Abstract
Training with haptic guidance has been proposed as a technique for learning complex movements in rehabilitation and sports, but it is unclear how to best deliver guidance-based training. Here, we hypothesized that breaking down a complex movement, similar to a tennis backhand, into simpler parts and then using haptic feedback from a robotic exoskeleton would help the motor system learn the movement. We also examined how the particular form of the decomposition affected learning. Three groups of unimpaired participants trained with the target arm movement broken down in three ways: 1) elbow flexion/extension and the unified shoulder motion independently (“anatomical” decomposition), 2) three component shoulder motions in Euler coordinates and elbow flexion/extension (“Euler” decomposition), or 3) the motion of the tip of the elbow and motion of the hand with respect to the elbow, independently (“visual” decomposition). A control group practiced the same number of movements, but experienced the target motion only, achieving eight times more direct practice with this motion. Despite less experience with the target motion, part training was better, but only when the arm trajectory was decomposed into anatomical components. Varying robotic movement training to include practice of simpler, anatomically-isolated motions may enhance its efficacy.
Index Terms: Haptic arm exoskeleton, motor learning, parallel mechanism, robot assisted movements, whole-part practice
I. Introduction
Learning complex limb movements is of interest for athletes in many sports and for patients undergoing rehabilitation training following neurologic injuries such as stroke and spinal cord injury. With the increasing emergence of robotic arm exoskeletons for human movement training [1]–[10], it is now possible to deliver haptic demonstrations of complex arm motions (Fig. 1) to try to aid in learning them [11]. However, it is currently not well understood how haptic input from an exoskeleton can be designed to optimally train a complex limb movement. Haptic guidance has sometimes been shown to have no effect or even reduce motor learning [5], [12]–[20] while in other tasks it may be beneficial [21]–[24].
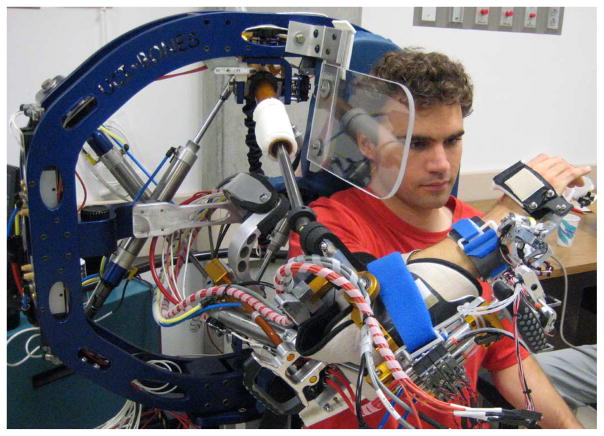
Fig. 1.
BONES (Biomimetic Orthosis for Neurorehabilitation of the Elbow and Shoulder) allows three DOF at the shoulder as well as elbow flexion/extension. A distal module accommodates forearm supination/pronation and wrist flexion/extension, but was not used in this study.
To date, haptic guidance has almost exclusively been used to demonstrate entire movements, but a strategy sometimes used by sports or rehabilitation trainers is to break a complex motion into component parts using haptic guidance, to allow the trainee to master an isolated motion that is simpler than the whole motion. This strategy relies on what the motor learning literature refers to as “part-whole transfer” [25]–[32]. Most previous studies on part-whole transfer have examined sequence learning, which refers to motor tasks that require the performance of a sequence of independent movements. Practicing parts of a sequence can improve performance on the whole task, but practicing the whole sequence is typically more effective [33], [34]. However, when coordination between parts is not a key element, part training can sometimes have more beneficial results towards the whole task if compared to whole training [35]. The few studies that have examined part-whole transfer in tasks that require simultaneous coordination of multiple joints, (also referred to as “continuous tasks” [30], which are common types of tasks in sports and rehabilitation) are ambiguous. One study suggested that the extent to which coordination is involved in a complex task (the task being tested was piloting a helicopter during liftoff, where four separate controls must be operated simultaneously) is directly related to the importance of training the task as a whole [36]. However, practice of individual elements of a complex task can sometimes transfer to the overall task, even though practice of the complex task is usually more effective [33].
In the experiment reported here, we studied part-whole transfer for complex arm movement training performed using haptic guidance. We hypothesized that breaking a movement down into movements with fewer degrees-of-freedom (DOF) would be more effective than simply practicing the whole movement. Further, we hypothesized that the specific form of the decomposition of the whole motion into individual joint motions would influence the amount of learning.
II. Experimental Methods
The experiment was approved by the Institutional Review Board of University of California-Irvine, and participants provided informed consent. For this experiment we used a four DOF arm exoskeleton developed in our laboratory called “BONES” (Fig. 1) [37] to guide the joints of the participant’s arm along desired movement trajectories. BONES is pneumatically actuated, and provides compliant assistance to multi-joint arm movements using a nonlinear control algorithm [38]. A total of 40 healthy adult volunteers (age: 28.6 ± 5.5, 57.5% male) attempted to learn a desired multi-joint arm movement with haptic guidance from BONES.
A. Experimental Protocol
Subjects tried to learn to perform a complex arm movement which we will call the “main motion”, and which we will denote as θ(t) or simply θ. The main motion involved simultaneous movement of the shoulder in abduction–adduction θ1, flexion–extension θ2, internal–external rotation θ3, and elbow in flexion–extension θ4 along sinusoidal trajectories.
To evaluate the ability of the subject to make the target arm movements, we characterized a four DOF movement using four coordinates. We chose to represent the three shoulder coordinates in Euler coordinates, which express rotations about orthogonal axes in a certain order. There are other shoulder coordinate systems that we could have used, such as Cardan angles, which express rotations in a different order [39]. We represented the elbow as a relative angle between the upper arm and forearm. The anatomical DOF corresponding to θ1, θ2, θ3, and θ4 are shown in Fig. 2. The main motion was defined as
Fig. 2.
