Abstract
The goal of synthetic chemists in the vitamin D field has been to produce an analog(s) of 1α,25-dihydroxyvitamin D3 (1,25-(OH)2D3) that is selective for a specific function. The accumulation of structure/function information has led to the synthesis of two analogs that are both selective and more potent than 1,25-(OH)2D3, that is, 2-methylene-19-nor-(20S)-1α,25-dihydroxyvitamin D3 (2MD) and 2α-methyl-19-nor-(20S)-1α,25-dihydroxyvitamin D3 (2AMD). In vivo, the efficacy of 2MD is approximately equal to that of 1,25-(OH)2D3 in intestinal calcium transport but is 30- to 100-fold more active in bone mobilization. In vitro, 2MD supports new bone synthesis at 10−12 M, whereas 1,25-(OH)2D3 is active at 10−8 M. Similarly, 2MD is two orders of magnitude more potent than 1,25-(OH)2D3 in stimulating osteoclastogenesis and osteoclastic bone resorption. 2MD also markedly increases bone mass and bone strength of ovariectomized female rats. In postmenopausal women, 2MD significantly increases markers of both bone formation and resorption but has minimal effect on bone mass. Thus, in patients who are undergoing primarily remodeling rather than modeling (rat), the increased resorption largely counteracts the increased bone formation. So far, 2MD has not been tested for reduction of fractures in this population. However, its selectivity includes the parathyroid gland. Thus in the 5/6-nephrectomy model of chronic renal failure, 2MD is much more potent than currently available vitamin D compounds used to suppress secondary hyperparathyroidism of renal failure without causing hypercalcemia. It is currently in phase 2B trials in patients on dialysis.
Introduction
Following the isolation, identification and demonstration of the importance of 1α,25-dihydroxyvitamin D3 (1,25-(OH)2D3) as the hormonal form of vitamin D, syntheses not only of the 1,25-(OH)2D3 but also of variations in that structure were carried out in an attempt to reduce the undesirable hypercalcemic effect of the native hormone.1,2 Many of these analogs have not been thoroughly tested to determine whether they have selective activity. Further, the assays in laboratories differ, and thus a comparison of their biological activities is difficult. Therefore, no attempt will be made in this review to compare the activity of 2-methylene-(20S)-19-nor-1α,25-dihydroxyvitamin D3 (2MD) with the activities of analogs other than 1,25-(OH)2D3 itself.
A major emphasis was placed on producing an analog that could suppress cancer because of the finding that 1,25-(OH)2D3 could suppress growth of cancer cells in vitro and promote differentiation.1,3,4,5 Many attempts were made to prepare analogs that would not increase serum calcium but instead would possess the ability to suppress growth of cancer cells in vitro.6,7,8 Although this was certainly successful, it is not clear whether in vitro suppression of cancer growth could be demonstrated in vivo. So far, no vitamin D analog has emerged with that property without producing lethal hypercalcemia. In fact, suppression of cancer growth by 1,25-(OH)2D3 or an analog in vivo has not been uniformly demonstrated.9 As discussed in the recent Dietary Reference Intakes Report, the relationship between vitamin D and cancer is far from clear.9
However, there is the possibility that modifications in the 1,25-(OH)2D3 structure may bring about selective activity on certain biochemical systems. One successful development has been the Leo Pharmaceutical compound, MC-903, also known as Dovonex.10,11 When applied topically, this compound clearly suppresses proliferation of the keratinocyte and is used as a therapy for the disease psoriasis. This compound does not produce hypercalcemia except under extreme circumstances because it is rapidly metabolized and eliminated with a lifetime of less than a few minutes in the blood stream.11,12 Thus, Dovonex is selective only when provided topically, and is ineffective when provided systemically.11,12 On the other hand, Chugai Pharmaceuticals' compound, 1,25-dihydroxy-22-oxavitamin D3 (OCT), when given systemically does suppress secondary hyperparathyroidism, but because it is rapidly metabolized and eliminated it has less of a calcemic effect.13 In both cases, the selectivity stems either from the route of administration or from rapid metabolism.
