Abstract
Even though osteoarthritis (OA) is mainly considered as a degradative condition of the articular cartilage, there is increasing body of data demonstrating the involvement of all branches of the immune system. Genetic, metabolic or mechanical factors cause an initial injury to the cartilage resulting in release of several cartilage specific auto-antigens, which trigger the activation of immune response. Immune cells including T cells, B cells and macrophages infiltrate the joint tissues, cytokines and chemokines are released from different kind of cells present in the joint, complement system is activated, cartilage degrading factors such as matrix metalloproteins (MMPs) and prostaglanding E2 (PGE2) are released, resulting in further damage to the articular cartilage. There is considerable success in the treatment of rheumatoid arthritis using anti-cytokine therapies. In OA, however, these therapies did not show much effect, highlighting more complex nature of pathogenesis of OA. This needs the development of more novel approaches to treat OA, which may include therapies that act on multiple targets. Plant natural products have this kind of properties and may be considered for future drug development efforts. Here we reviewed the studies implicating different components of the immune system in the pathogenesis of OA.
Keywords: Osteoarthritis, T cells, B cells, Complement system, Cytokines, Chemokines
1. Introduction
Osteoarthritis (OA) is a chronic disease and results from damage to articular cartilage induced by a complex interplay of genetic, metabolic, biochemical, and biomechanical factors followed by activation of inflammatory response involving the interaction of cartilage, subchondral bone, and synovium [1]. Many factors- some modifiable- contribute to an increased risk of OA and include obesity, genetics, aging and trauma to the joint. In most patients without a strong genetic predisposition, OA is thought to start as a result of damage to the joint tissue by physical forces as a single event of trauma or by repeated microtrauma due to altered mechanical loading of the joint [2]. Chondrocytes respond to the physical injury by stopping the production of anabolic factors and by releasing more catabolic enzymes such as MMPs, which results in further damage to the cartilage [3], and this further leads to the release of matrix components, which elicit inflammatory mechanisms [4]. Involvement of an immune response, both innate and adaptive, in OA is now widely accepted based on the following evidence:
An inflammatory synovium/synovitis has been linked to increased cartilage damage [5] and pain [6] in recent epidemiological studies on large number of OA patients.
Infiltrates of immune cells including T-cells, B-cells and macrophages have been detected in synovial tissue of OA patients [7,8,9].
Immunoglobulins and immune complexes against cartilage components are detected in cartilage, synoium and plasma in OA patients [4].
Key role of complement activation in OA synovium has been identified [10].
Here we provide a review and recent updates on the involvement of major aspects of immune system, including innate and adaptive immune responses, in the pathogenesis of OA.
2. Innate immunity
2.1 Cellular Factors: Monocytes/macrophages and other cells of innate immunity in OA
Macrophages are among the most abundant cell type present in the cellular infiltrates found in the inflamed synovium in OA [7,11,12]. Macrophage-derived cytokines, including IL-1β and TNF-α are the major players in the cartilage breakdown in OA [discussed later in this review]. Several chemokines responsible for chemotaxis of macrophages have been implicated in the development of OA. Using a collagenase-induced mouse model it was shown that depletion of synovial macrophages by injection of clodronate-laden liposomes resulted in decreased TGF-β-induced osteophyte formation [13]. Using the same model Blom et al showed that the activation of synovial macrophages is required for the production of MMPs and cartilage damage [14]. Bondeson et al developed a macrophage depleted synovial cell culture model by using CD14-conjugated magnetic beads. Specific removal of synovial macrophages from these cultures resulted in significantly decreased production of cytokines, IL-1β and TNF-α, indicating that the source of these cytokines were synovial macrophages. Further, macrophage depletion also resulted in decreased production of IL-6, IL-8, MMP-1 and MMP-3 [15].
Presence of natural killer cells was reported in synovial tissues obtained from patients undergoing total joint replacements, which constituted about 30% of the CD45+ mononuclear cell infiltrate [16]. These cells showed a quiescent phenotype consistent with post-activation exhaustion. Presence of low level of activated dendritic cells was also reported in OA synovium [17]. Recently, dendritic cell infiltrates were detected in the synovial tissue of rabbits with surgically induced OA in the early stages of the disease (2 and 4 weeks post-operation) [18]. However, the role of both NK cells and dendritic cells in OA pathogenesis has not yet been elucidated in detail.
2.2 Humoral Factors
2.2.1 Activation of complement system in OA
The complement system constitutes a crucial effector mechanism in the immune system to clear the pathogens and immune complexes and consists of a cascade of very tightly regulated array of proteins, improper regulation of which may lead to self tissue damage. The deposition and activation of complement factors in OA cartilage has been documented in several early studies in OA patients as well as in animal models of OA [19,20,21,22]. Tarkowski et al observed expression of decay accelerating factor (DAF) in the synovial lining cell layer both in rheumatoid arthritis (RA) and in osteoarthritis (OA) along with C5b-9 terminal complement complex suggesting an activation of complement-mediated response [23]. Corvetta et al found a correlation of terminal complement complex deposits in synovial tissue with the extent of inflammatory synovitis, irrespective of whether the synovitis was in RA or OA patients [24]. Doherty et al found C3 activation to be associated with RA and gout but not with OA [25]. A recent study looking specifically at the levels of lectin pathway proteins found a significant association of the level of these proteins in plasma and synoial fluid (SF) with RA and OA [26]. Several proteomic studies have identified components of complement system as differentially expressed proteins in synovial fluid [27,28], serum [29] and cartilage [30] samples from OA patients. A transcriptome analysis of damaged versus smooth articular cartilage obtained from OA patients revealed decay accelerating factor (DAF) and complement factor I (IF) as two of the six genes up-regulated in the affected cartilage [31]. Results obtained from several studies point to a local synthesis and secretion of these factors within SF instead of resulting from a leakage from the blood [30,31,32,33,34,35]. Chondrocytes have been shown to over-express the C5a receptor (C5aR/CD88) during RA and OA and C5aR/CD88 was found to be up-regulated upon treatment with IL-1 in RA chondrocytes but not in OA chondrocytes suggesting a differential role of C5aR/CD88 in RA and OA [36]. Functional studies have shown that one of the complement components 1s acts as a serine protease and degrades chondroprotective trophic factor IGFBP-5 in cartilage and inhibition of C1s results in improved OA symptoms [37,38]. In a recent report using three mouse models of OA, Wang et al showed that mice deficient in the ability to synthesize the membrane attack complex (MAC) are protected from developing OA while mice deficient in MAC-inhibitor CD59a developed more severe OA as compared to wild type mice [10]. This study also showed that MAC’s cartilage destruction was mediate by an over-expression of cartilage degradation enzymes and pro-inflammatory cytokines instead of lysis of the chondrocytes by the MAC itself [10].
2.2.2 Role of cytokines in OA
Inflammation of the synovial membrane/synovitis is emerging as the main feature of OA even in the early stages of the disease [reviewed recently in 39]. Several soluble factors such as cytokines and chemokines released from the inflamed synovium result in further progression of OA symptoms. During OA, the normally tightly regulated anabolic and catabolic process responsible for maintenance of cartilage homeostasis is disturbed due to the stimulation of inflammatory mechanisms and release of several cytokines. IL-1β and TNF-α are the two major pro-inflammatory cytokines responsible for the shift of cartilage homeostasis towards more catabolism and degradation of cartilage.
2.2.2.1 IL-1β
IL-1β is over-expressed during OA in cartilage as well as in the synovial tissue with a concomitant decrease in the expression of IL-1R antagonist (IL-1Ra) [40,41,42,43]. Over-expression of IL-1RI was observed in chondrocytes located in cartilage proximal to the macroscopic OA lesions resulting in increased binding of IL-1β making those cells more susceptible to the effects of this cytokine [44]. IL-1RI has also been reported to be over-expressed in human OA synovial fibroblasts [45]. Several studies in animal models of OA have also highlighted the important role played by IL-1β in development of symptoms associated with OA [46,47]. IL-1β has been shown to up-regulate the expression of MMP family of catabolic enzymes, MMP-1, -3 and -13 [48,49,50] and to stimulate the production of ADAMTS-4 and -5 (aggrecanases) in human chondrocytes [51], in human OA synovial fibroblasts [52] and bovine chondrocytes [53]. IL-1β also suppresses the anabolic mechanism in cartilage tissue by inhibiting the expression of type II collagen [54,55] and proteoglycans [56,57] in chondrocytes, the two major components of the cartilage extra-cellular matrix (ECM) in cartilage. The mechanism of action of IL-1β on the anabolism of proteoglycan was shown to involve the repression of galactose-beta-1,3-glucuronosyltransferase I (GlcAT-I), a key enzyme in the biosynthesis of glycosaminoglycans [58]. A decrease in the number of chondrocytes during OA has been reported and IL-1β has been attributed as the cause of apoptosis in chondrocytes. In fact, apoptotic features such as mitochondrial depolarization and upregulation of pro-apoptotic Bcl-2 family of proteins have been reported in chondrocytes treated with IL-1β [59,60]. IL-1β may induce apoptosis by increasing the production of NO via up-regulation of iNOS [61,62] or by generation of ROS in chondrocytes [63,64,65].
2.2.2.2 TNF-α
TNF-α shows similar effects on articular cartilage and may act synergistically with IL-1β during OA. TNF-α, similar to IL-1β, has been shown to be over-expressed in OA cartilage [66,67] and was detected in OA synovium [68]. TNF receptor expression is increased in OA cartilage and OA synovium [69,70]. TNF-α was shown to stimulate resorption and inhibit the synthesis of proteoglycan in cartilage explants [71]. TNF-α has also been shown to stimulate the expression of MMP-1, MMP-3 and MMP-13 [72,73]. mRNA expression of TNF-α converting enzyme (TACE), which mediates the conversion of pro-TNF-α to mature TNF-α, was also found to be elevated in OA cartilage [74]. Looking at the success of anti-TNF therapy in RA and given the important role played by IL-1β and TNF-α in OA, any optimism regarding the success of therapies aimed at blocking these cytokines in OA won’t be unjustified, but the few clinical trials conducted did not show much success in OA patients [reviewed recently in 75,76,77]. This may be attributed to a more complex interplay of various pathogenetic factors in OA, than those regulated by these cytokines alone.
