Abstract
Immaturity of gut-associated immunity may contribute to pediatric mortality associated with enteric infections. A murine model to parallel infantile enteric disease was used to determine the effects of probiotic, Lactobacillus acidophilus (La), prebiotic, inulin, or both (synbiotic, syn) on pathogen-induced inflammatory responses, NF-κB, and Smad 7 signaling. Newborn mice were inoculated bi-weekly for 4 weeks with La, inulin, or syn and challenged with Citrobacter rodentium (Cr) at 5 weeks. Mouse intestinal epithelial cells (CMT93) were exposed to Cr to determine temporal alterations in NF-Kappa B and Smad 7 levels. Mice with pretreatment of La, inulin, and syn show reduced intestinal inflammation following Cr infection compared with controls, which is associated with significantly reduced bacterial colonization in La, inulin, and syn animals. Our results further show that host defense against Cr infection correlated with enhanced colonic IL-10 and transforming growth factor-β expression and inhibition of NF-κB in syn-treated mice, whereas mice pretreated with syn, La, or inulin had attenuation of Cr-induced Smad 7 expression. There was a temporal Smad 7 and NF-κB intracellular accumulation post-Cr infection and post-tumor necrosis factor stimulation in CMT93 cells. These results, therefore, suggest that probiotic, La, prebiotic inulin, or synbiotic may promote host-protective immunity and attenuate Cr-induced intestinal inflammation through mechanisms affecting NF-κB and Smad 7 signaling.
Keywords: probiotics, prebiotics, synbiotic, enteric pathogens, cell signaling
Introduction
In the last two decades, diarrheal illnesses have accounted for approximately 4.6 million deaths of 1 billion episodes of diarrhea globally in children younger than 5 years (Snyder & Merson, 1982; Institute for World Health, 2010, http://www.oneworldhealth.org/diarrheal_disease). Pediatric mortality and morbidity remain a constant epidemiological problem owing to immaturity of gut-associated immunity (GAI) and subsequent resistance to enteric pathogens. These conditions predominate during early childhood and do not appear during any other stage of life (Snyder & Merson, 1982; Hoque et al., 1994), highlighting the particular vulnerability of the intestine during early development. Infections caused by enteric bacterial pathogens, such as diarrheagenic enterohemorrhagic (EHEC) and enteropathogenic (EPEC) Escherichia coli, the family of attaching and effacing (A/E) bacterial pathogens, are among the most important causative pathogens of severe infantile diarrhea (Donnenberg & Whittam, 2001; Hecht, 2001; Vallance et al., 2002). The mouse pathogen Citrobacter rodentium causes a similar A/E lesion in the murine intestine and has been used as a physiological model of human infection of EPEC and EHEC E. coli. Using the C. rodentium model, we have shown that preinoculation of murine gut with Lactobacillus acidophilus, a probiotic strain, early in life can enhance host defense against enteric bacterial infection and attenuate bacteria-mediated intestinal injury (Chen et al., 2005). We also observed that probiotic treatment stimulates regulatory cytokine expression in the colon transforming growth factor (TGF-β) (Chen et al., 2005). In line with these observations, it has been shown that breast-fed infants have a greater resistance to enteric pathogens owing to the transfer of commensal bacteria (Fanaro et al., 2003), nondigestible oligosaccharides (Newburg et al., 2005), TGF-β in maternal milk (Saito et al., 1993), and immunoglobulins (Brandtzaeg, 2010) which enhance development ofthe GAI. Moreover, targeted colonization of the neonate intestine with commensal microbiota has been shown to be effective in allergy prevention in later infancy (Lodinová-Zádníková et al., 2010). More specifically, the intestinal microbial communities predominately induce the maturation of the mucosal adaptive immune system in the human neonate (Kaplan et al., 2011). Conversely, formula-fed infants lack maternal transfer of commensal bacteria, nondigestive oligosaccharides, and TGF-β which results in the modification of gut microbial communities compounding the vulnerability of the neonatal intestine to enteric pathogens (Le Huërou-Luron et al., 2010).
TGF-β is a very potent negative regulator of mucosal inflammation (Letterio & Roberts, 1998) inhibiting T cell activation (Letterio, 2005) vital to maintaining tolerance to innocuous antigens found within the intestine. TGF-β mediates cell signaling by ligand-dependent activation of heterodimeric transmembrane serine/threonine kinases receptors (Piek et al., 1999). Downstream, the ligand-activated receptor directly phosphorylates Smad2 and Smad3 proteins, which associate with Smad 4 and translocate to the nucleus to participate in transcriptional control of targeted genes (Heldin et al., 1997). Disruption of TGF-β signaling occurs in the presence of antagonistic Smad 7, an inhibitor for TGFb signaling, which physically interferes with activation of Smad 2/Smad3 by preventing the interaction with TGF-β receptor (Hayashi et al., 1997) leaving epithelial cells of the intestine in a state of enhanced expression and production of pro-inflammatory cytokines (Maggio-Price et al., 2006).
Excessive Smad 7 protein blocks TGF-β signaling and maintains elevated pro-inflammatory cytokines in inflammatory bowel disease (IBD) patients, while silencing Smad7 expression restores the anti-inflammatory effects of TGF-β (Monteleone et al., 2001; Nguyen & Snapper, 2009). Additionally, IBD patients have high nuclear factor Kappa B (NF-κB) (Jobin and Sartor, 2000) and Smad7 protein expression (Monteleone et al., 2001, 2004a, b, c; Nguyen & Snapper, 2009), which may be correlated with enhanced chronic colonic inflammation. Several studies have suggested a strong correlation between NF-κB and TGF-β/Smad pathways (Bitzer et al., 2000; Nagarajan et al., 2000; Haller et al., 2003). In lamina propria mononuclear cells isolated from IBD patients, abrogation of Smad7 with antisense oligonucleotides allowed endogenous TGF-β to up-regulate inhibitor Kappa B-alpha (IκB-α) and lower NF-κB accumulation (Monteleone et al., 2004c).
The probiotic (commensal intestinal microorganisms)-induced effect on the NF-κB signaling pathway is well established (Yoon and Sun, 2011). Sougioultzis et al. (2006) reported that Saccharomyces boulardii, nonpathogenic yeast, inhibited interleukin 8 (IL-8) production, IκB-α degradation, reduced NF-κB DNA binding, and NF-κB reporter gene up-regulation of interleukin 1 (IL-1) in intestinal cells in vitro. Oral administration of probiotics attenuate intestinal inflammation (Petrof et al., 2004; Tien et al., 2006; Mañé et al., 2009) and NF-κB activation induced by infection (Murphy et al., 2008), stress, tumor necrosis factor (TNF-α), and interleukin 1 (Petrof et al., 2004).
Previously, we reported that inoculation of the probiotic L. acidophilus enhanced enteric protection to pathogens and reduced mucosal inflammation by enhancing TGF-β expression in mice (Chen et al., 2005). In the current study, by utilizing both in vivo (C. rodentium-mouse model, a model of human infection of EPEC and EHEC E. coli) and in vitro approaches, we tested the hypothesis that early inoculation of probiotic L. acidophilus may enhance host-protective immunity to enteric bacterial pathogens through promoting TGF-β response, which exerts its anti-inflammatory effect by reducing Smad 7 expression, allowing TGF-β to up-regulate IκB-α and lower NF-κB accumulation, and that co-administration of prebiotics, the nondigestible food ingredients, which can stimulate the growth and/or activity of beneficial probiotic bacteria, may promote probiotic-induced anti-inflammatory effects.
Materials and methods
Mice
Six- to 8-week-old female and male BALB/c ByJ mice were purchased from the Jackson Laboratory (Bar Harbor, ME), bred in a specific pathogen-free facility at Massachusetts General Hospital (Charlestown, MA), and provided mouse chow and sterile water ad libitum. Neonatal mice were born to pregnant female Balb/c ByJ mice. All animal experiments were approved by the Institutional Animal Care and Use Committee.
Bacterial cultures
Probiotic L. acidophilus (La) was cultured in deMan, Rogosa, and Sharpe broth (MRS; Difco, Detroit, MI) and grown at 37 °C for 20 h and re-suspended in PBS prior to oral inoculation (1 × 109 CFU per mouse). Citrobacter rodentium (strain DBS100; American Type Culture Collection number 51459) was grown overnight in Luria broth (LB) and subsequently re-suspended in PBS prior to dosing (0.5 mL per mouse; approximately 5 × 108 CFU of C. rodentium per mouse). Citrobacter rodentium (Cr) antigen was prepared by collecting an overnight culture of Cr in LB. The bacterial culture was washed in PBS and sonicated on ice. The homogenate was then centrifuged (6000 g) at 4 °C for 30 min. Supernatants were collected, and the protein concentration was determined.
