Abstract
Aims/Introduction
To evaluate the efficacy and safety of alogliptin added to treatment with glimepiride.
Materials and Methods
This multicenter, randomized, double‐blind, parallel‐group, 24‐week (12‐week observation and 12‐week treatment) study compared alogliptin 12.5 or 25 mg in combination with glimepiride (1–4 mg/day) vs placebo added to glimepiride monotherapy in Japanese patients with type 2 diabetes who had poor glycemic control despite treatment with diet and exercise plus a sulfonylurea. The primary end‐point was a change in glycated hemoglobin (HbA 1c) from baseline. A 40‐week open‐label extension study evaluated the long‐term safety and efficacy of the combination.
Results
Alogliptin 12.5 or 25 mg in combination with glimepiride significantly decreased HbA 1c compared with glimepiride monotherapy after 12 weeks' treatment (−0.59, −0.65 and 0.35%, respectively; P < 0.0001 for both combination groups vs glimepiride monotherapy). Alogliptin 12.5 and 25 mg combination therapy was also associated with significantly higher responder rates (HbA 1c <6.9%: 9.6% and 7.7%, HbA 1c <7.4%: 29.8% and 34.6%) compared with glimepiride monotherapy (HbA 1c <6.9%: 0%, HbA 1c <7.4%: 3.9%). The incidence of adverse events was comparable between glimepiride monotherapy and alogliptin combination treatment, with most reported adverse events being mild in severity. In the extension study, the incidence of adverse events was comparable between the combination groups, with the majority of adverse events being mild.
Conclusions
Once‐daily alogliptin was effective and generally well tolerated when given as add‐on therapy to glimepiride in Japanese patients with type 2 diabetes who had inadequate glycemic control on sulfonylurea plus lifestyle measures. Clinical benefits were maintained for 52 weeks. This trial was registered with ClinicalTrials.gov (double‐blind study no. NCT01318083; long‐term study no. NCT01318135).
Keywords: Alogliptin, Glimepiride, Type 2 diabetes
Introduction
The worldwide prevalence of diabetes mellitus continues to rise and the morbidity and mortality associated with it are also increasing. Current estimates indicate that more than 346 million people worldwide have diabetes, with this number projected to rise significantly by 20301. Indeed, an estimated 3.4 million people died as a consequence of hyperglycemia in 2004, and the World Health Organization forecasts that the rate of diabetes‐related deaths will double between 2005 and 20301. It is important to point out that over time diabetes can also cause damage to organs, such as blood vessels, eyes, heart, kidneys and nerves, and the overall risk of death in people with diabetes is at least double the risk of peer groups without diabetes1. The costs to global healthcare systems and society are enormous.
Approximately 90% of people with diabetes worldwide have type 2 diabetes, which is mainly the result of excess bodyweight and physical inactivity1. Thus, lifestyle measures are the cornerstone of initial treatment in these patients. However, progressive reductions in pancreatic β‐cell function and increased insulin resistance are pathogenic hallmarks of the disease, and pharmacotherapy becomes essential1. Furthermore, although diet, exercise and oral monotherapy are initially successful, the disease is associated with a secondary failure rate of 30–50% over a 3 to 5‐year period1. In the Japanese population, insulin hyposecretion is regarded as the main pathogenetic mechanism for the development of type 2 diabetes, and insulin secretagogues, such as the sulfonylureas, have been widely used in this clinical setting3. However, sulfonylureas produce a prolonged increase in insulin secretion, which increases the risk of hypoglycemia and secondary failure caused by exhaustion of pancreatic β‐cells. Combination therapy, commonly with oral hypoglycemic drugs with different mechanisms of action, is therefore the long‐term option for the majority of patients with type 2 diabetes4.
Incretin hormones, such as glucagon‐like peptide‐1 (GLP‐1) and glucose‐dependent insulinotropic polypeptide (GIP), potentiate glucose‐induced insulin secretion with their actions being dependent on plasma glucose concentrations. Incretin hormones are involved in the pathogenesis of type 2 diabetes, with their effects being severely reduced or absent in patients with the disease6. GIP fails to stimulate insulin secretion in patients with type 2 diabetes7, whereas GLP‐1 improves glucose homeostasis by enhancing glucose‐dependent stimulation of insulin secretion, suppressing glucose‐dependent glucagon secretion, and delaying gastric emptying8. GLP‐1 is rapidly metabolized and inactivated by the enzyme, dipeptidyl peptidase 4 (DPP‐4)12. In addition, very low active GLP‐1 levels in Japanese patients with type 2 diabetes have been reported13. Alogliptin is a highly selective inhibitor of DPP‐4, which spares GLP‐1 from degradation and thereby increases insulin secretion and reduces glucagon secretion8, and when administered as monotherapy or in combination with an α‐glucosidase inhibitor, it was found to be effective in the treatment of Japanese patients with type 2 diabetes14.
Given the wide use of sulfonylureas and the need for additional oral antidiabetic support in many patients, the present study was designed to evaluate the efficacy and safety of alogliptin in combination with glimepiride in Japanese patients with type 2 diabetes who had inadequate glycemic control despite treatment with a sulfonylurea.
Materials and Methods
Study Design
The present phase 2/3, multicenter, randomized, double‐blind, parallel‐group comparative study assessed the efficacy and safety of alogliptin at a dose of 12.5 or 25 mg in combination with glimepiride, and compared with glimepiride monotherapy in Japanese patients with type 2 diabetes mellitus. The study duration was 24 weeks, consisting of 12‐week observation and 12‐week treatment periods. It was followed by a 40‐week, open‐label extension study.
