Abstract
Aims/Introduction
The present study was undertaken to determine vascular endothelial impairment and endothelial progenitor cells (EPCs) in patients with type 2 diabetes mellitus and erectile dysfunction (ED).
Materials and Methods
A total of 100 type 2 diabetic men were enrolled. Flow‐mediated dilatation (FMD) and anaerobic threshold (AT) were measured. Also, EPCs in the peripheral blood were determined by flow cytometry.
Results
In the 42 ED diabetic patients, FMD and AT were significantly less than those in the 58 patients with normal erectile function (FMD 2.84 vs 3.82%, P = 0.038, and AT 11.2 vs 12.7 mL/kg/min, P = 0.022). Exercise tolerance significantly increased the number of EPCs in the patients with and without ED (49–60 cells/100 μL, P = 0.015, and 72–99 cells/100 μL, P = 0.003). In the diabetic patients without autonomic neuropathy, FMD was significantly reduced in the patients with ED than those without ED (P = 0.015). In response to exercise tolerance, the number of EPCs increased in both the diabetic patients with ED (P = 0.003) and without ED (P = 0.007). In contrast, in the diabetic patients with autonomic neuropathy, there was no difference in FMD between the patients with and without ED. The exercise tolerance increased the number of EPCs in the patients without ED (P = 0.023), but it disappeared in those with ED.
Conclusions
ED diabetic patients have endothelial impairment during the early period of diabetic complications, whose deranged endothelial function is concomitantly repaired by promoting bone marrow‐derived EPCs.
Keywords: Diabetes mellitus, Endothelial progenitor cells, Erectile dysfunction
Introduction
Cardiovascular disorders are major prognostic determinants for diabetic patients, because cardiovascular death occupies approximately 50% of mortality in diabetic patients. Atherosclerotic events are not always related to the duration of diabetes mellitus and progression of diabetic microangiopathic complications. Recent studies have shown that inflammatory changes in the vascular wall are involved in developing atherosclerosis. Damaged endothelial cells release cytokines and growth factors, and macrophages and adhesion molecules accumulate into subendothelial space of the injured region, promoting the atherogenic process1. There is a high prevalence of ischemic heart diseases in patients with erectile dysfunction (ED) as compared with those with normal erectile function2. It is not clear whether atherosclerotic disorders are profoundly accelerated in diabetic patients with ED. Thus, it is of value to evaluate the prevalence and progression of endothelial dysfunction and ED in patients with diabetes mellitus.
Bone marrow‐derived endothelial progenitor cells (EPC) can have beneficial effects on angiogenesis and vascular repair4. In ischemic heart diseases, EPC increased transiently in systemic circulation8. Also, transplanted bone marrow‐derived CD34+/133+ stem cells promote contraction of the left ventricle in animal models and patients with acute myocardial infarction9. Endothelial impairment is classified into an initial stage of vascular derangement. It is little known that induction of bone marrow‐derived EPC occurs in such an early stage of endothelial damage. Because exercise training can increase the number of circulating EPC in angina pectoris and acute myocardial infarction11, it is interesting to examine endothelial repair by EPC.
In the present study, we determined the relationship between endothelial dysfunction and ED in patients with type 2 diabetes mellitus. In addition, whether mobilization of EPC in systemic circulation occurs in diabetic patients with endothelial impairment was examined.
Materials and Methods
Patients
A total of 100 patients with type 2 diabetes mellitus were enrolled in the present study between May 2006 and February 2008. They were collected from the outpatient clinic of Jichi Medical University Saitama Medical Center in Saitama, Japan. They were all the male patients aged 62.8 ± 11.1 years (mean ± SD) ranging from 26 to 80 years. Type 2 diabetes mellitus was diagnosed by the Japan Diabetes Society criteria. Hemoglobin A1c (HbA1c; National Glycohemoglobin Standardization Program [NGSP]) was 7.8 ± 1.6%, and the duration of diabetes mellitus was 13.5 ± 8.2 years. A total of 45 patients had hypertension, 26 had dyslipdemia and 36 had obesity. A total of 48 patients were current smokers. A total of 42 patients had erectile dysfunction (ED). We excluded the following patients: (i) those receiving hemodialysis treatment, (ii) those taking maintenance medication of nitroglycerin; (iii) those with infectious diseases; (iv) those with a malignancy; and (v) those with a past history of intrapelvic system surgery. Blood samples were collected from the patients in the sitting position after an overnight fast to determine hemoglobin A1c (HbA1c), serum total cholesterol, high‐density lipoprotein (HDL) cholesterol, low‐density lipoprotein (LDL) cholesterol, triglyceride and creatinine at the time of visiting the outpatient clinic. Bone marrow‐derived CD34+/CD133+ cells in the peripheral blood were collected from the patients in the sitting position before and after a cardiopulmonary exercise stress test (CPX). Risk factors for atherosclerosis were defined as follows: hypertension was defined as systolic blood pressure of greater than 140 mmHg, diastolic blood pressure of greater than 90 mmHg, or the patient having taken antihypertentive agents. Dyslipidemia was defined as a total cholesterol level of greater than 220 mg/dL, a high‐density lipoprotein cholesterol level of less than 40 mg/dL and a triglyceride level of greater than 150 mg/dL, or the patient having taken either statins or fibrates. Obesity was defined as body mass index of greater than 25. A current smoker was defined as a patient who smoked more than one cigarette per day within 3 months. The patients who had erectile dysfunction were defined by the questionnaire survey, which included symptoms of diabetic neuropathy and erectile dysfunction. We examined the international index of erectile function (IIEF‐5; fifth version,) in the present patients13. The patients who had erectile dysfunction were defined as an IIEF‐5 score of less than 21 points. According to the questionnaire, 58 patients had normal erectile function, and 42 had ED. Diabetic retinopathy was diagnosed by an ophthalmologist. Diabetic nephropathy was defined as micro‐ and/or macroalbuminuria, or an estimated glomerular filtration rate (eGFR) level of less than 60 mL/min. According to the progression of nephropathy, the diabetic patients were divided into stages 1–5 based on the classification of diabetic nephropathy by the Research Committee of the Japanese Ministry of Health, Labor and Welfare for Disorders of diabetes mellitus14. Diabetic autonomic neuropathy was determined by composite parameter of the coefficient of variation of the R‐R interval (CVRR), eGFR and retinopathy. Namely, the diabetic patients who had a CVRR of less than 2%, and those who had a CVRR of greater than 2% in combination with an eGFR of less than 50 mL/min/1.73 m2 and the presence of retinopathy, resulting in a group of diabetic autonomic neuropathy. The numbers of patients taking medication for diabetes mellitus, hypertension and dyslipidemia are summarized in Table 1. All the patients had endothelial function tests of flow‐mediated dilatation (FMD) and nitroglycerin‐mediated dilatation (NMD) carried out. The patients carried out the CPX, and the number of CD34+/133+ cells before and after the CPX were measured. The present study was approved by the ethical committee of Jichi Medical University for Human Studies. We obtained informed consent from the patients who joined the present protocol.
