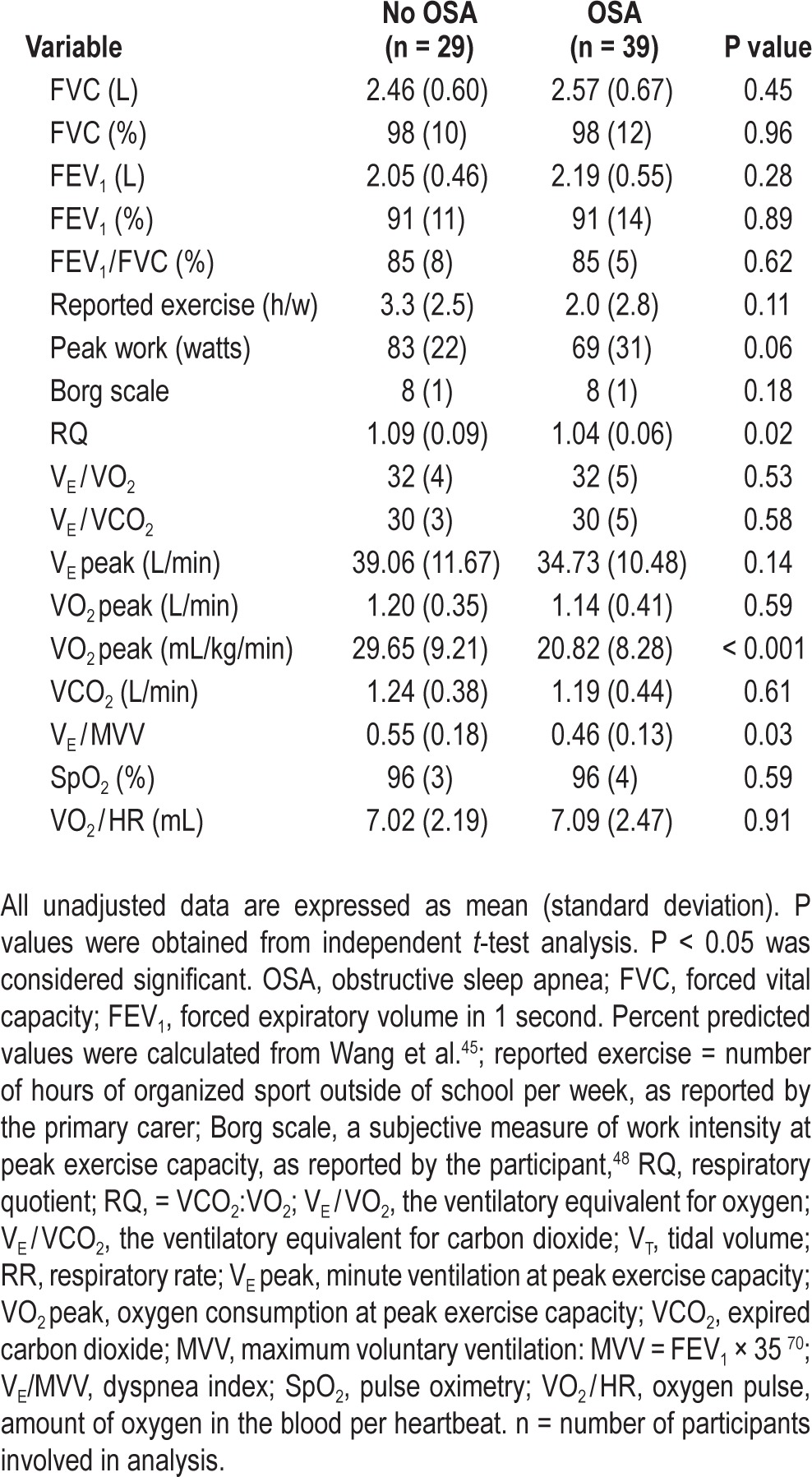
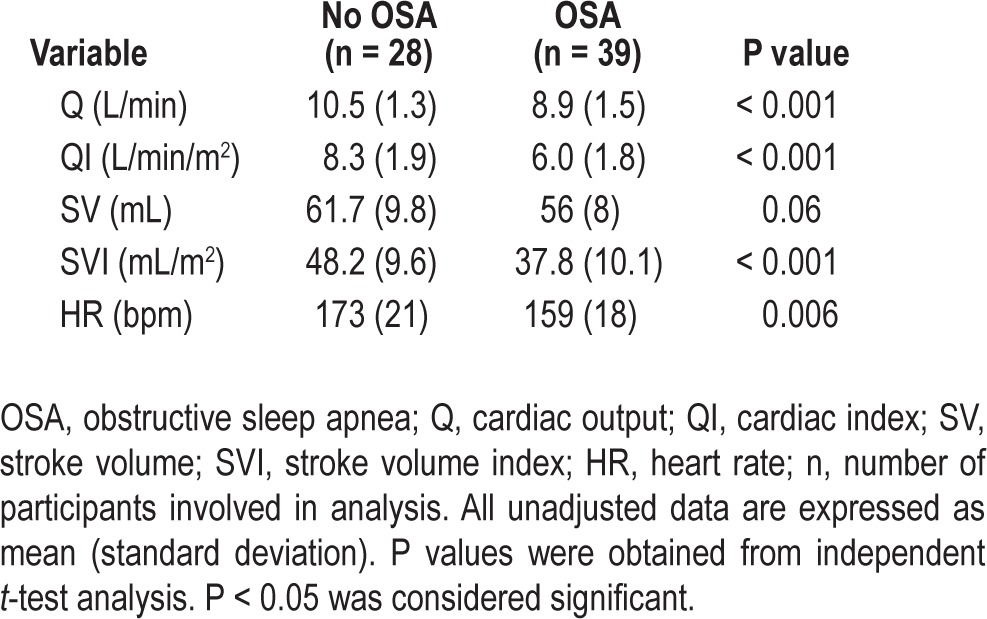
Table 3.