In vivo experimental design
Three independent experiments were conducted in which neonatal (3 days of age) mice and lactating dams were randomly divided into five groups of approximately 7–10 pups per treatment (Fig. 1): group A (nontreated normal control mice), group B (C. rodentium inoculated), group C (prebiotic inulin treated + C. rodentium), group D (probiotic L. acidophilus + C. rodentium), group E (synbiotic combination probiotic L. acidophilus + prebiotic inulin + C. rodentium). Mice of treatment group D were administered L. acidophilus (approximately 1 × 109 CFU per mouse) twice weekly by intragastric gavage for approximately 7 weeks. Sterile water was supplemented with prebiotic: inulin and oligofructose (1 g per 100 mL, Raftilose Synergy®) and administered by intragastric gavage three times weekly from 1 to 3 weeks of age and administered in drinking water provided ad libitum from weeks 3 to 7 weeks of age for mice of treatment group C, with fresh inulin-supplemented drinking water provided every 2 days. Mice of treatment group E were administered a synbiotic combination of L. acidophilus, approximately 1 × 109 CFU per mouse and prebiotic inulin (1 g per 100 mL) by intragastric gavage two times per week from 1 to 7 weeks of age. Control mice (group A) only received a saline vehicle bi-weekly over the duration of the experiment. At 5 weeks of age, mice of treatment groups B, C, D, and E were orally inoculated by intragastric gavage with enteric pathogen, C. rodentium. All mice were sacrificed at 7 weeks of age.
Fig. 1.
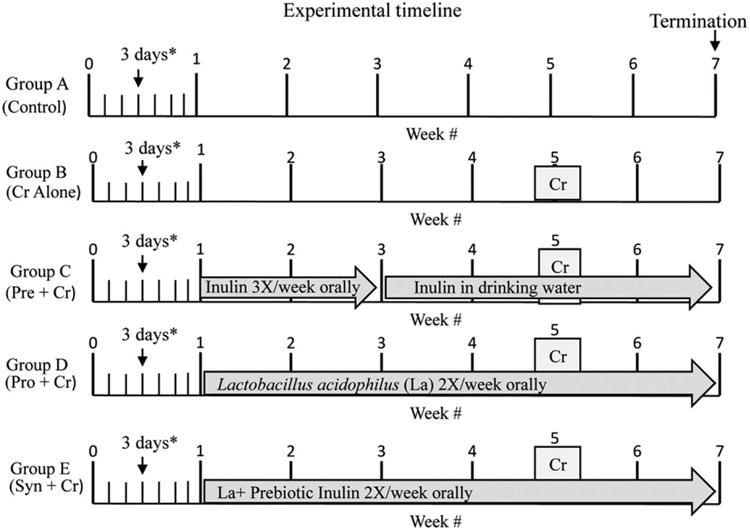
Experimental timeline. Three in vivo experiments were conducted in which 3-day-old mice along with lactating dams were randomly assigned to treatment groups of 7–10 mice pups per treatment. Group A (nontreated controls), group B (Citrobacter rodentium inoculated), group C (prebiotic inulin treated + C. rodentium), group D (probiotic Lactobacillus acidophilus + C. rodentium), group E (synbiotic combination probiotic L. acidophilus + prebiotic inulin + C. rodentium). Mice of treatment groups B, C, D, and E were inoculated with C. rodentium (Cr) at 5 weeks of age. All experimental procedures were terminated 2 weeks post-Cr pathogen inoculation.
Quantitation of clearance of C. rodentium
To assess the clearance of Cr, fecal pellets were collected from each mouse weekly postinfection. Fecal pellets were weighed, homogenized, serially diluted, and plated on selective MacConkey agar plates for gram-negative organisms (Chen et al., 2005; Johnson-Henry et al., 2005; Wu et al., 2008). Bacterial colonies were enumerated after overnight incubation at 37 °C. Citrobacter rodentium colonies were easily distinguished by appearance, using MacConkey agar–plated bacterial cultures from the manufacturer (strain DBS100; American Type Culture Collection number 51459) as a positive control. Bacterial counts are reported as colony-forming units per gram.
Lymphocyte isolation
Mice were sacrificed 2 weeks post-Cr infection. Lymphocyte suspensions were prepared from the mesenteric lymph nodes (MLN) and spleen as described previously (Shi et al., 2000; Chen et al., 2005). Cells (5 × 106 cells mL−1) were cultured on 48-well plates in the presence or absence of Cr antigen (50 μg mL−1) or plate-bound anti-CD3 MAb (10 μg mL−1). Culture supernatants were collected after 72 h and stored at −20 °C until assayed for cytokine production.
Measurement of interferon gamma, IL-10, and TNF-α production by ELISA
ELISA capture antibodies [R4-6A2, interferon gamma (IFN-γ); JESS-2A5, IL-10] and biotinylated secondary antibodies (XMG1.2, IFN-γ; SXC-1, IL-10) were purchased from PharMingen (San Diego, CA), whereas TNF-α ELISA capture antibodies (MP6-XT22) and biotinylated secondary antibodies (C1150-14) were purchased from BD Pharmingen, San Jose, CA. The biotinylated secondary antibodies were used as a second layer, and reactions were visualized with O-phenylenediamine at 492 nm (OPD; Zymed Labs, South San Francisco, CA). Standard curves were obtained using recombinant murine IFN-γ (Genzyme, Cambridge, MA), IL-10 (R&D Systems, Minneapolis, MN), and TNF-α (BD Pharmingen). Optical density values were converted to pg mL−1 for each cytokine by linear regression with Delta Soft II (Biometallics, Princeton, NJ).
Histopathological examinations
At necropsy, colonic tissues were isolated and small fragments were then frozen in Tissue-Tek® O.C.T. Compound (Miles Inc. Elkhart, IN) and stored at −80 °C. Some colonic fragments were snap-frozen in liquid nitrogen and then stored at −80 °C for detection of colonic cytokine gene expression. Seven-micrometer sections were cut on a 2800 Frigocut cryostat (Reichert-Jung, Germany) and stained with hematoxylin and eosin. Sections were analyzed without prior knowledge of treatment. Colonic pathology was scored using a modified histology scoring system based on previously published methods (Chen et al., 2005). The scoring system consists of two parts. Part 1 is the determination of the infiltration of inflammatory cells in the colon, with scores ranging from 0 to 4 (0, normal cell pattern; 1, scattered inflammatory cells in the lamina propria; 2, increased numbers of inflammatory cells in the lamina propria; 3, confluence of inflammatory cells extending into the submucosa; and 4, transmural extension of the infiltrative inflammatory cells). Part 2 is the evaluation of colon tissue damage, with scores that also range from 0 to 4 (0, normal tissue pattern; 1, minimal inflammation and colonic crypt hyperplasia; 2, mild colonic crypt hyperplasia with or without focal invasion of epithelium; 3, obvious colonic crypt hyperplasia, invasion of epithelium, and goblet cell depletion; and 4, extensive mucosal damage and extension through deeper structures of the bowel wall). The total colon pathology score equals the inflammatory cell score plus the tissue damage score (Fig. 3g).
Fig. 3.
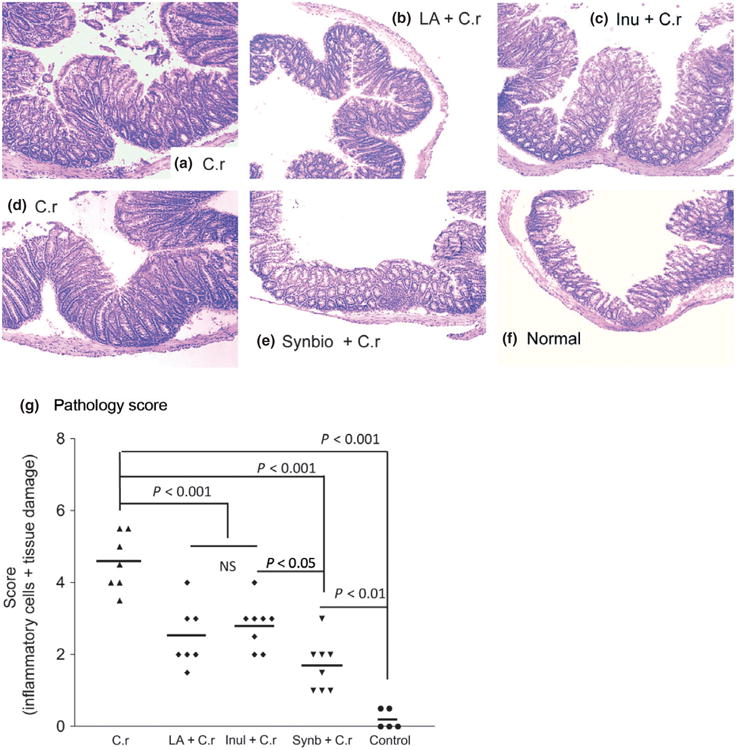
Representative histopathology of colonic tissues at 2 weeks post-Cr infection. Three independent experiments showing similar results with ≈ 7–10 mice per treatment. (a and d) Mice that were infected with Citrobacter rodentium only. (b) Mice pretreated with probiotic La and then infected with Cr. (c) Mice pretreated with prebiotic inulin and then infected with Cr. (e) Mice pretreated with synbiotic and then infected with Cr. (f) Normal mice. (g) The colonic pathology score of different groups of mice at 2 weeks after C rodentium infection. The scores were assessed by determination of inflammation and tissue damage. The figures shown are measurements of individual mice pooled from three independent experiments. The horizontal line represents the mean score of different groups. Data from the colonic pathology scores were analyzed using one-way analysis of variance (Tukey's multiple comparison test).