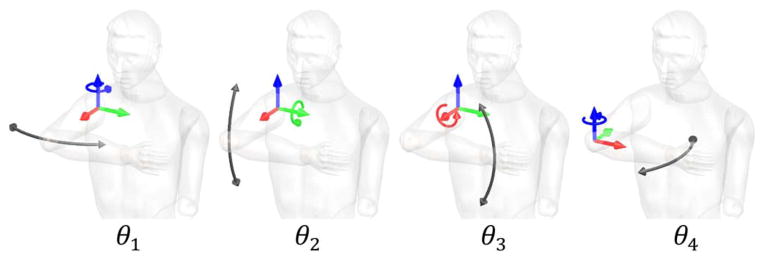
Definition of joint angles used in this paper.
| (1) |
| (2) |
Table I shows the values of A, T, φ, and B used for the main motion, which somewhat resembled a tennis backhand.
TABLE I.
Parameters of the Main Motion θ(t) and the Transfer Motion θ′(t)
| Component | A [deg] | T [sec] | φ [rad] | B [deg] |
|---|---|---|---|---|
| θ1 | 20.0 | 4.0 | 0 | −10 |
| θ2 | 12.5 | 4.0 | π/2 | −5 |
| θ3 | 15.0 | 4.0 | −π/2 | 35 |
| θ4 | 20.0 | 4.0 | π/2 | 55 |
| θ′1 | 15.0 | 3.3 | 0 | −10 |
| θ′2 | 7.5 | 3.3 | π/2 | −5 |
| θ′3 | 10.0 | 3.3 | −π/2 | 35 |
| θ′4 | 15.0 | 3.3 | π/2 | 55 |
We also assessed how learning the main motion θ affected the performance on a transfer movement θ′, which was also described by (1) and (2), but had a slightly shorter period T of its components, and different amplitude A, phase φ and offset B (Table I). The transfer motion θ′ was similar to a front crawl swimming stroke. We included this transfer movement in order to determine if the participants became more skilled at a movement substantially different from the trained movement; i.e., to assess specificity of training.
Each participant was assigned randomly to one of four training groups (for a total of 10 subjects in each group): 1-Whole (age: 28.1 ± 2.7, 80% male), 2-Euler (age: 31.9 ± 9.3, 40% male), 3-Anatomical (age: 28.0 ± 2.9, 50% male), and 4-Visual training techniques (age: 26.4 ± 3.4, 60% male). All groups received the same number of training movements (Table II). The “Whole” group served as a control group and always practiced the main motion in its entirety. For the other three training groups, subjects repeatedly practiced parts of the main motion, and then practiced a single example of the main motion in its entirety. The “Euler” training group experienced the parts of the main motion in Euler coordinates (Fig. 2). The “Anatomical” training group experienced the complete shoulder movement (i.e., θa1 = [θ1(t) θ2(t) θ3(t) 0]) as one component, and the elbow flexion/extension movement θa2 = [0 0 0 θ4(t)] as the other. The “Visual” training group experienced the shoulder motion, absent shoulder internal/external rotation (i.e., θv1 = [θ1(t) θ2(t) 0 0]) as one component, and then experienced shoulder internal/external rotation with elbow flexion/extension θv2 = [0 0 θ3(t) θ4(t)] as the other component. The reason we named this decomposition “Visual” is that it traces out the desired path of the tip of the elbow on the inside surface of a virtual sphere (with center at the shoulder), and the desired path of the hand on the inside surface of a second sphere (with center at the elbow). It is easy to visualize how to form the complete movement by summing these two paths.
TABLE II.
Experimental Protocol
| GROUP | BASELINE1 (x2) | TRAINING (x10) | ASSESS./RET.2 (x2) |
|---|---|---|---|
| 1-Whole | θ × 2/θ̂ | θ × 8/θ/θ̂ | θ̂/θ × 2/θ̂ |
| 2-Euler | θ × 2/θ̂ | rand{θ1/θ2/θ3/θ4} × 2/θ/θ̂ | θ̂/θ × 2/θ̂ |
| 3-Anatomical | θ × 2/θ̂ | rand{θa1/θa2} × 4/θ/θ̂ | θ̂/θ × 2/θ̂ |
| 4-Visual | θ × 2/θ̂ | rand{θv1/θv2} × 4/θ/θ̂ | θ̂/θ × 2/θ̂ |
θ refers to the target multi-joint movement that the robot guided the arm along, and θ̂ refers to unassisted assessment movement in which the participant tried to reproduce θ. rand{} indicates a block of the included motions presented in a randomized order.
The Baseline Transfer phase was performed after the Baseline phase shown in the table, and consisted of the same movement sequence pattern as Baseline replacing θ and θ̂ with θ′ and θ̂′ i.e. the target transfer movement.
The Assessment phase occurred 5–10 minutes after the training phase, and the Retention phase approximately 1 week later. The Assessment Transfer and Retention Transfer phases followed the same sequence as Assessment and Retention phases, using θ′ as the target movement, and occurred immediately after the Assessment and Retention phases, respectively.
The experiment consisted of 7 phases: 1-Baseline, 2-Baseline Transfer, 3-Training, 4-Assessment, 5-Assessment Transfer, 6-Retention, and 7-Retention Transfer. During the Baseline phase, the robot assisted the subjects in performing the main motion θ twice, and then the subjects were asked to try to reproduce the whole movement without assistance (Table II; see also Fig. 5). This process was repeated twice for a total of six movements, producing assessments 1 and 2. The Baseline Transfer phase repeated the protocol of the Baseline phase with the transfer motion θ′, producing assessments 3 and 4. After completing the Baseline Transfer phase, subjects rested for 5–10 min. During the resting periods subjects were not strapped into the robot and were allowed to leave the room.
