Abstract
Study Objectives:
Slow wave sleep (SWS) plays a critical role in body restoration and promotes brain plasticity; however, it markedly declines across the lifespan. Despite its importance, effective tools to increase SWS are rare. Here we tested whether a hypnotic suggestion to “sleep deeper” extends the amount of SWS.
Design:
Within-subject, placebo-controlled crossover design.
Setting:
Sleep laboratory at the University of Zurich, Switzerland.
Participants:
Seventy healthy females 23.27 ± 3.17 y.
Intervention:
Participants listened to an auditory text with hypnotic suggestions or a control tape before napping for 90 min while high-density electroencephalography was recorded.
Measurements and Results:
After participants listened to the hypnotic suggestion to “sleep deeper” subsequent SWS was increased by 81% and time spent awake was reduced by 67% (with the amount of SWS or wake in the control condition set to 100%). Other sleep stages remained unaffected. Additionally, slow wave activity was significantly enhanced after hypnotic suggestions. During the hypnotic tape, parietal theta power increases predicted the hypnosis-induced extension of SWS. Additional experiments confirmed that the beneficial effect of hypnotic suggestions on SWS was specific to the hypnotic suggestion and did not occur in low suggestible participants.
Conclusions:
Our results demonstrate the effectiveness of hypnotic suggestions to specifically increase the amount and duration of slow wave sleep (SWS) in a midday nap using objective measures of sleep in young, healthy, suggestible females. Hypnotic suggestions might be a successful tool with a lower risk of adverse side effects than pharmacological treatments to extend SWS also in clinical and elderly populations.
Citation:
Cordi MJ, Schlarb AA, Rasch B. Deepening sleep by hypnotic suggestion. SLEEP 2014;37(6):1143-1152.
Keywords: high-density EEG, hypnosis, sleep, slow wave sleep
INTRODUCTION
Sleep disturbances are highly common and present a major challenge for modern societies. Disturbed and insufficient sleep is strongly associated with several major diseases including hypertension, cardiovascular disease, obesity, depression, anxiety, bipolar disorders, and Alzheimer disease.1–6 In particular, slow wave sleep (SWS) has proven vital for health and well-being, and slow wave activity (SWA) during SWS benefits both the immune system as well as cognitive functions and brain plasticity.7–11 Importantly, both the amount of SWS and SWA are strongly reduced across the lifespan, and the reduction in SWS has been linked to age-related prefrontal brain atrophy and memory impairments.12,13 Furthermore, frequently prescribed sleep-inducing drugs typically hinder the occurrence of SWS, lose their efficacy during long-term treatment, have adverse side effects, and often are associated with a high risk of addiction.14,15 Thus, the development of efficient and risk-free approaches to improve sleep and particularly SWS are highly warranted.
One nonpharmacological approach to improve sleep is hypnosis.16–18 Although there are different definitions of hypnosis, Oakley and Halligan19 define hypnosis as a state of changed mental activity after an induction procedure that mainly encompasses a state of focused attention and absorption. Importantly, during the state of hypnosis, suggestible subjects respond more easily to hypnotic suggestions, which are statements given during induction or afterwards, intended to change or influence behavior. They can include decrease of pain, motor paralysis, or posthypnotic amnesia, and recent cognitive neuroscience research has successfully demonstrated effects of these suggestions on underlying brain activation using objective neuroimaging methods.20–25 In therapeutical contexts, hypnosis has been proven an effective tool in reducing pain, anxiety, and stress-related disorders,26,27 and several studies provide evidence for a beneficial effect of hypnosis on sleep disturbances and insomnias.16–18 However, most of these studies are either case reports or include only subjective measures of sleep quality, whereas well-controlled experimental studies including objective sleep parameters and standard polysomnography are lacking.28 In particular, no study has ever tested whether hypnotic suggestions are effective in increasing objective measures of sleep, like the amount of SWS or SWA. And finally, the possibility to induce SWS by hypnotic suggestions would be highly relevant in clinical terms as well as for healthy aging.
Here we tested whether a hypnotic suggestion to “sleep deeper” increases the amount of SWS and SWA using high-density electroencephalographic (EEG) recordings in a sleep laboratory (experiment 1). We show that the hypnotic suggestion increases the amount of SWS and SWA during a midday nap in healthy, nonhabitual nappers suggestible to hypnosis compared to a nonhypnotic control text. Two additional groups of suggestible females assured that the effects of the hypnotic agent were not purely the result of mere expectancy effects (experiment 2) or demand characteristics of the experiment (experiment 3). Furthermore, we observed no beneficial effects of the hypnotic suggestion on subsequent SWS in two groups of low suggestible participants who either normally listened to the hypnotic suggestion (experiment 4) or tried to simulate the effects of the hypnotic suggestion on subsequent sleep (experiment 5).
METHODS
Participants
A total of 70 healthy, German-speaking young females with a mean age (± standard deviation [SD]) of 23.27 ± 3.17 y (age range 18-35 y) took part in the five experiments. Only females were recruited to avoid gender effects. Suggestibility to hypnosis was verified by the Harvard Group Scale of Hypnotic Susceptibility (HGSHS) prior to the experiment (cutoff score for high suggestibility: HGSHS ≥ 7).29 Fourteen highly suggestible (HGSHS: 7.61 ± 0.2) females (mean age 23.36 ± 2.7 y) participated in the main (first) experiment. In experiments 2 and 3, 14 highly suggestible females (mean age 23.71 ± 3.0 y; HGSHS: 7.73 ± 0.2) and 12 highly suggestible females (mean age 23.92 ± 4.60 y, HGSHS: 7.09 ± 0.08) were included, respectively. In experiments 4 and 5, 15 low suggestible females (mean age 23.47 ± 3.0 y; HGSHS: 5.07 ± 0.3) and 12 low suggestible subjects (mean age 22.25 ± 2.60 y; HGSHS: 5.24 ± 0.25) participated. Three subjects were excluded due to sleep diaries indicating irregular sleep times or regular afternoon naps. Age did not differ between the five experimental groups (P > 0.70). None of the participants had shift work within the prior 6 w, nor a history of neurological or psychiatric disorders. Participants reported normal sleep (Pittsburgh Sleep Quality Index (PSQI) < 630), did not take any sleep influencing medication, did not regularly have an after-lunch nap, and were asked to refrain from caffeine and alcohol during the test day. Participants gave their written consent to take part in the study and were paid 140 Swiss francs for participation. The ethics committee of the University of Zurich approved the study.
Procedure
All participants had an adaptation nap and two experimental nap sessions in the sleep laboratory. The experimental sessions took place on the same day of the week, spaced exactly 7 days apart. One week before each of the experimental sessions, subjects started filling out a sleep diary. Except those from experiment 3, all subjects in all experimental groups were explicitly informed about the study purpose to deepen their sleep with the help of hypnosis. The experimental sessions started at 1:00 a.m. with attachment of 128 EEG electrodes, electromyographic (EMG), and electrocardiographic (ECG) electrodes for recording while listening to the text and subsequent napping. When participants were lying in bed, lights were turned off and the tape recording was started. Participants listened either to the tape including hypnotic suggestions or the control tape played over bedside speakers, in a randomized and balanced order. The duration of the tape recordings was 13 min. Participants were allowed to fall asleep during or directly after the record, and were, in all conditions, awakened after 90 min in bed (see Figure 1 for a summary of the procedure). After awakening, participants filled out a subjective sleep quality questionnaire.31 Before going to bed, participants performed a declarative (word-pairs)32 and a procedural memory task (sequence finger tapping),33 which they recalled after the nap. Parallel versions were used in a randomized order (see supplemental material and Table S1 for details). At the end of the second experimental session, participants filled out a general postexperimental questionnaire.
Figure 1.
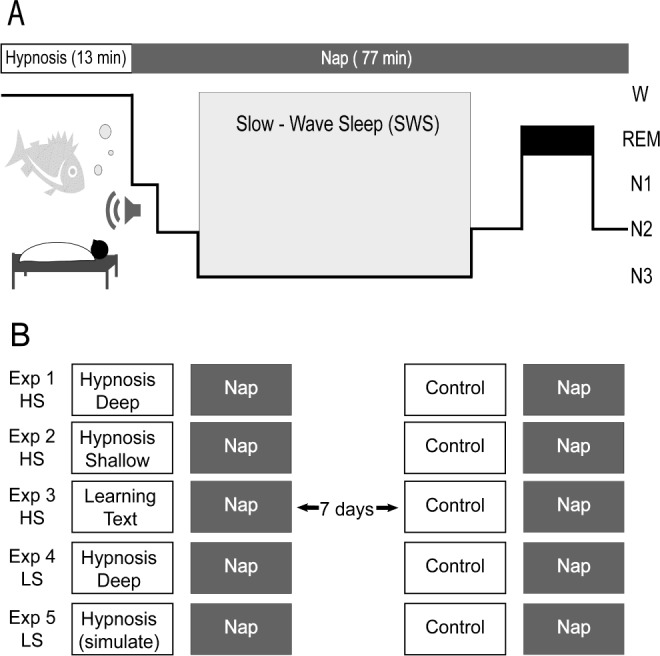
Overview of the experimental procedure. (A) Healthy young females listened either to a tape with hypnotic suggestions or a control tape while lying in bed, and were allowed to fall asleep afterward. The hypnosis tape included a standardized induction procedure, followed by a specifically developed metaphor of a fish swimming deep into the sea, repeatedly containing the suggestion to “sleep deeper” (see supplemental material, for a more detailed description). The control text had the same length and consisted of a documentation of natural mineral deposits. (B) All subjects participated in a hypnosis and a control condition, separated by 1 w. In the main experiment (experiment 1), participants suggestible to hypnosis (HS) listened to the hypnotic suggestion “to sleep deeper” (i.e., the fish). In experiment 2, only the hypnotic suggestion was altered now suggesting “to sleep shallower” (i.e., a boat, resting on the surface). In experiment 3 (demand characteristics), suggestible participants were informed that listening to verbal information before sleep increases subsequent slow wave sleep as the brain tries to consolidate the learned information. An incomprehensible version of the text was used as control condition. In experiment 4, low suggestible participants (LS) listened to the suggestion “to sleep deeper”. In experiment 5, LS participants were asked to simulate the effects of the hypnotic suggestion. In the control condition, all participants listened to the same neutral text (except in experiment 3).