The study was carried out at 33 centers across Japan in accordance with the Declaration of Helsinki and the International Conference on Harmonisation (ICH), Harmonised Tripartite Guideline on Good Clinical Practice15, and was approved by the Institutional Review Boards at each study site. All participants provided written informed consent. The double‐blind study was carried out between August 2008 and April 2009, whereas the extension study was completed in January 2010.
Patients
Patients were aged ≥20 years, had type 2 diabetes, and a glycated hemoglobin (HbA1c) level of between ≥7.4 and <10.4% at 8 weeks after the initiation of the observation period; with HbA1c levels at this time being within 10% of the level at 4 weeks after the initiation of the observation period. The patients had been taking a sulfonylurea for at least 4 weeks before the observation period, and they had received specific diet and exercise therapies, as well as glimepiride at a stable dosage during the observation period.
The main exclusion criteria were: diabetic medications other than glimepiride within 12 weeks before the initiation of the treatment period; requirement for insulin; hypersensitivity or allergy to glimepiride or sulfonamides; gastrointestinal disorder; history of cardiac failure; systolic/diastolic blood pressure ≥180/110 mmHg; hepatic or renal impairment (aspartate aminotransferase or alanine aminotransferase ≥2.5 × upper limit of normal or serum creatinine ≥2 mg/dL); serious cardiovascular, cerebrovascular, pancreatic or hematological disorders; any malignancy; drug abuse or dependency or excessive alcohol consumption; hypersensitivity to the component of glimepiride or sulfonamide drugs; treatment with any investigational drug within 12 weeks; and pregnancy or lactation (for women of child‐bearing age).
Treatment and Assessment
Eligible patients received alogliptin 12.5, 25 mg or placebo orally, once daily, during the treatment period; and glimepiride, given orally, at a stable dose regimen (1, 2, 3 or 4 mg/day, given on a once or twice daily basis) throughout the study. Patients were randomized 1:1:1 into each group with the daily dose of glimepiride being used as a randomization factor.
In the open‐label extension study, patients continued to receive combination alogliptin/glimepiride treatment at the same alogliptin dose as before. Patients in the glimepiride monotherapy group were randomized 1:1 into the alogliptin 12.5 mg or alogliptin 25 mg groups in combination with glimepiride given once or twice daily, at a dose of 1, 2, 3, 4, 5 or 6 mg/day.
During the observation period, assessments included medical and medication histories, physical examination, bodyweight, height, body mass index, vital signs, concomitant medications, concurrent medical conditions, clinical laboratory tests, pregnancy test in women of child‐bearing age, 12‐lead electrocardiogram (ECG), compliance with study treatment, diet and exercise therapies, HbA1c, and adverse effects. The evaluations listed were carried out during the treatment and extension studies, and additional assessments included fasting plasma glucose (FPG), fasting insulin, fasting glucagon, fasting C‐peptide, glycoalbumin, 1,5‐anhydroglucitol (1,5‐AG), fasting proinsulin, fasting serum lipids (total cholesterol, triglycerides, high density lipoprotein‐cholesterol, low density lipoprotein‐cholesterol and free fatty acids), homeostasis model assessment for insulin resistance (HOMA‐R), HOMA‐β cell function, insulinogenic index, proinsulin/insulin ratio, DPP‐4 activity, meal tolerance test, abdominal circumference, plasma drug concentration and blood sampling for pharmacogenomic assessment. Patients were assessed every 4 weeks in the observation period of the study; at weeks 0, 2, 4, 8 and 12 during the treatment period; and every 4 weeks during the extension study.
All clinical laboratory tests, including DPP‐4 activity (which was measured independently using a fluorescence‐based methodology), were carried out at a central independent laboratory (Mitsubishi Chemical Medience Corporation, Tokyo, Japan).
End‐Points
The primary end‐point was the change in HbA1c from baseline (start of double‐blind period) to the end of the 12‐week treatment period. Secondary end‐points included HbA1c and FPG at each assessment point; and plasma glucose measured by the meal tolerance test. Additional end‐points comprised parameters of glycemic control evaluated during the observation period (aforementioned) and bodyweight. Safety end‐points comprised adverse events, vital signs, 12‐lead ECGs and clinical laboratory test data. The primary end‐point in the extension study was adverse events with secondary and additional end‐points identical to those of the treatment period of the study.
Values for HbA1c (%) were estimated using the National Glycohemoglobin Standardization Program (NGSP) equivalent values (%), which were calculated using the formula HbA1c (%) = HbA1c (Japan Diabetes Society) (%) + 0.4%. This takes into consideration the relationship between HbA1c (Japan Diabetes Society) (%), determined using the previous Japanese standard measurement methods, and HbA1c (NGSP)16.
Sample Size and Statistical Methods
In a previous study, mean changes in HbA1c after 12 weeks' treatment with alogliptin 12.5, 25 mg and placebo were −0.50, −0.50 and 0.0%, respectively, and the standard deviation (SD) was assumed to be 0.80% for each treatment. Based on these assumptions, 65 patients per group were required to provide a 90% simultaneous power of detecting a statistically significant difference between the glimepiride monotherapy group and the alogliptin combination groups at the significance level of 2.5% (one‐sided) in the double‐blind study. Allowing for dropouts, it was planned to randomize 240 patients (~80 per group).