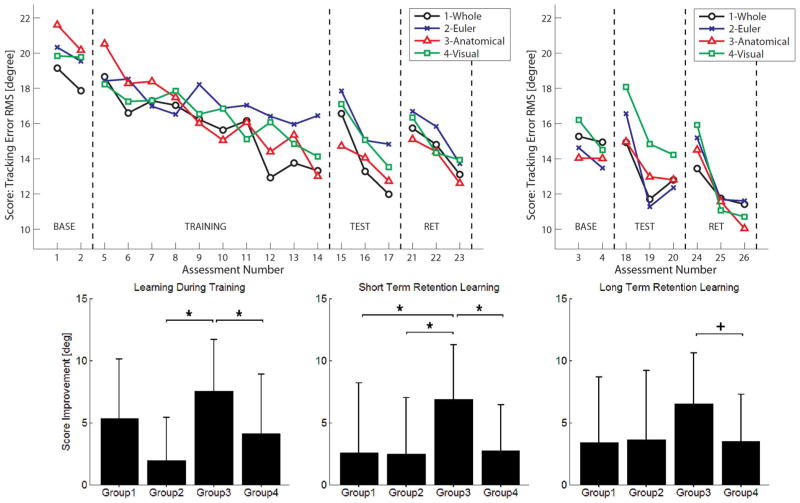
Fig. 5.
Top: Score during different training phases. The top left plot shows the assessments for the main motion. The top right shows the assessments for the transfer motion. Bottom: Amount of learning during training (assessment 5 versus 14, left), at short-term retention 5 min after training (assessment 1 versus 15, middle) and at long-term retention one week after training (assessment 1 versus 21, right). Group 1-Whole, 2-Euler, 3-Anatomical, and 4-Visual training techniques. Asterisks denote significance at p = 0.05, + denote marginal significance p < 0.1.
Then, in the Training phase, each group was taught the main motion using the group’s assigned training strategy. There were 10 training sets in this phase. For each set, each group received eight demonstrations of their respective joint components (with the component presentation order block randomized), or of the whole movement for the whole training group (see Table II). Then, for the ninth movement in the set for all groups, the robot showed the main motion θ. For all of these training movements (i.e., movements 1–9 in each training set) subjects were asked to follow along with the haptically displayed movement—i.e., to actively participate in learning. For the tenth movement of each set, the subject was asked to perform the main motion without assistance (a movement which we denote with θ̂). The transfer motion was not trained during this phase. Note that because of this protocol, the Whole group experienced the main motion eight times more than the part groups, although all groups performed a total number of training movements that were equal. During the training phase there were 10 assessments in which the subject performed the movement without assistance, which we number assessments 5–14.
After completing the 100 movements, and another 5–10 min rest, subjects started the Assessment phase and the Assessment Transfer phase, which duplicated the Baseline phase and the Baseline Transfer phase, accordingly, except they started with an assessment before experiencing the desired movement (producing assessments 15–20). Subjects returned approximately one week later to perform the Retention and Retention Transfer phases, which duplicated the Assessment and Assessment Transfer phases (producing assessments 21–26).
The specific training procedure for each movement attempt was as follows. At the beginning of each movement, a computer displayed a graphical countdown and provided an auditory alert to aid participants in synchronizing their movements with the desired trajectory, and subjects were allowed to watch the movement of their arm. During the assisted movements a semi-transparent rendered arm offered visual guidance on the monitor, following the desired arm trajectory. Simultaneously, a solid rendered arm displaying the actual position of the subject’s arm was overlapped over the semi-transparent “guide” arm. Thus, subjects could gauge how well they were performing the desired movement by watching the overlap of the semi-transparent and solid arms. Furthermore, during these assisted movements, the robot actively positioned the arm joints according to each group’s experimental condition while the unassisted arm joints were fixed in their corresponding initial position. For example, when the Anatomical group trained θa1, the robot actively assisted θ1, θ2, and θ3, while the unassisted joint (θ4) was locked at θ4(t) = θ4(0). Although the stiffness of the robot was set to be relatively high, we observed small average variations of θi(0) ± 3° on the locked joints.
During the assessment movements, the robot was set to fully operate in backdrive mode with gravity compensation, and provided no assistance for moving. Furthermore, during these unassisted assessments, the semi-transparent arm showing the desired trajectory was not displayed and only the solid arm showing the subject’s actual arm configuration was shown. At the end of each unassisted assessment, the subject’s performance evaluation was determined by computing the norm of the mean absolute tracking error (in degrees) for each joint and displayed on the computer monitor. The score was calculated as
| (3) |
where θ is the desired joint angle as a function of time from (1) and θ̂ is the measured joint angle. This score was only measured when each group performed the whole movement without assistance (assessment movements), therefore all groups were assessed under the same conditions. This performance evaluation not only reflected the accuracy of each DOF during the “Whole” motion, but also the synchronicity between them. The lower the score, the closer the performed movement was to the desired motion (both in space and time).
