Abstract
(J Diabetes Invest, doi: 10.1111/j.2040‐1124.2012.00224.x, 2012)
Aims/Introduction: The Japanese Red Cross Society introduced measurement of glycated albumin (GA) for all blood donors as a glycemic control marker. The GA levels were examined by sex and age.
Materials and Methods: GA was measured in 3.14 million blood donors who donated between April 2009 and March 2010. For the reference range for GA, values that were three times the reference range for glycated hemoglobin (Japan Diabetes Society value) were used. All donors were notified of their GA levels. For repeat donors, a comparison was made between the GA levels at the first and second donations to verify the GA change after notification.
Results: The mean GA was significantly lower in males than in females in donors aged <60 years. The mean GAs of both sexes increased with age and reached the same level of 14.8% in their 60s. The percentage of donors with prediabetes/diabetes (GA ≥16.5%) was 2.8% in males and 2.3% in females. In the normal high group (15.6% ≤ GA < 16.5%), the mean GA at the second donation was lower by 0.20% than at the first donation. In 42.4% of these donors, GA decreased to the normal range at the second donation.
Conclusions: Overall, 2.7% of otherwise healthy Japanese blood donors had a high GA (GA ≥16.5%). Donor blood screening for GA represents an effective measure to identify people at risk of diabetes. The decrease in the GA level after GA notification might indicate the potential usefulness of this strategy to improve glycemic control among people with high GA.
Keywords: Glycated albumin, prediabetes mellitus, screening
Introduction
The number of people with type 2 diabetes mellitus has been increasing worldwide1, and this disease has become the main public health challenge for this century. In Japan, a nationwide study on the prevalence of diabetes mellitus was carried out by the Ministry of Health, Labour and Welfare in 2007, and it was estimated that there were as many as 22.1 million people with diabetes or suspected diabetes2. Type 2 diabetes mellitus is thought to develop as a result of compound effects of hereditary factors, such as impaired insulin secretion or insulin resistance, and various lifestyle factors, such as overeating, lack of physical activity and obesity. The ability to identify and educate people who are predisposed to type 2 diabetes, and to prevent them from developing the disease and acquiring its complications is recognized not only as a medical issue, but also as a social problem that requires a well‐programmed, sociopolitical intervention. It is thus necessary to develop strategies that enable the efficient detection of individuals at risk of the disease that are accessible to a large proportion of the general population without much difficulty or cost3,4.
The Japanese Red Cross Society (JRCS) has a nationwide network of blood centers that deal with blood procurement and delivery of blood components to medical institutions. It collects blood from more than 5 million voluntary donors per year and returns results on blood tests to those donors. To help identify individuals with diabetes or those predisposed to the disease, the JRCS introduced the measurement of glycated albumin (GA) and the notification of the result for all blood donors from 15 March 2009.
Considering that glycated hemoglobin (HbA1c) measurement has become the gold standard for monitoring long‐term glycemic control, it would have been desirable for the JRCS to implement HbA1c measurement. However, this would have required another blood sample to be drawn from a donor, which would add a further burden on blood donors and also require independent testing machines in all JRCS testing laboratories. These factors made it highly infeasible to implement HbA1c testing in the JRCS system. The serum GA, in contrast, can be measured using the blood sample that is used for measuring other blood chemistry items. It is measured with the automatic clinical chemistry analyzer currently used in the blood centers without any change in the testing process. Furthermore, GA is not affected by the contents of meals taken on the day of the measurement5, which suits the blood donation environment, because blood donors are encouraged to take some refreshment before or after donation to mitigate or prevent vasovagal reactions characterized by sudden decreases in blood pressure and heart rate.
In the present study, GA levels of blood donors were analyzed by age and sex, and the validity of GA measurement as a screening tool for donors at risk for diabetes was examined. The change in GA levels over repeated donations was also examined, with special attention to the effect of notification of GA levels to blood donors.