2.2.2.3 IL-6
IL-6 is a pro-inflammatory cytokine, which activates the transcription of its target genes via formation of an IL-6 receptor complex involving a membrane bound IL-6 receptor (IL-6R), soluble IL-6R (sIL-6R) and gp130 followed by activation of STAT1/STAT3 pathway [78]. Guerne et al showed the release of IL-6 from synoviocytes in response to IL-1 and detected IL-6 activity in synovial fluids obtained from OA patients, though less than those from RA patients [79]. Later, the same group showed the release of IL-6 from human chondrocytes in response to cytokines, including IL-1 and TNF-α and certain growth factors [80]. Type II collagen stimulated the production of IL-6 from human chondrocytes [81]. PGE2 has also been shown to stimulate the synthesis of IL-6 in synovial fibroblasts [82] and human chondrocytes [83,84,85]. A prospective population study on a cohort of British women showed a correlation of higher BMI and elevated serum levels of IL-6 with development of radiographic knee OA [86]. IL-6 has been shown to exert its effect on catabolic mechanism in cartilage by upregulating the expression of MMP-1 and MMP-13 expression in combination with IL-1β and oncostatin M (OSM) in human and bovine cartilage explants [87,88]. IL-6 has also been shown to affect the anabolic process in cartilage by inhibiting the expression of type II collagen by inhibiting the binding of Sp1/Sp3 to the type II collagen promoter [89].
2.2.2.4 Other Cytokines
Role of other pro-inflammatory cytokines including oncostatin M (OSM), IL-7, IL-8, leukemia inhibitory factor (LIF), IL-11, IL-17 and IL-18 and anti-inflammatory cytokines including IL-4, IL-10 and IL-13 has also been reported in chondrocytes metabolism and development of OA [reviewed in 75,77].
2.2.3 Role of Chemokines in OA
Chemokines are small secretory molecules responsible for chemotaxis of immune cells. Chemokines are classified based on the motif displayed by the first two cysteines residues near the N-terminus into four groups: CC-, CXC, C- and CXXXC. Chemokines act through seven transmembrane Gi-protein coupled receptors [90]. Several chemokines including IL-8/CXCL-8, GROα/CXCL-1, MCP-1/CCL-2, RANTES/CCL-5, MIP-1α/CCL-3 and MIP-1β/CCL-4 were reported to be expressed by human chondrocytes and some of them were shown to be over-expressed in OA [91]. Pro-inflammatory cytokine IL-1β has been shown to stimulate the expression of chemokines in OA chondrocytes [92], while anti-inflammatory factors TGF-β and IL-10 did not show any effect on the expression of these molecules [93]. Expression of several chemokine receptors on the surface of chondrocytes was later reported, which also showed their functionality by inducing the release of MMPs upon binding with their lignads [94,95, 96]. Alaaeddine et al reported the over-expression of RANTES/CCL-5 and its receptors in OA chondrocytes and in normal chondrocytes upon stimulation with IL-1β. Treatment of normal chondrocytes with RANTES/CCL-5 induced the markers of cartilage degradation [69]. Expression of several chemokines and chemokine receptors were also reported in PBMCs and synovial tissue from RA, OA and reactive arthritis [97]. Endres et al reported the chemokine profile of synovial fluid from OA, RA and normal subjects and showed the presence of several chemokines in diseased synovium that may cause the migration of mesenchymal progenitor cells in the microfracture caused during arthritis [98]. A global gene expression analysis in rat model of OA revealed differential expression of MCP-1/CCL-2 and CXCR-4 in chondrocytes [99]. Interestingly, much higher levels of MIP-1β/CCL4 were found in synovial fluid of OA patients (18.0 +/− 8.9 ng/ml) as compared to RA patients (6.1 +/− 2.9 ng/ml) [100]. MIP-1γ/CCL-9 was shown to be produced from activated CD4+ T cells present in the synovium of a mouse model of OA, which also resulted in increased formation of osteoclasts in joints [101]. GRO-α/CXCL-1 and SDF-1/CXCL-12 have been shown to induce cell death in human chondrocytes in apoptotic and necrotic manner, respectively [102, 103]. SDF-1/CXCL-12 was also shown to stimulate IL-6 expression in human synovial fibroblasts [104]. Merz et al showed that IL-8/CXCL-8 and GRO-α/CXCL-1 induced hypertrophic differentiation and calcification in chondrocytes, highlighting the role of inflammation in altered differentiation of chondrocytes [105]. Expression of IL-8/CXCL-8/Kc was, recently, shown to be stimulated by mechanical, inflammatory and metabolic stresses [106]. Hsu et al reported higher levels of eotaxin-1/CCL-11 in OA patients compared to controls and there was an increase in expression of eotaxin-1/CCL-11 upon treatment of chondrocytes with IL-1β and TNF-α [107]. Further, treatment of chondrocytes with eotaxin-1/CCL-11 resulted in increased expression of its receptors CCR-3 and CCR-5 and cartilage degradation enzymes MMP-3 and MMP-13 [107]. Brul et al detected the expression of CCR-5 on synovial fibroblasts from OA and RA patients. Activation of CCR-5 with its ligands CCL-19 and CCL-21 resulted in cell migration and increased secretion of VEGF [108].
3. Adaptive immunity
3.1 T cells and cellular immunity in OA
Mononuclear cell infiltrates in synovial tissues have been reported in OA [40,109,110,111,112] and have been shown to contain primarily CD3+ T cells [113]. Both CD4+ and CD8+ cells were found in OA synovium at similar levels as in RA synovium. The Th1 subset of T cells were found to be about 5 times more than Th2 cells [113] and higher levels of Th1 cytokines, IL-2 and IFNγ, were detected in most of OA patients [110]. T-cells in lymphocytic aggregates in OA synovium were shown to bear early (CD69), intermediate (CD25 and CD38) and late (CD45RO) activation markers. These observations suggest the presence of an active cell-mediated immune response in majority of OA patients. Analysis of α/β T cell receptor diversity revealed the presence of oligoclonal populations of T cells in OA patients [9,114,115]. This suggested that those cells were undergoing clonal expansion in response to specific antigens within the synovium. Although there are no conclusive data on the antigens, which drive the immune response in OA, several candidate antigens have been proposed. T cells derived from peripheral blood and synovial fluid of OA patients showed a strong response to autologous chondrocyte and fibroblast membrane preparations [116]. In another study OA chondrocytes were shown to stimulate autologous T cell response in vitro [117]. Cellular immunity to type III collagen and proteoglycan was detected after partial meniscectomy in rabbits [118]. Higher cellular immunity was observed in OA patients compared to normal subjects when their peripheral blood lymphocytes were stimulated with human cartilage link protein and G1 globular domain of proteoglycan [119]. More specifically, peptides representing amino acid regions 16–31 and 263–280 located in G1 domain of proteoglycan were more frequently recognized by PBMCs isolated from OA patients compared to healthy controls [120]. These studies suggest a role for cartilage components as autoantigens responsible for oligoclonal T cell response observed in OA patients. The role of CD4+ T cells in OA was highlighted by a recent study in anterior cruciate ligament-transection (ACLT)-induced OA mice where these cells were found to be involved in increased production of MIP-1γ followed by increased infiltration of macrophages in synovium and increased expression of MMP-9 [101]. In another study, when chondrocytes from OA patients were co-culture with autologous T cells, they produced higher amounts of RANTES and MMP-1, MMP-3 and MMP-13 [121].
3.2 B cells and humoral immunity in OA
Cellular infiltrates in the inflamed OA synovium have been reported to contain activated B cells along with other cell types [7]. A clonal analysis of B cells in OA synovium revealed their oligoclonal nature suggesting an antigen driven activation instead of non-antigenic activation [122]. Moreover, several studies found antibodies against cartilage components highlighting the activation of humoral adaptive immune response in OA. When cartilage cell surface proteins were used as substrate in an ELISA and sera from OA patients were applied, an elevated antibody titer was detected compared to controls [123]. Similarly, autoantibodies were found in OA patients against cartilage derived proteins osteopontin [124], cartilage intermediate layer protein (CILP) [125], YKL-39, [126], fibulin-4 [127] and collagen [128]. Anti-CCP antibodies were detected in 7 out of 136 OA patients [129], while another group also detected them in OA patients but at significantly lower levels compared to RA patients [130]. Antibodies against native G1 domain of aggrecan core protein were found in synovial fluid of OA patients [131]. Using proteomic approach, Xiang et al identified triosephosphate isomerase (TPI) as an important antigen with autoantibodies present specifically in OA but not in RA [127]. Other studies have reported autoantibodies in animal models of OA including horses [132] and dogs [133]. The role of the autoantibodies against cartilage components in development of OA has been further highlighted by studies showing their deposition [134,135] and cytotoxic effects on cartilage [136], which may be one of the mechanisms playing important role in cartilage degeneration in OA.
4. Major signaling pathways involved in OA
4.1 Involvement of TLRs in OA
TLRs (TLR1 through 10 in humans) are a group of motif recognition receptors important in eliciting an initial innate response against pathogens. They are constitutively expressed on the surface of many immune cells including macrophages but their expression may be induced in other cell types. Upon tissue injury, TLRs may be activated by endogenous damage-associated molecular patterns (DAMPs) also called as alarmins such as hyaluronan, HMGB-1 and S100 family of proteins. The damage to the joint in OA resembles a chronic wound [137]. It involves the release of several DAMPs derived from the damaged extracellular matrix of the joint that act through activation of TLRs such as fibronectin [138], tenascin [139,140], hyaluronan [141,142,143] and biglycan [144 145,146].