Table 1. Baseline clinical and laboratory findings in the diabetic patients (all male patients.
| Non‐ED patients (n = 58) | ED patients (n = 42) | P‐value | |
|---|---|---|---|
| Age (years) | 61.8 ± 13.6 | 64.3 ± 8.0 | 0.26 |
| Height (cm) | 165.1 ± 6.3 | 166.5 ± 4.7 | 0.2 |
| Weight (kg) | 69.5 ± 15.1 | 67.3 ± 11.3 | 0.424 |
| BMI | 25.3 ± 4.2 | 24.2 ± 3.7 | 0.137 |
| HbA1c (%) | 7.9 ± 1.9 | 7.7 ± 1.2 | 0.56 |
| Duration of diabetes mellitus (years) | 12.5 ± 7.4 | 14.8 ± 8.4 | 0.204 |
| Systolic blood pressure (mmHg) | 134.3 ± 20.7 | 130.0 ± 16.1 | 0.253 |
| Diastolic blood pressure (mmHg) | 75.4 ± 9.0 | 73.8 ± 10.2 | 0.466 |
| Total cholesterol (mg/dL) | 188.8 ± 33.6 | 196.2 ± 40.0 | 0.32 |
| HDL cholesterol (mg/dL) | 50.0 ± 13.7 | 48.3 ± 13.5 | 0.533 |
| LDL cholesterol (mg/dL) | 113.9 ± 31.1 | 118.2 ± 34.3 | 0.511 |
| Triglyceride (mg/dL) | 123.7 ± 65.2 | 140.8 ± 60.1 | 0.184 |
| Serum creatinine (mg/dL) | 0.84 ± 0.22 | 0.83 ± 0.18 | 0.718 |
| Estimated GFR (mL/min/1.73 m2) | 77.9 ± 21.9 | 76.4 ± 19.1 | 0.723 |
| Clinical stages of diabetic nephropathy (1/2/3A/3B/4) (n) | 34/15/6/0/3 | 18/9/9/1/5 | 0.19 |
| CVRR (%) | 2.62 ± 1.77 | 2.63 ± 1.57 | 0.98 |
| Diabetic retinopaty (±) (n) | 46/12 | 24/18 | 0.017 |
| Smoking, n (%) | 25 (43.1%) | 25 (59.5%) | 0.198 |
| Treatment for diabetes mellitus, n (%) | |||
| Diet only | 4 (6.9) | 2 (4.8) | 0.592 |
| Sulfonylurea (gliclazide/glibenclamide/glimepride) | 3/2/32 (5.2/3.4/55.2) | 2/3/24 (4.8/7.1/57.1) | 0.908 |
| Biganide | 23 (39.7) | 16 (38.1) | 0.875 |
| Thiazolidinedione | 7 (12.1) | 7 (13.5) | 0.513 |
| α‐Glucosidase inhibitor | 12 (20.7) | 14 (33.3) | 0.155 |
| Insulin | 19 (32.8) | 13 (31.0) | 0.848 |
| Other current medication, n (%) | |||
| ACEI | 10 (17.2) | 9 (21.4) | 0.598 |
| ARB | 21 (36.2) | 15 (35.7) | 0.96 |
| Calcium channel blockers | 13 (22.4) | 14 (33.3) | 0.225 |
| Diuretics | 1 (1.7) | 1 (2.4) | 0.817 |
| Aspirin | 8 (13.8) | 8 (19.0) | 0.392 |
| Ticlopidine | 2 (3.4) | 3 (7.1) | 0.393 |
| Statins | 20 (34.5) | 10 (23.8) | 0.25 |
| Macroangiopathies, n (%) | |||
| None | 45 (77.6) | 31 (73.8) | 0.663 |
| Ischemic heart disease | 4 (6.9) | 2 (4.8) | 0.657 |
| Cerebral vascular disease | 8 (13.8) | 6 (14.2) | 0.944 |
| Arteriosclerosis obliterans | 1 (1.7) | 3 (7.1) | 0.172 |
Values are mean ± SD. Values are analyzed by Student's t‐test or χ2 for independence test. ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BMI, body mass index; CVRR, the coefficient of variation of the R‐R interval; GFR, glomerular filtration rate; HbA1c, hemoglobin A1c; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein.