Materials and Methods
Blood Donors
JRCS is the sole organization that deals with blood procurement, processing, testing and delivery in Japan. Healthy people aged 16–69 years are eligible for blood donation. Before donation, they complete questionnaires and are examined by a physician. With respect to diabetes, people requiring insulin injections and/or oral antidiabetic drugs or those with diabetic complications are not allowed to donate blood. The lowest weight eligible for blood donation is 45 kg for male and 40 kg for female donors. In the present study, the body mass index (BMI) was calculated for each donor as weight in kilograms divided by height in meters squared. The values for the height and weight of the donors that were used for analysis were principally self‐reported.
Measurement of Glycated Albumin
GA was measured by an enzymatic method using reagent Lucica GA‐L (Asahi Kasei Pharma, Tokyo, Japan) and the automatic clinical chemistry analyzer LABOSPECT (Hitachi High‐Technologies, Tokyo, Japan)6,7. GA measurement was carried out in 10 JRCS testing laboratories located nationwide. At each laboratory, measurement quality was assessed using control materials that were common for all laboratories. The assessment was carried out daily at the startup and the shutdown of the analyzer. Serum samples with a normal concentration and a high concentration of GA were used to determine the within‐run and between‐run coefficients of variation (CVs). CVs within 20 repeated runs were 0.77–1.07%, and CVs over nine different days’ runs were 0.76–1.11%. The reference range for GA on which diabetes is categorized has not yet been officially determined, therefore reports from several studies that dealt with the correlation between HbA1c and GA were referenced. It was found that most studies concentrated on an average ratio of 3:1 for the GA:HbA1c ratio8. The JRCS therefore adopted a tentative value for the reference range for GA that was three times the reference range for HbA1c (Japan Diabetes Society value)9. The HbA1c reference range used in the conversion was the one determined by the Japan Diabetes Society9. The GA reference range was categorized by the JRCS as follows: GA <15.6%, normal; 15.6% ≤ GA < 16.5%, normal high (health guidance required); 16.5% ≤ GA < 18.3%, prediabetes (consultation recommended); and GA ≥18.3%, diabetes. The category of ‘prediabetes’ described in the present study largely corresponds to that of impaired glucose tolerance.
Donor Notification and Data Analysis
GA was measured for 3,142,794 blood donors at the JRCS laboratories from April 2009 to March 2010. A notification letter was sent to all donors with the data for alanine aminotransferase, gamma‐glutamyl transpeptidase, total protein, albumin, albumin:globulin ratio, total cholesterol and GA attached. It was stated in the letter that the GA level reflects the average blood glucose level during approximately the preceding 2 weeks, that a normal GA level is <16.5%, and that consultation at a medical institution is recommended if the GA level exceeds the normal range. It also noted that attention was required if the GA exceeds 15.6%, even though it is within the normal range.
A total of 973,160 donors made more than one donation during this period, and a comparison was made between GA levels at their first donation and those at their second donation. Statistical analysis was carried out for the change in GA between the two sequential donations using the paired t‐test.
Results
Glycated Albumin in the Blood Donor Population
During the study period, 3,142,794 people donated blood to JRCS, of which 2,041,715 (65.0%) were males, and 1,101,079 (35.0%) were females. More than half of the male donors were in their 30s and 40s. Of the female donors, half were in their 20s and 30s.
Table 1 and Figure 1 show the GA levels by sex and age. Mean GA was significantly lower in males than in females in donors aged <60 years. The difference in GA between males and females was larger at younger ages. The mean GAs of both males and females increased with age and reached the same level of 14.8% in their 60s.
Table 1. Glycated albumin of blood donors (April 2009 to March 2010).
