Abstract
Heterogeneous loss of function mutations in the vitamin D receptor (VDR) interfere with vitamin D signaling and cause hereditary vitamin D-resistant rickets (HVDRR). HVDRR is characterized by hypocalcemia, secondary hyperparathyroidism and severe early-onset rickets in infancy and is often associated with consanguinity. Affected children may also exhibit alopecia of the scalp and total body. The children usually fail to respond to treatment with calcitriol; in fact, their endogenous levels are often very elevated. Successful treatment requires reversal of hypocalcemia and secondary hyperparathyroidism and is usually accomplished by administration of high doses of calcium given either intravenously or sometimes orally to bypass the intestinal defect in VDR signaling.
Introduction
Vitamin D, when formed in the skin utilizing the energy of sunlight or ingested as dietary vitamin D, is an inactive precursor requiring two hydroxylation steps, first in the liver and then the kidney, to be converted to 1α,25-dihydroxyvitamin D3 (1,25(OH)2D3 or calcitriol), the active hormone. As described in other chapters in this special issue.1,2,3,4 1,25(OH)2D3 then binds to the vitamin D receptor (VDR) to mediate the actions of the hormone. The VDR is present in selected cell types in most if not all tissues in the body and 1,25(OH)2D3/VDR complexes regulate multiple target genes in tissues containing the VDR.5 Although non-skeletal actions of vitamin D have been found in all tissues harboring a VDR, the most well-recognized actions of vitamin D take place in the intestine, kidney, parathyroids and bone, organs that regulate calcium and phosphate metabolism and that are responsible for normal mineralization of bone. In the absence of either adequate amounts of the active hormone (1,25(OH)2D3) or a functional receptor (VDR), calcium and phosphate absorption is impaired and hypocalcemia develops. This results in compensatory hyperparathyroidism, hypophosphatemia and skeletal defects in bone mineralization leading to under-mineralized portions of the bone matrix or osteoid. When this sequence of events occurs in children, the disease rickets develops; when it occurs in adults, osteomalacia develops. These conditions and the medical consequences for bone are discussed extensively in other chapters of this special issue.6,7,8,9 Nutritional vitamin D deficiency is the most common cause of rickets and osteomalacia worldwide. However, two rare genetic diseases due to mutations that interfere with synthesis of 1,25(OH)2D3 or the actions of the VDR also cause rickets in children. These diseases, and the knockout mouse models of the two human diseases, have provided exceptional insight into the metabolic pathway of synthesis and the mechanism of action of 1,25(OH)2D3.
The critical enzyme to synthesize 1,25(OH)2D from 25(OH)D (when written without a subscript indicates D2 or D3), the circulating hormone precursor, is 25-hydroxyvitamin D-1α-hydroxylase (1α-hydroxylase or CYP27B1). When this enzyme is defective due to various loss of function mutations, the result is an inability to synthesize adequate amounts of 1,25(OH)2D and the disease 1α-hydroxylase deficiency develops.10 The disease is also known as vitamin D-dependent rickets type 1 (VDDR-I) or pseudovitamin D deficiency rickets (PDDR) and is described by Glorieux and Pettifor in this special issue.8 When the VDR is defective due to a variety of loss of function mutations in the gene encoding the VDR, the result is impaired ability of the VDR to signal and to regulate target genes even in the presence of elevated 1,25(OH)2D concentrations and results in the development of the disease hereditary vitamin D-resistant rickets (HVDRR), also known as vitamin D-dependent rickets type II (VDDR II). Both diseases are rare autosomal recessive disorders characterized by hypocalcemia, secondary hyperparathyroidism and early-onset rickets. As will be discussed below in more detail, a crucial difference between the two diseases is that 1α-hydroxylase deficiency is characterized by extremely low serum 1,25(OH)2D levels while HVDRR, characteristically for a target organ resistant disease, is distinguished by elevated levels of 1,25(OH)2D, the ligand for the defective receptor. A second and critical difference between these diseases is that children with 1α-hydroxylase deficiency respond very well to calcitriol therapy while those with HVDRR are resistant to all forms of vitamin D therapy and require calcium treatment. In this chapter, we will focus on HVDRR but briefly discuss differences between these two genetic childhood diseases that present similarly with hypocalcemia and early-onset rickets.
We have recently reviewed the subjects of HVDRR10,11,12 and associated alopecia,13 and the current chapter adapts material from those papers with updates.
Overview of the structure of VDR relevant to HVDRR mutations
As discussed by Pike et al,1 the VDR is similar to the other members of the steroid–thyroid–retinoid receptor superfamily. Elucidation of the nature of naturally occurring mutations in children with HVDRR10,11,12,14 as well as laboratory-created mutations in the VDR15,16,17,18,19 has contributed to our understanding of the functional domains of the VDR protein.20 At the N terminus the VDR has a highly conserved DNA-binding domain (DBD) and in the C-terminal half of the protein a more variable ligand-binding domain (LBD). The DBD contains two finger-like structures of 12–13 amino acids each. Four cysteine residues bind one zinc atom to form each zinc-finger structure.21 Regions of the DBD are critical both for DNA binding and also serve as a dimerization interface for interaction with the retinoid X receptor α (RXRα).22,23 The hinge region (amino acids residues 93–120) connects the DBD and LBD.
The VDR LBD is formed by amino acids 123–427. X-ray crystallography of the VDR showed that the LBD is composed of 12 α-helices (H1–H12) and 3 β-sheets (S1–S3).20 Helix H12 forms a retractable lid that traps and holds 1,25(OH)2D in position. 1,25(OH)2D binding causes a conformational change in the VDR that promotes heterodimerization with RXRα. VDR heterodimerization with RXRα involves residues in H9, H10 and an E1 domain that overlaps H4 and H5 within the LBD. Activating function domain 2 (AF-2 domain) residues 416–424 of helix H12 and the region between amino acids 232–272 encompassing H3 and H4 are essential for transactivation.20 The repositioning of helix H12 after 1,25(OH)2D binding is critical for the formation of a hydrophobic groove that binds LxxLL motifs (where L is leucine and x is any amino acid) in the nuclear receptor-interacting domains of coactivators. Other regions of the VDR also act to recruit coactivator proteins or facilitate contact with proteins associated with the core transcriptional machinery such as TFIIB or the TAFs.24,25 Heterogeneous loss-of-function mutations in the VDR LBD that cause HVDRR have been shown to interfere with some aspect of 1,25(OH)2D binding or VDR signaling. The types of defects include ones that (1) completely abolish 1,25(OH)2D binding, (2) reduce VDR affinity for 1,25(OH)2D binding, (3) disrupt RXRα heterodimerization or (4) interfere with coactivator interactions.11,12 Mutations in the VDR DBD interfere with VDR binding to Vitamin D Response Elements (VDREs) in target genes and prevent the VDR/1,25(OH)2D complex from signaling target genes. Premature stop mutations prevent the formation of adequate VDR protein or in fact result in no detectable VDR at all.
The clinical appearance of children with HVDRR
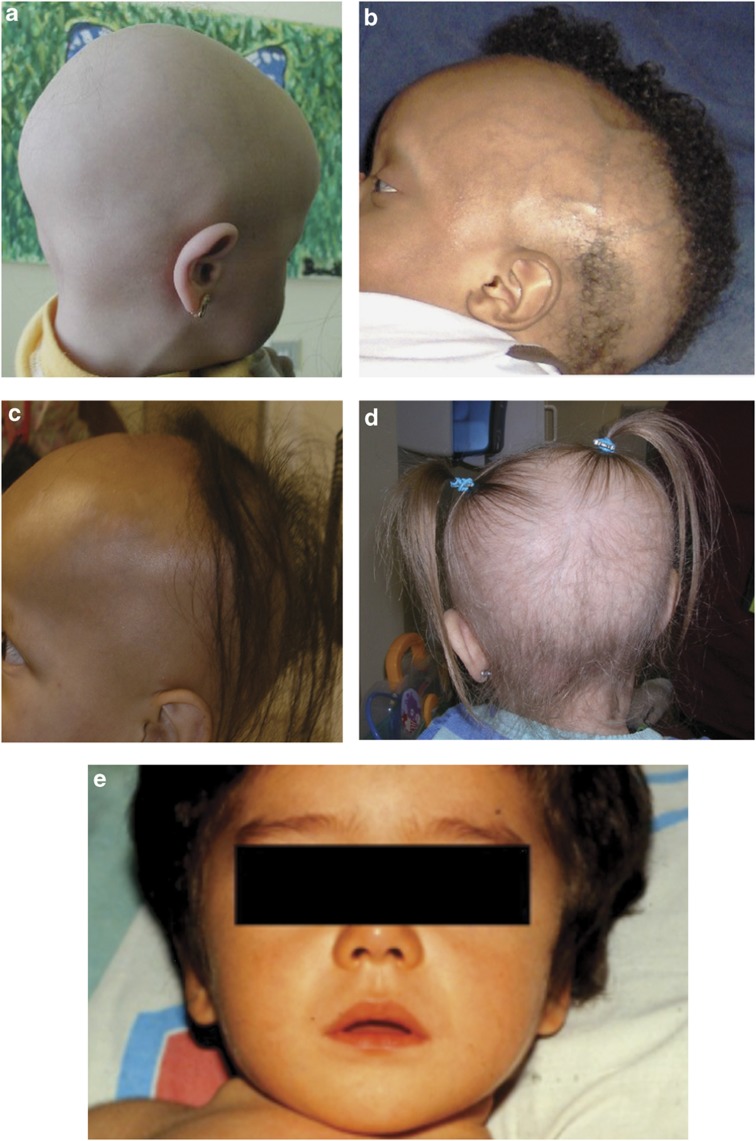
Although born with normal calcium levels, children with HVDRR develop hypocalcemia early in infancy, often within months of birth and clinical rickets soon follows.11,12,14,26 Affected children have bone pain, muscle weakness, hypotonia and occasionally may have convulsions due to severe hypocalcemia. Some children have died, often of respiratory complications due to difficulty with normal chest cage movement. Pain in the lower extremities often leads to delays in their development of the ability to walk. Fractures and pseudo-fractures are common. Affected children are usually growth-retarded and in many cases the children develop severe dental caries and/or hypoplasia of the teeth.12,14 Some children have alopecia either at birth or develop it in months after birth, which is an early sign of the disease and helps in the diagnosis especially in families that already have an affected child (Figure 1).
