Abstract
In a previous study, the Drosophila melanogaster OR67dGAL4;UAS system was used to functionally characterize the receptor for the major component of the sex pheromone in the tobacco budworm, Heliothis virescens Fabricius (Lepidoptera: Noctuidae), HvOR13. Electrophysiological and behavioral assays showed that transgenic flies expressing HvOR13 responded to (Z)-11-hexadecenal (Z11-16:Ald). However, tests were not performed to determine whether these flies would also respond to secondary components of the H. virescens sex pheromone. Thus, in this study the response spectrum of HvOR13 expressed in this system was examined by performing single cell recordings from odor receptor neuron in trichoid T1 sensilla on antennae of two Or67dGAL4 [1]; UAS-HvOR13 lines stimulated with Z11-16:Ald and six H. virescens secondary pheromone components. Fly courtship assays were also performed to examine the behavioral response of the Or67dGAL4[1]; UAS-HvOR13 flies to Z11-16:Ald and the secondary component Z9-14:Ald. Our combined electrophysiological and behavioral studies indicated high specificity and sensitivity of HvOR13 to Z11-16:Ald. Interestingly, a mutation leading to truncation in the HvOR13 C-terminal region affected but did not abolish pheromone receptor response to Z11-16:Ald. The findings are assessed in relationship to other HvOR13 heterologous expression studies, and the role of the C-terminal domain in receptor function is discussed. A third line expressing HvOR15 was also tested but did not respond to any of the seven pheromone components.
Keywords: Drosophila melanogaster, HvORI3, T1 sensilla
Introduction
Studies on pheromone processing by male moths have greatly contributed to the understanding of the mechanisms involved in animal sensory perception (Rützler and Zwiebel 2005; Touhara and Vosshall 2009; Kaupp 2010). Work in this area has been focused mainly on two moth species, Bombyx mori and Heliothis virescens Fabricius (Lepidoptera: Noctuidae), with the former having a simple two-component pheromone blend (Kaissling and Kasang 1978) and the latter a more complex pheromone blend (Vetter and Baker 1983). Molecular aspects of male pheromone reception in these two moths have been examined using different approaches (Sakurai et al. 2004, 2011; Nakagawa et al. 2005; Grosse-Wilde et al. 2007; Kurtovic et al. 2007) for which differences in the level of pheromone receptor response specificity and sensitivity have been observed.
The GAL4/UAS targeted gene expression system in Drosophila melanogaster (Brand and Perrimon 1993) has been used to functionally characterize odorant receptors (ORs) in insects (Dobritsa et al. 2003; Hallem et al. 2004a; Jones et al. 2005; Carey et al. 2010). The “empty neuron” in the ab3 basiconic sensilla (▵halo;OR22a-Gal4/UAS) has been a useful tool for characterization of general odorant receptors as well as sex pheromone receptors (Hallem et al. 2004b; Hallem and Carlson 2006; Syed et al. 2006), while the knock-in mutant in trichoid T1 sensilla (OR67dGAL4;UAS) has been used to functionally characterize the D. melanogaster receptor for the pheromone cVA (Z11-18:OAc) (Ha and Smith 2006) and two moth sex pheromone receptors (Kurtovic et al. 2007). Recently, Syed et al. (2010) expressed a pheromone receptor from the silkworm moth, B. mori, BmORl, in both of these expression systems (i.e., basiconic and trichoid) and found a more sensitive and specific receptor response in fly trichoid sensilla Tl than in the ab3 basiconic sensilla. The latter system not only needed higher doses of bombykol to stimulate the receptor, but was also unusually sustained, suggesting that this system is less suitable for testing pheromone receptors.
