Abstract
Aims
To determine if diabetes or pre-diabetes is associated with monofilament insensitivity and peripheral neuropathy symptoms.
Methods
The 10-g Semmes-Weinstein monofilament test and Michigan Neuropathy Screening Instrument symptom questionnaire were administered to participants in the Study of Women’s Health Across the Nation – Michigan site (n=396). We determined the concordance of monofilament insensitivity and symptoms and used chi-square tests, ANOVA, and logistic regression to quantify the relationships among diabetes status, monofilament insensitivity, and symptoms.
Results
The prevalence of monofilament insensitivity was 14.3%, and 19.4% of women reported symptoms of peripheral neuropathy. With monofilament testing, 11.7% of women with normal fasting glucose, 14.4% of women with impaired fasting glucose (IFG), and 18.3% of women with diabetes had monofilament insensitivity (p-value=0.33). For symptoms, 14.0% of women with normal fasting glucose, 16.5% of women with IFG, and 31.2% of women with diabetes reported symptoms of peripheral neuropathy. Women who reported symptoms of small fiber nerve dysfunction alone were unlikely to have monofilament insensitivity. Compared to women with normal fasting glucose, women with diabetes were more likely to report peripheral neuropathy symptoms [OR 2.8 (95% CI: 1.5, 5.1)]. Women with diabetes were also more likely to report symptoms than women with IFG (p=0.02). There was no difference in the frequency of symptoms between women with normal fasting glucose and IFG.
Conclusions
Women with diabetes were more likely to report peripheral neuropathy symptoms. The prevalence of monofilament insensitivity and peripheral neuropathy symptoms did not differ between women with normal fasting glucose and IFG.
Keywords: Peripheral neuropathy, monofilament testing, symptoms
Introduction
Peripheral neuropathy is a complication of overt type 1 and type 2 diabetes, but the question of whether impaired fasting glucose causes peripheral neuropathy remains open to debate [1–6], especially as there is controversy about the best definition of peripheral neuropathy in epidemiologic investigations. We assessed the prevalence and concordance of monofilament insensitivity and peripheral neuropathy symptoms, and described the association of peripheral neuropathy with diabetes and pre-diabetes in a population-based cohort.
Materials and Methods
The Study of Women’s Health Across the Nation [7] – Michigan site conducted a peripheral neuropathy sub-study in study year 12 (2008) among 396 participants. Monofilament insensitivity was assessed using a 10-gram Semmes-Weinstein monofilament [8] on the dorsal side of the great toe, midway between the nail fold and the distal interphalangeal joint briefly placed (<1 second) for 10 repetitions [9] and defined as 80% or fewer correct responses to the brief sensation in either foot [10]. The Michigan Neuropathy Screening Instrument (MNSI) symptom questionnaire [9,11] acquired information about the presence of 15 neuropathy signs and symptoms. Participants who reported ≥4 symptoms [12] were further classified into women with symptoms of small fiber nerve dysfunction only (burning pain, sensitivity, prickling feelings, lack of hot/cold differentiation, and severe dryness in the foot), or women with symptoms of large fiber nerve dysfunction (numbness, open sores, and amputations) with or without symptoms of small fiber nerve dysfunction (see Appendix A) [13–16]. The MNSI symptom questionnaire was evaluated by a panel of four neuropathy experts to determine which questions identified small and large fiber symptoms, respectively. Monofilament and questionnaire assessments were selected based on previous use in large-scale epidemiologic investigations [10, 17–19] and acceptability to our observational cohort.
Race/ethnicity was self-identified. Body mass index (BMI) was calculated as weight (kg)/height (m2). Waist circumference (cm) was measured using a non-stretching tape measure. Current smokers were defined as smoking regularly since their last study visit. Less than 5% of our study population reported more than one alcoholic drink per day, so current alcohol consumers were defined as consumers of more than one drink per month (>180 grams/year). Participants were classified as hypertensive if their average systolic blood pressure was ≥140mmHg, average diastolic blood pressure was ≥90mmHg, or if they reported current use of antihypertensive medications. Diabetes was defined as fasting glucose ≥7.0 mmol/L or diagnosis, treatment, or current use of diabetes medication; impaired fasting glucose was defined as fasting glucose 5.6 – 7.0 mmol/L; and normal was defined as fasting glucose <5.6 mmol/L.