Figure 1.
Variations in patterns of scalp hair in children with alopecia. (a) Total alopecia, (b) areas of normal hair adjacent to bald areas, (c and d) different patterns of alopecia despite the children having the same VDR mutation, and (e) child with a full head of hair despite severe hypocalcemia and rickets. Figure from Mol Cell Endocrinol 2011; 347:90–96 used with permission.13
The laboratory findings include low serum concentrations of calcium and phosphate and often strikingly elevated serum alkaline phosphatase activity. The children exhibit secondary hyperparathyroidism with markedly elevated PTH levels. The serum 25(OH)D values are usually normal except if concommitant vitamin D deficiency is also present. Serum 1,25(OH)2D levels are usually substantially elevated. This clinical pattern distinguishes HVDRR from 1α-hydroxylase deficiency in which case the serum 1,25(OH)2D values are low or absent. Examination of the children reveals classical signs of rickets, which are confirmed by X-rays.
Many children with HVDRR also have alopecia. The molecular mechanisms that differentiate those with and without alopecia will be discussed below. When present, the extent of alopecia can vary from sparse scalp hair to total baldness and full-body alopecia including eyebrows and in some cases eyelashes (Figure 1).13 However, even when children have the identical VDR mutation, they may exhibit different patterns or extent of alopecia.27 When present, alopecia is a useful sign to distinguish HVDRR from 1α-hydroxylase deficiency and other causes of rickets. Most affected children who exhibit alopecia are resistant to therapy with supra-physiologic doses of all forms of vitamin D including calcitriol, a critical difference compared with the responsiveness of children with 1α-hydroxylase deficiency.
HVDRR is an autosomal recessive disease with males and females equally affected. The parents of affected children, who are heterozygous carriers of the genetic trait, usually show no symptoms of the disease and have normal bone development. In one recently published study, a child harboring a heterozygotic mutation in the gene encoding the VDR (Glu420Ala) developed clinically severe HVDRR due to dominant negative suppression of the wild-type allele.28 The father, who carried the heterozygotic mutation, also had some unknown bone disease when young and as an adult had modestly elevated 1,25(OH)2D and PTH levels with low serum calcium levels. The mother was entirely normal and carried no VDR mutation. The E420A mutant VDR protein was expressed at higher levels than the WT VDR in the patient and father's fibroblasts. The higher amount of E420A mutant VDR protein most likely caused the resistance as studies of cells transfected with combinations of the E420A mutant VDR along with the WT VDR demonstrated silencing of VDR signaling via the WT VDR.28 However, in most studied cases, consanguinity is associated with the disease, with each parent contributing a defective gene. Occasionally in non-cansanguinous cases, a different mutation has been found on each VDR allele (compound mutations) in the affected children so that the child has no normal VDR allele.29,30,31
Mutations in the VDR gene as the molecular basis for HVDRR
Over 100 cases of HVDRR have been recorded and a number of these have been analyzed at the biochemical and molecular level.11,12,14,32 As discussed in our recent reviews,10,11,12,14 45 unique mutations have been identified inthe VDR gene as the cause of HVDRR. Mutations in the DBD prevent the VDR from binding to DNA, causing total resistance to 1,25(OH)2D even though 1,25(OH)2D binding to the VDR is normal. On the other hand, mutations in the LBD may disrupt ligand binding or heterodimerization with RXRα, or prevent coactivators from binding to the VDR and cause partial or total hormone resistance. Other mutations that have been identified in the VDR that cause 1,25(OH)2D resistance include nonsense mutations, insertions/substitutions, insertions/duplications, deletions and splice site mutations. Some of these VDR mutations will be described below.
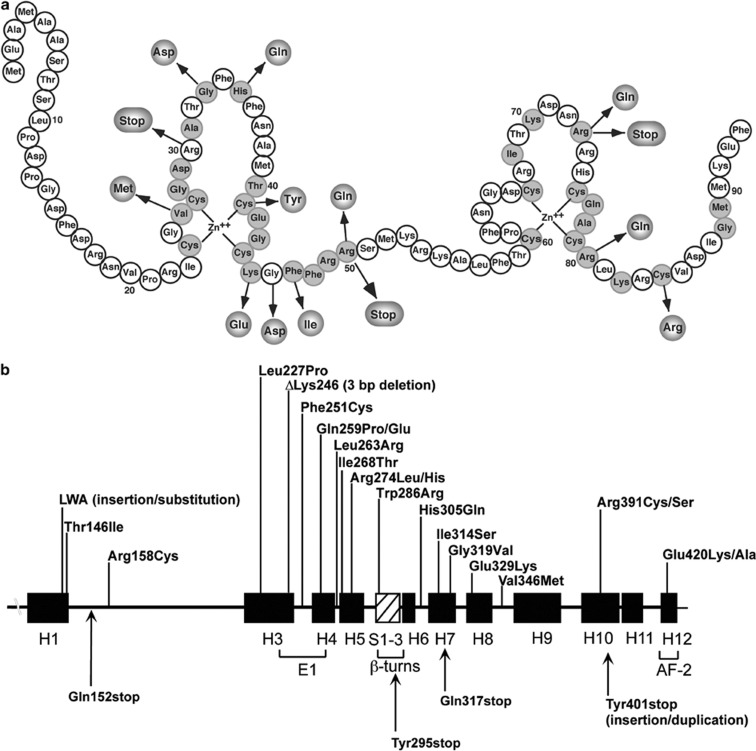
We are aware of 11 missense mutations that have been identified in the VDR DBD as the cause of HVDRR (Figure 2). These mutant VDRs exhibit normal ligand binding but defective DNA binding.11,12,14 A common feature of patients with mutations in the DBD is that they all have alopecia. The heterozygotic parents with a single mutant allele are usually asymptomatic.
Figure 2.
Mutations in the VDR that cause HVDRR. (a) Location of mutations in the DNA-binding domain (DBD). Conserved amino acids are shaded. (b) Location of mutations in the ligand-binding domain (LBD). The α-helices are shown as black boxes and the β-turns as hatched box. Missense mutations are on top and nonsense mutations on bottom. E1 and AF-2 (activation function 2) represent helices important for transactivation; adapted from Feldman et al.10
Several nonsense mutations have been identified in the VDR gene that truncate the VDR protein.11,12,14 Fibroblasts from patients with VDR nonsense mutations exhibit no ligand binding and often the truncated VDR protein is not detected by immunoblotting. One particular mutation, a single base change in exon 8 that introduced a premature termination codon (Tyr295stop), was identified in several families that comprise a large kindred where consanguineous marriages were common.33 The VDR mRNA was undetectable by northern blot analysis, indicating that the Tyr295stop mutation led to nonsense-mediated mRNA decay. Interestingly, in one family with an Arg30stop mutation, the parents of the affected child had somewhat elevated serum 1,25(OH)2D levels, indicating some resistance to 1,25(OH)2D.34 All of the affected children with nonsense mutations had alopecia.
Splice site mutations in the VDR gene have also been identified as the cause of HVDRR. These mutations usually cause a frameshift and eventually introduce a downstream premature stop signal, resulting in a non-functional VDR. Splice site mutations in the VDR gene cause exons to be skipped,35,36 or cause incorporation of an intron into the VDR mRNA.37 In one case, a cryptic 5′ donor splice site was generated in exon 6, which deleted 56 nucleotide bases and led to a frameshift in exon 7.38
At this time we are aware of 20 missense mutations that have been identified in the VDR LBD (Figure 2).39,40 Two patients had slightly different mutations in Arg274 (Arg274Leu and Arg274His), a contact point for the 1α-hydroxyl group of 1,25(OH)2D3 that lowered the binding affinity of the VDR for 1,25(OH)2D3.20,39,41 A second patient had an His305Gln mutation that altered the contact point for the 25-hydroxyl group of 1,25(OH)2D3.20,42 This mutation also lowered the binding affinity of the VDR for 1,25(OH)2D3. These cases illustrate the importance of critical amino acids as contact points for 1,25(OH)2D3 and demonstrate that mutations of these residues can often be the basis for HVDRR.