In male moths, neurons expressing pheromone receptors are housed in trichoid sensilla dedicated to pheromone reception (Christensen and Hildebrand 2002). Therefore, the higher sensitivity and specificity of the D. melanogaster trichoid Tl sensilla system expressing BmORl may be due to the innate biochemical machinery and structural features of sensilla for detection of the sex pheromones, in which case a similarly sensitive and specific response is expected for other moth sex pheromone receptors. For example, the H. virescens major pheromone component receptor, HvOR13, expressed in D. melanogaster trichoid Tl sensilla was found to respond to its putative ligand (Z)-11-hexadecenal (Z11-16:Ald) (Kurtovic et al. 2007). However, it is unclear whether this receptor, expressed in D. melanogaster, has high specificity for Z11-16:Ald or could also respond to some or all of the secondary components of the H. virescens sex pheromone. Functional analyses of other moth pheromone receptors by Wanner et al. (2010) and Miura et al. (2010) both found that some pheromone receptors of Ostrinia nubilalis were broadly tuned, while one receptor appeared to be highly specific for one pheromone component. Our study was therefore designed to determine the degree of specificity of HvOR13 expressed in this system by performing single cell recordings from odor receptor neurons in trichoid Tl sensilla on antennae of two Or67dGAL4 [1]; UAS-HvORl3 lines and a control line (Or67d+[1]) stimulated with Z11-16:Ald and six H. virescens secondary pheromone components. The electrophysiological response of another construct, Or67dGAL4 [1];UAS-HvOR15, to the seven H. virescens pheromone components was also examined. HvOR15 was considered a candidate receptor for Z9-14:Ald (Baker 2009; Krieger et al. 2009, Gould et al. 2010); however, it has been shown that it does not respond to this pheromone component in a Xenopus laevis oocyte system (Wang et al. 2011), a finding that we expect to corroborate in the D. melanogaster trichoid Tl sensilla system. In addition, fly courtship assays were performed to examine the behavioral response of the Or67dGALA [1];UAS-HvORl3 flies to Z11-16:Ald and Z9-14:Ald. Expression levels of HvOR13 in both Or67dGAL4 [1]; UAS-HvOR13 lines were measured by qRT-PCR to determine if HvOR13 expression was associated to differential electrophysiological and behavioral responses between the two Or67dGAL4 [1]; UAS-HvORl3 lines. The combined electrophysiological and behavioral studies indicated that HvOR13 showed high specificity and sensitivity for Z11-16:Ald, with results comparable to those observed for HvOR13 heterologously expressed in X. laevis oocytes (Wang et al. 2011), a useful finding considering that expression of other insect odorant receptors in these two systems do not always produce similar results (Carey et al. 2010; Wang et al. 2010). Interestingly, a truncation of the HvOR13 C-terminal region appeared to affect, but did not completely abolish, pheromone receptor function, a finding that could be linked to the functional importance of the C-terminal domain in the formation of the odorant receptor/Orco heteromeric complex (Benton et al. 2006; de Bruyne et al. 2009; Vosshall and Hansson 2011).
Materials and Methods
Drosophila melanogaster stocks
Drosophila melanogaster stocks Or67d+[1] Or67dGAL4 [1], and Or67dGAL4[1];UAS-HvOR13 (Kurtovic et al. 2007) were provided by A. Widmer, Research Institute of Molecular Pathology (Vienna, Austria). UAS-HvOR13* and UAS-HvOR15 were prepared by subcloning an H. virescens OR13* cDNA and an H. virescens OR15 cDNA, respectively, into pUAS (donated by J. Mahaffey, North Carolina State University, Department of Biological Sciences, Raleigh, NC) using Novagen pSTBlue-1 Acceptor Vector Kit (EMD Millipore, www.emdmillipore.com). The HvOR13* cDNA contained two amino acid polymorphisms and an early termination codon (Figure 1). Transgenic fly lines containing UAS-HvOR13* and UAS-HvOR15 were generated by Rainbow Transgenic Flies, Inc. (www.rainbowgene.com). To express the HvOR13* and HvOR15 in the trichoid Tl sensilla, six UAS-HvOR13* lines were crossed to Or67dGAL4 [1] and four UAS-HvOR15 lines were crossed to Or67dGAL4 [1].
Figure 1.
Alignment of HvOR13 translated sequences from Heliothis virescens and Drosophila melanogaster Or67dGAL4[1];UAS-HvOR13 lines. Conserved and major amino acid changes in Or67dGAL4[1];UAS-HvOR13* are boxed in black. Motifs A, B and C in the C-terminal region of HvOR13 are highlighted in yellow. High quality figures are available online.
Single sensillum recordings
Recordings were performed as described by Syed et al. (2006). In brief, a D. melanogaster adult was restrained, a glass reference electrode was placed in the eye, and the recording electrode was brought into contact with the base of the trichoid sensillum. Recorded extracellular action potentials (spontaneous and upon stimulation) were amplified, fed into an IDAC4-USB box (Syntech, www.syntech.nl), recorded, and analyzed with Auto Spike version 3.7 (Syntech). AC signals (action potentials or spikes) were band-pass filtered between 100 and 10,000 Hz. The preparation was held in a humidified air stream delivered at 20 mL/sec, to which a stimulus pulse of 2 mL/sec was added for 500 msec. Unless specified otherwise, signals were recorded for 10 sec starting 2 sec before stimulation, and spikes were counted off-line in a 500 msec period before and during the stimulation. Responses from individual neurons were calculated as the increase in spike frequency (spikes/sec) relative to the pre-stimulus frequency. At least three flies of each of the four genotypes (Or67d+[1], Or67dGAL4 [1]; UAS-HvOR13, Or67dGAL4 [1];UAS-HvORl3*, and Or67dGAL4 [1]\UAS-HvORl5) were examined, and recordings were made from up to five sensilla from each fly tested. Data were pooled after observing no significant differences between sensilla, sexes, or age groups (1- to 5-day-old flies) were observed.