TLR-2 and TLR-4 were detected in OA synovial membrane, though less than in RA synovium [147]. However, in vitro, synovial cells from OA and RA patients were equally responsive to the TLR-4 agonist lipopolysaccharides [148] and to the TLR-2 agonist bacterial peptidoglycan [149]. Human chondrocytes have also been shown to express TLRs [150,151] and activation of the TLRs by their ligands leads to the activation of catabolic pathways in chondrocytes [152]. TLR-2 and TLR-4 were found to be upregulated in damaged cartilage in patients with advanced osteoarthritis [150,151]. Zhang et al showed that activation of different TLRs had a differential effect on collagenase gene expression [153]. TLR-4 gene expression was increased in the synovial tissue of stifle joints with OA induced by cranial cruciate ligament transection in dogs, but expression of TLR-2 remained unchanged [154]. MyD88 dependent TLR2/TLR4 signaling was demonstrated as crucial in mediating catabolic responses to low molecular weight hyaluronan (LMW-HA) and HMG-B1 in murine cartilage explants [155]. Alarmins S100A8 and S100A9 were shown to induce cartilage catabolism in human OA chondrocytes by activating TLR-4 [156]. In this study, OA chondrocytes were found to be more sensitive to S100 stimulation compared to normal chondrocytes. Plasma proteins found in the synovial fluid of OA patients Gc-globulin, α1-microglobulin, and α2-macroglobulin were shown to stimulate pro-inflammatory cytokine production from macrophages via TLR-4 mediated pathway [157]. More recently, CD14, a co-receptor for TLRs, was shown to sensitize the synovial fibroblasts from OA patients to TLR-2 and TLR-4 lignads [158].
4.2 Role of NF-κB pathway in OA
Transcription factor NF-κB is the master regulator involved in control of expression of several proteins involved in inflammation, immune response and apoptosis. It is present in the cytoplasm in inactive form associated with the inhibitory κB (IκB) proteins. In response to a broad range of stimuli, including TNF-α, IL-1β, bacterial and viral products, UV radiation and free radicals, a cascade of signaling events result in phosphorylation of IκB by activated IKKs through ubiquitination-dependent degradation by the proteasome leading to the activation and nuclear translocation of NF-κB. NF-κB then binds to the DNA elements present in its target genes and facilitates their transcription. Numerous studies have reported a central role for NF-κB proteins in cartilage metabolism and development of OA [reviewed in 159]. More direct evidence for the involvement of NF-κB in OA development came from a series of studies in cultured synovial fibroblasts from OA patients [52,160]. Using adenoviral vector over-expressing IκB in cultured synovial fibroblasts resulted in decreased baseline expression of IL-6, IL-8, MCP-1/CCL-2 and MMPs [160]. In a subsequent study, IL-1β induced expression of ADAMTS-4 was inhibited by IκB over-expression while ADAMTS-5 expression remained unaffected [52]. In a rat model of surgically induced OA, NF-κB was inhibited by adenoviral vector mediated delivery of the siRNA specific for NF-κB-p65 [161]. This approach resulted in inhibition of the expression of p65, reduced stimulation of IL-1β and TNF-α in synovial fluid, reduced inflammation of the synovium and reduced cartilage damage [161].
Due to its central role in the regulation of genes involved in OA, NF-κB pathway has been an important target of several strategies aimed at developing novel therapies for the treatment of OA [162]. NF-κB activity can be inhibited by inhibiting different steps of the NF-κB activation pathway [159]. Inhibition of IKKβ, proteasomal machinery and DNA binding activity of NF-κB subunits using small molecules or siRNA are some of the most popular approaches under study in OA drug discovery. The biggest challenge in these approaches is the side effects of these drugs because of the involvement of NF-κB in normal cellular functioning [163]. A number of natural products including curcumin and resveratrol have been studied as modulators of NF-κB pathway without showing severe side effects [164]. Studies from our group showed a cartilage protective role of a polyphenol rich extract from pomegranate via inhibition of the NF-κB pathway [165].
5. Conclusion
The accumulating evidence suggesting the involvement of all aspects of the immune response in OA, along with other mechanical and biochemical factors, put OA into the category of one of the most complex disorders (Fig. 1). This is contrary to earlier perception of OA as a simple degradation of the joint cartilage due to advanced age. The complexity of the pathogenesis makes it extremely difficult to develop therapeutic approaches to treat OA. Most of the current approaches employed for the treatment of OA provide symptomatic relief from pain and inflammation and effective disease modifying OA drugs (DMOADs) are still elusive. Success of anti-cytokine therapy in RA raised enthusiasm in the field of OA also, but all the recent trials showed limited or no success in modifying the disease condition in OA patients [75] (Table 1). Therefore, it becomes imperative to look for some novel approaches to treat this complex disease. There should be more research and human trials on natural health products with multiple targets and low side effects, which are supported by traditional wisdom as well as new scientific data.
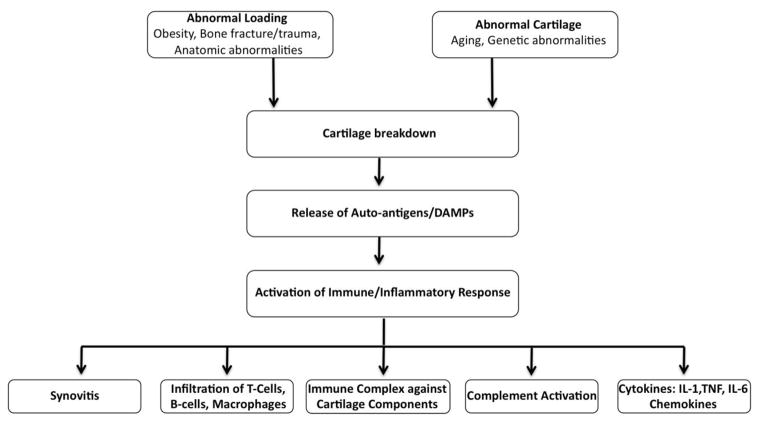
Figure 1. Schematic representation of immunopathogenesis of OA.
Several genetic, metabolic as well as environmental factors lead to the damage of cartilage resulting in the release of cartilage specific autoantigens, which in turn activate the immune/inflammatory response. There is increased infiltration of T-cells, B-cells and macrophages in the joint tissues. These immune cells along with other cells of the joint tissue get activated and release several molecules such as cytokines, chemokines and other cartilage degrading factors such as MMPs and PGE2 resulting in further degradation in the cartilage. DAMPs: damage-associated molecular patterns
Table 1.
Selected in vivo studies targeting IL-1β, TNF-α and NF-κB for the treatment of OA.
| Target | Authors [Ref.] | Therapy | Type of study (model) | Main findings |
|---|---|---|---|---|
| IL-1β | ||||
| Pelletier et al [166] | IL-1Ra gene therapy | Pre-clinical (dogs) | Reduced lesion severity and progression | |
| Fernandes et al [167] | IL-1Ra gene therapy | Pre-clinical (rabbits) | Reduced lesion severity and progression | |
| Frisbie et al [168] | IL-1Ra gene therapy | Pre-clinical (horses) | Significant improvement in pain, disease activity and cartilage preservation | |
| Zhang et al [169] | IL-1Ra+IL-10 gene therapy | Pre-clinical (rabbits) | Significant reduction in cartilage breakdown | |
| Caron et al [170] | Recombinant IL-1Ra (Anakinra) | Pre-clinical (dogs) | Protection from OA lesions and reduction in collagenase-I expression | |
| Chevalier et al [171] | Recombinant IL-1Ra (Anakinra) | Clinical trial | Drug found to be safe and well-tolerated | |
| Chevalier et al [172] | Recombinant IL-1Ra (Anakinra) | Clinical trial | Not effective in relieving disease symptoms | |
| Bacconnier et al [173] | Recombinant IL-1Ra (Anakinra) | Case report (3 female patients) | Improvement in pain and global handicap in erosive OA of the hand | |
| TNF-α | ||||
| Grunke et al [174] | Monoclonal anti-TNF antibody (Adalimumab) | Case report (single 68 yr old male patient) | Relief from pain, improved movement and improved disease symptoms | |
| Magnano et al [175] | Monoclonal anti-TNF antibody (Adalimumab) | Pilot clinical trial (12 patients) | Well-tolerated, modest improvement in disease symptoms in erosive OA of the hand | |
| Fioravanti et al [176] | Monoclonal anti-TNF antibody (Infliximab) | Pilot clinical trial (10 female patients) | Well-tolerated and relief from pain but modest improvement in lesion in erosive OA of the hand | |
| NF-κB | ||||
| Hashimoto H et al [177] | NF-κB decoy oligodeoxynucleotde | Pre-clinical (rats) | Decrease in OA lesion (assessed by Mankin score), significant decrease in IL-1β and TNF-α levels in synovium and cartilage | |
| Chen et al [161] | Adenoviral vector-mediated NF-κBp65-specific siRNA | Pre-clinical (rats) | Reduced synovial inflammation, cartilage degradation and IL-1β and TNF-α levels in the synovium in early phase of experimental OA | |
Highlights.
OA is the most common and painful disease of the joints among the elderly.
Pathogenesis of OA is complex and both the innate and adaptive immunity play a role.
Use of biologics to treat OA has not met with the same success as in RA.