Experimental Design
A total of five separate experiments were conducted. Each experiment contained a within-subject comparison of two experimental naps according to a placebo-controlled crossover design. In the main experiment (experiment 1), participants suggestible to hypnosis (HS) either listened to a tape containing hypnotic suggestions to “sleep deeper” or a control text. The hypnotic tape contained a standard hypnotic induction section followed by a hypnotic suggestion section (i.e., a metaphor of a fish swimming deeper and deeper into the water). The control text contained a neutral documentation on mineral deposits. While the hypnotic text was spoken in a soft, slow, hypnotic, calming voice, frequently containing relaxing words such as “deep” “easily”, “relax”, “let go”, the control text was spoken in a normal voice and normal speed containing neither relaxing nor arousing words (see supplemental material for further details on the texts). In experiment 2, highly suggestible participants also listened to a hypnotic and a control tape, but the hypnotic tape was altered now suggesting to “sleep shallower” (i.e., a metaphor of a boat resting on the surface). Importantly, the hypnotic induction procedure, the voice, the slow-relaxing way of speaking and the inclusion of relaxing words was identical to the hypnotic tape used in experiment 1. In experiment 3 (demand characteristics), no hypnotic induction procedure or hypnotic suggestions were used. Here, suggestible participants were simply informed that listening to verbal information before sleep increases subsequent SWS as the brain tries to consolidate the learned information. The tape on mineral deposits (used as control tapes in all other experiments) was used to “induce” SWS in this experiment, and an incomprehensible version of the text was used as control condition. In experiments 4 and 5, low suggestible participants (LS) listened to identical hypnotic and control tapes used in the main experiment (experiment 1). The procedure and instructions in experiment 4 were identical to experiment 1. In experiment 5, low suggestible participants were asked to simulate the effects of the hypnotic suggestion. All tape recordings used in the different experiments were spoken by the same male voice (see supplemental material for details on the tape recordings).
Statistical Analyses
Sleep was scored by two experts blind to experimental condition and analyzed using a repeated- measures analysis of variance (ANOVA) using the repeated factor “text” (hypnosis versus control) and the between subject factor “experiment” (experiment 1 versus experiment 2, experiment 1 versus experiment 3; experiment 1 versus experiment 4; experiment 4 versus experiment 5, respectively). For identification of sleep stage specificity, the repeated factor “sleep stage” was included. For EEG power analyses, the repeated factor “topography” (frontal, central, parietal) was used. Significant main effects and interactions were further explored using paired sample t-tests. Associations were explored with Pearson correlations. The level of significance was set to P = 0.05. In case variance homogeneity was not fulfilled, values were Greenhouse-Geisser corrected.
RESULTS
Influence of the Hypnotic Suggestion on Subsequent SWS in Suggestible Females (Experiment 1)
As predicted, the hypnotic suggestion to “sleep deeper” strongly increased the amount of SWS during the subsequent nap. After listening to the text with hypnotic suggestions, participants showed an SWS amount of 181.2 ± 28.95% (mean ± standard error of the mean [SEM]), with percentage of SWS after the control text set to 100%. Thus, participants almost doubled their amount of SWS during the nap after the hypnotic suggestion compared to the control condition, indicating a very strong influence of the hypnotic suggestion to sleep deeper on later SWS amounts. This increase in SWS by hypnotic suggestions was statistically significant (t(13) = 2.90, P = 0.013, Cohen's d = 0.77 see Figure 2A). In addition to successfully increasing SWS, the influence of the hypnotic suggestion was highly specific: although the SWS percentage of total sleep time increased from 16.89 ± 4.38% after the control text to 30.60 ± 4.89% after hypnotic suggestions, we observed no changes in the percentages of sleep stages N1, N2, or rapid eye movement (REM) sleep after hypnotic suggestions compared to the control condition (all P > 0.40, see Figure 2B; see Table S2 for descriptive values). Additionally, the percentage of time awake after sleep onset was marginally reduced after hypnosis condition (7.34 ± 3.88%) as compared to the control condition (22.34 ± 7.60%; t(13) = -2.02, P = 0.065, d = 0.54). The sleep stage specificity of the influence of hypnotic suggestions on sleep architecture was confirmed by a significant interaction between the experimental condition (hypnosis versus control text) and sleep stage (W, N1, N2, SWS, REM; F(2.0, 26.06) = 3.73, P = 0.037, η2 = 0.22). Total sleep time did not differ between experimental conditions (74.11 ± 4.89 min versus 75.68 ± 4.86 min, respectively; P > 0.80).
Figure 2.
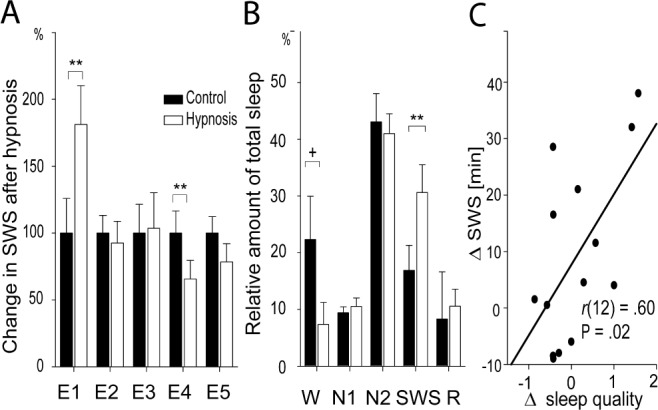
Effects of the hypnotic suggestions on sleep. (A) Highly suggestible subjects in experiment 1 almost doubled their amount of slow wave sleep (SWS) after the hypnotic suggestion “to sleep deeper” (white bar), with the SWS amount after the control tape set to 100% (black bar). Using the suggestion “to sleep shallower” the beneficial effect of hypnotic suggestions on SWS in a group of highly suggestible subjects was completely abolished (experiment 2). Similarly in experiment 3, no increase of SWS was observed after listening to verbal information, even though participants were informed previously that listening to verbal information should increase subsequent SWS (demand characteristics). In low suggestible subjects, the suggestion “to sleep deeper” even decreased the amount of SWS (experiment 4), and the direction of the effect was similar when subjects were asked to simulate the effect of hypnotic suggestion on subsequent sleep (experiment 5). (B) The hypnotic suggestion “to sleep deeper” specifically increased the amount of SWS in experiment 1, whereas time awake after sleep onset (W) was marginally reduced, leaving the other sleep stages unaffected (N1/N2: nonrapid eye movement sleep stage 1 and 2, R,: rapid eye movement sleep). Means ± standard error of the mean are indicated. +: P ≤ 0.08; *: P ≤ 0.05; **: P ≤ 0.01. (C) The hypnosis-induced increases in SWS significantly correlated with subjective increases in sleep quality in experiment 1.
Specificity of the Hypnotic Suggestion: Expectancy and Demand Characteristics (Experiments 2 and 3)
One might argue that the beneficial effect of hypnotic suggestion on SWS is actually due to a placebo effect caused by the expectancy or belief of the participants. Prior to the experiment, all participants were explicitly informed that hypnosis is effective and should result in deeper sleep, which might have deepened subsequent sleep independent of the hypnotic suggestion itself. To exclude this alternative, we recruited a second group of suggestible females, which also received the information that hypnosis will result in deeper sleep prior to the experiment. After the identical hypnotic induction procedure, however, the hypnotic suggestion was altered, now suggesting that the participants should sleep shallower (i.e., metaphor of a boat that rested on the surface of the sea; see supplemental material). The results of experiment 2 clearly indicate that the subjective belief of the participants is not sufficient to induce SWS, and that the type of suggestion during hypnosis is critical: After listening to the hypnotic text suggesting sleeping shallower, participants exhibited a relative decrease in SWS amount (92.45 ± 16.19%) as compared to the control condition (set to 100%). However, this decrease was not statistically significant (P > 0.60, see Figure 2A). Other sleep stages were also not affected (all P > 0.40, see Table S2). A direct comparison between experiments 1 and 2 confirmed that SWS increases were only observed after the suggestion to “sleep deeper” (ANOVA experiment 1 versus 2 * hypnosis versus control text, F(1, 26) = 5.70, P = 0.024, η2 = 0.18, see Table S2).
In addition, we attempted to exclude that the reported benefits of hypnotic suggestion on SWS are solely caused by demand characteristics of the experimental situation. We conducted a third experiment (experiment 3) with suggestible females, informing them that listening to verbal information before sleep increases subsequent SWS as the brain aims at consolidating this information during deep sleep. In a design identical to that of the two previous experiments, participants either listened to verbal information (i.e., the control text used in experiments 1 and 2) and an incomprehensible version of the control text. Incomprehensibility was achieved by low-pass filtering the audio file of the control text, leaving the intonation and length of the text intact but rendering comprehension of the words impossible. All participants were informed that listening to the comprehensible version of the text before sleep should increase subsequent SWS, whereas listening to the incomprehensible version should not. Again, we did not observe any effect of the demand characteristics of the experimental situation on subsequent sleep. Participants in the “SWS-induction” condition exhibited 103.59 ± 26.55% SWS, with the amount of SWS in the incomprehensible text condition set to 100% (P > 0.80). In addition, no other sleep parameters differed between the two conditions (all P > 0.15). Directly comparing induced changes in SWS between experiment 1 and experiment 3 confirmed that an increase in SWS required listening to an audio file with a hypnotic suggestion (ANOVA experiment 1 versus 3 * hypnosis versus control text, F(1, 24) = 4.31, P = 0.049, η2 = 0.15, see Table S2 for descriptive values), safely excluding that the beneficial effects of hypnotic suggestion on SWS are due to demand characteristics of the experimental situation.
