Abstract
Allelic variation in the vitamin D receptor was the first non-structural gene to be associated with osteoporosis, and together with the effects of the vitamin D system on bone homeostasis, suggested that this vitamin might have a strong role in bone health. However, controversy exists regarding what level of serum 25-hydroxyvitamin D (25(OH)D) is optimal. Current data from biochemical, observational and randomized controlled trials (RCTs), indicate serum 25(OH)D levels of at least 50 nmol l−1 are required for normalization of parathyroid hormone (PTH) levels, to minimize the risk of osteomalacia and for optimal bone cell function. The skeletal consequences of 25(OH)D insufficiency include secondary hyperparathyroidism, increased bone turnover and bone loss and an increased risk of minimal trauma fractures. In large scale epidemiological studies, serum 25(OH)D levels are associated with bone mineral density in both men and women. However, there is mixed evidence on the effectiveness of optimizing serum 25(OH)D levels for the prevention of bone loss and minimal trauma fractures in postmenopausal women and older men. There may be some benefit on primary fracture prevention for those who have inadequate serum levels of 25(OH)D, particularly in institutionalised elderly patients, but only when combined with calcium supplements. For optimal bone health, evidence from RCTs suggests vitamin D may be considered a threshold nutrient with few further bone benefits observed at levels of 25(OH)D above which PTH is normalized. An adequate calcium intake is also imperative to gain optimum benefit from an improved vitamin D status in those with insufficient 25(OH)D levels, with an increased calcium intake being associated with suppression of PTH levels.
The role of vitamin D genetic factors in bone health
Recent studies report metabolic interactions between the variants of the vitamin D receptor, vitamin D binding protein and calcium sensing receptor genes in determining response to vitamin D therapy.1,2,3 New genetic data might explain why there is considerable inter-individual variability of serum 25-hydroxyvitamin D (25(OH)D) levels,4 with only 25% of this variability accounted for by season, latitude or vitamin D intake.
A large, multicentre, genome-wide association study of 15 cohorts, comprising about 30 000 white people from European descent, found that polymorphisms at three different loci involved in vitamin D metabolism affect serum 25(OH)D levels and the risk of vitamin D deficiency.4 After accounting for age, sex, body mass index and season, polymorphisms in at least three, and perhaps four loci, influenced serum 25(OH)D levels: (1) 4p12 polymorphisms near or within the GC gene, which encodes vitamin D binding protein, the main transporter of vitamin D metabolites in the blood; (2) 11q12 polymorphisms near DHCR7/NADSYN1, encoding the enzyme 7-dehydrocholesterol reductase, which converts 7-dehydrocholesterol into cholesterol in the skin thereby removing the substrate for production of vitamin D3; (3) 11p15 polymorphisms near CYP2R1, which encodes an enzyme responsible for 25-hydroxylation of vitamin D in the liver; (4) CYP24A1 encoding 24-hydroxylase, which initiates degradation of 25(OH)D and 1,25(OH)2D. Participants with a genotype score (combining the three main variants) in the top quartile had twice the risk of having vitamin D insufficiency (<50 or 75 nmol l−1) than those in the lowest quartile. It was also associated with a 1.5-fold risk of severe vitamin D deficiency (<20 nmol l−1). These findings are important, as they confirm that common genetic variants may contribute to the inter-individual variability of serum 25(OH)D levels and may either predispose to, or protect against, vitamin D deficiency.
Optimal serum levels of 25(OH)D for bone health—why the controversy?