There is a need to identify novel therapeutic targets and agents for treating OA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Creamer P, Hochberg MC. Osteoarthritis. Lancet. 1997;350:503–508. doi: 10.1016/S0140-6736(97)07226-7. [DOI] [PubMed] [Google Scholar]
- 2.Brandt KD, Dieppe P, Radin E. Etiopathogenesis of osteoarthritis. Med Clin North Am. 2009;93:1–24. doi: 10.1016/j.mcna.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 3.Lane Smith R, Trindade MC, Ikenoue T, et al. Effects of shear stress on articular chondrocyte metabolism. Biorheology. 2000;37:95–107. [PubMed] [Google Scholar]
- 4.Jasin HE. Immune mediated cartilage destruction. Scand J Rheumatol Suppl. 1988;76:111–116. doi: 10.3109/03009748809102960. [DOI] [PubMed] [Google Scholar]
- 5.Ayral X, Pickering EH, Woodworth TG, et al. Synovitis: a potential predictive factor of structural progression of medial tibiofemoral knee osteoarthritis - results of a 1 year longitudinal arthroscopic study in 422 patients. Osteoarthritis Cartilage. 2005;13:361–367. doi: 10.1016/j.joca.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 6.Hill CL, Hunter DJ, Niu J, et al. Synovitis detected on magnetic resonance imaging and its relation to pain and cartilage loss in knee osteoarthritis. Ann Rheum Dis. 2007;66:1599–1603. doi: 10.1136/ard.2006.067470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Revell PA, Mayston V, Lalor P, Mapp P. The synovial membrane in osteoarthritis: a histological study including the characterisation of the cellular infiltrate present in inflammatory osteoarthritis using monoclonal antibodies. Ann Rheum Dis. 1988;47:300–307. doi: 10.1136/ard.47.4.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sakkas LI, Scanzello C, Johanson N, et al. T cells and T-cell cytokine transcripts in the synovial membrane in patients with osteoarthritis. Clin Diagn Lab Immunol. 1998;5:430–437. doi: 10.1128/cdli.5.4.430-437.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakamura H, Yoshino S, Kato T, et al. T-cell mediated inflammatory pathway in osteoarthritis. Osteoarthritis Cartilage. 1999;7:401–402. doi: 10.1053/joca.1998.0224. [DOI] [PubMed] [Google Scholar]
- 10.Wang Q, Rozelle AL, Lepus CM, et al. Identification of a central role for complement in osteoarthritis. Nat Med. 2011;17:1674–1679. doi: 10.1038/nm.2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walsh DA, Bonnet CS, Turner EL, et al. Angiogenesis in the synovium and at the osteochondral junction in osteoarthritis. Osteoarthritis Cartilage. 2007;15:743–751. doi: 10.1016/j.joca.2007.01.020. [DOI] [PubMed] [Google Scholar]
- 12.Benito MJ, Veale DJ, FitzGerald O, et al. Synovial tissue inflammation in early and late osteoarthritis. Ann Rheum Dis. 2005;64:1263–1267. doi: 10.1136/ard.2004.025270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Lent PL, Blom AB, van der Kraan P, et al. Crucial role of synovial lining macrophages in the promotion of transforming growth factor β–mediated osteophyte formation. Arthritis Rheum. 2004;50:103–111. doi: 10.1002/art.11422. [DOI] [PubMed] [Google Scholar]
- 14.Blom AB, van Lent PL, Libregts S, et al. Crucial role of macrophages in matrix metalloproteinase-mediated cartilage destruction during experimental osteoarthritis: involvement of matrix metalloproteinase 3. Arthritis Rheum. 2007;56:147–157. doi: 10.1002/art.22337. [DOI] [PubMed] [Google Scholar]
- 15.Bondeson J, Wainwright SD, Lauder S, et al. The role of synovial macrophages and macrophage-produced cytokines in driving aggrecanases, matrix metalloproteinases, and other destructive and inflammatory responses in osteoarthritis. Arthritis Res Ther. 2006;8:R187. doi: 10.1186/ar2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huss RS, Huddleston JI, Goodman SB, et al. Synovial tissue-infiltrating natural killer cells in osteoarthritis and periprosthetic inflammation. Arthritis Rheum. 2010;62:3799–3805. doi: 10.1002/art.27751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pettit AR, Ahern MJ, Zehntner S, et al. Comparison of differentiated dendritic cell infiltration of autoimmune and osteoarthritis synovial tissue. Arthritis Rheum. 2001;44:105–110. doi: 10.1002/1529-0131(200101)44:1<105::AID-ANR14>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 18.XE, Cao Y, Meng H, et al. Dendritic cells of synovium in experimental model of osteoarthritis of rabbits. Cell Physiol Biochem. 2012;30:23–32. doi: 10.1159/000339046. [DOI] [PubMed] [Google Scholar]
- 19.Gabay R, Micheli A, Fallet GH. Behaviour of synovial complement C3 and C4 components in inflammatory and degenerative joint diseases, before and after synoviorthesis. Ann Rheum Dis. 1975;34:166–170. doi: 10.1136/ard.34.2.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cooke TD, Bennett EL, Ohno O. The deposition of immunoglobulins and complement in osteoarthritic cartilage. Int Orthop. 1980;4:211–217. doi: 10.1007/BF00268158. [DOI] [PubMed] [Google Scholar]
- 21.Moskowitz RW, Kresina TF. Immunofluorescent analysis of experimental osteoarthritic cartilage and synovium: evidence for selective deposition of immunoglobulin and complement in cartilaginous tissues. J Rheumatol. 1986;13:391–396. [PubMed] [Google Scholar]
- 22.Cantatore FP, Benazzo F, Ribatti D, et al. Early alteration of synovial membrane in osteoarthrosis. Clin Rheumatol. 1988;7:214–219. doi: 10.1007/BF02204457. [DOI] [PubMed] [Google Scholar]
- 23.Tarkowski A, Trollmo C, Seifert PS, Hansson GK. Expression of decay-accelerating factor on synovial lining cells in inflammatory and degenerative arthritides. Rheumatol Int. 1992;12:201–205. doi: 10.1007/BF00302153. [DOI] [PubMed] [Google Scholar]
- 24.Corvetta A, Pomponio G, Rinaldi N, et al. Terminal complement complex in synovial tissue from patients affected by rheumatoid arthritis, osteoarthritis and acute joint trauma. Clin Exp Rheumatol. 1992;10:433–438. [PubMed] [Google Scholar]
- 25.Doherty M, Richards N, Hornby J, Powell R. Relation between synovial fluid C3 degradation products and local joint inflammation in rheumatoid arthritis, osteoarthritis, and crystal associated arthropathy. Ann Rheum Dis. 1988;47:190–197. doi: 10.1136/ard.47.3.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ammitzboll CG, Thiel S, Ellingsen T, et al. Levels of lectin pathway proteins in plasma and synovial fluid of rheumatoid arthritis and osteoarthritis. Rheumatol Int. 2012;32:1457–1463. doi: 10.1007/s00296-011-1879-x. [DOI] [PubMed] [Google Scholar]
- 27.Gobezie R, Kho A, Krastins B, et al. High abundance synovial fluid proteome: distinct profiles in health and osteoarthritis. Arthritis Res Ther. 2007;9:R36. doi: 10.1186/ar2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mateos J, Lourido L, Fernández-Puente P, et al. Differential protein profiling of synovial fluid from rheumatoid arthritis and osteoarthritis patients using LC-MALDI TOF/TOF. J Proteomics. 2012;75:2869–2878. doi: 10.1016/j.jprot.2011.12.042. [DOI] [PubMed] [Google Scholar]
- 29.Fernández-Puente P, Mateos J, Fernández-Costa C, et al. Identification of a panel of novel serum osteoarthritis biomarkers. J Proteome Res. 2011;10:5095–5101. doi: 10.1021/pr200695p. [DOI] [PubMed] [Google Scholar]
- 30.Rosenthal AK, Gohr CM, Ninomiya J, Wakim BT. Proteomic analysis of articular cartilage vesicles from normal and osteoarthritic cartilage. Arthritis Rheum. 2011;63:401–411. doi: 10.1002/art.30120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Geyer M, Grässel S, Straub RH, et al. Differential transcriptome analysis of intraarticular lesional vs intact cartilage reveals new candidate genes in osteoarthritis pathophysiology. Osteoarthritis Cartilage. 2009;17:328–335. doi: 10.1016/j.joca.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 32.Ruddy S, Colten HR. Rheumatoid arthritis. Biosynthesis of complement proteins by synovial tissues. N Engl J Med. 1974;290:1284–1288. doi: 10.1056/NEJM197406062902304. [DOI] [PubMed] [Google Scholar]
- 33.Katz Y, Strunk RC. Synovial fibroblast-like cells synthesize seven proteins of the complement system. Arthritis Rheum. 1988;31:1365–1370. doi: 10.1002/art.1780311104. [DOI] [PubMed] [Google Scholar]
- 34.Firestein GS, Paine MM, Littman BH. Gene expression (collagenase, tissue inhibitor of metalloproteinases, complement, and HLA-DR) in rheumatoid arthritis and osteoarthritis synovium. Quantitative analysis and effect of intraarticular corticosteroids. Arthritis Rheum. 1991;34:1094–1105. doi: 10.1002/art.1780340905. [DOI] [PubMed] [Google Scholar]
- 35.Breitner S, Storkel S, Reichel W, Loos M. Complement components C1q, C1r/C1s, and C1INH in rheumatoid arthritis. Correlation of in situ hybridization and northern blot results with function and protein concentration in synovium and primary cell cultures. Arthritis Rheum. 1995;38:492–498. doi: 10.1002/art.1780380406. [DOI] [PubMed] [Google Scholar]
- 36.Onuma H, Masuko-Hongo K, Yuan G, et al. Expression of the anaphylatoxin receptor C5aR (CD88) by human articular chondrocytes. Rheumatol Int. 2002;22:52–55. doi: 10.1007/s00296-002-0199-6. [DOI] [PubMed] [Google Scholar]