No Beneficial Effects of the Hypnotic Suggestion in Low Suggestible Females (Experiments 4 and 5)
Because the first three experiments only included participants with high hypnotic suggestibility, in experiments 4 and 5 we tested whether a hypnotic suggestion to sleep deeper is also effective in participants who exhibit low hypnotic suggestibility. The experimental procedure was identical to experiment 1, including the hypnotic suggestion to “sleep deeper”. In experiment 4, the instructions to listen to the tape with the hypnotic suggestion were identical to those in experiment 1. In experiment 5, low suggestible females were asked to ‘simulate’ the effects of the hypnotic suggestion on subsequent sleep. In contrast to highly suggestible subjects, low suggestible subjects in experiment 4 did not exhibit an increase in SWS during the nap after listening to the hypnotic text. In fact, the amount of SWS decreased to 65.70 ± 13.77% in the hypnosis condition, with the amount of SWS in the control condition set to 100% (t(14) = -3.26, P = 0.006, d = 0.84; see Figure 2A). Again, the effect was specific to SWS, as no other sleep stage was significantly altered in the hypnosis as compared to the control condition (all P > 0.30, see Table S3 for descriptive values). A direct comparison between experiments 1 and 4 confirmed that high suggestibility is substantial for the beneficial effect of hypnotic suggestions on SWS (ANOVA experiment 1 and experiment 4 * hypnosis versus control text, F(1, 27) = 18.02, P ≤ 0.001, η2 = 0.40, see Tables S2 and S3). These results also held when the single participant who did not fall asleep during both sessions was excluded from the analyses.
Also in experiment 5, we observed no beneficial effects of the hypnotic suggestion, even though low suggestible females were asked to ‘simulate’ the effects of the hypnotic suggestions on subsequent sleep. As in experiment 4, the amount of SWS was decreased in the hypnosis condition (78.29 ± 13.73%, with the amount of SWS in the control condition set to 100%), although the difference did not reach significance (P > 0.30). No other sleep parameters significantly differed between the conditions (all P > 0.08), except a reduction in REM sleep after simulation of the effects of the hypnotic suggestion (P = 0.02, see Table S3 for descriptive values). A direct comparison between experiments 1 and 5 with respect to the changes in SWS confirmed that even simulation of high suggestibility is not sufficient to achieve beneficial effects of a hypnotic suggestion on subsequent SWS (ANOVA experiment 1 and experiment 5 * hypnosis versus control text, F(1, 24) = 5.89, P = 0.023, η2 = 0.20, see Tables S2 and S3). Interestingly, combining the results of the effects of the hypnotic suggestion to sleep deeper in low suggestible females (experiments 4 and 5) revealed a significant main effect of the type of text (hypnosis versus control), indicating a significant decrease in SWS after listening to the hypnotic suggestion in experiments 4 and 5 (F(1, 25) = 5.10, P = 0.033, η2 = 0.17), but no interaction (P > 0.70).Thus, subjects with low suggestibility might possibly even actively counteract the beneficial effects of hypnotic suggestions on sleep architecture, whether they are listening normally to the hypnotic suggestion or trying to ‘simulate’ its effects.
Analysis of Control Variables (Experiments 1-5)
To exclude the possibility that the different results of the five experimental groups are in fact caused by differences in falling asleep during the listening period, we compared average sleep latency between groups and conditions. However, sleep latency was on average larger than the duration of the audio tape (13 min) and did neither differ between experiments (P > 0.60) nor between conditions (15.18 ± 4.06 versus 15.29 ± 3.70 min, P > 0.90; 14.93 ± 2.60 versus 20.18 ± 4.12 min, P > 0.20; 19.92 ± 8.56 versus 9.54 ± 2.20 min, P > 0.20; 14.70 ± 6.07 versus 16.37 ± 6.34 min, P > 0.60; 9.88 ± 2.31 versus 7.63 ± 1.85 min, P > 0.30; for experiments 1-5, hypnosis versus control, respectively). While listening to audio tapes, subjects neither differed in min spent in N1 between text conditions (3.18 ± 0.6 versus 2.21 ± 0.5 min, P > 0.20; 2.64 ± 0.67 versus 2.18 ± 0.76 min, P > 0.40; 2.21 ± 0.72 versus 1.96 ± 0.49, P > 0.70; 2.93 ± 0.75 versus 2.6 ± 0.50, P > 0.70; 1.92 ± 0.55 versus 2.00 ± 0.36 min, P > 0.90; for experiments 1-5, hypnosis versus control, respectively) nor was there a difference between experiments 1-5 (P > 0.60). Moreover, most subjects indicated that they had listened to the audio tapes, particularly when the hypnotic suggestions were given (see Table S4).
We also analyzed whether the changes in SWS in the five experimental groups resulted in differences in subjective sleep quality. Despite the robust increases in SWS in experiment 1, these changes were not reflected in averaged subjectively rated sleep quality (3.45 ± 0.18 versus 3.34 ± 0.20; in hypnosis and control condition, respectively, P > 0.50). However, on the individual level, differences in the duration of SWS between the hypnosis and control session reliably predicted differences in subjective sleep quality between the hypnosis and control session (r(12) = 0.60, P = 0.023, see Figure 2C). Participants in experiments 2-5 did not indicate any changes in sleep quality (3.58 ± 0.19 versus 3.68 ± 0.16; 3.37 ± 0.24 versus 3.11 ± 0.22; 3.46 ± 0.26 versus 3.45 ± 0.31; 3.21 ± 0.35 versus. 3.64 ± 0.20, all P > 0.20).
In addition, we analyzed whether the differences in amounts of SWS resulted in differences in memory consolidation across sleep. Although consolidation of declarative memories has been previously suggested to depend on SWS, we did not see any significant changes in consolidation measures across the nap between the hypnosis and the control condition in experiment 1 (102.92 ± 3.34% versus 100.41 ± 1.30% remembered word pairs, with learning performance before sleep set to 100%, P > 0.40). Likewise, consolidation in the procedural finger-tapping task did not differ between the hypnosis and the control condition (118.82 ± 6.14% versus 114.23 ± 4.14% correctly tapped sequences, with learning performance before sleep set to 100%, P > 0.40). Similarly, no condition effects on changes in memory performance were observed for declarative and procedural memory consolidation in experiments 2-5 (all P > 0.30, see Table S5). The order in which the parallel versions were presented had no influence in none of the experiments (all P > 0.20).
Finally, additional analyses confirmed for experimental groups 1, 2, and 4 that hypnotic suggestions did neither influence the proportion of subjects reaching REM sleep (see Table S6), nor the number of nonrapid eye movement (NREM) sleep cycles (see Table S7) nor spindle density or sigma power (see Table S8).
Influence of the Hypnotic Suggestion on SWA During Sleep (Experiments 1, 2, and 4)
To further specify the effect of the hypnotic suggestion on objective sleep parameters, we calculated spectral power values for SWA (0.5-4.5 Hz) during NREM sleep. We focused on experiments 1, 2, and 4 because only in these three experiments we used a real (and not simulated) hypnotic suggestion. After suggesting sleeping deeper in experiment 1, we observed a widespread increase in SWA that was most pronounced in central and parietal regions (Figures 3A and 3B, see Figure 3C for an illustrated power spectrum). Statistical analysis with grouped electrodes in six topographical regions (left/right frontal, central, and parietal, respectively, see supplemental methods and Figure S1) revealed a significant main effect of type of text (hypnosis versus control, F(1, 13) = 5.67, P = 0.03, η2 = 0.30) and a significant interaction between type of text (hypnosis versus control) and topography (frontal, central, parietal) (F(1.14, 14.8) = 5.48, P = 0.03, η2 = 0.30, Greenhouse-Geisser corrected, see Figure 3D for post hoc contrasts). The overall increase in SWA after hypnotic suggestions in all electrodes was strongly correlated with the increase in time spent in SWS (r(14) = 0.88, P < 0.001, see Table S9). In addition to the significant interaction, a main effect of topography (frontal, central parietal, F(1.22, 15.9) = 90.50, P < 0.001, η2 = 0.87, Greenhouse-Geisser corrected) occurred, revealing the well-known SWA distribution of higher SWA in frontal as compared to central and parietal regions in young adults. The same ANOVA for experiment 2 and experiment 4 did not reveal any differences in SWA between the hypnosis and control conditions (all P > 0.12).
Figure 3.
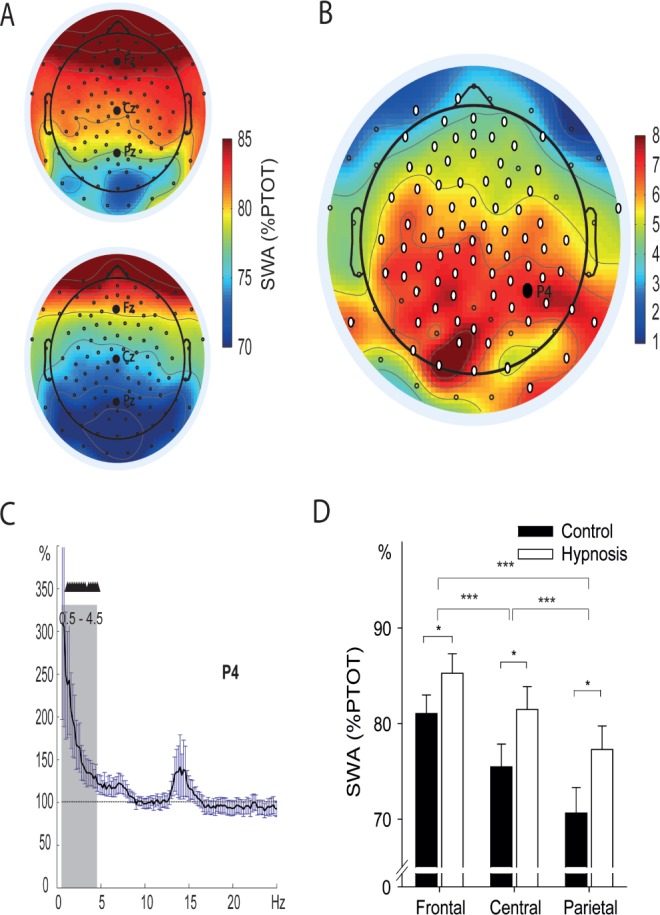
Changes in slow wave activity (SWA) after hypnosis and control conditions in experiment 1. (A) Topographical distribution of SWA (0.5–4.5Hz) during nonrapid eye movement (NREM) sleep after listening to the hypnotic suggestion (upper panel) and the control text (lower panel). SWA is indicated as percent of total power (% PTOT; 0.5–50 Hz). Black dots represent electrode positions of the 128-channel electroencephalography (EEG) cap. (B) Topographical distribution of the difference of SWA %PTOT between hypnosis and control condition. White dots represent significant differences between the conditions, indicating a widespread increase in SWA during NREM sleep after the hypnotic suggestion “to sleep deeper”. (C) Representative spectrogram of electrode P4. Black triangles indicate significant increases (P < 0.05) in SWA after hypnosis as compared to the control condition, which are highly specific for SWA (gray area). Mean increases ± standard error of the mean (SEM) are indicated for the hypnosis condition, with the control condition set to 100% (dotted line). (D) Averaged SWA over three topographical regions (frontal, central, parietal, see supplemental material), for the hypnosis and control conditions. SWA is significantly increased after hypnosis as compared to the control condition (P < 0.03), and the effect is stronger over central and parietal as compared to frontal regions (interaction type of text * topography: P = 0.03). Means ± SEM are indicated. * P ≤ 0.05; *** P ≤ 0.001, for post hoc contrasts.