Measurements
Physical examinations included FMD, CPX and CVRR. Biochemical examinations included HbA1c, serum total cholesterol, HDL cholesterol, LDL cholesterol, triglyceride, blood urea nitrogen, creatinine, adiponectin, retinol‐binding protein (RBP) 4, vascular endothelial growth factor (VEGF), placental growth factor (PlGF), C‐reactive protein (CRP), CD146 and high mobility group box‐1 (HMGB‐1). Also, the number of CD34+/133+ stem cells was determined.
Blood Samples and Assays
Blood samples were collected into tubes and centrifuged at 2000 g at 4°C for 15 min. The supernatants were decanted and frozen at −80°C until assayed. The value for HbA1c (%) was estimated as an National Glycohemoglobin Standardization Program (NGSP) equivalent value (%) calculated by the formula HbA1c (%) = HbA1c (Japan Diabetes Society [JDS]) (%) + 0.4%, considering the relational expression of HbA1c (JDS) (%) measured by the previous Japanese standard substance and measurement methods and HbA1c (NGSP)15. Serum RBP4 was measured by the methods of ELISA using Human RBP4 ELISA kits (AdipoGen, Seoul, Korea). Serum adiponectin was measured using Human adiponectin ELISA kits (Otsuka Pharmaceutical Co., Tokyo, Japan). Serum VEGF was measured using human VEGF ELISA system (GE Healthcare, Buckinghamshire, UK). Serum PlGF was measured using Quantikine® Human PlGF Immunoassay (R&D Systems, Minneapolis, MN, USA). Serum HMGB‐1 was measured using HMGB1 ELISA kit II (SHINO‐TEST Corporation, Kanagawa, Japan). Serum CD146 was measured using CY‐QUANT™ ELISA sCD146 (Biocytex, Marseille, France). Urine samples were collected in the morning at the outpatient clinic. Urinary excretion of albumin was determined by latex agglutination immunoassay (Eiken, Tokyo, Japan). Renal function was calculated as the eGFR by the Modification of Diet in Renal Disease equation (MDRD) revised for Japanese by the Japan Society of Nephrology16. The measurement of the number of endothelial progenitor cells (EPCs) were defined as CD34+/133+ cells by flow cytometry. Trained technicians of Special Reference Laboratories (SRL; Tokyo, Japan), blind to the patients' information, measured the number of CD34+/45+/133+ cells17, using fluorescence‐activated cell sorting (FACS) (FACS Calibur™; BD Biosciences, San Jose, CA, USA) with the following antibodies: anti‐CD34 FITC (Beckman Coulter Inc., Marseille, France), anti‐CD45‐PerCP (BD Biosciences) and anti‐CD133/2 (293C3)‐PE (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany). CD34+ cells were analyzed using sequential gating strategies. CD45+ cells were gated on a forward scatter vs side scatter to confirm mononuclear cell fraction. At least 2,000,000 events were measured in the CD45+ gate. Data were analyzed using CELLQuest (BD Biosciences). After gating CD45+ from whole blood, we measured the number of cells that were double‐positive for CD34 and CD133.
Flow‐Mediated Dilatation
Endothelial function was evaluated by flow‐mediated dilatation (FMD). FMD is an arterial response to reactive hyperemia, causing endothelial‐dependent dilatation18. According to the procedure previously described, FMD of the right brachial artery was determined by one investigator, the first author, between 16:00 hours and 18:00 hours after 15 min rest using 15‐MHz ultrasound equipment (SONOS 5500; Philips Medical Systems, Best, the Netherlands). The brachial artery in the longitudinal section just above the antecubital fossa was imaged, and internal diameters from anterior to posterior intimal interfaces were measured at the end‐diastole using B‐mode imaging as baseline. The diameter was determined by the mean value of three points on the ultrasound screen. Doppler measurement provided the waveforms of intravascular blood flow. Computer software installed in the ultrasound apparatus traced the waveforms automatically and calculated mean flow velocities. A pneumatic cuff was inflated on the forearm at a pressure of over 50 mmHg higher than systolic blood pressure for 5 min. The intravascular blood flow velocities and vessel diameter were measured at 20 and 60 s after cuff deflation, respectively, the same way as that at baseline. FMD was calculated as the percent increase in vessel diameter after reactive hyperemia ([maximum diameter after reactive hyperemia–baseline diameter]/baseline diameter × 100). Nitroglycerin‐mediated dilatation (NMD), which is endothelium‐independent dilatation, was carried out 15 min after FMD examination by using sublingual nitroglycerin 0.3‐mg spray. The basal vessel diameter was measured in the same manner noted above. The maximum vessel diameter by nitroglycerin was measured 4 min after the administration, and then NMD was calculated.
Exercise Capacity
Cardiopulmonary exercise stress test was carried out between 14:00 and 15:00 hours to measure oxygen consumption at the anaerobic threshold (AT) and peak oxygen consumption (peak VO2) using an electronically braked cycle ergometer (Ergometer 232CR; Minato Medical Science, Osaka, Japan) at a constant rate of 60 r.p.m. The mean exercise time was approximately 8 min. During the exercise test, blood pressure and 12‐lead electrocardiogram (ECG) were monitored. The work rate was increased using a 15‐W/min ramp until leg fatigue, shortness of breath, significant ST depression or maximum heart rate (220 b.p.m. – age) arrival. Expired air gas was analyzed continuously using a metabolic cart (AE‐300SR; Minato Medical Science). The anaerobic threshold was determined by the V‐slope method by the starting point of the non‐linear increase in carbon dioxide output20.