An Institute of Medicine report5 concluded that vitamin D deficiency was defined as 25(OH)D <50 nmol l−1. From meta-analyses of vitamin D supplementation for falls and fracture prevention, the serum 25(OH)D thresholds are 60 and 75 nmol l−1, respectively. Australian guidelines recommend serum 25(OH)D ⩾50 nmol l−1 at the end of winter/early spring, or 60 nmol l−1 in summer for optimal bone health.6 To aim for a target level ⩾50 nmol l−1 at the end of winter, most individuals will require between 800–2000 (20–45 μg) vitamin D3 per day. A recent US Endocrine Society guideline was consistent with this and recommends that all adults aged 50–70 years and >70 years will require at least 600 and 800 IU (15–20 μg) of vitamin D3 per day, respectively, to maximize bone health and muscle function.7 However, to raise the serum level of 25(OH)D above 75 nmol l−1, as both the Endocrine Society7 and the International Osteoporosis Foundation8 recommend, individuals may require at least 1500–2000 IU (37.5–50 μg) per day of supplemental vitamin D, whereas doses of up to 10 000 IU (250 μg) per day have proven to be safe.7
Effects of season on bone health
Seasonal variations in serum 25(OH)D occur with a decline during the winter months.9,10,11,12,13,14,15 More importantly, seasonal variations in serum 25(OH)D concentrations are accompanied by responsive changes in serum parathyroid hormone (PTH) concentrations, later increases in bone resorption markers and bone formation markers, and decreases in bone mineral density (BMD). In 43 German subjects during the winter months, bone turnover was significantly accelerated, and lumbar spine and femoral BMD declined by 0.3–0.9%. Supplementation with oral 500 mg calcium and 500 IU cholecalciferol per day for one year either reversed or abolished these seasonal changes. In the subjects receiving oral cholecalciferol and calcium, lumbar and femoral BMD also increased significantly, whereas controls continued to lose bone.16
Rates of hip fracture also vary annually, with a winter peak in both Northern and Southern hemispheres.17,18 Inadequate vitamin D levels have been demonstrated in patients with osteoporosis,19 including in hip fracture patients in many countries, although low levels may be influenced by the fracture itself.20,21,22 It is also important to note that vitamin D deficiency increases with ageing and that this contributes independently to secondary hyperparathyroidism.23 In addition, serum androgen levels in men are associated with serum 25(OH)D levels and both hormones have a concordant annual periodicity being lowest in late winter,24 suggesting important, and as yet unidentified, links between the vitamin D and sex–steroid axes.
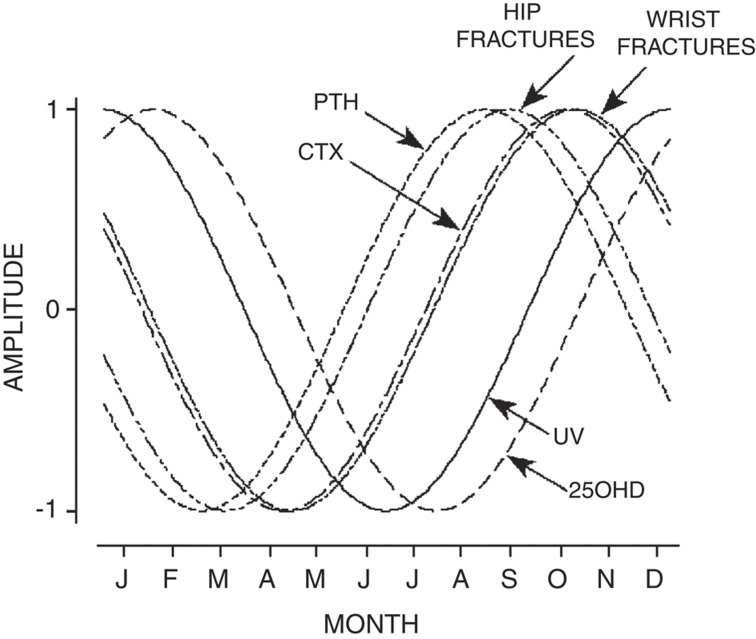
Similarly in 287 southeastern Australian (latitude 38–39°S) women, drawn from an observational, cross-sectional, population-based study,25 annual periodicities of ultraviolet radiation, serum 25(OH)D, serum PTH, a bone resorption marker (C-telopeptide), BMD, falls and fractures were measured. Cyclic variations in serum 25(OH)D lagged one month behind ultraviolet radiation, peaking in summer and dipping in winter (Figure 1). The periodicity of serum PTH was the inverse of serum 25(OH)D, with a phase shift delay of 1 month. Peak serum C-telopeptide lagged behind peak serum PTH by 1–2 months. In late winter, a greater proportion of falls resulted in fracture. Seasonal periodicity in 439 hip and 307 wrist fractures also followed a simple harmonic model, peaking 1.5–3 months after the winter trough in serum 25(OH)D.
Figure 1.
Sine curves showing the periodicity of UV, serum 25(OH)D, PTH, CTx, and hip and wrist fractures in the southern hemisphere. Amplitude set a maximum of +1 and a minimum of −1 (Pasco et al.25). 25(OH)D, 25-hydroxyvitamin D; CTx, C-telopeptide; PTH, parathyroid hormone; UV, ultraviolet radiation.