Cell culture
Mouse intestinal epithelial cell line CMT93 was grown in six-well plates with complete DMEM [10% fetal calf serum, 10 mM HEPES, 2 mM l-glutamine, 100 U penicillin mL−1, 100 μg streptomycin mL−1, 50 μM 2-mercaptoethanol, 0.1 mM nonessential amino acids, and 1 mM sodium pyruvate (Life Technologies, Grand Island, NY)]. All cultures were maintained at 37 °C in a humidity-controlled incubator with 5% CO2 and were grown to confluence over 5–6 days before addition of pathogenic bacteria C. rodentium. The cells were washed and placed in antibiotic-free medium for 1 h. Confluent stock monolayers were subcultured by trypsinization.
In vitro experimental design
In this study, we utilized mouse intestinal epithelial cell line CMT93 to better elucidate cell signaling responses to enteric pathogens in vitro. Nine experiments were conducted independently with similar results. To determine the time-dependent intracellular changes of NF-κB and Smad 7 in response to pathogen exposure, CMT93 cells were exposed with Cr (2.5 × 107 CFU per well) for 1 h in antibiotic-free DMEM at 37 °C. Subsequently, the media and cell lysates were collected at 0, 15, 30, 60, 90, and 120 min and 14 and 24 h postpathogen exposure. Cells were washed and lysed [(1% Triton-X-100 supplemented with 0.1 μM phenylmethylsulphonyl fluoride, 0.1 μM sodium orthovanadate, and Halt protease inhibitor (10 μL mL−1, Pierce cat# 78410, Thermo Scientific, Rockford, IL)]. The lysates were kept at −80 °C for future Western blot analysis. The culture supernatants were stored at −20 °C for future measurement of TNF-α cytokine production.
Detection of colonic cytokine expression (quantitative real-time PCR)
Total RNA was isolated from frozen colonic tissue (distal part of the colon) and treated CMT93 cells using TRIzol (Invitrogen Life Technologies, Carlsbad, CA) following the manufacturer's protocol. First-strand cDNA was synthesized using 2 μg of extracted total RNA (Ready-to-Go kit; Amersham Pharmacia Biotech, Piscataway, NJ). IL-10 and TGF-β colonic expression was determined by realtime PCR using QuantiTect SYBR green real-time PCR kit (Qiagen, Valencia, CA) on the Opticon II DNA thermocylcer (MJ Research, Waltham, MA). A PCR master mix was prepared according to the manufacturer's protocol with a reaction volume of 50 μL, using the following real-time cycler conditions: 95 °C for 15 min, 94 °C for 15 s, 55 °C for 30 s, 72 °C for 30 s for 38 cycles. GAPDH was used as internal controls. LightCycler relative quantification software was used to normalize data to the same GAPDH mRNA level. Samples were run in duplicate. Mouse IL-10 and TGF-β commercially available PCR primers were purchased from Biosource International, Inc. (Camarillo, CA) for detection, while GAPDH commercially available upstream and downstream PCR primers were utilized for detection (R&D Systems, Minneapolis, MN).
Protein extraction and Western blot analysis
Mouse colonic tissue and treated CMT93 cells were homogenized with lysis buffer prepared as previously mentioned. The suspensions were centrifuged at 4 °C, and the supernatant was collected, and protein content was determined using DC protein assay (Bio-Rad Laboratories, Hercules, CA). Proteins were separated by SDS-PAGE for Western blot analysis. Pooled samples per treatment [equal protein amounts (μg) from each mouse within a treatment] from colonic tissue were separated by SDS-PAGE for Western blot analysis, while lysates of 2-well replicates of treated CMT93 cells were pooled per treatment and separated by SDS-PAGE for Western blot analysis. Smad7 and IκB-α protein expression was determined using polyclonal rabbit anti-mouse Smad7 (sc-11392) and IκB-α (sc-847) primary antibodies, respectively (Santa Cruz Biotech, Santa Cruz, CA). Bio-detection was determined utilizing secondary antibody goat anti-rabbit IgG conjugated with horseradish peroxidase (sc-2004, Santa Cruz). Each blot was stripped and analyzed for GAPDH protein expression, as an internal loading control, using a specific rabbit anti-mouse GAPDH antibody (sc25778, Santa Cruz), followed by a goat anti-rabbit antibody conjugated to horseradish peroxidase.
Statistical analysis
All results were expressed as the mean ± SEM. Statistical differences were determined using one-way analysis of variance test (Tukey's multiple comparison test) with GRAPHPAD PRISM. A value for P < 0.05 was considered significant.
Results
Probiotic La and/or prebiotic inulin administration reduces C. rodentium-induced early morbidity in mice pups
Numerous reports have demonstrated the various health benefits of probiotic administration in mature animals (Tien et al., 2006; Damaskos & Kolios, 2008; Farnworth, 2008; Gill & Prasad, 2008). However, few studies have examined the effects of administration of probiotics and/or prebiotics on early development, survivability, and resistance to enteric pathogens in young animals. To determine how early inoculation of probiotic, La, and/or prebiotic inulin may alter the developmental patterns of the GAI affecting host resistance to enteric pathogens, we pre-inoculated the mice with and without La, inulin, and both and infected them with C. rodentium. During the experimental period, the clinical symptoms, change in body weight and survival of the animals were monitored. As expected, mice infected only with Cr showed signs of Citrobacter-associated disease, such as soft stool, a hunched posture, disturbed body hair, and a marked body weight loss during the initial period of infection. The body weight remained significantly lower in mice with Cr infection alone throughout the experiment period compared with groups that were uninfected normal control (P < 0.01), C. rodentium-infected with pretreatment of probiotic La (P < 0.05), and synbiotic combination (P < 0.05) (Fig. 2a). Pretreatment of mice with prebiotic inulin alone showed limited effect on host body weight gain during C. rodentium infection, as the body weight changes of these mice did not differ significantly with all other treatment groups (P > 0.05 for all comparisons: Inu + Cr vs. Cr; Inu + Cr vs. La + Cr; Inu + Cr vs. Synb + Cr; and Inu + Cr vs. control).
Fig. 2.
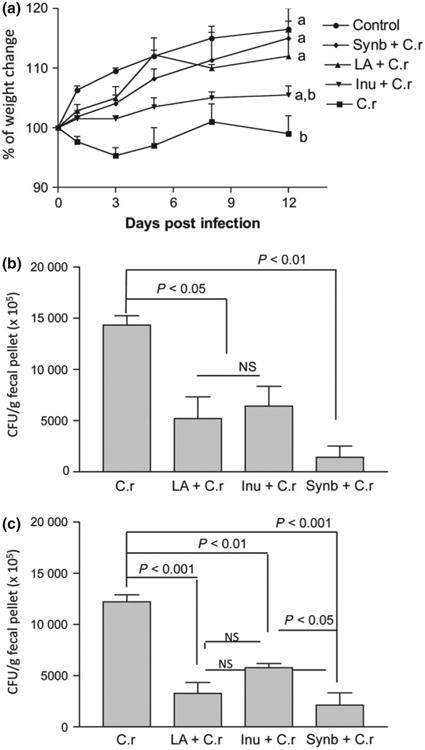
Early administration of probiotic Lactobacillus acidophilus (La) and/or prebiotic inulin reduces Citrobacter rodentium (Cr)-induced morbidity in mice. (a) Body weight changes of noninfected control mice (○) and mice pretreated with a combination of probiotic La and prebiotic (synbiotics ◆), probiotic La (▲), or prebiotic inulin (∇) and infected with C. rodentium, normal mice infected with C. rodentium alone (■) during the course of the experiments. Different letters represent significant difference (P < 0.05) based on one-way ANOVA (Tukey's multiple comparison test). (b and c) Mice that were pretreated with probiotic (La), prebiotic (inulin), and both and then infected with C. rodentium have significantly lower bacterial output in the fecal pellets at 1 and 2 weeks postinfection. The C. rodentium counts in fecal pellet were significantly higher in C. rodentium-infected group compared with the intermediate levels of bacteria recovery from mice pretreated with probiotic La or prebiotic inulin at 1 week (P < 0.05) or 2 weeks (P < 0.01 and P < 0.001, respectively) postinfection. The lowest level of bacterial output was detected in synbiotics group at 1 week (P < 0.01) and 2 week postinfection compared with C. rodentium-infected only group. Significant difference was also detected in the fecal bacterial output of in mice with symbiotic treatment compared with inulin-treated and C. rodentium-infected mice at 2 week postinfection (P < 0.05), based on one-way ANOVA (Tukey's multiple comparison test).
Moreover, a 10% mortality rate was detected in the group that was infected with Cr alone, and no mortality was observed in any other groups (data not shown). These results provide evidence to suggest a protective effect of pretreatment of neonatal mice with probiotic La and synbiotic treatment (La + inulin) on host response against enteric bacterial infection.