Cardiac output at peak exercise capacity for children with and without obstructive sleep apnea
Among children with and without OSA, no differences existed for the number of hours of organized exercise per week (Table 2), pulmonary lung function, VE, gas exchange (results not shown), or Q at rest (4.6 ± 1.2 L/min versus 4.5 ± 1.1 L/min, not significant).
The decrease in Q at peak exercise capacity was caused by both a lower peak HR and SVI. The HR for children with OSA increased by 66 ± 20 bpm in response to exercise, to achieve a peak HR of 159 ± 18 bpm. In children without OSA, HR increased by 87 ± 24 bpm from rest (P < 0.001) to reach a peak HR of 173 ± 21 bpm (P < 0.05). SVI was significantly lower at rest and peak exercise for children with versus without OSA; SVI at rest was 33.5 ± 9.1 mL versus 41.1 ± 7.2 mL (P < 0.001), and at peak exercise it was 37.8 ± 10.1 mL versus 48.2 ± 9.6 mL (P < 0.001). There was no difference in VE or ventilatory responses at peak exercise between the two groups.
Effects of Obesity Versus OSA on Cardiopulmonary Exercise Function
Effects of OSA versus obesity on cardiopulmonary function are summarized in Table 4. When corrected for BMI z-score, children with OSA still had cardiovascular dysfunction during exercise, with a significantly lower VE/MVV, HR, SVI, Q, QI and VO2peak than children without OSA. When corrected for BMI z-score, Q at peak exercise capacity in children with and without OSA was 9.0 ± 0.2 L/min and 10.4 ± 0.3 L/min (P = 0.001). This impaired Q at peak exercise was associated with a SVI 4.5 mL lower (95% CI -8.2 to -0.8 mL; P < 0.05) and a HR response 14.9 bpm less (95% CI -26.2 to -3.6 bpm; P = 0.01) than those without OSA. VO2peak for children with and without OSA was 22.22 mL/kg/min and 27.33 mL/kg/min (P = 0.02).
Table 4.
Cardiopulmonary function at peak exercise capacity between groups: A: healthy weight versus obese (adjusted for obstructive apnea-hypopnea index) or B: obstructive sleep apnea versus non obstructive sleep apnea (adjusted for body mass index z-score).
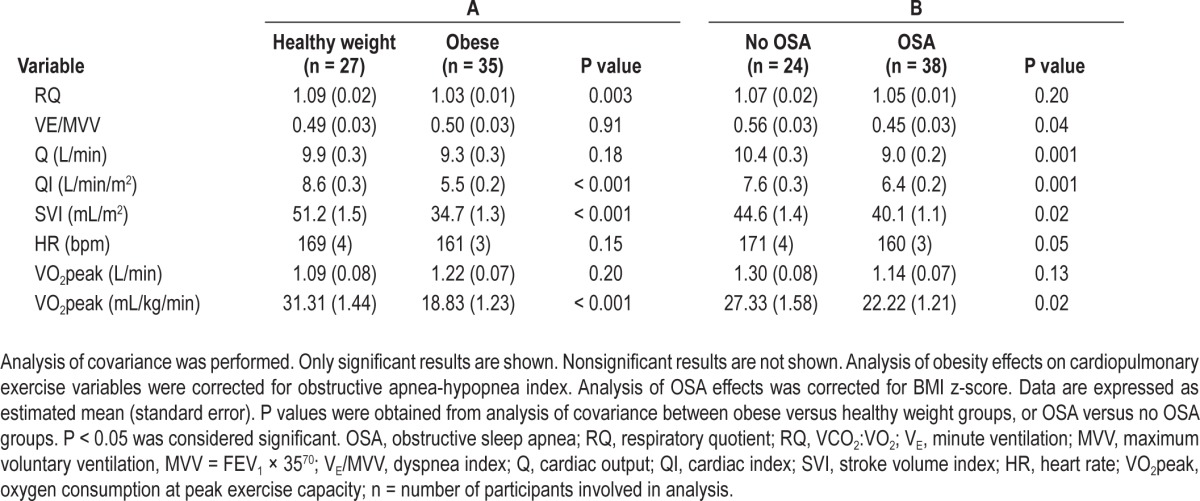
Conversely, when exercise function was corrected for OAHI, obesity was only associated with a deceased RQ and VO2peak (Table 4). Differences in VO2peak between healthy-weight and obese children were only seen if VO2peak was expressed ‘per kilogram’ (31.31 mL/kg/min and 18.83 mL/kg/min, respectively; P < 0.001). Pulmonary and cardiovascular function was not different between healthy-weight and obese children (results not shown).
Determinants of Exercise Function
Significant correlations among anthropometric, sleep, and cardiopulmonary function variables indicated that exercise function was inversely associated with weight status, central obesity, OSA severity, arousals, intermittent hypoxia, and HR during sleep. Specifically, RQ correlated with BMI z-score and WHtR. HR, SVI, Q, and VO2peak at peak exercise capacity correlated with BMI z-score, OAHI, the respiratory arousal index, and hypoxia during sleep (Figure 1). SVI, HR and VO2peak at peak exercise capacity also correlated with WHtR, and mean HR during sleep.