Mutations have also been identified in the VDR that disrupt VDR:RXRα protein interaction (heterodimerization) and thereby cause HVDRR. For example, the Arg391Cys mutation in the VDR LBD had no affect on ligand binding but reduced its transactivation activity. Arg391 is located in helix H10 where the RXRα dimerization interface is formed from helix H9 and helix H10 and the interhelical loops between H7–H8 and H8–H9 in VDR.20 Arg391 was also mutated to serine (Arg391Ser).30 Several other mutations have been identified in the VDR that affect VDR:RXRα heterodimerization including Gln259Pro and Phe251Cys.38,43 Gln259 was also mutated to glutamic acid (Gln259Glu).44 A Val346Met mutation was identified in a patient with HVDRR that may be important in RXRα heterodimerization.45 All of the patients with defects in VDR:RXRα heterodimerization had alopecia.
A very informative mutation has also been identified in the VDR that prevented coactivator recruitment that is critical for transcriptional activity.46 An Glu420Lys mutation located in helix H12 caused no defect in multiple steps in VDR gene regulation including ligand binding, VDR:RXRα heterodimerization or DNA binding. However, the Glu420Lys mutation abolished VDR binding by the coactivators SRC-1 and DRIP205. Interestingly, the child with the Glu420Lys mutation, although full-blown rickets was present, did not have alopecia.46 Importantly, this finding suggested that ligand-mediated transactivation and coactivator recruitment by the VDR, which is crucial to prevent rickets, is not required for normal hair growth.
Several compound heterozygous mutations in the VDR gene have been identified in children with HVDRR. In these cases each heterozygotic parent harbored a different mutant VDR and parental consanguinity was not involved. One patient was heterozygous for a Glu329Lys mutation and also had a second mutation on the other VDR allele that deleted a single nucleotide 366 (366delC) in exon 4.47 The single-base deletion resulted in a frameshift creating a premature termination signal that truncated most of the LBD. Glu329 in helix 8 is important for heterodimerization with RXRa and the Glu329Lys mutation likely disrupts this critical step in the activation pathway. Leu263Arg and Arg391Ser compound heterozygous mutations were also identified in the VDR gene in a child with HVDRR and early-childhood-onset type 1 diabetes.30 The mutant VDRs in this case exhibited differential effects on 24-hydroxylase and RelB promoters. The 24-hydroxylase responses were abolished in the Leu263Arg mutant but only partially altered in the Arg391Ser mutant. On the other hand, RelB responses were normal for the Leu263Arg mutant but the Arg391Ser mutant was defective in this response.30 The reason for the differential activities of these VDR mutants is unknown. Compound heterozygous mutations were also found in the VDR gene in a patient with HVDRR and alopecia.29 The patient was heterozygous for a nonsense mutation Arg30stop and a 3-bp deletion in exon 6 that deleted the codon for lysine at amino acid 246 (ΔLys246). The ΔLys246 mutation did not affect ligand binding but abolished heterodimerization with RXRα and binding to coactivators.29 Compound heterozygous mutations were also found in the VDR gene in a patient with HVDRR without alopecia.31 The patient was heterozygous for two missense mutations Thr146Ile and Arg158Cys in the VDR LBD (Figure 2).
Two cases of insertions/duplications in the VDR gene causing HVDRR have been reported. In one case, a unique 5-bp deletion/8-bp insertion was found in the VDR gene.48 The mutation deleted amino acids Lys141 and Thr142 and inserted three amino acids (Leu141, Trp142 and Ala143). Only the Ala143 insertion into the WT VDR disrupted transactivation to the same extent as the natural mutation. The patient with this mutation did not have alopecia. In the second case, a 102-bp insertion/duplication was found in the VDR gene, which introduced a premature stop (Tyr401stop) and deleted helix H12.49 The truncated VDR was able to heterodimerize with RXRα, bind to DNA and interact with the corepressor hairless (HR) but failed to bind coactivators and was transactivation-defective. The patient with this mutation had patchy alopecia.
There is only a single reported case where investigators failed to detect a mutation in the VDR as the basis of HVDRR.50 In this case the authors speculated that the resistance to the action of 1,25(OH)2D was due to abnormal expression of hormone response element-binding proteins belonging to the hnRNP family, which prevented the VDR:RXRα complex from binding to VDREs in target genes.51
Animal models of HVDRR: the VDR-null mouse
Mouse models of HVDRR have been created in the laboratory by several groups using targeted ablation of the VDR52,53,54,55 and lessons for the human disease learned from the mouse models are discussed by Eisman and Bouillon in this special issue.4 The targeted region of the VDR to create the knockdown has been the DBD domain. The VDR-null mice (VDRKO) recapitulate the findings in the children with HVDRR. The VDR-null mice appear normal at birth and become hypocalcemic and their PTH levels rise sometime after weaning. Bone mineralization is severely impaired and the changes of rickets develop over time. The VDR-null mice have normal hair at birth but develop progressive alopecia, thickened skin, enlarged sebaceous glands and epidermal cysts.52,53 The metabolic derangements are usualy fatal. In one VDR-null mouse model uterine hypoplasia with impaired folliculogenesis was found in female reproductive organs but it was not completely clear whether this was secondary to hypocalcemia or the VDR defect.53 When the VDR-null mice are fed a ‘rescue' diet, their calcium can be normalized and the rickets reversed or prevented as was described for children with HVDRR successfully treated with intravenous or oral calcium supplements.10,11,12,14 Many non-skeletal abnormalities seen in the hypocalcemic mice are prevented by the rescue diet, indicating that many abnormalities resulted from the hypocalcemia and were not directly caused by the absence of a functional VDR. However, again as in children with HVDRR, the alopecia is not reversed or prevented by normalization of calcium homeostasis.13
A spontaneous case of HVDRR in a Pomeranian dog was recently described.56 The dog had characteristic findings of HVDRR with marked hypocalcemia, elevated 1,25(OH)2D levels and partial alopecia. The VDR mutation was a deletion causing a frameshift at Arg175 and a premature termination of the VDR protein. The dog had severe bone disease and developed a spinal fracture of T11 causing acute spinal cord compression requiring euthanization.
Treatment of HVDRR
Mutations in the VDR that cause HVDRR result in partial or total resistance to the action of 1,25(OH)2D.11,12,14 Despite elevated levels of 1,25(OH)2D, the patients become hypocalcemic. The mechanism appears to be predominately due to a lack of VDR signaling in the intestine to promote calcium absorption by a lack of upregulation of a series of intestinal target genes including TRPV6 and calbindin-9k57,58,59,60 (and see Christakos et al in this special issue3). The hypocalcemia leads to secondary hyperparathyroidism and hypophosphatemia, causing a decrease in bone mineralization and the development of rickets. Some HVDRR patients improve both clinically and radiologically when treated with pharmacological doses of vitamin D ranging from 5,000–40 000 IU per day; 20–200 μg per day of 25(OH)D3 and 17–20 μg per day of 1,25(OH)2D3.11,12,14 Some patients also responded to 1α(OH)D3.11,12,14 The patient with the His305Gln mutation, a contact point for the 25-hydroxyl group of 1,25(OH)2D3, showed improvement with 12.5 μg per day calcitriol treatment.42,61 On the other hand, the patient with the Arg274Leu mutation, a contact point for the 1α-hydroxyl group of 1,25(OH)2D3, was unresponsive to treatment with 600 000 IU vitamin D, up to 24 μg per day of 1,25(OH)2D3 or 12 μg per day 1α (OH)D3.62 Mutations that may respond to treatment with vitamin D and its metabolites are usually in the LBD, which cause a reduced binding affinity for 1,25(OH)2D that can be overcome by increasing the concentration of ligand. Mutations that cause premature termination of the protein, that prevent DNA binding or RXRα dimerization or that prevent coactivator binding do not respond to hormone therapy. Except for coactivator-binding defects these are the same mutations that are associated with alopecia.10,11,12,13,14
When patients fail to respond to vitamin D or calcitriol, as in most cases, intensive calcium therapy is used.11,12,14 Oral calcium can be absorbed in the intestine by both vitamin D-dependent and vitamin D-independent pathways.3 In children with non-functional VDR, the vitamin D-independent pathway becomes critical. When oral calcium therapy is successful, the calcium levels in the gut have been raised high enough so that passive diffusion or other non-vitamin D-dependent absorption is adequate to achieve and maintain normocalcemia. The high calcium may in fact induce expression of transport components like calbindin 9k and TRPV6.63
Intravenous (IV) calcium infusions are used to treat children with HVDRR who failed prior treatments with large doses of vitamin D derivatives and/or oral calcium.64,65,66,67,68 Delivery of calcium by the IV route bypasses the calcium absorption defect in the intestine caused by the lack of action of the mutant VDR.11,12,27 However, in affected children receiving IV calcium, when the IV therapy is discontinued the syndrome recurs slowly over time. Some children have been managed with intermittent IV calcium regimens using oral calcium in the intervals.27,36 Once the child is older, perhaps when the skeleton has finished its major growth or when puberty initiates sex hormone secretion, oral calcium often suffices to maintain normocalcemia and the IV calcium regimen can be discontinued.12,68 Oral calcium alone has sometimes been successfully used as a therapy for HVDRR patients from the outset.10,11,12,69 In one study calcium delivered via the enteral route using a gastric tube was successful in reversing the metabolic abnormalities in a case of HVDRR when maintaining an IV became problematic in a young child due to repeated infections of the intravenous line.70
A recent study has suggested the use of Cinacalcet as an adjunctive therapy for treatment of the secondary hyperparathyroidism in HVDRR.71 In this study the authors emphasized that secondary hyperparathyroidism from inadequate calcium absorption in the intestine contributed to the underlying pathophysiology for rachitic changes in HVDRR. They described a novel use of Cinacalcet to treat a child with HVDRR in whom conventional modes of therapy had to be discontinued. This drug acts as a calcimimetic and is used most often in renal disease to suppress secondary hyperparathyroidism. Cinacalcet therapy along with high-dose oral calcium effectively normalized the hyperparathyroidism and other metabolic abnormalities in their patient. The authors suggested that the relative ease of administration of the calcimimetic as a once- or twice-daily oral preparation, compared with traditional intravenous calcium administration, should encourage its move to the frontline of treatment of HVDRR. Although only reported in this single case of HVDRR thus far, the concept has an excellent rationale and appears that it would be especially useful in those cases where suppression of elevated PTH is hard to achieve with calcium alone.