Influence of the Hypnotic Suggestion on Theta Activity During Listening (Experiments 1, 2, and 4)
In addition to the effects of hypnotic suggestion on SWA during NREM sleep, we tested whether hypnosis induced changes in theta activity (4.5–8 Hz) during listening to the hypnotic suggestion. Again we focused on experiments 1, 2, and 4 because only in these three experiments we used a real (and not simulated) hypnotic suggestion. We specifically focused on theta activity because hypnotic trance states are typically associated with a general slowing of the EEG from alpha to theta frequencies.34,35 In addition, increases in theta activity are related to feelings of drowsiness and falling asleep.36 As expected, suggestible participants in experiment 1 exhibited a significant increase in theta activity during listening to the suggestion part of the hypnosis text, as compared to an identical time period of the control text (13.46 ± 2.16% versus 11.05 ± 1.82%, main effect “text type” F(1, 13) = 8.39, P = 0.013, η2 = 0.39, see Figures 4A-4C). Generally, theta activity was higher in parietal (13.07 ± 2.17%) and central (13.25 ± 2.16%) as compared to frontal recording sites (10.44 ± 1.63%, main effect “topography” F(1.37, 17.84) = 8.94, P = 0.004, η2 = 0.41, Greenhouse-Geisser corrected), whereas no significant interaction between text-type and topography occurred (P > 0.40). No differences in theta activity during listening to the hypnotic suggestion were observed in the other experiments (P > 0.40) (see Tables S2 and S3). Remarkably, the increase in theta activity in parietal regions during listening to the hypnotic suggestions as compared to the control text reliably predicted the increase in subsequent SWS duration (r(12) = 0.60, P = 0.023, See Figure 4D and Table S9) and almost reached significance with respect to the overall increase in SWA (r(12) = 0.51, P = 0.06), suggesting a strong association between the immediate effects of the hypnotic suggestions during listening as indicated by theta activity and its later effects on SWS duration.
Figure 4.
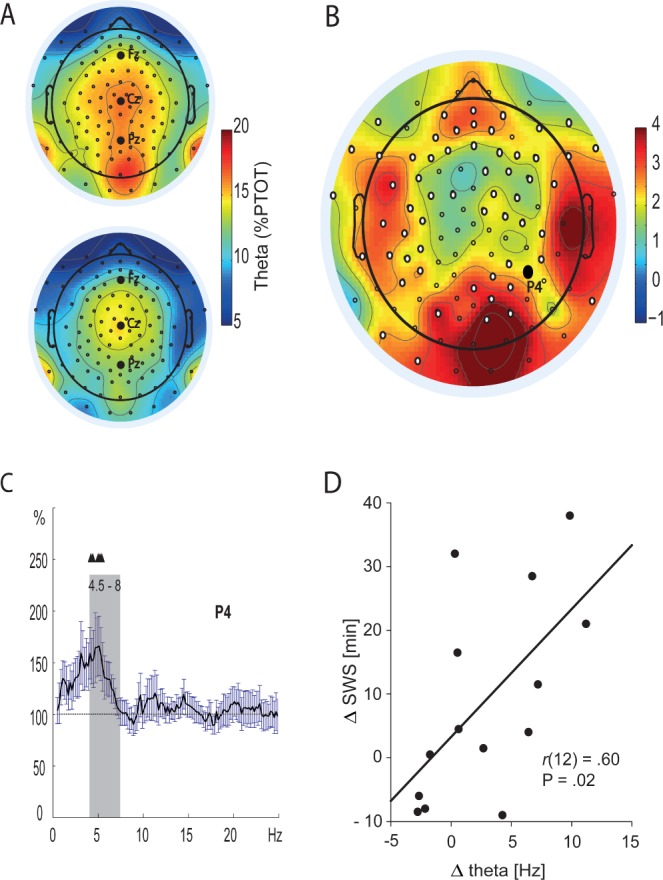
Theta activity during listening to the hypnosis versus the control text before sleep. (A) Topographical distribution of theta activity (4.5–8.0 Hz) during listening to the hypnotic suggestion (upper panel) and the control text (lower panel). Theta power is indicated as percent of total power (% PTOT; 0.5 - 50 Hz). Black dots represent electrode positions of the 128-channel electroencephalography (EEG) cap. (B) Topographical distribution of the difference of theta % PTOT between the hypnosis and the control condition. White dots represent significant differences between the conditions (P < 0.05), indicating a widespread increase in theta power during listening to the hypnotic suggestion “to sleep deeper”. (C) Representative spectrogram of electrode P4. Black triangles indicate significant increases (P < 0.05) in theta power during listening to the hypnosis as compared to the control text, which occur mainly in the lower theta band (gray area). Mean increases ± standard error of the mean are indicated for the hypnosis condition, with the control condition set to 100% (dotted line). (D) Increases in parietal theta activity during listening to the text with hypnotic suggestions as compared to the control text are highly predictive for subsequent changes in slow wave sleep duration between the experimental conditions.
DISCUSSION
Our results show for the first time that a hypnotic suggestion to “sleep deeper” selectively extends the amount of SWS in suggestible females. In addition, control experiments indicate that the suggestion during hypnosis is essential and that the effect does not occur in low suggestible participants. Furthermore, hypnotic suggestions induce an increase in SWA during subsequent NREM sleep and increases in theta activity during listening to audio tapes with hypnotic suggestions predict subsequent increases in SWS.
Our finding that a hypnotic suggestion before sleep increases the amount of SWS is highly relevant because SWS plays a critical role in the optimal functioning of our immune system,8 metabolism,37 and optimal brain functioning,38 in particular with regard to memory consolidation and brain plasticity.9,10 The National Heart, Lung, and Blood Institute has estimated that 50 to 70 million Americans suffer from a chronic disorder of sleep,39 which has severe consequences on daytime functioning40 and is associated with morbidities including hyper-tension, cardiovascular disease, depression, and anxiety.2,5 Importantly, sleep-inducing drugs usually hinder the occurrence of SWS, lose their efficacy during long-term treatment, and have a high risk of addiction and adverse side effects.14,15 Although our study sample was limited to healthy participants, our results strongly suggest that suggestions given during hypnosis might be an efficient tool to improve sleep and SWS, also with respect to sleep disturbances. Increasing SWS by hypnotic suggestion might also have a beneficial effect in primary insomnia, as a recent meta-analysis by Baglioni and colleagues showed that primary insomnia is associated with significant SWS reductions,41 although this was not consistently confirmed for patients with chronic insomnia. Furthermore, the increase in SWS by hypnotic suggestion reported here was even more pronounced than increases in SWS induced by pharmacological treatments as reported previously.32 Previous studies have already provided evidence for a positive effect of hypnosis on different sorts of sleep disturbances,16–18 and concluded that hypnosis is an effective treatment option for insomnias.42 However, most of the studies included only a small sample size43 and used subjective measures of sleep such as questionnaires or sleep diaries.16,18 Here we showed that a hypnotic suggestion is also effective in increasing objective measures of SWS using standard polysomnography and high-density EEG, and that the increase in SWS predicted individual improvements in subjective sleep quality. Comparable to negative influences of emotional or cognitive factors that can influence sleep as stress or rumination,44 hypnosis might represent a positive example thereof, maybe influencing SWS by a calming effect on the arousal system. Although our sample only consisted of females, limiting generalization of the results, the possibility to improve SWS might be also highly relevant for healthy aging, because aging is strongly associated with a reduction in the amount of SWS, and the possibility to use hypnotic suggestions to increase SWS could prove critical for maintaining optimal cognitive functioning and health in old age.12,45,46 Although, for instance, Ehrenreich47 reported a slight decline in hypnotizability score as a function of age, there is evidence that the elderly are as suggestible as young adults and robust test-retest correlations exist.48 A limitation of our study is that we did not include an intervention-free control group or a control group not performing memory tests before sleep. Thus, we cannot completely rule out the possibility that listening to the control text or working on memory tests might also have affected the amount of SWS. However, amounts of SWS in other intervention-free nap studies using a comparable sample and design are close to the amount we observed in the control condition (i.e., 18.09% in the study by Mednick et al.49) and hypnosis condition (i.e., 33.1% in the study by Hofer-Tinguely et al.50), which suggests that the amount of SWS in our study varied within the normal range. Please note that we only included nonhabitual nappers who might not sleep as deeply as subjects used to having midday naps.