Figure 1.
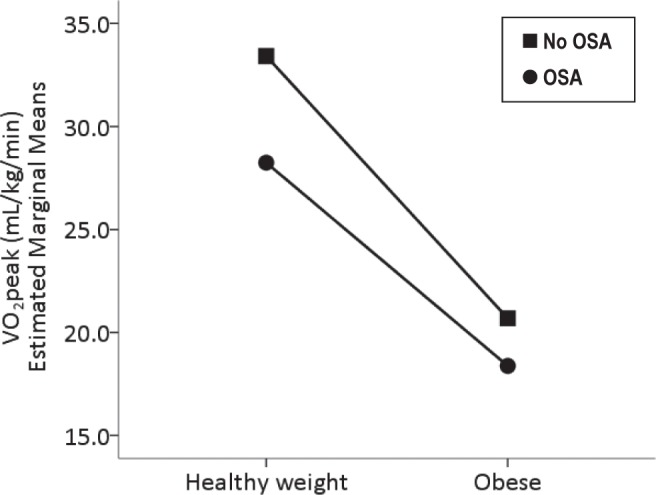
The effect of obesity and obstructive sleep apnea (OSA) on oxygen consumption at peak exercise capacity (VO2peak). The results are displayed as estimated marginal means. Covariates appearing in the model are evaluated at the following values: Obstructive apnea-hypopnea index = 8.9 h-1 and body mass index z-score = 1.14.
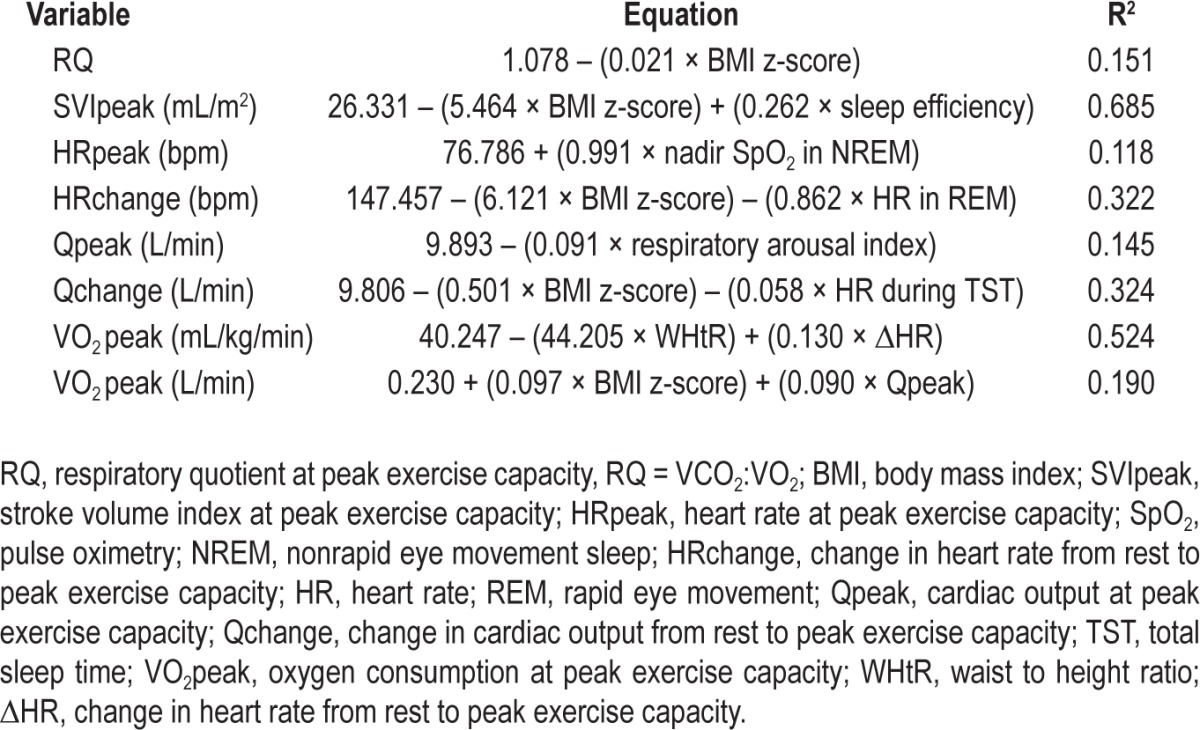
After multiple regression modeling (Table 5), OSA severity (i.e., OAHI) was not a strong predictor of cardiac output or exercise function. Instead, physical conditioning was strongly dependent on BMI z-score, and cardiac function was most dependent on BMI z-score, sleep efficiency, the respiratory arousal index, hypoxia, and HR during sleep. The strongest predictor for RQ was BMI z-score. Peak cardiac output was most dependent on the respiratory arousal index. The strongest model to predict cardiac output's response from rest to exercise included BMI z-score and mean HR during total sleep time. The strongest model to predict SVI at peak exercise included BMI z-score and sleep efficiency. The strongest model to predict HR at peak exercise capacity included nadir SpO2 in non-rapid eye movement (NREM) sleep. The strongest model to predict HR response from rest to peak exercise included BMI z-score and mean HR during REM sleep. The strongest model for VO2peak included WHtR and the HR response to exercise.
Table 5.
Regression models involving anthropometric and sleep variables to predict cardiopulmonary function.