| Sex, age (years) | n (%) | Mean BMI† ± SD | Mean GA ± SD | Donor percentage‡ | ||
|---|---|---|---|---|---|---|
| GA ≥15.6% | GA ≥16.5% | GA ≥18.3% | ||||
| Total | ||||||
| 16–19 | 226,046 (7.2) | 21.7 ± 3.0 | 13.5 ± 1.0 | 1.9 | 0.2 | 0.0 |
| 20–29 | 687,235 (21.9) | 22.1 ± 3.1 | 13.5 ± 1.1 | 2.6 | 0.5 | 0.1 |
| 30–39 | 819,949 (26.1) | 23.1 ± 3.3 | 13.6 ± 1.4 | 3.9 | 1.1 | 0.5 |
| 40–49 | 727,986 (23.2) | 23.5 ± 3.1 | 13.9 ± 1.8 | 7.2 | 2.8 | 1.3 |
| 50–59 | 499,586 (15.9) | 23.6 ± 2.8 | 14.4 ± 2.1 | 13.7 | 6.3 | 2.7 |
| 60–69 | 181,992 (5.8) | 23.6 ± 2.6 | 14.8 ± 2.4 | 20.8 | 10.5 | 4.3 |
| 16–69 | 3,142,794 (100.0) | 23.0 ± 3.2 | 13.8 ± 1.7 | 6.8 | 2.7 | 1.1 |
| Male | ||||||
| 16–19 | 121,260 (5.9) | 22.0 ± 3.2 | 13.3 ± 1.0 | 0.8 | 0.1 | 0.0 |
| 20–29 | 404,260 (19.8) | 22.7 ± 3.2 | 13.2 ± 1.1 | 1.1 | 0.2 | 0.1 |
| 30–39 | 547,316 (26.8) | 23.8 ± 3.2 | 13.4 ± 1.5 | 2.7 | 1.0 | 0.5 |
| 40–49 | 513,474 (25.1) | 24.0 ± 3.0 | 13.8 ± 1.9 | 6.5 | 3.0 | 1.6 |
| 50–59 | 340,405 (16.7) | 23.9 ± 2.7 | 14.3 ± 2.3 | 13.1 | 6.7 | 3.3 |
| 60–69 | 115,000 (5.6) | 23.8 ± 2.5 | 14.8 ± 2.5 | 20.7 | 11.5 | 5.2 |
| 16–69 | 2,041,715 (100.0) | 23.5 ± 3.1 | 13.7 ± 1.8 | 6.0 | 2.8 | 1.4 |
| Female | ||||||
| 16–19 | 104,786 (9.5) | 21.3 ± 2.6 | 13.8 ± 1.0 | 3.2 | 0.4 | 0.0 |
| 20–29 | 282,975 (25.7) | 21.4 ± 2.9 | 13.9 ± 1.1 | 4.7 | 0.8 | 0.1 |
| 30–39 | 272,633 (24.8) | 21.8 ± 3.2 | 13.9 ± 1.3 | 6.5 | 1.4 | 0.3 |
| 40–49 | 214,512 (19.5) | 22.3 ± 3.1 | 14.1 ± 1.5 | 8.9 | 2.5 | 0.7 |
| 50–59 | 159,181 (14.5) | 22.8 ± 2.8 | 14.4 ± 1.8 | 14.9 | 5.3 | 1.5 |
| 60–69 | 66,992 (6.1) | 23.3 ± 2.7 | 14.8 ± 2.2 | 20.9 | 8.8 | 2.7 |
| 16–69 | 1,101,079 (100.0) | 22.0 ± 3.0 | 14.1 ± 1.5 | 8.3 | 2.3 | 0.6 |
†Body mass index (BMI) calculated as weight in kilograms divided by height in meters squared. ‡Percentage of donors with indicated glycated albumin (GA) range within the shown age group.
Figure 1.
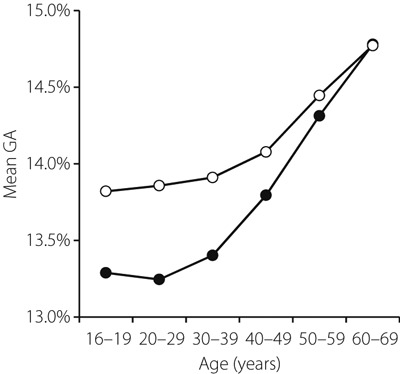
Mean glycated albumin (GA) by donor age. Closed circles indicate GA (%) in the male group, and open circles indicate GA (%) in the female group.