We reported characteristics of the total study population and by diabetes status using frequencies and means. Chi-square tests, ANOVA, and logistic regression were used to compare the prevalence of monofilament insensitivity and symptoms by diabetes categories. SAS (version 9.3) was used for all data management and analyses. Statistical tests were 2-sided with the level of significance defined as p-value <0.05.
Results
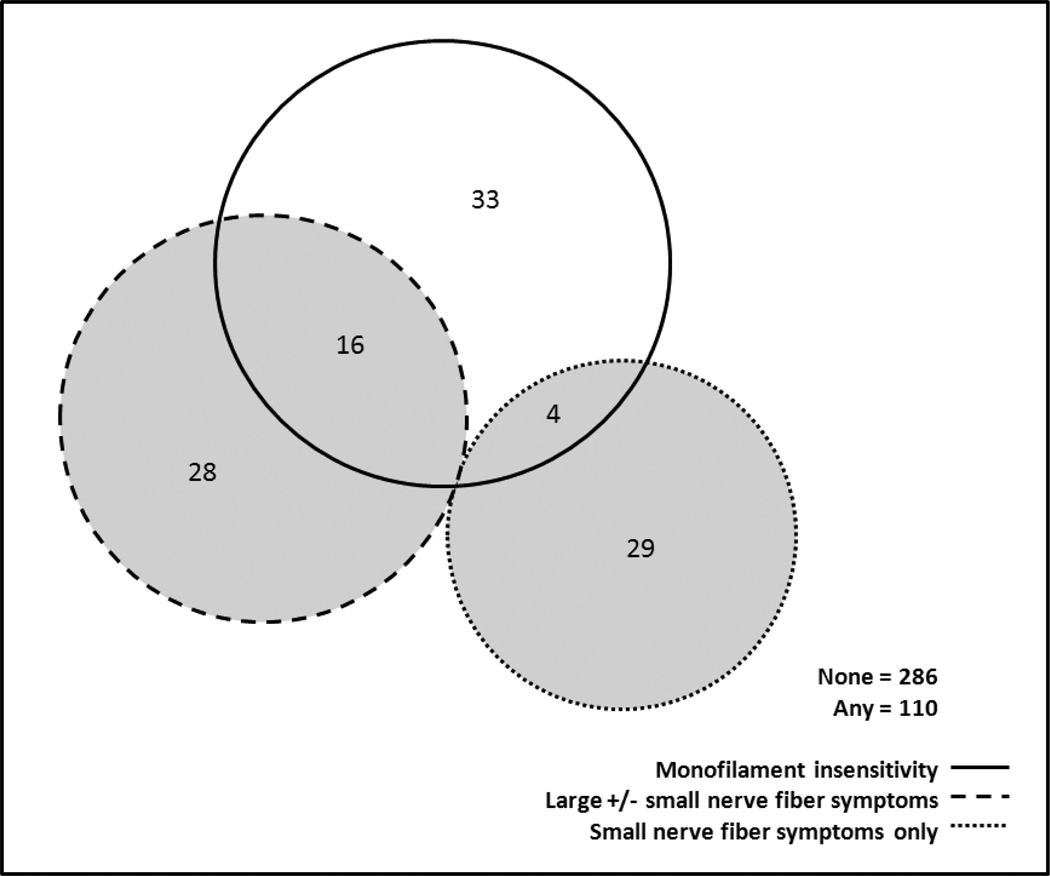
The prevalence of monofilament insensitivity was 14.3% (n=53), and 19.4% (n=77) of women reported peripheral neuropathy symptoms. Thirty-three (43%) reported only small nerve fiber symptoms and 44 (57%) reported large fiber with or without small fiber symptoms. Of the 53 women with monofilament insensitivity, 33 (62%) did not report any peripheral neuropathy symptoms and 20 (38%) were identified by the symptom questionnaire (Figure 1). Women with monofilament insensitivity were significantly more likely to report large nerve fiber symptoms (p=0.02) and significantly less likely to report small nerve fiber symptoms (p=0.01).
Figure 1.
Counts of coinciding peripheral nerve dysfunction assessments using three measures, Peripheral Neuropathy Substudy, Michigan SWAN, 2008.
