Abstract
Study Objective:
To contrast the effects of slow wave sleep (SWS) disruption and age on daytime functioning.
Design:
Daytime functioning was contrasted in three age cohorts, across two parallel 4-night randomized groups (baseline, two nights of SWS disruption or control, recovery sleep).
Setting:
Sleep research laboratory.
Participants:
44 healthy young (20-30 y), 35 middle-aged (40-55 y), and 31 older (66-83 y) men and women.
Interventions:
Acoustic stimulation contingent on appearance of slow waves.
Measurements and Results:
Cognitive performance was assessed before sleep latency tests at five daily time-points. SWS disruption resulted in less positive affect, slower or impaired information processing and sustained attention, less precise motor control, and erroneous implementation, rather than inhibition, of well-practiced actions. These performance impairments had far smaller effect sizes than the increase in daytime sleepiness and differed from baseline to the same extent for each age group. At baseline, younger participants performed better than older participants across many cognitive domains, with largest effects on executive function, response time, sustained attention, and motor control. At baseline, the young were sleepier than other age groups.
Conclusions:
SWS has been considered a potential mediator of age-related decline in performance, although the effects of SWS disruption on daytime functioning have not been quantified across different cognitive domains nor directly compared to age-related changes in performance. The data imply that two nights of SWS disruption primarily leads to an increase in sleepiness with minor effects on other aspects of daytime functioning, which are different from the substantial effects of age.
Citation:
Groeger JA, Stanley N, Deacon S, Dijk DJ. Dissociating effects of global sws disruption and healthy aging on waking performance and daytime sleepiness. SLEEP 2014;37(6):1127-1142.
Keywords: slow wave sleep, aging, daytime functioning, cognition, sleep
INTRODUCTION
Slow wave sleep (SWS) and slow wave activity (SWA) are key markers of homeostatic sleep pressure and associated sleep propensity and play a central role in conceptual and mathematical models for the regulation of sleep timing.1–3 Neurophysiological mechanisms underlying the slow waves have been elucidated,4 and molecular processes associated with SWS have been identified.5 Slow waves can be recorded from all locations on the scalp and intracranially and show a frontal predominance both under baseline conditions and in response to sleep loss,6 and the dramatic effects of aging on SWA are most marked in frontal EEG derivations.7 SWS has been shown to be correlated with measures of gray matter density,8 and SWA undergoes a shift towards more anterior sites as adolescence proceeds, mirroring the developmental trajectory of more frontal cortical structures.9 At the other end of the spectrum, age-related medial prefrontal cortex gray matter atrophy has recently been shown to be associated with reduced SWA in older adults.10 SWS/ SWA changes in response to manipulations of the waking experience11,12 and have been reported to be correlated with consolidation of what is learned during waking.13–15 Slow waves have been hypothesized to play a role in the restoration of optimal connectivity (synaptic homeostasis) required for adequate waking performance16 and in impaired long-term memory, resulting from diminished SWA following atrophy of the medial prefrontal cortex.10 The evidence for a relationship between SWS/SWA and daytime functioning is, as we will see below, scant. Nor is it clear whether the pervasive effects of age on SWS are related to age-related changes in cognition. This paper has two broad aims: to assess the effects of reducing SWS on daytime functioning, using a sufficiently large range of tasks and a sample large enough to detect even relatively small effects in particular areas of functioning; and to assess whether these effects, if any, differ across groups of healthy young, middle-aged, and older individuals, whose typical sleep is, given their age, likely to differ in the proportion of sleep time spent in SWS. A supplementary aim was to demonstrate that the particular tasks used were not simply insensitive, and thus we also report the effects of age on performance of these tasks, where participants were well slept.
The functional significance of SWS has been investigated by selective SWS deprivation experiments. Selective deprivation of SWS/SWA leads to a highly predictable rebound of SWS/SWA within the same17 or the subsequent undisturbed sleep episode,18 indicating that this manipulation leads to an increased pressure for SWS. Increases in daytime sleep propensity following SWS deprivation have been reported repeatedly,19,20 but not in every case.21 We recently reported an increase in sleep propensity as assessed by the multiple sleep latency test (MSLT), after SWS disruption22 in a large age-structured sample with 44 participants aged 20-30 years, 35 participants aged 40-55 years, and 31 participants aged 66 and older. At baseline, older people produced less SWS and were less sleepy; after SWS disruption, increase in sleep propensity was approximately similar in all three age groups.
In contrast to the robust positive findings with regard to sleep propensity, failures to detect robust effects of SWS deprivation on subsequent waking performance are the norm in the literature and were first reported by Webb and coworkers in the 1960s. Using a tone or mild electric stimulation in response to the ongoing EEG SWS was significantly reduced, using tone to effect deprivation, for 2 consecutive nights in 5 subjects23 and, using shock to effect deprivation, for 7 consecutive nights in 6 subjects.24 Both studies reduced stage 4 sleep effectively and demonstrated increases in the amount of stage 4 during recovery sleep. Although an increase in negative somatic feelings was noted, there was no effect on tests of reaction time and addition tests administered on waking, or pursuit tracking or grip strength tests administered immediately prior to bedtime.24
In the subsequent four decades, several SWS deprivation experiments aimed at identifying the contribution of SWS to waking function by using acoustic stimulation or frequent awakenings to disrupt SWS, and evaluating the effects on waking performance on the day following one or two nights of sleep during which SWS was reduced.20,21 No general or specific contribution of SWS to waking performance was reported in any of these experiments. More recently, effects of SWS disruption have been reinvestigated using paradigms in which simple waking performance or memory consolidation was considered.14,25 While these studies offer further indication of a role for SWS in supporting cognition, in our view, the evidence is not compelling (see Discussion for further consideration of these studies).
This pessimistic view of the role SWS may play in supporting waking function and how this may be associated with age-related changes in cognition must be tempered by a number of important considerations: small sample sizes, absense of age contrasts, limited age-ranges studied, and restricted range of tasks used may underestimate both the scale and range of effects SWS disruption has on cognition. With the substantial caveat that studies may not have had the statistical power to detect them, relatively simple reaction time tasks, more complex addition tasks, and motor control (tracking, grip strength) have not shown effects of SWS disruption—tasks in which very robust effects of age have been consistently reported.26,27 Implicit memory fails to show effects of SWS disuption, consistent with what is found in the aging literature, while mood and attentional lapses appear to be affected by both SWS disruption and age.28,29 However, the effects of SWS disruption have not been quantified on what are perhaps the leitmotifs of age-related cognitive decline: less effective executive functioning, working memory deficits, slowing of information processing speed, and poor reasoning.30 Because these and other cognitive functions may begin to deteriorate in middle age,31,32 in the study below we contrasted the effects of SWS disruption on the widest range of performance assessments used until now in this literature, in order to characterize the nature and extent of any daytime functioning detrioration following SWS disruption in three healthy adult age cohorts—each larger than any previous sample used to investigate the effects of SWS disruption on daytime function.
METHODS
Subjects, Screening, and Ethical Approval
The protocol was favorably reviewed by the Quorn Independent Ethics Committee, a legally constituted regulatory ethics committee in the UK, external to the university, and all participants provided written consent after having been informed about the protocol and all study procedures. The study was conducted at the University of Surrey's Clinical Research Centre (CRC). As described before,22 participants entered the study following 2 laboratory visits to allow for a general medical screening for health, psychiatric symptoms (MINI, Mini International Neuropsychiatric Interview33) and mild cognitive impairment (Mini Mental State Examination ([MMSE] ≤ 25, elderly only)34; overnight polysomnography (PSG) for laboratory habituation and sleep screening (no sleep disorders or sleep complaints (Pittsburgh Sleep Questionnaire Index [PSQI]35 ≤ 5; satisfactory PSG, apnea-hypopnea index < 15/h or periodic leg movements arousal index < 15/h), and familiarization and assessment of daytime functioning (see Appendix). In all, 110 participants—44 healthy young (20-30 y; 23F), 35 middle-aged (40-55 y; 20F), and 31 older (66-83 y; 25F; see endnote A)—met these criteria. Younger (Mean 2.20; SD 1.13), middle-aged (Mean 2.09; SD 1.22), and older subjects (Mean 2.39; SD 1.05) scored similarly on the PSQI (F2,107 = 0.579, ns). Younger subjects scored significantly lower on the NART (National Adult Reading Test36) with a mean full-scale intelligence quotient (FSIQ) = 107.15; SD 9.13—lower than both the middle-aged (FSIQ = 114.89; SD 9.29) and older subjects (FSIQ = 118.68; SD 6.54; F2,107 = 17.97, P < 0.001).