The percentage of donors that fell into the diabetes category defined by GA >18.3% was 1.1% of all blood donors, and the percentage of donors with GA ≥16.5% was 2.8% among males and 2.3% among females (Table 1, Figure 2). The percentage of donors with GA ≥16.5% increased steadily with age from 0.1% for males and 0.4% for females in their teens, to 11.5% in males and 8.8% in females in their 60s, reversing the order between the sexes over the age of 40 years (Table 1, Figure 2). The mean BMI was 23.5 kg/m2 for male donors and 22.0 kg/m2 for female donors. Although within the normal range, the mean BMI also increased with age (Table 1).
Figure 2.
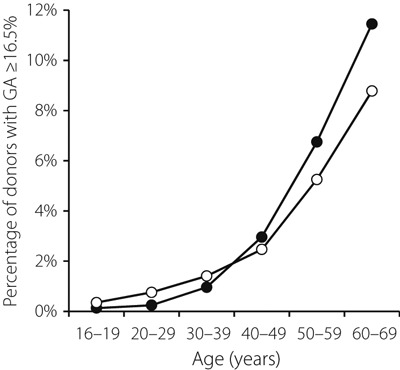
Percentages of individuals with glycated albumin (GA) ≥16.5% by donor age. Closed circles indicate percentage of males with GA ≥16.5% and open circles indicate percentage of females with GA ≥16.5%.
Changes of Glycated Albumin Levels
GA levels obtained from 973,160 donors who donated multiple times during the study period were analyzed. Donors were classified into three categories by GA levels at the first donation, and a comparison was made between GA at the first donation and that at the second donation for each category (Table 2). The interval between the two blood donations in each group was approximately 4.5 months. At the first donation, 92.4% of the donors had a normal GA (GA <15.6%), 4.7% had a normal high level (15.6% ≤ GA < 16.5%), and 2.9% had prediabetes/diabetes (GA ≥16.5%). In the normal group, GA was slightly higher at the second donation than at the first donation (P < 0.001). In contrast, GA was significantly lower at the second donation in the normal high group and the prediabetes/diabetes group than at the first donation. The mean decrease in GA was 0.20% (P < 0.001) and 0.14% (P < 0.001) in the normal high group and the prediabetes/diabetes group, respectively. There was a trend that the decrease was more evident in females than in males (data not shown).
Table 2. Comparison of glycated albumin at the first and second donations.
| First donation | Second donation | Difference in GA† | Intervals (days)‡ | P‐value* | ||
|---|---|---|---|---|---|---|
| Groups§ | n (%) | Mean GA ± SD | Mean GA ± SD | |||
| Normal | 899,248 (92.4) | 13.61 ± 1.0 | 13.63 ± 1.0 | 0.02 | 136 | <0.001 |
| Normal high | 45,612 (4.7) | 15.90 ± 0.2 | 15.70 ± 0.8 | −0.20 | 132 | <0.001 |
| Prediabetes/diabetes | 28,300 (2.9) | 19.59 ± 4.9 | 19.45 ± 5.0 | −0.14 | 138 | <0.001 |
†Glycated albumin (GA) at the second donation minus GA at the first donation (%). ‡Mean intervals between the first donation and the second donation. §GA levels for each group are as follows: normal group, GA <15.6%; normal high group, 15.6% ≤ GA < 16.5%; prediabetes/diabetes group, GA ≥16.5%. *P‐values determined by the paired t‐test for the difference in the mean GAs between the first and second donations.
In the normal high group, GA decreased in 62.6% of the donors and reached the normal range in 42.4% at the second donation (Table 3). In the prediabetes/diabetes group, in contrast, GA decreased in 56.6%, but just 4.1% showed a normal GA at the second measurement. The number of donors with a lower GA at the second donation was thus greater than that with a higher GA at the second donation in both the normal high and prediabetes/diabetes groups (Table 3).