Another study did not show a seasonal variation in bone turnover markers.26 Season appears to be more important than latitude in determining serum 25(OH)D in many countries. However behavioural factors, such as sun avoidance and genetic factors, may also be important. In Australia, both season and latitude accounted for only <20% of the variation in serum 25(OH)D levels,27 supporting the importance of behavioural factors. In Australian women aged 20–92 years, high vitamin D status was associated with healthy body habitus and active lifestyles. In contrast, excessive weight and smoking were associated with lower vitamin D status. Women with high vitamin D were less likely to have elevated PTH, hypertension or osteopenia than women with poor levels.28 Despite the importance of behavioural factors, seasonal declines in vitamin D metabolites and the associated increases in fracture rates make interventions with vitamin D a logical intervention to prevent fractures, particularly in winter months.
Determinants of optimal serum 25(OH)D concentrations for bone health
Global vitamin D status
A systematic review of 195 studies from 44 countries and involving >168 000 participants showed that mean population-level 25(OH)D values varied considerably across the studies with 37.3% of the studies reporting mean values below 50 nmol l−1.29 Age-related differences were observed in the Asia/Pacific and Middle East/Africa regions, whereas sex-related differences were not observed in any region. The substantial heterogeneity between studies precluded drawing conclusions on overall vitamin D status at the population level. However, newborns and institutionalised elderly appeared to be at generally higher risk of lower 25(OH)D values. This study indicates that serum 25(OH)D levels <50nmol l−1 are likely to be common worldwide and involve both extremes of age.
Parathyroid hormone and calcium absorption
Previous attempts to define an optimal serum 25(OH)D for bone health used indirect measures such as the relationships between serum 25(OH)D and PTH concentrations in normal adult populations with a plateau of serum PTH above 31 ng ml−1 (78 nmol l−1).30 A major role for active vitamin D metabolites is in stimulating gut calcium absorption. In humans there is evidence of an age-related intestinal resistance to 1,25(OH)2D that may be secondary to reduced levels of intestinal vitamin D receptor.31 This gut defect could lead to compensatory increases in PTH secretion and 1,25(OH)2D3 production, which maintain calcium absorption and serum calcium, but at the expense of increased bone loss. Serum 25(OH)D concentrations are also weakly related to active calcium absorption,32 and one study suggested a plateau in active intestinal calcium absorption at serum 25(OH)D concentrations ⩾32 ng ml−1 (80 nmol l−1).
Prevention of osteomalacia
In a study of iliac crest bone biopsies from 675 women and men from northern Europe, mineralization defects were present in 25.6% patients.33 The latter were found independent of bone volume per trabecular volume throughout all ages and affected both sexes equally. Although a minimum 25(OH)D level that was inevitably associated with mineralization defects could not be identified, there was no pathologic accumulation of osteoid in any patient with serum 25(OH)D >75 nmol l−1, and in only very few patients with serum 25(OH)D between 50 and 75 nmol l−1.
Increased fracture risk
Others have related serum 25(OH)D concentrations to fracture risk. In a nested, case–control study of 7.1 years duration, baseline serum 25(OH)D levels in 400 hip fracture patients and 400 controls were compared. Lower serum 25(OH)D concentrations were associated with increased hip fracture risk (adjusted odds ratio for each 25 nmol l−1 decrease, 1.33). Women with the lowest 25(OH)D concentrations (⩽47.5 nmol l−1) had a higher hip fracture risk than did those with the highest concentrations (⩾70.7 nmol l−1) (adjusted odds ratio, 1.71). Importantly, this increase in fracture risk was independent of the number of falls, physical function, frailty, renal function and sex–steroid hormone levels but was, in part, mediated by increased bone resorption. Thus, serum 25(OH)D concentrations⩽20 ng ml−1 (50 nmol l−1) are associated with a higher risk for hip fracture.34
In another study of 1311 community-dwelling older Dutch men and women followed for 6 years, a low serum 25(OH)D level (<12 ng/ml or 30 nmol/l) increased the risk of fracture in those individuals aged 65–75 years (HR=3.1; 95% confidence interval 1.4–6.9), but not in older individuals (75–89 years).35
Randomised controlled trials—fractures
The above inferences about optimal serum 25(OH)D levels are drawn from association studies. Stronger evidence relates to the effects of vitamin D supplementation on the reduction in fractures and the serum 25(OH)D threshold concentrations required for these outcomes.
The anti-fracture efficacy of oral vitamin D supplementation in women and men ⩾65 years old has been assessed in a meta-analysis of 12 double-blind randomized controlled trials (RCTs) for nonvertebral fractures (n=42 279) and 8 RCTs for hip fractures (n=40 886).36 The pooled relative risks were 0.86 (95% confidence interval, 0.77–0.96) and 0.91 (95% confidence interval, 0.78–1.05) for the prevention of nonvertebral fractures or hip fractures, respectively. However, there was significant heterogeneity for both endpoints. Factors explaining the heterogeneity were the daily dose and achieved serum 25(OH)D concentrations. When examining the trials with a high (482–770 IU per day) received dose (that is, dose adjusted for adherence) of vitamin D, nonvertebral fractures were reduced by 20% and hip fractures by 18%, whereas doses <400 IU per day did not show any effect.