Treatment Visit and Procedure Scheduling
Participants were randomized within each age group, to a control or SWA/SWS disruption condition. This 2-day/night intervention or control phase was preceded by a baseline and followed by a recovery night/day. Subjects remained in the CRC throughout the experimental intervention, during which time they were not allowed to drink caffeinated beverages or alcohol, did not engage in strenuous activity, and were under continuous supervision. All sleep episodes were scheduled for 23:00-07:00, a schedule which participants had been required to observe over the 2 weeks prior to the laboratory visit, and no naps were allowed except for the few epochs of sleep that may have occurred during the MSLT. Each day subjects conducted waking performance tests starting at 08:00, 10:00, 12:00, 14:00, and 16:00, and MSLTs at 09:00, 11:00, 13:00, 15:00, and 17:00. All participants were screened for hearing difficulties, and a hearing test on admission to the laboratory established that all individuals were reliably able to detect the lowest volume sounds presented during the study.
SWS Disruption
During the treatment phase, trained technicians monitored EEG during nocturnal sleep episodes, and disrupted SWS using acoustic stimulation as described before.22 Following detection of delta waves, a 1000 Hz tone, duration 1 sec, was delivered through an overhead loudspeaker at an initial intensity of 40 dB (and increased by 5dB for each subsequent delta wave until a response was observed, or the sound reached 110 dB), using a custom-made system (Glensound Electronics Ltd, Maidstone UK). Each sound-attenuated bedroom was configured to ensure that each bed received the same level of sound. Control group of subjects slept in identical conditions except in silence. Details of PSG recording procedures, subjective assessments of sleep quality administered soon after waking from nocturnal sleep, as well as the detailed procedures followed for the MSLT have been presented previously.22
Measurement of State and Performance
Daytime functioning was assessed by repeated administration of a wide range of computer controlled tests which challenged participants across a variety of cognitive domains: Mood & Affect, Sustained Attention & Arousal, Decision & Response Time, Motor & Sequence Control, Working Memory, and Executive Function. Task characteristics and implementation details are provided in the Appendix. Except where bespoke equipment was required (e.g., Critical Flicker Fusion, see Appendix), tests ran on identical computers with screen refresh rates of 60 Hz, running Active X, C#, and Exactics code to control stimulus presentation, response detection, and related timing. Except in the case of overnight retention tests, training for which was scheduled for the evenings preceding the first and second nights of SWS disruption with next day mid-morning assessments, the test battery was administered 5 times, at intervals of 2 h from 08:00. Each test battery administration was followed 1 h later by the MSLT,37,38 during which subjects lay in their bedroom and were asked to fall asleep as soon as the lights went out. The Karolinska Sleepiness Scale (KSS)39 was administered as part of the computerized test battery before each sleep latency test.
Statistical Analyses
All data and statistical analyses were conducted by the researchers and/or statisticians at the Surrey Sleep Research Centre and Clinical Research Centre of the University of Surrey. Effects of SWS deprivation and age on performance were assessed using PROC MIXED (SAS versions 8-9.1), and time courses, Cohen's d effect size measures of changes of the simple differences from baseline, and other procedures were calculated by one of the authors (JAG, SAS versions 8-9.1/ SPSS version 18). The effects of SWS disruption on daytime functioning were quantified using 2 approaches: a PROC MIXED with Fixed Effects of Age and Treatment groups with Baseline/Day 2 as a repeated measure (see endnote B), with Satterthwaite correction of degrees of freedom, and a statistical model in which individual differences at baseline are taken into account when contrasting difference from baseline in the SWS disruption and control groups (i.e., contrast between treatments, D2 - D-1, Baseline as co-variate, D1 and D2 in model). Since a large number of comparisons were made, analyses are shown with and without the traditional 5% significance level corrected for the false discovery rate (FDR40; see endnote C). Effect sizes were scaled using conventional Cohen's d effect-size criteria41 [i.e., small: 0.2 < d > 0.6; medium: 0.6 < d > 0.8; large: d > 0.8]. Age-related effects at baseline were also quantified using a mixed model approach in which absolute performance in age groups was treated as a Fixed factor and Cohen's f2 was used to quantify age-related effect sizes,42,43 using conventional qualitative cutoffs for small (0.02), medium (0.15), and large (0.35) effects. Age-group contrasts were Bonferroni corrected (see endnote C). In order to more accurately characterize effect sizes of the unique contributions of age and SWS disruption, we adopted a recently reported approach to calculating a “local effect size” which relies on using PROC MIXED to quantify the variance terms associated with the null model, combined age-mediated/SWS disruption model, and models of the separate effects of age and SWS disruption on Day 2, thus allowing calculation of Cohen's f2 to reflect the independent contributions of age group and SWS disruption. For all analyses, other than where stated explicitly below, raw performance averaged over the 5 daily time points was used to quantify performance, and thus the data reported relate to daytime performance in general, rather than to particular times of the day (see endnote D).
RESULTS
Acoustic stimulation following slow wave onset substantially changed sleep, and details on these changes in sleep are reported elsewhere in considerable depth.22 We focus here on the differences in daytime functioning which may result from these changes, the size of these effects, and on the sensitivity of the tasks used and their relationship to age-related performance differences observed in healthy adults. Thus, although our primary concern here is daytime functioning, some aspects of the sleep of those taking part may be of assistance to readers. At baseline, as expected, age groups differed significantly in total minutes spent asleep (young: 433.5 ± 23.7; middle-aged: 409.9 ± 40.9; older: 390 ± 38.5). During baseline sleep, a similar percentage of total sleep time was spent in REM sleep (20, 20, and 19%, respectively), but as would be expected, younger subjects spent proportionally longer in SWS (27.3% ± 12%) than did middle-aged (20.9% ± 14%) and older (21.5% ± 14%) participants (F2,100 = 8.12, P < 0.001), and this age-related difference slow waves sleep was substantially larger when SWA was compared across age groups.22 Reduction in total sleep duration between baseline and Night 2 was slightly over 2 minutes (2.2 ± 6 min), and this change from baseline was similar for both the control and SWS disruption (F1,94 = 0.002) in the 3 age groups (F2,94 = 0.170), and were of the same extent in intervention and control groups in each age group (F2,94 = 0.006). Change from baseline percentage REM sleep showed a reduction of 2% (± 7%). This was similar for control and intervention groups (F1,94 = 1.01) and age groups (F2,94 = 0.721), but middle-aged control group participants showed a larger reduction in %REM, resulting in a statistically reliable age and treatment interaction (F2,94 = 3.835, P < 0.05). SWS on the second intervention night was almost identical to that at baseline for the control group (100%, 94%, 97% of young, middle-aged, and older baseline SWS for those participants), but substantially lower in each of the intervention age groups, (69%, 67%, 54% of young, middle-aged, and older SWS baseline for those participants), resulting in a significant effect of the SWS disruption intervention (F1,94 = 36.202; P < 0.001), but no effect of age group or interaction (both F < 1). As previously reported, this change in SWS was achieved at the cost of increased numbers of PSG awakenings—approximately 10 more for each age group than at their baseline. The number awakenings reported next day following SWS disruption was fewer than this, averaging 7 and 6 more than baseline on Nights 1 and 2, respectively. On Night 2, both objective and subjective awakenings differed significantly from the age-matched controls, but as noted above, these differences in numbers of awakenings did not result in a significant difference in sleep duration between the control and disruption groups. In summary, as intended, at baseline the age groups each had different amounts of SWS. This was successfully reduced by acoustic SWS disruption to a similar extent in each group, but neither sleep duration nor extent of REM was affected.