To further determine effects of pretreatment of La, inulin, or both on host protection, we examined whether these treatments affected bacterial output from C. rodentium-infected mice by collecting the fecal pellets during the experimental periods, homogenizing, and plating them onto the commonly used selective MacConkey agar plates for the determination of the number of C. rodentium (Chen et al., 2005; Johnson-Henry et al., 2005; Wu et al., 2008). Our results show that bacterial output was significantly lower in mice pretreated with probiotic La (P < 0.05), prebiotic inulin (P < 0.05), or with both (synbiotic) (P < 0.01) at both 1 week postinfection (Fig. 2b). The same trend was consistent through 2 weeks postinfection (Fig. 2c) in all treatment groups with the difference in bacterial output being more pronounced in synbiotic and La group (P < 0.001) and prebiotic inulin treatment (P < 0.01). These results provide evidence indicating that the probiotic, prebiotic, and symbiotic treatments alter the dynamics of the enteric bacterial infection.
Microscopic examination showed that mice infected with C. rodentium showed typical pathological changes associated with this bacterial infection in the intestine, including colonic epithelial cell hyperplasia, crypt elongation, extensive inflammatory cellular infiltration, and disruption of the epithelial surface (Fig. 3a and d). Colonic tissue of mice pretreated with either probiotic La (Fig. 3b) or prebiotic inulin (Fig. 3c) showed less severe pathology (Fig. 3g) compared with mice infected with Cr alone (Fig. 3a and d). This is evidenced by milder colonic crypt elongation, less cellular infiltration of the colonic lamina propria, and epithelial damage detected in La- or inulin-treated mice (Fig. 3b and c) in comparison with Cr-infected mice (Fig. 3a and d). The pathology scores for inflammation and intestinal damage were significantly lower in probiotic La-, prebiotic inulin- and La plus inulin-treated mice, as compared to mice only infected with C. rodentium (Fig. 3g). These observations suggest that pretreatment of probiotic La or prebiotic inulin resulted in a reduction in bacteria-induced intestinal damage. No significant differences were detected in colonic pathology score between La- and inulin-treated mice (Fig. 3g). Furthermore, pathological analysis of colonic tissue revealed that mice pretreated with synbiotics had the most significant reduction in intestinal inflammation and intestinal damage (Fig. 3e and g), as evidenced by the mildest degree of colonic inflammation post-Cr infection in comparison with all the other treatments, with the exception of the controls (Fig. 3f). These results demonstrate that pretreatment of neonatal mice with probiotic La and/or prebiotic inulin may attenuate Cr-induced colon injury with synbiotic treatment being more effective.
Pretreatment with probiotic, La, and/or prebiotic inulin alters cytokine responses in the MLN and intestine of mice
Colonization of C. rodentium on the intestinal epithelial surface resulted in a Th1-type immune response, and Th1 cytokines play a role in host-protective immunity (Simmons et al., 2002); Chen et al., 2005; Gonçalves et al., 2001). To test the hypothesis that early inoculation of probiotic La and/or prebiotic inulin may alter developmental patterns of the GAI, Th1, Th2, and T reg cytokine production and expression in the intestine- and gutassociated lymphoid tissue in young mice following pathogen challenge were determined. Analysis of bacterial (Cr) antigen (Cr-Ag)-specific cytokine production of the MLN revealed that the lymphocytes from mice pretreated with probiotic La, prebiotic inulin, or the synbiotic combination of probiotic La and prebiotic inulin had significantly enhanced Cr-Ag-specific IL-10 secretion (Fig. 4a) compared with that detected in mice with C. rodentium infection alone. Pretreatment of mice with the synbiotic combination of probiotic La and prebiotic inulin resulted in a more pronounced IL-10 production by the MLN cells compared with other groups (Fig. 4a). In contrast, the MLN of mice pretreated with the synbiotic combination of probiotic La and prebiotic inulin had significantly reduced Cr-Ag-specific IFN-γ response (Fig. 4b) at 2 weeks post-Cr infection.
Fig. 4.
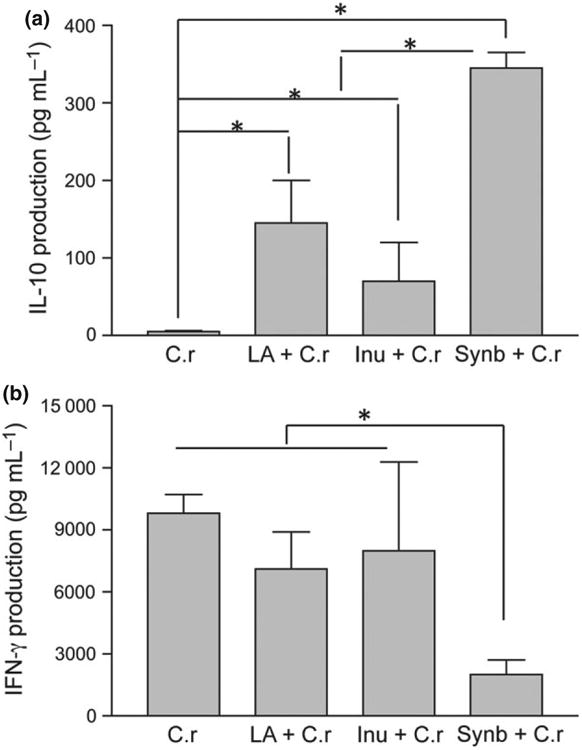
Pretreatment with probiotic Lactobacillus acidophilus (La), prebiotic inulin, or synbiotics alters cytokine responses in the MLN of mice. Cells from the MLN were isolated from mice 2 weeks post-Citrobacter rodentium infection of each treatment and exposed ex vivo to C rodentium antigen (C-Ag) in culture. Culture supernatants were collected after 72 h and assayed for cytokine production. (a) Synbiotic group showed a significant impact on the response of IL-10. (b) Pretreatment with synbiotic (probiotic La + prebiotic inulin) significantly reduced IFN-γ levels. *P < 0.05 when compared to other treatment groups, based on one-way anova (Tukey's multiple comparison test). The results were displayed as means ± SEM and were representative of three independent experiments with N = 7–10 pups per treatment.
To further determine the impact of La, inulin, and combined treatments on pro-inflammatory and regulatory cytokine responses in the colonic tissue, we measured gene expression of IL-10 and TGF-β, the regulatory cytokines, using real-time PCR. The results showed that mice of the synbiotic combination treated group had significantly greater colonic expression of TGF-β, in comparison with C. rodentium-infected control, prebiotic- and probiotictreated groups (Fig. 5a), and pretreatment of mice with La only resulted in an increase in colonic TGF-β expression. These observations, therefore, suggest that probiotic La and synbiotics enhance the expression and production of TGF-β, a key regulator of immunity and vital for the suppression of enteric pathogen-induced inflammatory responses. Similarly, probiotic La and synbiotic combination treatments resulted in a significant increase in colonic IL-10 expression (Fig. 5b) in comparison with Cr infected alone.
Fig. 5.
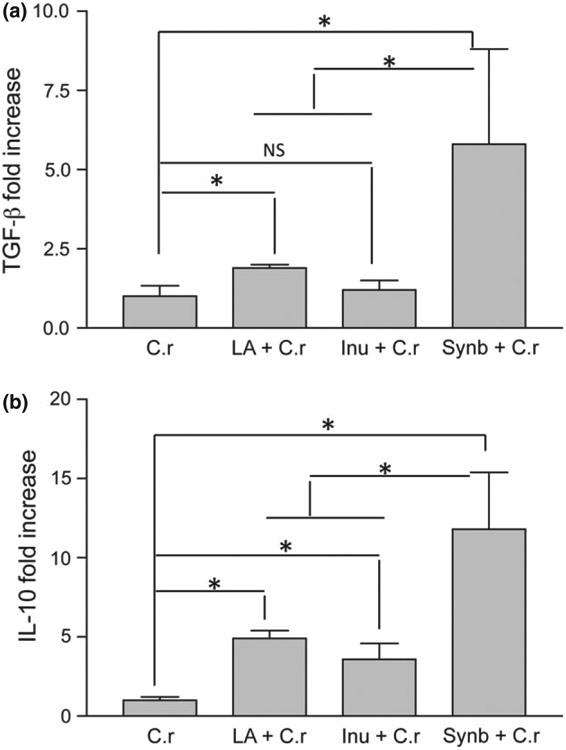
Probiotic (La) and synbiotic treatments promote regulatory cytokine expression in colonic tissue. Mice were pretreated with synbiotics (combination of probiotic La + prebiotic inulin), or probiotic La or prebiotic inulin and infected with Citrobacter rodentium. Cytokine mRNA expression in colon tissue was measured by real-time PCR at 2 weeks after Cr bacterial infection. Values represent the average fold change in mRNA expression in reference to the expression levels in Cr alone-infected mice with GAPDH mRNA expression as an internal control. (a) TGF-β response. (b) IL-10 response. *P < 0.05 when compared with that of Cr-infected group, based on one-way anova (Tukey's multiple comparison test). The results were displayed as means ± SEM and were representative of three independent experiments with N = 7–10 pups per treatment.