The full analysis set (FAS), defined as all patients who were randomized and received at least one dose of study medication, was the primary efficacy analysis set. For this data set, summary statistics (number, mean, SD; maximum, minimum and quartile values) and two‐sided 95% confidence intervals (CI) of the mean were calculated for each group. Adjusted means (least square [LS] means), standard error (SE), and two‐sided 95% CI of the LS means were calculated for the three treatment groups using an analysis of covariance (ancova) model. In this model, change in HbA1c (at the completion of the treatment period) was a dependent variable; the daily dose of glimepiride during the observation period was a block factor; HbA1c at the completion of the observation period was a covariate, and the treatment group was an independent variable. A comparison between each alogliptin group and the glimepiride monotherapy group was carried out in accordance with a closed testing procedure using the ancova model. A secondary efficacy analysis was carried out with the per protocol set (PPS) using methods described above to assess the robustness and sensitivity of the results. Area under the curve (AUC) time profiles were evaluated for parameters measured during meal tolerance tests. Adverse events were coded using MedDRA (version 12.0, Pharmaceutical and Medical Device Regulatory Science Society of Japan, Tokyo, Japan) and summarized using preferred term and system organ class. Significance levels for contrast tests in the ancova model were 2.5 (one‐sided tests) and 5% for other statistical tests (two‐sided tests).
Results
Baseline Data
Of the 455 enrolled patients, 312 were randomized into the present double‐blind study: with 105 and 104 in the alogliptin 12.5 and 25 mg groups, respectively, and 103 in the glimepiride monotherapy group (Figure 1). The reasons for not undergoing randomization into one of the treatment groups included, failure to meet the entry criteria (n = 133), suffered a pretreatment event (n = 6) and voluntary withdrawal (n = 4). The PPS groups comprised 99, 102 and 98 patients, in the alogliptin 12.5 mg, alogliptin 25 mg and glimepiride monotherapy groups, respectively. Baseline data for each treatment group are shown in Table 1.
Figure 1.
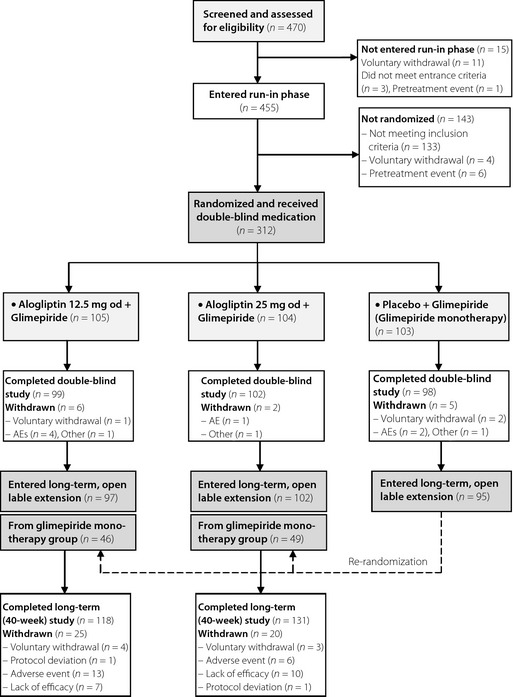
Disposition of patients in the 12‐week double‐blind study and 40‐week open‐label extension. AE, adverse event.
Table 1. Baseline characteristics (12‐week double‐blind study) in glimepiride monotherapy (+ placebo), alogliptin 12.5 mg and alogliptin 25 mg groups.
| Placebo + glimepiride n = 103 | Alogliptin 12.5 mg + glimepiride n = 105 | Alogliptin 25 mg + glimepiride n = 104 | |
|---|---|---|---|
| Male/female (n) | 71/32 | 67/44 | 70/43 |
| Age (years) | 60.3 (9.11) | 60.5 (9.50) | 59.8 (9.10) |
| Weight (kg) | 65.19 (12.58) | 65.02 (12.79) | 64.28 (13.73) |
| Height (cm) | 161.7 (8.15) | 161.2 (8.50) | 162.4 (9.16) |
| BMI (kg/m2) | 24.84 (3.90) | 24.89 (3.81) | 24.29 (4.40) |
| Diabetes duration (years) | 9.37 (7.38) | 9.59 (7.03) | 10.38 (6.86) |
| HbA1c (%) | 8.62 (0.78) | 8.54 (0.81) | 8.54 (0.79) |
| Fasting C‐peptide (ng/mL) | 1.77 (0.79) | 1.86 (0.79) | 1.65 (0.93) |
| 2‐h postprandial PG (mg/dL) | 279.2 (62.19) | 276.7 (58.06) | 277.7 (64.13) |
| Glimepiride dose (mg/day), No. (%) patients | |||
| 1 | 37 (35.9) | 36 (34.3) | 37 (35.6) |
| 2 | 31 (30.1) | 34 (32.4) | 32 (30.8) |
| 3 | 13 (12.6) | 13 (12.4) | 13 (12.5) |
| 4 | 22 (21.4) | 22 (21.0) | 22 (21.2) |
Values shown are mean (SD). BMI, body mass index; HbA1c, glycosylated hemoglobin; PG, plasma glucose.
Efficacy
The primary end‐point, mean change in HbA1c (from baseline to the completion of the double‐blind treatment period; LS mean ± SE), was 0.35 ± 0.059% in the glimepiride monotherapy group, −0.59 ± 0.058% in the alogliptin 12.5 mg group and −0.65 ± 0.059% in the alogliptin 25 mg group. Figure 2 shows mean ± SD changes in HbA1c for each treatment group. There were highly significant differences in the change in HbA1c between each alogliptin combination group and the glimepiride monotherapy group (P < 0.0001), whilst the different dosages of alogliptin produced similar reductions in HbA1c.