B. Data Analysis
We characterized the subjects’ performance with a score that represented the trajectory error for each movement without penalizing time delays between the desired and the actual starting time. To minimize the effect of any initial time delay, we defined the post hoc score (ScorePH, in degrees) as follows:
| (4) |
where θ and θ̂ are the same as in (3), and d is a time delay applied to all four movement components simultaneously. To determine the value of d that minimized the score, we used the Matlab function fminsearch [40], an unconstrained function minimum value finder. For each movement, the initial time shift was set to 0 ms (with respect to the beginning of the target motion). The iteration step was set to 4 ms, corresponding to the period of the sampling rate of the data acquisition system (250 Hz). We repeated the search of the global minima with two different initial time delays (3 s before and 3 s after the actual movement start, confirming that the algorithm converged to the same values (and not local minima) with different initial conditions. For instance, an optimal value d = 0.4 s would indicate that if the subject had started moving 0.4 s later, the movement θ̂ would have been closer to the desired trajectory θ (Fig. 3).
Fig. 3.
Time shifting example. A 0.38 s delay is applied simultaneously to the four components of the original measured movement, θ̂ (dashed line). The resulting delayed movement (solid line) is, overall, closer to the desired trajectory, θ (dotted line).
The primary outcome measure was the post-hoc score. The Shapiro–Wilk (S-W) normality test was performed on the scores at the key assessments as well as on the differences in scores for each group during training, short term retention and long term retention. The Mann–Whitney U-Test (M-W) was used to compare differences between groups and the Wilcoxon Signed Rank Test (WSR) was performed to analyze score improvements within each group. The statistical analysis was processed using PASW version 18.0 (formerly SPSS). The statistical significance level in all tests was set at p ≤ 0.05.
To gain further insight on the performance of each individual movement component θ̂i, we determined a least squares optimal spatiotemporal fit for the measured trajectory of each joint θ̂i as a function of amplitude Â, period T̂, phase delay φ̂ and offset B̂ using
| (5) |
The optimal parameters (Â, T̂, φ̂, B̂) were determined using Matlab’s fminsearch, as described earlier. The initial values for Â, T̂, φ̂, and B̂ were set to the ones described in Table I. An example of a sinusoidal fit to θ̂1 is shown in Fig. 4.
Fig. 4.
A sinusoidal function (solid line) closely fits the subject’s measured trajectory (dashed line). The subject started about 0.3 s late and did not reach the desired amplitude levels compared to that of the desired trajectory for this joint (dotted line).
III. Results
The baseline and first training assessment scores of Group 4 failed the Shapiro–Wilk normality test (p = 0.01). Moreover, the difference in scores during training for Group 4 also failed the normality test (p = 0.02). Therefore, nonparametric statistics were used.
There were no significant differences in ScorePH between groups at the beginning of training. All training groups significantly improved their ScorePH during training (Fig. 5). However, Group 3 (the anatomical decomposition group) exhibited the greatest improvements (7.5° ± 4.2°, WSR p = 0.049) during training (Fig. 5).
When we analyzed the task performance 5–10 minutes after training (assessment 15) and compared it to the first Baseline assessment (assessment 1) we observed a significant effect of training technique, with the anatomical decomposition group again improving the most compared to groups 1, 2, and 4 (M-W, p = 0.043, p = 0.063, and p = 0.042, respectively). Group 3 also performed the best score improvement at the retention test one week later (6.5° ± 4.1°, WSR, p = 0.007). This improvement was marginally significant compared to Group 4 (M-W, p = 0.080) but not significant compared to the rest.
In order to gain insight into why Group 3 tended to perform better, we looked at the results from the optimized movement component analysis. During the training phase, short term retention test and long term retention test, Group 3 presented the best amplitude (Âi) learning in all four components , and out of all groups. At the same time, Group 3 showed the best angle offset (B̂i) learning in , and . No significant differences were observed regarding the learning of the period (T̂i) and phase shift (φ̂i) of the four motion components.
A possible reason why Group 3 may have performed better is that during the decomposed movements they may have held the joints in positions that may have given clues about how to hold the arm during the main motion. We, therefore, calculated the distance in joint angle space between the time-averaged arm position of the main motion and the time-averaged arm position for each of the decomposed movements experienced during the training techniques (i.e., one movement for the group that performed the main motion only, four movements for Euler decomposition, two for anatomical decomposition, and two for visual decomposition). The scores for each group’s movement components were: S1,1 = 0°, S2,1 = 28.0°, S2,2 = 28.0°, S2,3 = 26.8°, S2,4 = 23.3°, S3,a1 = 20.0°, S3,a2 = 23.3°, S4,v1 = 25.0°, and S4,v2 = 17.8°, where Si,j is the score, i is the group number and j is the movement component code (as noted in Table II). For example, S3,a1 is the score that Group 3 would obtain if they were to repeat θa1 during the assessment phase. Thus, Groups 3 (the anatomical decomposition group) and 4 (the visual decomposition group) did on average hold the arm closer to the main motion during training. Similar results apply to the transfer motion.
The ability to perform the untrained movement θ̂′ did not improve significantly following training or during the short-term retention test, or at the beginning or the long-term retention test (assessment 24), indicating the specificity of training with haptic guidance. Nevertheless, all groups improved their ScorePH for the untrained movement significantly by the end of the long-term retention test (assessment 26). Group 3, again, presented the smallest tracking error in the last long-term retention test, although differences between groups were not significant.
IV. Discussion
We found that breaking a complex motion into parts benefited trainees, but only when the parts were presented in the anatomical decomposition—i.e., as individual shoulder and elbow movements. Note that this benefit was gained in comparison to the “Whole” group (Group 1), which, although it practiced the same number of total movements, practiced the main motion eight times more than the part training group. We first discuss implications of these findings for mechanisms of human motor learning and then for design of robotic movement training.