Spontaneous healing of rickets has been observed in some HVDRR patients as they get older and all therapy sometimes can be discontinued.10,12,72,73,74 In all of the cases regardless of the therapy, if alopecia is present, it remains unchanged by the treatment despite normalization of calcium and all metabolic parameters including healing of rickets.10,11,12,13,14
An interesting finding is that raising the serum calcium to normal concentrations by IV or oral calcium administration reversed all aspects of HVDRR including hypocalcemia, hypophosphatemia, secondary hyperparathyroidism, rickets, elevated alkaline phosphatase, and so on, except for alopecia.10,11,12,13,14 Correcting the hypocalcemia often corrects the hypophosphatemia without the need for phosphate supplements, although some children with extremely decreased serum phosphate have been treated with phosphate.36 This finding indicates that the low phosphate was predominantly caused by secondary hyperparathyroidism, which normalizes with correction of the hypocalcemia even in the absence of VDR action. The role of the FGF 23/Klotho axis on phosphate metabolism and 1,25(OH)2D levels75 (and Fukomoto in this special issue76) has not been examined in HVDRR.
One important conclusion from these studies is that the most important actions of 1,25(OH)2D on calcium and bone homeostasis occur in the intestine on calcium absorption and not in the bone. The ability of the rachitic bone abnormality to normalize in the absence of VDR-mediated vitamin D action in bone was surprising. The data are incomplete in patients about whether the bones are entirely normal and careful studies of the bone histology in VDR-null mice suggest that subtle defects remain in the bones of VDR-null mice whose serum calcium had been corrected by a rescue diet.77 However, the reversal of all clinical aspects of HVDRR with IV calcium does indicate that healing of the bones and reversal of secondary hyperparathyroidism and hypophosphatemia can take place without normal VDR-mediated vitamin D action.12,14 There is no doubt that vitamin D has important actions on bone and parathyroid cells,5 as discussed in many chapters in special issue.78,79,80 However, these actions can apparently be compensated for in vivo if the calcium level is normalized. That high doses of oral calcium can improve hypocalcemia in children with HVDRR suggests that VDR-independent pathways of calcium absorption must come into play in the absence of functional VDR. Intestinal calcium absorption is known to have paracellular as well as transcellular pathways, and the paracellular route is dependent on high concentrations of calcium within the intestine.81 This mechanism may contribute to the benefits of oral calcium supplementation in the children with HVDRR.
However, some children with HVDRR appear to actually ‘outgrow' their requirement for calcium supplementation as they grow older, and they can do well with no supplementation at all requiring an additional explanation to just raising the concentration of intestinal calcium. This phenomenon occurs around the time of puberty or even before. In a recent paper Chaturvedi et al.82 described the clinical course of three children with the previously reported Arg73X mutation leading to an early truncation in the VDR. The authors detail their findings and support the idea that VDR-independent pathways become operative as children grow up.82 As described by Fudge and Kovacs,83 vitamin D receptor-independent mechanisms of intestinal calcium absorption are operative in the placenta and thus may supply normal calcium to the fetus because mineral metabolism is apparently normal at birth in HVDRR infants.82 As has been noticed in multiple cases of HVDRR, as the affected children grow up, their requirement for calcium supplementation diminishes and calcium can even be discontinued with continued normal mineral metabolism, suggesting that an adaptation occurs with a transition to a VDR-independent pathway to regulate intestinal calcium absorption. One possible explanation is a role for estrogens, which have been shown to upregulate calcium transporter genes by VDR-independent mechanisms.84,85 Some HVDRR children appear to ‘outgrow' their metabolic defect sometime toward the end of puberty when they no longer require calcium supplementation to normalize their serum calcium levels and prevent secondary secondary hyperparathyroidism. To understand the mechanism for the spontaneous normalization of calcium metabolism Tiosano et al.86 used stable calcium isotopes to study calcium absorption. Fractional calcium absorption (FCA) in HVDRR patients aged 1.5–17 years was 34.9±11.2% compared with 57.3±2.0% in age-matched controls (P<0.00004), whereas in HVDRR patients aged 18–26 years, it was 82.0±7.8 in subjects and 53.6±1.2% in controls (P<0.001). FCA of HVDRR patients older than 29 years was comparable to controls. Femoral-neck BMD Z-score was −2.38±0.3 in patients under 18 years and improved to 0.28±0.87 in post-pubertal patients (P<0.0001). Bone structure by high-resolution magnetic resonance imaging and bone parameters of HVDRR patients and controls were similar. The authors concluded that calcium absorption is highly vitamin D-dependent during infancy until the end of puberty, after which time there is a period of ∼10 years in which mechanisms other than vitamin D-dependent ones are substantially involved in calcium absorption and are responsible for the improvement in metabolic status of HVDRR children who are metabolically normal without supplemental calcium.
Alopecia
The molecular analysis of the VDR from HVDRR patients with and without alopecia has provided several clues to the functions of the VDR that are important for normal hair growth.10,11,12,13,14 For example, children with vitamin D deficiency or with 1α-hydroxylase deficiency do not develop alopecia, suggesting that the absence of vitamin D action is not the cause of alopecia. Patients with premature stop mutations have alopecia, suggesting that the intact VDR protein is critical for renewed hair growth after birth.52,53 Forced expression of the wild-type VDR in keratinocytes of VDR-null mice prevented alopecia, a finding that further supports a role for the VDR itself (ligand-independent) in regulating hair growth and a critical role for keratinocytes.87 Patients with DBD mutations, similar to some VDR-null mice, also have alopecia indicating that VDR binding to DNA is critical to prevent alopecia. Patients with VDR mutations that inhibit RXRα heterodimerization have alopecia, indicating an essential role for VDR:RXRα heterodimers in hair growth.38,40,43 Inactivation of RXRα in keratinocytes in mice also caused alopecia, clearly demonstrating a role for RXRα in normal hair growth.88 On the other hand, patients with VDR mutations that abolish ligand binding do not develop alopecia, suggesting that a ligand-independent action of the VDR may be critical to regulate the normal hair cycle.13,46,55,89,90,91 The patient with the Glu420Lys mutation that abolished coactivator binding (but not ligand binding, RXRα heterodimerization or DNA binding) did not have alopecia despite having severe metabolic defects and rickets, indicating that VDR actions to regulate hair growth were independent of coactivator interactions.46 Also, when ligand-binding-defective or coactivator-binding-defective mutant VDRs were specifically expressed in keratinocytes in VDR-null mice that have alopecia, hair growth was fully or partially restored.91 Cumulatively, the data indicate that unliganded VDR is the essential factor that acts to prevent alopecia.
As shown in Figure 1, various patterns of alopecia occur ranging from total absence of hair to diffuse sparse hair, to areas of absent hair adjacent to areas with hair. There does not appear to be a correlation of the alopecia pattern and the type of mutation present. In fact, unrelated children with the same mutation showed different patterns of alopecia (Figure 1).27 The mechanism behind the development of these diverse patterns of alopecia is unknown.
Regarding the mechanism by which unliganded VDR prevents alopecia, the definitive answer is still unclear. The alopecia associated with HVDRR is clinically and pathologically indistinguishable from the generalized disease atrichia with papular lesions (APL, OMIM#209500) found in patients with mutations in the hairless (hr) gene.47,92,93 APL is a rare autosomal recessively inherited form of irreversible alopecia characterized by alopecia and by papular lesions of keratin-filled cysts on various regions of the body caused by mutations in the hr gene that encodes the HR protein. Similar skin lesions can be seen in HVDRR patients, although generally less prominently.47 HR acts as a VDR corepressor that directly interacts with the VDR and suppresses 1,25(OH)2D3-mediated transactivation.89,93,94 It has been hypothesized that the role of the VDR in the hair cycle is to repress the expression of a gene(s) in a ligand-independent manner.46,55,89,91,93 The ligand-independent activity requires that the VDR heterodimerize with RXRα and bind to DNA.46,90 The corepressor actions of HR may also be required in order for the unliganded VDR to repress gene transcription during the hair cycle. Mutations in the VDR that disrupt the ability of the unliganded VDR to suppress gene transcription are hypothesized to lead to the derepression of a gene(s) whose product, when expressed inappropriately, disrupts the hair cycle that ultimately leads to alopecia.46,55,89,91,93 Inhibitors of the Wnt signaling pathway are possible candidates.13,95,96,97
Recent data on children with HVDRR as they grow up
In recent years there have been many new actions attributed to vitamin D that mediate important and wide-spread effects on a number of target organs and diseases that are unrelated to calcium and bone homeostasis.5 These extra-skeletal effects include actions to reduce the risk of cancer, autoimmune disease, infection, neurodegeneration, hypertension and cardiac disease, and so on. At this time a trend toward an increased risk for any of these potential problems in the children with HVDRR has not been detected.12 However, there are very few cases of HVDRR and most of the cases are detected in infants and young children, so that it may be too early in their life to detect an increased tendency toward any of these potential health problems.