DISCUSSION
This study is the first to assess the relative contributions of OSA and obesity to exercise function in children. Regardless of weight status, OSA was independently associated with exercise dysfunction in children, and this was attributable to cardiovascular dysfunction. Specifically, at peak exercise capacity, children with OSA have a lower HR, stroke volume index, cardiac output, and VO2peak than those without OSA. Furthermore, obesity and OSA had compounded effects on exercise capacity, more than either obesity or OSA alone.
The Effect of OSA on Cardiopulmonary Function During Exercise
The most significant outcome from this study was that cardiac output at peak exercise is affected by the presence of OSA in children. No equivalent data exist in children, but research in adults have demonstrated impaired cardiovascular performance is linked to OSA, although the mechanism remains debated, with possibilities including lower stroke volume at peak exercise53 and lower peak HR.54,55 Przybylowski et al. reported peak HR was not significantly different between patients with mild to moderate or severe OSA,56 but our subject numbers did not allow this analysis. Of interest, the HR characteristics we found in children are similar to the pattern of change seen in adult patients with heart failure where HR at baseline is equivalent but at peak exercise capacity it is significantly lower for those with chronic heart failure compared with healthy controls.21 This pattern suggests a significant role of the sympathetic nervous system.
There was an independent effect of OSA on VO2peak. The magnitude of reduction in VO2peak in children with OSA is similar to that seen in children with congenital heart disease compared with age- and sex-matched controls.57 Results from adult studies vary, with some showing no differences,53,54 whereas others report that OSA is associated with lower VO2peak in a dose-dependent manner.55,56,58 Results from Lin et al. is consistent with our study results, whereby they reported adults with OSA had a mean VO2peak of 21.64 mL/kg/min compared to 30.10 mL/kg/min for those without OSA.59
VE/MVV is a ratio used to describe how much breathing reserve an individual has when peak exercise capacity is achieved. For example, a VE/MVV of 0.70 indicates that the individual has used 70% of his or her breathing capacity at peak exercise (and they have 30% in reserve). We found that children with OSA had a lower VE/MVV, and thus a greater breathing reserve at peak exercise capacity than those without OSA. This supports the notion that pulmonary function is not a limiting factor for children with OSA, and instead, as our other assessments of cardiac output suggest, the limitations were more likely to be cardiac in nature. The only equivalent studies available were undertaken in adults, in whom results are inconsistent, although some show the same pattern as in children.54,59
The difference in exercise function occurred despite equivalent reported hours of organized sport and physical activity per week, and equivalent modified ESS scores in the children with or without OSA. The peak exercise capacity in those with OSA was 14 watts lower than those without OSA (95% CI -28.8 to 0.7 watts, P = 0.06). At peak workload, there was no difference in the respiratory quotient or the degree of perceived exertion between groups; these findings are similar to adult-based studies that report no difference in peak workload53,54 or the degree of perceived exertion54 between adults with and without OSA.
The Effect of Obesity on Cardiopulmonary Exercise Function
Obese children achieved peak exercise capacity at a lower respiratory quotient and had a lower VO2peak (per kilogram), indicating fatigue and physical deconditioning. Rowland et al. also found that obese children ceased exercise at a lower respiratory quotient although the children in their study had higher absolute VO2peak.32 Other studies of obese children having low VO2peak32 report VO2peak as per kilogram, which adjusts VO2peak for total body mass including fat mass and fat-free mass. This allows bias, predisposing obese children to an artificially poorer outcome.60 Nonetheless, we found that weight status, not OSA, alters physical conditioning. Obese children are predisposed to a higher VO2peak (i.e. L/min) because they have a larger left ventricle, stroke volume, and thus cardiac output than healthy-weight children.32 However, correcting VO2peak for total body mass (i.e. mL/kg/min; fat mass and fat-free mass) can skew aerobic function for obese children as fat mass does not metabolize oxygen.
This study reinforces the concept that physical decondition is associated with obesity and not OSA. Compared with healthy-weight children, obese children had a lower respiratory quotient at peak exercise capacity. In particular, this study showed the respiratory quotient was dependent on BMI z-score and waist-to-height ratio, indicating that physical deconditioning is related to body mass and central obesity. Children with OSA achieved similar respiratory quotients at peak exercise capacity as weight-matched children without OSA.
Giordano et al. found that cardiac output at peak exercise is not affected by obesity61; however, when indexed for body surface, cardiac index and stroke volume index were significantly lower in obese compared to healthy weight children. Correcting cardiovascular function for body surface area is common, but doing so obscures the effect of obesity on cardiac output and stroke volume.62 Our study found obesity does not affect cardiac output at peak exercise capacity.
Correlations and Proposed Mechanisms Between Sleep, Anthropometry, and Exercise Function
The major influences on cardiovascular function and VO2peak were weight status, central obesity, cortical arousals, hypoxia and mean HR during sleep. VO2peak (mL/kg/min) is dependent upon the HR response to exercise and is inversely associated with central obesity.
Oxygen consumption and aerobic metabolism are regulated by three mechanisms: (1) ventilation, (2) cardiac output, and (3) metabolism in the mitochondria.19,63 Because OSA was not linked to any impairment of ventilation or ventilatory responses to exercise, the decreased VO2peak must be a consequence of cardiovascular or mitochondrial dysfunction. In adult literature, the low VO2peak is attributed to the down regulation of cardiac beta-receptors or an altered baroreflex set-point.54 A second hypothesis is that OSA impairs aerobic metabolism in muscle tissue as an adaptive measure to nocturnal hypoxia.64,65
Our findings suggest that cardiovascular function is the major limiting factor for exercise in children with OSA. OSA in children has been linked to increased sympathetic activity at rest and a blunted response to stress,11 resulting in increased secretion of adrenaline and noradrenaline12,66 that correlates with AHI and nadir SpO2.12,14 Catecholamines were not measured in this study, but elevated catecholamine concentrations at rest may be associated with a blunted sensitivity response to stress, thus impairing the HR and cardiac output's response to exercise.