Pooled participant-level data from 11 double-blind RCTs of oral vitamin D supplementation (daily, weekly or every 4 months), with or without calcium, were recently compared with placebo or calcium alone in persons 65 years of age or older.37 A total of 31 022 persons (mean age, 76 years; 91% women) with 1111 incident hip fractures and 3770 nonvertebral fractures were included. The participants who were randomly assigned to receive vitamin D, as compared with those assigned to control groups, had a nonsignificant 10% reduction in the risk of hip fracture and a significant 7% reduction in the risk of nonvertebral fracture. Fracture risk reduction was shown only at the highest intake level (median, 800 IU daily), with a 30% reduction in the risk of hip fracture and a 14% reduction in the risk of any nonvertebral fracture. Benefits of vitamin D intake were fairly consistent across subgroups defined by age group, type of dwelling, baseline 25-(OH)D level and additional calcium intake. High-dose vitamin D supplementation (⩾800 IU daily) had beneficial effects in the prevention of hip fracture and any nonvertebral fracture in persons aged ⩾65 years.
A meta-analysis showed that sunlight exposure reduced the risk of hip fractures in patients with Alzheimer's disease, Parkinson's disease, stroke and co-existing vitamin D deficiency by 77%.38 Sunlight exposure also increased serum 25(OH)D and BMD.
Very high dose vitamin D is not recommended. Two trials have shown that very high annual doses of either intramuscular ergocalciferol (300 000 IU) or oral cholecalciferol (500 000 IU) resulted in an increase in falls and/or fractures in elderly postmenopausal women compared with placebo.39
Randomised controlled trials—bone mineral density (BMD)
The 2011 Institute of Medicine report considered the relationship between baseline or attained 25(OH)D status and changes in BMD for healthy adults;5 five out of six studies included found no association or did not report such findings. Regarding the effect of vitamin D supplementation on BMD, studies of daily doses of 800 IU (20 μg) of vitamin D (or lower equivalent doses of vitamin D3 or D2) reported equivocal findings. However, this dose is now considered suboptimal with respect to bone health, with doses of >2000 IU (50 μg) per day viewed as the level at which beneficial effects on BMD will result. Because most study patients also received calcium supplementation, the Institute of Medicine concluded that both supplements were required for all age groups to achieve gains in BMD.
A systematic review of 17 randomised controlled trials showed small positive effects of vitamin D3 plus calcium on lumbar spine, femoral neck and total body BMD in late menopausal women.40 The Women's Health Initiative (WHI) trial found a significant benefit of vitamin D3 400 IU plus 1000, mg of calcium on total hip BMD.41 Although there were no effects on spine BMD, this large study had several potential cofounders including 30% of participants already taking a calcium supplement, 30% having a high baseline dietary calcium intake (>1200, mg per day), 52% receiving current hormone replacement therapy, a low daily dose of vitamin D3 and a large proportion of patients taking a bisphosphonate at study end, and poor adherence with supplements.
However, in combined trials of vitamin D3 plus calcium versus calcium, a significant increase in BMD was not observed, suggesting vitamin D3 may be of less benefit in calcium-replete postmenopausal women. Nor did vitamin D3 treatment alone versus placebo result in any significant increases in BMD, except in one trial that noted an increase in femoral neck BMD.42 In the few trials reporting the impact of baseline serum 25(OH)D concentrations on BMD, a lower baseline serum 25(OH)D was not associated with a greater BMD increase. Overall, there is good evidence that vitamin D3 plus calcium results in small increases in BMD of the spine, total body, femoral neck and total hip.
A further meta-analysis examined effects of calcium or calcium combined with vitamin D on BMD and fractures in men and women aged >50 years.43 Of the 23 trials (n=41 419) reporting BMD as an outcome, calcium and calcium in combination with vitamin D were associated with a reduced bone loss of 0.54% at the hip, and 1.19% at the spine, whereas there was no difference in fracture reduction between calcium combined with vitamin D or calcium alone.