A. Part-Whole Learning Mechanisms
Why did breaking the movement into parts improve motor learning? Note first that the observed effect was not a generalized learning effect, as subjects did not improve performance on the transfer motion; training affected the main motion performance. When we analyzed which movement parameters of the main motion the participants improved, we found significantly greater improvements in the amplitude and angle offset of shoulder internal/external rotation and elbow flexion/extension respectively ( , , , and ). We found no significant improvements related to the temporal parameters of the movement components ( and ). Thus, we speculate that individual joint guidance alerted the motor system to the desired range and average position in space for the exercised joint, presumably using both haptic and visual sensory channels. Given such amplitude/range templates for individual joints, the motor system was apparently then able to shape the multi-joint movement. We note, however, that the temporal cues for these movements were relatively simple and very similar between joints. Looking at movements with more complex temporal components may be of interest for future studies.
A key point is that the experience of component movement features helped in learning the target motion more than practicing the target motion itself, which is a somewhat counterintuitive result. Somehow, the information added from the part practice was more valuable than the repetitive experience of the target motion. One possibility is that the motor system has trouble determining where the problems lie in making, accurate, complex movements; breaking the movements down may allow better identification and then more focused practice on key problems.
It is significant, however, that the benefit of adding part training was gained only when the whole arm motion was decomposed into the anatomical decomposition—i.e., the shoulder and elbow moving through their complete motions, one at a time. The Euler decomposition was complex and “unnatural,” but the Visual decomposition was intuitive in visual space, requiring addition of two viewed trajectories. These results suggest that the spatiotemporal summation mechanism that facilitates part-whole transfer operates in joint and not visual coordinates. This finding is consistent with other research that has found that the motor system represents arm movement in joint-based or shoulder-based coordinate frames [41]–[44].
It should be noted that the average position of the arm during the decomposed movements was closer to the average position of the arm during the main motion for the anatomical and visual decompositions. The average position of the arm during training may have been a cue that helped subjects perform the main motion better. However, the average position was about equally close to the main motion for the anatomical and visual decompositions, so this explanation does not distinguish the differential benefit seen for training with the anatomical decomposition.
A possible caveat is that we measured the performance using Euler coordinates. Thus, one might speculate that training with the Euler decomposition would have allowed subjects to learn to perform better by this outcome measure. However, the results still favored the anatomical decomposition, and thus the conclusion that anatomical decomposition was the best training technique remains unchanged. It is interesting to note that the human motor learning system is very different from a typical robotic movement learning system. A robot that experienced individual joint movements once could typically learn a whole movement immediately afterwards simply by memorizing and then simultaneously replaying the individual movements. The human motor system took many movements to integrate the information contained in the part movements; thus its performance is much less than what is theoretically possible.
B. Using Robots to Enhance Motor Learning
There is increasing interest in using physically-interacting robotic devices to create dynamic training environments, such that when people practice moving in these environments, they learn motor skills more quickly or better [45], [46]. An obvious approach, but not the only approach [47], is to use a robot to guide the person’s limbs through a desired movement trajectory to help learn the target movement. Results with this haptic guidance strategy have been mixed. For unimpaired people, haptic guidance can help in learning the timing of a complex trajectory [21] or steering or hitting task [22], [47], [48], but has provided little measurable benefit or a learning decrement, compared to visual demonstration [19], [20]. In a clinical setting, robotic guidance of the arms during rehabilitation of stroke patients has been found to provide no additional benefit compared to a matched amount of unguided, active exercise [5], [49], although there is at least one exception with a hand robot [23], and another study found almost significantly better improvement on at least one key functional outcome (Frenchay Arm Test) with robot guidance [24]. Interestingly, both of these latter studies guided the hand or the arm along simplified joint movements. Robotic guidance during gait training of ambulatory stroke patients reduced clinical benefit compared to walking with assistance from a therapist [50].
The present study demonstrated that varying the type of guidance was more effective than always guiding along the final, integrated, goal movement. As stated above, this is a somewhat counterintuitive result: the “Whole” training group experienced the main motion eight times more than the part groups, but this experience did not avail them as much as experiencing specific components of the main motion. Thus, we conclude that practicing the same thing again and again, even if it is the thing one wishes to learn, is not always the best practice. Instead, practicing simpler parts of the thing one wishes to learn may be better, if the simpler parts are designed appropriately. This observation is consistent with the long-standing practice in sports and rehabilitation training of isolating individual joint movements to facilitate learning of complex multi-joint movements, but to our knowledge this study provides quantitative evidence that supports this practice for the first time. Note however, that, since mechanisms of learning with and without haptic guidance may not be identical, the benefit of decomposition noted here may only exist with haptic guidance. Future studies should examine if a similar benefit of decomposition exists when the decomposition is provided using visual feedback only.
The implications for robotic movement training are that training on a variety of target motions may in fact improve the outcomes of robot-assisted training, if the target motions are selected appropriately, which, according to our results, means in “anatomical bundles.” Practically, this means that simpler robotic devices may be able to play an important role in training complex movements (for example [51], [52]). One could imagine a wearable device, for example, that focuses on the appropriate use of an individual joint in relation to a complex task. Finally, we expect the results described here to inform rehabilitation therapy in as much as normal motor learning mechanisms continue to operate in rehabilitation, a possibility which seems highly likely.
Acknowledgments
This report summarizes work supported by Federal Contract N01-HD-3-3352 by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and co-funded by the National Institute of Biomedical Imaging and Bioengineering (NIBIB) and under NCRR M01RR00827. The findings and conclusions in the report are those of the authors and do necessarily represent the views of NICHD, NIBIB, or NCRR.
Biographies
 Julius Klein (M’08) received the B.S. degree in industrial engineering from Universitat de Girona, Catalunya, Spain, in 2003, and the M.S. degree in mechanical and aerospace engineering and the Ph.D. degree in mechanical engineering from the Department of Mechanical and Aerospace Engineering, both from the University of California, Irvine, in 2005 and 2009, respectively.