Heart Rate Variability (the Coefficient of Variation of the R‐R Interval)
After 15 min rest in the supine position, 12‐lead ECG was recorded over 100 beats at rest, and the CVRR was analyzed automatically (Cardiofax V; Nihon Kohden, Tokyo, Japan). CVRR was determined by dividing the standard deviation of all R‐R intervals (SD) by the mean R‐R interval (M) for the ECG recording. CVRR (%) = (SD/M) × 100.
Statistical Analysis
All values are expressed as mean ± SEM. The values were analyzed by Student's t‐test to compare the differences between the groups. Corresponding data were analyzed by paired t‐test. Categorical data were analyzed by χ2 for independence test. The statistical package of Statcel Statistical Software (Second Edition; OMS Publishing, Inc., Saitama, Japan) was used for the present analysis. A P‐value <0.05 was considered significant.
Results
Table 1 shows the clinical and laboratory characteristics of the diabetic patients in the presence and absence of ED. According to the questionnaire, 58 patients had normal erectile function and 42 had ED. Except for diabetic retinopathy, there were no differences in any parameter between the two groups of patients.
Physical and biochemical examinations are shown in Table 2. FMD was 2.84 ± 0.34% in the diabetic patients with ED, a value significantly less than that of 3.82 ± 0.39% in those without ED (P = 0.038). However, NMD remained normal, and there was no difference in NMD between the patients with and without ED. Oxygen consumption at AT of 11.2 ± 0.4 mL/kg/min in the diabetic patients with ED was significantly lower than that of 12.7 ± 0.5 mL/kg/min in those without ED (P = 0.022). Basal levels of CD34+/133+ cells were comparable in both the diabetic patients with and without ED, but the exercise load significantly augmented CD34+/133+ cells in both groups (P = 0.0003 and P = 0.015). Biochemical studies showed that there were no differences in all the variables, including serum RBP4, adiponectin, VEGF, PlGF, CRP, HMGB1 and CD146 between the two groups of patients.
Table 2. Physical and biochemical studies in the diabetic patients.
| Non‐ED patients | ED patients | P‐value | |
|---|---|---|---|
| FMD (%) | 3.82 ± 0.39 | 2.84 ± 0.34 | 0.038 |
| NMD (%) | 13.8 ± 0.79 | 12.2 ± 0.83 | 0.175 |
| n | 58 | 42 | |
| CPX | |||
| VO2 at AT (mL/kg/min) | 12.7 ± 0.5 | 11.2 ± 0.4 | 0.022 |
| Peak VO2 (mL/kg/min) | 21.4 ± 0.8 | 19.4 ± 0.9 | 0.055 |
| n | 27 | 33 | |
| EPCs (cells/100 mL) | |||
| Before CPX | 72 ± 12 | 49 ± 6 | 0.119 |
| After CPX | 99 ± 18** | 60 ± 8* | |
| n | 23 | 22 | |
| Serum RBP4 (µg/mL) | 53.2 ± 4.1 | 63.0 ± 6.1 | 0.178 |
| Serum Adiponectin (µg/mL) | 9.8 ± 1.5 | 8.8 ± 1.0 | 0.595 |
| Serum VEGF (pg/mL) | 245 ± 21 | 275 ± 32 | 0.422 |
| Serum PlGF (pg/mL) | 10.2 ± 0.7 | 10.7 ± 0.5 | 0.608 |
| Serum hs‐CRP (mg/L) | 1.37 ± 0.35 | 2.25 ± 0.78 | 0.279 |
| Serum HMGB1 (pg/mL) | 3.8 ± 0.1 | 3.7 ± 0.2 | 0.791 |
| Serum CD146 (ng/mL) | 585 ± 23 | 553 ± 23 | 0.331 |
| n | 38 | 35 | |
Values are mean ± SEM. Values are analyzed by Student's t‐test or paired t‐test. *P < 0.05, ** P < 0.001 vs the value before cardiopulmonary exercise stress test (CPX). AT, anaerobic threshold; EPCs, endothelial progenitor cells; FMD, flow‐mediated dilatation; HMGB1, high mobility group box 1; hs‐CRP, high sensitive C‐reactive protein; NMD, nitroglycerin‐mediated dilatation; PlGF, placental growth factor; RBP4, retinol‐binding protein 4; VEGF, vascular endothelial growth factor; VO2, oxygen consumption.