Related to the increase in SWS, we also showed that the hypnotic suggestion induced a widespread increase in SWA during NREM sleep. SWA is a more precise quantitative measure of sleep depth, reflecting the reduction of sleep pressure across sleep and has been implicated in brain plasticity, synaptic downscaling, immune function, and memory consolidation.9,51–53 Local differences in SWA during sleep have been related to plastic changes in these brain regions.51,52,54 In particular, changes in SWA are associated with changes in prefrontal atrophy and memory consolidation in the elderly.12 In our study, increases in SWA were particularly strong in parietal brain areas, although significant increases were also observed over frontal and central brain regions. Note that we included the average of all NREM sleep episodes and did not analyze changes in SWA across NREM episodes or changes in slow oscillations slope. This limits the possibility to exclude that the SWS increase had resulted from mechanisms different from natural ones. Please note that our sleep scorers who were blind to the experimental condition did not report anything unusual with respect to SWS. Because we did not observe any improvement in memory functions in experiment 1, the benefits for cognitive functioning of the hypnosis -induced increase in SWS, however, remain to be determined, particularly considering that other studies observed an increase in memory performance in similar tasks after enhancing SWA by oscillatory stimulation.55 Interestingly, however, Mednick et al.49 recently reported that pharmacologically enhancing SWS only benefit declarative memory consolidation when sleep spindle density was also increased. Here, we did not observe a concomitant increase in sleep spindles number and density or power in the sigma band, which might explain the lack of an effect on declarative memory consolidation.
In addition to the effects on SWS and SWA, we observed increases in theta activity during listening to the hypnotic audio tapes, which were predictive for the subsequent beneficial effect of hypnotic suggestions on the duration of SWS and SWA. Previous EEG studies on hypnosis have frequently reported a general slowing of the EEG and an increase in theta activity during brain states of hypnotic trance.35,56 In addition, alpha activity (indicative for quiet resting with eyes closed) characteristically decreases at the onset of light sleep stages and feelings of drowsiness.36 However, no differences in sleep latency occurred between our experimental conditions and we did not observe any differences in sleep stage N1 during listening, indicating that participants did not fall asleep earlier while listening to the hypnotic suggestions as compared to the control text. Thus, the increase in theta activity might be indeed indicative for processes related to the hypnosis, particularly because it predicted the later effects of the hypnotic suggestion, i.e., the increase in SWS and SWA. Please note that we did not include any questionnaire or behavioral test to ensure that subjects were in the hypnotic state during or after listening to the hypnotic text.
In recent years, interest in the mechanisms and effects of hypnosis as well as hypnotic suggestion is growing in neuroscience research.57,58 According to Oakley and Halligan,19 these studies “illustrate the potential of hypnotic suggestion as a powerful cognitive tool to explore in a controlled way selective phenomena directly relevant to cognitive and clinical neuroscience.” Several recent studies have examined the underlying brain effects of hypnosis and hypnotic suggestion with respect to motor inhibition, hypnotic analgesia, the default mode network and posthypnotic amnesia20–22,59,60. In general, studies on the effects of hypnosis during wakefulness have the disadvantage that they need to safely exclude that participants do not just “simulate” and conform to the demands of the experiment or the experimenter (i.e., demand characteristics). In our study, the argument is not valid, as the effects of the hypnotic suggestion are observed after the hypnosis during sleep without waking consciousness using objective EEG parameters. Thus, effects of hypnotic suggestions on sleep might be an elegant way to examine aftereffects of posthypnotic suggestions.
Generally, it is well known that our thoughts and subjective beliefs can influence sleep. Stress and particularly rumination of negative thoughts diminish sleep quality and sleep efficiency.61,62 In addition, the anticipation of a certain wake up time has consequences on sleep length that can be objectively measured, e.g., by earlier increase in cortisol during sleep.63 Thus, changing inappropriate beliefs concerning sleep is one major target in clinical approaches to treat sleep disturbances.64 However, intentionally “wanting” to fall asleep is often counterproductive; therefore, paradoxical interventions are sometimes more helpful to induce sleep.65,66 Thus, inducing sleep or extending SWS under hypnosis might bypass the explicit and voluntary intention, inducing subsequent sleep effects on a more subconscious level, not involving willing decision processes. In particular, the effects of the hypnotic suggestion were highly specific in our study: The suggestion to “sleep deeper” specifically extended duration of SWS, leaving other sleep stages unaffected. It remains to be tested whether different hypnotic suggestions may be capable of changing other sleep stages such as REM sleep or N2 sleep, which we expect to be possible in case an adequate metaphor can be developed.
The specificity of the hypnotic suggestion was further confirmed by showing that the beneficial effects of the hypnotic tape disappeared with a changed hypnotic suggestion. Although the suggestion to sleep deeper effectively increased the duration of SWS, the suggestion to sleep “shallower” had no effect on SWS. As the participants in experiment 2 were also told prior to the experiment that the hypnotic suggestions should result in deeper sleep, the absence of the effects in this condition clearly shows that the previous belief of the participants is not sufficient to elicit SWS changes and that the type of the hypnotic suggestion during the hypnosis is critical for the beneficial effect. Despite this apparently controversial input, a postexperimental questionnaire indicated that subjects did not assume that the hypothesis was different from what we told them and that they were not irritated by the content of the tape. It seems that subjects do not question the content of the hypnotic suggestion so critically. One might argue that the suggestion to sleep “shallower” should have induced an increase in light sleep stages in the control experiment. However, although the type of suggestion is critical, it might still be possible that the participants' belief plays some role in the effectiveness of hypnotic suggestions and that the belief to “sleep deeper” might have weakened the effectiveness of the hypnotic suggestion to sleep “shallower”. Importantly, the null result in experiment 2 also excludes that the effects of hypnotic suggestions on deep sleep are simply caused by unspecific differences between the hypnotic tape and the control tape. For example, the hypnotic tape was spoken in a slow, relaxing, and calming voice, whereas the control tape was spoken in a neutral voice at normal speed and contained neither relaxing nor arousing words. Because these unspecific differences in the “relaxing nature” between the tapes are identical in experiments 1 and 2, they cannot explain why a beneficial effect on SWS occurred solely in experiment 1. In an additional control group, we tested the pure effect of expectancy and examined whether a neutral (not hypnotic) text will also increase deep sleep. SWS was not affected by this belief alone without being given hypnotic suggestions. Alternatively, it might simply be less effective to induce lighter sleep as compared to deeper sleep using a hypnotic suggestion.
Finally, our experiments 4 and 5 indicate that the effectiveness of the hypnotic suggestion depends on the suggestibility of the participants. People strongly vary in their responsiveness to hypnotic suggestions, and the degree to which suggestibility remains stable over time might be partly the result of genetic differences.48,67,68 Moreover, high suggestibility is associated with openness to experience23 and focused attentional abilities,69,70 which might be related to the fact that suggestibility is highly predictive for the success or failure of effects of hypnotic suggestions,71 and the inclusion of high versus low suggestible participants is common practice in experimental studies on hypnosis.32,48,67,68 Interestingly, in our study, not only did low suggestible participants fail to increase SWS after the hypnotic suggestion, but in fact spent less time in SWS after the text including hypnotic suggestions, both when they normally listened to the hypnotic suggestion as well as when they were asked to simulate the effects of the hypnotic suggestion. This negative subject effect has already been described in previous studies, indicating that low suggestible subjects tend to counteract the implications of the suggestion instead of only failing to responding to them.72 A possible explanation is the definition of the context within which the hypnosis is presented. Changing the setting from a hypnosis session into a test of imagination increases the score in a consecutive hypnotizability test for low suggestible subjects.73 Thus, for low suggestible subjects it might be advantageous to emphasize a relaxing instead of a hypnotic state for the extension of SWS to avoid negative effects. Of note, the inverse effect in low suggestible participants renders it very unlikely that simply the relaxing nature of the hypnotic tape (i.e., voice, intonation, words, etc.) as compared to the control text was responsible for the observed changes in SWS in experiment 1.
Limitations
Our study included a highly selective sample of healthy, young females, which limits the generalizability of our results. Furthermore, our study was designed as a nap study, and further studies should test the effectiveness of hypnotic suggestions on SWS during nighttime sleep. Moreover, future studies should include an intervention-free control condition without listening to any text before sleep and control groups without presleep memory tests. Additionally, further studies should characterize the hypnosis -induced increases in SWA in more detail (e.g., slope analyses, changes in SWA across NREM episodes, etc.). Finally, further simulator studies are required to answer the important question whether the reported effects of hypnotic suggestions on deep sleep are specific to the state of hypnosis per se, or whether they might similarly occur with more unspecific procedures involving prior expectations (i.e., demand characteristics), suggestions without hypnosis, and relaxation.
In summary, we show that a hypnotic suggestion to sleep deeper is effective in extending subsequent SWS and SWA in healthy participants. Our results imply that hypnotic suggestions are an efficient tool to deepen sleep and strongly indicate that hypnotic suggestions might prove an efficient non-pharmacological tool with a lower risk of adverse side effects than pharmacological treatments to also deepen sleep in patients with sleep disturbances or in the elderly, thereby improving health and well-being.
DISCLOSURE STATEMENT
This was not an industry supported study. Financial support was from a Grant of the Swiss National Foundation (SNF) (PP00P1_133685) and the Clinical Research Priority Program (CRPP) “Sleep and Health” of the University of Zurich. The work was performed at the University of Zurich, Institute of Psychology, Department of Biopsychology, Zurich, Switzerland. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank Sandra Perner, Isabelle Braham, Sarah Schoch, Benjamin Roth, Mirjam Stieger, Melissa Maeder, Andrea Schmidt for assistance in data collection and Sandra Ackermann for helpful comments.