Effects of Cumulative SWS Disruption on Next Day Functioning
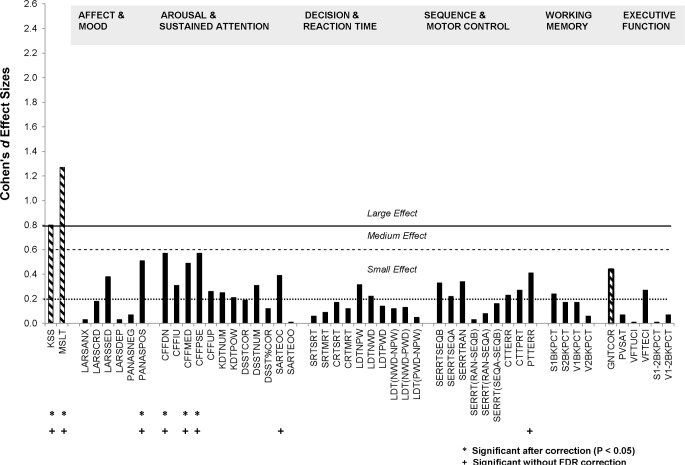
SWS disruption was associated with statistically significant deterioration in daytime functioning across a substantial number of measures (Figure 1). For convenience, outcome measures for each task are considered under a number of broad headings: Affect & Mood; Arousal & Sustained Attention; Decision & Reaction Time; Sequence & Motor Control; Working Memory; and Executive Function.
Figure 1.
Cohen's d effect sizes of daytime performance impairment across cognitive domains following two nights of SWS disruption. The only simple performance improvement from baseline (GNTCOR), is shown with a diagonal filled bar. Significance of planned contrasts is shown with (*) and without (+) correction for False Discovery Rate.
Affect & Mood
In general, negative affect (e.g., anxiety, depression) was similar in the SWS disruption and control groups. The exception to this was a small effect-sized increase in Sedation (LARSSED) in the treatment group (P = 0.054), which did not survive FDR correction. In contrast, the medium effect-sized deterioration in Positive Affect (PANASPOS) in the treatment group was statistically significant (P < 0.01), and survived correction. All effects were similar in each age group (see Table 1).
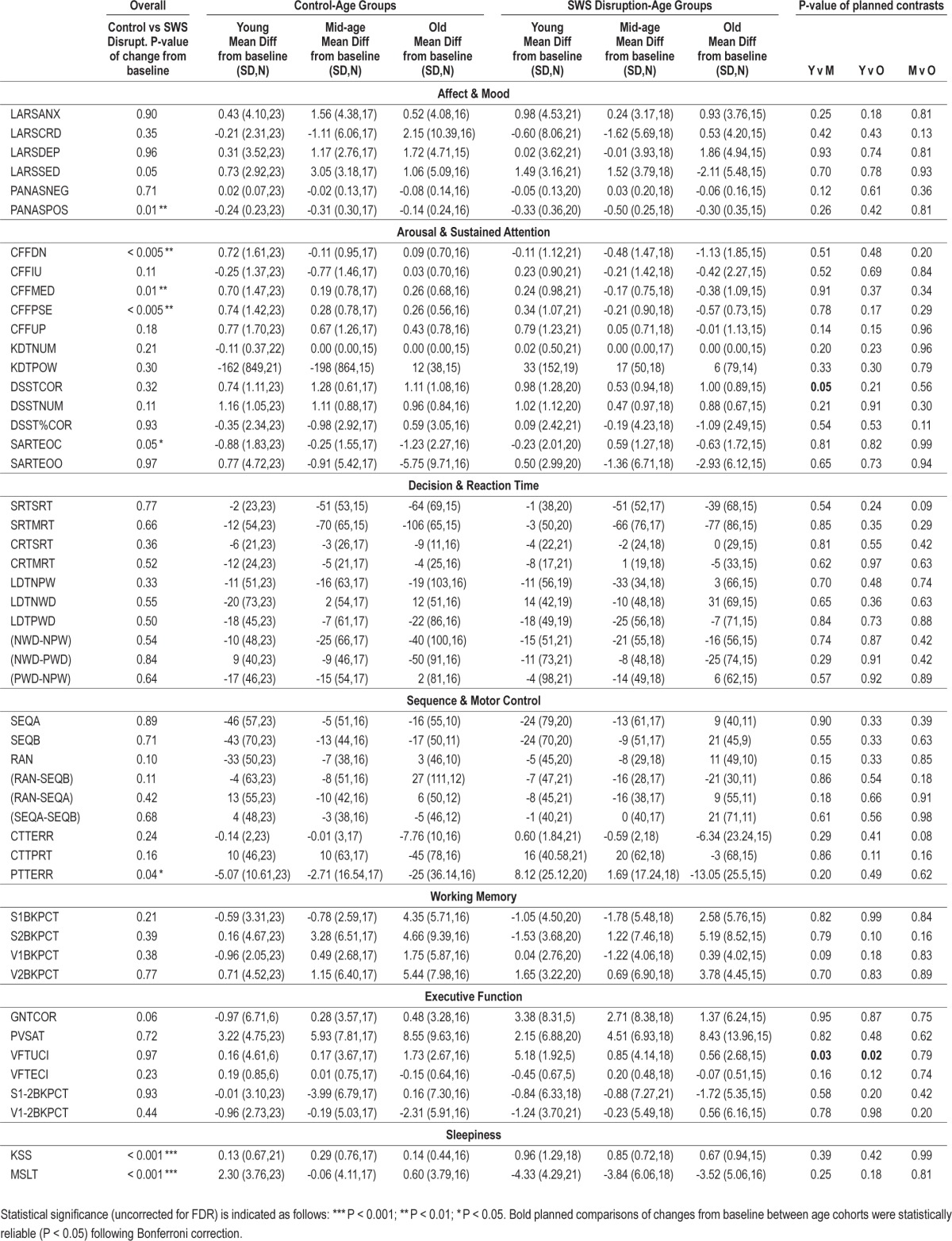
Table 1.
Changes from baseline as a function of SWS disruption and age.
Arousal & Sustained Attention
At least one index from each of the very different measures of Arousal and Sustained Attention showed small or larger effect sizes of SWS disruption, but only those from Critical Flicker Fusion survived FDR correction. Of those that did not survive correction, only SART errors of commission (i.e., failing to stop a highly practiced and thus harder to inhibit response, SARTEOC), increased substantially after SWS disruption (P < 0.05) reached conventional levels of significance. Mean Descending threshold (CFFDN, P < 0.005), Median CFF threshold (CFFMED, P < 0.01), and Point of Subjective Equality (CFFPSE, P < 0.005) revealed statistically reliable deterioration of function, which survived FDR correction. In the baseline-controlled change from baseline analyses, these findings were similar in each age group. The head-to-head analysis revealed statistically significant 3-way interactions between treatment group, age group, and day (baseline vs Day 2) for CFFDN, CFFIU, CFFPSE. In each case, in part because these 3 measures are interdependent, differences between baseline performance between the control and treatment groups rather than differential effects of SWS disruption in each age groups, underlie the interactions observed.
Decision & Reaction Time
A range of tasks required participants to respond as quickly as possible to simple and more complex stimuli. Some of these tasks, specifically Simple (SRT) and Choice Reaction time (CRT), allowed the customary separation of 2 components of response time—the time taken to realize that a response must be made (S/CRTSRT), as indexed by the latency between stimulus presentation and first movement, and the movement time taken to complete the response (S/CRTMRT). The other reaction time measures assessed the time taken to determine whether a rapidly presented and subsequently masked stimulus was or was not a word (LDT). Correct response times to non-words (LDTNWD) and words (LDTPWC, LDTNPW), and the subtractions of these which allow us to quantify lexical access time (LDT NWD-NPW) and semantic priming LDT (NPWPWD, see the Appendix for further explanation). SWS disruption had no substantial effect on response time measures, nor, in the baseline controlled analyses did the age groups differ in their change from baseline if that baseline was followed by SWS disruption (Table 1). The 3-way interaction observed in the head to head analysis appears to have been the effect of baseline differences, rather than to differences due to treatment.
Sequence & Motor Control
Tasks grouped under this heading share the characteristic that performance is largely continuous. With the exception of the start and end of each task, the next action required is spatially and/or sequentially determined by what has gone before. Thus these tasks, uniquely in the test battery, depend both on continuous attention and anticipating what will be required next. SWS disruption was associated with a small but consistent deterioration across tasks. For the most complex pursuit tracking task (PTT), this results in a significant decrement in performance following SWS disruption (P < 0.05), which did not survive correction for false discovery rate. Planned comparisons between age groups revealed no age-related differences in this general pattern (Table 1).
Working Memory
Working memory, at least as measured by 1- and 2-back tasks, appeared unaffected by SWS disruption, and this was so in each of the age groups.