For example, ordinary vitamin D deficiency has been linked to hypertension and an increased prevalence of cardiovascular risk factors and disease.98,99 Studies in VDR-null mice have revealed an overactive renin–angiotensin system (RAS) and consequent high blood pressure and cardiac hypertrophy.55,100 This aspect of the cardiovascular system was studied in 17 age- and gender-matched HVDRR subjects (9 males, 8 females, aged 6–36 years).101,102 In addition to the vitamin D axis, RAS was studied. Serum calcium, phosphorus and alkaline phosphatase values were normal. Serum 1,25(OH)2D and PTH were elevated but not plasma renin activity or angiotensin-converting enzyme (ACE). Angiotensin II levels were higher than normal in the HVDRR patients but not significantly different from those of the controls. Aldosterone levels were normal in all HVDRR patients. No HVDRR patient had hypertension or echocardiographic pathology. These findings reveal that 6- to 36-year-old humans with HVDRR have normal renin and ACE activity, mild but non-significant elevation of angiotensin II, normal aldosterone levels and no hypertension or gross heart abnormalities. Whether these young subjects will eventually develop an increased risk for hypertension and cardiac disease remains to be seen. However, the number of subjects is very small and they are still very young. Only further study over time will reveal whether adults with HVDRR have increased risk for the many non-skeletal abnormalities associated with vitamin D deficiency.
HVDRR cells used as reagents to study actions of the VDR
For many years the nuclear receptor field has gradually come to recognize that steroid receptors mediate non-genomic actions that occur too rapidly to be caused by induction of new mRNA and translation of new proteins by the classic genomic pathway.103 In the vitamin D field the exploration of rapid actions started with the finding of transcaltachia, a rapid action of 1,25(OH)2D on intestinal calcium transport.104 However, despite many advances in this field with other nuclear receptors, acceptance of the concept that 1,25(OH)2D mediated non-genomic actions was viewed with some skepticism. We recognized that HVDRR mutant human fibroblasts would be an ideal reagent to study whether VDR indeed mediated non-genomic actions and in collaboration with Rebecca Mason's group we undertook experiments to clarify the role of the 1,25(OH)2D/VDR pathway in the rapid actions in the skin that Mason's group had discovered to protect against DNA damage due to ultra-violet radiation (UVR) exposure.105 Our findings indicated that human fibroblasts harboring wild-type VDR could mediate protection against UVR DNA damage. However, a VDR with a DBD point mutation that failed to induce genomic actions could still mediate photoprotection, while a mutant VDR with early termination and no VDR failed to mediate photoprotection.106 Photoprotection also remained in HVDRR fibroblasts expressing a VDR with a mutation in helix H1 of the classical LBD. Both DBD and LBD defects that resulted in a failure to mediate genomic responses could still mediate photoprotection, implicating non-genomic responses for photoprotection.
These findings provide evidence that mutant VDRs that were unable to mediate classic genomic activity were still capable of inducing the rapid actions of DNA photoprotection. This evidence provides strong support for the hypothesis that the VDR is required and involved in rapid, non-genomic actions. The story is more complicated in that ERp57 is also involved and is essential for the rapid action of DNA photoprotection. Evidence for an alternate 1,25(OH)2D membrane receptor, endoplasmic reticulum stress protein 57 (ERp57), also known as membrane-associated rapid response steroid-binding protein (MARRS) and protein disulfide isomerase family A, member 3, has also been reported.107,108 The data implicate both the VDR and ERp57 as critical components for actions of 1,25(OH)2D against DNA photodamage, but the VDR does not require normal DNA binding or classical ligand binding to mediate photoprotection.106
Previous observations in mice showed that the subcutaneous and visceral white adipose tissue depots were smaller in VDRKO mice than WT mice.109 The fat that was present had features of brown fat including multilocular fat droplets and elevated levels of uncoupling protein 1 (UCP1), the critical protein for uncoupling fatty acid oxidation in brown fat and burning energy, implicating the VDR in adipocyte metabolism and lipid storage. However, the relevence of these findings for humans was unclear. Malloy and Feldman110 used the HVDRR fibroblasts to examine the role of the VDR in regulating adipocyte metabolism in human cells. The HVDRR and normal human fibroblasts were differentiated into adipocyte-like cells that accumulated many lipid droplets and expressed the pan fat cell marker fatty acid binding protein 4 (FABP4). UCP1 was found to be over-expressed in the HVDRR cells including cells that had no VDR. In contrast, UCP1 levels were suppressed in normal control cells with WT VDR, suggesting that the VDR represses UCP1 expression. Chromatin immunoprecipitation analyses showed that the inhibition is enforced by VDR occupancy of a negative response element in the promoter proximal region of the UCP1 gene. These studies were performed in the absence of 1,25(OH)2D3, indicating that the unligand VDR regulates UCP1 expression.
Conclusions
The biochemical and genetic analyses of the VDR in HVDRR patients has yielded important insights into the structure and function of the receptor that mediates 1,25(OH)2D action. Mechanistic studies using the VDR-null mouse, a model of the human disease, have added much to our understanding of the molecular biology of the 1,25(OH)2D/VDR mechanism of action.55 Study of the affected children with HVDRR continues to provide a more complete understanding of the biological role of 1,25(OH)2D in vivo in humans. A concerted investigative approach of HVDRR at the clinical, cellular and molecular level has proven exceedingly valuable in gaining knowledge about the functions of the domains of the VDR protein and in elucidating a detailed understanding of the mechanism of action of 1,25(OH)2D. These studies have been essential to promote the well being of the families with HVDRR and in improving the diagnostic and clinical management of this rare genetic disease.
Footnotes
The authors declare no conflict of interest.