We divided the diabetic patients according to the development of autonomic neuropathy. Physical and biochemical studies are shown in Table 3 and Figure 1. In the diabetic patients without autonomic neuropathy, FMD was significantly reduced in the diabetic patients with ED than those without ED (2.43 ± 0.38% vs 3.92 ± 0.41%, P = 0.015). There was not any difference in oxygen consumption at AT and peak VO2 between the diabetic patients with and without ED in the exercise tolerance study. In response to exercise load, the numbers of CD34+/133+ cells significantly increased from 56 ± 10 to 72 ± 15 cells/100 μL in the diabetic patients with ED (P = 0.012) and from 92 ± 21 to 125 ± 29 cells/100 μL in those without ED (P = 0.007; Figure 1). There was not any difference in serum RBP4, adiponectin, VEGF, PlGF, CRP, HMGB1 and CD146 between the presence and absence of ED in the diabetic patients. In contrast, in the diabetic patients with autonomic neuropathy, there was no difference in FMD and NMD between the diabetic patients with and without ED. The exercise tolerance study showed that oxygen consumption at AT and peak VO2 was significantly less in the diabetic patients with ED than those without ED (P = 0.026 and P = 0.036, respectively). In both the patients with and without ED, the numbers of CD34+/133+ cells before and after the exercise load seemed likely to be comparable levels. The exercise tolerance significantly increased the numbers of CD34+/133+ cells in the patients without ED (P = 0.023), but it disappeared in those with ED. Otherwise, there was not any difference in serum RBP4, adiponectin, VEGF, PlGF, CRP, HMGB1 and CD146 between the diabetic patients in the presence and absence of ED.
Table 3. Physical and biochemical studies in diabetic patients according to the development of autonomic neuropathy.
| No autonomic neuropathy | Autonomic neuropathy† | |||||
|---|---|---|---|---|---|---|
| Non‐ED patients | ED patients | P‐value | Non‐ED patients | ED patients | P‐value | |
| FMD (%) | 3.92 ± 0.41 | 2.43 ± 0.38 | 0.015 | 3.65 ± 0.50 | 2.26 ± 0.50 | 0.065 |
| NMD (%) | 13.1 ± 1.1 | 11.2 ± 1.2 | 0.251 | 13.7 ± 1.2 | 13.3 ± 1.3 | 0.816 |
| n | 29 | 19 | 24 | 16 | ||
| CPX | ||||||
| VO2 at AT (mL/kg/min) | 12.2 ± 0.6 | 11.5 ± 0.5 | 0.385 | 13.1 ± 0.9 | 10.7 ± 0.6 | 0.026 |
| Peak VO2 (mL/kg/min) | 20.2 ± 0.8 | 20.2 ± 0.9 | 0.922 | 22.6 ± 1.7 | 18.3 ± 1.5 | 0.036 |
| n | 16 | 16 | 10 | 16 | ||
| EPCs (cells/100 μl) | ||||||
| before‐CPX | 92 ± 21 | 56 ± 10 | 0.151 | 45 ± 9 | 47 ± 7 | 0.872 |
| after‐CPX | 125 ± 29† | 72 ± 15† | 60 ± 12* | 55 ± 9 | ||
| n | 12 | 11 | 10 | 12 | ||
| Serum RBP4 (µg/mL) | 52.7 ± 4.5 | 61.5 ± 7.7 | 0.337 | 53.6 ± 6.8 | 62.1 ± 8.8 | 0.446 |
| Serum Adiponectin (µg/mL) | 7.7 ± 1.1 | 7.3 ± 0.7 | 0.721 | 11.3 ± 2.5 | 10.8 ± 1.7 | 0.877 |
| Serum VEGF (pg/mL) | 216 ± 27 | 250 ± 43 | 0.497 | 264 ± 29 | 298 ± 45 | 0.513 |
| Serum PlGF (pg/mL) | 11.0 ± 1.1 | 10.8 ± 0.7 | 0.827 | 9.5 ± 0.8 | 10.2 ± 0.7 | 0.515 |
| Serum hs‐CRP (mg/L) | 1.13 ± 0.18 | 1.09 ± 0.18 | 0.872 | 1.61 ± 0.69 | 3.65 ± 1.66 | 0.218 |
| Serum HMGB1 (pg/mL) | 3.7 ± 0.2 | 3.6 ± 0.3 | 0.6 | 3.9 ± 0.2 | 3.9 ± 0.4 | 0.959 |
| Serum CD146 (ng/mL) | 569 ± 25 | 545 ± 31 | 0.553 | 603 ± 35 | 572 ± 31 | 0.528 |
| n | 17 | 17 | 19 | 16 | ||
Values are mean ± SEM. Values are analyzed by Student's t‐test or paired t‐test. *P < 0.05 vs the value before cardio‐pulmonary exercise stress test (CPX). †Diabetic autonomic neuropathy was determined by composite parameters of coefficient of variation of the R‐R interval (CVRR), estimated glomerular filtration rate and the presence of retinopathy. AT, anaerobic threshold; EPC, endothelial progenitor cells; FMD, flow‐mediated dilatation; HMGB1, high mobility group box 1; hs‐CRP, high sensitive C‐reactive protein; NMD, nitroglycerin‐mediated dilatation; PlGF, placental growth factor; RBP4, retinol‐binding protein 4; VEGF, vascular endothelial growth factor; VO2, oxygen consumption.
Figure 1.
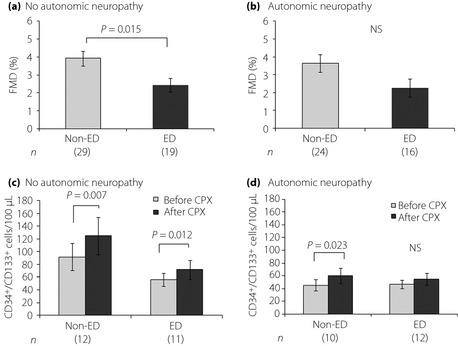
(a,b) Flow‐mediated dilatation (FMD) in the right brachial artery in diabetic patients with and without erectile dysfunction (ED). The patients were subgrouped into two groups by progression of autonomic neuropathy. (c,d) Alterations in bone marrow‐derived endothelial progenitor cells before and after the cardiopulmonary exercise stress test in diabetic patients with and without ED. Values are means ± SEM. The numbers of patients are shown in brackets.