Executive Function
Three specific measures of executive functioning were included—Paced Visual Serial Addition (PVSAT), Goal Neglect (GNT), and Verbal Fluency (VFT) —as were 2 others which assess working memory with different levels of executive demand (difference between 1 and 2-back performance). Because of equipment malfunction, two tasks—Goal Neglect, which required switching between different rules to govern performance, and Verbal Fluency, which required participants to say aloud as many words as possible from a specified semantic category in 30 sec—the sample sizes in age groups were substantially lower than intended. None of these standard measures of Executive functioning were affected by having had 2 nights of disrupted slow wave sleep. This is consistent with the lack of effect of SWS disruption on performance when N-back increases in task difficulty from 1- to 2-back. The Goal Neglect Task (GNT) revealed nonsignificant improvement in performance following SWS disruption, suggesting that rule compliance may be enhanced by SWS disruption.
Sleepiness
In contrast to the relatively few effects of SWS disruption on daytime functioning, subjects were significantly sleepier following 2 nights of SWS disruption. Times to fall asleep were significantly shorter (MSLT, P < 0.001) and subjective sleepiness was significantly higher (KSS, P < 0.001) in disrupted participants, even when the correction for false discovery rate is applied.
Overnight Memory Consolidation
Before retiring on the first SWS disruption night, participants learned a new visual (Texture Discrimination, TDTPCT) or motor (Finger Opposition, FOTPCT) task and, in a counterbalanced fashion, learned the other task before retiring the following night. When data were collapsed across study night, performance on the TDT was found to have markedly improved overnight (F1,51 = 18.440, P < 0.001; ηp2 = 0.266), although there was no difference in the level of overnight improvement as a function of SWS disruption (F1,51 = 0.102; P = 0.8; ηp2 = 0.002) or age (F2,51 = 1.892; P = 0.2; ηp2 = 0.069). Performance on the motor task was subject to a substantial amount of data loss but did not appear to change overnight (F1,40 = 0.007, P = 0.9; ηp2 = 0.000), an outcome which was independent of both SWS disruption (F1,51 = 0.102; P = 0.8; ηp2 = 0.002) and age (F2,40 = 1.833; P = 0.2; ηp2 = 0.084).
Summary
Of the measures reported here for the first time, (i.e. excluding MLST and KSS), changes from baseline with probabilities < 5% were observed for 6 of 47 measures. Just 4 effects survived correction for the false discovery rate (lowered Positive Affect, slower Critical Flicker Fusion Mean Descending Threshold, and the correlated measures Point of Subjective Equality (PSE) and Median (MED). Two did not (increased Errors of Commission in Sustained Attention to Response, less accurate Pursuit Tracking). Twenty-two of the contrasts between baseline and D2 performance in the control and SWS disruption groups show at least “small” effects (i.e. d > 0.2), all but one of these changes reflect worsening performance (GNTCOR). In terms of our classification of measures, the majority of those relating to sustained attention (9 of 12) and those relating to motor control (6 of 9) show small effects. In contrast, only a minority of measures from the other domains resulted in “small” effects: 2 mood arousal related indices (2 of 6), one measure each of executive functioning (2 of 6) and working memory (1 of 4); the extent of slowing of reaction times was similarly negligible (2 of 10). Just 3 of these deteriorations—reduced positive affect and slower resolution of flicker-fusion and fusion-flicker— approach the size required for a “medium” effect. Three age-related planned comparisons yielded statistically significant differences (P < 0.05, uncorrected for multiple comparisons). These suggested that SWS disruption enhanced verbal fluency in the young to a greater extent than for middle-aged and older participants (Table 1), and that middle-aged subjects witnessed an impairment of Digit-Symbol substitution following SWS disruption not apparent in the younger or older subjects.
In short, approximately half the measures taken show a deterioration of performance following SWS disruption. This deterioration is largely confined to measures of arousal and tasks which require sustained attention, including sequence monitoring and motor control. The extent of this deterioration is independent of age. Since this is perhaps surprising, in the next section, we consider whether the performance measures used were simply insensitive to age.
Age-Related Differences in Baseline Daytime Functioning
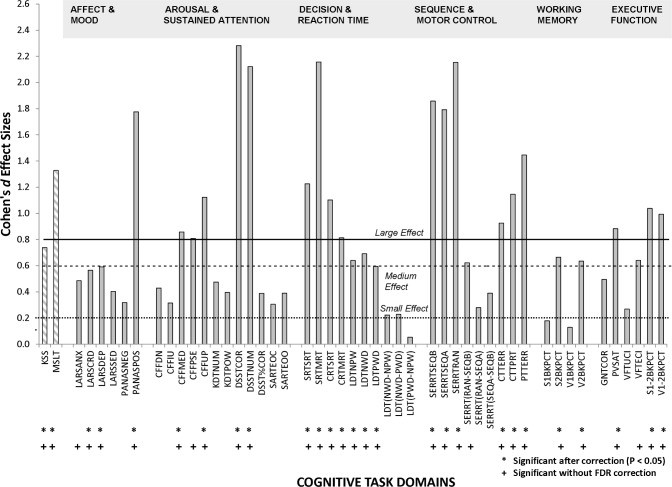
Baseline performance across age groups was contrasted for each of the tasks and measures described above. The outcomes of mixed effect analyses and Bonferroni corrected age group contrasts are presented in Table 2, with Cohen's d effect size measures plotted in Figure 2, with indications of significance (P < 0.05) with and without FRD correction included in each case.
Table 2.
Age-related differences in baseline daytime functioning

Figure 2.
Cohen's d effect sizes of age-related decline in daytime performance across cognitive domains. Lower subjective and objective sleepiness in older participants are shown with diagonal filled bars. Significance of planned contrasts is shown with (*) and without (+) correction for False Discovery Rate.
Mood & Affect
Each of the 6 affect measures revealed at least small (d > 0.2) effects of age at baseline, 3 of which survived correction for false discovery rate. Positive affect (PANASPOS) was substantially lower in younger participants than middle-aged and older participants. Depression (LARSDEP) was higher in younger than in older participants, but the latter had higher feelings of clumsiness and dizziness (LARSCRD).
Sustained Attention & Arousal
Four measures from 4 sustained attention tasks (CFF, KDT, DSST, SART) differed significantly across age groups, four after correction for the false discovery rate, and almost all revealed small or larger effect sizes. The Digit-Symbol task was performed more successfully by younger participants, in terms of both the total number of responses made (DSSTNUM) and the number of these which were correct (DSSTCOR). The large effect sizes of age in both cases would seem to be partly a function of a slowing of encoding and response times with age, although when the percentage of accurate responses was considered (DSSTPCT), age groups did not differ. Critical Flicker Fusion, which requires individuals to distinguish between flicker and fusion but does not require speeded responding, is less well preserved in older subjects (e.g., CFFMED), who cannot make reliable discriminations at as high flicker rates as their younger counterparts, especially when the rates are moving from slower to faster (i.e., CFFUP, see Table 2).
Decision & Reaction Time
All Simple and Choice Reaction time measures, and simple Lexical Decision measures (LDTNPW, LDTPWD, LDTNWD) differed significantly across age groups; all 7 measures survived correction for false discovery rate. Younger participants were faster than older subjects on both components of simple and choice reaction time (SRTSRT, SRTMRT, CRTSRT, CRTMRT), and faster also than middle-aged subjects in each case, except the motor component of choice reaction time (CRTMRT). Middle-aged subjects were faster than older participants on each motor component but not registering that a simple stimulus required responding to (SRTSRT, CRTSRT). The other reaction time measure included in Table 2 related to the time taken to determine whether a rapidly presented and subsequently masked stimulus was a word (LDT). Correct response times to non-words (LDTNWD) and words (LDTPWC, LDTNPW), were all faster for younger participants, but middle-aged participants were also faster than older participants determining whether a stimulus was a word (LDTNWD). Younger and middle-aged participants did not differ. As described in the Appendix, the Lexical Decision Task can also be used to measure priming implicit memory, in this case by comparing lexical decision times for words from previously thought about semantic categories and similar semantic categories that have not been recently thought about (LDT [NPW-PWD]). While there is a highly reliable priming effect for each age group (i.e., in each case the faster response time to primed categories was significantly different from zero, P < 0.01 in each case), as might be expected, implicit memory showed no difference across age groups and was the only reaction time-based measure to have a negligible effect size.