The most recent meta-analysis of 23 studies (mean duration 23.5 months, comprising 4082 participants, 92% women, average age 59 years) showed a small increase in femoral neck BMD (0.8%, 95% confidence interval 0.2–1.4) with heterogeneity among trials and no effect at any other site.44
Calcium and vitamin D supplementation during winter results in increases in spinal and femoral neck BMD.16 One randomised controlled trial of 302 elderly women examined the effect of the combination of calcium and vitamin D2 (ergocalciferol) on bone structure and bone turnover compared with calcium alone.45 Serum 25(OH)D increased only in the vitamin D group from 18 (44 nmol l−1) to 24 ng ml−1 (60 nmol l−1) after 1 year. Total hip and total body BMD increased significantly. In addition, this increase in serum 25(OH)D had no extra effect on active fractional intestinal calcium absorption, which fell equally in both groups by 15–17%. Thus, ergocalciferol (vitamin D2) 1000 IU for 1 year had no extra beneficial effect on bone structure, bone formation markers or intestinal calcium absorption over an additional 1000, mg of calcium.
Effect of withdrawal of calcium and vitamin D
A 5-year randomized, controlled, double-blind trial of 120 community-dwelling women aged 70–80 years compared 1200, mg per day calcium with placebo vitamin D (Ca group) or with 1000 IU per day ergocalciferol (vitamin D2) (CaD group) or double placebo (control).46 Hip BMD was preserved in both intervention groups, but not in controls at year 1 and maintained in the CaD group only over the long-term at years 3 and 5. At year 1, compared with controls, the Ca and CaD groups had lower bone turnover markers. At 5 years, suppression of bone turnover markers was maintained only in the CaD group, and was associated with reductions in PTH at 3 and 5 years compared with controls. Thus, although the combination of calcium with vitamin D had no additional effect over calcium alone in the short-term (at 1 year), continuing skeletal benefits appeared to be greater with the combination over the long-term (up to 5 years).
Another study examined effects of calcium and vitamin D fortified milk compared with normal diet over 24 months in older men.47 Men received milk containing 1000, mg of calcium plus 800 IU of vitamin D3 or no additional milk. After 2 years, the mean percent decrease in BMD was 0.9–1.6% less in the milk supplementation compared with control group at the femoral neck, total hip and radius. There was a greater increase in lumbar spine BMD in the milk supplementation group after 12 and 18 months, but not after 2 years. Serum 25(OH)D increased and PTH decreased in the milk supplementation relative to control group after both the first and second years of the study. After a further 18 months of follow-up, the net beneficial effects of fortified milk on femoral neck and radius BMD at the end of the intervention (24 months) were sustained48 suggesting, once again, the possibility of sustained skeletal benefits after withdrawal.
Thus, there is no evidence that vitamin D alone has beneficial effects on BMD. Treatment with the combination of calcium and vitamin D either prevents bone loss or results in only small increases in BMD. For long-term maintenance of BMD up to 5 years, the combination of calcium and vitamin D appears to be better than calcium alone. These skeletal benefits of calcium and vitamin D may be maintained at some, but not all, skeletal sites after withdrawal.
Conclusion
Although much controversy exists regarding what level of serum 25(OH)D is optimal,6,8,49,50,51 current data from biochemical and observational studies, and RCTs, indicate serum 25(OH)D levels of at least 50nmol l−1 are required for normalization of PTH levels,30 to minimize the risk of osteomalacia33 and for optimal bone cell function. Mixed evidence exists on the effectiveness of optimising serum 25(OH)D levels for the prevention of bone loss and reducing minimal trauma fractures in postmenopausal women and older men.43,44 An adequate calcium intake is also imperative to gain optimum benefit from an improved vitamin D status in those with insufficient 25(OH)D levels, with an increased calcium intake being associated with suppression of PTH levels.52,53 There may be a small benefit on primary fracture prevention and preventing bone loss for those who have inadequate serum levels of 25(OH)D, particularly in institutionalised elderly patients, but only when vitamin D supplements are combined with calcium supplements.
Footnotes
The author declares no conflict of interest.