References
- Pike JW, Lee SM, Meyer MB. Regulation of gene expression by 1,25-dihydroxyvitamin D3 in bone cells: exploiting new approaches and defining new mechanisms. BoneKEy Reports 3: Article number: 482 (2014); 10.1038/bonekey.2013.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Driel M, van Leeuwen JPTM. Vitamin D endocrine system and osteoblasts. BoneKEy Reports 3: Article number: 493 (2014); 10.1038/bonekey.2013.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christakos S, Lieben L, Masuyama R, Carmeliet G. Vitamin D endocrine system and the intestine. BoneKEy Reports 3: Article number: 496 (2014); 10.1038/bonekey.2013.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisman JA, Bouillon R. Vitamin D: direct effects of vitamin D metabolites on bone: lessons from genetically modified mice. BoneKEy Reports 3: Article number: 499 (2014); 10.1038/bonekey.2013.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman D, Pike JW, Adams JS (eds). Vitamin D. Third Edn Elsiever: San Diego, CA, USA, 2011. [Google Scholar]
- O'Riordan JLH, Bijvoet OLM. Rickets before the discovery of Vitamin D. BoneKEy Reports 3: Article number: 478 (2014); 10.1038/bonekey.2013.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouillon R, Suda T. Vitamin D: calcium and bone homeostasis during evolution. BoneKEy Reports 3: Article number: 480 (2014); 10.1038/bonekey.2013.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glorieux FH, Pettifor JM. Vitamin D/dietary calcium deficiency rickets and pseudo-vitamin D deficiency rickets. BoneKEy Reports 3: Article number: 524 (2014); 10.1038/bonekey.2014.19 (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebeling PR. Vitamin D and bone health: epidemiologic studies. BoneKEy Reports 3: Article number: 511 (2014); 10.1038/bonekey.2014.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman D, Malloy PJ, Miller WL. Genetic disorders of vitamin D synthesis and action. In: Thakker RV, Whyte MP, Eisman JA, Igarashi T (eds).Genetics of Bone Biology and Skeletal Disease. Elsevier: San Diego, CA, USA, 2013, pp 537–552. [Google Scholar]
- Malloy PJ, Feldman D. Genetic disorders and defects in vitamin D action. Endocrinol Metab Clin N Am 2010;39:333–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malloy PJ, Tiosano D, Feldman D. Hereditary 1,25-dihydroxyvitamin D resistant rickets. In: Feldman D, Pike JW, Adams JS (eds).Vitamin D 3 Edn Elsevier: San Diego, CA, USA, 2011, pp1197–1232. [Google Scholar]
- Malloy PJ, Feldman D. The role of vitamin D receptor mutations in the development of alopecia. Mol Cell Endocrinol 2011;347:90–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malloy PJ, Pike JW, Feldman D. The vitamin D receptor and the syndrome of hereditary 1,25-dihydroxyvitamin D-resistant rickets. Endocr Rev 1999;20:156–188. [DOI] [PubMed] [Google Scholar]
- Hsieh JC, Jurutka PW, Selznick SH, Reeder MC, Haussler CA, Whitfield GK et al. The T-box near the zinc fingers of the human vitamin D receptor is required for heterodimeric DNA binding and transactivation. Biochem Biophys Res Commun 1995;215:1–7. [DOI] [PubMed] [Google Scholar]
- Hsieh JC, Whitfield GK, Oza AK, Dang HT, Price JN, Galligan MA et al. Characterization of unique DNA-binding and transcriptional-activation functions in the carboxyl-terminal extension of the zinc finger region in the human vitamin D receptor. Biochemistry 1999;38:16347–16358. [DOI] [PubMed] [Google Scholar]
- Jin CH, Kerner SA, Hong MH, Pike JW. Transcriptional activation and dimerization functions in the human vitamin D receptor. Mol Endocrinol 1996;10:945–957. [DOI] [PubMed] [Google Scholar]
- Jurutka PW, Hsieh JC, Remus LS, Whitfield GK, Thompson PD, Haussler CA et al. Mutations in the 1,25-dihydroxyvitamin D3 receptor identifying C-terminal amino acids required for transcriptional activation that are functionally dissociated from hormone binding, heterodimeric DNA binding, and interaction with basal transcription factor IIB, in vitro. J Biol Chem 1997;272:14592–14599. [DOI] [PubMed] [Google Scholar]
- Whitfield GK, Hsieh JC, Nakajima S, MacDonald PN, Thompson PD, Jurutka PW et al. A highly conserved region in the hormone-binding domain of the human vitamin D receptor contains residues vital for heterodimerization with retinoid X receptor and for transcriptional activation. Mol Endocrinol 1995;9:1166–1179. [DOI] [PubMed] [Google Scholar]
- Rochel N, Wurtz JM, Mitschler A, Klaholz B, Moras D. The crystal structure of the nuclear receptor for vitamin D bound to its natural ligand. Mol Cell 2000;5:173–179. [DOI] [PubMed] [Google Scholar]
- Berg JM. Proposed structure for the zinc-binding domains from transcription factor IIIA and related proteins. Proc Natl Acad Sci USA 1988;85:99–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mader S, Kumar V, de Verneuil H, Chambon P. Three amino acids of the oestrogen receptor are essential to its ability to distinguish an oestrogen from a glucocorticoid-responsive element. Nature 1989;338:271–274. [DOI] [PubMed] [Google Scholar]
- Umesono K, Evans RM. Determinants of target gene specificity for steroid/thyroid hormone receptors. Cell 1989;57:1139–1146. [DOI] [PubMed] [Google Scholar]
- Blanco JC, Wang IM, Tsai SY, Tsai MJ, O'Malley BW, Jurutka PW et al. Transcription factor TFIIB and the vitamin D receptor cooperatively activate ligand-dependent transcription. Proc Natl Acad Sci USA 1995;92:1535–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald PN, Sherman DR, Dowd DR, Jefcoat SC Jr, DeLisle RK. The vitamin D receptor interacts with general transcription factor IIB. J Biol Chem 1995;270:4748–4752. [DOI] [PubMed] [Google Scholar]
- Malloy PJ, Feldman D. Molecular defects in the vitamin D receptor associated with hereditary 1,25-dihydroxyvitamin D resistant rickets. In: Holick MF (ed).Vitamin D: Physiology, Molecular Biology, and Clinical Applications Humana Press: Totowa, NJ, USA, 2010;. [Google Scholar]
- Forghani N, Lum C, Krishnan S, Wang J, Wilson DM, Blackett PR et al. Two new unrelated cases of hereditary 1,25-dihydroxyvitamin D-resistant rickets with alopecia resulting from the same novel nonsense mutation in the vitamin D receptor gene. J Pediatr Endocrinol Metab 2010;23:843–850. [DOI] [PubMed] [Google Scholar]
- Malloy PJ, Zhou Y, Wang J, Hiort O, Feldman D. Hereditary vitamin D-resistant rickets (HVDRR) owing to a heterozygous mutation in the vitamin D receptor. J Bone Miner Res 2011;26:2710–2718. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Wang J, Malloy PJ, Dolezel Z, Feldman D. Compound heterozygous mutations in the vitamin D receptor in a patient with hereditary 1,25-dihydroxyvitamin D-resistant rickets with alopecia. J Bone Miner Res 2009;24:643–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen M, d'Alesio A, Pascussi JM, Kumar R, Griffin MD, Dong X et al. Vitamin D-resistant rickets and type 1 diabetes in a child with compound heterozygous mutations of the vitamin D receptor (L263R and R391S): dissociated responses of the CYP-24 and rel-B promoters to 1,25-dihydroxyvitamin D3. J Bone Miner Res 2006;21:886–894. [DOI] [PubMed] [Google Scholar]
- Song JK, Yoon KS, Shim KS, Bae CW. Novel compound heterozygous mutations in the vitamin D receptor gene in a Korean girl with hereditary vitamin D resistant rickets. J Korean Med Sci 2011;26:1111–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malloy PJ, Feldman D. Molecular defects in the vitamin D receptor associated with hereditary 1,25-dihydroxyvitamin D resistant rickets. In: Holick MF (ed).Vitamin D: Physiology, Molecular Biology, and Clinical Applications Humana Press: Totowa, NJ, USA, 1998;. [Google Scholar]
- Malloy PJ, Hochberg Z, Tiosano D, Pike JW, Hughes MR, Feldman D. The molecular basis of hereditary 1,25-dihydroxyvitamin D3 resistant rickets in seven related families. J Clin Invest 1990;86:2071–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechica JB, Leite MO, Mendonca BB, Frazzatto ES, Borelli A, Latronico AC. A novel nonsense mutation in the first zinc finger of the vitamin D receptor causing hereditary 1,25-dihydroxyvitamin D3-resistant rickets. J Clin Endocrinol Metab 1997;82:3892–3894. [DOI] [PubMed] [Google Scholar]
- Hawa NS, Cockerill FJ, Vadher S, Hewison M, Rut AR, Pike JW et al. Identification of a novel mutation in hereditary vitamin D resistant rickets causing exon skipping. Clin Endocrinol 1996;45:85–92. [PubMed] [Google Scholar]
- Ma NS, Malloy PJ, Pitukcheewanont P, Dreimane D, Geffner ME, Feldman D. Hereditary vitamin D resistant rickets: identification of a novel splice site mutation in the vitamin D receptor gene and successful treatment with oral calcium therapy. Bone 2009;45:743–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katavetin P, Wacharasindhu S, Shotelersuk V. A girl with a novel splice site mutation in VDR supports the role of a ligand-independent VDR function on hair cycling. Horm Res 2006;66:273–276. [DOI] [PubMed] [Google Scholar]