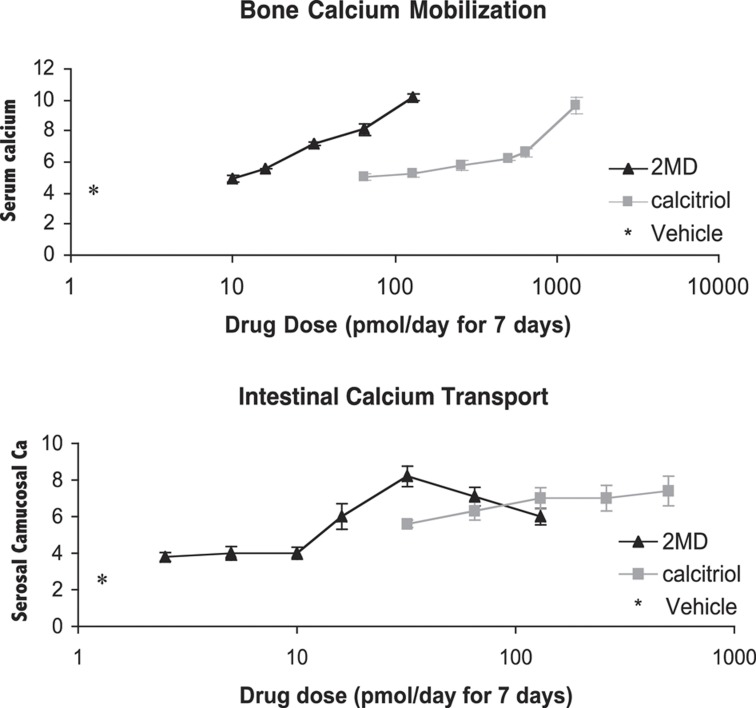
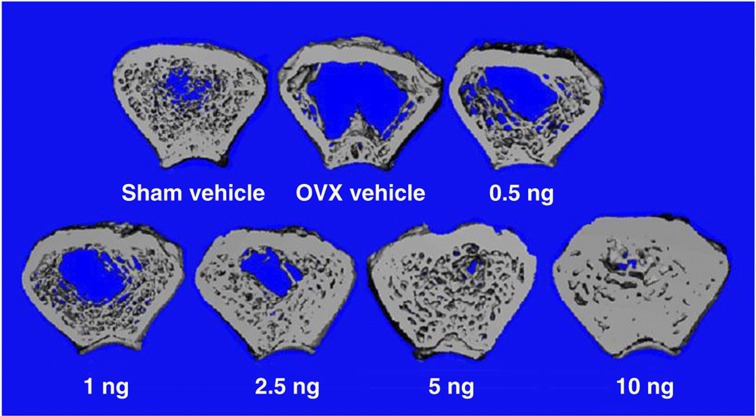
In order for an analog to function selectively, it must be able to alter the vitamin D receptor (VDR) in a differential way. The VDR is a single receptor found in many organs of the body.14,15,16 Thus, it might be expected that a targeted specific form of 1,25-(OH)2D might influence the structure of the VDR in such a way that it would bind certain coactivators and not others and may lend specificity with regard to target gene responses. Rochel et al.17,18 and subsequently Vanhooke et al.19 have crystallized the ligand-binding domain (LBD) of the VDR with different analogs. Both groups found that, even with analogs of very divergent structures and activities, the crystalline structure of the LBD is unchanged by the ligand. On the basis of the X-ray structure, it is not possible to see how an analog can be selective in its actions. However, recently the LBD has been studied in solution using nuclear magnetic resonance spectroscopy, in which, in fact, analogs do have different effects on the solution structure of the receptor.20 It is, therefore, feasible that an analog might alter the VDR structure such that it selectively binds coactivators or corepressors that might result in selective activity. This idea has not yet reached fruition, but there is evidence in vivo that there could be analogs with such selective activity. One such analog is 2MD (Figure 1).21 So far, we have not been able to translate these findings into a mechanism that would reveal why 2MD has preferential activity on bone or the parathyroid gland.20 2MD and 1,25-(OH)2D3 bind the VDR with equal affinity.21 However, when given in vivo by intravenous injection, 2MD is 30–100 times more effective than 1,25-(OH)2D3 in mobilizing calcium from bone.22 In the same animals, its activity is either equal to or even slightly less than that of 1,25-(OH)2D3 in inducing intestinal calcium transport by the everted sac method of measuring this system (Figure 2).22 As bone calcium mobilization is believed to be the result of the vitamin D hormone interacting with the receptor in the osteoblast to bring about the transcription of the RANK ligand and as the RANK ligand then stimulates the osteoclasts to mobilize bone, this initial result seemed to suggest that 2MD might be selective for activity on the osteoblast. When tested in human osteoblasts in culture, 2MD proved to be two orders of magnitude more effective than 1,25-(OH)2D3 in osteoclastogenesis and in inducing osteoclast-mediated bone resorption.22 Furthermore, when tested in the synthesis of new