Abbreviations: SWAN, Study of Women’s Health Across the Nation
In this population, 44% had normal fasting glucose, 26% had impaired fasting glucose, and 30% had diabetes. There were no statistically significant differences in the prevalence of monofilament insensitivity, but the prevalence of peripheral neuropathy symptoms increased with fasting glucose categories (p-value=0.002; Table 1). Women with diabetes had almost three times the odds of peripheral neuropathy symptoms (OR=2.8; 95% CI: 1.5, 5.1; p-value<0.001) compared to women with normal fasting glucose, and women with diabetes were more likely to report peripheral neuropathy symptoms than women with impaired fasting glucose (p-value= 0.02). There were no statistically significant differences in monofilament insensitivity or symptoms between the pre-diabetes and the normal glucose group.
Table 1.
Prevalence of monofilament insensitivity, prevalence of peripheral neuropathy symptoms, and characteristics of study population by diabetes status, Peripheral Neuropathy Substudy, Michigan SWAN, 2008.
| Total | Fasting Glucose* | ||||
|---|---|---|---|---|---|
| Normal | IFG | Diabetes | P value† | ||
| Monofilament insensitivity | 14.3 | 11.7 | 14.4 | 18.3 | 0.33 |
| Peripheral neuropathy symptoms | 19.4 | 14.0 | 16.5 | 31.2 | 0.002 |
| Age, years | 57.6 (2.8) | 57.5 (2.8) | 57.6 (2.7) | 57.6 (2.8) | 0.99 |
| Race/ethnicity, % | |||||
| Caucasian | 40.4 | 40.9 | 38.1 | 38.7 | 0.89 |
| African American | 59.6 | 59.2 | 61.9 | 61.3 | |
| Weight, kg | 89.9 (22.0) | 82.5 (19.6) | 93.3 (21.7) | 99.2 (21.1) | <0.001 |
| Height, cm | 162.7 (6.1) | 162.9 (6.0) | 163.1 (6.3) | 161.8 (6.4) | 0.22 |
| BMI, kg/m2 | 34.0 (8.3) | 31.1 (7.5) | 35.1 (7.8) | 37.9 (7.9) | <0.001 |
| Waist circumference, cm | 101.3 (17.2) | 94.5 (16.3) | 103.2 (16.2) | 111.1 (14.2) | <0.001 |
| Hypertension, % | 57.5 | 40.2 | 56.7 | 80.7 | <0.001 |
| Current smoker, % | 20.9 | 20.1 | 21.7 | 20.2 | 0.95 |
| Alcohol consumer, % | 31.7 | 37.2 | 35.1 | 24.3 | 0.07 |
Abbreviations: BMI, body mass index; IFG, impaired fasting glucose; SWAN, Study of Women’s Health Across the Nation.
Normal fasting glucose <5.6 mmol/L; IFG 5.6–7.0 mmol/L; Diabetes ≥7.0 mmol/L or diagnosis or current use of medications.
Calculated by ANOVA for continuous variables or chi-square test for categorical variables.
Conclusions
In this bi-racial cohort of older community-dwelling women, monofilament insensitivity and peripheral neuropathy symptoms were common. Almost 20% of women in our study reported ≥4 symptoms of peripheral neuropathy. Symptoms of peripheral neuropathy were significantly associated with diabetes but we did not observe significant differences in signs or symptoms between women with normal and impaired fasting glucose groups. Our results are consistent in direction with a study that identified the presence of neuropathic pain [19], but of increased magnitude, perhaps due to the relatively large body sizes of our cohort. Monofilament testing has been used to identify feet of people with diabetes at high risk for ulceration and amputation [20], but recent research has applied monofilament testing to people without diabetes as well [10,17,21,22]. Consistent with our results, these studies reported no significant differences between normal and impaired glycemic groups [18].
Monofilament insensitivity correlated relatively well with symptoms of larger fiber nerve dysfunction but had little overlap with symptoms of small fiber nerve dysfunction. Low correlation of MNSI-defined symptoms with other neuropathy assessments has been previously reported [9], but may be explained in part by fiber size. We would expect transient hyperglycemia/pre-diabetes to affect small nerve fibers first, but investigations using only monofilament testing may overlook small fiber involvement. In keeping with this hypothesis, we did not observe a significant association between monofilament insensitivity and impaired fasting glucose.