Sequence & Motor & Control
All of the direct measures from these tasks revealed statistically reliable differences across age groups, even after correction for false discovery rate. In the Serial Reaction Task, response times to the practiced (SERRTSEQA, SERRTSEQB) and random (SERRTRAN) stimulus sequences showed significant age effects. In each case reaction time increased significantly across age groups. As described earlier, the Serial Reaction Task also allows the level of sequence learning to be measured—typically indexed by the difference in latencies between response time to sequential and random stimulus presentations—and younger participants were more slowed when encountering the random block, indicating perhaps greater sequence learning on their part. The 2 motor control tasks used, a complex pursuit tracking task in which the target moved both horizontally and vertically as determined by a very complex but learnable formula (PTT), and a more simple tracking task (CTT) in which the stimulus moved horizontally only, with occasional distracting peripheral stimuli to which an additional simple motor response had to be made. In the more demanding pursuit tracking task, younger participants were closer to the moving target throughout (PTTERR) than middle-aged participants; both groups were more accurate than their older counterparts. Error on the simpler tracking task (CTTERR) was also lower in younger and middle-aged participants than older subjects, but younger and middle-aged participants performed similarly. Table 2 also shows that the reaction time to a secondary task performed during the continuous tracking task (CTTPRT) exhibited the younger age advantage in reaction time tasks, and in this case, it is worth noting, in a dual task situation. It is also noteworthy that even with the additional difficulty imposed by occasional responding to the secondary task, the simpler horizontal tracking task (CTT) did not discriminate as effectively between younger and middle-aged adults as did the more complex pursuit tracking task (PTT).
Working Memory
Simple working memory tasks (1-back), revealed no age-related differences, whether these assessed verbal (VBK) or visuo-spatial (SBK) working memory. More difficult, but perceptually identical working memory tasks (2-back), showed statistically significant differences in accuracy across age groups for both verbal and spatial versions, even after FDR correction. Planned age contrasts showed an advantage in accuracy for younger participants over older participants in both spatial and verbal tasks, and for young over middle-aged participants with respect to S2BKPCT.
Executive Function
Each of the 6 measures of executive functioning revealed at least small effect sizes, and in 3 cases statistically significant age differences after correction for false discovery. As noted above, the deterioration in performance as the n-back task became more difficult (which helps to isolate the more executive components of the n-back task) also showed considerable age sensitivity. Paced Visual Serial Addition (PVSAT) showed a similar advantage for younger over older participants and for middle-aged participants over the older group, and also survived FDR correction. Goal Neglect (GNT), which here is compromised by a lack of statistical power, revealed a similar trend. Surprisingly, younger participants had more Verbal Fluency errors (VFTECI, i.e., mentioning non-category items or repeating items already said, P < 0.05), than older participants, and also reported marginally fewer items, which is consistent with the difference in Verbal Intelligence/NART scores across the groups.
Sleepiness
As noted above, we have included, for comparative purposes, previously reported data showing age-related effects on sleepiness. At baseline, there were substantial differences across of age groups for daytime sleepiness as measured objectively (MSLT, significant, large effect) and subjectively (KSS, medium effect, which also survived the false discovery correction). In both cases, younger subjects were sleepier than the older participants. As previously reported,22 various subjective assessments of sleep were provided by participants soon after waking. Only subjective duration of baseline sleep awakenings and feelings of being refreshed on waking following baseline sleep showed main effects of age, with post hoc comparisons showing that older participants were less refreshed and reported being awake for longer than their younger counterparts.
Summary
Some 63% of the measures analyzed revealed statistically significant differences across age groups, even after the significance level was adjusted for false discovery rate, and almost all (44 of 47) had small (18), medium (6), or large effect sizes (19), with just 3 measures failing to reach Cohen's criterion for a “small” effect. With respect to reaction time, motor control, sustained attention, and executive functioning, where there was an age-related difference in performance, younger participants outperformed their older counterparts. Simple tests of working memory, and derived measures of implicit memory (i.e., semantic priming and sequence learning), were insensitive to age. Exceptions to the general age-related deterioration pattern were tasks which were related to sleepiness and positive affect, where older subjects were less sleepy and more positive.
Dissimilarity of Performance Impairment Arising Age and SWS Disruption
As the foregoing sections show, although relatively few of our performance measures showed significantly different effects of SWS disruption across age groups, this is certainly not because the tasks used were themselves insensitive to age differences.
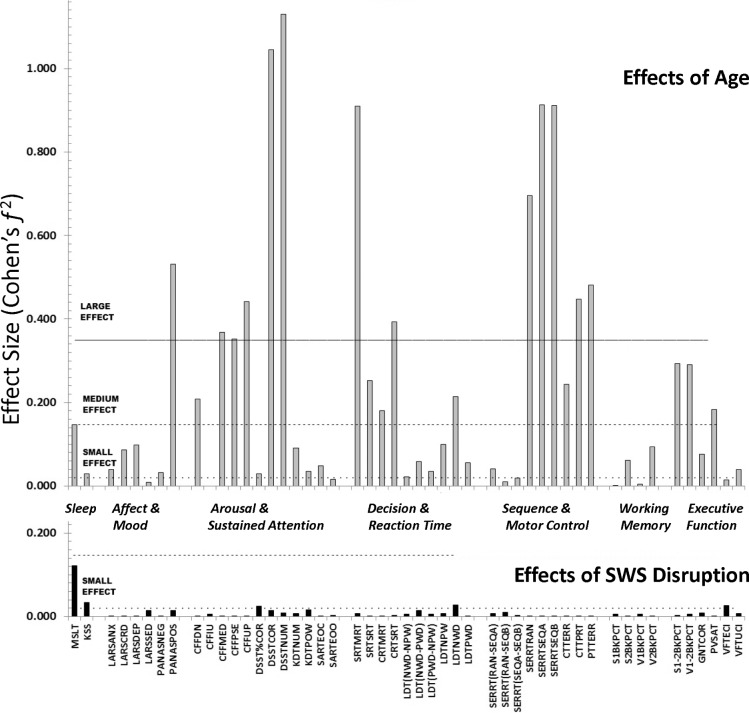
As pointed out above, a recently reported adaptation of PROC MIXED allows the calculation of a “local effect size,” which enables us to assess the separate effect of age and SWS disruption on performance at a particular point in time. The resulting Cohen's f2 allows us to compare the effects of age and SWS disruption while minimizing potential confounds. Doing so shows that the average Day 2 age effect size (Mean Cohen's f2 = 0.24, SD 0.30) and SWS disruption effect size (Mean = 0.01, SD 0.02) are very different (t49 = 5.53; P < 0.001). The lack of a statistically reliable parametric correlation (r(50) = -0.10; P = 0.48) indicates that age and SWS disruption have very little variance in common. Figure 3 shows that while the effect sizes of age and SWS disruption are very similar for daytime sleepiness (but the direction of change is opposite), those for the other task groupings are very different. That is, the sizes of effects of SWS disruption are not just smaller than those of age, but differ across tasks.
Figure 3.
Contrasting effect sizes of age-related decline and SWS disruption across cognitive domains.
DISCUSSION
The primary purpose of this paper is to provide an assessment of the effects of short-term SWS disruption on daytime function. Previous attempts to assess the effects of SWS disruption have had relatively small sample sizes, limited assessment of cognitive function, and restricted age ranges. The results reported above show that people felt less positive affect, and information processing slowed following SWS disruption; motor control became less precise, and incorrect proponent actions were carried out rather than inhibited. The sizes of these effects on performance are dwarfed by the very large effect that SWS disruption has on daytime sleepiness, a pattern noted in our recent study of acute total and seven nights of partial sleep deprivation (6 h time in bed vs 10 h time in bed) in young adults.44 The rather limited evidence of performance deterioration following SWS disruption was similar for each age group, even though the majority of performance measures used were evidently sensitive to age.
The serendipitous finding that the effects of SWS deprivation and age-cohort differences on baseline daytime sleepiness were very similar in size allows us to conclude that SWS disruption causes small but widespread deterioration of cognitive function of too small a scale to have been identified in any previous study of the effects of SWS disruption. Orders of magnitude of larger numbers of participants would be required to adequately assess whether such effects are statistically robust. Thus, while few deleterious effects of SWS disruption have been reported in the literature, except perhaps with respect to reductions in positive affect45 and increased lapses during vigilance tasks,25 we show that such effects are indeed present, but so small that the lack of reported effects is unsurprising.
