Recently, a large number of studies that used large administrative databases have reported risk associations between cancer and the use of drugs among patients with diabetes, with increased, unchanged and decreased risks of cancer for the same individual drugs. These reports, especially those reporting increased cancer risks with the use of certain drugs, might have negatively impacted clinical practice in the management of patients with diabetes. For example, a study from the large Kaiser Permanente Northern California Diabetes Registry in the USA reported that the use of pioglitazone for 2 years or more was associated with increased bladder cancer risk1. Mainly based on the study from the Kaiser Permanente Northern California Diabetes Registry, the US Food and Drug Administration warned the public that pioglitazone use for more than 1 year might be associated with increased bladder cancer risk2. Many of these studies share two common features: (i) very large sample sizes, and thus an excellent power to detect a small change in the cancer risk; and (ii) incomplete collection of clinical and biochemical data, which are essential to estimate whether those users themselves (but not the drug effects) are a group at increased risk of cancer. It is well known that observational studies are prone to suffer from various biases or systematic errors, and incomplete collection of important clinical variables might make it impossible to control for these biases. Thus, a question remains debatable: are the observed increased risks of cancer with the use of these drugs a result of biased methods used in these investigations or use of those drugs are really to increase cancer risk?
A recent review on major biases has questioned the validity of many observational studies examining associations between drug use and cancer risk in diabetes3. The illustration with the use of statins and their proven cardiovascular disease (CVD) benefit from large clinical trials as an example clearly showed several important biases including drug use indication bias and prevalent user bias, which are particularly relevant when addressing drug use effects in diabetes with the use of administrative databases3. For example, failing to consider drug use indication bias (that belongs to selection bias) and incomplete adjustment for CVD covariables led to an incorrect conclusion that the use of statins did not reduce CVD risk. In a similar way, the use of prevalent users to address the effect of statins on CVD risk led to an incorrect conclusion that the use of statins might ‘increase’ CVD risk. More importantly, immortal time, which has been believed to introduce significant bias; that is, immortal time bias4, resulted in a neutral effect on the estimated effect of statins on CVD (Figure 1). The use of a time‐fixed Cox model analysis with the exclusion of prevalent users and consideration of drug use indication, but ignoring immortal time among users, resulted in an estimate of statins' effect on CVD that was closer to those obtained from large clinical trials. In contrast, the use of suggested methods, such as the use of a time‐dependent statin exposure Cox model and artificial inclusion of immortal time periods of the drug users in their matched non‐drug users to handle the bias as suggested4, had led to severe inflation or deflation of the hazard ratio of statins for CVD, respectively. If we examine many of those studies from large administrative databases, we would find that one or more biases were not taken into consideration. For example, the Kaiser Permanente Northern California diabetes registry study used a time‐dependent pioglitazone exposure Cox model analysis1; using the Hong Kong Diabetes Registry, the hazard ratio of insulin usage for cancer with use of a time‐fixed Cox model analysis was 0.48 (95% confidence interval [CI]: 0.32–0.73). Whereas, when immortal time periods of insulin users in both the user group and their matched non‐insulin user group were excluded, the hazard ratio was reduced to 0.17 (95% CI 0.09–0.32)5. Therefore, in a sense, whether the use of a drug increases or decreases cancer risk in diabetes is dependent on the study design, data analysis and availability of important covariables, but not the drug effects on cancer. Potentially, non‐consideration of drug use indication bias, prevalent user bias and/or incorrect use of some proposed methods for removal of immortal time bias might have contributed to the current controversial findings about the effects of drug use on the risk of cancer in diabetes.
Figure 1.
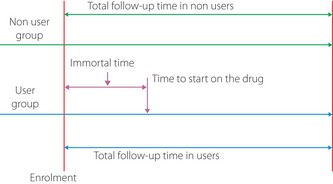
Illustration of immortal time in drug users in cohort study designs.
There has been a consensus to use propensity score to control for drug indication bias and to use new user designs to remove prevalent user bias in observational studies that aim to address drug effects outside clinical trial settings3. In the case addressing drug‐use effects on cancer in diabetes, we know that hyperglycemia is associated with an increased risk of cancer5, and it is recommended that antidiabetes drugs be used in a roughly sequential way; that is, first‐line antidiabetes drugs, second‐line antidiabetes drugs and then insulin. Nevertheless, many studies still suffered from non‐consideration of drug use indication bias and prevalent user bias.