Even among persons with diabetes there is a background rate of other etiologies for neuropathy. Of note, we report that women with diabetes were marginally less likely to consume alcohol. Since extremely high alcohol consumption is associated with peripheral neuropathy, our findings suggest alternate etiologies, but we did not differentiate between other possible disease-causing pathways. Nevertheless, the use of simple screening methods is desirable and often overlooked in the clinical setting. Our results are consistent with recent reports of nerve conduction studies demonstrating no association between impaired fasting glucose and neuropathy [2,3]. Recent reports of diagnostic practice patterns for neuropathy have suggested that too many patients undergo high-cost, low-yield testing before cost-effective history and examination practices are employed [23,24]. While the complexity of the disease suggests a single non-invasive test may not completely capture peripheral neuropathy, a combined approach, using both monofilament testing and the symptom questionnaire, is useful for peripheral neuropathy screening of at-risk adults. Insensitivity in the extremities and burdensome symptoms are important quality-of-life indicators and detecting peripheral neuropathy in its early stages may prevent or delay consequences like falls and disability.
Summary.
Monofilament insensitivity corresponded to symptoms of large fiber nerve dysfunction. Compared to women without diabetes, women with diabetes were more likely to report peripheral neuropathy symptoms. The prevalence of monofilament insensitivity and peripheral neuropathy symptoms did not differ between women with normal and impaired fasting glucose.
Acknowledgments
The Study of Women's Health Across the Nation (SWAN) has grant support from the National Institutes of Health (NIH), DHHS, through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR) and the NIH Office of Research on Women’s Health (ORWH) (Grants NR004061; AG012505, AG012535, AG012531, AG012539, AG012546, AG012553, AG012554, AG012495, as well as AG017719 (SWAN Repository)). The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH or the NIH.
Clinical Centers: University of Michigan, Ann Arbor – Siobán Harlow, PI 2011 – present, MaryFran Sowers, PI 1994– 2011; Massachusetts General Hospital, Boston, MA – Joel Finkelstein, PI 1999 – present; Robert Neer, PI 1994 – 1999; Rush University, Rush University Medical Center, Chicago, IL – Howard Kravitz, PI 2009 – present; Lynda Powell, PI 1994 – 2009; University of California, Davis/Kaiser – Ellen Gold, PI; University of California, Los Angeles – Gail Greendale, PI; Albert Einstein College of Medicine, Bronx, NY – Carol Derby, PI 2011 – present, Rachel Wildman, PI 2010 – 2011; Nanette Santoro, PI 2004 – 2010; University of Medicine and Dentistry – New Jersey Medical School, Newark – Gerson Weiss, PI 1994 – 2004; and the University of Pittsburgh, Pittsburgh, PA – Karen Matthews, PI.
NIH Program Office: National Institute on Aging, Bethesda, MD – Sherry Sherman 1994 – present; Marcia Ory 1994 – 2001; National Institute of Nursing Research, Bethesda, MD – Program Officers.
Central Laboratory: University of Michigan, Ann Arbor – Daniel McConnell (Central Ligand Assay Satellite Services).
SWAN Repository: University of Michigan, Ann Arbor – Dan McConnell 2011; MaryFran Sowers 2000 – 2011.
Coordinating Center: University of Pittsburgh, Pittsburgh, PA – Kim Sutton-Tyrrell, PI 2001 – present; New England Research Institutes, Watertown, MA - Sonja McKinlay, PI 1995 – 2001.
Steering Committee: Susan Johnson, Current Chair
Chris Gallagher, Former Chair
We thank the study staff at each site and all the women who participated in SWAN.
APPENDIX A
The frequency of symptoms and characteristics of peripheral neuropathy are shown below. Questions 1, 8, 13, and 15 were considered indicators of large fiber nerve dysfunction. Questions 2, 3, 5, 6, 7, and 14 were considered indicators of small fiber nerve dysfunction. The most commonly reported symptoms were burning pain (23%), prickling feelings (26%), and muscle cramps in legs and/or feet (54%). Thirty percent of women reported no symptoms, while almost 20% of women reported at least four symptoms of neuropathy. Of the 77 women reporting ≥4 signs or symptoms, 42% reported only small fiber symptoms and 58% reported large and small fiber symptoms.
Table A.1.