Table 3. Number of donors who showed a change in glycated albumin levels at the second donation.
| First donation | GA at the second donation | ||||
|---|---|---|---|---|---|
| Increased | No change | Decreased (GA ≥15.6) | Decreased (GA <15.6) | ||
| Groups† | n | n (%) | n (%) | n (%) | n (%) |
| Normal high | 45,612 | 14,181 (31.1) | 2899 (6.4) | 9196 (20.2) | 19,336 (42.4) |
| Prediabetes/diabetes | 28,300 | 11,156 (39.4) | 1127 (4.0) | 14,853 (52.5) | 1164 (4.1) |
†Glycated albumin (GA) levels for each group are as follows: normal high group, 15.6% ≤ GA < 16.5%; prediabetes/diabetes group, GA ≥16.5%.
Discussion
Universal GA measurement for all blood donors was introduced by the JRCS as a screening tool for donors at risk of type 2 diabetes, which is the first time that it has been implemented by a blood service anywhere in the world. As there is not yet an internationally agreed reference range for GA, the normal GA range and the four categories for GA levels were decided on the basis of the HbA1c range used by the Japan Diabetes Society.
GA levels were measured in approximately 5 million donations obtained from approximately 3 million donors over a 1‐year period using the same, appropriately calibrated, chemical analyzers. The number of donors, 3 million, corresponds to approximately 3% of the population eligible for blood donation. It was found that blood donors who were supposed to be healthy at the time of donation included individuals with a high GA; 2.7% of blood donors had GAs falling into the prediabetes/diabetes category (GA ≥16.5%). If this figure is applied to the general population in Japan, it is estimated that the number of people with prediabetes/diabetes in the blood donor age population (16–69 years‐of‐age) is approximately 3.5 million. If the exponential curve for the percentage of donors with GA ≥16.5 (Figure 2) is extrapolated to people aged ≥70 years, the number of people with prediabetes/diabetes would be 6.5 million in total. There are two possible reasons for the considerable difference in the projected number of people with prediabetes/diabetes between the current study and the governmental research report2 based on HbA1c levels (22.1 million). First, the reference range for GA was determined as three times the reference range for HbA1c in the present study. Although this was based on the results from several previous reports8, the actual conversion coefficient between GA and HbA1c might be smaller than 3.0. A slight change in the GA level for categorizing prediabetes/diabetes would seriously affect the projected number of such persons. Second, the governmental research included data obtained from inpatients and outpatients. The prevalence of prediabetes/diabetes is likely higher among patients who visit medical facilities because of health problems than among volunteer blood donors. The present data do not include data from people with overt cardiovascular diseases or metabolic diseases. Thus, the projected number of people with prediabetes/diabetes in Japan (6.5 million) could be a very conservative one. It is very important to recognize that, even among seemingly healthy blood donors who visit a donation site, there are over 3 million people with prediabetes/diabetes. It is of note that individuals with GA ≥16.5% were also found in 0.2% of teenage donors and in 0.5% of donors in their 20s. It could be extrapolated from these figures that there are 15,000 and 72,000 people with prediabetes/diabetes in the 16–19 years and 20–29 years age groups, respectively.
It has been reported that the HbA1c level increases with age10,11. The present data show that mean GAs and the percentage of donors with GA ≥16.5% also increases with age. The mean GA increased in proportion to the square of the age; the coefficient of determination R2 between GA and age was remarkably high (0.992 for males and 0.994 for females). This finding might indicate that the increase in GA is a result of synergistic effects of multiple factors, such as the decrease in insulin secretion12 or the increase in insulin resistance13, both of which are known to correlate with aging. It is possible that both HbA1c and GA increase with age as a result of the direct effects of changes in insulin secretion and insulin resistance. This is in contrast with the fructosamine level that does not increase with age14, although fructosamine is a component of glycated serum protein. This contradiction might result from the difference in the calculation method: the GA level indicates the ratio of glycated albumin to total albumin, whereas the fructosamine level indicates the concentration of glycated protein. Fructosamine is thus affected by the concentration of total serum protein, which decreases with age. The GA level is independent of the change in serum protein level as a denominator. The measurements of approximately 200,000 blood donors showed that total serum protein decreased with age from 7.5 g/dL in their teens to 7.2 g/dL in their 60s (JRCS data).