The following compounds were used as stimuli: (Z)-ll-hexadecenal (Z11-16:Ald), (Z)-9-tetradecenal (Z9-14:Ald), (Z)-9-hexadecenal (Z9-16:Ald), hexadecanal (16:Ald), (Z)-11-hexadecenyl acetate (Z11-16:OAc), (Z)-11-hexadecen-1-ol (Z11-16:OH), and (Z)-9-hexadecen-l-ol (Z9-16:OH) (all 93–95% minimum purity) and cVA ( ⩾98% purity) . All compounds were purchased from Bedoukian (www.bedoukian.com) except for cVA, which was purchased from Cayman Chemical (www.caymanchem.com). A 10 μL aliquot of a stimulus dissolved in double distilled hexane at the desired dose was loaded on a filter paper strip, the solvent evaporated for 30 sec, and the strip was placed in a disposable Pasteur pipette. Hexane loaded on a filter paper strip alone and an empty Pasteur pipette served as controls. All pheromone components were tested at 10 μg source dose for initial screening and subsequent recordings were performed with varying doses ranging from 10 ng to 10 μg. Source doses indicated throughout the manuscript for electrophysiology data represent the dilutions of the solution deposited onto the filter paper of the stimulus cartridge. Thus, a source dose of 10 μg means a 10 μL solution of 10 μg/μL was deposited.
Courtship assays
Single pair courtship assays were performed following Kurtovic et al. (2007). In brief, Or67d+[1] virgin female flies (5–8 days old) were treated by applying 0.2 μL of Z11-16:Ald (4 μg), Z9-14:Ald (2 μg), or acetone (solvent control) onto their dorsal abdomens while lightly anaesthetized with CO2. Treated females were placed individually in a round chamber (10 mm diameter and 4 mm height) with moistened nitrocellulose paper and paired with single Or67d+[1], Or67dGAL4 [1];UAS-HvORl3, or Or67dGAL4 [1];UAS-HvOR13* male flies (5*8 days old). A clear acetate sheet prevented contact between female and male flies. Flies were allowed to recover for 1 hr before behavioral assays were performed. Courtship index, the percentage of time for which the male courts the female during a 10-min assay, was used to quantify male courtship behavior. In these assays, Or67d+[1] male flies were expected to be avid courters in greater than 70% of all treated female flies. In the courtship ritual, the male orients toward and follows the female, taps her with his forelegs, sings a courtship song by extending and vibrating one wing, licks her genitalia, and finally curls his abdomen for copulation (Demir and Dickson 2005). In contrast, Or67dGAL4 [1]; UAS-HvOR13 and Or67dGAL4 [1];UAS-HvORl3* male flies were expected to display a reduced courtship index when paired with females treated with Z11-16:Ald, as this compound would inhibit male courtship behavior in these transgenic flies, which have HvOR13 replacing Or67d, the receptor for cVA, a male sex pheromone that inhibits male courtship.
HvOR13 expression levels by qRT-PCR
HvOR13 mRNA expression levels were measured in heads of Or67dGAL4 [1];UAS-HvOR13 and Or67dGAL4 [1];UAS-HvORl3* male flies by qRT-PCR. Total RNA from each sample (pool of five adult heads) was isolated and purified using the RNeasy Plus Mini Kit (Qiagen, www.qiagen.com). RNA quality and quantity were determined using a NanoDrop ND-1000 Spectrophotometer (NanoDrop Technologies, www.nanodrop.com). Total RNA (150 ng) was converted to cDNA using Ambion ArrayScript reverse transcriptase (Ambion, Invitrogen, www.invitrogen.com). Random hexamers (Applied Biosystems, Invitrogen) were used as cDNA synthesis primers in a reaction mix that included 10X Array Script buffer (Ambion), RNAseOUT (Invitrogen), and 10 mM dNTP (Invitrogen). Primers targeting exons of HvOR13 and the housekeeping gene RP49 were designed with PRIMER EXPRESS 2.0 software (Applied Biosystems) set to select for an optimal primer annealing temperature of 59° C (58–60° C), amplicon sizes of 40–150 bp, a -3′GC clamp = 0, and a minimum and maximum GC content of 30% and 80%, respectively. Primers were designed based on H. virescens OR13 and B. mori RP49 mRNA sequences obtained from GenBank database. Quantitative RT-PCR was performed with an ABI Prism 7900 sequence detector and 96-well optical reaction plates (Applied Biosystems). All reactions were performed in triplicate in a total volume of 10 μL containing 5 μL of SYBR Green PCR Master Mix (Applied Biosystems) and 10 μM of each primer under the following conditions: 50° C for 2 min, 95° C for 10 min followed by 40 cycles of denaturation at 95° C for 15 sec, annealing and extension at 60° C for 1 min, followed by 95° C for 15 sec and 60° C for 15 sec. A dissociation curve and negative control (cDNA reaction without reverse transcriptase enzyme) were used to ensure primer specificity and lack of contamination. Six samples per genotype were examined, and the same samples were run on separate plates twice (two runs). A standard curve was generated for each primer set using dilutions of genomic DNA to calculate the relative quantities of mRNA levels. For each sample, the ratio of the expression level of the target gene to that of the control gene (RP49) was calculated (ABI User Bulletin 2, Applied Biosystems) and used for data analysis.