- 37.Clemmons DR, Busby WH, Jr, Garmong A, et al. Inhibition of insulin-like growth factor binding protein 5 proteolysis in articular cartilage and joint fluid results in enhanced concentrations of insulin-like growth factor 1 and is associated with improved osteoarthritis. Arthritis Rheum. 2002;46:694–703. doi: 10.1002/art.10222. [DOI] [PubMed] [Google Scholar]
- 38.Busby WH, Jr, Yocum SA, Rowland M, et al. Complement 1s is the serine protease that cleaves IGFBP-5 in human osteoarthritic joint fluid. Osteoarthritis Cartilage. 2009;17:547–555. doi: 10.1016/j.joca.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scanzello CR, Goldring SR. The role of synovitis in osteoarthritis pathogenesis. Bone. 2012;51:249–257. doi: 10.1016/j.bone.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith MD, Triantafillou S, Parker A, et al. Synovial membrane inflammation and cytokine production in patients with early osteoarthritis. J Rheumatol. 1997;24:365–371. [PubMed] [Google Scholar]
- 41.Kubota E, Imamura H, Kubota T, et al. Interleukin 1 beta and stromelysin (MMP3) activity of synovial fluid as possible markers of osteoarthritis in the temporomandibular joint. J Oral Maxillofac Surg. 1997;55:20–27. doi: 10.1016/s0278-2391(97)90438-9. [DOI] [PubMed] [Google Scholar]
- 42.Loeser RF, Carlson CS, Del Carlo M, Cole A. Detection of nitrotyrosine in aging and osteoarthritic cartilage: Correlation of oxidative damage with the presence of interleukin 1β and with chondrocyte resistance to insulin-like growth factor 1. Arthritis Rheum. 2002;46:2349–2357. doi: 10.1002/art.10496. [DOI] [PubMed] [Google Scholar]
- 43.Pelletier JP, McCollum R, Cloutier JM, Martel-Pelletier J. Synthesis of metalloproteases and interleukin 6 (IL-6) in human osteoarthritic synovial membrane is an IL-1 mediated process. J Rheumatol. 1995;43:109–114. [PubMed] [Google Scholar]
- 44.Shlopov BV, Gumanovskaya ML, Hasty KA. Autocrine regulation of collagenase 3 (matrix metalloproteinase 13) during osteoarthritis. Arthritis Rheum. 2000;43:195–205. doi: 10.1002/1529-0131(200001)43:1<195::AID-ANR24>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 45.Sadouk MB, Pelletier JP, Tardif G, et al. Human synovial fibroblasts coexpress IL-1 receptor type I and type II mRNA. The increased level of the IL-1 receptor in osteoarthritic cells is related to an increased level of the type I receptor. Lab Invest. 1995;73:347–355. [PubMed] [Google Scholar]
- 46.Wheaton AJ, Borthakur A, Dodge GR, et al. Sodium magnetic resonance imaging of proteoglycan depletion in an in vivo model of osteoarthritis. Acad Radiol. 2004;11:21–28. doi: 10.1016/s1076-6332(03)00574-9. [DOI] [PubMed] [Google Scholar]
- 47.Lai YC, Shaftel SS, Miller JN, et al. Intraarticular induction of interleukin-1β expression in the adult mouse, with resultant temporomandibular joint pathologic changes, dysfunction, and pain. Arthritis Rheum. 2006;54:1184–1197. doi: 10.1002/art.21771. [DOI] [PubMed] [Google Scholar]
- 48.Tetlow LC, Adlam DJ, Woolley DE. Matrix metalloproteinase and proinflammatory cytokine production by chondrocytes of human osteoarthritic cartilage: associations with degenerative changes. Arthritis Rheum. 2001;44:585–594. doi: 10.1002/1529-0131(200103)44:3<585::AID-ANR107>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 49.Inoue K, Masuko-Hongo K, Okamoto M, Nishioka K. Induction of vascular endothelial growth factor and matrix metalloproteinase-3 (stromelysin) by interleukin-1 in human articular chondrocytes and synoviocytes. Rheumatol Int. 2005;26:93–98. doi: 10.1007/s00296-004-0513-6. [DOI] [PubMed] [Google Scholar]
- 50.Kobayashi M, Squires GR, Mousa A, et al. Role of interleukin-1 and tumor necrosis factor α in matrix degradation of human osteoarthritic cartilage. Arthritis Rheum. 2005;52:128–135. doi: 10.1002/art.20776. [DOI] [PubMed] [Google Scholar]
- 51.Fan Z, Bau B, Yang H, et al. Freshly isolated osteoarthritic chondrocytes are catabolically more active than normal chondrocytes, but less responsive to catabolic stimulation with interleukin-1β. Arthritis Rheum. 2005;52:136–143. doi: 10.1002/art.20725. [DOI] [PubMed] [Google Scholar]
- 52.Bondeson J, Lauder S, Wainwright S, et al. Adenoviral gene transfer of the endogenous inhibitor IkappaBalpha into human osteoarthritis synovial fibroblasts demonstrates that several matrix metalloproteinases and aggrecanases are nuclear factor-kappaB-dependent. J Rheumatol. 2007;34:523–533. [PubMed] [Google Scholar]
- 53.Cortial D, Gouttenoire J, Rousseau CF, et al. Activation by IL-1 of bovine articular chondrocytes in culture within a 3D collagen-based scaffold. An in vitro model to address the effect of compounds with therapeutic potential in osteoarthritis. Osteoarthritis Cartilage. 2006;14:631–640. doi: 10.1016/j.joca.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 54.Goldring MB, Birkhead J, Sandell LJ, et al. Interleukin 1 suppresses expression of cartilage-specific types II and IX collagens and increases types I and III collagens in human chondrocytes. J Clin Invest. 1988;82:2026–2037. doi: 10.1172/JCI113823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chadjichristos C, Ghayor C, Kypriotou M, et al. Sp1 and Sp3 transcription factors mediate interleukin-1β down-regulation of human type II collagen gene expression in articular chondrocytes. J Biol Chem. 2003;278:39762–39772. doi: 10.1074/jbc.M303541200. [DOI] [PubMed] [Google Scholar]
- 56.Nietfeld JJ, Wilbrink B, Den Otter W, et al. The effect of human interleukin 1 on proteoglycan metabolism in human and porcine cartilage explants. J Rheumatol. 1990;17:818–826. [PubMed] [Google Scholar]
- 57.Stöve J, Huch K, Günther KP, Scharf HP. Interleukin-1β induces different gene expression of stromelysin, aggrecan and tumor-necrosis-factor-stimulated gene 6 in human osteoarthritic chondrocytes in vitro. Pathobiology. 2000;68:144–149. doi: 10.1159/000055915. [DOI] [PubMed] [Google Scholar]
- 58.Gouze JN, Bordji K, Gulberti S, et al. Interleukin-1beta down-regulates the expression of glucuronosyltransferase I, a key enzyme priming glycosaminoglycan biosynthesis: influence of glucosamine on interleukin-1beta-mediated effects in rat chondrocytes. Arthritis Rheum. 2001;44:351–360. doi: 10.1002/1529-0131(200102)44:2<351::AID-ANR53>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 59.Heraud F, Heraud A, Harmand MF. Apoptosis in normal and osteoarthritic human articular cartilage. Ann Rheum Dis. 2000;59:959–965. doi: 10.1136/ard.59.12.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lopez-Armada MJ, Carames B, Lires-Dean M, et al. Cytokines, tumor necrosis factor-α and interleukin-1β, differentially regulate apoptosis in osteoarthritis cultured human chondrocytes. Osteoarthritis Cartilage. 2006;14:660–669. doi: 10.1016/j.joca.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 61.Pelletier JP, Mineau F, Ranger P, et al. The increased synthesis of inducible nitric oxide inhibits IL-1ra synthesis by human articular chondrocytes: possible role in osteoarthritic cartilage degradation. Osteoarthritis Cartilage. 1996;4:77–84. doi: 10.1016/s1063-4584(96)80009-4. [DOI] [PubMed] [Google Scholar]
- 62.Tenor H, Hedbom E, Hauselmann HJ, et al. Phosphodiesterase isoenzyme families in human osteoarthritis chondrocytes- functional importance of phosphodiesterase 4. Br J Pharmacol. 2002;135:609–618. doi: 10.1038/sj.bjp.0704480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Afonso V, Champy R, Mitrovic D, et al. Reactive oxygen species and superoxide dismutases: role in joint diseases. Joint Bone Spine. 2007;74:324–329. doi: 10.1016/j.jbspin.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 64.Mathy-Hartert M, Hogge L, Sanchez C, et al. Interleukin-1β and interleukin-6 disturb the antioxidant enzyme system in bovine chondrocytes: a possible explanation for oxidative stress generation. Osteoarthritis Cartilage. 2008;16:756–763. doi: 10.1016/j.joca.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 65.Scott JL, Gabrielides C, Davidson RK, et al. Superoxide dismutase downregulation in osteoarthritis progression and end-stage disease. Ann Rheum Dis. 2010;69:1502–1510. doi: 10.1136/ard.2009.119966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Moos V, Fickert S, Müller B, et al. Immunohistological analysis of cytokine expression in human osteoarthritic and healthy cartilage. J Rheumatol. 1999;26:870–879. [PubMed] [Google Scholar]
- 67.Melchiorri C, Meliconi R, Frizziero L, et al. Enhanced and coordinated in vivo expression of inflammatory cytokines and nitric oxide synthase by chondrocytes from patients with osteoarthritis. Arthritis Rheum. 1998;41:2165–2174. doi: 10.1002/1529-0131(199812)41:12<2165::AID-ART11>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 68.Schlaak JF, Pfers I, Meyer Zum Buschenfelde KH, Marker-Hermann E. Different cytokine profiles in the synovial fluid of patients with osteoarthritis, rheumatoid arthritis and seronegative spondylarthropathies. Clin Exp Rheumatol. 1996;14:155–162. [PubMed] [Google Scholar]
- 69.Alaaeddine N, Olee T, Hashimoto S, et al. Production of the chemokine RANTES by articular chondrocytes and role in cartilage degradation. Arthritis Rheum. 2001;44:1633–1643. doi: 10.1002/1529-0131(200107)44:7<1633::AID-ART286>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 70.Silvestri T, Pulsatelli L, Dolzani P, et al. In vivo expression of inflammatory cytokine receptors in the joint compartments of patients with arthritis. Rheumatol Int. 2006;26:360–368. doi: 10.1007/s00296-005-0586-x. [DOI] [PubMed] [Google Scholar]