References
- Arabi A, Zahed L, Mahfoud Z, El-Onsi L, Nabulsi M, Maalouf J et al. Vitamin D receptor gene polymorphisms modulate the skeletal response to vitamin D supplementation in healthy girls. Bone 2009;45:1091–1097. [DOI] [PubMed] [Google Scholar]
- Giroux S, Elfassihi L, Clement V, Bussieres J, Bureau A, Cole DE et al. High-density polymorphisms analysis of 23 candidate genes for association with bone mineral density. Bone 2010;47:975–981. [DOI] [PubMed] [Google Scholar]
- Laaksonen MM, Outila TA, Karkkainen MU, Kemi VE, Rita HJ, Perola M et al. Associations of vitamin D receptor, calcium-sensing receptor and parathyroid hormone gene polymorphisms with calcium homeostasis and peripheral bone density in adult Finns. J Nutrigenet Nutrigenomics 2009;2:55–63. [DOI] [PubMed] [Google Scholar]
- Wang TJ, Zhang F, Richards JB, Kestenbaum B, van Meurs JB, Berry D et al. Common genetic determinants of vitamin D insufficiency: a genome-wide association study. Lancet 2010;376:180–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocr Metab 2011;96:53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowson CA, McGrath JJ, Ebeling PR, Haikerwal A, Daly RM, Sanders KM et al. Vitamin D and health in adults in Australia and New Zealand: a position statement. Med J Aust 2012;196:686–687. [DOI] [PubMed] [Google Scholar]
- Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocr Metab 2011;96:1911–1930. [DOI] [PubMed] [Google Scholar]
- Dawson-Hughes B, Mithal A, Bonjour JP, Boonen S, Burckhardt P, Fuleihan GE et al. IOF position statement: vitamin D recommendations for older adults. Osteoporosis Int 2010;21:1151–1154. [DOI] [PubMed] [Google Scholar]
- Stamp TC, Round JM. Seasonal changes in human plasma levels of 25-hydroxyvitamin D. Nature 1974;247:563–565. [DOI] [PubMed] [Google Scholar]
- Bouillon RA, Auwerx JH, Lissens WD, Pelemans WK. Vitamin D status in the elderly: seasonal substrate deficiency causes 1,25-dihydroxycholecalciferol deficiency. Am J Clin Nutr 1987;45:755–763. [DOI] [PubMed] [Google Scholar]
- Webb AR, Pilbeam C, Hanafin N, Holick MF. An evaluation of the relative contributions of exposure to sunlight and of diet to the circulating concentrations of 25-hydroxyvitamin D in an elderly nursing home population in Boston. Am J Clin Nutr 1990;51:1075–1081. [DOI] [PubMed] [Google Scholar]
- Holick MF. McCollum Award Lecture, 1994: vitamin D--new horizons for the 21st century. Am J Clin Nutr 1994;60:619–630. [DOI] [PubMed] [Google Scholar]
- Scharla SH, Scheidt-Nave C, Leidig G, Woitge H, Wuster C, Seibel MJ et al. Lower serum 25-hydroxyvitamin D is associated with increased bone resorption markers and lower bone density at the proximal femur in normal females: a population-based study. Exp Clin Endocrinol Diabetes 1996;104:289–292. [DOI] [PubMed] [Google Scholar]
- Harris SS, Dawson-Hughes B. Seasonal changes in plasma 25-hydroxyvitamin D concentrations of young American black and white women. Am J Clin Nutr 1998;67:1232–1236. [DOI] [PubMed] [Google Scholar]
- Pasco JA, Henry MJ, Nicholson GC, Sanders KM, Kotowicz MA. Vitamin D status of women in the Geelong Osteoporosis Study: association with diet and casual exposure to sunlight. Med J Aust 2001;175:401–405. [DOI] [PubMed] [Google Scholar]
- Meier C, Woitge HW, Witte K, Lemmer B, Seibel MJ. Supplementation with oral vitamin D3 and calcium during winter prevents seasonal bone loss: a randomized controlled open-label prospective trial. J Bone Miner Res 2004;19:1221–1230. [DOI] [PubMed] [Google Scholar]
- Jacobsen SJ, Goldberg J, Miles TP, Brody JA, Stiers W, Rimm AA. Regional variation in the incidence of hip fracture. US white women aged 65 years and older. JAMA 1990;264:500–502. [PubMed] [Google Scholar]
- Lau EM, Gillespie BG, Valenti L, O'Connell D. The seasonality of hip fracture and its relationship with weather conditions in New South Wales. Aust J Public Health 1995;19:76–80. [DOI] [PubMed] [Google Scholar]