bone by the osteoblast, it also proved to be two or three orders of magnitude more effective than the native hormone.21,22 This surprising result raises the question of whether vitamin D compounds are in fact bone anabolic. Until very recently, it had been believed that the primary, if not only, role of the vitamin D hormone in bone was to elevate calcium and phosphorus to levels that cause mineralization of the skeleton, but there was no strong evidence that increased bone synthesis occurred in response to the vitamin D hormone. However, the work of Xue et al.23 provided secure evidence that in fact 1,25-(OH)2D3 does have bone anabolic activity in CYP27B1- and parathyroid-ablated mice. This encouraging result led to the testing of whether 2MD given to female, ovariectomized rats might in fact show an increase in bone synthesis, and hence an increase in bone mass. Retired female breeders were ovariectomized and treated with 2MD, and the results were quite marked, showing an increase in bone mass.24,25 That 2MD is bone anabolic in the rat is evident by the experiments by Pfizer's scientists (Figure 3).25 However, it is well known that the rat is primarily a bone-modeling organism and has very little bone remodeling, whereas the human skeleton is largely a remodeling skeleton.26 Thus, it was not certain whether the bone mass effect in the rat would be translated into an increase in bone mass in humans. When a 1-year clinical trial in postmenopausal women with osteopenia was carried out, 2MD markedly increased the bone biomarkers of both bone resorption and formation.27 However, little change in bone mass occurred. But again in rats, bone strength, determined by three-point bending measurements and other measurements, showed that bone strength was improved by 2MD.24 In developing toxicity studies in monkeys and rats in preparation for clinical trials, a clinical research organization found that the bones of animals given 2MD were much harder than normal and were difficult to cut. This suggested that 2MD increased bone turnover and as a result may have provided increased bone strength. However, the fact that 2MD did not increase bone mass in postmenopausal women with osteopenia prevented the expenditure of $1 billion for a human fracture study required by the FDA for approval. Thus, it is unknown whether 2MD might be beneficial in postmenopausal osteoporosis. Because of the position taken by the FDA that a fracture study in postmenopausal women will be required for approval, it is not clear whether this question will ever be answered.
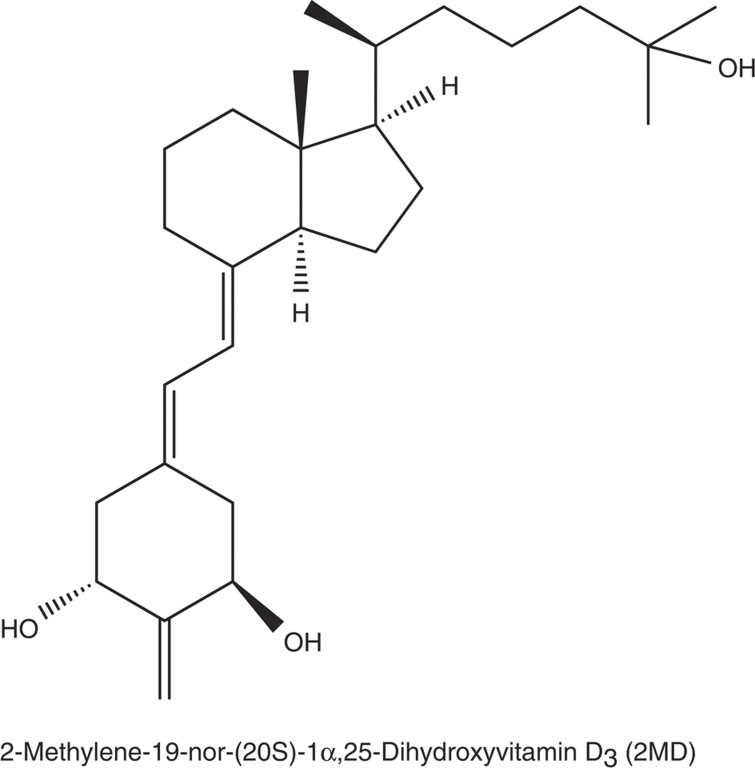
Figure 1.