Frequency of participant-reported affirmative responses from the Michigan Neuropathy Screening Instrument symptom questionnaire, Peripheral Neuropathy Substudy, Michigan SWAN, 2008.
| MNSI symptom questionnaire | % |
| 1. Are your legs and/or feet numb? | 11.8 |
| 2. Do you ever have any burning pain in your legs and/or feet? | 23.1 |
| 3. Are your feet too sensitive to touch? | 4.0 |
| 4. Do you get muscle cramps in your legs and/or feet? | 54.5 |
| 5. Do you ever have any prickling feelings in your legs or feet? | 26.7 |
| 6. Does it hurt when the bed covers touch your skin? | 2.5 |
| 7. When you get into the tub or shower, are you able to tell the hot water from the cold water? | 96.5 |
| 8. Have you ever had an open sore on your foot? | 4.5 |
| 9. Has your doctor ever told you that you have diabetic neuropathy? | 5.8 |
| 10. Do you feel weak all over most of the time? | 8.0 |
| 11. Are your symptoms worse at night? | 13.1 |
| 12. Do your legs hurt when you walk? | 21.9 |
| 13. Are you able to sense your feet when you walk? | 96.7 |
| 14. Is the skin on your feet so dry that it cracks open? | 9.6 |
| 15. Have you ever had an amputation? | 1.0 |
| Total number of reported symptoms* | |
| 0 | 29.8 |
| 1 | 24.8 |
| 2 | 13.9 |
| 3 | 12.1 |
| ≥ 4 | 19.4 |
Abbreviations: SWAN, Study of Women’s Health Across the Nation.
Total number of reported symptoms based on composite score of MNSI symptom questionnaire "yes" responses with questions 7 and 13 reverse-scored.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures: None
Conflict of Interest
The work poses no conflict of interest or financial benefit to any party.
K.R.Y. analyzed data and wrote the manuscript. W.H.H. contributed to the discussion and reviewed/edited the manuscript. S.D.H. reviewed/edited the manuscript. K.R.Y. is the guarantor of this work and, as such, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Katon JG, Reiber GE, Nelson KM. Peripheral neuropathy defined by monofilament insensitivity and diabetes status: NHANES 1999–2004. Diabetes Care. 2012 Dec 28; doi: 10.2337/dc12-1102. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dyck PJ, Clark VM, Overland CJ CJ, et al. Impaired glycemia and diabetic polyneuropathy: the OC IG Survey. Diabetes Care. 2012;35:584–591. doi: 10.2337/dc11-1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pourhamidi K, Dahlin LB, Englund E, Rolandsson O. No difference in small or large nerve fiber function between individuals with normal glucose tolerance and impaired glucose tolerance. Diabetes Care. 2013;36:962–964. doi: 10.2337/dc12-1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singer MA, Vernino SA, Wolfe GI. Idiopathic neuropathy: new paradigms, new promise. JPNS. 2012;17:S43–S49. doi: 10.1111/j.1529-8027.2012.00395.x. [DOI] [PubMed] [Google Scholar]
- 5.Papanas N, Vinik AI, Ziegler D. Neuropathy in prediabetes: Does the clock start ticking early? Nat Rev Endocrinol. 2011;7:682–690. doi: 10.1038/nrendo.2011.113. [DOI] [PubMed] [Google Scholar]
- 6.Smith AG. Impaired glucose tolerance and the metabolic syndrome in idiopathic neuropathy. JPNS. 2012;17:S15–S21. doi: 10.1111/j.1529-8027.2012.00390.x. [DOI] [PubMed] [Google Scholar]
- 7.Sowers MF, Crawford S, Sternfeld B B, et al. SWAN: a multi-center, multi-ethnic, community-based cohort study of women and the menopause. In: Lobo R, Kelsey J, Marcus R, editors. Menopause: Biology and Pathobiology. San Diego: Academic Press; 2000. pp. 175–178. [Google Scholar]
- 8.Mueller MJ. Identifying patients with diabetes mellitus who are at risk for lower-extremity complications: use of Semmes-Weinstein monofilaments. Phys Ther. 1996;76:68–71. doi: 10.1093/ptj/76.1.68. [DOI] [PubMed] [Google Scholar]