- 71.Saklatvala J. Tumour necrosis factor alpha stimulates resorption and inhibits synthesis of proteoglycan in cartilage. Nature. 1986;322:547–549. doi: 10.1038/322547a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lefebvre V, Peeters-Joris C, Vaes G. Modulation by interleukin 1 and tumor necrosis factor α of production of collagenase, tissue inhibitor of metalloproteinases and collagen types in differentiated and dedifferentiated articular chondrocytes. Biochim Biophys Acta. 1990;1052:366–378. doi: 10.1016/0167-4889(90)90145-4. [DOI] [PubMed] [Google Scholar]
- 73.Reboul P, Pelletier JP, Tardif G, et al. The new collagenase, collagenase-3, is expressed and synthesized by human chondrocytes but not by synoviocytes. A role in osteoarthritis. J Clin Invest. 1996;97:2011–2019. doi: 10.1172/JCI118636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Patel IR, Attur MG, Patel RN, et al. TNF-a convertase enzyme from human arthritis-affected cartilage: isolation of cDNA by differential display, expression of the active enzyme, and regulation of TNF-a. J Immunol. 1998;160:4570–4579. [PubMed] [Google Scholar]
- 75.Malemud CJ. Anticytokine therapy for osteoarthritis: evidence to date. Drugs Aging. 2010;27:95–115. doi: 10.2165/11319950-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 76.Calich AL, Domiciano DS, Fuller R. Osteoarthritis: can anti-cytokine therapy play a role in treatment? Clin Rheumatol. 2010;29:451–455. doi: 10.1007/s10067-009-1352-3. [DOI] [PubMed] [Google Scholar]
- 77.Kapoor M, Martel-Pelletier J, Lajeunesse D, et al. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 2011;7:33–42. doi: 10.1038/nrrheum.2010.196. [DOI] [PubMed] [Google Scholar]
- 78.Zhong Z, Wen Z, Darnell JE., Jr Stat3: a STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Science. 1994;264:95–98. doi: 10.1126/science.8140422. [DOI] [PubMed] [Google Scholar]
- 79.Guerne PA, Zuraw BL, Vaughan JH, et al. Synovium as a source of interleukin 6 in vitro. Contribution to local and systemic manifestations of arthritis. J Clin Invest. 1989;83:585–592. doi: 10.1172/JCI113921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Guerne PA, Carson DA, Lotz M. IL-6 production by human articular chondrocytes. Modulation of its synthesis by cytokines, growth factors and hormones in vitro. J Immunol. 1992;144:499–505. [PubMed] [Google Scholar]
- 81.Klatt AR, Zech D, Kuhn G, et al. Discoidin domain receptor 2 mediates the collagen II-dependent release of interleukin- 6 in primary human chondrocytes. J Pathol. 2009;218:241–247. doi: 10.1002/path.2529. [DOI] [PubMed] [Google Scholar]
- 82.Inoue H, Takamori M, Shimoyama Y, et al. Regulation by PGE2 of the production of interleukin-6, macrophage colony stimulating factor, and vascular endothelial growth factor in human synovial fibroblasts. Br J Pharmacol. 2002;136:287–295. doi: 10.1038/sj.bjp.0704705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tetlow LC, Woolley DE. Histamine and PGE(2) stimulate the production of interleukins -6 and -8 by human articular chondrocytes in vitro. Inflamm Res. 2006;55(Suppl 1):S73–4. doi: 10.1007/s00011-005-0049-6. [DOI] [PubMed] [Google Scholar]
- 84.Li X, Ellman M, Muddasani P, et al. Prostaglandin E2 and its cognate EP receptors control human adult articular cartilage homeostasis and are linked to the pathophysiology of osteoarthritis. Arthritis Rheum. 2009;60:513–523. doi: 10.1002/art.24258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang P, Zhu F, Konstantopoulos K. Prostaglandin E2 induces interleukin-6 expression in human chondrocytes via cAMP/protein kinase A- and phosphatidylinositol 3-kinase-dependent NF-kappaB activation. Am J Physiol Cell Physiol. 2010;298:C1445–1456. doi: 10.1152/ajpcell.00508.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Livshits G, Zhai G, Hart DJ, et al. Interleukin-6 is a significant predictor of radiographic knee osteoarthritis: the Chingford study. Arthritis Rheum. 2009;60:2037–2045. doi: 10.1002/art.24598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cawston TE, Curry VA, Summers CA, et al. The role of oncostatin M in animal and human connective tissue collagen turnover and its localization within the rheumatoid joint. Arthritis Rheum. 1998;41:1760–1771. doi: 10.1002/1529-0131(199810)41:10<1760::AID-ART8>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 88.Rowan AD, Koshy PJ, Shingleton WD, et al. Synergistic effects of glycoprotein 130 binding cytokines in combination with interleukin-1 on cartilage collagen breakdown. Arthritis Rheum. 2001;44:1620–1632. doi: 10.1002/1529-0131(200107)44:7<1620::AID-ART285>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 89.Porée B, Kypriotou M, Chadjichristos C, et al. Interleukin- 6 (IL-6) and soluble IL-6 receptor down-regulation of human type II collagen gene expression in articular chondrocytes requires a decrease of Sp1.Sp3 ratio and of the binding activity of both factors to the COL2A1 promoter. J Biol Chem. 2008;283:4850–4865. doi: 10.1074/jbc.M706387200. [DOI] [PubMed] [Google Scholar]
- 90.Sallusto F, Mackay CR, Lanzavecchia A. The role of chemokine receptors in primary, effector, and memory immune responses. Annu Rev Immunol. 2000;18:593–620. doi: 10.1146/annurev.immunol.18.1.593. [DOI] [PubMed] [Google Scholar]
- 91.Borzi RM, Mazzetti I, Macor S, et al. Flow cytometric analysis of intracellular chemokines in chondrocytes in vivo: constitutive expression and enhancement in osteoarthritis and rheumatoid arthritis. FEBS Lett. 1999;455:238–242. doi: 10.1016/s0014-5793(99)00886-8. [DOI] [PubMed] [Google Scholar]
- 92.Akhtar N, Haqqi TM. Epigallocatechin-3-gallate suppresses the global interleukin-1beta-induced inflammatory response in human chondrocytes. Arthritis Res Ther. 20113;13:R93. doi: 10.1186/ar3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pulsatelli L, Dolzani P, Piacentini A, et al. Chemokine production by human chondrocytes. J Rheumatol. 1999;26:1992–2001. [PubMed] [Google Scholar]
- 94.Borzì RM, Mazzetti I, Cattini L. Human chondrocytes express functional chemokine receptors and release matrix-degrading enzymes in response to C-X-C and C-C chemokines. Arthritis Rheum. 2000;43:1734–1741. doi: 10.1002/1529-0131(200008)43:8<1734::AID-ANR9>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 95.Mazzetti I, Magagnoli G, Paoletti S, et al. A role for chemokines in the induction of chondrocyte phenotype modulation. Arthritis Rheum. 2004;50:112–22. doi: 10.1002/art.11474. [DOI] [PubMed] [Google Scholar]
- 96.Yuan GH, Masuko-Hongo K, Sakata M, et al. The role of C-C chemokines and their receptors in osteoarthritis. Arthritis Rheum. 2001;44:1056–1070. doi: 10.1002/1529-0131(200105)44:5<1056::AID-ANR186>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 97.Haringman JJ, Smeets TJ, Reinders-Blankert P, Tak PP. Chemokine and chemokine receptor expression in paired peripheral blood mononuclear cells and synovial tissue of patients with rheumatoid arthritis, osteoarthritis, and reactive arthritis. Ann Rheum Dis. 2006;65:294–300. doi: 10.1136/ard.2005.037176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Endres M, Andreas K, Kalwitz G, et al. Chemokine profile of synovial fluid from normal, osteoarthritis and rheumatoid arthritis patients: CCL25, CXCL10 and XCL1 recruit human subchondral mesenchymal progenitor cells. Osteoarthritis Cartilage. 2010;18:1458–1466. doi: 10.1016/j.joca.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 99.Appleton CT, Pitelka V, Henry J, Beier F. Global analyses of gene expression in early experimental osteoarthritis. Arthritis Rheum. 2007;56:1854–1868. doi: 10.1002/art.22711. [DOI] [PubMed] [Google Scholar]
- 100.Koch AE, Kunkel SL, Shah MR, et al. Macrophage inflammatory protein-1 beta: a C-C chemokine in osteoarthritis. Clin Immunol Immunopathol. 1995;77:307–14. doi: 10.1006/clin.1995.1157. [DOI] [PubMed] [Google Scholar]
- 101.Shen PC, Wu CL, Jou IM, et al. T helper cells promote disease progression of osteoarthritis by inducing macrophage inflammatory protein-1γ. Osteoarthritis Cartilage. 2011;19:728–736. doi: 10.1016/j.joca.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 102.Borzi RM, Mazzetti I, Magagnoli G, et al. Growth-related oncogene alpha induction of apoptosis in osteoarthritis chondrocytes. Arthritis Rheum. 2002;46:3201–3211. doi: 10.1002/art.10650. [DOI] [PubMed] [Google Scholar]
- 103.Wei L, Sun X, Kanbe K, et al. Chondrocyte death induced by pathological concentration of chemokine stromal cell-derived factor-1. J Rheumatol. 2006;33:1818–1826. [PubMed] [Google Scholar]
- 104.Chen HT, Tsou HK, Hsu CJ, et al. Stromal cell-derived factor-1/CXCR4 promotes IL-6 production in human synovial fibroblasts. J Cell Biochem. 2011;112:1219–1227. doi: 10.1002/jcb.23043. [DOI] [PubMed] [Google Scholar]
- 105.Merz D, Liu R, Johnson K, Terkeltaub R. IL-8/CXCL8 and growth-related oncogene alpha/CXCL1 induce chondrocytes hypertrophic differentiation. J Immunol. 2003;171:4406–4415. doi: 10.4049/jimmunol.171.8.4406. [DOI] [PubMed] [Google Scholar]