- Cockerill FJ, Hawa NS, Yousaf N, Hewison M, O'Riordan JL, Farrow SM. Mutations in the vitamin D receptor gene in three kindreds associated with hereditary vitamin D resistant rickets. J Clin Endocrinol Metab 1997;82:3156–3160. [DOI] [PubMed] [Google Scholar]
- Rut AR, Hewison M, Rowe P, Hughes M, Grant D, O'Riordan JLH. A novel mutation in the steroid binding region of the vitamin D receptor (VDR) gene in hereditary vitamin D resistant rickets (HVDRR). In: Norman AW, Bouillon R, Thomasset M (eds).Vitamin D: Gene Regulation, Structure-Function Analysis, and Clinical Application Eighth workshop on Vitamin D Walter de Gruyter: New York, NY, USA, 1991, pp94–95. [Google Scholar]
- Whitfield GK, Selznick SH, Haussler CA, Hsieh JC, Galligan MA, Jurutka PW et al. Vitamin D receptors from patients with resistance to 1,25-dihydroxyvitamin D3: point mutations confer reduced transactivation in response to ligand and impaired interaction with the retinoid X receptor heterodimeric partner. Mol Endocrinol 1996;10:1617–1631. [DOI] [PubMed] [Google Scholar]
- Aljubeh JM, Wang J, Al-Remeithi SS, Malloy PJ, Feldman D. Report of two unrelated patients with hereditary vitamin D resistant rickets due to the same novel mutation in the vitamin D receptor. J Pediatr Endocrinol Metab 2011;24:793–799. [DOI] [PubMed] [Google Scholar]
- Malloy PJ, Eccleshall TR, Gross C, Van Maldergem L, Bouillon R, Feldman D. Hereditary vitamin D resistant rickets caused by a novel mutation in the vitamin D receptor that results in decreased affinity for hormone and cellular hyporesponsiveness. J Clin Invest 1997;99:297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malloy PJ, Zhu W, Zhao XY, Pehling GB, Feldman D. A novel inborn error in the ligand-binding domain of the vitamin D receptor causes hereditary vitamin D-resistant rickets. Mol Genet Metab 2001;73:138–148. [DOI] [PubMed] [Google Scholar]
- Macedo LC, Soardi FC, Ananias N, Belangero VM, Rigatto SZ, De-Mello MP et al. Mutations in the vitamin D receptor gene in four patients with hereditary 1,25-dihydroxyvitamin D-resistant rickets. Arq Bras Endocrinol Metabol 2008;52:1244–1251. [DOI] [PubMed] [Google Scholar]
- Arita K, Nanda A, Wessagowit V, Akiyama M, Alsaleh QA, McGrath JA. A novel mutation in the VDR gene in hereditary vitamin D-resistant rickets. Br J Dermatol 2008;158:168–171. [DOI] [PubMed] [Google Scholar]
- Malloy PJ, Xu R, Peng L, Clark PA, Feldman D. A novel mutation in helix 12 of the vitamin D receptor impairs coactivator interaction and causes hereditary 1,25-dihydroxyvitamin D-resistant rickets without alopecia. Mol Endocrinol 2002;16:2538–2546. [DOI] [PubMed] [Google Scholar]
- Miller J, Djabali K, Chen T, Liu Y, Ioffreda M, Lyle S et al. Atrichia caused by mutations in the vitamin D receptor gene is a phenocopy of generalized atrichia caused by mutations in the hairless gene. J Invest Dermatol 2001;117:612–617. [DOI] [PubMed] [Google Scholar]
- Malloy PJ, Xu R, Cattani A, Reyes L, Feldman D. A unique insertion/substitution in helix H1 of the vitamin D receptor ligand binding domain in a patient with hereditary 1,25-dihydroxyvitamin D-resistant rickets. J Bone Miner Res 2004;19:1018–1024. [DOI] [PubMed] [Google Scholar]
- Malloy PJ, Wang J, Peng L, Nayak S, Sisk JM, Thompson CC et al. A unique insertion/duplication in the VDR gene that truncates the VDR causing hereditary 1,25-dihydroxyvitamin D-resistant rickets without alopecia. Arch Biochem Biophys 2007;460:285–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewison M, Rut AR, Kristjansson K, Walker RE, Dillon MJ, Hughes MR et al. Tissue resistance to 1,25-dihydroxyvitamin D without a mutation of the vitamin D receptor gene. Clin Endocrinol 1993;39:663–670. [DOI] [PubMed] [Google Scholar]
- Chen H, Hewison M, Hu B, Adams JS. Heterogeneous nuclear ribonucleoprotein (hnRNP) binding to hormone response elements: A cause of vitamin D resistance. Proc Natl Acad Sci USA 2003;100:6109–6114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YC, Pirro AE, Amling M, Delling G, Baron R, Bronson R et al. Targeted ablation of the vitamin D receptor: an animal model of vitamin D-dependent rickets type II with alopecia. Proc Natl Acad Sci USA 1997;94:9831–9835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshizawa T, Handa Y, Uematsu Y, Takeda S, Sekine K, Yoshihara Y et al. Mice lacking the vitamin D receptor exhibit impaired bone formation, uterine hypoplasia and growth retardation after weaning. Nat Genet 1997;16:391–396. [DOI] [PubMed] [Google Scholar]
- Panda DK, Miao D, Bolivar I, Li J, Hendy GN, Goltzman D. Comparison of skeletal phenotypes and mineral metabolism of mice with targeted disruption of the 25OH vitamin D3-1α hydroxylase (1αOHase) and of the vitamin D receptor (VDR). In: Norman AW, Bouillon R, Thomasset M (eds).Vitamin D: Gene Regulation, Structure-Function Analysis, and Clinical Application Twelveth Workshop on Vitamin D Walter de Gruyter: New York, NY, USA, 2003;. [Google Scholar]
- Bouillon R, Carmeliet G, Verlinden L, van Etten E, Verstuyf A, Luderer HF et al. Vitamin D and human health: lessons from vitamin D receptor null mice. Endocr Rev 2008;29:726–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeVine DN, Zhou Y, Ghiloni RJ, Fields EL, Birkenheuer AJ, Gookin JL et al. Hereditary 1,25-dihydroxyvitamin D-resistant rickets in a Pomeranian dog caused by a novel mutation in the vitamin D receptor gene. J Vet Intern Med 2009;23:1278–1283. [DOI] [PubMed] [Google Scholar]
- Renkema KY, Alexander RT, Bindels RJ, Hoenderop JG. Calcium and phosphate homeostasis: concerted interplay of new regulators. Ann Med 2008;40:82–91. [DOI] [PubMed] [Google Scholar]
- Fleet JC, Schoch RD. Molecular mechanisms for regulation of intestinal calcium absorption by vitamin D and other factors. Crit Rev Clin Lab Sci 2010;47:181–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JY, Swami S, Krishnan AV, Feldman D. Combination of calcitriol and dietary soy exhibits enhanced anticancer activity and increased hypercalcemic toxicity in a mouse xenograft model of prostate cancer. Prostate 2012;72:1628–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christakos S. Recent advances in our understanding of 1,25-dihydroxyvitamin D3 regulation of intestinal calcium absorption. Arch Biochem Biophys 2012;523:73–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Maldergem L, Bachy A, Feldman D, Bouillon R, Maassen J, Dreyer M et al. Syndrome of lipoatrophic diabetes, vitamin D resistant rickets, and persistent müllerian ducts in a Turkish boy born to consanguineous parents. Am J Med Genet 1996;64:506–513. [DOI] [PubMed] [Google Scholar]
- Fraher LJ, Karmali R, Hinde FR, Hendy GN, Jani H, Nicholson L et al. Vitamin D-dependent rickets type II: extreme end organ resistance to 1,25-dihydroxy vitamin D3 in a patient without alopecia. Eur J Pediatr 1986;145:389–395. [DOI] [PubMed] [Google Scholar]
- Ko SH, Lee GS, Vo TT, Jung EM, Choi KC, Cheung KW et al. Dietary calcium and 1,25-dihydroxyvitamin D3 regulate transcription of calcium transporter genes in calbindin-D9k knockout mice. J Rep Dev 2009;55:137–142. [DOI] [PubMed] [Google Scholar]
- Balsan S, Garabedian M, Liberman UA, Eil C, Bourdeau A, Guillozo H et al. Rickets and alopecia with resistance to 1,25-dihydroxyvitamin D: two different clinical courses with two different cellular defects. J Clin Endocrinol Metab 1983;57:803–811. [DOI] [PubMed] [Google Scholar]
- Balsan S, Garabedian M, Larchet M, Gorski AM, Cournot G, Tau C et al. Long-term nocturnal calcium infusions can cure rickets and promote normal mineralization in hereditary resistance to 1,25-dihydroxyvitamin D. J Clin Invest 1986;77:1661–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisman Y, Bab I, Gazit D, Spirer Z, Jaffe M, Hochberg Z. Long-term intracaval calcium infusion therapy in end-organ resistance to 1,25-dihydroxyvitamin D. Am J Med 1987;83:984–990. [DOI] [PubMed] [Google Scholar]
- Bliziotes M, Yergey AL, Nanes MS, Muenzer J, Begley MG, Viera NE et al. Absent intestinal response to calciferols in hereditary resistance to 1,25-dihydroxyvitamin D: documentation and effective therapy with high dose intravenous calcium infusions. J Clin Endocrinol Metab 1988;66:294–300. [DOI] [PubMed] [Google Scholar]
- Hochberg Z, Tiosano D, Even L. Calcium therapy for calcitriol-resistant rickets. J Pediatr 1992;121:803–808. [DOI] [PubMed] [Google Scholar]
- Sakati N, Woodhouse NJY, Niles N, Harfi H, de Grange DA, Marx S. Hereditary resistance to 1,25-dihydroxyvitamin D: clinical and radiological improvement during high-dose oral calcium therapy. Hormone Res 1986;24:280–287. [DOI] [PubMed] [Google Scholar]
- Huang K, Malloy P, Feldman D, Pitukcheewanont P. Enteral calcium infusion used successfully as treatment for a patient with hereditary vitamin D resistant rickets (HVDRR) without alopecia: a novel mutation. Gene 2013;512:554–559. [DOI] [PubMed] [Google Scholar]
- Srivastava T, Alon US. Cinacalcet as adjunctive therapy for hereditary 1,25-dihydroxyvitamin D-resistant rickets. J Bone Miner Res 2013;28:992–996. [DOI] [PubMed] [Google Scholar]