Although obesity is one of the major risk factors for diabetes, the majority of Japanese patients with diabetes are not obese. It has been reported that the mean BMI of Japanese type 2 diabetes patients is 23.1 kg/m215,16. There is also a report suggesting that Japanese with a lean constitution might have some susceptibility genes that predispose them to type 2 diabetes mellitus17. In fact, the present study showed that the mean BMI of donors with GA ≥16.5% was 24.1 kg/m2, a value within the normal range. If a mass screening program is carried out for people at diabetic risk in Japan, a screening that mainly targets obese people would miss a considerable number of people at risk; instead, universal screening, such as that presented here, would be more appropriate. It also implies that lean Japanese with a high GA should consider the adoption of some preventive measures for diabetes.
In the present study, the mean GA at the second donation was lower than that at the first donation among the donors with GA ≥15.6%. It was also verified that 56.6–62.6% of the donors in this category had decreased GA levels at the second measurement. Although the precise reason for the decrease is not clear, it is tempting to speculate that the blood donors who were informed of their high GA levels afterwards paid attention to their health status regarding diabetes and improved their dietary habits or increased their physical activity, which might have resulted in the decreased GA at the second measurement. The decrease in GA was more evident in the normal high group than in the prediabetes/diabetes group. This might indicate either that the decrease in GA is more visible in the normal high group than in the prediabetes/diabetes group even with the equivalent degree of effort to decrease their GA level, or that the clinical significance of a high GA could be taken more seriously among people with a normal high GA than among those with a prediabetes/diabetes GA. However, it remains to be elucidated whether the decrease in GA is a true effect of GA notification, which might be verified through a collection of donor responses to questionnaires on their efforts to improve their health condition after being informed of their GA levels or through a study that might include a control arm without GA notification, though it would be undesirable to carry out such a study with a backward control arm.
The JRCS sends the results of the GA measurement to all blood donors by postcards accompanied by the results of other biochemical parameters described earlier. For donors with high GA levels, a recommendation for an individual consultation at medical facilities is also included in the postcard. It would be desirable to add an explanatory leaflet that describes not only general information on GA and diabetes, but also an individual evaluation of health status for each donor integrating all of his or her biochemical test results. However, to carry out effective consultations, education and follow up for people with high GA levels, it is necessary to establish an efficient system integrating national and local governments, local hospitals, general physicians and paramedical personnel.
In summary, universal GA measurement for Japanese blood donors showed a surprisingly high number of people with prediabetes/diabetes. The cross‐sectional analysis verified that the change in the GA level with age was similar to that for HbA1c. Through the measurement and notification of GA levels for blood donors, we are able to screen approximately 3 million people annually without the need for an additional blood sample or a special analyzer for this purpose in the blood center laboratories. GA measurement of blood donors thus has potential value for identifying individuals at risk for type 2 diabetes mellitus among the general population who are apparently healthy. It is also suggested that GA notification educates blood donors about the risks of diabetes and its prevention. If this effect is proven, then GA measurement and notification of blood donors might represent a very efficient strategy to improve the metabolic control status of a large proportion of the general population. It will be necessary to explore whether there is a true association between notification and improvement of GA levels, and whether the strategy described here might lead to the future prevention of diabetes development and improve the health status of a large segment of the population while saving costs related to the management of diabetes‐related complications. Finally, it is expected that consensus GA ranges for normal, normal high, prediabetes and diabetes will be determined formally by an appropriate organization or committee.
Acknowledgements
Introduction of glycated albumin measurement by the Japanese Red Cross Society was supported by Dr T Kadowaki of the Japan Diabetes Society, Dr A Kashiwagi of Shiga University of Medical Science Hospital, Dr M Nakabayashi and Dr Y Oomori of the Japanese Society of Diabetes and Pregnancy, and Dr Y Kanazawa of the Japan Diabetes Foundation. The authors are very grateful to them. No potential conflicts of interest relevant to this article were reported.