An interesting finding was that cortical arousals may be more influential than hypoxia because the respiratory arousal index correlated highly with cardiac output at peak exercise capacity. This hypothesis is supported by our results, whereby healthy-weight children with mild OSA had a significantly higher arousal index but their nadir SpO2 was not significantly different from that of weight-matched children without OSA (results not shown). The concept that cardiovascular activity is influenced by arousals and cortical activity is also supported by O'Driscoll et al.,67 who reported that the surge in HR activity immediately after obstructive events during sleep correlates with subcortical arousals and not oxygen desaturation.
Impaired cardiac output response to stress may also be a consequence of hypoxia during sleep as other research has shown that individuals have a blunted HR response when exposed to hypoxic environments. For instance, individuals exposed to high altitude conditions have an elevated resting HR and a decreased peak HR.68 Lundby et al. compared cardiopulmonary exercise tests at sea level and at high altitude (highest elevation 6,300 m).69 With increasing altitude, workload and peak HR significantly decreased from 191 ± 3.3 bpm at sea level to 165 ± 1.3 bpm at 6,300 m altitude with no change in adrenaline, noradrenaline, or lactate concentrations from sea level to altitude.
There are several limitations to this study. Although the study was performed at a tertiary pediatric hospital over 2.5 years, it was difficult to identify healthy-weight children with OSA, and obese children without OSA between age 7–12 y. A second limitation was the difficulty recruiting nonsnorers and our ‘no OSA’ group included snorers without OSA but the small study numbers did not permit comparisons between snorers and nonsnorers without OSA. Only children who successfully completed the exercise test were included in the analysis, so some children were excluded because they could not reach the pedals, became too anxious during the exercise test, or could not ride a bike. Surprisingly, a number of children had never ridden a bike prior to this study and were excluded because of this. No child required a bronchodilator following the exercise test, but no child repeated spirometry after the test to formally exclude exercise-induced asthma.
This is the first study to evaluate cardiac output during exercise in children with OSA and to evaluate whether exercise dysfunction in obese children is influenced by the presence of OSA. Obesity and OSA were found to be independently linked to exercise restriction in children. Obesity reduces a child's exercise tolerance because they have to carry a heavier body mass and are physically deconditioned. Our hypothesis that OSA reduces a child's exercise capacity through a blunted cardiac response to stress is supported by our findings that these children have reduced peak HR, stroke volume index, and cardiac output at peak exercise capacity compared to those without OSA. Both conditions also independently reduce VO2peak. Thus, children who are obese and have OSA are more exercise limited and have a lower cardiac output and VO2peak than children who have OSA or obesity. Longitudinal research is needed to evaluate if these findings translate into risk for early cardiovascular disease.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Evans was supported by a National Health and Medical Research Council Biomedical Postgraduate Scholarship (#571242). Dr. Evans is currently employed with ResMed Ltd., Bella Vista NSW Australia. This employment commenced after the completion of this project (including participant recruitment, data collection and analysis). ResMed Ltd. has no affiliation with this specific project or manuscript. The other authors have indicated no financial conflicts of interest. The work was performed at the Children's Hospital at Westmead, Westmead, NSW Australia
ACKNOWLEDGMENTS
We would like to thank Professor Jennifer Peat, Mr Ihsan Savran, and the staff from the David Read Sleep Unit, Respiratory Function Unit, The Children's Hospital Institute of Sport's Medicine and Weight Management Service at The Children's Hospital at Westmead for contributing and supporting this study. We would also like to thank the children and families who participated in this study.
REFERENCE
- 1.Redline S, Tishler PV, Schluchter M, Aylor J, Clark K, Graham G. Risk factors for sleep-disordered breathing in children. Associations with obesity, race, and respiratory problems. Am J Respir Crit Care Med. 1999;159:1527–32. doi: 10.1164/ajrccm.159.5.9809079. [DOI] [PubMed] [Google Scholar]