The weak effects of SWS disruption on cognition may seem at variance with studies mentioned earlier, which appear to imply that effects should be stronger and more pervasive, than we observed. For example, effects of SWS disruption have been reinvestigated using paradigms in which both simple waking performance and memory consolidation was considered.14,25 In the latter study, 13 participants with an average age of 60 years, underwent two nights of SWA reduction, during which an increasingly loud tone was presented when SWA activity exceeded an individually determined threshold. While SWA was reduced significantly, the duration of stage 3 and stage 4 sleep was not. A variant of the psychomotor vigilance task, and a “more complex vigilance task” which required responses to one of two letters repeatedly presented, both showed more lapses following SWA reduction, but no effect on false alarms or task complexity. Effects of SWS disruption were also reported for overnight recognition memory, thought to reflect a SWS-disruption induced encoding deficit, but, reminiscent of what we reported above, not for implicit memory in a serial reaction task.25,46 The study also claims to show no effect of SWA reduction on reaction time, although it should be noted that reaction time was quantified on the basis of responses to stimuli presented as part of the vigilance tasks, and is thus confounded.
Other studies show that consolidation of texture discrimination skills was reduced for nine young participants who underwent acoustic SWA reduction, compared with six young participants whose sleep was undisturbed, during a 4-hour overnight sleep opportunity,47 although originally consolidation on this task was reported to be dependent on REM sleep.48 However, although the authors report that improvement in performance “correlated with EEG power density during NREM sleep in the frequency range of SWA (maximum r = 0.75 at 0.75-1.0 Hz)” during a subsequent 8-hour undisturbed overnight sleep opportunity, performance improvement from baseline was similar irrespective of whether SWA was reduced in the first sleep post-training when, one would assume, consolidation would be most beneficial. A causal role for SWS in overnight learning is also suggested in a study of 12 young participants showing that only when acoustic stimulation during overnight sleep was independent of the appearance of slow waves did motor control of learned movements improve overnight.14 While we also show effects of improved next day performance on the Texture Discrimination task, the “consolidation” was similar irrespective of whether participants' SWS was disrupted. While more carefully controlled studies are warranted in order to determine what causal role, if any, is played by SWS in overnight consolidation, we would observe that reducing SWA overall and not ensuring that increases in the volume of acoustic stimulation are consistently contingent on the appearance of slow waves is unlikely to produce a satisfactory diminution of SWS, particularly if one assumes that the effect of a particular acoustic stimulus is to suppress an individual slow wave.
Studies in which SWS was not directly manipulated but changes because individuals' overall sleep duration was consistently restricted over a 7-day49 or 14-day50 day period, show that despite substantial sleep duration dose-related effects on daytime impairment, there was no associated change in SWS or delta-power. Similarly, during total sleep deprivation, delta-wave activity increased as a function of time awake, but no concomitant effect was observed on performance.51 These studies, while influencing a broad range of sleep-related parameters, are also suggestive of a lack of effect of slow wave activity and next day performance.
As reviewed above, the apparent relationship between waxing and waning executive functioning, cortical maturation, growth hormone secretion, reductions in SWS, and the predominance of anterior activity in EEG during SWS have suggested a seductively integrated account of how sleep and cognitive development and decline may be intimately linked. One variant of this suggests that SWS may be particularly important for preserving or restoring executive functioning.52,53 While the current study does not preclude a crucial role of SWS in cortical maturation and decline, the data reported above seriously challenge the view that global SWS is crucial for conservation or demonstration of effective daytime functioning in the shorter term.16 Instead, what we see when SWS is disrupted is arguably more similar to the slowing of performance which is associated with normal healthy aging—that is, the sheer speed at which information is processed may slow—but SWS disruption does not appear to result in additional effects on executive functioning which are readily apparent when we age.30 This is consistent with the effects of SWS disruption on Critical Flicker Fusion, which depends on speed of integration of perceptual stimuli rather than response time per se. A slowing of processing speed would also, obviously, result in slower reaction time—whether as the result of slower stimulus detection, response generation, or both. However, in tasks where one's current response is partly determined by one's previous action (e.g., continuous tracking, serial reaction), slowed information processing speed would also result in impaired performance. As with the effects of slowed information processing in older participants, deteriorating performance may not necessarily be apparent in the sheer accuracy of performance. In some cases, for example, words may be well or badly recalled, but the speed at which this is done may not be relevant or measured in the study. This study sought to establish whether SWS disruption impaired next day functioning, and in order to do so deliberately used a broad range of tasks widely used in age-related studies of cognition. Clearly issues arise as the result of the findings reported above, such as whether general slowing, or whether what results is a quite specific compromising of particular cognitive functions or neural substrates. Ultimately, careful empirical manipulation of task requirements would be more compelling than arguments along the lines we have provided above.54,55
However, the widespread but small deteriorations in cognitive functioning which follow SWS disruption are not simply weaker than, but are qualitatively different from, those of age. In particular, while deterioration in executive functioning is both prominent in, and characteristic of, non-pathological aging, effects of SWS disruption on executive functioning are far weaker, if present at all. Accounts of healthy cognitive decline that see this as linked to SWS, and that see SWS as particularly restorative of executive functions are challenged by these findings. It may be that other aspects of sleep changes, such as duration and wake after sleep onset are associated with cognitive decline, but the data reported here neither support nor weaken such claims. Short-term SWS disruption makes people more sleepy, but does not make them perform worse on most measures of functioning—being sleepy does not inevitably result in poor performance. This, together with what we might call the age-sleep conundrum, i.e., that age has contradictory effects on cognition and sleepiness, requires a more subtle exposition of the relationships between cognition, performance, sleep, and age than is currently available.
ENDNOTES
This disparity in gender balance is reflective of the general population of the UK among those aged 66 and older, 76% of whom are female (Office of National Statistics, 2006). Analyses not reported here show no consistent effects of gender on performance measures.
This head-to-head analysis was suggested by an anonymous reviewer, and while the analyses showed many statistically significant main effects of Age, Treatment Group and Day, none are materially different to those observed in other analyses reported here. Crucially, only one Group * Day interaction was statistically significant (F2,103 = 7.206; P < 0.001), which showed, as reported elsewhere, that sleep latencies increased significantly in the SWS disruption group from baseline, but the Control group showed no such change, and four 3-way interactions (CFFDN, CFFMED, CFFPSE, CRTMRT) suggested that the effects of SWS disruption were not consistent across age groups in these small proportion of cases. These performance effects are considered as part of the overall statistical treatment, which we prefer, because baseline differences in performance are more fully taken into account when evaluating the effects of SWS disruption, and the effects of age on baseline performance use all of the data available.
We consider that FDR correction is more appropriate for the current analyses, since the sample sizes provide substantially more power than in previous studies. The more permissive nature of FDR, when contrasted with Bonferroni correction, is also, we believe more appropriate when essentially exploring whether any effects might exist, rather than when confirming those we might already anticipate in the basis of extant literature. Hence, for age-effect analyses we use Bonferroni correction to compare age groups, but FDR when assessing effects of SWS disruption on daytime function.
The analyses referred to above have also been carried out including individual time points, but as these reveal nothing different from the averaged daytime performance, the additional complexity and length of reporting individual time points is eschewed in favour of the average measure, which reflects performance across the span of what would be a typical working day.
DISCLOSURE STATEMENT
This study was sponsored by H. Lundbeck A/S, but involved no off-label or investigational substance use. Study design was collaborative between the investigators and sponsor. Data were analyzed by Dr. Groeger and statisticians at the Clinical Research Centre, under supervision of Dr. Dijk. Manuscript written by Drs. Groeger and Dijk with input and approval on the final version by all co-authors. Dr. Deacon was employed by sponsor during data collection. Dr. Dijk has received research support/contracts and/or consulted for Ferring Pharmaceuticals, Ono Pharmaceuticals, Lilly, Philips, Viropharma, Servier, UCB, and H Lundbeck A/S. Dr. Groeger has consulted for Ferring Pharmaceuticals. Dr. Stanley has received honoraria from Ferring, Novartis, and Pfizer. Dr. Deacon's present address is ONO PHARMA, MidCity Place, 71 High Holborn, London, WC1V 6EA, UK.
ACKNOWLEDGMENT
The authors thank the staff of CRC and SSRC, past and present, for their many and varied contributions to the execution of the study, and particularly Dr. Sigurd Johnson for support with statistical analyses.