Structure of 2MD.
Figure 2.
In vivo selective activity of 2MD in vitamin D-deficient rats. Rats (male) were placed on a vitamin D-free purified diet containing 0.47% calcium/0.3% phosphorus for 4 weeks, and then placed on the same diet devoid of calcium (0.02%) for 3 weeks and dosed for 7 days. Four hours after the last dose, animals were killed to determine intestinal calcium transport by the everted sac method, and serum calcium was used to determine bone mobilization (see Shevde et al.22 for details).
Figure 3.
Cross-sections of femoral metaphysics from ovariectomized female rats on the indicated daily dose of 2MD. Sections of femoral metaphysics of 1-mm thickness were scanned by a pQCT machine.
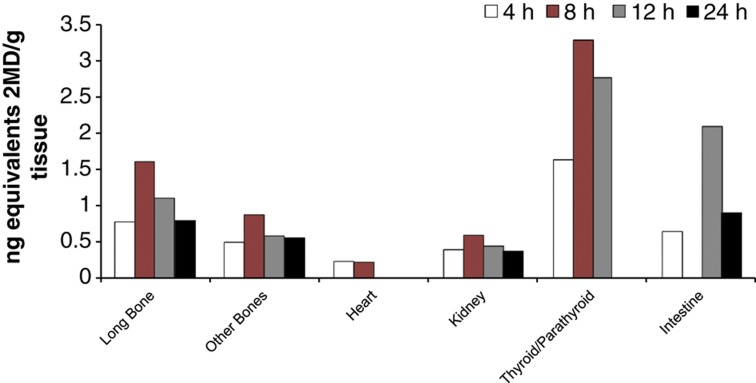
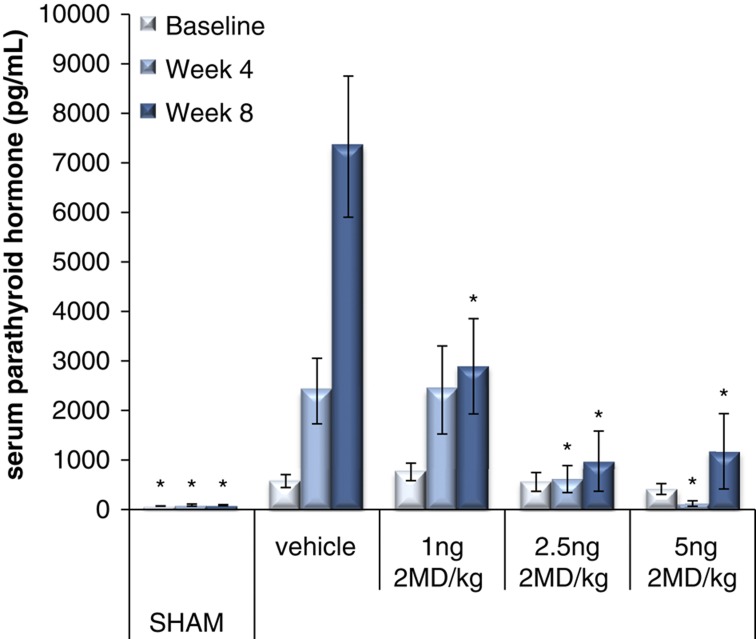
One of the findings that resulted from the preclinical studies done in the Pfizer laboratory is that 2MD preferentially localizes in the parathyroid glands, as shown in Figure 4. Furthermore, when tested in the 5,6-nephrectomy model of rats, 2MD seemed extremely effective without raising serum calcium in suppressing secondary hyperparathyroidism (Figure 5). In fact, in the 5,6-nephrectomy model, 2MD appears 10 times more active than 1α-hydroxyvitamin D2 (Hectorol) or 19-nor-1α,25-dihyroxyvitamin D2 (Zemplar) in the suppression of parathyroid hormone levels (Zella J et al., unpublished results). Thus, 2MD has been introduced into the dialysis world as a possible replacement of vitamin D analogs already in use. A clinical trial underway will largely determine whether this compound provides a significant improvement over existing therapies and whether it will be useful in controlling secondary hyperparathyroidism.
Figure 4.