ABBREVIATIONS
- ECG
electrocardiogram
- EEG
electroencephalography
- EMG
electromyogram
- REM
rapid eye movement
- SWA
slow wave activity
- SWS
slow wave sleep
SUPPLEMENTAL MATERIAL
SUPPLEMENTAL METHODS
1. Hypnosis and Control Texts
The hypnosis texts were written by A. Schlarb, a coauthor and professional hypnotherapist treating sleep problems and sleep disorders with hypnosis. All texts were spoken and recorded by B. Rasch. The hypnosis texts started with a 4-min progressive induction technique, including 10 steps while each step indicated a further step into relaxation, leading the listener into the state of hypnotic trance. In the main hypnotic text used in experiment 1, the induction was followed by a hypnotic suggestion to sleep deeper. More specifically, the auditors were invited to imagine a picture of a sea and to follow a fish swimming in the water, progressively swimming deeper and deeper. The picture of the swimming fish and the sea was used as a metaphor to symbolize the depth of sleep. In addition, it was suggested that swimming deeper and deeper is safe and without any risk. Finally, the fish arrived at the bottom of the sea, whereby the auditors were further induced to sleep deeply. The tape with hypnotic suggestions stopped here and did not bring the listener out of the hypnotic trance. Instead, subjects were invited to fall asleep at any time afterward. In total, duration of the tape was 13 min in which 932 words were spoken with a soft, slow, hypnotic, calming voice, frequently containing relaxing words such as “sleep deep” “easily”, “relax”, “let go”.
The control text was a documentation concerning natural mineral deposits taken from Wikipedia (http://de.wikipedia.org/wiki/Lagerstättenkunde) and was also spoken and recorded by B. Rasch. The control text was matched to the hypnotic texts with respect to length in minutes and volume. In this text, 1,712 words were spoken with an everyday intonation and speed. The text was not designed to contain relaxing or arousing words in particular, but be as neutral and objective as possible.
In the second hypnotic text used in experiment 2, the identical induction of hypnotic trance from experiment 1 spoken by B. Rasch was followed by a hypnotic suggestion to sleep shallower. More specifically, the auditor was invited to imagine a picture of a sea and to be on a ship swimming on the surface of the sea. The picture of the ship on the surface was used as a metaphor to symbolize light and shallow sleep, and it was suggested that it is safer to rest on the surface and not to go deep under water. The recording sounded almost identical to the recording used in experiment 1, containing 967 words that were spoken with a soft, slow, hypnotic, and calming voice, frequently containing words such as “sleep shallow” “easily”, “relax”, “let go”. As in experiment 1, the tape with hypnotic suggestions ended without bringing the listener out of the hypnotic trance.
The audio files with hypnotic suggestions (deep and shallow) as well as the control text are accessible on our homepage: http://www.psychologie.uzh.ch/fachrichtungen/allgpsy/biopsy/links.html
2. Assessment of Suggestibility
Prior to participation in the experiment, suggestibility was assessed for all candidates using the Harvard Group Scale of Hypnotic Susceptibility Test, Form A (HGSHS: A;1 German translation,2), which represents a widely used standard measure of hypnotic suggestibility on a scale from 0 to 12. Of the initially screened 112 females, 68 turned out to be low suggestible according to the classification proposed by Bongartz.2 In the literature, suggestibility is reported to be normally distributed into high (49%) and low (51%) suggestible subjects2,3 and quite robust test-retest correlations are reported.4 In experiments 1 and 2, only females with a suggestibility index from 7 to 12 were included (highly suggestible subjects), whereas females with a suggestibility of 0 to 6 were included in experiment 3 (low suggestible subjects). Mean suggestibility was significantly lower in experiment 3 F(1, 41) = 83.58, P < 0.001.
3. Behavioral Tasks
In the Psychomotor Vigilance Test (PVT), a millisecond counter was displayed at random intervals and subjects had to press the space bar on the keyboard as quickly as possible after it began to count upward. The achieved reaction time in ms was displayed thereafter for 1 sec. The test is highly sensitive to measure the effects of tiredness on vigilance.5
The Word Pair Associate Learning Task (PAL) consisted of 80 pairs of semantically related words that were taken from Rasch et al.6 Two randomized and parallel lists were constructed according to concreteness, imagery, meaningfulness, valence, and arousal ratings as well as association strength of the words (see Table S1 for the word lists). Word pairs (e.g., clock-church) were presented in black font on a white background with EPrime on a computer screen. After a fixation cross, present for 500 ms, word pairs were presented sequentially for 1 sec per word, separated by a blank interval of 200 ms. A blank interval of 500 ms preceded the next fixation cross. The order of the word pairs was at random. Subjects were asked to learn the association between the two words for later cued recall, meaning recall of the second word, when only the first one was presented. The order of words during recall did not correspond to the one during learning. Response time was not restricted and no feedback was given. Retrieval was tested immediately and after the nap while word pairs were presented in the same order during both recall phases. Memory performance was measured using the number of correctly recalled words at retrieval after napping relative to the correctly recalled words after the learning phase. As a consequence, values can exceed 100%.
In the Procedural Finger Sequence Tapping Task,7 the term “memory consolidation” refers to a process whereby a memory becomes increasingly resistant to interference from competing or disrupting factors with the continued passage of time. Recent findings regarding the learning of skilled sensory and motor tasks (“procedural learning”) subjects were asked to replicate a five-element finger sequence with their nondominant hand on a keyboard as fast and as accurately as possible. Learning period contained nine 30-sec trials interrupted by 30-sec breaks during which the sequence did not change and was displayed during the whole trial. The recall period after the nap contained only three trials. Each subject randomly conducted each of two created number sequences (4-1-3-2-4 versus 4-2-3-1-4) in either of the experimental sessions. Feedback about the number of completed sequences and the error rate was given after each block. The average score of correct sequences during the final three blocks was taken as measure of procedural memory performance before sleep while recall performance after sleep was measured by the average score of correctly completed sequences of the entire three blocks that were presented. “Overnight” changes in speed (number of entered sequences) and percentage of error rate (amount of errors per correct sequence), were calculated in percent, with performance before sleep set to 100%.
Parallel versions of the paired associate task involved words, balanced according to concreteness, imagery, arousal, meaningfulness, association strength, frequency in use, and word length6
4. Polysomnographic Recordings
Sleep was recorded with electromyographic (EMG), electrocardiographic (ECG), and 128 electroencephalographic (EEG) electrodes (Electrical Geodesics, Inc.) referenced against the Cz channel using a sampling rate of 500 Hz. Data were preprocessed with VisionAnalyzer 2.0 (Brain Products, Germany), filtered using a notch filter (50 Hz) and standard filter settings suggested by the American Academy of Sleep Medicine (AASM) (e.g., EEG 0.3–35 Hz8) and referenced against the mastoids. Sleep was visually scored based on derivations F4, C4, O4, HEOG, VEOG, and EMG using 30-sec periods according to standard criteria of the AASM8 by two sleep experts blind to condition. In case of disagreement, a third expert was consulted who was also blind to condition. Stages 1-3, rapid eye movement (REM) sleep, and wake after sleep onset (WASO) were scored.
Sleep parameters for the first three experiments including highly suggestible subjects
Sleep parameters for the last two experiments including low suggestible subjects.
5. Analysis of EEG Data
For a more fine-grained exploratory analysis of the effects of hypnotic suggestions on oscillatory brain activity, high-density EEG recordings were subjected to power spectral analysis. For the analysis of direct effects of the hypnosis or control text, data from the 13 min during tape listening were segmented into segments of 4,096 data points (≈ 4 sec) with an overlap of 409 between segments. For the analysis of the effect of hypnotic suggestions on sleep, only nonrapid eye movement (NREM) sleep segments of N2 and N3 were selected and similarly segmented in periods of 4,096 data points with an overlap of 409 between segments. Participants lacking NREM sleep were excluded from the analysis. In both analyses, movement artefacts were controlled by automatically removing segments during which EMG activity was above ± 200 μV. A Hanning window (10%) was applied on each 4,096-point block of EEG data before calculating the power spectra using fast Fourier transform with a resolution of 0.2 Hz. Individual mean power was determined in the slow wave band (0.5–4.5 Hz) averaged across all NREM sleep episodes and in the theta band (4.5–8.0Hz) during listening to the auditory tape. Data were normalized on the average total power between 0.5 and 50 Hz. SWA was defined as power density between 0.5–4.5 Hz during NREM sleep, theta activity as power in the 4.5–8 Hz range during listening to the texts.
Overview of how many subjects reported to have listened or not focused on the text or tried to ignore the voice
Performance on memory tasks
For statistical analysis, six topographical regions were defined: right frontal (electrodes 1-5, 8-10, 14, 116-118, 124, 121-123), left frontal (electrodes 12, 17, 19-26, 28, 32-34, 38), right central (electrodes 79, 80, 87, 93, 102-106, 108-112, 114, 115), left central (electrodes 7, 13, 29-31, 35-37, 39-42, 44-46, 54), right parietal (electrodes 76-78, 82-86, 89-92, 95-98, 100, 101), and left parietal (electrodes 47, 50-53, 57-61, 64-67, 69-71, 74) (see Figure S1). We used the within-subjects factors “topography” (frontal, central, parietal), “laterality” (left, right), and “text” (hypnosis versus control) separately in each experiment for the repeated-measures analysis of variance on slow wave activity during NREM sleep and on theta activity during listening to the audio tape. In addition, we show results for a representative single electrode (electrode P4) for an analysis of the specificity of the effects on the power spectrum.
Overview how many subjects reached rapid eye movement sleep separately for condition, including the results of the McNemar test for paired proportions
Number of nonrapid eye movement cycles until the first occurrence of rapid eye movement sleep and total amount of nonrapid eye movement cycles in the 90 min nap (± standard error of the mean).
Data of fast spindle density in Pz, slow spindle density in Fz and electroencephalographic sigma power (averaged across all electrodes) during nonrapid eye movement
Correlations between hypnosis-induced increases in minutes spent in slow wave sleep and changes in slow wave activity during nonrapid eye movement sleep and changes in theta activity during listening in experiment 1
Topographical regions used for the statistical analysis of the EEG data. We defined six topographical regions (as indicated by gray areas): frontal left (FL), frontal right (FR), central left (CL), central right (CR), parietal left (PL), parietal right (PR).