Citrobacter rodentium activate NF-κB and Smad 7 intracellular signaling in colonic epithelial cells in vitro
TGF-β can act as a potent negative regulator of mucosal inflammation. However, Smad 7, by physically interfering with activation of Smad2/Smad 3 and preventing their interaction with TGF-β, causes disruption of TGF-β signaling. This may contribute to the enhanced pro-inflammatory responses in the intestine (Hayashi et al, 1997; Maggio-Price et al., 2006). Studies have suggested that NF-κB (Jobin & Sartor, 2000) and Smad 7 (Monteleone et al., 2001, 2004b) are up-regulated in IBD patients and may be responsible for colonic inflammation. NF-κB plays a key role in regulating the immune response to infection and inflammation. In unstimulated cells, NF-κB is complexed with inhibitor, IκB-α, thereby retaining NF-κB within the cytoplasm. Upon induction of the NF-κB pathway by inflammatory signals (IL-1, TNF-α, lipopolysaccharides, stress), IκB-α is degraded; leaving NF-κB free to translocate to the nucleus to elicit transcriptional response (Gosh, 2007). Thus, we next determined the kinetics of NF-κB by measuring IκB-α protein abundance at different time points after C. rodentium exposure using CMT93 cells. NF-κB activation was observed at 60 min post-C. rodentium infection, as indicated by IκB-α degradation (Fig. 6a) in CMT93 cells. This response occurs between 30–60 min postpathogen exposure, with IκB-α levels returning to baseline within 120 min in CMT93 cells. Western blot analysis of the effects of C. rodentium infection on Smad 7 signaling showed a gradual increase in intracellular Smad 7 (between 0–24 h postinfection) in mouse epithelial cells (Fig. 6b), providing evidence to suggest that enteric bacterial infections induce Smad 7 expression in intestinal epithelial cells. Our analysis of TNF-α production reveals that Cr bacteria-induced NF-κB activation and Smad 7 response correlate with pro-inflammatory cytokine responses in intestinal epithelial cells. As shown in Fig. 6b, TNF-α production was enhanced at 1 h postinfection and peaked at 1.5 h post-Cr infection in CMT93 cells (Fig. 6b).
Fig. 6.
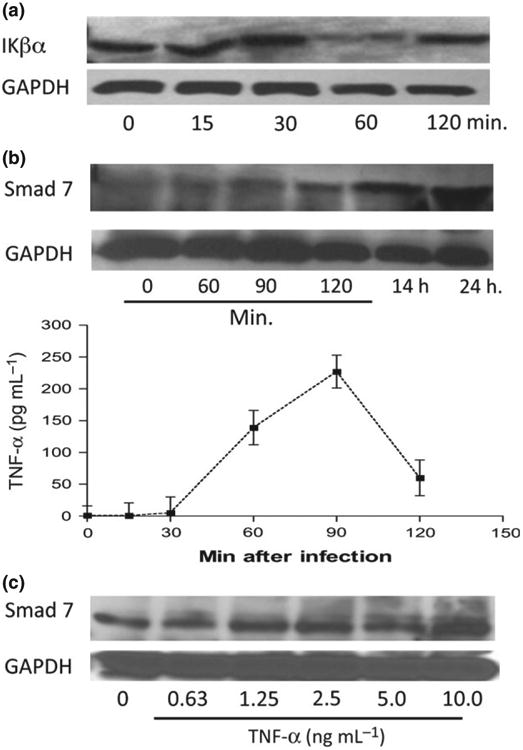
Activation of intracellular NF-kB and Smad 7 in mouse epithelial cells. (a) Confluent CMT93 (p23) cells were incubated with Citrobacter rodentium (Cr, 2.5 × 107 CFU per well) in a six-well plate for 1 h. Cells were subsequently washed in PBS and lysed. (a) Cell lysate (40 μg of protein per lane) was analyzed by Western blotting to determine temporal IkB-α degradation at 0, 15, 30, 60, and 120 min post-Cr pathogen exposure. (b) CMT93 (p25) cells were inoculated with Cr (2.5 × 107 CFU per well) in a six-well plate for 1 h, and 75 μg of protein per lane of cell lysate was analyzed by Western blotting at 0, 1, 1.5, 2, 14, and 24 h post-Cr pathogen exposure to determine temporal Smad 7 induction. Culture media was collected at each time point and assayed for TNF-α secretion (pg mL−1) at each time point. (c) CMT93 cells (p28) were stimulated with TNF-α for 4 h in complete DMEM media, washed 2× in PBS, and 75 μg of protein/lane of cell lysate was analyzed by Western blotting to determine intracellular Smad 7 levels at various TNF-α doses. All data shown here are representative of nine independent in vitro experiments showing similar results.
We next determined whether pro-inflammatory cytokine secretion downstream of NF-Kappa B signaling may be responsible for the induction of Smad 7 and other inflammatory signaling responses. To test this idea, CMT93 cells were stimulated with TNF-α at doses 0.63–10.0 ng mL−1 for 3 h and Smad 7 levels were examined using immunoblot. As indicated in Fig. 6c, a modest increase in the levels of Smad 7 was detected in most of TNF-α-treated cells (1.25, 2.5 and 5 ng mL−1) in comparison with the baseline levels detected in control cells. The effect of TNF-α treatment was found to be more pronounced in cells treated with high doses of TNF-α ng mL−1 CMT93 cells. These results, therefore, suggest a role of pro-inflammatory cytokines in the induction of Smad 7 expression.
Probiotic (La) and/or prebiotic (inulin) inhibit induction of the NF-Kappa B pathway and Smad 7 signaling in vivo
Our data from in vitro experiments suggest that enteric pathogen, C. rodentium induced intracellular NF-κB and Smad 7 signaling in intestinal epithelial cells (Fig. 6). Therefore, in our next set of studies we determine whether probiotic La, prebiotic inulin, or synbiotic pre-treatment will alter pathogen-induced NF-κB and Smad 7 signaling in vivo. We pretreated mice with probiotic La, prebiotic inulin, or both and infected the mice with C. rodentium at 5 weeks of age. Mouse colonic tissues from each group of mice were collected for immunoblotting. Our Western blot analysis revealed that mice infected with Cr alone or in combination with probiotic La or prebiotic inulin pretreatment had decreased levels of IκB-α in comparison with uninfected mice, which indicates an activation of the NF-κB pathway; suggesting that pretreatment with the probiotic La, or prebiotic inulin alone had no clear effect on attenuating NF-κB activation. By contrast, synbiotic treatment restored IκB-α to levels similar to those observed in uninfected animals (Fig. 7). The results further imply that Cr infection induces Smad 7 expression, which is inhibited in mice with pretreatment of probiotic La, prebiotic inulin, or both (Fig. 7). These results suggest that synbiotic combination of probiotic La and prebiotic inulin treatment result in the inhibition of bacteria-induced NF-κB activation and up-regulation of Smad 7 in vivo.
Fig. 7.
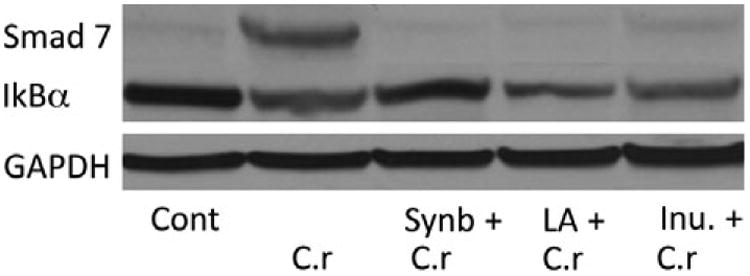
Probiotic La pretreatment attenuates Smad 7 and NF-kB activation post-Citrobacter rodentium exposure in the colon of mice pups. BALB/c ByJ mice were inoculated bi-weekly after birth with, probiotic La, prebiotic inulin, or a combination of both (synbiotic) and challenged with pathogen Cr at 5 weeks of age. Lysates from the colon were prepared 2 weeks post-Cr infection. Seventy-five micrograms of protein per lane of pooled lysates per treatment (≈ 7.5 μg to 10 μg of protein per mouse) from colonic tissue was analyzed by Western blotting to determine the effects of La, inulin, and synbiotic treatment, and Cr on Smad 7 and IkB-α signaling. Control mice only received a saline vehicle bi-weekly. The data shown here are the representative one of three in vivo experiments performed with N= 7–10 pups per treatment, showing similar results.
Discussion
During the early neonatal period, the human infant has a deficiency in antigen presenting cell functions (Tonon et al., 2002; Darmochwal-Kolarz et al., 2004; Upham et al., 2009) and altered T cell-mediated immune responses (Liu et al., 2001; Darmochwal-Kolarz et al., 2004). However, it is during the early neonatal period that the intestine is colonized with approximately 100 trillion bacteria (Ogra & Welliver, 2008). Early exposure to environmental microorganisms promotes the maturation and development of the infant's gut and GAI and may determine the outcome to induced mucosal inflammation (Sjögren et al., 2009), resistance to enteric pathogens, disease development (Hoque et al., 1994), autoimmunity and allergic disorders (Isolauri & Salminen, 2008; Rodriguez et al., 2010) in later life. The diversity of acquired neonatal microbiota is dependent upon the external environment microbial communities, breastfeeding (Kaplan et al., 2011), use of antibiotics, and the presence of nondigestible sugars (prebiotics) in the maternal milk (Newburg et al., 2005; Newburg, 2009). Upon transit to the lower gut, nondigestible oligosaccharides (prebiotics) alter the intestinal luminal environment favorable to support the growth and proliferation of commensal microorganisms. Hence, early exposure to commensal organisms (probiotics) in the breast-fed neonate enhances development and maturation of the gut and GAI and resistance to enteric pathogens (Chen et al., 2005; Salminen & Isolauri, 2008). However, the precise mechanisms by which the microbial communities influence the maturation of the mucosal immunity are not fully understood. In this current study, we utilized the murine C. rodentium model, a physiological model of human infection of EPEC and EHEC E. coli, to determine how early inoculation of probiotic La and/or prebiotic (inulin) affects intestinal innate and adaptive immunity and cell signaling molecules postpathogen exposure.