- 9.Feldman EL, Stevens MJ, Thomas PK, et al. A practical two-step quantitative clinical and electrophysiological assessment for the diagnosis and staging of diabetic neuropathy. Diabetes Care. 1994;17:1281–1289. doi: 10.2337/diacare.17.11.1281. [DOI] [PubMed] [Google Scholar]
- 10.Nang EEK, Khoo CM, Tai ES, et al. Is there a clear threshold for fasting plasma glucose that differentiates between those with and without neuropathy and chronic kidney disease? The Singapore prospective study program. Am J Epidemiol. 2009;169:1454–1462. doi: 10.1093/aje/kwp076. [DOI] [PubMed] [Google Scholar]
- 11.Feldman EL, Stevens MJ. Clinical testing in diabetic peripheral neuropathy. Can J Neurol Sci. 1994;21:S3–S7. doi: 10.1017/s0317167100040671. [DOI] [PubMed] [Google Scholar]
- 12.Herman WH, Pop-Busui R, Braffett BH, et al. Use of the Michigan Neuropathy Screening Instrument as a measure of distal symmetrical peripheral neuropathy in type 1 diabetes: Results from the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications. Diabet Med. 2012;29:937–944. doi: 10.1111/j.1464-5491.2012.03644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lacomis D. Small-fiber neuropathy. Muscle Nerve. 2002;26:173–188. doi: 10.1002/mus.10181. [DOI] [PubMed] [Google Scholar]
- 14.Hoitsma E, Reulen JP, de Baets M, et al. Small fiber neuropathy: a common and important clinical disorder. J Neurol Sci. 2004;227:119–130. doi: 10.1016/j.jns.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 15.Devigili G, Tugnoli V, Penza P, et al. The diagnostic criteria for small fibre neuropathy: from symptoms to neuropathology. Brain. 2008;131:1912–1925. doi: 10.1093/brain/awn093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tavee J, Zhou L. Small fiber neuropathy: a burning problem. Cleve Clin J Med. 2009;76:297–305. doi: 10.3949/ccjm.76a.08070. [DOI] [PubMed] [Google Scholar]
- 17.Gregg EW, Sorlie P, Paulose-Ram R, et al. Prevalence of lower-extremity disease in the US adult population ≥40 years of age with and without diabetes: 1999–2000 National Health and Nutrition Examination Survey. Diabetes Care. 2004;27:1591–1597. doi: 10.2337/diacare.27.7.1591. [DOI] [PubMed] [Google Scholar]
- 18.Gregg EW, Gu Q, Williams D, et al. Prevalence of lower extremity diseases associated with normal glucose levels, impaired fasting glucose, and diabetes among US adults aged 40 or older. Diabetes Res Clin Pract. 2007;77:485–488. doi: 10.1016/j.diabres.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 19.Ziegler D, Rathmann W, Dickhaus T, et al. Neuropathic pain in diabetes, prediabetes, and normal glucose tolerance: The MONICA/KORA Augsburg Surveys S2 and S3. Pain Med. 2009;10:393–400. doi: 10.1111/j.1526-4637.2008.00555.x. [DOI] [PubMed] [Google Scholar]
- 20.Smieja M, Hunt DL, Edelman D, et al. Clinical examination for the detection of protective sensation in the feet of diabetic patients. J Gen Intern Med. 1999;14:418–424. doi: 10.1046/j.1525-1497.1999.05208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng YJ, Gregg EW, Kahn HS, et al. Peripheral insensate neuropathy- a tall problem for US adults? Am J Epidemiol. 2006;164:873–880. doi: 10.1093/aje/kwj281. [DOI] [PubMed] [Google Scholar]
- 22.Ylitalo KR, Sowers MF, Heeringa S. Peripheral vascular disease and peripheral neuropathy in individuals with cardiometabolic clustering and obesity. Diabetes Care. 2011;34:1642–1647. doi: 10.2337/dc10-2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Callaghan B, McCammon R, Kerber K, et al. Tests and expenditures in the initial evaluation of peripheral neuropathy. Arch Intern Med. 2012;172:127–132. doi: 10.1001/archinternmed.2011.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gordon Smith A. Diagnosis of neuropathy: Comment on “Tests and expenditures in the initial evaluation of peripheral neuropathy”. Arch Intern Med. 2012;172:132–133. doi: 10.1001/archinternmed.2011.1718. [DOI] [PubMed] [Google Scholar]