- 2.Shi Z, Taylor AW, Gill TK, Tuckerman J, Adams R, Martin J. Short sleep duration and obesity among Australian children. BMC Public Health. 2010;10:609. doi: 10.1186/1471-2458-10-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silva GE, Goodwin JL, Parthasarathy S, et al. Longitudinal association between short sleep, body weight, and emotional and learning problems in Hispanic and Caucasian children. Sleep. 2011;34:1197–205. doi: 10.5665/SLEEP.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amin RS, Kimball TR, Bean JA, et al. Left ventricular hypertrophy and abnormal ventricular geometry in children and adolescents with obstructive sleep apnea. Am J Respir Crit Care Med. 2002;165:1395–9. doi: 10.1164/rccm.2105118. [DOI] [PubMed] [Google Scholar]
- 5.Amin RS, Kimball TR, Kalra M, et al. Left ventricular function in children with sleep-disordered breathing. Am J Cardiol. 2005;95:801–4. doi: 10.1016/j.amjcard.2004.11.044. [DOI] [PubMed] [Google Scholar]
- 6.Tal A, Leiberman A, Margulis G, Sofer S. Ventricular dysfunction in children with obstructive sleep apnea: radionuclide assessment. Pediatr Pulmonol. 1988;4:139–43. doi: 10.1002/ppul.1950040304. [DOI] [PubMed] [Google Scholar]
- 7.Shiomi T, Guilleminault C, Stoohs R, Schnittger I. Obstructed breathing in children during sleep monitored by echocardiography. Acta Paediatr. 1993;82:863–71. doi: 10.1111/j.1651-2227.1993.tb17629.x. [DOI] [PubMed] [Google Scholar]
- 8.Amin R, Somers VK, McConnell K, et al. Activity-adjusted 24-hour ambulatory blood pressure and cardiac remodeling in children with sleep disordered breathing. Hypertension. 2008;51:84–91. doi: 10.1161/HYPERTENSIONAHA.107.099762. [DOI] [PubMed] [Google Scholar]
- 9.Snow AB, Khalyfa A, Serpero LD, et al. Catecholamine alterations in pediatric obstructive sleep apnea: effect of obesity. Pediatr Pulmonol. 2009;44:559–67. doi: 10.1002/ppul.21015. [DOI] [PubMed] [Google Scholar]
- 10.McConnell K, Somers VK, Kimball T, et al. Baroreflex gain in children with obstructive sleep apnea. Am J Respir Crit Care Med. 2009;180:42–8. doi: 10.1164/rccm.200808-1324OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chaicharn J, Lin Z, Chen ML, Ward SL, Keens T, Khoo MC. Model-based assessment of cardiovascular autonomic control in children with obstructive sleep apnea. Sleep. 2009;32:927–38. doi: 10.1093/sleep/32.7.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O'Driscoll DM, Horne RS, Davey MJ, et al. Increased sympathetic activity in children with obstructive sleep apnea: cardiovascular implications. Sleep Med. 2011;12:483–8. doi: 10.1016/j.sleep.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 13.McConnell K, Somers VK, Kimball T, et al. Baroreflex gain in children with obstructive sleep apnea. Am J Respir Crit Care Med. 2009;180:42–8. doi: 10.1164/rccm.200808-1324OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaditis AG, Alexopoulos EI, Damani E, et al. Urine levels of catecholamines in Greek children with obstructive sleep-disordered breathing. Pediatr Pulmonol. 2009;44:38–45. doi: 10.1002/ppul.20916. [DOI] [PubMed] [Google Scholar]
- 15.Dubern B, Tounian P, Medjadhi N, Maingot L, Girardet JP, Boule M. Pulmonary function and sleep-related breathing disorders in severely obese children. Clin Nutr. 2006;25:803–9. doi: 10.1016/j.clnu.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 16.Marcus CL, Curtis S, Koerner CB, Joffe A, Serwint JR, Loughlin GM. Evaluation of pulmonary function and polysomnography in obese children and adolescents. Pediatr Pulmonol. 1996;21:176–83. doi: 10.1002/(SICI)1099-0496(199603)21:3<176::AID-PPUL5>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 17.Marcus CL, Lutz J, Carroll JL, Bamford O. Arousal and ventilatory responses during sleep in children with obstructive sleep apnea. J Appl Physiol. 1998;84:1926–36. doi: 10.1152/jappl.1998.84.6.1926. [DOI] [PubMed] [Google Scholar]
- 18.Marcus CL, Gozal D, Arens R, et al. Ventilatory responses during wakefulness in children with obstructive sleep apnea. Am J Respir Crit Care Med. 1994;149:715–21. doi: 10.1164/ajrccm.149.3.8118641. [DOI] [PubMed] [Google Scholar]
- 19.Weisman IM, Beck KC, Casaburi R, et al. ATS/ACCP statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med. 2003;167:211–77. doi: 10.1164/rccm.167.2.211. [DOI] [PubMed] [Google Scholar]
- 20.Sarullo FM, Fazio G, Brusca I, et al. Cardiopulmonary exercise testing in patients with chronic heart failure: prognostic comparison from peak VO2 and VE/VCO2 slope. Open Cardiovasc Med J. 2010;4:127–34. doi: 10.2174/1874192401004010127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ingle L, Sloan R, Carroll S, Goode K, Cleland JG, Clark AL. Prognostic significance of different measures of the ventilation-carbon dioxide relation in patients with suspected heart failure. Eur J Heart Fail. 2011;13:537–42. doi: 10.1093/eurjhf/hfq238. [DOI] [PubMed] [Google Scholar]
- 22.Myers J, Gullestad L, Vagelos R, et al. Cardiopulmonary exercise testing and prognosis in severe heart failure: 14 mL/kg/min revisited. Am Heart J. 2000;139:78–84. doi: 10.1016/s0002-8703(00)90312-0. [DOI] [PubMed] [Google Scholar]
- 23.Nixon PA, Orenstein DM, Kelsey SF, Doershuk CF. The prognostic value of exercise testing in patients with cystic fibrosis. N Engl J Med. 1992;327:1785–8. doi: 10.1056/NEJM199212173272504. [DOI] [PubMed] [Google Scholar]