Figure 2.
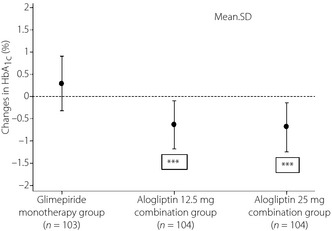
Changes in glycated hemoglobin (HbA 1c; mean and SD) at the completion of the double‐blind treatment period for glimepiride monotherapy, alogliptin 12.5 mg and alogliptin 25 mg groups in the full analysis set. ***P < 0.0001 vs placebo.
Analysis of secondary end‐points showed statistically significant differences in the mean change in HbA1c between each alogliptin group and the glimepiride monotherapy group at all assessment points during the treatment period. There was a significant difference in the proportion of patients achieving the HbA1c goal of <6.9 or <7.4%, between each alogliptin group and the glimepiride monotherapy group. Values for HbA1c <6.9% were: 0% in the glimepiride monotherapy group, 9.6 and 7.7% in the alogliptin 12.5 and 25 mg groups, respectively; and for HbA1c <7.4%: 3.9%, 29.8% and 34.6%, respectively.
Changes in glycemic/metabolic parameters including the primary, secondary and other end‐points from baseline to the completion of the 12‐week treatment period are summarized in Table 2. Statistically significant differences between each alogliptin group and the glimepiride monotherapy group were observed for mean changes in all three of these parameters (Table 2). Glucose, insulin and glucagon responses during a meal tolerance test at baseline (week 0) and end of double‐blind treatment (week 12) in the glimepiride monotherapy, and alogliptin 12.5 and 25 mg groups are available online (Figure S1).
Table 2. Changes in glycemic/metabolic parameters from baseline to the completion of the 12‐week treatment period in glimepiride monotherapy, alogliptin 12.5 mg and alogliptin 25 mg groups.
| Placebo + glimepiride (n = 103) | Alogliptin 12.5 mg + glimepiride (n = 105) | Alogliptin 25 mg + glimepiride (n = 104) | |
|---|---|---|---|
| ∆HbA1c (%) | 0.35 (0.059) | −0.59 (0.058)*** | −0.65 (0.059)*** |
| Responders (%): HbA1c <6.9% | 0 (0.0) | 10 (9.6)* | 8 (7.7)* |
| Responders (%): HbA1c <7.4% | 4 (3.9) | 31 (29.8)* | 36 (34.6)* |
| ∆ Fasting plasma glucose (mg/dL) | 6.0 (32.97) | −22.3 (31.05)* | −15.9 (28.12)* |
| ∆ Fasting C peptide (ng/mL) | −0.00 (0.597) | 0.06 (0.402) | 0.02 (0.375) |
| ∆ Fasting insulin (μU/mL) | −0.12 (3.315) | 0.57 (2.580) | 0.53 (2.292) |
| ∆ Fasting glucagon (pg/mL) | −12.4 (33.21) | −12.0 (24.59) | −10.4 (22.32) |
| ∆ Glycoalbumin (%) | 0.99 (2.353) | −2.60 (2.467)* | −3.05 (2.504)* |
| ∆ 1,5‐AG (μg/mL) | −0.33 (1.418) | 2.43 (2.269)* | 2.77 (2.976)* |
| ∆ Fasting proinsulin (pmol/L) | −1.82 (5.864) | −3.19 (6.074) | −2.82 (4.415) |
| ∆ Insulinogenic index | −0.07 (0.720) | 0.11 (0.504) | 0.05 (0.363) |
| ∆ Proinsulin/insulin ratio | −0.403 (0.9485) | −0.582 (0.7921) | −0.701 (0.7084)* |
| Inhibition rate of DPP‐4 activity (%) at week 4 | 1.25 (7.930) | 74.88 (5.817)* | 81.22 (8.593)* |
| Inhibition rate of DPP‐4 activity (%) at week 8 | 2.37 (7.293) | 75.45 (6.965)* | 81.47 (11.134)* |
| Inhibition rate of DPP‐4 activity (%) at week 12 | 0.23 (9.661) | 75.09 (7.223)* | 81.15 (11.974)* |
| ∆ HOMA‐R | 0.02 (2.064) | −0.17 (1.255) | −0.01 (1.345) |
| ∆ HOMA‐β | −0.94 (9.813) | 10.73 (20.162)* | 5.70 (10.252)* |
| ∆ Bodyweight (kg) | −0.37 (1.213) | 0.27 (1.225)* | 0.56 (1.105)* |
| ∆ Abdominal circumference (cm) | −1.00 (2.749) | 0.01 (3.303)* | 0.24 (3.736)* |
| Meal tolerance test | |||
| ∆ Plasma glucose 2‐h (mg/dL) | 8.1 (42.69) | −44.4 (52.18)* | −33.3 (47.78)* |
| ∆ Plasma glucose AUC0–2 h (mg·h/dL) | 13.7 (66.33) | −77.6 (78.36)* | −61.0 (69.73)* |
| ∆ Insulin AUC0–2 h (μU.hr/mL) | 0.79 (17.366) | 3.63 (21.726) | 4.53 (12.085) |
| ∆ C‐peptide AUC0–2 h (ng.hr/mL) | −0.08 (1.300) | 0.17 (1.442) | 0.29 (1.309) |