References
- 1.Zimmer P, Albert KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature 2001; 414: 782–787 [DOI] [PubMed] [Google Scholar]
- 2.Harada N. Present condition of diabetes and its treatment – from the perspective of a hospital pharmacist. Yakugaku Zasshi 2011; 131: 901–907 [DOI] [PubMed] [Google Scholar]
- 3.Tuomilehto J, Lindström J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001; 344: 1343–1350 [DOI] [PubMed] [Google Scholar]
- 4.Kahn R, Alperin P, Eddy D, et al. Age at initiation and frequency of screening to detect type 2 diabetes: a cost‐effectiveness analysis. Lancet 2010; 375: 1365–1374 [DOI] [PubMed] [Google Scholar]
- 5.Tahara Y, Shima K. Kinetics of HbA1c, glycated albumin, and fructosamine and analysis of their weight functions against preceding plasma glucose level. Diabetes Care 1995; 18: 440–447 [DOI] [PubMed] [Google Scholar]
- 6.Kouzuma T, Uemastu Y, Usami T, et al. Study of glycated amino acid elimination reaction for an improved enzymatic glycated albumin measurement method. Clin Chim Acta 2004; 346: 135–143 [DOI] [PubMed] [Google Scholar]
- 7.Roohk HV, Zaidi AR. A review of glycated albumin as an intermediate glycation index for controlling diabetes. J Diabetes Sci Technol 2008; 2: 1114–1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inaba M, Okuno S, Kumeda Y, et al. Glycated albumin is a better glycemic indicator than glycated hemoglobin values in hemodialysis patients with diabetes: effect of anemia and erythropoietin injection. J Am Soc Nephrol 2007; 18: 896–903 [DOI] [PubMed] [Google Scholar]
- 9.The Committee of the Japan Diabetes Society on the Diagnostic Criteria of Diabetes Mellitus , Seino Y, Nanjo K, et al. Report of the Committee on the Classification and Diagnostic Criteria of Diabetes Mellitus. J Diabetes Invest 2010; 1: 212–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pani LN, Korenda L, Meigs JB, et al. Effect of aging on A1C levels in individuals without diabetes: evidence from the Framingham Offspring Study and the National Health and Nutrition Examination Survey 2001‐2004. Diabetes Care 2008; 31: 1991–1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hashimoto Y, Futamura A, Ikushima M. Effect of aging on HbA1c in a working male Japanese population. Diabetes Care 1995; 18: 1337–1340 [DOI] [PubMed] [Google Scholar]
- 12.Iozzo P, Beck‐Nielsen H, Laakso M, et al. Independent influence of age on basal insulin secretion in nondiabetic humans. European Group for the Study of Insulin Resistance. J Clin Endocrinol Metab 1999; 84: 863–868 [DOI] [PubMed] [Google Scholar]
- 13.Ferrannini E, Vichi S, Beck‐Nielsen H, et al. European Group for the Study of Insulin Resistance (EGIR). Insulin action and age. Diabetes 1996; 45: 947–953 [DOI] [PubMed] [Google Scholar]
- 14.Kilpatrick ES, Dominiczak MH, Small M. The effects of ageing on glycation and the interpretation of glycaemic control in Type 2 diabetes. QJM 1996; 89: 307–312 [DOI] [PubMed] [Google Scholar]
- 15.Sone H, Ito H, Ohashi Y, et al. Obesity and type 2 diabetes in Japanese patients. Lancet 2003; 361: 85. [DOI] [PubMed] [Google Scholar]
- 16.Sone H, Katagiri A, Ishibashi S, et al. Effects of lifestyle modifications on patients with type 2 diabetes: the Japan Diabetes Complications Study (JDCS) study design, baseline analysis and three year‐interim report. Horm Metab Res 2002; 34: 509–515 [DOI] [PubMed] [Google Scholar]
- 17.Okamoto K, Iwasaki N, Nishimura C, et al. Identification of KCNJ15 as a Susceptibility Gene in Asian Patients with Type 2 Diabetes Mellitus. Am J Hum Genet 2010; 86: 54–64 [DOI] [PMC free article] [PubMed] [Google Scholar]