Localization of 2MD in the parathyroid glands, bone and intestine. Radiolabeled 2MD (26,27-3H; 160 Ci/mmol) was given, sections of tissue were taken and radioactivity was detected by autoradiography.
Figure 5.
Suppression of secondary hyperparathyroidim in 5/6-nephrectomized rats. The nephrectomies were carried out as described in the study by Brown et al.13 Following 4 weeks of recovery, the indicated daily oral dose or vehicle was administered. Parathyroid hormone (PTH) measurements were taken at the indicated times.
Summary
2MD is a promising analog of 1,25-(OH)2D3 that may prove to be an improvement in the treatment of secondary hyperparathyroidism in dialysis patients and may actually be an excellent agent for improving bone strength in postmenopausal women, thereby aiding in the treatment of osteoporosis.
Acknowledgments
This work was funded by the Wisconsin Alumni Research Foundation.
Footnotes
The author is the CEO and President of Deltanoid Pharmaceuticals, Inc., which is developing 2MD.
References
- Binderup L, Binderup E, Godtfredsen WO. Development of new vitamin D analogs. In: Feldman D, Glorieux FH, Pike JW (eds).Vitamin D. Academic Press: San Diego, CA, USA, 1997;Chapter 61:1027–1043. [Google Scholar]
- Uskokovic MR, Studzinski P, Reddy SG. The 16-ene vitamin D analogs. In: Feldman D, Glorieux FH, Pike JW (eds).Vitamin D. Academic Press: San Diego, CA, USA, 1997;Chapter 62:1045–1070. [Google Scholar]
- Colston K. Vitamin D and breast cancer: therapeutic potential of new vitamin D analogs. In: Feldman D, Glorieux FH, Pike JW (eds).Vitamin D. Academic Press: San Diego, CA, USA, 1997;Chapter 65:1107–1123. [Google Scholar]
- Gross C, Peehl DM, Feldman D. Vitamin D and prostate cancer. In: Feldman D, Glorieux FH, Pike JW (eds).Vitamin D. Academic Press: San Diego, CA, USA, 1997; Chapter 66:1125–1139. [Google Scholar]
- Brasitus TA, Sitrin MD. Chemoprevention of colon cancer by vitamin D3 and its metabolites/analogs. In: Feldman D, Glorieux FH, Pike JW (eds).Vitamin D. Academic Press: San Diego, CA, USA, 1997;Chapter 67:1141–1154. [Google Scholar]
- Posner GH, Kahraman M. Overview: rational design of 1α,25-dihydroxyvitamin D3 analogs (deltanoids). In: Feldman D, Glorieux FH, Pike JW (eds).Vitamin D. Elsevier Academic Press: Burlington, MA, USA, 2005;Chapter 80:1405–1422. [Google Scholar]
- Binderup L, Binderup E, Godtfredsen WO, Kissmeyer A-M. Development of new vitamin D analogs. In: Feldman D, Glorieux FH, Pike JW (eds).Vitamin D. Elsevier Academic Press: Burlington, MA, USA, 2005;Chapter 84:1489–1510. [Google Scholar]
- Uskokovic MR, Maehr H, Reddy SG, Li YC, Adorini L, Holick MF. Gemini: the 1,25-dihydroxy vitamin D analogs with two side-chains. In: Feldman D, Glorieux FH, Pike JW (eds).Vitamin D. Elsevier Academic Press: Burlington, MA, USA, 2005;Chapter 85:1511–1524. [Google Scholar]
- Ross AC, Taylor CL, Yaktine AL, Del Valle HB (eds).Dietary reference intakes for calcium and vitamin D. Institute of Medicine of the National Academies. The National Academies Press: Washington, DC, USA, 2001; pp 1115. [PubMed] [Google Scholar]
- Calverley MJ. Synthesis of MC 903, a biologically active vitamin D metabolite analogue. Tetrahedron 1987;43:4609–4619. [Google Scholar]
- Binderup L, Bramm E. Effects of a novel vitamin D analogue MC903 on cell proliferation and differentiation in vitro and on calcium metabolism in vivo. Biochem Pharmacol 1988;37:889–895. [DOI] [PubMed] [Google Scholar]
- Binderup L. Vitamin D analogues: new regulators of cancer cell growth and differentiation. Bioorg Med Chem Lett 1993;3:1891–1896. [Google Scholar]