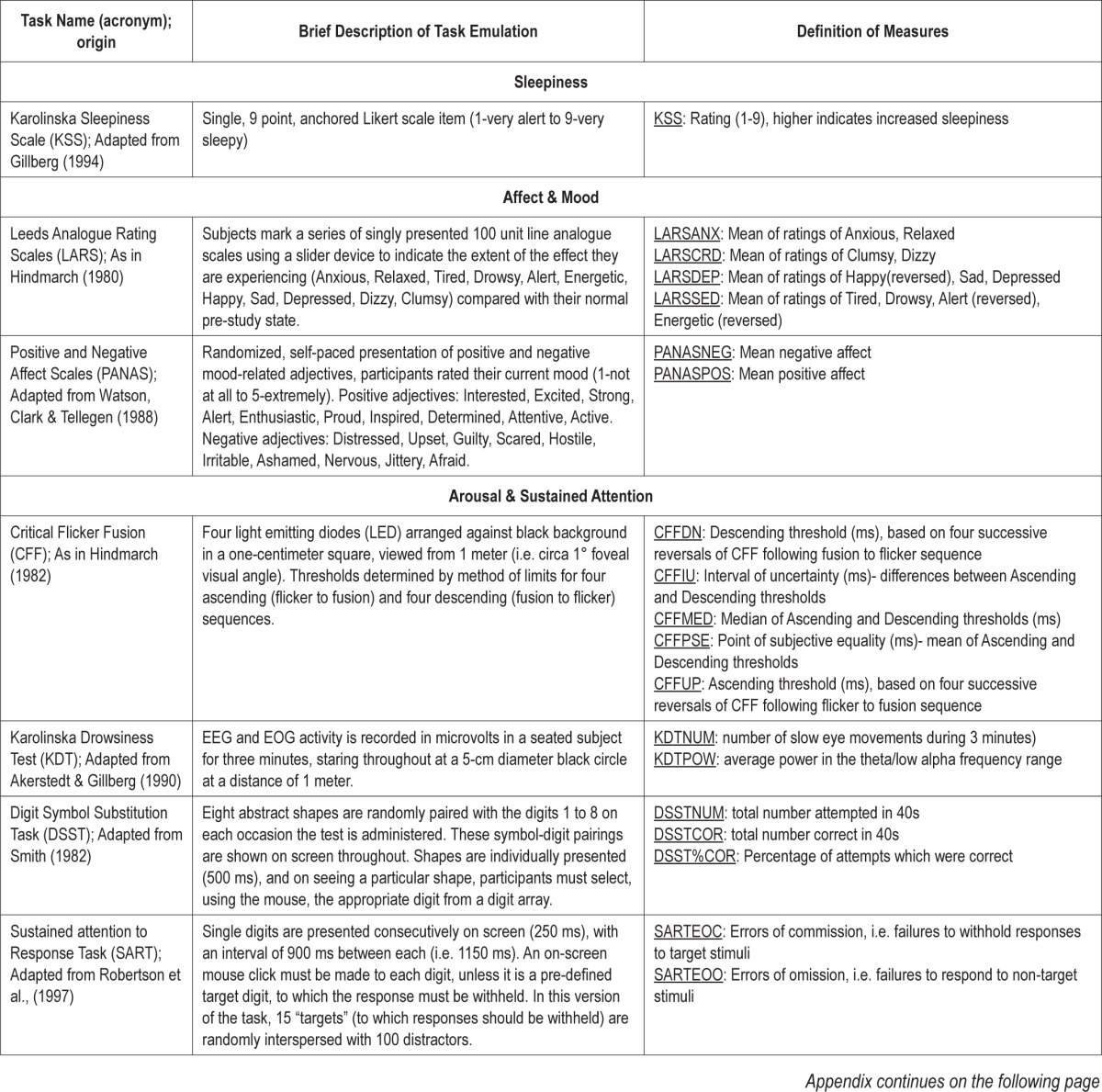
Appendix.
Computer-based task details, measures and procedures.


REFERENCE
- 1.Dijk DJ, Czeisler CA. Contribution of the circadian pacemaker and the sleep homeostat to sleep propensity, sleep structure, electroencephalographic slow waves, and sleep spindle activity in humans. J Neurosci. 1995;15:3526–38. doi: 10.1523/JNEUROSCI.15-05-03526.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dijk DJ, Brunner DP, Beersma DG, Borbely AA. Electroencephalogram power density and slow wave sleep as a function of prior waking and circadian phase. Sleep. 1990;13:430–40. doi: 10.1093/sleep/13.5.430. [DOI] [PubMed] [Google Scholar]
- 3.Achermann P, Borbely AA. Mathematical models of sleep regulation. Front Biosci. 2003;8:s683–93. doi: 10.2741/1064. [DOI] [PubMed] [Google Scholar]
- 4.Crunelli V, Hughes SW. The slow (< 1 Hz) rhythm of non-REM sleep: a dialogue between three cardinal oscillators. Nat Neurosci. 2010;13:9–17. doi: 10.1038/nn.2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krueger JM, Rector DM, Roy S, Van Dongen HP, Belenky G, Panksepp J. Sleep as a fundamental property of neuronal assemblies. Nat Rev Neurosci. 2008;9:910–9. doi: 10.1038/nrn2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cajochen C, Foy R, Dijk DJ. Frontal predominance of a relative increase in sleep delta and theta EEG activity after sleep loss in humans. Sleep Research Online. 1999;2:65–9. [PubMed] [Google Scholar]
- 7.Munch M, Knoblauch V, Blatter K, et al. The frontal predominance in human EEG delta activity after sleep loss decreases with age. Eur J Neurosci. 2004;20:1402–10. doi: 10.1111/j.1460-9568.2004.03580.x. [DOI] [PubMed] [Google Scholar]
- 8.Buchmann A, Ringli M, Kurth S, et al. EEG sleep slow-wave activity as a mirror of cortical maturation. Cereb Cortex. 2011;21:607–15. doi: 10.1093/cercor/bhq129. [DOI] [PubMed] [Google Scholar]
- 9.Kurth S, Ringli M, Geiger A, LeBourgeois M, Jenni OG, Huber R. Mapping of cortical activity in the first two decades of life: a high-density sleep electroencephalogram study. J Neurosci. 2010;30:13211–9. doi: 10.1523/JNEUROSCI.2532-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mander BA, Rao V, Lu B, et al. Prefrontal atrophy, disrupted NREM slow waves and impaired hippocampal-dependent memory in aging. Nat Neurosci. 2013;16:357–64. doi: 10.1038/nn.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kattler H, Dijk DJ, Borbely AA. Effect of unilateral somatosensory stimulation prior to sleep on the sleep EEG in humans. J Sleep Res. 1994;3:159–64. doi: 10.1111/j.1365-2869.1994.tb00123.x. [DOI] [PubMed] [Google Scholar]
- 12.Huber R, Ghilardi MF, Massimini M, et al. Arm immobilization causes cortical plastic changes and locally decreases sleep slow wave activity. Nat Neurosci. 2006;9:1169–76. doi: 10.1038/nn1758. [DOI] [PubMed] [Google Scholar]
- 13.Plihal W, Born J. Effects of early and late nocturnal sleep on declarative and procedural memory. J Cogn Neurosci. 1997;9:534–47. doi: 10.1162/jocn.1997.9.4.534. [DOI] [PubMed] [Google Scholar]
- 14.Landsness EC, Crupi D, Hulse BK, et al. Sleep-dependent improvement in visuomotor learning: a causal role for slow waves. Sleep. 2009;32:1273–84. doi: 10.1093/sleep/32.10.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diekelmann S, Born J. The memory function of sleep. Nat Rev Neurosci. 2010;11:114–26. doi: 10.1038/nrn2762. [DOI] [PubMed] [Google Scholar]
- 16.Tononi G, Cirelli C. Sleep function and synaptic homeostasis. Sleep Med Rev. 2006;10:49–62. doi: 10.1016/j.smrv.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 17.Dijk DJ, Beersma DG, Daan S, Bloem GM, Van den Hoofdakker RH. Quantitative analysis of the effects of slow wave sleep deprivation during the first 3 h of sleep on subsequent EEG power density. Eur Arch Psychiatry Neurol Sci. 1987;236:323–8. doi: 10.1007/BF00377420. [DOI] [PubMed] [Google Scholar]
- 18.Ferrara M, De Gennaro L, Bertini M. Selective slow-wave sleep (SWS) deprivation and SWS rebound: do we need a fixed SWS amount per night? Sleep Research Online. 1999;2:15–9. [PubMed] [Google Scholar]
- 19.Walsh JK, Hartman PG, Schweitzer PK. Slow-wave sleep deprivation and waking function. J Sleep Res. 1994;3:16–25. doi: 10.1111/j.1365-2869.1994.tb00099.x. [DOI] [PubMed] [Google Scholar]
- 20.Gillberg M, Akerstedt T. Sleep restriction and SWS-suppression: effects on daytime alertness and night-time recovery. J Sleep Res. 1994;3:144–51. doi: 10.1111/j.1365-2869.1994.tb00121.x. [DOI] [PubMed] [Google Scholar]
- 21.Bonnet MH. Performance and sleepiness as a function of frequency and placement of sleep disruption. Psychophysiology. 1986;23:263–71. doi: 10.1111/j.1469-8986.1986.tb00630.x. [DOI] [PubMed] [Google Scholar]
- 22.Dijk DJ, Groeger JA, Stanley N, Deacon S. Age-related reduction in daytime sleep propensity and nocturnal slow wave sleep. Sleep. 2010;33:211–23. doi: 10.1093/sleep/33.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Agnew HW, Jr., Webb WB, Williams RL. The effects of stage four sleep deprivation. Electroencephalogr Clin Neurophysiol. 1964;17:68–70. doi: 10.1016/0013-4694(64)90011-2. [DOI] [PubMed] [Google Scholar]
- 24.Agnew HW, Jr., Webb WB, Williams RL. Comparison of stage four and 1-rem sleep deprivation. Percept Mot Skills. 1967;24:851–8. doi: 10.2466/pms.1967.24.3.851. [DOI] [PubMed] [Google Scholar]
- 25.Van Der Werf YD, Altena E, Vis JC, Koene T, Van Someren EJ. Reduction of nocturnal slow-wave activity affects daytime vigilance lapses and memory encoding but not reaction time or implicit learning. Prog Brain Res. 2011;193:245–55. doi: 10.1016/B978-0-444-53839-0.00016-8. [DOI] [PubMed] [Google Scholar]
- 26.Deary IJ, Penke L, Johnson W. The neuroscience of human intelligence differences. Nat Rev Neurosci. 2010;11:201–11. doi: 10.1038/nrn2793. [DOI] [PubMed] [Google Scholar]