HvOR13 cDNA sequence comparison between Or67dGAL4[1];UAS-HvOR13 lines
HvOR13 was amplified by RT-PCR from H. virescens male antennae and from heads of Or67dGAL4 [1]; UAS-HvOR13 and Or67dGAL4 [1];UAS-HvORl3* flies using gene specific primers designed based on published H. virescens cDNA sequences (Krieger et al. 2004). H. virescens male antennal RNA, and RNA from heads of Or67dGAL4 [1];UAS-HvOR13 and Or67dGAL4 [1];UAS-HvOR13* lines, were isolated using Qiagen RNeasy Plus. cDNA was synthesized using Qiagen QuantiTect Reverse Transcription kit with a total RNA concentration of 0.5 μg. PCR was performed using the FastStart High-fidelity PCR system (Roche, www.roche.com) under the following conditions: 94° C for 3 min followed by 19 cycles of denaturation at 94° C for 1 min, annealing at 57° C for 1 min with 0.5° C decreasing per cycle, extension at 72° C for 2 min followed by 19 cycles of denaturation at 94° C for 1 min, annealing at 47° C for 1 min and extension at 72° C for 2 min, followed by 72° C for 7 min. PCR products were purified and sequenced by Genewiz (www.genewiz.com). Nucleotide and translated sequences were aligned using ClustalW2 (EMBL-EBI, www.ebi.ac.uk).
Results
Electrophysiological assays
These experiments were designed to examine whether HvOR13 expressed in D. melanogaster Tl sensilla have high specificity and sensitivity for Z11-16:Ald, as compared to Wang et al. (2011). Or67dGAL4 [1];UAS-HvOR13 T1 sensilla responded to varying doses (0.01–10 μg) of Z11-16:Ald in a dosedependent manner (Figure 2A, B) while T1 sensilla of Or67dGAL4 [1];UAS-HvOR13* responded only to the highest dose of Z11-16:Ald tested (10 μg) (Figure 2B). The control Or67d+[1] did not respond to any of the H. virescens pheromone components, except for Z11-16:OAc at the highest dose (10 μg) tested (87 ± 15.87 spikes/sec, n = 7), and responded to doses of cVA between 0.1–10 μg (Figure 3). In addition, Or67dGAL4 [1];UAS-HvORl3 Tl sensilla responded only to high doses (10 μg) of Z9-14:Ald and Z9-16:Ald, and no response to 16:Ald, Z11-16:OAc, Z11-16:OH, or Z9-16:OH was observed at the same high dose (Figure 4). Or67dGAL4[1];UAS-HvOR13* Tl sensilla did not respond to any of the other pheromone compounds tested. Or67dGAL4 [1];UAS-HvOR15 T1 sensilla did not respond to any of the pheromone compounds tested.
Figure 2.
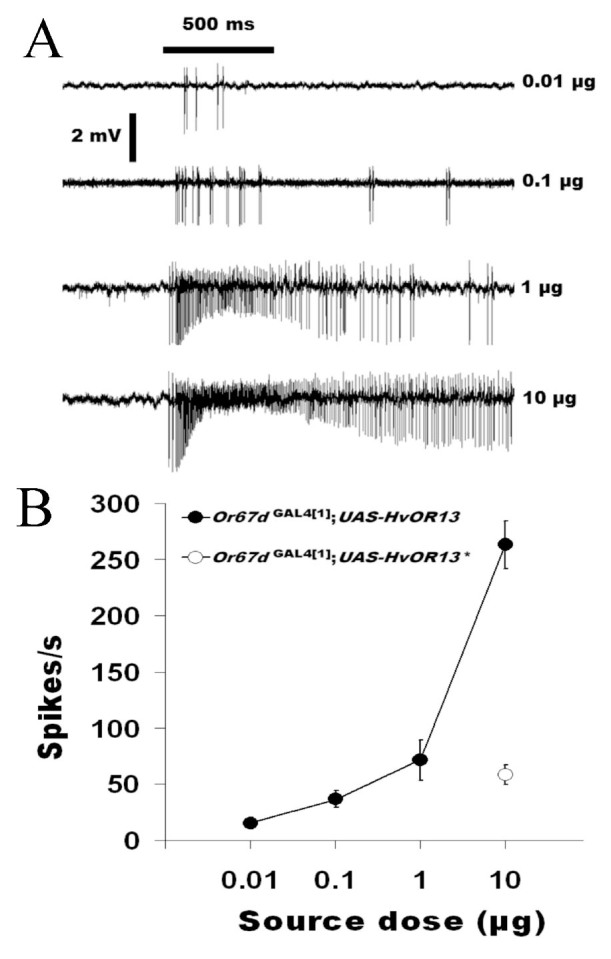
A major component of Heliothis virescens pheromone Z11-16:Ald induces dose dependent excitatory responses from an olfactory receptor neuron expressing the H. virescens moth pheromone receptor HvORl3 in a trichoid sensillum of Drosophila melanogaster Or67dGAL4[1];UAS-HvOR13 but not from one expressing HvORl3* in a trichoid sensillum of D. melanogaster Or67dGAL4[1];UAS-HvOR13*. A. Traces of the excitatory responses recorded from Or67dGAL4[1];UAS-HvOR13 Tl sensilla to increasing pheromone dose. B. Dose-response curve for Or67dGAL4[1];UAS-HvOR13 (n = 11) and response to 10 μg for Or67dGAL4[1];UAS-HvOR13* (n = 9). No differences between males and female responses were recorded. High quality figures are available online.