Similarly, Table 4 shows physical and biochemical studies in association with the progression of diabetic nephropathy. In the patients whose eGFR was greater than 60 mL/min/1.73 m2, the ED patients had lower FMD than the non‐ED patients (2.82 ± 0.39% vs 3.94 ± 0.37%, P = 0.044). After exercise tolerance, the numbers of CD34+/133+ cells significantly increased in both the patients with ED (51 ± 7 to 63 ± 10 cells/100 μL, P = 0.003) and without ED (84 ± 14 to 117 ± 20 cells/100 μL, P = 0.0003). In contrast, in the patients who had eGFR less than 60 mL/min/1.73 m2, there was no difference in FMD and oxygen consumption at AT and peak VO2 between the presence and absence of ED. The exercise load also did not increase the numbers of CD34+/133+ cells in the patients with and without ED. However, a limitation of the study was the small number of patients with nephropathy in relation to the analysis of CD34+/133+ cells. Also, there were no differences in serum adiponectin, RBP4, VEGF, CRP, HMGB‐1 and CD146 between the two groups with and without ED.
Table 4. Physical and biochemical studies in diabetic patients in association with the progression of diabetic nephropathy.
| eGFR ≥ 60 mL/min/1.73 m2 | eGFR < 60 mL/min/1.73 m2 | |||||
|---|---|---|---|---|---|---|
| Non‐ED patients | ED patients | P‐value | Non‐ED patients | ED patients | P‐value | |
| FMD (%) | 3.94 ± 0.37 | 2.82 ± 0.39 | 0.044 | 3.49 ± 0.55 | 2.82 ± 0.56 | 0.458 |
| NMD (%) | 14.2 ± 0.9 | 12.6 ± 1.0 | 0.235 | 12.7 ± 1.4 | 10.8 ± 2.0 | 0.431 |
| n | 42 | 33 | 16 | 9 | ||
| CPX | ||||||
| VO2 at AT (mL/kg/min) | 12.8 ± 0.5 | 11.5 ± 0.5 | 0.065 | 12.7 ± 1.5 | 10.2 ± 0.6 | 0.106 |
| Peak VO2 (mL/kg/min) | 21.6 ± 1.0 | 19.4 ± 1.1 | 0.159 | 20.7 ± 1.8 | 19.2 ± 1.3 | 0.517 |
| n | 20 | 22 | 6 | 9 | ||
| EPCs (cells/100 μL) | ||||||
| Before CPX | 84 ± 14 | 51 ± 7 | 0.049 | 28 ± 11 | 43 ± 6 | 0.292 |
| After CPX | 117 ± 20** | 63 ± 10* | 33 ± 10 | 46 ± 10 | ||
| n | 18 | 18 | 5 | 4 | ||
| Serum RBP4 (µg/mL) | 46.5 ± 3.6 | 51.5 ± 4.6 | 0.399 | 64.8 ± 8.7 | 93.1 ± 14.0 | 0.085 |
| Serum Adiponectin (µg/mL) | 8.3 ± 1.3 | 8.1 ± 1.0 | 0.893 | 12.3 ± 3.5 | 10.5 ± 2.4 | 0.714 |
| Serum VEGF (pg/mL) | 255 ± 22 | 264 ± 33 | 0.829 | 222 ± 48 | 308 ± 80 | 0.343 |
| Serum PlGF (pg/mL) | 10.8 ± 1.0 | 10.2 ± 0.5 | 0.635 | 9.1 ± 0.6 | 11.9 ± 0.8 | 0.009 |
| Serum hs‐CRP (mg/L) | 1.54 ± 0.52 | 2.17 ± 1.00 | 0.571 | 1.05 ± 0.23 | 2.47 ± 0.98 | 0.097 |
| Serum HMGB1 (pg/mL) | 3.8 ± 0.2 | 3.9 ± 0.3 | 0.797 | 3.8 ± 0.2 | 3.3 ± 0.3 | 0.151 |
| Serum CD146 (ng/mL) | 568 ± 31 | 523 ± 27 | 0.282 | 615 ± 31 | 633 ± 29 | 0.699 |
| n | 26 | 26 | 12 | 9 | ||
Values are mean ± SEM. Values are analyzed by Student's t‐test or paired t‐test. *P < 0.05, **P < 0.001 vs the value before cardiopulmonary exercise stress test (CPX). AT, anaerobic threshold; CPX, cardio‐pulmonary exercise stress test; eGFR, estimated glomerular filtration rate; EPC, endothelial progenitor cells; FMD, flow‐mediated dilatation; hs‐CRP, high sensitive C‐reactive protein; HMGB1, high mobility group box 1; NMD, nitroglycerin‐mediated dilatation; PlGF, placental growth factor; RBP4, retinol‐binding protein 4; VEGF, vascular endothelial growth factor; VO2, oxygen consumption.