- 24.Damianidou L, Eboriadou M, Giannopoulos A, et al. Reduced exercise capacity in Greek children with mild to moderate obstructive sleep apnea syndrome. Pediatr Pulmonol. 2013;48:1237–45. doi: 10.1002/ppul.22730. [DOI] [PubMed] [Google Scholar]
- 25.Mallory GB, Jr., Fiser DH, Jackson R. Sleep-associated breathing disorders in morbidly obese children and adolescents. J Pediatr. 1989;115:892–7. doi: 10.1016/s0022-3476(89)80738-3. [DOI] [PubMed] [Google Scholar]
- 26.Verhulst SL, Schrauwen N, Haentjens D, et al. Sleep-disordered breathing in overweight and obese children and adolescents: prevalence, characteristics and the role of fat distribution. Arch Dis Child. 2007;92:205–8. doi: 10.1136/adc.2006.101089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wing YK, Hui SH, Pak WM, et al. A controlled study of sleep related disordered breathing in obese children. Arch Dis Child. 2003;88:1043–7. doi: 10.1136/adc.88.12.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu Z, Jiaqing A, Yuchuan L, Shen K. A case-control study of obstructive sleep apnea-hypopnea syndrome in obese and nonobese Chinese children. Chest. 2008;133:684–9. doi: 10.1378/chest.07-1611. [DOI] [PubMed] [Google Scholar]
- 29.Li AM, Chan MH, Chan DF, et al. Insulin and obstructive sleep apnea in obese Chinese children. Pediatr Pulmonol. 2006;41:1175–81. doi: 10.1002/ppul.20508. [DOI] [PubMed] [Google Scholar]
- 30.Goran M, Fields DA, Hunter GR, Herd SL, Weinsier RL. Total body fat does not influence maximal aerobic capacity. Int J Obes Relat Metab Disord. 2000;24:841–8. doi: 10.1038/sj.ijo.0801241. [DOI] [PubMed] [Google Scholar]
- 31.Zanconato S, Baraldi E, Santuz P, et al. Gas exchange during exercise in obese children. Eur J Pediatr. 1989;148:614–7. doi: 10.1007/BF00441512. [DOI] [PubMed] [Google Scholar]
- 32.Rowland T, Bhargava R, Parslow D, Heptulla RA. Cardiac response to progressive cycle exercise in moderately obese adolescent females. J Adolesc Health. 2003;32:422–7. doi: 10.1016/s1054-139x(03)00051-x. [DOI] [PubMed] [Google Scholar]
- 33.Rowland TW. Effect of obesity on cardiac function in children and adolescents: a review. J Sports Sci Med. 2007;6:319–26. [PMC free article] [PubMed] [Google Scholar]
- 34.Unnithan VB, Baynard T, Potter CR, et al. An exploratory study of cardiac function and oxygen uptake during cycle ergometry in overweight children. Obesity (Silver Spring) 2007;15:2673–82. doi: 10.1038/oby.2007.319. [DOI] [PubMed] [Google Scholar]
- 35.Schuster I, Karpoff L, Perez-Martin A, et al. Cardiac function during exercise in obese prepubertal boys: effect of degree of obesity. Obesity. 2009;17:1878–83. doi: 10.1038/oby.2009.197. [DOI] [PubMed] [Google Scholar]
- 36.Franco RA, Jr., Rosenfeld RM, Rao M. First place--resident clinical science award 1999. Quality of life for children with obstructive sleep apnea. Otolaryngol Head Neck Surg. 2000;123:9–16. doi: 10.1067/mhn.2000.105254. [DOI] [PubMed] [Google Scholar]
- 37.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. CDC growth charts: United States. Adv Data. 2000:1–27. [PubMed] [Google Scholar]
- 38.Garnett SP, Baur LA, Cowell CT. Waist-to-height ratio: a simple option for determining excess central adiposity in young people. Int J Obes. 2008;32:1028–30. doi: 10.1038/ijo.2008.51. [DOI] [PubMed] [Google Scholar]
- 39.Loughlin GM, Brouillette RT, Brooke LJ, et al. Standards and indications for cardiopulmonary sleep studies in children. Am J Respir Crit Care Med. 1996;153:866–78. doi: 10.1164/ajrccm.153.2.8564147. [DOI] [PubMed] [Google Scholar]
- 40.Iber C, Ancoli-Israel S, Chesson AL, Quan SF. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. [Google Scholar]
- 41.Katz ES, Marcus CL. Diagnosis of obstructive sleep apnea syndrome in infants and children. In: Sheldon SH, Ferber R, Kryger MH, editors. Principles and Practice of Pediatric Sleep Medicine. Philadelphia, PA: Elsevier Saunders; 2005. pp. 197–210. [Google Scholar]
- 42.Melendres MC, Lutz JM, Rubin ED, Marcus CL. Daytime sleepiness and hyperactivity in children with suspected sleep-disordered breathing. Pediatrics. 2004;114:768–75. doi: 10.1542/peds.2004-0730. [DOI] [PubMed] [Google Scholar]
- 43.Johns MW. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 44.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–38. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 45.Wang X, Dockery DW, Wypij D, Fay ME, Ferris BG., Jr Pulmonary function between 6 and 18 years of age. Pediatr Pulmonol. 1993;15:75–88. doi: 10.1002/ppul.1950150204. [DOI] [PubMed] [Google Scholar]
- 46.USCOM. Sydney, NSW, Australia: USCOM; 2008. USCOM the measure of life: the basics; pp. 1–52. 1.8.0.0 ed. [Google Scholar]
- 47.Boyd E. Minneapolis, MN: University of Minnesota Press; 1935. The Growth of the Surface Area of the Human Body. [Google Scholar]
- 48.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14:377. [PubMed] [Google Scholar]