| ∆ Glucagon AUC0–2 h (pg.hr/mL) | −24.0 (60.73) | −28.3 (43.27)* | −25.2 (41.26)* |
| ∆ Active GLP‐1 concentrations (pg.hr/mL) | 0.17 (3.61) | 12.71 (21.98)* | 11.56 (11.20)* |
| Serum lipids | |||
| ∆ Total cholesterol (mg/dL) | −0.1 (26.83) | −2.6 (24.31) | −1.7 (24.80) |
| ∆ LDL‐cholesterol (mg/dL) | −1.6 (22.59) | −3.1 (19.65) | −1.8 (20.13) |
| ∆ HDL‐cholesterol (mg/dL) | −1.6 (7.68) | −1.4 (8.64) | −2.7 (7.18) |
| ∆ Triglycerides (mg/dL) | 13.0 (214.59) | −10.0 (67.14) | 0.2 (48.43) |
| ∆ Free fatty acids (mEg/L) | 0.057 (0.2151) | −0.070 (0.2031) | −0.059 (0.2020) |
1,5‐AG, 1,5‐anhydroglucitol; AUC0–2 h, area under the blood glucagon concentration time curve from 0 to 2 hours; DDP‐4, dipeptidyl peptidase IV; FPG, fasting plasma glucose; GLP‐1, glucagon‐like peptide‐1; HbA1c, glycated hemoglobin; HDL, high‐density lipoprotein; HOMA‐R, homeostasis model assessment of insulin resistance; HOMA‐β, homeostasis model assessment of β‐cell function; LDL, low‐density lipoprotein. Values shown are mean (SD) or (95% CI); ∆, change from baseline. *P < 0.05 vs placebo. ***P < 0.0001 vs placebo.
There were also significant differences between the glimepiride monotherapy group and the alogliptin groups for DPP‐4 inhibitory activity at weeks 4, 8 and 12 as well as for mean changes in glycoalbumin, 1,5‐AG, HOMA‐β, glucagon AUC0–2 h, active GLP‐1 concentrations, bodyweight and abdominal circumference. However, the differences in change in bodyweight and abdominal circumference were not considered to be clinically significant. There was a significant difference in change in proinsulin/insulin ratio between the glimepiride monotherapy group and the alogliptin 25 mg group (but not the alogliptin 12.5 mg group). Serum lipids remained relatively stable after the addition of alogliptin with small, but not statistically significant, reductions for most parameters (Table 2). There was no significant difference between the glimepiride monotherapy group and the alogliptin groups for HOMA‐R, and this suggests no change of insulin resistance in the fasting state. There were also no significant differences for fasting insulin and fasting C‐peptide (Table 2); but after 52 weeks, there were statistically significant differences for these parameters (data not shown). These findings suggest that fasting insulin secretion might be improved during long‐term administration of alogliptin as a result of sustained inhibition of DPP‐4 activity.
The FAS in the open‐label extension study comprised 150 and 152 patients in the alogliptin 12.5 and 25 mg groups, respectively. Efficacy comparisons for the open‐label extension study were made from baseline; that is, over 52 weeks' treatment. The profile of mean HbA1c levels over this time period is shown in Figure 3, and the mean changes from baseline in HbA1c, for the alogliptin 12.5 and 25 mg groups, were statistically significant at all time‐points. At the end of the study, HbA1c levels were reduced by −0.42 and −0.58%, respectively. The proportions of participants in the alogliptin 12.5 and 25 mg groups achieving a HbA1c of <6.9% were 6.0 and 4.6%; and for HbA1c <7.4%, they were 22.1 and 26.3%, respectively. In the alogliptin 12.5 and 25 mg groups, statistically significant mean changes in FPG (−16.0 and −13.0 mg/dL), 2‐h postprandial plasma glucose (−31.0 and −27.3 mg/dL) and plasma glucose AUC0–2 h (−58.5 and −53.6 mg.h/dL), respectively, were recorded.
Figure 3.
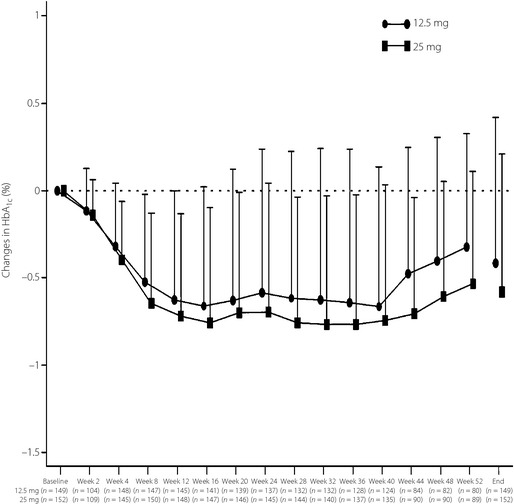
Time profiles of changes in glycated hemoglobin (HbA 1c; mean ± SD) in the alogliptin 12.5 and 25 mg groups during the course of the 52‐week clinical study (the period in which placebo was given is excluded).