In this study, neonatal (3 days) mice pups were orally dosed with probiotic bacteria La and/or prebiotic inulin and then exposed to enteric bacterial pathogen C. rodentium to parallel a period of critical early development of GAI and subsequent enteric pathogen exposure in the human neonate. Our results provide evidence that protection activated by synbiotic combination of probiotic La and prebiotic inulin in Citrobacter-infected mice is associated with enhanced mucosal immune responses evidenced by an increase in mucosal IL-10 secretion, up-regulation of IL-10 and TGF-β mRNA expression, reduction in pro-inflammatory cytokine IFN-γ secretion, and reduced bacterial loads, which parallels findings of Steed et al. (2010) demonstrating a significant reduction in intestinal pro-inflammatory TNF-α expression in synbiotic-treated patients. Moreover, the results from this investigation provide evidence to suggest that early treatment with synbiotic combination of probiotic La and prebiotic inulin can effectively prevent pathogen-induced intestinal inflammation by affecting NF-κB and Smad 7 signaling within the intestinal epithelium.
Prebiotics are known to help colonization of beneficial probiotics. While early administration of a synbiotic combination of probiotic La and prebiotic inulin attenuated the secretion and expression of pro-inflammatory cytokines and inflammation, supporting a potential indirect role of prebiotic inulin in regulating mucosal immune response by modulating the colonic microbial communities. Our results are supported by previous observations showing that a diet supplemented with Fructooligosaccharides (FOS) and inulin can trigger and stimulate the gut mucosal immune system (Benyacoub et al., 2008). Our observations also are in line with the results of randomized controlled trials, which provide evidence to suggest that synbiotic therapy can be more effective in the treatment IBD than therapies limited to probiotics or prebiotics (Fujimori et al., 2009; Macfarlane et al., 2009; Steed et al., 2010). In the current study, we found that prebiotic (inulin) treatment of young mice resulted in a reduction in fecal C. rodentium output after the bacterial infection (Fig. 2b and c). It was reported previously that feeding rats with an inulin-oligofructose diet resulted in reduced numbers of Salmonella Typhimurium in the content of ileum and cecum (Kleessen & Blaut, 2005). However, contradicting results have also been reported. Petersen et al. (2009) reported that BALB/c mice fed diets containing prebiotics (FOS or xylo-oligosaccharide) had significantly higher numbers of S. Typhimurium, translocated into liver, spleen, and MLN compared with mice fed with control diet. In contrast, no increased translocation of S. Typhimurium was found in mice fed inulin (Petersen et al., 2009), in that same study. Nevertheless, most prebiotics and/or probiotics have not been shown to cause illness, but additional research is needed to determine the safety of prebiotics and probiotics in young children or people whose immune system is compromised.
The observations showing an enhanced colonic TGF-β and IL-10 responses in mice with early synbiotic or probiotic treatments provided evidence to support the idea that these treatments may modulate gut mucosal inflammatory responses by promoting immunological regulatory mechanisms, which parallel results by Roller et al. (2004) demonstrating that synbiotic and prebiotic supplementation stimulated IL-10 production in the gut-associated lymphoid tissues of azoxymethane treated rats. The protective role of IL-10 and TGF-β/Smad cascade is supported by a study showing that colonization with gram-positive Enterococcus faecalis in IL-10-deficient mice resulted in the development of persistent activation of TLR/NF-κB signaling and inflammation in intestinal epithelial cells, which completely lack Smad 7 expression (Ruiz et al., 2005).
Smad 7 can cause disruption of TGF-β signaling by physically interfering with activation of Smad2/Smad 3 and preventing their interaction with TGF-β receptor. In the current study, we observed that mice infected with C. rodentium alone had significantly enhanced Smad 7 expression and pro-inflammatory cytokine secretion. These responses were reduced in mice pretreated with probiotic La, prebiotic inulin, and synbiotic combination. The association between the attenuation of pathogeninduced colitis and abolished pro-inflammatory Smad 7 signaling in colonic tissues of Cr pathogen-infected mice provide evidence to suggest that probiotic La, prebiotic inulin, and a synbiotic combination may enhance host protection from enteric pathogens by modulating regulatory immunological responses within the gut, which is supported by recent evidence demonstrating a direct effect of Smad 7 on NF-κB (Grau et al., 2006). Hegazy & El-Bedewy (2010) demonstrated that oral probiotic supplementation ameliorated colonic pro-inflammatory cytokine secretion and TNF-α and NF-κB expression in IBD patients. Moreover, we demonstrate that in vitro with CMT93 cells that Smad 7 and NF-κB induction parallels pro-inflammatory cytokine secretion (TNF-α), which imply that colonic Smad 7 and NF-κB induction may be correlated with the production of inflammatory cytokines contributing to the pathological changes attributed to pathogen invasion. Other studies have also shown a correlation between chronic inflammation, pro-inflammatory cytokines, and Smad 7 in patients with autoimmune disease (Monteleone et al., 2004a; Hegazy & El-Bedewy, 2010). Thus, we can conjecture that pro-inflammatory cytokines produced in vivo by the early responding antigen presenting cells may perpetuate Smad 7 signaling culminating in a chronic inflammatory response.
Studies have demonstrated that lamina propria mononuclear cells isolated from IBD patients had enhanced Smad 7 protein levels and pro-inflammatory cytokine secretion, which was not reduced by TGF-β, whereas inhibition of Smad 7 restores the ability of TGF-β to inhibit pro-inflammatory cytokine production (Monteleone et al., 2001), implying that the effects of TGF-β in the microenvironment are not linearly related to its relative abundance. Inhibitory Smads, such as Smad 7, control the strength of the signal from the cell surface to the nucleus and thus control cell function (Monteleone et al., 2001). These studies raise the exciting possibility that resolution of chronic inflammation in the gut might be accomplished by enabling endogenous immunosuppressive mechanisms to function, rather than blocking proinflammatory pathways directly. Reducing Smad 7 enables the abundant TGF-β in inflamed tissues to become functional. Consequently, in this study, we demonstrate that synbiotics not only enhanced TGF-β expression, but also reduced Smad 7 protein levels in colonic tissue of Crinfected mice, resulting in an attenuated mucosal inflammatory and immune responses. Thus, this study may help additionally to identify Smad 7 as a key pro-inflammatory cell signaling molecule altered by probiotic La, prebiotic inulin, and synbiotic administration in the presence of enteric pathogens and gut-associated inflammation.
Acknowledgments
This work was supported by R21DK074727 and R01DK082427 (to H.N.S.) and the Clinical Nutrition Research Center at Harvard (P30 DK040561) (to W.A.W.). I-F.H. is sponsored by Kaohsiung Veterans General Hospital, National Yang-Ming University, Taiwan. The authors also acknowledge Drs Bobby J. Cherayil and Michelle Conroy for their critical review of the manuscript.
Footnotes
Authors' contribution: O.T.F. and I.-F.H. contributed equally to this work.