- 27.Akerstedt T, Hume K, Minors D, Waterhouse J. Good sleep--its timing and physiological sleep characteristics. J Sleep Res. 1997;6:221–9. doi: 10.1111/j.1365-2869.1997.00221.x. [DOI] [PubMed] [Google Scholar]
- 28.Deary IJ, Corley J, Gow AJ, et al. Age-associated cognitive decline. Br Med Bull. 2009;92:135–52. doi: 10.1093/bmb/ldp033. [DOI] [PubMed] [Google Scholar]
- 29.Der G, Allerhand M, Starr JM, Hofer SM, Deary IJ. Age-related changes in memory and fluid reasoning in a sample of healthy old people. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2010;17:55–70. doi: 10.1080/13825580903009071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deary IJ, Johnson W, Starr JM. Are processing speed tasks biomarkers of cognitive aging? Psychol Aging. 2010;25:219–28. doi: 10.1037/a0017750. [DOI] [PubMed] [Google Scholar]
- 31.Park DC, Reuter-Lorenz P. The adaptive brain: aging and neurocognitive scaffolding. Annu Rev Psychol. 2009;60:173–96. doi: 10.1146/annurev.psych.59.103006.093656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hedden T, Gabrieli JD. Insights into the ageing mind: a view from cognitive neuroscience. Nature reviews. Neuroscience. 2004;5:87–96. doi: 10.1038/nrn1323. [DOI] [PubMed] [Google Scholar]
- 33.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. quiz 4-57. [PubMed] [Google Scholar]
- 34.Folstein MF, Folstein SE, Mchugh PR. Mini-Mental State - Practical Method for Grading Cognitive State of Patients for Clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 35.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 36.Nelson HE. London: NFER-Nelson; 1982. The National Adult Reading Test (NART): test manual. [Google Scholar]
- 37.Arand D, Bonnet M, Hurwitz T, Mitler M, Rosa R, Sangal RB. The clinical use of the MSLT and MWT. Sleep. 2005;28:123–44. doi: 10.1093/sleep/28.1.123. [DOI] [PubMed] [Google Scholar]
- 38.Littner MR, Kushida C, Wise M, et al. Practice parameters for clinical use of the multiple sleep latency test and the maintenance of wakefulness test. Sleep. 2005;28:113–21. doi: 10.1093/sleep/28.1.113. [DOI] [PubMed] [Google Scholar]
- 39.Gillberg M, Kecklund G, Akerstedt T. Relations between performance and subjective ratings of sleepiness during a night awake. Sleep. 1994;17:236–41. doi: 10.1093/sleep/17.3.236. [DOI] [PubMed] [Google Scholar]
- 40.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate - a practical and powerful approach to multiple testing. J Roy Stat Soc B Met. 1995;57:289–300. [Google Scholar]
- 41.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 42.Fritz CO, Morris PE, Richler JJ. Effect Size estimates: current use, calculations, and interpretation. J Exp Psychol Gen. 2012;141:2–18. doi: 10.1037/a0024338. [DOI] [PubMed] [Google Scholar]
- 43.Selya AS, Rose JS, Dierker LC, Hedeker D, Mermelstein RJ. A practical guide to calculating Cohen's f(2), a measure of local effect size, from PROC MIXED. Front Psychol. 2012;3:111. doi: 10.3389/fpsyg.2012.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lo JC, Groeger JA, Santhi N, et al. Effects of partial and acute total sleep deprivation on performance across cognitive domains, individuals and circadian phase. PLoS One. 2012;7:e45987. doi: 10.1371/journal.pone.0045987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Williams RL, Agnew HW, Jr., Webb WB. Effects of prolonged stage four and 1-REM sleep deprivation. EEG, task performance, and psychologic responses.SAM-TR-67-59. [Technical report] SAM-TR. USAF School of Aerospace Medicine. 1967:1–10. [PubMed] [Google Scholar]
- 46.Vandewalle G, Archer SN, Wuillaume C, et al. Effects of light on cognitive brain responses depend on circadian phase and sleep homeostasis. J Biol Rhythms. 2011;26:249–59. doi: 10.1177/0748730411401736. [DOI] [PubMed] [Google Scholar]
- 47.Aeschbach D, Cutler AJ, Ronda JM. A role for non-rapid-eye-movement sleep homeostasis in perceptual learning. J Neurosci. 2008;28:2766–72. doi: 10.1523/JNEUROSCI.5548-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Karni A, Tanne D, Rubenstein BS, Askenasy JJ, Sagi D. Dependence on REM sleep of overnight improvement of a perceptual skill. Science. 1994;265:679–82. doi: 10.1126/science.8036518. [DOI] [PubMed] [Google Scholar]
- 49.Belenky G, Wesensten NJ, Thorne DR, et al. Patterns of performance degradation and restoration during sleep restriction and subsequent recovery: a sleep dose-response study. J Sleep Res. 2003;12:1–12. doi: 10.1046/j.1365-2869.2003.00337.x. [DOI] [PubMed] [Google Scholar]
- 50.Van Dongen HP, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26:117–26. doi: 10.1093/sleep/26.2.117. [DOI] [PubMed] [Google Scholar]
- 51.Leproult R, Colecchia EF, Berardi AM, Stickgold R, Kosslyn SM, Van Cauter E. Individual differences in subjective and objective alertness during sleep deprivation are stable and unrelated. Am J Physiol Regul Integr Comp Physiol. 2003;284:R280–90. doi: 10.1152/ajpregu.00197.2002. [DOI] [PubMed] [Google Scholar]
- 52.Harrison Y, Horne JA, Rothwell A. Prefrontal neuropsychological effects of sleep deprivation in young adults--a model for healthy aging? Sleep. 2000;23:1067–73. [PubMed] [Google Scholar]
- 53.Wilckens KA, Erickson KI, Wheeler ME. Age-related decline in controlled retrieval: the role of the PFC and sleep. Neural Plast. 2012 doi: 10.1155/2012/624795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tucker AM, Whitney P, Belenky G, Hinson JM, Van Dongen HP. Effects of sleep deprivation on dissociated components of executive functioning. Sleep. 2010;33:47–57. doi: 10.1093/sleep/33.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jackson ML, Gunzelmann G, Whitney P, et al. Deconstructing and reconstructing cognitive performance in sleep deprivation. Sleep Med Rev. 2013;17:215–25. doi: 10.1016/j.smrv.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]