- Hochberg Z, Benderli A, Levy J, Vardi P, Weisman Y, Chen T et al. 1,25-Dihydroxyvitamin D resistance, rickets, and alopecia. Am J Med. 1984;77:805–811. [DOI] [PubMed] [Google Scholar]
- Hirst MA, Hochman HI, Feldman D. Vitamin D resistance and alopecia: a kindred with normal 1,25-dihydroxyvitamin D binding, but decreased receptor affinity for deoxyribonucleic acid. J Clin Endocrinol Metab 1985;60:490–495. [DOI] [PubMed] [Google Scholar]
- Chen TL, Hirst MA, Cone CM, Hochberg Z, Tietze HU, Feldman D. 1,25-dihydroxyvitamin D resistance, rickets, and alopecia: analysis of receptors and bioresponse in cultured fibroblasts from patients and parents. J Clin Endocrinol Metab 1984;59:383–388. [DOI] [PubMed] [Google Scholar]
- Martin A, David V, Quarles LD. Regulation and function of the FGF23/klotho endocrine pathways. Physiol Rev 2012;92:131–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumoto S. Phosphate metabolism and vitamin D. BoneKEy Reports 3: Article number: 497 (2014); 10.1038/bonekey.2013.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda DK, Miao D, Bolivar I, Li J, Huo R, Hendy GN et al. Inactivation of the 25-hydroxyvitamin D 1α-hydroxylase and vitamin D receptor demonstrates independent and interdependent effects of calcium and vitamin D on skeletal and mineral homeostasis. J Biol Chem 2004;279:16754–16766. [DOI] [PubMed] [Google Scholar]
- Sims NA, Martin TJ. Coupling the activities of bone formation and resorption: a multitude of signals within the basic multicellular unit. BoneKEy Reports 3: Article number: 481 (2014); 10.1038/bonekey.2013.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigwekar SU, Tamez H, Thadani RI. Vitamin D and chronic kidney disease–mineral bone disease (CKD–MBD). BoneKEy Reports 3: Article number: 498 (2014); 10.1038/bonekey.2013.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLuca HF. The development of a bone- and parathyroid-specific analog of vitamin D: 2-methylene-19-Nor-(20S)-1α,25-dihydroxyvitamin D3. BoneKEy Reports 3: Article number: 514 (2014); 10.1038/bonekey.2014.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronner F. Recent developments in intestinal calcium absorption. Nutr Rev 2009;67:109–113. [DOI] [PubMed] [Google Scholar]
- Chaturvedi D, Garabedian M, Carel JC, Leger J. Different mechanisms of intestinal calcium absorption at different life stages: therapeutic implications and long-term responses to treatment in patients with hereditary vitamin D-resistant rickets. Horm Res Paediatr 2012;78:326–331. [DOI] [PubMed] [Google Scholar]
- Fudge NJ, Kovacs CS. Pregnancy up-regulates intestinal calcium absorption and skeletal mineralization independently of the vitamin D receptor. Endocrinology 2010;151:886–895. [DOI] [PubMed] [Google Scholar]
- Van Cromphaut SJ, Rummens K, Stockmans I, Van Herck E, Dijcks FA, Ederveen AG et al. Intestinal calcium transporter genes are upregulated by estrogens and the reproductive cycle through vitamin D receptor-independent mechanisms. J Bone Miner Res 2003;18:1725–1736. [DOI] [PubMed] [Google Scholar]
- Colin EM, Van Den Bemd GJ, Van Aken M, Christakos S, De Jonge HR, Deluca HF et al. Evidence for involvement of 17β-estradiol in intestinal calcium absorption independent of 1,25-dihydroxyvitamin D3 level in the rat. J Bone Miner Res 1999;14:57–64. [DOI] [PubMed] [Google Scholar]
- Tiosano D, Hadad S, Chen Z, Nemirovsky A, Gepstein V, Militianu D et al. Calcium absorption, kinetics, bone density, and bone structure in patients with hereditary vitamin D-resistant rickets. J Clin Endocrinol Metab 2011;96:3701–3709. [DOI] [PubMed] [Google Scholar]
- Chen CH, Sakai Y, Demay MB. Targeting expression of the human vitamin D receptor to the keratinocytes of vitamin D receptor null mice prevents alopecia. Endocrinology 2001;142:5386–5389. [DOI] [PubMed] [Google Scholar]
- Li M, Chiba H, Warot X, Messaddeq N, Gerard C, Chambon P et al. RXR-α ablation in skin keratinocytes results in alopecia and epidermal alterations. Development 2001;128:675–688. [DOI] [PubMed] [Google Scholar]
- Hsieh JC, Sisk JM, Jurutka PW, Haussler CA, Slater SA, Haussler MR et al. Physical and functional interaction between the vitamin D receptor and hairless corepressor, two proteins required for hair cycling. J Biol Chem 2003;278:38665–38674. [DOI] [PubMed] [Google Scholar]
- Malloy PJ, Feldman D. Hereditary 1,25-Dihydroxyvitamin D-resistant rickets. Endocr Dev 2003;6:175–199. [DOI] [PubMed] [Google Scholar]
- Skorija K, Cox M, Sisk JM, Dowd DR, MacDonald PN, Thompson CC et al. Ligand-independent actions of the vitamin D receptor maintain hair follicle homeostasis. Mol Endocrinol 2005;19:855–862. [DOI] [PubMed] [Google Scholar]
- Ahmad W, Faiyaz ul Haque M, Brancolini V, Tsou HC, ul Haque S, Lam H et al. Alopecia universalis associated with a mutation in the human hairless gene. Science 1998;279:720–724. [DOI] [PubMed] [Google Scholar]
- Wang J, Malloy PJ, Feldman D. Interactions of the vitamin D receptor with the corepressor hairless: analysis of hairless mutants in atrichia with papular lesions. J Biol Chem 2007;282:25231–25239. [DOI] [PubMed] [Google Scholar]
- Xie Z, Chang S, Oda Y, Bikle DD. Hairless suppresses vitamin D receptor transactivation in human keratinocytes. Endocrinology 2006;147:314–323. [DOI] [PubMed] [Google Scholar]
- Beaudoin GM 3rd, Sisk JM, Coulombe PA, Thompson CC. Hairless triggers reactivation of hair growth by promoting Wnt signaling. Proc Natl Acad Sci USA 2005;102:14653–14658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson CC, Sisk JM, Beaudoin GM 3rd. Hairless and Wnt signaling: allies in epithelial stem cell differentiation. Cell Cycle 2006;5:1913–1917. [DOI] [PubMed] [Google Scholar]
- Luderer HF, Demay MB. The vitamin D receptor, the skin and stem cells. J Steroid Biochem Mol Biol 2010;121:314–316. [DOI] [PubMed] [Google Scholar]
- Wang L, Song Y, Manson JE, Pilz S, Marz W, Michaelsson K et al. Circulating 25-hydroxy-vitamin D and risk of cardiovascular disease: a meta-analysis of prospective studies. Circ Cardiovasc Qual Outcomes 2012;5:819–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilz S, Kienreich K, Tomaschitz A, Lerchbaum E, Meinitzer A, Marz W et al. Vitamin D and cardiovascular disease: update and outlook. Scand J Clin Lab Invest Suppl 2012;243:83–91. [DOI] [PubMed] [Google Scholar]
- Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP. 1,25-Dihydroxyvitamin D3 is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest 2002;110:229–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiosano D, Schwartz Y, Braver Y, Hadash A, Gepstein V, Weisman Y et al. The renin-angiotensin system, blood pressure, and heart structure in patients with hereditary vitamin D-resistance rickets (HVDRR). J Bone Miner Res 2011;26:2252–2260. [DOI] [PubMed] [Google Scholar]
- Tiosano D, Gepstein V. Vitamin D action: lessons learned from hereditary 1,25-dihydroxyvitamin-D-resistant rickets patients. Curr Opin Endocrinol Diabetes Obes 2012;19:452–459. [DOI] [PubMed] [Google Scholar]
- Haussler MR, Jurutka PW, Mizwicki M, Norman AW. Vitamin D receptor (VDR)-mediated actions of 1α,25(OH)2vitamin D3: genomic and non-genomic mechanisms. Best Pract Res Clin Endocrinol Metab 2011;25:543–559. [DOI] [PubMed] [Google Scholar]
- Norman AW, Mizwicki MT, Norman DP. Steroid-hormone rapid actions, membrane receptors and a conformational ensemble model. Nat Rev Drug Discov 2004;3:27–41. [DOI] [PubMed] [Google Scholar]
- Song EJ, Gordon-Thomson C, Cole L, Stern H, Halliday GM, Damian DL et al. 1α,25-Dihydroxyvitamin D3 reduces several types of UV-induced DNA damage and contributes to photoprotection. J Steroid Biochem Mol Biol 2013;136:131–138. [DOI] [PubMed] [Google Scholar]
- Sequeira VB, Rybchyn MS, Tongkao-On W, Gordon-Thomson C, Malloy PJ, Nemere I et al. The role of the vitamin D receptor and ERp57 in photoprotection by 1α,25-dihydroxyvitamin D3. Mol Endocrinol 2012;26:574–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemere I, Garbi N, Hammerling GJ, Khanal RC. Intestinal cell calcium uptake and the targeted knockout of the 1,25D3-MARRS (membrane-associated, rapid response steroid-binding) receptor/PDIA3/Erp57. J Biol Chem 2010;285:31859–31866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemere I, Ray R, McManus W. Immunochemical studies on the putative plasmalemmal receptor for 1, 25(OH)2D3. I. Chick intestine. Am J Physiol Endocrinol Metab 2000;278:E1104–E1114. [DOI] [PubMed] [Google Scholar]
- Narvaez CJ, Matthews D, Broun E, Chan M, Welsh J. Lean phenotype and resistance to diet-induced obesity in vitamin D receptor knockout mice correlates with induction of uncoupling protein-1 in white adipose tissue. Endocrinology 2009;150:651–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malloy PJ, Feldman BJ. Cell autonomous regulation of brown fat identity gene UCP1 by unliganded vitamin D receptor. Mol Endocrinol 2013;27:1632–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]