ABBREVIATIONS
- PVT
psychomotor vigilance test
- PAL
word pair associate learning task
- N1 and N2
stage 1 and 2 sleep
- SWS
slow-wave sleep
- REM
rapid eye movement sleep
- TST
total sleep time
- SWS latency
slow wave sleep latency
REFERENCE
- 1.Shor RE, Orne EC. Norms on the Harvard group scale of hypnotic susceptibility, form A. Int J Clin Exp Hypn. 1963;11:39–47. doi: 10.1080/00207146308409226. [DOI] [PubMed] [Google Scholar]
- 2.Bongartz W. German norms for the Harvard Group Scale of Hypnotic Susceptibility, Form A. Int J Clin Exp Hypn. 1985;33:131–9. doi: 10.1080/00207148508406643. [DOI] [PubMed] [Google Scholar]
- 3.Oakley DA, Halligan PW. Hypnotic suggestion: opportunities for cognitive neuroscience. Nat Rev Neurosci. 2013;14:565–76. doi: 10.1038/nrn3538. [DOI] [PubMed] [Google Scholar]
- 4.Piccione C, Hilgard ER, Zimbardo PG. On the degree of stability of measured hypnotizability over a 25-year period. J Pers Soc Psychol. 1989;56:289–95. doi: 10.1037//0022-3514.56.2.289. [DOI] [PubMed] [Google Scholar]
- 5.Dinges DF, Powell JW. Microcomputer analyses of performance on a portable, simple visual RT task during sustained operations. Behav Res methods, instruments, Comput. 1985;17:652–55. [Google Scholar]
- 6.Rasch B, Born J, Gais S. Combined blockade of cholinergic receptors shifts the brain from stimulus encoding to memory consolidation. J Cogn Neurosci. 2006;18:793–802. doi: 10.1162/jocn.2006.18.5.793. [DOI] [PubMed] [Google Scholar]
- 7.Walker MP, Brakefield T, Hobson JA, Stickgold R. Dissociable stages of human memory consolidation and reconsolidation. Nature. 2003;425:616–20. doi: 10.1038/nature01930. [DOI] [PubMed] [Google Scholar]
- 8.Iber C, Ancoli-Israel S, Chessonn A, Quan SF. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. [Google Scholar]
REFERENCE
- 1.Ohayon MM, Vecchierini M-F. Normative sleep data, cognitive function and daily living activities in older adults in the community. Sleep. 2005;28:981–9. [PubMed] [Google Scholar]
- 2.Gangwisch JE, Feskanich D, Malaspina D, Shen S, Forman JP. Sleep duration and risk for hypertension in women: results from the nurses' health study. Am J Hypertens. 2013;26:903–11. doi: 10.1093/ajh/hpt044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ju Y-ES. Sleep quality and preclinical alzheimer disease. JAMA Neurol. 2013;70:587–93. doi: 10.1001/jamaneurol.2013.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vorona RD, Winn MP, Babineau TW, Eng BP, Feldman HR, Ware JC. Overweight and obese patients in a primary care population report less sleep than patients with a normal body mass index. Arch Intern Med. 2005;165:25–30. doi: 10.1001/archinte.165.1.25. [DOI] [PubMed] [Google Scholar]
- 5.Newman AB, Enright PL, Manolio TA, Haponik EF, Wahl PW. Sleep disturbance, psychosocial correlates, and cardiovascular disease in 5201 older adults: the Cardiovascular Health Study. J Am Geriatr Soc. 1997;45:1–7. doi: 10.1111/j.1532-5415.1997.tb00970.x. [DOI] [PubMed] [Google Scholar]
- 6.Aydin A, Selvi Y, Besiroglu L, et al. Mood and metabolic consequences of sleep deprivation as a potential endophenotype' in bipolar disorder. J Affect Disord. 2013;150:284–94. doi: 10.1016/j.jad.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 7.Anderson C, Horne JA. Prefrontal cortex: links between low frequency delta EEG in sleep and neuropsychological performance in healthy, older people. Psychophysiology. 2003;40:349–57. doi: 10.1111/1469-8986.00038. [DOI] [PubMed] [Google Scholar]
- 8.Lange T, Dimitrov S, Born J. Effects of sleep and circadian rhythm on the human immune system. Ann N Y Acad Sci. 2010;1193:48–59. doi: 10.1111/j.1749-6632.2009.05300.x. [DOI] [PubMed] [Google Scholar]
- 9.Tononi G, Cirelli C. Sleep function and synaptic homeostasis. Sleep Med Rev. 2006;10:49–62. doi: 10.1016/j.smrv.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 10.Rasch B, Born J. About sleep's role in memory. Physiol Rev. 2013;93:681–766. doi: 10.1152/physrev.00032.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bryant PA, Trinder J, Curtis N. Sick and tired: Does sleep have a vital role in the immune system? Nat Rev Immunol. 2004;4:457–67. doi: 10.1038/nri1369. [DOI] [PubMed] [Google Scholar]
- 12.Mander BA, Rao V, Lu B, et al. Prefrontal atrophy, disrupted NREM slow waves and impaired hippocampal-dependent memory in aging. Nat Neurosci. 2013;16:357–64. doi: 10.1038/nn.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Backhaus J, Born J, Hoeckesfeld R, Fokuhl S, Hohagen F, Junghanns K. Midlife decline in declarative memory consolidation is correlated with a decline in slow wave sleep. Learn Mem. 2007;14:336–41. doi: 10.1101/lm.470507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Riemann D, Perlis ML. The treatments of chronic insomnia: a review of benzodiazepine receptor agonists and psychological and behavioral therapies. Sleep Med Rev. 2009;13:205–14. doi: 10.1016/j.smrv.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 15.Hajak G, Rüther E. Therapie von Ein- und Durchschlafstörungen. In: Möller A, editor. Therapie psychischer Erkrankungen. Stuttgart: Thieme; 2006. pp. 1015–55. [Google Scholar]
- 16.Schlarb AA. Verhaltenstherapie und Hypnotherapie bei primärer Insomnie. 2005 [Google Scholar]
- 17.Borkovec TD, Fowles Don C. Controlled investigation of the effects of progressive and hypnotic relaxation on insomnia. J Abnorm Psychol. 1972;82:153–8. doi: 10.1037/h0034970. [DOI] [PubMed] [Google Scholar]
- 18.Stanton HE. Hypnotic relaxation and the reduction of sleep onset insomnia. Int J Psychosom. 1989;36:64–8. [PubMed] [Google Scholar]
- 19.Oakley DA, Halligan PW. Hypnotic suggestion and cognitive neuroscience. Trends Cogn Sci. 2009;13:264–70. doi: 10.1016/j.tics.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 20.Mendelsohn A, Chalamish Y, Solomonovich A, Dudai Y. Mesmerizing memories: brain substrates of episodic memory suppression in posthypnotic amnesia. Neuron. 2008;57:159–70. doi: 10.1016/j.neuron.2007.11.022. [DOI] [PubMed] [Google Scholar]
- 21.Cojan Y, Archimi A, Cheseaux N, Waber L, Vuilleumier P. Time-course of motor inhibition during hypnotic paralysis: EEG topographical and source analysis. Cortex. 2013;49:423–36. doi: 10.1016/j.cortex.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 22.Cojan Y, Waber L, Schwartz S, Rossier L, Forster A, Vuilleumier P. The brain under self-control: modulation of inhibitory and monitoring cortical networks during hypnotic paralysis. Neuron. 2009;62:862–75. doi: 10.1016/j.neuron.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 23.Kihlstrom JF. Neuro-hypnotism: prospects for hypnosis and neuroscience. Cortex. 2013;49:365–74. doi: 10.1016/j.cortex.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Posner MI, Rothbart MK. Brain states and hypnosis research. Conscious Cogn. 2011;20:325–7. doi: 10.1016/j.concog.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bell V, Oakley DA, Halligan PW, Deeley Q. Dissociation in hysteria and hypnosis: evidence from cognitive neuroscience. J Neurol Neurosurg Psychiatry. 2011;82:332–9. doi: 10.1136/jnnp.2009.199158. [DOI] [PubMed] [Google Scholar]
- 26.Bongartz W, Flammer E, Schwonke R. Die Effektivität der Hypnose. Psychotherapeut. 2002:67–76. [Google Scholar]
- 27.Flammer E, Bongartz W. On the efficacy of hypnosis: a meta-analytic study. Contemp Hypn. 2003;20:179–97. [Google Scholar]
- 28.Schlarb AA, Gulewitsch MD. Wenn der Sandmann kommt. Wirkt Hypnotherapie bei Kindern mit Schlafstörungen? Hypnose. 2011;6:189–98. [Google Scholar]
- 29.Bongartz W. German norms for the Harvard Group Scale of Hypnotic Susceptibility, Form A. Int J Clin Exp Hypn. 1985;33:131–9. doi: 10.1080/00207148508406643. [DOI] [PubMed] [Google Scholar]
- 30.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 31.Görtelmeyer R. Göttingen: Hogrefe; 2011. SF-A/R und SF-B/R - Schlaffragebogen A und B -Revidierte Fassung. [Google Scholar]
- 32.Rasch B, Born J, Gais S. Combined blockade of cholinergic receptors shifts the brain from stimulus encoding to memory consolidation. J Cogn Neurosci. 2006;18:793–802. doi: 10.1162/jocn.2006.18.5.793. [DOI] [PubMed] [Google Scholar]
- 33.Walker MP, Brakefield T, Hobson JA, Stickgold R. Dissociable stages of human memory consolidation and reconsolidation. Nature. 2003;425:616–20. doi: 10.1038/nature01930. [DOI] [PubMed] [Google Scholar]
- 34.Crawford HJ, Gruzelier JH. A midstream view of the neuropsychophysiology of hypnosis. Recent research and future directions. In: Fromm E, Nash MR, editors. Contemporary hypnosis research. New York: Guilford Press; 1992. pp. 227–66. [Google Scholar]
- 35.Sabourin ME, Cutcomb SD, Crawford HJ, Pribram K. EEG correlates of hypnotic susceptibility and hypnotic trance: spectral analysis and coherence. Int J Psychophysiol. 1990;10:125–42. doi: 10.1016/0167-8760(90)90027-b. [DOI] [PubMed] [Google Scholar]
- 36.Schacter DL. EEG theta waves and psychological phenomena: A review and analysis. Biol Psychol. 1977;5:47–82. doi: 10.1016/0301-0511(77)90028-x. [DOI] [PubMed] [Google Scholar]