Discussion
The present study showed the decreases in FMD and oxygen consumption at AT in type 2 diabetic patients with ED as compared with those who had normal erectile function. In particular, vascular endothelial dysfunction was obvious in the diabetic patients with ED whose diabetic autonomic neuropathy and nephropathy were minimal. There is a close association of endothelial dysfunction with ED in diabetic patients with minimal chronic complications. We further analyzed the past history of cardiovascular diseases in these patients, and did not find any high prevalence of ischemic heart disease and cerebral infarction in the ED diabetic patients, even if the number of patients was small. However, recent studies have reported the relationship of ED with cardiovascular diseases. Chiurlia et al.2 showed that coronary atherosclerosis is severe in patients with ED. A mass study showed that ED was associated with a hazard ratio of 1.25 for subsequent cardiovascular events during the 5‐year follow up in 2420 patients with ED3. We collected the diabetic patients with ED in the present study, and showed endothelial impairment preceded atherosclerotic disorders in the ED diabetic patients with modest chronic complications. As aforementioned, 65% of the ED patients had 5–7 points in the IIEF‐5 score, thus resulting in advanced impairment of erectile function. There was no correlation between IIEF‐5 score and FMD (data not shown). These findings might implicate that endothelial dysfunction links to future development of atherosclerotic disorders in the diabetic patients with ED. There was no difference in average ages between the ED and non‐ED diabetic patients. We suspected that several factors, including severity of vascular endothelial dysfunction and autonomic neuropathy and others, as well as aging, could be involved in diabetic patients developing ED.
We further analyzed EPC characterized by CD34+/133+ mononuclear cells in the systemic circulation of patients with diabetes mellitus. Bone marrow‐derived EPC can have beneficial effects on vascular repair and angiogenesis, and inhibit atherosclerotic process4. In the basal condition, there was no difference in the numbers of CD34+/133+ cells between the diabetic patients in the presence and absence of ED. After exercise tolerance, the numbers of CD34+/133+ cells significantly increased in the ED diabetic patients having no autonomic neuropathy or having eGFR more than 60 mL/min, and the study might suggest that bone marrow‐derived stem cells protect from further development of endothelial impairment. However, bone marrow‐derived stem cells no longer altered endothelial dysfunction in the ED diabetic patients who had advanced microangiopathy. Several reports showed that physical exercise induces mobilization of EPCs from bone marrow7. The mechanism for exercise‐promoted EPCs is not evident, but an undetermined factor might be derived during the exercise load, and promote bone marrow to derive stem cells (EPCs). When the patients have advanced microangiopathy, this activation might disappear. Taken together, the present study might indicate that endothelial dysfunction could occur with manifestation of ED, and that also endothelial repair concomitantly proceeds by promoting bone marrow‐derived EPC during the relatively early period of diabetic complications.
We measured several adipokines, growth factors and inflammatory cytokines in patients with diabetes mellitus. There was no alteration in the synthesis of endothelial growth factors under chronically developed impairment of the endothelium. No change in plasma VEGF and PlGF levels was dissociated from the findings of diminished dilating capability of the endothelium and the induction of EPC. As noted earlier, in this situation, bone marrow‐derived EPC promptly promoted to repair the endothelium itself and improve endothelial function in the ED diabetic patients as well as in the non‐ED diabetic patients. Endothelial derangement had a different progression among the physiological and biochemical properties in the diabetic patients. Alteration in serum RBP4 and adiponectin, which are derived from adipose tissues and partly the liver, are closely associated with diabetes mellitus and atherosclerotic disorders24. In the present study, there was no change in serum RBP4 and adiponectin levels in the ED and non‐ED diabetic patients who had either autonomic neuropathy or not. The present study noted that the ED patients had no increment in atherosclerotic diseases. Endothelial impairment should reside in the preclinical state of atherosclerosis in ED diabetic patients, and might not affect serum levels of adipokines.
In conclusion, the present study showed reduced FMD and anaerobic threshold after exercise in ED diabetic patients who had no or modest autonomic neuropathy and/or nephropathy. Also, the exercise‐induced increase in EPC was found in ED diabetic patients, a finding similar to those without ED. In contrast, in advanced diabetic microangiopathy, there was a reduction in FMD and exercise‐induced EPC in the ED diabetic patients. These findings might indicate that ED diabetic patients have vascular endothelial impairment during the early period of diabetic complications, who concomitantly repair endothelial function by promoting bone marrow‐derived EPC. Furthermore, endothelial derangement has different progression between the physiological and biochemical properties in diabetic patients.
Acknowledgement
The authors have nothing to disclose.