- Lips P, Hosking D, Lippuner K, Norquist JM, Wehren L, Maalouf G et al. The prevalence of vitamin D inadequacy amongst women with osteoporosis: an international epidemiological investigation. J Intern Med 2006;260:245–254. [DOI] [PubMed] [Google Scholar]
- Boonen S, Aerssens J, Dequeker J. Age-related endocrine deficiencies and fractures of the proximal femur. II implications of vitamin D deficiency in the elderly. J Endocrinol 1996;149:13–17. [DOI] [PubMed] [Google Scholar]
- LeBoff MS, Kohlmeier L, Hurwitz S, Franklin J, Wright J, Glowacki J. Occult vitamin D deficiency in postmenopausal US women with acute hip fracture. JAMA 1999;281:1505–1511. [DOI] [PubMed] [Google Scholar]
- Pieper CF, Colon-Emeric C, Caminis J, Betchyk K, Zhang J, Janning C et al. Distribution and correlates of serum 25-hydroxyvitamin D levels in a sample of patients with hip fracture. Am J Geriatr Pharmacother 2007;5:335–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khosla S, Atkinson EJ, Melton LJ 3rd, Riggs BL. Effects of age and estrogen status on serum parathyroid hormone levels and biochemical markers of bone turnover in women: a population-based study. J Clin Endocrinol Metab 1997;82:1522–1527. [DOI] [PubMed] [Google Scholar]
- Wehr E, Pilz S, Boehm BO, Marz W, Obermayer-Pietsch B. Association of vitamin D status with serum androgen levels in men. Clin Endocrinol (Oxf) 2010;73:243–248. [DOI] [PubMed] [Google Scholar]
- Pasco JA, Henry MJ, Kotowicz MA, Sanders KM, Seeman E, Pasco JR et al. Seasonal periodicity of serum vitamin D and parathyroid hormone, bone resorption, and fractures: the Geelong Osteoporosis Study. J Bone Miner Res 2004;19:752–758. [DOI] [PubMed] [Google Scholar]
- Blumsohn A, Naylor KE, Timm W, Eagleton AC, Hannon RA, Eastell R. Absence of marked seasonal change in bone turnover: a longitudinal and multicenter cross-sectional study. J Bone Miner Res 2003;18:1274–1281. [DOI] [PubMed] [Google Scholar]
- van der Mei IA, Ponsonby AL, Engelsen O, Pasco JA, McGrath JJ, Eyles DW et al. The high prevalence of vitamin D insufficiency across Australian populations is only partly explained by season and latitude. Environ Health Perspect 2007;115:1132–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasco JA, Henry MJ, Nicholson GC, Brennan SL, Kotowicz MA. Behavioural and physical characteristics associated with vitamin D status in women. Bone 2009;44:1085–1091. [DOI] [PubMed] [Google Scholar]
- Hilger J, Friedel A, Herr R, Rausch T, Roos F, Wahl DA et al. A systematic review of vitamin D status in populations worldwide. Br J Nutr 2014;111:23–45. [DOI] [PubMed] [Google Scholar]
- Chapuy MC, Preziosi P, Maamer M, Arnaud S, Galan P, Hercberg S et al. Prevalence of vitamin D insufficiency in an adult normal population. Osteoporos Int 1997;7:439–443. [DOI] [PubMed] [Google Scholar]
- Ebeling PR, Sandgren ME, DiMagno EP, Lane AW, DeLuca HF, Riggs BL. Evidence of an age-related decrease in intestinal responsiveness to vitamin D: relationship between serum 1,25-dihydroxyvitamin D3 and intestinal vitamin D receptor concentrations in normal women. J Clin Endocrinol Metab 1992;75:176–182. [DOI] [PubMed] [Google Scholar]
- Heaney RP. Functional indices of vitamin D status and ramifications of vitamin D deficiency. Am J Clin Nutr 2004;80:1706S–1709S. [DOI] [PubMed] [Google Scholar]
- Priemel M, von Domarus C, Klatte TO, Kessler S, Schlie J, Meier S et al. Bone mineralization defects and vitamin D deficiency: histomorphometric analysis of iliac crest bone biopsies and circulating 25-hydroxyvitamin D in 675 patients. J Bone Miner Res 2010;25:305–312. [DOI] [PubMed] [Google Scholar]
- Cauley JA, Lacroix AZ, Wu L, Horwitz M, Danielson ME, Bauer DC et al. Serum 25-hydroxyvitamin D concentrations and risk for hip fractures. Ann Intern Med 2008;149:242–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Schoor NM, Visser M, Pluijm SM, Kuchuk N, Smit JH, Lips P. Vitamin D deficiency as a risk factor for osteoporotic fractures. Bone 2008;42:260–266. [DOI] [PubMed] [Google Scholar]