References
- Benyacoub J, Rochat F, Saudan KY, Rochat I, Antille N, Cherbut C, von der Weid T, Schiffrin EJ, Blum S. Feeding a diet containing a fructooligosaccharide mix can enhance Salmonella vaccine efficacy in mice. J Nutr. 2008;138:123–129. doi: 10.1093/jn/138.1.123. [DOI] [PubMed] [Google Scholar]
- Bitzer M, Von Gersdorff G, Liang D, Dominguez-Rosales A, Beg AA, Rojkind M, Böttinger EP. A mechanism of suppression of TGF-beta/SMAD signaling by NF-Kappa B/RelA. Genes Dev. 2000;14:187–197. [PMC free article] [PubMed] [Google Scholar]
- Brandtzaeg P. The mucosal immune system and its integration with the mammary glands. J Pediatr. 2010;156:S8–S15. doi: 10.1016/j.jpeds.2009.11.014. [DOI] [PubMed] [Google Scholar]
- Chen CC, Louie S, Shi HN, Walker WA. Preinoculation with the probiotic Lactobacillus acidophilus early in life effectively inhibits murine Citrobacter rodentium colitis. Pediatr Res. 2005;58:1185–1191. doi: 10.1203/01.pdr.0000183660.39116.83. [DOI] [PubMed] [Google Scholar]
- Damaskos D, Kolios G. Probiotics and prebiotics in inflammatory bowel disease: microflora on the scope. Br J Clin Pharmacol. 2008;65:453–467. doi: 10.1111/j.1365-2125.2008.03096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darmochwal-Kolarz D, Rolinski J, Buczkowski J, Tabarkiewicz J, Leszczynska-Gorzelak B, Zych I, Oleszczuk J. CD1c+ immature myeloid dendritic cells are predominant in cord blood of healthy neonates. Immunol Lett. 2004;91:71–74. doi: 10.1016/j.imlet.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Donnenberg MS, Whittam TS. Pathogenesis and evolution of virulence in enteropathogenic and enterohemorrhagic Escherichia coli. J Clin Investig. 2001;107:539–548. doi: 10.1172/JCI12404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanaro S, Chierici R, Guerrini P, Vigi V. Intestinal microflora in early infancy: composition and development. Acta Paediatr Suppl. 2003;91:48–55. doi: 10.1111/j.1651-2227.2003.tb00646.x. [DOI] [PubMed] [Google Scholar]
- Farnworth ER. The evidence to support health claims for probiotics. J Nutr. 2008;138:1250S–1254S. doi: 10.1093/jn/138.6.1250S. [DOI] [PubMed] [Google Scholar]
- Fujimori S, Gudis K, Mitsui K, Seo T, Yonezawa M, Tanaka S, Tatsuguchi A, Sakamoto C. A randomized controlled trial on the efficacy of synbiotic versus probiotic or prebiotic treatment to improve the quality of life in patients with ulcerative colitis. Nutrition. 2009;25:520–525. doi: 10.1016/j.nut.2008.11.017. [DOI] [PubMed] [Google Scholar]
- Gill H, Prasad J. Probiotics, immunomodulation, and health benefits. J Adv Exp Med Biol. 2008;606:423–454. doi: 10.1007/978-0-387-74087-4_17. [DOI] [PubMed] [Google Scholar]
- Gonçalves NS, Ghaem-Maghami M, Monteleone G, Frankel G, Dougan G, Lewis DJ, Simmons CP, MacDonald TT. Critical role for tumor necrosis factor alpha in controlling the number of lumenal pathogenic bacteria and immunopathology in infectious colitis. Infect Immun. 2001;69:6651–6659. doi: 10.1128/IAI.69.11.6651-6659.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosh S. Handbook of Transcription Factor NF-Kappa G. CRC Press, Taylor and Francis Group; Boca Raton, FL: 2007. The NF-kappa B pathway: a paradigm for inducible gene expression; pp. 4–7. [Google Scholar]
- Grau AM, Datta PK, Zi J, Halder SK, Beauchamp RD. Role of Smad proteins in the regulation of NF-KappaB by TGF-beta in colon cancer cells. Cell Signal. 2006;18:1041–1050. doi: 10.1016/j.cellsig.2005.08.021. [DOI] [PubMed] [Google Scholar]
- Haller D, Holt L, Kim SC, Schwabe RF, Sartor RB, Jobin C. Transforming growth factor-beta 1 inhibits nonpathogenic Gram negative bacteria-induced NF-Kappa B recruitment to the interleukin-6 gene promoter in intestinal epithelial cells through modulation of histone acetylation. J Biol Chem. 2003;278:23851–23860. doi: 10.1074/jbc.M300075200. [DOI] [PubMed] [Google Scholar]
- Hayashi H, Abdollah S, Qiu Y, et al. The MAD-related protein Smad7 associates with the TGF receptor and functions as an antagonist of TGF signaling. Cell. 1997;89:1165–1173. doi: 10.1016/s0092-8674(00)80303-7. [DOI] [PubMed] [Google Scholar]
- Hecht G. Microbes and microbial toxins: paradigms for microbialmucosal interactions VII. Enteropathogenic Escherichia coli: physiological alterations from an extracellular position. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1–G7. doi: 10.1152/ajpgi.2001.281.1.G1. [DOI] [PubMed] [Google Scholar]
- Hegazy SK, El-Bedewy MM. Effect of probiotics on pro-inflammatory cytokines and NF-KappaB activation in ulcerative colitis. World J Gastroenterol. 2010;16:4145–4151. doi: 10.3748/wjg.v16.i33.4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldin CH, Kohei M, Ten Dijke P. TGF-beta signaling from cell membrane to nucleus through SMAD proteins. Nature. 1997;390:465–471. doi: 10.1038/37284. [DOI] [PubMed] [Google Scholar]
- Hoque SS, Faruque AS, Mahalanabis D, Hasnat A. Infectious agents causing acute watery diarrhoea in infants and young children in Bangladesh and their public implications. J Trop Pediatr. 1994;40:351–354. doi: 10.1093/tropej/40.6.351. [DOI] [PubMed] [Google Scholar]
- Isolauri E, Salminen S. Probiotics: use in allergic disorders: a Nutrition, Allergy, Mucosal Immunology, and Intestinal Microbiota (NAMI) Research Group Report. J Clin Gastroenterol. 2008;42:S91–S96. doi: 10.1097/MCG.0b013e3181639a98. [DOI] [PubMed] [Google Scholar]
- Jobin C, Sartor RB. NF-kappaB signaling proteins as therapeutic targets for inflammatory bowel diseases. Inflamm Bowel Dis. 2000;6:206–213. doi: 10.1097/00054725-200008000-00007. [DOI] [PubMed] [Google Scholar]
- Johnson-Henry KC, Nadjafi M, Avitzur Y, Mitchell DJ, Ngan BY, Galindo-Mata E, Jones NL, Sherman PM. Amelioration of the effects of Citrobacter rodentium infection in mice by pretreatment with probiotics. J Infect Dis. 2005;191:2106–2117. doi: 10.1086/430318. [DOI] [PubMed] [Google Scholar]
- Kaplan JL, Shi HN, Walker WA. The role of microbes in developmental immunologic programming. Pediatr Res. 2011;69:465–472. doi: 10.1203/PDR.0b013e318217638a. [DOI] [PubMed] [Google Scholar]
- Kleessen B, Blaut M. Modulation of gut mucosal biofilms. Br J Nutr. 2005;93:S35–S40. doi: 10.1079/bjn20041346. [DOI] [PubMed] [Google Scholar]
- Le Huërou-Luron IL, Blat S, Boudry G. Breast- v. formula-feeding: impacts on the digestive tract and immediate and long-term health effects. Nutr Res Rev. 2010;23:23–26. doi: 10.1017/S0954422410000065. [DOI] [PubMed] [Google Scholar]
- Letterio JJ. TGF-beta signaling in T cells: roles in lymphoid and epithelial neoplasia. Oncogene. 2005;24:5701–5712. doi: 10.1038/sj.onc.1208922. [DOI] [PubMed] [Google Scholar]
- Letterio JJ, Roberts AB. Regulation of immune responses by TGF-beta. Annu Rev Immunol. 1998;16:137–161. doi: 10.1146/annurev.immunol.16.1.137. [DOI] [PubMed] [Google Scholar]
- Liu E, Tu W, Law HK, Lau YL. Decreased yield, phenotypic expression and function of immature monocyte-derived dendritic cells in cord blood. Br J Haematol. 2001;113:240–246. doi: 10.1046/j.1365-2141.2001.02720.x. [DOI] [PubMed] [Google Scholar]
- Lodinová-Zádníková R, Prokesová L, Kocourková I, Hrdý J, Zizka J. Prevention of allergy in infants of allergic mothers by probiotic Escherichia coli. Int Arch Allergy Immunol. 2010;153:201–206. doi: 10.1159/000312638. [DOI] [PubMed] [Google Scholar]
- Macfarlane GT, Blackett KL, Nakayama T, Steed H, Macfarlane S. The gut microbiota in inflammatory bowel disease. Curr Pharm Des. 2009;15:1528–1536. doi: 10.2174/138161209788168146. [DOI] [PubMed] [Google Scholar]
- Maggio-Price L, Treuting P, Zeng W, Tsang M, Bielefeldt-Ohmann H, Iritani BM. Helicobacter infection is required for inflammation and colon cancer in SMAD3-deficient mice. Cancer Res. 2006;66:828–838. doi: 10.1158/0008-5472.CAN-05-2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mañé J, Lorén V, Pedrosa E, Ojanguren I, Xaus J, Cabré E, Domènech E, Gassull MA. Lactobacillus fermentum CECT 5716 prevents and reverts intestinal damage on TNBS-induced colitis in mice. Inflamm Bowel Dis. 2009;15:1155–1163. doi: 10.1002/ibd.20908. [DOI] [PubMed] [Google Scholar]