References
- 1.Ross R. Atherosclerosis – an inflammatory disease. N Engl J Med 1999; 340: 115–126 [DOI] [PubMed] [Google Scholar]
- 2.Chiurlia E, D'Amico R, Ratti C, et al Subclinical coronary artery atherosclerosis in patients with erectile dysfunction. J Am Coll Cardiol 2005; 46: 1503–1506 [DOI] [PubMed] [Google Scholar]
- 3.Thompson IM, Tangen CM, Goodman PJ, et al Erectile dysfunction and subsequent cardiovascular disease. JAMA 2005; 294: 2996–3002 [DOI] [PubMed] [Google Scholar]
- 4.Asahara T, Murohara T, Sullivan A, et al Isolation of putative progenitor endothelial cells for angiogenesis. Science 1997; 275: 964–967 [DOI] [PubMed] [Google Scholar]
- 5.Asahara T, Masuda H, Takahashi T, et al Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res 1999; 85: 221–228 [DOI] [PubMed] [Google Scholar]
- 6.Asahara T, Takahashi T, Masuda H, et al VEGF contributes to postnatal neovascularization by mobilizing bone marrow‐derived endothelial progenitor cells. EMBO J 1999; 18: 3964–3972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laufs U, Werner N, Link A, et al Physical training increases endothelial progenitor cells, inhibits neointima formation, and enhances angiogenesis. Circulation 2004; 109: 220–226 [DOI] [PubMed] [Google Scholar]
- 8.Shintani S, Murohara T, Ikeda H, et al Mobilization of endothelial progenitor cells in patients with acute myocardial infarction. Circulation 2001; 103: 2776–2779 [DOI] [PubMed] [Google Scholar]
- 9.Kawamoto A, Gwon HC, Iwaguro H, et al Therapeutic potential of ex vivo expanded endothelial progenitor cells for myocardial ischemia. Circulation 2001; 103: 634–637 [DOI] [PubMed] [Google Scholar]
- 10.Assmus B, Schächinger V, Teupe C, et al Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction (TOPCARE‐AMI). Circulation 2002; 106: 3009–3017 [DOI] [PubMed] [Google Scholar]
- 11.Steiner S, Niessner A, Ziegler S, et al Endurance training increases the number of endothelial progenitor cells in patients with cardiovascular risk and coronary artery disease. Atherosclerosis 2005; 181: 305–310 [DOI] [PubMed] [Google Scholar]
- 12.Ikeda N, Yasu T, Kubo N, et al Daily exercise and bone marrow‐derived CD34+/133+ cells after myocardial infarction treated by bare metal stent implantation. Circ J 2008; 72: 897–901 [DOI] [PubMed] [Google Scholar]
- 13.Rosen RC, Cappelleri JC, Smith MD, et al Development and evaluation of an abridged, 5‐item version of the International Index of Erectile Function (IIEF‐5) as a diagnostic tool for erectile dysfunction. Int J Impot Res 1999; 11: 319–326 [DOI] [PubMed] [Google Scholar]
- 14.The research committee of diabetic nephropathy . The renewal of classification by Research Committee of the Japanese Ministry of Health, Labor and Welfare for disorders of diabetes mellitus. J Jpn Diabetes Soc 2001; 44: 623 [Google Scholar]
- 15.The Committee of the Japan Diabetes Society on the Diagnostic Criteria of Diabetes Mellitus . Report of the Committee on the Classification and Diagnostic Criteria of Diabetes Mellitus. J Diabetes Invest 2010; 1: 212–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsuo S, Imai E, Horio M, et al Collaborators developing the Japanese equation for estimated GFR Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 2009; 53: 982–992 [DOI] [PubMed] [Google Scholar]
- 17.Morishita T, Uzui H, Nakano A, et al Number of endothelial progenitor cells in peripheral artery disease as a marker of severity and association with pentraxin‐3, malondialdehyde‐modified low‐density lipoprotein and membrane type‐1 matrix metalloproteinase. J Atheroscler Thromb 2012; 19: 149–158 [DOI] [PubMed] [Google Scholar]
- 18.Celermajer DS, Sorensen KE, Gooch VM, et al Non‐invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet 1992; 340: 1111–1115 [DOI] [PubMed] [Google Scholar]
- 19.Corretti MC, Anderson TJ, Benjamin EJ, et al International Brachial Artery Reactivity Task Force. Guidelines for the ultrasound assessment of endothelial‐dependent flow‐mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol 2002; 39: 257–265 [DOI] [PubMed] [Google Scholar]
- 20.Beaver WL, Wasserman K, Whipp BJ. A new method for detecting anaerobic threshold by gas exchange. J Appl Physiol 1986; 60: 2020–2027 [DOI] [PubMed] [Google Scholar]
- 21.Hill JM, Zalos G, Halcox JP, et al Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med 2003; 348: 593–600 [DOI] [PubMed] [Google Scholar]
- 22.Werner N, Kosiol S, Schiegl T, et al Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med 2005; 353: 999–1007 [DOI] [PubMed] [Google Scholar]
- 23.Rehman J, Li J, Parvathaneni L, et al Exercise acutely increases circulating endothelial progenitor cells and monocyte‐/macrophage‐derived angiogenic cells. J Am Coll Cardiol 2004; 43: 2314–2318 [DOI] [PubMed] [Google Scholar]
- 24.Graham TE, Yang Q, Blüher M, et al Retinol‐binding protein 4 and insulin resistance in lean, obese, and diabetic subjects. N Engl J Med 2006; 354: 2552–2563 [DOI] [PubMed] [Google Scholar]
- 25.Cabré A, Lázaro I, Girona J, et al Retinol‐binding protein 4 as a plasma biomarker of renal dysfunction and cardiovascular disease in type 2 diabetes. J Intern Med 2007; 262: 496–503 [DOI] [PubMed] [Google Scholar]
- 26.Hotta K, Funahashi T, Arita Y, et al Plasma concentrations of a novel, adipose‐specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol 2000; 20: 1595–1599 [DOI] [PubMed] [Google Scholar]
- 27.Kumada M, Kihara S, Sumitsuji S, et al Osaka CAD Study Group Coronary artery disease. Association of hypoadiponectinemia with coronary artery disease in men. Arterioscler Thromb Vasc Biol 2003; 23: 85–89 [DOI] [PubMed] [Google Scholar]
- 28.Kawano T, Saito T, Yasu T, et al Close association of hypoadiponectinemia with arteriosclerosis obliterans and ischemic heart disease. Metabolism 2005; 54: 653–656 [DOI] [PubMed] [Google Scholar]
- 29.Sasaki M, Otani T, Kawakami M, et al Elevation of plasma retinol‐binding protein 4 and reduction of plasma adiponectin in subjects with cerebral infarction. Metabolism 2010; 59: 527–532 [DOI] [PubMed] [Google Scholar]