- Bischoff-Ferrari HA, Willett WC, Wong JB, Stuck AE, Staehelin HB, Orav EJ et al. Prevention of nonvertebral fractures with oral vitamin D and dose dependency: a meta-analysis of randomized controlled trials. Arch Intern Med 2009;169:551–561. [DOI] [PubMed] [Google Scholar]
- Bischoff-Ferrari HA, Willett WC, Orav EJ, Lips P, Meunier PJ, Lyons RA et al. A pooled analysis of vitamin D dose requirements for fracture prevention. New Engl J Med 2012;367:40–49. [DOI] [PubMed] [Google Scholar]
- Iwamoto J, Takeda T, Matsumoto H. Sunlight exposure is important for preventing hip fractures in patients with Alzheimer's disease, Parkinson's disease, or stroke. Acta Neurol Scand 2012;125:279–284. [DOI] [PubMed] [Google Scholar]
- Sanders KM, Nicholson GC, Ebeling PR. Is high dose vitamin D harmful? Calcif Tissue Int 2013;92:191–206. [DOI] [PubMed] [Google Scholar]
- Cranney A, Horsley T, O'Donnell S, Weiler H, Puil L, Ooi D et al. Effectiveness and safety of vitamin D in relation to bone health. Evid Rep Technol Assess (Full Rep) 2007;158:1–235. [PMC free article] [PubMed] [Google Scholar]
- Jackson RD, LaCroix AZ, Gass M, Wallace RB, Robbins J, Lewis CE et al. Calcium plus vitamin D supplementation and the risk of fractures. New Engl J Med 2006;354:669–683. [DOI] [PubMed] [Google Scholar]
- Ooms ME, Roos JC, Bezemer PD, van der Vijgh WJ, Bouter LM, Lips P. Prevention of bone loss by vitamin D supplementation in elderly women: a randomized double-blind trial. J Clin Endocrinol Metab 1995;80:1052–1058. [DOI] [PubMed] [Google Scholar]
- Tang BM, Eslick GD, Nowson C, Smith C, Bensoussan A. Use of calcium or calcium in combination with vitamin D supplementation to prevent fractures and bone loss in people aged 50 years and older: a meta-analysis. Lancet 2007;370:657–666. [DOI] [PubMed] [Google Scholar]
- Reid IR, Bolland MJ, Grey A. Effects of vitamin D supplements on bone mineral density: a systematic review and meta-analysis. Lancet 2014;383:146–155. [DOI] [PubMed] [Google Scholar]
- Zhu K, Bruce D, Austin N, Devine A, Ebeling PR, Prince RL. Randomized controlled trial of the effects of calcium with or without vitamin D on bone structure and bone-related chemistry in elderly women with vitamin D insufficiency. J Bone Miner Res 2008;23:1343–1348. [DOI] [PubMed] [Google Scholar]
- Zhu K, Devine A, Dick IM, Wilson SG, Prince RL. Effects of calcium and vitamin D supplementation on hip bone mineral density and calcium-related analytes in elderly ambulatory Australian women: a five-year randomized controlled trial. J Clin Endocrinol Metab 2008;93:743–749. [DOI] [PubMed] [Google Scholar]
- Daly RM, Brown M, Bass S, Kukuljan S, Nowson C. Calcium- and vitamin D3-fortified milk reduces bone loss at clinically relevant skeletal sites in older men: a 2-year randomized controlled trial. J Bone Miner Res 2006;21:397–405. [DOI] [PubMed] [Google Scholar]
- Daly RM, Petrass N, Bass S, Nowson CA. The skeletal benefits of calcium- and vitamin D3-fortified milk are sustained in older men after withdrawal of supplementation: an 18-mo follow-up study. Am J Clin Nutr 2008;87:771–777. [DOI] [PubMed] [Google Scholar]
- Henry HL, Bouillon R, Norman AW, Gallagher JC, Lips P, Heaney RP et al. 14th Vitamin D Workshop consensus on vitamin D nutritional guidelines. J Steroid Biochem Mol Biol 2010;121:4–6. [DOI] [PubMed] [Google Scholar]
- Heaney RP, Holick MF. Why the IOM recommendations for vitamin D are deficient. J Bone Miner Res 2011;26:455–457. [DOI] [PubMed] [Google Scholar]
- Lips P, Bouillon R, van Schoor NM, Vanderschueren D, Verschueren S, Kuchuk N et al. Reducing fracture risk with calcium and vitamin D. Clin Endocrinol (Oxf) 2010;73:277–285. [DOI] [PubMed] [Google Scholar]
- Elders P, Lips P, Netelenbos J, Van Grinkel F, Khoe E, Van der Vijgh W et al. Long-term effect of calcium supplementation on bone loss in perimenopausal women. J Bone Miner Res 1994;9:963–970. [DOI] [PubMed] [Google Scholar]
- Reid I, Mason B, Horne A, Ames R, Reid H, Bava U et al. Randomized controlled trial of calcium in healthy older women. Am J Med 2006;119:777–785. [DOI] [PubMed] [Google Scholar]