Safety
Of the 312 participants randomized in this double‐blind study, 13 participants withdrew prematurely: five in the glimepiride monotherapy group, six in the alogliptin 12.5 mg group and two in the alogliptin 25 mg group. The incidence of reported adverse events including subcategories was similar between treatment groups. In the glimepiride monotherapy group, alogliptin 12.5 mg group and alogliptin 25 mg group, the incidence of adverse events was 48.5, 46.7 and 56.7%, respectively. The majority of reported adverse events were mild in severity. The incidence of adverse events assessed as drug‐related in the glimepiride monotherapy group, alogliptin 12.5 mg group and alogliptin 25 mg group was: 5.8, 6.7 and 7.7%; of serious adverse events, 0.0, 2.9 and 1.0%; and of adverse events leading to drug discontinuation 1.9, 3.8 and 1.0%, respectively.
The most common adverse event in each group was nasopharyngitis, with an incidence of 21.4, 19.0 and 19.2% in the glimepiride monotherapy group, alogliptin 12.5 mg group and alogliptin 25 mg group. Upper respiratory tract inflammation (11.5%), which was classified as mild and assessed as ‘not related’ to the treatment and back pain (3.8%), was also reported in the alogliptin 25 mg group; headache (3.9%) and fall (3.9%) was reported in the glimepiride monotherapy group; and gastroenteritis (3.8%) was reported in the alogliptin 12.5 mg group. Hypoglycemia was reported in three participants: one patient in the glimepiride monotherapy group and two patients in the alogliptin 25 mg group. These adverse events were mild and assessed as drug‐related. No clinically significant changes were found in clinical laboratory tests, vital signs or 12‐lead ECG findings.
Three patients (one from the alogliptin 12.5 mg group and two from the alogliptin 25 mg group) were excluded from the randomized treatment study before the open‐label extension study. A total of 150 and 152 patients, respectively, comprised the alogliptin 12.5 and 25 mg groups in the safety analysis set.
In the extension study, the incidence of adverse events was 81.3 and 88.2% in the 12.5 and 25 mg groups, respectively, and most were considered to be of mild intensity. The incidence of adverse events assessed as drug‐related in these groups was 18.0 and 17.8%; of serious adverse events 10.7 and 2.0%; and of adverse events leading to drug discontinuation 12.7 and 5.3%. Two patients receiving alogliptin 12.5 mg died during the study; one as a result of gas gangrene and one sudden death. Serious adverse events assessed as drug‐related (which included the two deaths) were cerebral infarction in one patient (alogliptin 12.5 mg), gas gangrene in one patient (alogliptin 12.5 mg), acute myocardial infarction in one patient (alogliptin 12.5 mg), sciatica and sudden death in one patient (occurred in the same patient), malignant neoplasm of renal pelvis in one patient (alogliptin 12.5 mg), and vertigo in one patient (alogliptin 25 mg).
Adverse events that occurred at an incidence of ≥3% in either group during the 52‐week treatment period comprised nasopharyngitis (30.7 and 37.5% for alogliptin 12.5 and 25 mg groups, respectively), gastroenteritis (4.7, 2.6), cystitis (3.3, 3.3), cataract (4.0, 4.6), diabetic retinopathy (2.7, 5.3), upper respiratory tract inflammation (6.7, 10.5), constipation (4.7, 4.6), gastritis (3.3, 5.3), diarrhea (3.3, 2.0), dental caries (4.0, 0.7), urticaria (1.3, 3.3), back pain (5.3, 7.2), periarthritis (4.7, 2.6), arthralgia (4.0, 2.6), osteoarthritis (4.0, 0.7) and fall (5.3%, 7.9%). Hypoglycemia was reported as an adverse event in 4 (2.7) and 8 (5.3%) patients, respectively, and was mild in all cases. The incidence of ‘skin and subcutaneous tissue disorders’ adverse events was 12.7 and 9.9%, respectively. In three patients it resulted in drug discontinuation: mild eczema in one patient (alogliptin 12.5 mg), rash in one patient (alogliptin 25 mg) and urticaria in another (alogliptin 25 mg); both of these latter cases were rated as moderate. There were no clinically relevant changes in mean values for laboratory parameters, vital signs or 12‐lead ECG during the study.
Discussion
In the present phase 2/3 multicenter, randomized, double‐blind, parallel‐group comparative study, the addition of alogliptin to continued treatment with glimepiride was associated with a statistically and clinically significant reduction in the primary end‐point, HbA1c at 12 weeks, which was maintained during a 40 week open‐label extension study. The target set for diabetic therapy by the Japanese Diabetes Society is a reduction of HbA1c levels to <6.9%5. In the present study, significantly more patients in the 12.5 and 25 mg alogliptin groups achieved HbA1c levels <7.4 and <6.9% in comparison with glimepiride monotherapy. A significant long‐term beneficial effect of alogliptin therapy was also demonstrable after 52 weeks with 22–26% of patients having HbA1c levels <7.4%.
Other markers of glycemic control including FPG, 2‐h postprandial glucose and postprandial glucose AUC0–2 h were also statistically significantly reduced in both alogliptin combination groups. These positive effects were maintained during the 40‐week extension study. Glycemic benefits were attained irrespective of age, sex and duration of disease. Furthermore, they were achieved in patients with uncontrolled type 2 diabetes, despite being highly compliant with diet/exercise therapy and glimepiride monotherapy.
A study of Japanese patients showed that alopliptin improved glycemic control as monotherapy18 and in combination with voglibose or pioglitazone in patients with uncontrolled type 2 diabetes19. International trials have reported similar efficacy findings for alogliptin given as monotherapy21, or in combination with metformin22, glyburide23, pioglitazone24 or insulin25. Alogliptin was also effective and well tolerated in elderly patients (≥65 years‐of‐age) when given as monotherapy or in combination with metformin, glyburide, pioglitazone or insulin26.