- 106.Chauffier K, Laiguillon MC, Bougault C, et al. Induction of the chemokine IL-8/Kc by the articular cartilage: Possible influence on osteoarthritis. Joint Bone Spine. 2012 Feb 15; doi: 10.1016/j.jbspin.2011.12.013. [In Press] [DOI] [PubMed] [Google Scholar]
- 107.Hsu YH, Hsieh MS, Liang YC, et al. Production of the chemokine eotaxin-1 in osteoarthritis and its role in cartilage degradation. J Cell Biochem. 2004;93:929–939. doi: 10.1002/jcb.20239. [DOI] [PubMed] [Google Scholar]
- 108.Brühl H, Mack M, Niedermeier M, et al. Functional expression of the chemokine receptor CCR7 on fibroblast-like synoviocytes. Rheumatology (Oxford) 2008;47:1771–1774. doi: 10.1093/rheumatology/ken383. [DOI] [PubMed] [Google Scholar]
- 109.Lindblad S, Hedfors E. Arthroscopic and immunohistologic characterization of knee joint synovitis in osteoarthritis. Arthritis Rheum. 1987;30:1081–1088. doi: 10.1002/art.1780301001. [DOI] [PubMed] [Google Scholar]
- 110.Sakkas LI, Scanzello C, Johanson N, et al. T cells and T-cell cytokine transcripts in the synovial membrane in patients with osteoarthritis. Clin Diagn Lab Immunol. 1988;5:430–437. doi: 10.1128/cdli.5.4.430-437.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kennedy TD, Plater-Zyberk C, Partridge TA, et al. Morphometric comparison of synovium from patients with osteoarthritis and rheumatoid arthritis. J Clin Pathol. 1988;41:847–852. doi: 10.1136/jcp.41.8.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Haraoui B, Pelletier JP, Cloutier JM, et al. Synovial membrane histology and immunopathology in rheumatoid arthritis and osteoarthritis: in vivo effects of antirheumatic drugs. Arthritis Rheum. 1991;34:153–163. doi: 10.1002/art.1780340205. [DOI] [PubMed] [Google Scholar]
- 113.Ishii H, Tanaka H, Katoh K, et al. Characterization of infiltrating T cells and Th1/Th2-type cytokines in the synovium of patients with osteoarthritis. Osteoarthritis Cartilage. 2002;10:277–281. doi: 10.1053/joca.2001.0509. [DOI] [PubMed] [Google Scholar]
- 114.Zwillich SH, Fang Q, Kieber-Emmons T, et al. Vα gene usage in rheumatoid compared with osteoarthritic synovial tissue T cells. DNA Cell Biol. 1994;13:923–931. doi: 10.1089/dna.1994.13.923. [DOI] [PubMed] [Google Scholar]
- 115.Scanzello CR, Sakkas LI, Johanson NA, Platsoucas CD. Oligoclonal populations of T-cells infiltrate the synovial membrane (SM) of patients with osteoarthritis (OA) Arthritis Rheum. 1999;42(Suppl 59):S257. [Google Scholar]
- 116.Alsalameh S, Mollenhauer J, Hain N, et al. Cellular immune response toward human articular chondrocytes: T cell reactivities against chondrocyte and fibroblast membranes in destructive joint diseases. Arthritis Rheum. 1990;33:1477–1486. doi: 10.1002/art.1780331004. [DOI] [PubMed] [Google Scholar]
- 117.Sakata M, Masuko-Hongo K, Nakamura H, et al. Osteoarthritic articular chondrocytes stimulate autologous T cell responses in vitro. Clin Exp Rheumatol. 2003;21:704–710. [PubMed] [Google Scholar]
- 118.Champion BR, Poole AR. Immunity to homologous type III collagen after partial meniscectomy and sham surgery in rabbits. Arthritis Rheum. 1982;25:274–287. doi: 10.1002/art.1780250305. [DOI] [PubMed] [Google Scholar]
- 119.Guerassimov A, Zhang Y, Cartman A, et al. Immune responses to cartilage link protein and the G1 domain of proteoglycan aggrecan in patients with osteoarthritis. Arthritis Rheum. 1999;42:527–533. doi: 10.1002/1529-0131(199904)42:3<527::AID-ANR18>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 120.de Jong H, Berlo SE, Hombrink P, et al. Cartilage proteoglycan aggrecan epitopes induce proinflammatory autoreactive T-cell responses in rheumatoid arthritis and osteoarthritis. Ann Rheum Dis. 2010;69:255–262. doi: 10.1136/ard.2008.103978. [DOI] [PubMed] [Google Scholar]
- 121.Nakamura H, Tanaka M, Masuko-Hongo K, et al. Enhanced production of MMP-1, MMP-3, MMP-13 and RANTES by interaction of chondrocytes with autologous T cells. Rheumatol Int. 2006;26:984–90. doi: 10.1007/s00296-006-0116-5. [DOI] [PubMed] [Google Scholar]
- 122.Shiokawa S, Matsumoto N, Nishimura J. Clonal analysis of B cells in the osteoarthritis synovium. Ann Rheum Dis. 2001;60:802–805. doi: 10.1136/ard.60.8.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mollenhauer J, von den Mark K, Burmester G, et al. Serum antibodies against chondrocyte cell surface proteins in osteoarthritis and rheumatoid arthritis. J Rheumatol. 1988;15:1811–1817. [PubMed] [Google Scholar]
- 124.Sakata M, Tsuruha JI, Masuko-Hongo K, et al. Autoantibodies to osteopontin in patients with osteoarthritis and rheumatoid arthritis. J Rheumatol. 2001;28:1492–1495. [PubMed] [Google Scholar]
- 125.Tsuruha J, Masuko-Hongo K, Kato T, et al. Implication of cartilage intermediate layer protein in cartilage destruction in subsets of patients with osteoarthritis and rheumatoid arthritis. Arthritis Rheum. 2001;44:838–845. doi: 10.1002/1529-0131(200104)44:4<838::AID-ANR140>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 126.Tsuruha J, Masuko-Hongo K, Kato T, et al. Autoimmunity against YKL-39, a human cartilage derived protein, in patients with osteoarthritis. J Rheumatol. 2002;29:1459–1466. [PubMed] [Google Scholar]
- 127.Xiang Y, Sekine T, Nakamura H, et al. Proteomic surveillance of autoimmunity in osteoarthritis: identification of triosephosphate isomerase as an autoantigen in patients with osteoarthritis. Arthritis Rheum. 2004;50:1511–1521. doi: 10.1002/art.20189. [DOI] [PubMed] [Google Scholar]
- 128.Charrière G, Hartmann DJ, Vignon E, et al. Antibodies to types I, II, IX, and XI collagen in the serum of patients with rheumatic diseases. Arthritis Rheum. 1988;31:325–332. doi: 10.1002/art.1780310303. [DOI] [PubMed] [Google Scholar]
- 129.Du H, Masuko-Hongo K, Nakamura H, et al. The prevalence of autoantibodies against cartilage intermediate layer protein, YKL-39, osteopontin, and cyclic citrullinated peptide in patients with early-stage knee osteoarthritis: evidence of a variety of autoimmune processes. Rheumatol Int. 2005;26:35–41. doi: 10.1007/s00296-004-0497-2. [DOI] [PubMed] [Google Scholar]
- 130.Caspi D, Anouk M, Golan I, et al. Synovial fluid levels of anti-cyclic citrullinated peptide antibodies and IgA rheumatoid factor in rheumatoid arthritis, psoriatic arthritis, and osteoarthritis. Arthritis Rheum. 2006;55:53–56. doi: 10.1002/art.21691. [DOI] [PubMed] [Google Scholar]
- 131.Karopoulos C, Rowley MJ, Ilic MZ, Handley CJ. Presence of antibodies to native G1 domain of aggrecan core protein in synovial fluids from patients with various joint diseases. Arthritis Rheum. 1996;39:1990–1997. doi: 10.1002/art.1780391207. [DOI] [PubMed] [Google Scholar]
- 132.Niebauer GW, Wolf B, Yarmush M, Richardson DW. Evaluation of immune complexes and collagen type-specific antibodies in sera and synovial fluids of horses with secondary osteoarthritis. Am J Vet Res. 1988;49:1223–1227. [PubMed] [Google Scholar]
- 133.De Rooster H, Cox E, van Bree H. Prevalence and relevance of antibodies to type-I and -II collagen in synovial fluid of dogs with cranial cruciate ligament damage. Am J Vet Res. 2000;61:1456–1461. doi: 10.2460/ajvr.2000.61.1456. [DOI] [PubMed] [Google Scholar]
- 134.Jasin HE. Autoantibody specificities of immune complexes sequestered in articular cartilage of patients with rheumatoid arthritis and osteoarthritis. Arthritis Rheum. 1985;28:241–248. doi: 10.1002/art.1780280302. [DOI] [PubMed] [Google Scholar]
- 135.Cooke TD. Significance of immune complex deposits in osteoarthritic cartilage. J Rheumatol. 1987;14:77–79. [PubMed] [Google Scholar]
- 136.Takagi T, Jasin HE. Interactions between anticollagen antibodies and chondrocytes. Arthritis Rheum. 1992;35:224–230. doi: 10.1002/art.1780350217. [DOI] [PubMed] [Google Scholar]
- 137.Scanzello CR, Plaas A, Crow MK. Innate immune system activation in osteoarthritis: is osteoarthritis a chronic wound? Curr Opin Rheumatol. 2008;20:565–572. doi: 10.1097/BOR.0b013e32830aba34. [DOI] [PubMed] [Google Scholar]
- 138.Chevalier X, Claudepierre P, Groult N, et al. Presence of ED-A containing fibronectin in human articular cartilage from patients with osteoarthritis and rheumatoid arthritis. J Rheumatol. 1996;23:1022–1030. [PubMed] [Google Scholar]
- 139.Chevalier X, Groult N, Larget-Piet B, et al. Tenascin distribution in articular cartilage from normal subjects and from patients with osteoarthritis and rheumatoid arthritis. Arthritis Rheum. 1994;37:1013–1022. doi: 10.1002/art.1780370706. [DOI] [PubMed] [Google Scholar]
- 140.Midwood K, Sacre S, Piccinini AM, et al. Tenascin-C is an endogenous activator of Toll-like receptor 4 that is essential for maintaining inflammation in arthritic joint disease. Nat Med. 2009;15:774–780. doi: 10.1038/nm.1987. [DOI] [PubMed] [Google Scholar]
- 141.Belcher C, Yaqub R, Fawthrop F, et al. Synovial fluid chondroitin and keratan sulphate epitopes, glycosaminoglycans, and hyaluronan in arthritic and normal knees. Ann Rheum Dis. 1997;56:299–307. doi: 10.1136/ard.56.5.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Termeer C, Benedix F, Sleeman J, et al. Oligosaccharides of Hyaluronan activate dendritic cells via toll-like receptor 4. J Exp Med. 2002;195:99–111. doi: 10.1084/jem.20001858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Scheibner KA, Lutz MA, Boodoo S, et al. Hyaluronan fragments act as an endogenous danger signal by engaging TLR2. J Immunol. 2006;177:1272–1281. doi: 10.4049/jimmunol.177.2.1272. [DOI] [PubMed] [Google Scholar]