Figure 3.
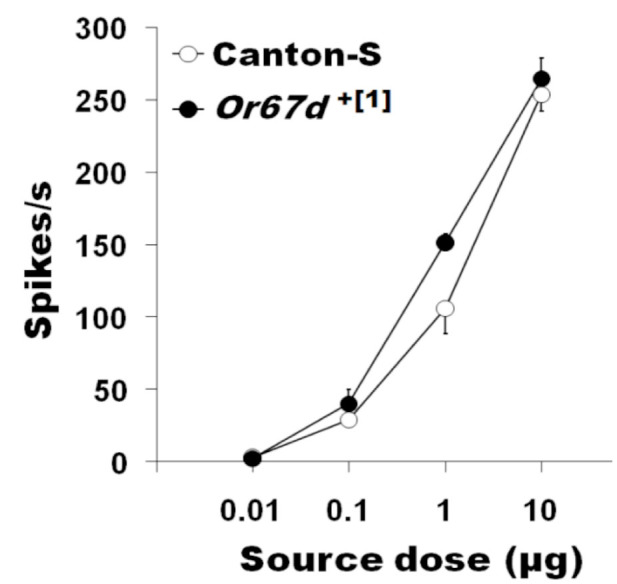
Drosophila melanogaster trichoid sensilla respond with high sensitivity to the cVA sex pheromone in Canton S and the Or67d+[1] control line. High quality figures are available online.
Figure 4.
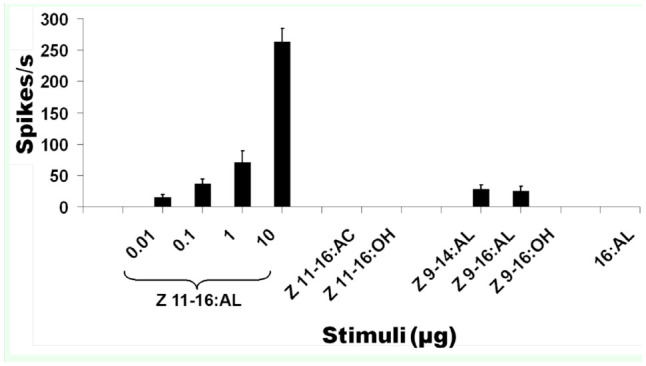
Response spectrum of the pheromone receptor HvORl 3 expressed in an olfactory receptor neuron housed in a trichoid sensillum of Drosophila melanogaster Or67dGAL4 [1];UAS-HvOR13. All compounds (Z11-l6:Ald, Z9-l4:Ald, Z9-16:Ald, 16:Ald, Z11-16:OAc, Z11-16:OH, and Z9-16:OH) were tested at 10 μg source dose, except Z11-16:Ald, which was tested at 0.01, 0.1, 1, and 10 μg source doses (n = 11).High quality figures are available online.
Behavioral assays
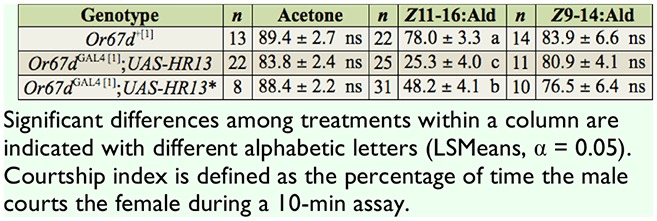
In single pair courtship assays, the genotypes Or67d+[1]; Or67dGAL4 [1]; UAS-HvOR13, and Or67dGAL4 [1];UAS-HvORl3* had comparable responses when paired with Or67d+[1] virgin female flies treated with acetone (F2,40 = 1.60, p = 0.2152) (Table 1). Comparable responses were also recorded when females were treated with Z9-14:Ald (F2,32 = 0.58, p = 0.5634), a H. virescens pheromone component for which no electrophysiological responses were observed in the transgenic fly strains. In contrast, Or67dGAL4 [1]; UAS-HvOR13 and Or67dGAL4 [1];UAS-HvORl3* males exhibited a lower courtship index than that of the Or67d+[1] males when paired with female flies treated with Z11-16:Ald (F2,44.7 = 63.67, p < 0.0001) (Table 1). The lower courtship index indicates that male flies expressing HvOR13 detected Z11-16:Ald and responded accordingly.
Table 1.