Alogliptin in combination therapy with glimepiride was well tolerated throughout the 52‐week study. In particular, alogliptin did not increase the risk of hypoglycemia compared with glimepiride monotherapy. Similar findings were previously reported with sitagliptin in Japanese patients with type 2 diabetes27. The overall incidence of adverse events was comparable between the alogliptin 12.5 and 25 mg groups.
The rationale for giving a DPP‐4 inhibitor with a low dose of sulfonylurea, both of which act as insulin secretagogues, is counter‐intuitive with the generally accepted practice of giving agents with different mechanisms of action to achieve optimal glycemic control. However, it was previously shown that sulfonylureas had little effect on incretin (GLP‐1 and GIP) secretion28, and it seems unlikely that alogliptin stimulates GLP‐1 secretion. Recently, Mukai et al.29 have shown that exendin‐4, an incretin mimetic, improves impaired glucose metabolism in diabetic pancreatic β‐cells and, in addition, it increased adenosine triphosphate (ATP) production. Therefore, improved glucose metabolism was associated with elevated ATP production, creating a physiological environment in which sulfonylureas have been shown to be more effective, as their actions are ATP dependent30. Furthermore, Zhang et al.31 reported that there was an interaction between incretin and sulfonylureas through the Epac system in pancreatic β‐cells. These interactions involving incretin mimetics and sulfonylureas might provide a rationale for combination therapy with alogliptin and a sulfonylurea, as co‐administration might provide additive effects and result in more appropriate insulin secretion.
In conclusion, add‐on therapy with alogliptin was well tolerated and improved glycemic control in Japanese patients with type 2 diabetes who were uncontrolled on glimepiride plus diet and exercise therapies. Furthermore, the benefits of combination therapy were maintained over a 1‐year period. Thus, alogliptin will be a useful treatment option for patients with type 2 diabetes currently managed with glimeperide and who become less responsive to the antihyperglycemic effects of the sulfonylurea.
Supplementary Material
Figure S1 | Glucose, insulin and glucagon responses during a meal tolerance test at baseline (week 0) and end of double‐blind treatment (week 12) in the glimepiride monotherapy and alogliptin 12.5 and 25 mg groups.
Acknowledgements
The authors thank all investigators for their dedication and support, and also the patients who participated in the study. This study, including editorial assistance provided by ContentEdNet (Spain), was supported by Takeda Pharmaceutical Company, Limited (Japan). KK has received honoraria for lectures from Takeda and has indicated no other conflicts of interest regarding the content of this article.
References
- 1.World Health Organisation . Diabetes. Fact Sheet No 312, August 2011. Available at http://www.who.int/mediacentre/factsheets/fs312/en/ (accessed on 2012, March 5).
- 2.Wild S, Roglic G, Green A, et al Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004; 27: 1047–1053 [DOI] [PubMed] [Google Scholar]
- 3.Arai K, Matoba K, Hirao K, et al Present status of sulfonylurea treatment for type 2 diabetes in Japan: second report of a cross‐sectional survey of 15,652 patients. Endocr J 2010; 57: 499–507 [DOI] [PubMed] [Google Scholar]
- 4.Turner RC, Cull CA, Frighi V, et al Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). UK Prospective Diabetes Study (UKPDS) Group. JAMA 1999; 281: 2005–2012 [DOI] [PubMed] [Google Scholar]
- 5.Japanese Diabetes Society . Treatment Guide for Diabetes. Japanese Diabetes Society, Tokyo, Japan, 2007 [Google Scholar]
- 6.Holst JJ, Vilsbøll T, Deacon CF. The incretin system and its role in type 2 diabetes mellitus. Mol Cell Endocrinol 2009; 297: 127–136 [DOI] [PubMed] [Google Scholar]
- 7.Nauck MA, Heimesaat MM, Orskov C, et al Preserved incretin activity of glucagon‐like peptide 1 [7‐36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type‐2 diabetes mellitus. J Clin Invest 1993; 91: 301–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pratley RE. Alogliptin: a new, highly selective dipeptidyl peptidase‐4 inhibitor for the treatment of type diabetes. Expert Opin Pharmacother 2009; 10: 503–512 [DOI] [PubMed] [Google Scholar]
- 9.Neumiller JJ. Differential chemistry (structure), mechanism of action, and pharmacologyof GLP‐1 receptor agonists and DPP‐4 inhibitors. J Am Pharm Assoc 2009; 49(Suppl 1): S16–S29 [DOI] [PubMed] [Google Scholar]
- 10.White J. Efficacy and safety of incretin based therapies: clinical trial data. J Am Pharm Assoc 2009; 49(Suppl 1): S30–S40 [DOI] [PubMed] [Google Scholar]
- 11.Seino Y, Fukushima M, Yabe D. GIP and GLP‐1, the two incretin hormones: similarities and differences. J Diabetes Invest 2010; 1: 8–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holst JJ. The physiology of glucagon‐like peptide 1. Physiol Rev 2007; 87: 1409–1439 [DOI] [PubMed] [Google Scholar]
- 13.Yabe D, Kuroe D, Lee S, et al Little enhancement of meal‐induced glucagon‐like peptide 1 secretion in Japanese: comparison of type 2 diabetes patients and healthy controls. J Diabetes Invest 2010; 1: 56–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seino Y, Kaku K. Efficacy and safety of alogliptin, a potent and highly selective DPP‐4 inhibitor, in Japanese patients with type 2 diabetes mellitus. European Association for the Study of Diabetes Annual Meeting 2010; 2010. A‐10‐1252‐EASD.