Julius Klein (M’08) received the B.S. degree in industrial engineering from Universitat de Girona, Catalunya, Spain, in 2003, and the M.S. degree in mechanical and aerospace engineering and the Ph.D. degree in mechanical engineering from the Department of Mechanical and Aerospace Engineering, both from the University of California, Irvine, in 2005 and 2009, respectively.
His research interests include robotics, design optimization, fabrication processes, motor learning, and neurorehabilitation. Since March 2010, he has been a Post Doctoral Researcher at Human Robotics Group at Imperial College London.
 Steven J. Spencer (M’08) received the B.S. degree in mechanical engineering from the University of Maryland, in 2005, and the M.S. and Ph.D. degrees in mechanical and aerospace engineering at the University of California, Irvine, in 2008 and 2011, respectively.
Steven J. Spencer (M’08) received the B.S. degree in mechanical engineering from the University of Maryland, in 2005, and the M.S. and Ph.D. degrees in mechanical and aerospace engineering at the University of California, Irvine, in 2008 and 2011, respectively.
He is currently researching control algorithms for rehabilitative body weight support devices at Aretech LLC. His research interests include robotics, nonlinear control, kinematics, dynamics, motor learning, and machine learning.
 David Reinkensmeyer (M’93) received the B.S. degree in electrical engineering from the Massachusetts Institute of Technology, Cambridge, and the M.S. and Ph.D. degrees in electrical engineering from the University of California, Berkeley, in 1988, 1991, and 1993, respectively, focusing on robotics and the neuroscience of human movement. He carried out postdoctoral studies at the Rehabilitation Institute of Chicago and Northwestern University Medical School from 1994 to 1997.
David Reinkensmeyer (M’93) received the B.S. degree in electrical engineering from the Massachusetts Institute of Technology, Cambridge, and the M.S. and Ph.D. degrees in electrical engineering from the University of California, Berkeley, in 1988, 1991, and 1993, respectively, focusing on robotics and the neuroscience of human movement. He carried out postdoctoral studies at the Rehabilitation Institute of Chicago and Northwestern University Medical School from 1994 to 1997.
He became an Assistant Professor at the University of California, Irvine, in 1997, establishing a research program that develops robotic and sensor-based systems for movement training and assessment following neurologic injuries such as stroke and spinal cord injury. He recently served as the Chair of the National Science Foundation International Study on Technology for Mobility, and is currently the lead for iMove, a collaborative effort at UCI focused on using technology to help restore human mobility.
References
- 1.Coote S, Stokes EK, Murphy BT, Harwin WS. The effect of GENTLE/s robot mediated therapy on upper extremity function post stroke. Proc Int Conf Rehabil Robot. 2003:59–61. [Google Scholar]
- 2.Frisoli A, Borelli L, Montagner A, Marcheschi S, Procopio C, Salsedo F, Bergamasco M, Carboncini MC, Tolaini M, Rossi B. Arm rehabilitation with a robotic exoskeleleton in virtual reality. Proc. IEEE 10th Int. Conf. Rehabil. Robot. (ICORR); Noordwijk, The Netherlands. 2007. pp. 631–642. [Google Scholar]
- 3.Hesse S, Werner C, Pohl M, Rueckriem S, Mehrholz J, Lingnau ML. Computerized arm training improves the motor control of the severely affected arm after stroke: A single-slinded randomized trial in two centers. Stroke. 2005 Sep 1;36:1960–1966. doi: 10.1161/01.STR.0000177865.37334.ce. [DOI] [PubMed] [Google Scholar]
- 4.Hogan N, Krebs HI, Sharon A, Charnnarong J. Interactive Robot Therapist. 1995:5466213. [Google Scholar]
- 5.Kahn L, Zygman ML, Rymer WZ, Reinkensmeyer DJ. Robot-assisted reaching exercise promotes arm movement recovery in chronic hemiparetic stroke: A randomized controlled pilot study. J Neuroeng Rehabil. 2006;3:12. doi: 10.1186/1743-0003-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klein J, Spencer SJ, Allington J, Minakata K, Wolbrecht ET, Smith R, Bobrow JE, Reinkensmeyer DJ. Biomimetic orthosis for the neurorehabilitation of the elbow and shoulder (BONES). presented at the 2nd IEEE RAS & EMBS Int. Conf. Biomed. Robot. Biomechatron; Scottsdale, AZ. 2008. [Google Scholar]
- 7.Nef T, Mihelj M, Kiefer G, Perndl C, Muller R, Riener R. ARMin—Exoskeleton for arm therapy in stroke patients. Proc. IEEE 10th Int. Conf. Rehabil. Robot. (ICORR); 2007. pp. 68–74. [Google Scholar]
- 8.Riener R, Nef T, Colombo G. Robot-aided neurorehabilitation of the upper extremities. Med Biol Eng Comput. 2005 Jan;43:2–10. doi: 10.1007/BF02345116. [DOI] [PubMed] [Google Scholar]
- 9.Sanchez RJ, Wolbrecht ET, Smith R, Liu J, Rao S, Cramer SC, Rahman T, Bobrow JE, Reinkensmeyer DJ. A pneumatic robot for re-training arm movement after stroke: Rationale and mechanical design. 9th Int. Conf. Rehabil. Robot; 2005. pp. 500–504. [Google Scholar]