Courtship indices (mean ± SE) for males of the indicated genotypes paired with Or67d+[1]; virgin female Drosophila melanogaster treated with acetone, Z11-16:Ald, or Z9-14:Ald.
HvOR13 expression and cDNA sequence comparison
HvOR13 mRNA expression levels in Or67dGAL4 [1]; UAS-HvOR13 (HvOR13/RP49 = 0.038 ± 0.005) and Or67dGAL4 [1];UAS-HvOR13* (HvOR13/RP49 = 0.043 ± 0.005) flies were examined, but no differences in transcript abundance were found between these lines (t = 0.41, p = 0.91), indicating that differences in electrophysiological and behavioral responses are not related to differences in gene expression. HvOR13 coding sequence comparison between H. virescens and the Or67dGAL4 [1];UAS-HvOR13, and Or67dGAL4[1];UAS-HvOR13* flies indicated some sequence variation that may represent nucleotide polymorphisms (122A>C; 287C>T; 387C>T; 504A>G; 714T>C; 717C>T;732G>A;861T>C) or single point mutations (160G>C; 261T>C;606C>T;801G>A;877A>G; 947T>A;1150C>T). Point mutations led to two conserved amino acid changes at position 54 (V → L) and 293 (M → V), a major amino acid change at position 316 (L → Q), and an early termination codon at position 384 (Q → stop codon) in Or67dGAL4 [1]; UAS-HR13* flies (Figure 1).
Discussion
The combined electrophysiological and behavioral data showed that the response of HvORl3 expressed in D. melanogaster T1 sensilla (OR67dGAL4;UAS) was highly sensitive and specific and in agreement with the findings of Syed et al. (2010) for BmORl in the same heterologous expression system. Expression of HvOR13 and other H. virescens pheromone receptors in Flp-In T-Rex293/Gα15 cells (Grosse-Wilde et al. 2007) indicated that this heterologous expression system is not as specific as the D. melanogaster system that we used. Moreover, pheromone binding proteins and an organic solvent had to be used in the cell system to increase sensitivity and specificity of the H. virescens receptors tested. It is possible that D. melanogaster biochemical components involved in cVA detection may increase the sensitivity and specificity of moth pheromone receptors expressed in the OR67dGAL4;UAS system.
Recently, Wang et al. (2011) expressed HvORl 3 in X. laevis oocytes and found a level of specificity of the receptor response based on their electrophysiological results that is comparable to the results reported in our study. In the same study, it was found that HvORl 5 expressed in X. laevis oocytes did not respond to H. virescens pheromone components and 50 general odorants. Thus, the lack of response of Or67dGAL4 [1];UAS-HvOR15 flies to the seven pheromone components tested in our study supports the findings of Wang et al. (2011). Despite the technical differences between the D. melanogaster and the X. laevis systems, both are useful for characterization of pheromone receptors in moths and possibly other insect taxa. It is important to note that when X. laevis oocytes and D. melanogaster empty neurons were used for functional characterization of a large set of Anopheles gambiae odorant receptors, the two methods did not always produce similar results (Carey et al. 2010; Wang et al. 2010). This emphasizes the need to conduct both types of assays in order to make firm conclusions about functional specificity of important receptors. In addition, expressing moth odorant receptors in fly trichoids offers a system to test the behavioral output in response to cognate pheromone ligands.
The results of our study also suggest that a point mutation leading to a major amino acid change at position 316 (L → Q), and another resulting in an early termination codon at 384 (Q → stop codon), in Or67dGAL4 [1];UAS-HvOR13* flies may have affected HvORl3 structure and function, which could be associated with differences in electrophysiological and behavioral responses observed between Or67dGAL4 [1]; UAS-HvOR13 lines. It has been shown that the C-terminal domain of odorant receptors plays a an important role in the formation of the odorant receptor/Orco heteromeric complex (Benton et al. 2006; de Bruyne et al. 2009), and that the three conserved motifs (A, B, and C) within the last 70–90 amino acid residues of this region appear to have major functional importance (Miller and Tu 2008). As shown in Figure 1, Or67dGAL4 [1];UAS-HvORl3* flies have an incomplete HvORl3 C-terminal region. It is possible that the lack of motif C and the partial motif B may be affecting heterodimer formation and the localization and stability of HvORl3 in OR neuron dendrites (Benton et al. 2006; de Bruyne et al. 2009). However, HvORl3 response was not completely obliterated, indicating that a C-terminal missing the last 42 amino acids could affect but not necessarily abolish receptor function. Also, the N-terminal half of odorant receptors has been suggested to be involved in odor binding (de Bruyne et al. 2009), which may explain how this mutated pheromone receptor could possibly bind to its ligand, Z11-16:Ald. Thus, we suggest that the Or67dGAL4-UAS system is not only a powerful tool for characterization of insect pheromone receptors, but is also very useful for testing mutations that could affect pheromone receptor function.