- 144.Poole AR, Rosenberg LC, Reiner A, et al. Contents and distributions of the proteoglycans decorin and biglycan in normal and osteoarthritic human articular cartilage. J Orthop Res. 1996;14:681–689. doi: 10.1002/jor.1100140502. [DOI] [PubMed] [Google Scholar]
- 145.Bock HC, Michaeli P, Bode C, et al. The small proteoglycans decorin and biglycan in human articular cartilage of late-stage osteoarthritis. Osteoarthritis Cartilage. 2001;9:654–663. doi: 10.1053/joca.2001.0420. [DOI] [PubMed] [Google Scholar]
- 146.Schaefer L, Babelova A, Kiss E, et al. The matrix component biglycan is proinflammatory and signals through Toll-like receptors 4 and 2 in macrophages. J Clin Invest. 2005;115:2223–2233. doi: 10.1172/JCI23755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Radstake TR, Roelofs MF, Jenniskens YM, et al. Expression of toll-like receptors 2 and 4 in rheumatoid synovial tissue and regulation by proinflammatory cytokines interleukin-12 and interleukin-18 via interferon-gamma. Arthritis Rheum. 2004;50:3856–3865. doi: 10.1002/art.20678. [DOI] [PubMed] [Google Scholar]
- 148.Ozawa T, Koyama K, Ando T, et al. Thymic stromal lymphopoietin secretion of synovial fibroblasts is positively and negatively regulated by Toll-like receptors/ nuclear factor-kappaB pathway and interferon-gamma/dexamethasone. Mod Rheumatol. 2007;17:459–463. doi: 10.1007/s10165-007-0620-9. [DOI] [PubMed] [Google Scholar]
- 149.Kyburz D, Rethage J, Seibl R, et al. Bacterial peptidoglycans but not CpG oligodeoxynucleotides activate synovial fibroblasts by toll-like receptor signaling. Arthritis Rheum. 2003;48:642–650. doi: 10.1002/art.10848. [DOI] [PubMed] [Google Scholar]
- 150.Su SL, Tsai CD, Lee CH, et al. Expression and regulation of Toll-like receptor 2 by IL-1β and fibronectin fragments in human articular chondrocytes. Osteoarthritis Cartilage. 2005;13:879–886. doi: 10.1016/j.joca.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 151.Liu-Bryan R, Pritzker K, Firestein GS, Terkeltaub R. TLR2 signaling in chondrocytes drives calcium pyrophosphate dihydrate and monosodium urate crystal-induced nitric oxide generation. J Immunol. 2005;174:5016–5023. doi: 10.4049/jimmunol.174.8.5016. [DOI] [PubMed] [Google Scholar]
- 152.Kim HA, Cho ML, Choi HY, et al. The catabolic pathway mediated by Toll-like receptors in human osteoarthritic chondrocytes. Arthritis Rheum. 2006;54:2152–2163. doi: 10.1002/art.21951. [DOI] [PubMed] [Google Scholar]
- 153.Zhang Q, Hui W, Litherland GJ, et al. Differential Toll-like receptor dependent collagenase expression in chondrocytes. Ann Rheum Dis. 2008;67:1633–1641. doi: 10.1136/ard.2007.079574. [DOI] [PubMed] [Google Scholar]
- 154.Kuroki K, Stoker AM, Sims HJ, Cook JL. Expression of Toll-like receptors 2 and 4 in stifle joint synovial tissues of dogs with or without osteoarthritis. Am J Vet Res. 2010;71:750–754. doi: 10.2460/ajvr.71.7.750. [DOI] [PubMed] [Google Scholar]
- 155.Liu-Bryan R, Terkeltaub R. Chondrocyte innate immune myeloid differentiation factor 88-dependent signaling drives procatabolic effects of the endogenous Toll-like receptor 2/Toll-like receptor 4 ligands low molecular weight hyaluronan and high mobility group box chromosomal protein 1 in mice. Arthritis Rheum. 2010;62:2004–2012. doi: 10.1002/art.27475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Schelbergen RF, Blom AB, van den Bosch MH, et al. Alarmins S100A8 and S100A9 elicit a catabolic effect in human osteoarthritic chondrocytes that is dependent on Toll-like receptor 4. Arthritis Rheum. 2012;64:1477–1487. doi: 10.1002/art.33495. [DOI] [PubMed] [Google Scholar]
- 157.Sohn DH, Sokolove J, Sharpe O, et al. Plasma proteins present in osteoarthritic synovial fluid can stimulate cytokine production via Toll-like receptor 4. Arthritis Res Ther. 2012;14:R7. doi: 10.1186/ar3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Nair A, Kanda V, Bush-Joseph C, et al. Synovial fluid from patients with early osteoarthritis modulates fibroblast-like synoviocyte responses to toll-like receptor 4 and toll-like receptor 2 ligands via soluble CD14. Arthritis Rheum. 2012;64:2268–2277. doi: 10.1002/art.34495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Marcu KB, Otero M, Olivotto E, et al. NF-kappaB signaling: multiple angles to target OA. Curr Drug Targets. 2010;11:599–613. doi: 10.2174/138945010791011938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Amos N, Lauder S, Evans A, et al. Adenoviral gene transfer into osteoarthritis synovial cells using the endogenous inhibitor IkappaBalpha reveals that most, but not all, inflammatory and destructive mediators are NFkappaB dependent. Rheumatology (Oxford) 2006;45:1201–1209. doi: 10.1093/rheumatology/kel078. [DOI] [PubMed] [Google Scholar]
- 161.Chen LX, Lin L, Wang HJ, et al. Suppression of early experimental osteoarthritis by in vivo delivery of the adenoviral vector-mediated NF-kappaBp65-specific siRNA. Osteoarthritis Cartilage. 2008;16:174–184. doi: 10.1016/j.joca.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 162.Roman-Blas JA, Jimenez SA. NF-kappaB as a potential therapeutic target in osteoarthritis and rheumatoid arthritis. Osteoarthritis Cartilage. 2006;14:839–848. doi: 10.1016/j.joca.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 163.Khalifé S, Zafarullah M. Molecular targets of natural health products in arthritis. Arthritis Res Ther. 2011;13:102. doi: 10.1186/ar3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Bremner P, Heinrich M. Natural products as targeted modulators of the nuclear factor-kappaB pathway. J Pharm Pharmacol. 2002;54:453–472. doi: 10.1211/0022357021778637. [DOI] [PubMed] [Google Scholar]
- 165.Rasheed Z, Akhtar N, Haqqi TM. Pomegranate extract inhibits the interleukin-1beta-induced activation of MKK-3, p38alpha-MAPK and transcription factor RUNX-2 in human osteoarthritis chondrocytes. Arthritis Res Ther. 2010;12:R195. doi: 10.1186/ar3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Pelletier JP, Caron JP, Evans C, et al. In vivo suppression of early experimental osteoarthritis by interleukin-1 receptor antagonist using gene therapy. Arthritis Rheum. 1997;40:1012–1019. doi: 10.1002/art.1780400604. [DOI] [PubMed] [Google Scholar]
- 167.Fernandes JC, Tardif G, Martel-Pelletier J, et al. In vivo transfer of interleukin-1 receptor antagonist gene in osteoarthritic rabbit knee joints: prevention of osteoarthitic progression. Am J Pathol. 1999;154:1535–1544. doi: 10.1016/S0002-9440(10)65368-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Frisbie DD, Ghivizzani SC, Robbins PD, Evans CH, McIlwraith CW. Treatment of experimental equine osteoarthritis by in vivo delivery of the equine interleukin-1 receptor antagonist gene. Gene Ther. 2002;9:12–20. doi: 10.1038/sj.gt.3301608. [DOI] [PubMed] [Google Scholar]
- 169.Zhang X, Mao Z, Yu C. Suppression of early experimental osteoarthritis by gene transfer of interleukin-1 receptor antagonist and interleukin-10. J Orthop Res. 2004;22:742–750. doi: 10.1016/j.orthres.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 170.Caron JP, Fernandes JC, Martel-Pelletier J, et al. Chondroprotective effect of intra-articular injections of interleukin-1 receptor antagonist in experimental osteoarthritis. Suppression of collagenase-1 expression. Arthritis Rheum. 1996;39:1535–1544. doi: 10.1002/art.1780390914. [DOI] [PubMed] [Google Scholar]
- 171.Chevalier X, Girardeau B, Conrozier T, et al. Safety study of intraarticular injection of interleukin 1 receptor antagonist in patients with painful knee osteoarthritis: a multicenter study. J Rheumatol. 2005;32:1317–1323. [PubMed] [Google Scholar]
- 172.Chevalier X, Goupille P, Beaulieu AD, et al. Intraarticular injection of anakinra in osteoarthritis of the knee: a multicenter, randomized, double-blind, placebo-controlled study. Artthritis Rheum. 2009;61:344–352. doi: 10.1002/art.24096. [DOI] [PubMed] [Google Scholar]
- 173.Bacconnier L, Jorgensen C, Fabre S. Erosive osteoarthritis of the hand: clinical experience with anakinra. Ann Rheum Dis. 2009;68:1078–1079. doi: 10.1136/ard.2008.094284. [DOI] [PubMed] [Google Scholar]
- 174.Grunke M, Schulze-Koops H. Successful treatment of inflammatory knee osteoarthritis with tumor necrosis factor blockade. Ann Rheum Dis. 2006;65:555–556. doi: 10.1136/ard.2006.053272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Magnano MD, Chakravarty EF, Broudy C, et al. A pilot study of tumor necrosis factor inhibition in erosive/inflammatory osteoarthritis of the hands. J Rheumatol. 2007;34:1323–1327. [PubMed] [Google Scholar]
- 176.Fioravanti A, Fabbroni M, Cerase A, Galeazzi M. Treatment of erosive osteoarthritis of the hands by intra-articular infliximab injections: a pilot study. Rheumatol Int. 2009;29:961–965. doi: 10.1007/s00296-009-0872-0. [DOI] [PubMed] [Google Scholar]
- 177.Hashimoto H, Tomita T, Kunugiza Y, et al. NF-kB decoy oligodeoxynucleotides suppressed the progression of osteoarthritis in rat arthritis. Arthritis Rheum. 2003;48:S630. [Google Scholar]