- Brown AJ, Ritter CR, Finch JL, Morrissey J, Martin KJ, Murayama E et al. The noncalcemic analog of vitamin D, 22-oxacalcitriol, suppresses parathyroid hormone synthesis and secretion. J Clin Invest 1989;84:728–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumpf WE, Sar M, Reid FA, Tanaka Y, DeLuca HF. Target cells for 1,25-dihydroxyvitamin D3 in intestinal tract, stomach, kidney, skin, pituitary and parathyroid. Science 1979;206:1188–1190. [DOI] [PubMed] [Google Scholar]
- Sandgren ME, Bronnegard M, DeLuca HF. Tissue distribution of the 1,25-dihydroxyvitamin D3 receptor in the male rat. Biochem Biophys Res Commun 1991;181:611–616. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhu J, DeLuca HF. Where is the vitamin D receptor? Arch Biochem Biophys 2012;523:123–133. [DOI] [PubMed] [Google Scholar]
- Rochel N, Wurtz JM, Mitschler A, Klaholz B, Moras D. The crystal structure of the nuclear receptor for vitamin D bound to its natural ligand. Mol Cell 2000;5:173–179. [DOI] [PubMed] [Google Scholar]
- Rochel N, Hourai S, Perez-Garcia X, Rumbo A, Mourino A, Moras D. Crystal structure of the vitamin D nuclear receptor ligand binding domain in complex with a locked side chain analog of calcitriol. Arch Biochemn Biophys 2007;460:172–176. [DOI] [PubMed] [Google Scholar]
- Vanhooke JL, Benning MM, Bauer CB, Pike JW, DeLuca HF. Molecular structure of the rat vitamin D receptor ligand binding domain complexed with 2-carbon-substituted vitamin D3 hormone analogues and a LXXLL-containing coactivator peptide. Biochemistry 2004;43:4104–4110. [DOI] [PubMed] [Google Scholar]
- Singarapu KK, Zhu J, Tonelli M, Rao H, Assadi-Porter FM, Westler WM et al. Ligand-specific structural changes in the vitamin D receptor in solution. Biochemistry 2011;50:11025–11033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicinski JRR, Prahl JM, Smith CM, DeLuca HF. New 1α,25-dihydroxy-19-norvitamin D3 compounds of high biological activity: Synthesis and biological evaluation of 2-hydroxymethyl, 2-methyl and 2-methylene analogs. J Med Chem 1998;4:4662–4674. [DOI] [PubMed] [Google Scholar]
- Shevde NK, Plum LA, Clagett-Dame M, Yamaoto H, Pike JW, DeLuca HF. A novel potent analog of 1α,25-dihydroxyvitamin D3 selectively induces bone formation. Proc Natl Acad Sci USA 2002;99:13487–13491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Y, Karaplis AC, Hendy GN, Goltzman D, Miao D. Exogenous 1,25-dihydroxyvitamin D3 exerts a skeletal anabolic effect and improve mineral ion homeostasis in mice that are homozygous for both the 1α-hydroxylase and parathyroid hormone null alleles. Endocrinology 2006;147:4801–4810. [DOI] [PubMed] [Google Scholar]
- Plum LA, Fitzpatrick LA, Ma X. 2MD, a new anabolic agent for osteoporosis treatment. Osteoporos Int 2006;17:704–715. [DOI] [PubMed] [Google Scholar]
- Ke HZ, Qi H, Crawford DT, Simmons HA, Xu G, Li M et al. A new vitamin D analog, 2MD, restores trabecular and cortical bone mass and strength in ovariectomized rats with established osteopenia. J Bone Miner Res 2005;20:1742–1755. [DOI] [PubMed] [Google Scholar]
- Frost HM. Bone Dynamics in Osteoporosis and Osteomalacia. Henry Ford Hospital Surgical Monograph Series Charles A. Thomas Co: Springfield, MA, USA, 1966;. [Google Scholar]
- DeLuca HF, Bedale W, Binkley N, Gallagher JC, Bolognese M, Peacock M et al. The vitamin D analogue 2MD increases bone turnover but not BMD in postmenopausal women with osteopenia: Results of a 1-year phase 2 double-blind, placebo-controlled, randomized clinical Trial. J Bone Miner Res 2011;26:538–545. [DOI] [PubMed] [Google Scholar]