- 10.Sugar TG, Jiping H, Koeneman EJ, Koeneman JB, Herman R, Huang H, Schultz RS, Herring DE, Wanberg J, Balasubramanian S, Swenson P, Ward JA. Design and control of RUPERT: A device for robotic upper extremity repetitive therapy. IEEE Trans Neural Syst Rehabil Eng. 2007 Sep;15(3):336–346. doi: 10.1109/TNSRE.2007.903903. [DOI] [PubMed] [Google Scholar]
- 11.Liu J, Emken JL, Cramer SC, Reinkensmeyer DJ. Learning to perform a novel movement pattern using haptic guidance: Slow learning, rapid forgetting, and attractor paths. Proc. 9th Int. Conf. Rehabil. Robot; 2005. pp. 37–40. [Google Scholar]
- 12.Hagman JD. Presentation and test-trial effects on acquisition and retention of distance and location. J Exp Psychol Learn Mem Cogn. 1983;9:334–345. doi: 10.1037//0278-7393.9.2.334. [DOI] [PubMed] [Google Scholar]
- 13.Winstein CJ, Pohl PS, Lewthwaite R. Effects of physical guidance and knowledge of results on motor learning: support for the guidance hypothesis. Res Q Exerc Sport. 1994;65:316–323. doi: 10.1080/02701367.1994.10607635. [DOI] [PubMed] [Google Scholar]
- 14.Armstrong TR. Training for the production of memorized movement patterns. Dept. Psychol., Univ. Michigan; Ann Arbor: 1970. [Google Scholar]
- 15.Tsutsui S, Imanaka K. Effect of manual guidance on acquiring a new bimanual coordination pattern. Res Q Exerc Sport. 2003;74:104–109. doi: 10.1080/02701367.2003.10609069. [DOI] [PubMed] [Google Scholar]
- 16.Gillespie RB, O’Modhrain MS, Tang P, Zaretsky D, Pham C. The virtual teacher. presented at the ASME Int. Mechan. Eng. Conf. Expo; Anaheim, CA. 1998. [Google Scholar]
- 17.O’Malley MK, Gupta A, Gen M, Li Y. Shared control in haptic systems for performance enhancement and training. J Dyn Syst Meas Control. 2006;128:75–85. [Google Scholar]
- 18.Wallis R, Elliott B, Koh M. The effect of a fast bowling harness in cricket: An intervention study. J Sports Sci. 2002;20:495–506. doi: 10.1080/02640410252925161. [DOI] [PubMed] [Google Scholar]
- 19.Liu J, Cramer SC, Reinkensmeyer DJ. Learning to perform a new movement with robotic assistance: Comparison of haptic guidance and visual demonstration. J Neuroeng Rehabil. 2006;3:20. doi: 10.1186/1743-0003-3-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Asseldonk EHF, Wessels M, Stienen AHA, Van der Helm FCT, Van der Kooij H. Influence of haptic guidance in learning a novel visuomotor task. J Physiol (Paris) 2009;103:276–285. doi: 10.1016/j.jphysparis.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 21.Feygin D, Keehner M, Tendick R. Haptic guidance: Experimental evaluation of a haptic training method for a perceptual motor skill. Proc. 10th Symp. Haptic Interfaces Virtual Environ. Teleoperator Syst. HAPTICS; 2002. pp. 40–47. [Google Scholar]
- 22.Marchal-Crespo L, Reinkensmeyer DJ. Haptic guidance can enhance motor learning of a steering task. J Motor Behav. 2008;40:545–557. doi: 10.3200/JMBR.40.6.545-557. [DOI] [PubMed] [Google Scholar]
- 23.Takahashi CD, Der-Yeghiaian L, Le V, Motiwala RR, Cramer SC. Robot-based hand motor therapy after stroke. Brain. 2008 Feb;131:425–437. doi: 10.1093/brain/awm311. [DOI] [PubMed] [Google Scholar]
- 24.Masiero S, Armani M, Rosati G. Upper-limb robot-assisted therapy in rehabilitation of acute stroke patients: focused review and results of new randomized controlled trial. J Rehabil Res Develop. 2011;48:355–366. doi: 10.1682/jrrd.2010.04.0063. [DOI] [PubMed] [Google Scholar]
- 25.Brydges R, Carnahan H, Backstein D, Dubrowski A. Application of motor learning principles to complex surgical tasks: Searching for the optimal practice schedule. J Mot Behav. 2007 Jan;39:40–48. doi: 10.3200/JMBR.39.1.40-48. [DOI] [PubMed] [Google Scholar]
- 26.Dubrowski A, Backstein D, Abughaduma R, Leidl D, Carnahan H. The influence of practice schedules in the learning of a complex bone-plating surgical task. Am J Surg. 2005;190:359–363. doi: 10.1016/j.amjsurg.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 27.Hansen S, Tremblay L, Elliott D. Part and whole practice: Chunking and online control in the acquisition of a serial motor task. Res Q Exerc Sport. 2005;76:60–66. doi: 10.1080/02701367.2005.10599262. [DOI] [PubMed] [Google Scholar]
- 28.Hommel B, Müsseler J, Aschersleben G, Prinz W. The theory of event coding (TEC): A framework for perception and action planning. Behav Brain Sci. 2001;24:849–878. doi: 10.1017/s0140525x01000103. [DOI] [PubMed] [Google Scholar]
- 29.Klapp ST, Martin ZE, McMillan GG, Brock DT. Whole-task and part-task training in dual motor tasks. In: Mark LS, Warm JS, Huston RL, editors. Ergonomics and Human Factors. New York: Springer-Verlag; 1987. pp. 125–130. [Google Scholar]
- 30.Schmidt RA, Lee TD. Motor Control and Learning: A Behavioral Emphasis. Champaign, IL: Human Kinetics Publishers; 1998. [Google Scholar]