- 15.International Conference on Harmonisation . ICH Harmonised Tripartite Guideline. Guideline for good clinical practice, E6(R1). Current Step 4 version, dated 10 June 1996. Available at: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6_R1/Step4/E6_R1__Guideline.pdf (accessed on 2011, January 24).
- 16.Seino Y, Nanjo K, Tajima N, et al The committee of the Japan Diabetes Society on the diagnostic criteria of diabetes mellitus. Report of the committee on the classification and diagnostic criteria of diabetes mellitus. J Diabetes Invest 2010; 1: 212–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seino Y, Nanjo K, Tajima N, et al The committee of the Japan Diabetes Society on the diagnostic criteria of diabetes mellitus. Report of the committee on the classification and diagnostic criteria of diabetes mellitus. Diabetology Int 2010; 1: 2–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seino Y, Fujita T, Hiroi S, et al Efficacy and safety of alogliptin in Japanese patients with type 2 diabetes mellitus: a randomized, double‐blind, dose‐ranging comparison with placebo, followed by a long‐term extension study. Curr Med Res Opin 2011; 27: 1781–1792 [DOI] [PubMed] [Google Scholar]
- 19.Seino Y, Fujita T, Hiroi S, et al Alogliptin plus voglibose in Japanese patients with type 2 diabetes: a randomized, double‐blind, placebo‐controlled trial with an open‐label, long‐term extension. Curr Med Res Opin 2011; 27(Suppl 3): 21–29 [DOI] [PubMed] [Google Scholar]
- 20.Kaku K, Itayasu T, Hiroi S, et al Efficacy and safety of alogliptin added to pioglitazone in Japanese patients with type 2 diabetes: a randomized, double‐blind, placebo‐controlled trial with an open‐label long‐term extension study. Diabetes Obes Metab 2011; 13: 1028–1035 [DOI] [PubMed] [Google Scholar]
- 21.DeFronzo RA, Fleck PR, Wilson CA, et al Efficacy and safety of the dipeptidyl peptidase‐4 inhibitor alogliptin in patients with type 2 diabetes and inadequate glycemic control: a randomized, double‐blind, placebo‐controlled study. Diabetes Care 2008; 31: 2315–2317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nauck MA, Ellis GC, Fleck PR, et al Efficacy and safety of adding the dipeptidyl peptidase‐4 inhibitor alogliptin to metformin therapy in patients with type 2 diabetes inadequately controlled with metformin monotherapy: a multicentre, randomised, double‐blind, placebo‐controlled study. Int J Clin Pract 2009; 63: 46–55 [DOI] [PubMed] [Google Scholar]
- 23.Pratley RE, Kipnes MS, Fleck PR, et al Efficacy and safety of the dipeptidyl peptidase‐4 inhibitor alogliptin in patients with type 2 diabetes inadequately controlled by glyburide monotherapy. Diabetes Obes Metab 2009; 11: 167–176 [DOI] [PubMed] [Google Scholar]
- 24.Pratley RE, Reusch JE, Fleck PR, et al Efficacy and safety of the dipeptidyl peptidase‐4 inhibitor alogliptin added to pioglitazone in patients with type 2 diabetes: a randomized, double‐blind, placebo‐controlled study. Curr Med Res Opin 2009; 25: 2361–2371 [DOI] [PubMed] [Google Scholar]
- 25.Rosenstock J, Rendell MS, Gross JL, et al Alogliptin added to insulin therapy in patients with type 2 diabetes reduces HbA(1C) without causing weight gain or increased hypoglycaemia. Diabetes Obes Metab 2009; 11: 1145–1152 [DOI] [PubMed] [Google Scholar]
- 26.Pratley RE, McCall T, Fleck PR, et al Alogliptin use in elderly people: a pooled analysis from phase 2 and 3 studies. J Am Geriatr Soc 2009; 57: 2011–2019 [DOI] [PubMed] [Google Scholar]
- 27.Kubota A, Matsuba I, Saito T, et al Secretory units of islets in transplantation index is a useful clinical marker to evaluate the efficacy of sitagliptin in treatment of type 2 diabetes mellitus. J Diabetes Invest 2011; 2: 377–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yabe D, Watanabe K, Sugawara K, et al Comparison of incretin immunoassays with or without plasma extraction: incretin secretion in Japanese patients with type 2 diabetes. J Diabetes Invest 2012; 3: 70–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mukai E, Fujimoto S, Sato H, et al Exendin‐4 suppresses src activation and reactive oxygen species production in diabetic goto‐kakιzaki rat islets in an epac‐dependent manner. Diabetes 2010; 60: 218–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mukai E, Ishida H, Kato S, et al Metabolic inhibition impairs ATP‐sensitive K+ channel block by sulfonylurea in pancreatic β‐cells. Am J Physiol Endocrinol Metab 1998; 274: E38–E44 [DOI] [PubMed] [Google Scholar]
- 31.Zhang CL, Katoh M, Shibasaki T, et al The cAMP sensor Epac2 is a direct target of antidiabetes sulfonylurea drugs. Science 2009; 325: 607–610 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 | Glucose, insulin and glucagon responses during a meal tolerance test at baseline (week 0) and end of double‐blind treatment (week 12) in the glimepiride monotherapy and alogliptin 12.5 and 25 mg groups.