The new user design requires only inclusion of patients who start the drug therapy being studied after enrolment as the drug users, and the design also requests moving the start point of follow up from the enrolment time to a point just before starting the drug therapy or the end of immortal time periods. In that case, the covariables measured on the drug users at the time‐point just before the drug therapy should also be available for adjustment. New user designs organized in this way and with full consideration of drug indication bias as well as with adjustment of known cancer risk factors including their interactions are, theoretically, expected to result in an estimate free of prevalent user bias and largely free from drug use indication bias (that depends on the c‐statistics of the propensity score). Nevertheless, this approach still needs to be validated using a drug with its known effect before actual use. Alternatively, the time‐fixed Cox model analysis with exclusion of prevalent users and taking into account drug use indication bias might also be an acceptable approach when measurements of covariables at the time just before the drug therapy on the users are not accessible to the analysis3. Although this approach can generate an effect size of statins on CVD in the Hong Kong Diabetes Registry, it remains unknown whether it works well in other databases, and thus requires further drug‐effect pair validations before use in other cohorts.
Notwithstanding the use of a validated study design and analysis approach, complete removal of drug use indication bias, prevalent user bias and other potential biases in examination of drugs with administrative databases is almost impossible. In this regard, the c‐statistics of propensity scores of drug usage are an indicator of how well the propensity score works, but few investigations of drug effects on cancer in diabetes can reach a level of excellence; that is, ≥ 0.90, thus there being residual drug use indication bias. Readers, including the media, should not overinterpret an ‘association’ as a drug effect and thus to change or imply to chance practices based on these reported associations, but we should wait for the results of clinical trials that address these important issues.
The diabetes–cancer link is complex and our understanding of the underlying mechanism is very limited. Observational studies with simultaneous consideration of all these major biases are very important and would contribute to generating new hypotheses for further testing by mechanistic investigations and clinical trials. In contrast, reporting biased results will do harm rather than contributing to our understanding of the plausible biological links. Although immortal time bias is an established concept, we call for more studies on immortal time bias to examine and ascertain whether immortal time really introduces substantial bias, and whether those suggested methods can really remove the so‐called immortal time bias or they themselves might introduce substantial biases. Confronted with these uncertainties, we call for validation of the method to be used with a drug‐effect pair that has been proved by large clinical trials, which might be a working way to avoid making major erroneous conclusions about drug use and cancer risk in diabetes. Although the use of a validated method cannot ensure unbiased results, this conservative approach will minimize the reporting of misleading results, and will eventually lead us onto the right road to generating hypotheses about biological links between diabetes and cancer for further testing.
Acknowledgement
There was no funding support for this article and the authors have declared no conflict of interest.
References
- 1.Lewis JD, Ferrara A, Peng T, et al Risk of bladder cancer among diabetic patients treated with pioglitazone: Interim report of a longitudinal cohort study. Diabetes Care 2011; 34: 916–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.U.S. Food and Drug Administration . FDA Drug Safety Communication: Update to ongoing safety review of Actos (pioglitazone) and increased risk of bladder cancer. [Updated 15 June 2011; accessed 14 March 2012]. Available from URL: http://www.fda.gov/Drugs/DrugSafety/ucm259150.htm
- 3.Yang XL, Ma RC, So WY, et al Addressing different biases in analysing drug use on cancer risk in diabetes in non‐clinical trial settings‐what, why and how? Diabetes Obes Metab 2012; 14: 579–585 [DOI] [PubMed] [Google Scholar]
- 4.Levesque LE, Hanley JA, Kezouh A, et al Problem of immortal time bias in cohort studies: Example using statins for preventing progression of diabetes. BMJ 2010; 340: b5087. [DOI] [PubMed] [Google Scholar]
- 5.Yang X, Ko GT, So WY, et al Associations of hyperglycemia and insulin usage with the risk of cancer in type 2 diabetes: The Hong Kong diabetes registry. Diabetes 2010; 59: 1254–1260 [DOI] [PMC free article] [PubMed] [Google Scholar]