- 31.Swinnen S, Walter CB, Shapiro DC. The coordination of limb movements with different kinematic patterns. Brain Cogn. 1988;8:326–347. doi: 10.1016/0278-2626(88)90058-9. [DOI] [PubMed] [Google Scholar]
- 32.Wenderoth N, Puttemans V, Vangheluwe S, Swinnen SP. Bi-manual training reduces spatial interference. J Mot Behav. 2003;35:296–308. doi: 10.1080/00222890309602142. [DOI] [PubMed] [Google Scholar]
- 33.Briggs GE, Brogden WJ. The effect of component practice on performance of a lever-positioning skill. J Exp Psychol. 1954;48:375. doi: 10.1037/h0060580. [DOI] [PubMed] [Google Scholar]
- 34.Kurtz S, Lee TD. Part and whole perceptual-motor practice of a polyrhythm. Neurosci Lett. 2003;338:205–208. doi: 10.1016/s0304-3940(02)01394-0. [DOI] [PubMed] [Google Scholar]
- 35.Mané AM. Acquisition of a perceptual-motor skill: Asaptive and part-whole training. Proc Human Factors Ergonom Soc Annu Meet. 1984;28:522–526. [Google Scholar]
- 36.Zavala A, Locke EA, Van Cott HP, Fleishman EA. The Analysis of Helicopter Pilot Performance. American Institutes for Research; Washington, DC: 1965. Tech. Rep. AIR-E-29-6/65-TR. [Google Scholar]
- 37.Klein J, Spencer SJ, Allington J, Bobrow JE, Reinkensmeyer DJ. Optimization of a parallel shoulder mechanism to achieve a high force, low mass, robotic arm exoskeleton. IEEE Trans Robot. 2010:1–6. [Google Scholar]
- 38.Wolbrecht ET, Chan V, Le V, Cramer SC, Reinkensmeyer DJ, Bobrow JE. Real-time computer modeling of weakness following stroke optimizes robotic assistance for movement therapy. Proc. 3rd Int. IEEE/EMBS Conf. Neural Eng; 2007. pp. 152–158. [Google Scholar]
- 39.Tupling SJ, Pierrynowski MR. Use of cardan angles to locate rigid bodies in three-dimensional space. Med Biol Eng Comput. 1987 Sep;25:527–532. doi: 10.1007/BF02441745. [DOI] [PubMed] [Google Scholar]
- 40.Matlab Mathworks. R2009A. 2009. [Google Scholar]
- 41.Shadmehr R, Mussa-Ivaldi FA. Adaptive representation of dynamics during learning of a motor task. J Neurosci. 1994;14:3208–3224. doi: 10.1523/JNEUROSCI.14-05-03208.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Soechting JF, Flanders M. Sensorimotor representations for pointing to targets in three-dimensional space. J Neurophysiol. 1989;62:582–594. doi: 10.1152/jn.1989.62.2.582. [DOI] [PubMed] [Google Scholar]
- 43.Morrow MM, Miller LE. Prediction of muscle activity by populations of sequentially recorded primary motor cortex neurons. J Neurophysiol. 2003 Apr;89:2279–2288. doi: 10.1152/jn.00632.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scott SH, Kalaska JF. Reaching movements with similar hand paths but different arm orientations. I. Activity of individual cells in motor cortex. J Neurophysiol. 1997 Feb;77:826–852. doi: 10.1152/jn.1997.77.2.826. [DOI] [PubMed] [Google Scholar]
- 45.Reinkensmeyer DJ, Patton JL. Can robots help the learning of skilled actions? Exerc Sport Sci Rev. 2009;37 doi: 10.1097/JES.0b013e3181912108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang F, Patton J, Mussa-Ivaldi F. Interactive priming enhanced by negative damping aids learning of an object manipulation task. Proc Eng Med Biol Soc. 2007:4011–4014. doi: 10.1109/IEMBS.2007.4353213. [DOI] [PubMed] [Google Scholar]
- 47.Marchal-Crespo L, Reinkensmeyer DJ. Review of control strategies for robotic movement training after neurologic injury. J Neuroeng Rehabil. 2009;6:20. doi: 10.1186/1743-0003-6-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Milot MH, Marchal-Crespo L, Green C, Cramer SC, Reinkensmeyer DJ. Comparison of error-amplification and haptic-guidance training techniques for learning of a timing-based motor task by healthy individuals. Exp Brain Res. 2010;201:119–131. doi: 10.1007/s00221-009-2014-z. [DOI] [PubMed] [Google Scholar]
- 49.Volpe BT, Lynch D, Rykman-Berland A, Ferraro M, Galgano M, Hogan N, Krebs HI. Intensive sensorimotor arm training mediated by therapist or robot improves hemiparesis in patients with chronic stroke. Neurorehabil Neural Repair. 2008;22:305–310. doi: 10.1177/1545968307311102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hidler J, Nichols D, Pelliccio M, Brady K, Campbell DD, Kahn JH, Hornby TG. Multicenter randomized clinical trial evaluating the effectiveness of the lokomat in subacute stroke. Neurorehabil Neural Repair. 2009;23:5–13. doi: 10.1177/1545968308326632. [DOI] [PubMed] [Google Scholar]
- 51.Masia L, Krebs HI, Cappa P, Hogan N. Design and characterization of hand module for whole-arm rehabilitation following stroke. IEEE-ASME Trans Mechatron. 2007 Aug;12:399–407. doi: 10.1109/TMECH.2007.901928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lambercy O, Dovat L, Gassert R, Burdet E, Teo CL, Milner T. A haptic knob for rehabilitation of hand function. IEEE Trans Neural Syst Rehabil Eng. 2007 Sep;15(3):356–366. doi: 10.1109/TNSRE.2007.903913. [DOI] [PubMed] [Google Scholar]