Acknowledgements
We thank Fran Haire, Denise Reaves, Laura Smith, and Whitney Bailey for technical assistance. This work was supported by the National Science Foundation (DEB-1025217 to F. Gould), the United States Department of Agriculture (NRI-2007-35607-17824 to F. Gould), and a W.M. Keck Post-doctoral Fellowship to G.M. Vásquez.
Glossary
Abbreviations
- OR,
odorant receptor;
- Z9-14:Ald,
(Z)-9-tetradecenal;
- Z9-16:Ald,
(Z)-9-hexadecenal;
- Z9-16:OH,
(Z)-9-hexadecen-1-ol;
- Z11-16:Ald,
(Z)-11-hexadecenal;
- Z11-16:OAc,
(Z)-11-hexadecenyl acetate;
- Z11-16:OH,
(Z)-11-hexadecen-1-ol
References
- Baker TC. Nearest Neural Neighbors: Moth Sex Pheromone Receptors HR11 and HR13. Chemical Senses. 2009;34:465–468. doi: 10.1093/chemse/bjp025. [DOI] [PubMed] [Google Scholar]
- Benton R, Sachse S, Michnick SW, Vosshall LB. Atypical membrane topology and heteromeric function of Drosophila odorant receptors in vivo. PLOS Biology. 2006;4:240–257. doi: 10.1371/journal.pbio.0040020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Carey AF, Wang G, Su C-Y, Zwiebel LJ, Carlson JR. Odorant reception in the malaria mosquito Anopheles gambiae. Nature. 2010;464:66–72. doi: 10.1038/nature08834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen TA, Hildebrand JG. Pheromonal and host-odor processing in the insect antennal lobe: how different? Current Opinion in Neurobiology. 2002;12:393–399. doi: 10.1016/s0959-4388(02)00336-7. [DOI] [PubMed] [Google Scholar]
- de Bruyne M, Smart R, Zammit E. Functional and molecular evolution of olfactory neurons and receptors for aliphatic esters across the Drosophila genus. Journal of Comparative Physiology A. 2009;196:97–109. doi: 10.1007/s00359-009-0496-6. [DOI] [PubMed] [Google Scholar]
- Demir E, Dickson BJ. fruitless splicing specifies male courtship behavior in Drosophila. Cell. 2005;121:785–794. doi: 10.1016/j.cell.2005.04.027. [DOI] [PubMed] [Google Scholar]
- Dobritsa AA, van der Goes van Naters W, Warr CG, Steinbrecht RA, Carlson JR. Integrating the molecular and cellular basis of odor coding in the Drosophila antennae. Neuron. 2003;37:827–841. doi: 10.1016/s0896-6273(03)00094-1. [DOI] [PubMed] [Google Scholar]
- Grosse-Wilde E, Gohl T, Bouche E, Breer H, Krieger J. Candidate pheromone receptors provide the basis for the response of distinct antennal neurons to pheromonal compounds. European Journal of Neuroscience. 2007;25:2364–2373. doi: 10.1111/j.1460-9568.2007.05512.x. [DOI] [PubMed] [Google Scholar]
- Gould F, Estock M, Hillier NK, Powell R, Groot AT, Ward CM, Emerson J, Schal C, Vickers NJ. Sexual isolation of male moths explained by a single QTL containing four receptor genes. Proceedings of the National Academy of Sciences USA. 2010;107:8660–8665. doi: 10.1073/pnas.0910945107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha TS, Smith DP. A pheromone receptor mediates 11-cis-vaccenyl acetate-induced responses in Drosophila. The Journal of Neuroscience. 2006;26:8727–8733. doi: 10.1523/JNEUROSCI.0876-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallem EA, Fox AN, Zwiebel LJ, Carlson JR. Mosquito receptor for human-sweat odorant. Nature. 2004a;427:212–213. doi: 10.1038/427212a. [DOI] [PubMed] [Google Scholar]
- Hallem EA, Ho MG, Carlson JR. The molecular basis of odor coding in the Drosophila antenna. Cell. 2004b;117:965–979. doi: 10.1016/j.cell.2004.05.012. [DOI] [PubMed] [Google Scholar]
- Hallem EA, Carlson JR. Coding of odors by a receptor repertoire. Cell. 2006;125:143–160. doi: 10.1016/j.cell.2006.01.050. [DOI] [PubMed] [Google Scholar]
- Jones WD, Nguyen TAT, Kloss B, Lee KJ, Vosshall LB. Functional conservation of an insect odorant receptor gene across 250 million years of evolution. Current Biology. 2005;15:R119–R121. doi: 10.1016/j.cub.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Kaissling KE, Kasang G. New pheromone of silkworm moth Bombyx mori— sensory pathway and behavioral effect. Naturwissenschaften. 1978;65:382–384. [Google Scholar]
- Kaupp UB. Olfactory signalling in vertebrates and insects: differences and commonalities. Nature Reviews Neuroscience. 2010;11:188–200. doi: 10.1038/nrn2789. [DOI] [PubMed] [Google Scholar]