- 37.Benedict C, Hallschmid M, Lassen A, et al. Acute sleep deprivation reduces energy expenditure in healthy men. Am J Clin Nutr. 2011;93:1229–36. doi: 10.3945/ajcn.110.006460. [DOI] [PubMed] [Google Scholar]
- 38.Hobson JA. Sleep is of the brain, by the brain and for the brain. Nature. 2005;437:1254–6. doi: 10.1038/nature04283. [DOI] [PubMed] [Google Scholar]
- 39.National Heart, Lung and BI. Bethesda, MD: National Institutes of Health; 2003. National sleep disorders research plan. [Google Scholar]
- 40.Goldman SE, Stone KL, Ancoli-Israel S, et al. Poor sleep is associated with poorer physical performance and greater functional limitations in older women. Sleep. 2007;30:1317–24. doi: 10.1093/sleep/30.10.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baglioni C, Regen W, Teghen A, et al. Sleep changes in the disorder of insomnia: A meta-analysis of polysomnographic studies. Sleep Med Rev. 2013:1–19. doi: 10.1016/j.smrv.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 42.Hauri PJ, Silber MH, Boeve BF. The treatment of parasomnias with hypnosis: a 5-year follow-up study. J Clin Sleep Med. 2007;3:369–73. [PMC free article] [PubMed] [Google Scholar]
- 43.Ng B-Y, Lee TS. Hypnotherapy for sleep disorders. Ann Acad Med Singapore. 2008;37:683–8. [PubMed] [Google Scholar]
- 44.Van Reeth O, Weibel L, Spiegel K, Leproult R, Dugovic C, Maccari S. Physiology of sleep (review)—Interactions between stress and sleep: from basic research to clinical situations. Sleep Med Rev. 2000;4:201–19. [Google Scholar]
- 45.Wilckens KA, Erickson KI, Wheeler ME. Age-related decline in controlled retrieval: the role of the PFC and sleep. Neural Plast. 2012;2012:15. doi: 10.1155/2012/624795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Harand C, Bertran F, Doidy F, et al. How aging affects sleep-dependent memory consolidation? Front Neurol. 2012;3:8. doi: 10.3389/fneur.2012.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ehrenreich GA. The relationship of certain descriptive factors to hypnotizability. Trans Kans Acad Sci. 1949;52:24–7. [PubMed] [Google Scholar]
- 48.Piccione C, Hilgard ER, Zimbardo PG. On the degree of stability of measured hypnotizability over a 25-year period. J Pers Soc Psychol. 1989;56:289–95. doi: 10.1037//0022-3514.56.2.289. [DOI] [PubMed] [Google Scholar]
- 49.Mednick SC, Cai DJ, Kanady J, Drummond SP a. Comparing the benefits of caffeine, naps and placebo on verbal, motor and perceptual memory. Behav Brain Res. 2008;193:79–86. doi: 10.1016/j.bbr.2008.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hofer-Tinguely G, Achermann P, Landolt H-P, et al. Sleep inertia: performance changes after sleep, rest and active waking. Cogn Brain Res. 2005;22:323–31. doi: 10.1016/j.cogbrainres.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 51.Huber R, Ghilardi MF, Massimini M, et al. Arm immobilization causes cortical plastic changes and locally decreases sleep slow wave activity. Nat Neurosci. 2006;9:1169–76. doi: 10.1038/nn1758. [DOI] [PubMed] [Google Scholar]
- 52.Huber R, Ghilardi MF, Massimini M, Tononi G. Local sleep and learning. Nature. 2004;430:78–81. doi: 10.1038/nature02663. [DOI] [PubMed] [Google Scholar]
- 53.Besedovsky L, Lange T, Born J. Sleep and immune function. Pflugers Arch. 2012;463:121–37. doi: 10.1007/s00424-011-1044-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huber R, Määttä S, Esser SK, et al. Measures of cortical plasticity after transcranial paired associative stimulation predict changes in electroencephalogram slow-wave activity during subsequent sleep. J Neurosci. 2008;28:7911–8. doi: 10.1523/JNEUROSCI.1636-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marshall L, Helgadóttir H, Mölle M, Born J. Boosting slow oscillations during sleep potentiates memory. Nature. 2006;444:610–3. doi: 10.1038/nature05278. [DOI] [PubMed] [Google Scholar]
- 56.Deivanayagi S, Manivannan M, Fernandez P. Spectral analysis of EEG signals during hypnosis. Int J Syst Cybern Informatics. 2007:75–80. [Google Scholar]
- 57.Oakley DA, Halligan PW. Using hypnosis to gain insights into healthy and pathological cognitive functioning. Conscious Cogn. 2011;20:328–31. doi: 10.1016/j.concog.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 58.Halligan PW, Oakley DA. Hypnosis and cognitive neuroscience: Bridging the gap. Cortex. 2013;49:359–64. doi: 10.1016/j.cortex.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 59.Deeley Q, Oakley DA, Toone B, et al. Modulating the default mode network using hypnosis. Int J Clin Exp Hypn. 2012;60:206–28. doi: 10.1080/00207144.2012.648070. [DOI] [PubMed] [Google Scholar]
- 60.Crawford HJ, Knebel T, Vendemia JMC. The nature of hypnotic analgesia: neurophysiological foundation and evidence. Contemp Hypn. 1998;15:22–33. [Google Scholar]
- 61.Vandekerckhove M, Weiss R, Schotte C, et al. The role of presleep negative emotion in sleep physiology. Psychophysiology. 2011;48:1738–44. doi: 10.1111/j.1469-8986.2011.01281.x. [DOI] [PubMed] [Google Scholar]
- 62.Vandekerckhove M, Cluydts R. The emotional brain and sleep: an intimate relationship. Sleep Med Rev. 2010;14:219–26. doi: 10.1016/j.smrv.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 63.Born J, Hansen K, Marshall L, Mölle M, Fehm HL. Timing the end of nocturnal sleep. Nature. 1999;397:29–30. doi: 10.1038/16166. [DOI] [PubMed] [Google Scholar]
- 64.Morin CM, Blais F, Savard J. Are changes in beliefs and attitudes about sleep related to sleep improvements in the treatment of insomnia? Behav Res Ther. 2002;40:741–52. doi: 10.1016/s0005-7967(01)00055-9. [DOI] [PubMed] [Google Scholar]
- 65.Ascher LM, Turner R. Paradoxical intention and insomnia: an experimental investigation. Behav Res Ther. 1979;17:408–11. doi: 10.1016/0005-7967(79)90015-9. [DOI] [PubMed] [Google Scholar]
- 66.Ascher LM, Efran JS. Use of paradoxical intention in a behavioral program for sleep onset insomnia. J Consult Clin Psychol. 1978;46:547–50. doi: 10.1037//0022-006x.46.3.547. [DOI] [PubMed] [Google Scholar]
- 67.Kirsch I, Lynn SJ. The altered state of hypnosis: changes in the theoretical landscape. Am Psychol. 1995;50:846–58. [Google Scholar]
- 68.Szekely A, Kovacs-Nagy R, Bányai EI, et al. Association between hypnotizability and the catechol-O-methyltransferase (COMT) polymorphism. Int J Clin Exp Hypn. 2010;58:301–15. doi: 10.1080/00207141003760827. [DOI] [PubMed] [Google Scholar]
- 69.Lichtenberg P, Bachner-Melman R, Ebstein RP, Crawford HJ. Hypnotic susceptibility: multidimensional relationships with Cloninger's Tridimensional Personality Questionnaire, COMT polymorphisms, absorption, and attentional characteristics. Int J Clin Exp Hypn. 2004;52:47–72. doi: 10.1076/iceh.52.1.47.23922. [DOI] [PubMed] [Google Scholar]
- 70.Hoeft F, Gabrieli JDE, Whitfield-Gabrieli S, et al. Functional brain basis of hypnotizability. Arch Gen Psychiatry. 2012;69:1064–72. doi: 10.1001/archgenpsychiatry.2011.2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ray WJ, Keil A, Mikuteit A, Bongartz W, Elbert T. High resolution EEG indicators of pain responses in relation to hypnotic susceptibility and suggestion. Biol Psychol. 2002;60:17–36. doi: 10.1016/s0301-0511(02)00029-7. [DOI] [PubMed] [Google Scholar]
- 72.Jones B, Spanos NP. Suggestions for altered auditory sensitivity, the negative subject effect and hypnotic susceptibility: a signal detection analysis. J Pers Soc Psychol. 1982;43:637–47. doi: 10.1037//0022-3514.43.3.637. [DOI] [PubMed] [Google Scholar]
- 73.Spanos NP, Gabora NJ, Jarrett LE, Gwynn MI. Contextual determinants of hypnotizability and of relationships between hypnotizability scales. J Pers Soc Psychol. 1989;57:271–8. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Parallel versions of the paired associate task involved words, balanced according to concreteness, imagery, arousal, meaningfulness, association strength, frequency in use, and word length6
Sleep parameters for the first three experiments including highly suggestible subjects
Sleep parameters for the last two experiments including low suggestible subjects.
Overview of how many subjects reported to have listened or not focused on the text or tried to ignore the voice
Performance on memory tasks
Overview how many subjects reached rapid eye movement sleep separately for condition, including the results of the McNemar test for paired proportions
Number of nonrapid eye movement cycles until the first occurrence of rapid eye movement sleep and total amount of nonrapid eye movement cycles in the 90 min nap (± standard error of the mean).
Data of fast spindle density in Pz, slow spindle density in Fz and electroencephalographic sigma power (averaged across all electrodes) during nonrapid eye movement
Correlations between hypnosis-induced increases in minutes spent in slow wave sleep and changes in slow wave activity during nonrapid eye movement sleep and changes in theta activity during listening in experiment 1
Topographical regions used for the statistical analysis of the EEG data. We defined six topographical regions (as indicated by gray areas): frontal left (FL), frontal right (FR), central left (CL), central right (CR), parietal left (PL), parietal right (PR).