- 49.Lamb KL. Exercise regulation during cycle ergometry using the children's effort rating table (CERT) and rating of perceived exertion (RPE) scales. Pediatric Exercise Science. 1996;8:337–50. [Google Scholar]
- 50.Hommerding PX, Donadio MV, Paim TF, Marostica PJ. The Borg scale is accurate in children and adolescents older than 9 years with cystic fibrosis. Respir Care. 2010;55:729–33. [PubMed] [Google Scholar]
- 51.Cooper D, Weiler-Ravell D. Gas exchange response to exercise in children. Am Rev Respir Dis. 1984;129:S47–8. doi: 10.1164/arrd.1984.129.2P2.S47. [DOI] [PubMed] [Google Scholar]
- 52.Tabachnick BG, Fidell LS. Using Multivariate Statistics. 4th ed. Boston, MA: Allyn and Bacon; 2001. [Google Scholar]
- 53.Alonso-Fernandez A, Garcia-Rio F, Arias MA, et al. Obstructive sleep apnoea-hypoapnoea syndrome reversibly depresses cardiac response to exercise. Eur Heart J. 2006;27:207–15. doi: 10.1093/eurheartj/ehi621. [DOI] [PubMed] [Google Scholar]
- 54.Kaleth AS, Chittenden TW, Hawkins BJ, et al. Unique cardiopulmonary exercise test responses in overweight middle-aged adults with obstructive sleep apnea. Sleep Med. 2007;8:160–8. doi: 10.1016/j.sleep.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 55.Vanhecke TE, Franklin BA, Zalesin KC, et al. Cardiorespiratory fitness and obstructive sleep apnea syndrome in morbidly obese patients. Chest. 2008;134:539–45. doi: 10.1378/chest.08-0567. [DOI] [PubMed] [Google Scholar]
- 56.Przybylowski T, Bielicki P, Kumor M, et al. Exercise capacity in patients with obstructive sleep apnea syndrome. J Physiol Pharmacol. 2007;58(Suppl 5):563–74. [PubMed] [Google Scholar]
- 57.Fredriksen P, Ingjer F, Nystad W, Thaulow E. A comparison of VO2peak between patients with congenital heart disease and healthy subjects, all aged 8-17 years. Eur J Appl Physiol. 1999;80:409–16. doi: 10.1007/s004210050612. [DOI] [PubMed] [Google Scholar]
- 58.Ucok K, Aycicek A, Sezer M, et al. Aerobic and anaerobic exercise capacities in obstructive sleep apnea and associations with subcutaneous fat distributions. Lung. 2009;187:29–36. doi: 10.1007/s00408-008-9128-0. [DOI] [PubMed] [Google Scholar]
- 59.Lin CC, Hsieh WY, Chou CS, Liaw SF. Cardiopulmonary exercise testing in obstructive sleep apnea syndrome. Respir Physiol Neurobiol. 2006;150:27–34. doi: 10.1016/j.resp.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 60.Rowland T, Goff D, Martel L, Ferrone L. Influence of cardiac functional capacity on gender differences in maximal oxygen uptake in children. Chest. 2000;117:629–35. doi: 10.1378/chest.117.3.629. [DOI] [PubMed] [Google Scholar]
- 61.Giordano U, Ciampalini P, Turchetta A, et al. Cardiovascular hemodynamics: relationships with insulin resistance in obese children. Pediatr Cardiol. 2003;24:548–52. doi: 10.1007/s00246-003-0368-8. [DOI] [PubMed] [Google Scholar]
- 62.de Simone G, Devereux RB, Daniels SR, et al. Stroke volume and cardiac output in normotensive children and adults. Assessment of relations with body size and impact of overweight. Circulation. 1997;95:1837–43. doi: 10.1161/01.cir.95.7.1837. [DOI] [PubMed] [Google Scholar]
- 63.Gallagher CG, Marciniuk DD. Postgraduate course 16: Interpretation of clinical exercise testing for the clinician. In: Dempsey JA, editor. 1st ed. San Francisco, CA: American Thoracic Society; 1997. pp. 1–18. [Google Scholar]
- 64.Bonanni E, Pasquali L, Manca ML, et al. Lactate production and catecholamine profile during aerobic exercise in normotensive OSAS patients. Sleep Med. 2004;5:137–45. doi: 10.1016/j.sleep.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 65.Vanuxem D, Badier M, Guillot C, Delpierre S, Jahjah F, Vanuxem P. Impairment of muscle energy metabolism in patients with sleep apnoea syndrome. Respir Med. 1997;91:551–7. doi: 10.1016/s0954-6111(97)90089-5. [DOI] [PubMed] [Google Scholar]
- 66.Snow AB, Khalyfa A, Serpero LD, et al. Catecholamine alterations in pediatric obstructive sleep apnea: effect of obesity. Pediatr Pulmonol. 2009;44:559–67. doi: 10.1002/ppul.21015. [DOI] [PubMed] [Google Scholar]
- 67.O'Driscoll DM, Foster AM, Ng ML, et al. Acute cardiovascular changes with obstructive events in children with sleep disordered breathing. Sleep. 2009;32:1265–71. doi: 10.1093/sleep/32.10.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gamboa A, Leon-Velarde F, Rivera-Ch M, Vargas M, Palacios JA, Monge CC. Ventilatory and cardiovascular responses to hypoxia and exercise in Andean natives living at sea level. High Alt Med Biol. 2001;2:341–7. doi: 10.1089/15270290152608516. [DOI] [PubMed] [Google Scholar]
- 69.Lundby C, Araoz M, van Hall G. Peak heart rate decreases with increasing severity of acute hypoxia. High Alt Med Biol. 2001;2:369–76. doi: 10.1089/15270290152608543. [DOI] [PubMed] [Google Scholar]
- 70.Hansen JE, Sue DY, Wasserman K. Predicted values for clinical exercise testing. Am Rev Respir Dis. 1984;129:S49–55. doi: 10.1164/arrd.1984.129.2P2.S49. [DOI] [PubMed] [Google Scholar]