- Krieger J, Grosse-Wilde E, Gohl T, Dewer YM, Raming K, Breer H. Genes encoding candidate pheromone receptors in a moth (Heliothis virescens). Proceedings of the National Academy of Sciences USA. 2004;101:11845–11850. doi: 10.1073/pnas.0403052101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger J, Gondesen I, Forstner M, Gohl T, Dewer Y, Breer H. HR11 and HR13 receptor-expressing neurons are housed together in pheromone-responsive sensilla trichodea of male Heliothis virescens. Chemical Senses. 2009;34:469–477. doi: 10.1093/chemse/bjp012. [DOI] [PubMed] [Google Scholar]
- Kurtovic A, Widmer A, Dickson BJ. A single class of olfactory neurons mediates behavioural responses to a Drosophila sex pheromone. Nature. 2007;446:542–546. doi: 10.1038/nature05672. [DOI] [PubMed] [Google Scholar]
- Miller R, Tu Z. Odorant receptor c-terminal motifs in divergent insect species. Journal of Insect Science. 2008;8:53. Available online: http://www.insectscience.org/8.53. [Google Scholar]
- Miura N, Nakagawa T, Touhara K, Ishikawa Y. Broadly and narrowly tuned odorant receptors are involved in female sex pheromone reception in Ostrinia moths. Insect Biochemistry and Molecular Biology. 2010;40:64–73. doi: 10.1016/j.ibmb.2009.12.011. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Sakurai T, Nishioka T, Touhara K. Insect sex-pheromone signals mediated by specific combinations of olfactory receptors. Science. 2005;307:1638–1642. doi: 10.1126/science.1106267. [DOI] [PubMed] [Google Scholar]
- Rützler M, Zwiebel LJ. Molecular biology of insect olfaction: recent progress and conceptual models. Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology. 2005;191:777–790. doi: 10.1007/s00359-005-0044-y. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Nakagawa T, Mitsuno H, Mori H, Endo Y, Tanoue S, Yasukochi Y, Touhara K, Nishioka T. Identification and functional characterization of a sex pheromone receptor in the silkmoth Bombyx mori. Proceedings of the National Academy of Sciences USA. 2004;101:16653–16658. doi: 10.1073/pnas.0407596101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai T, Mitsuno H, Haupt SS, Uchino K, Yokohari F. A single sex pheromone receptor determines chemical response secificity of sexual behavior in the silkmoth Bombyx mori. PLOS Genetics. 2011;7:e1002115. doi: 10.1371/journal.pgen.1002115. doi: 10.1371/journal.pgen.1002115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed Z, Ishida Y, Taylor K, Kimbrell DA, Leal WS. Pheromone reception in fruit flies expressing a moth's odorant receptor. Proceedings of the National Academy of Sciences USA. 2006;103:16538–16543. doi: 10.1073/pnas.0607874103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed Z, Kopp A, Kimbrell DA, Leal WS. Bombykol receptors in the silkworm moth and the fruit fly. Proceedings of the National Academy of Sciences USA. 2010;107:9436–9439. doi: 10.1073/pnas.1003881107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touhara K, Vosshall LB. Sensing odorants and pheromones with chemosensory receptors. Annual Review of Entomology. 2009;71:307–332. doi: 10.1146/annurev.physiol.010908.163209. [DOI] [PubMed] [Google Scholar]
- Vetter RS, Baker TC. Behavioral responses of male Heliothis virescens in a sustained-flight tunnel to combination of seven compounds identified from female sex pheromone glands. Journal of Chemical Ecology. 1983;9:747–719. doi: 10.1007/BF00988780. [DOI] [PubMed] [Google Scholar]
- Vosshall LB, Hansson BS. A unified nomenclature system for the insect olfactory coreceptor. Chemical Senses. 2011;36:497–498. doi: 10.1093/chemse/bjr022. [DOI] [PubMed] [Google Scholar]
- Wang G, Carey AF, Carlson JR, Zwiebel LJ. Molecular basis of odor coding in the malaria vector mosquito Anopheles gambiae. Proceedings of the National Academy of Sciences USA. 2010;107:4418–4423. doi: 10.1073/pnas.0913392107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Vásquez GM, Schal C, Zwiebel LJ, Gould F. Functional characterization of pheromone receptors in the tobacco budworm Heliothis virescens. Insect Molecular Biology. 2011;20:125–133. doi: 10.1111/j.1365-2583.2010.01045.x. [DOI] [PubMed] [Google Scholar]
- Wanner KV, Nichols AS, Allen JE, Bunger PL, Garczynski SF, Linn CE, Robertson HM, Luetje CW. Sex pheromone receptor specificity in the European corn borer moth, Ostrinia nubilalis. PLOS One. 2010;5:e8685. doi: 10.1371/journal.pone.0008685. doi: 10.1371/journal.pone.0008685. [DOI] [PMC free article] [PubMed] [Google Scholar]