- Monteleone G, Kumberova A, Croft NM, McKenzie C, Steer HW, MacDonald TT. Blocking Smad7 restores TGF-b1 signaling in chronic inflammatory bowel disease. J Clin Invest. 2001;108:601–609. doi: 10.1172/JCI12821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteleone G, Pallone F, MacDonald TT. Smad7 in TGF-beta-mediated negative regulation of gut inflammation. Trends Immunol. 2004a;25:513–517. doi: 10.1016/j.it.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Monteleone G, Del Vecchio Blanco G, Palmieri G, et al. Induction and regulation of Smad7 in the gastric mucosa of patients with Helicobacter pylori infection. Gastroenterology. 2004b;126:674–682. doi: 10.1053/j.gastro.2003.11.048. [DOI] [PubMed] [Google Scholar]
- Monteleone G, Mann J, Monteleone I, et al. A failure of transforming growth factor-beta1 negative regulation maintains sustained NF-Kappa B activation in gut inflammation. J Biol Chem. 2004c;279:3925–3932. doi: 10.1074/jbc.M303654200. [DOI] [PubMed] [Google Scholar]
- Murphy K, Travers P, Walport M. Janeways Immunobiology. 7th. Gaylord Science, Taylor and Francis Group LLC; New York and London: 2008. The mucosal immune system; pp. 482–490. [Google Scholar]
- Nagarajan RP, Chen F, Li W, Vig E, Harrington MA, Nakshatri H, Chen Y. Repression of transforming-growth-factor-beta-mediated transcription by nuclear factor KappaB. Biochem J. 2000;348:591–596. [PMC free article] [PubMed] [Google Scholar]
- Newburg DS. Neonatal protection by an innate immune system of human milk consisting of oligosaccharides and glycans. J Anim Sci. 2009;87:26–34. doi: 10.2527/jas.2008-1347. [DOI] [PubMed] [Google Scholar]
- Newburg DS, Ruiz-Palacios GM, Morrow AL. Human milk glycans protect infants against enteric pathogens. Annu Rev Nutr. 2005;25:37–58. doi: 10.1146/annurev.nutr.25.050304.092553. [DOI] [PubMed] [Google Scholar]
- Nguyen DD, Snapper SB. Targeting Smads to restore transforming growth factor-beta signaling and regulatory T-cell function in inflammatory bowel disease. Gastroenterology. 2009;136:1161–1164. doi: 10.1053/j.gastro.2009.02.024. [DOI] [PubMed] [Google Scholar]
- Ogra PL, Welliver RC., Sr Effects of early environment on mucosal immunologic homeostasis, subsequent immune responses and disease outcome. Nestle Nutr Workshop Ser Pediatr Program. 2008;61:145–181. doi: 10.1159/000113492. [DOI] [PubMed] [Google Scholar]
- Petersen A, Heegaard PM, Pedersen AL, Andersen JB, Sorensen RB, Frøkiaer H, Lahtinen SJ, Ouwehand AC, Poulsen M, Licht TR. Some putative prebiotics increase the severity of Salmonella enterica serovar Typhimurium infection in mice. BMC Microbiol. 2009;9:245–255. doi: 10.1186/1471-2180-9-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrof EO, Kojima K, Ropeleski MJ, Musch MW, Tao Y, De Simone C, Chang EB. Probiotics inhibit nuclear factor KappaB and induce heat shock proteins in colonic epithelial cells through proteasome inhibition. Gastroenterology. 2004;127:1474–1487. doi: 10.1053/j.gastro.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Piek E, Heldin CH, ten Dijke P. Specificity, diversity, and regulation in TGF-beta superfamily signaling. FASEB J. 1999;13:2105–2124. [PubMed] [Google Scholar]
- Rodriguez B, Prioult G, Bibiloni R, Nicolis I, Mercenier A, Butel MJ, Waligora-Dupriet AJ. Germ-free status and altered caecal subdominant microbiota are associated with a high susceptibility to cow's milk allergy in mice. FEMS Microbiol Ecol. 2010;76:133–144. doi: 10.1111/j.1574-6941.2010.01035.x. [DOI] [PubMed] [Google Scholar]
- Roller M, Pietro Femia A, Caderni G, Rechkemmer G, Watzl B. Intestinal immunity of rats with colon cancer is modulated by oligofructose-enriched inulin combined with Lactobacillus rhamnosus and Bifidobacterium lactis. Br J Nutr. 2004;92:931–938. doi: 10.1079/bjn20041289. [DOI] [PubMed] [Google Scholar]
- Ruiz PA, Shkoda A, Kim SC, Sartor RB, Haller D. IL-10 gene-deficient mice lack TGF-/Smad signaling and fail to inhibit pro-inflammatory gene expression in intestinal epithelial cells after the colonization with colitogenic Enterococcus faecalis. J Immunol. 2005;174:2990–2999. doi: 10.4049/jimmunol.174.5.2990. [DOI] [PubMed] [Google Scholar]
- Saito S, Yoshida M, Ichijo M, Ishizaka S, Tsujii T. Transforming growth factor-beta (TGF-beta) in human milk. Clin Exp Immunol. 1993;94:220–224. doi: 10.1111/j.1365-2249.1993.tb06004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen S, Isolauri E. Opportunities for improving the health and nutrition of the human infant by probiotics. Nestle Nutr Workshop Ser Pediatr Program. 2008;62:223–233. doi: 10.1159/000146350. [DOI] [PubMed] [Google Scholar]
- Shi HN, Liu HY, Nagler-Anderson C. Enteric infection acts as an adjuvant for the response to a model food antigen. J Immunol. 2000;165:6174–6182. doi: 10.4049/jimmunol.165.11.6174. [DOI] [PubMed] [Google Scholar]
- Simmons CP, Goncalves NS, Ghaem-Maghami M, Bajaj-Elliott M, Clare S, Neves B, Frankel G, Dougan G, MacDonald TT. Impaired resistance and enhanced pathology during infection with a noninvasive, attaching-effacing enteric bacterial pathogen, Citrobacter rodentium, in mice lacking IL-12 or IFN-gamma. J Immunol. 2002;168:1804–1812. doi: 10.4049/jimmunol.168.4.1804. [DOI] [PubMed] [Google Scholar]
- Sjögren YM, Tomicic S, Lundberg A, Böttcher MF, Björkstén B, Sverremark-Ekström E, Jenmalm MC. Influence of early gut microbiota on the maturation of childhood mucosal and systemic immune responses. Clin Exp Allergy. 2009;39:1842–1851. doi: 10.1111/j.1365-2222.2009.03326.x. [DOI] [PubMed] [Google Scholar]
- Snyder JD, Merson MH. The magnitude of the global problem of acute diarrhoeal disease: a review of active surveillance data. Bull World Health Organ. 1982;60:605–613. [PMC free article] [PubMed] [Google Scholar]
- Sougioultzis S, Simeonidis S, Bhaskar KR, Chen X, Anton PM, Keates S, Pothoulakis C, Kelly CP. Saccharomyces boulardii produces a soluble anti-inflammatory factor that inhibits NF-KappaB-mediated IL-8 gene expression. Biochem Biophys Res Commun. 2006;343:69–76. doi: 10.1016/j.bbrc.2006.02.080. [DOI] [PubMed] [Google Scholar]
- Steed H, Macfarlane GT, Blackett KL, Bahrami B, Reynolds N, Walsh SV, Cummings JH, Macfarlane S. Clinical trial: the microbiological and immunological effects of synbiotic consumption – a randomized double-blind placebo-controlled study in active Crohn's disease. Aliment Pharmacol Ther. 2010;32:872–883. doi: 10.1111/j.1365-2036.2010.04417.x. [DOI] [PubMed] [Google Scholar]
- Tien MT, Girardin SE, Regnault B, Le Bourhis L, Dillies MA, Coppée JY, Bourdet-Sicard R, Sansonetti PJ, Pédron T. Anti-inflammatory effect of Lactobacillus casei on Shigella-infected human intestinal epithelial cells. J Immunol. 2006;176:1228–1237. doi: 10.4049/jimmunol.176.2.1228. [DOI] [PubMed] [Google Scholar]
- Tonon S, Goriely S, Aksoy E, Pradier O, Del Giudice G, Trannoy E, Willems F, Goldman M, De Wit D. Bordetella pertussis toxin induces the release of inflammatory cytokines and dendritic cell activation in whole blood: impaired responses in human newborns. Eur J Immunol. 2002;32:3118–3125. doi: 10.1002/1521-4141(200211)32:11<3118::AID-IMMU3118>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Upham JW, Zhang G, Rate A, Yerkovich ST, Kusel M, Sly PD, Holt PG. Plasmacytoid dendritic cells during infancy are inversely associated with childhood respiratory tract infections and wheezing. J Allergy Clin Immunol. 2009;124:707–713. doi: 10.1016/j.jaci.2009.07.009. [DOI] [PubMed] [Google Scholar]
- Vallance BA, Chan C, Robertson ML, Finlay BB. Enteropathogenic and enterohemorrhagic Escherichia coli infections: emerging themes in pathogenesis and prevention. Can J Gastroenterol. 2002;16:771–778. doi: 10.1155/2002/410980. [DOI] [PubMed] [Google Scholar]
- Wu X, Vallance BA, Boyer L, Bergstrom KS, Walker J, Madsen K, O'Kusky JR, Buchan AM, Jacobson K. Saccharomyces boulardii ameliorates Citrobacter rodentium-induced colitis through actions on bacterial virulence factors. Am J Physiol Gastrointest Liver Physiol. 2008;294:G295–G306. doi: 10.1152/ajpgi.00173.2007. [DOI] [PubMed] [Google Scholar]
- Yoon SS, Sun J. Probiotics, nuclear receptor signaling, and anti-inflammatory pathways. Gastroenterol Res Pract. 2011;2011:971938. doi: 10.1155/2011/971938. [DOI] [PMC free article] [PubMed] [Google Scholar]


