Abstract
Interleukin-1 (IL-1) is a cytokine critical to inflammation, immunological activation, response to infection, and bone marrow hematopoiesis. Cyclophosphamide downmodulates immune suppressor cells and is cytotoxic to a variety of tumors. A phase I trial of IL-1 and cyclophosphamide was conducted by the Eastern Cooperative Oncology Group. This study evaluated 3 dose levels and 3 schedules in patients with solid tumors. The goal was to evaluate the hematopoietic supportive care effect and possible antitumor effect. Toxicity was fever, chills, hypotension, nausea/emesis, hepatic, and neutropenia. Toxicity increased with dose increases of interleukin-1. Treatment at all dose levels resulted in significant increases in total white blood cell (WBC) counts above baseline. Nadir WBC and nadir absolute neutrophil counts were not significantly different by dose level of IL-1 or schedule of IL-1. Toxicity due to IL-1 at higher doses prohibited further evaluation of this agent for hematopoietic support, particularly in view of the activity and tolerability of more lineage-specific hematopoietic cytokines. Therapeutic interventions in the role of IL-1 in inflammatory conditions and cancer may be further informed by our definition of its clinical and biological effects in this evaluation of dose and schedule.
Introduction
Interleukin-1 (IL-1) is a family of polypeptide cytokines produced by human cells that has pleiotropic biological effects. IL-1 is a critical component of inflammation, immunological activation, response to infection, and bone marrow hematopoiesis (Dinarello 1988, 1991, 1996, 2010, 2011; Dinarello and others 1986, 2012). Human IL-1 is produced by almost every nucleated cell type, although predominantly by monocytes and macrophages. The release of IL-1 is induced by antigens, toxins, injury, the inflammatory process, infections, and other cytokines (Dinarello 1988, 1991, 1996, 2010, 2011; Dinarello and others 1986, 2012).
IL-1 directly activates T-lymphocytes to proliferate, differentiate, increase their production of IL-2, and increase the number of IL-2 cell surface receptors, and also augments the cytotoxic function of T-cells (Farran and others 1980; Dempsey and others 1982). IL-1 is released during IL-2 therapy and believed to contribute to the fever, capillary leak, and possibly other toxic and immunologic effects of this treatment (Numerof and others 1988; Tilg and others 1994, 1995; Vannier and others 1999). IL-1 alone and in combination with other cytokines causes the proliferation of resting and activated B-cells as well as stimulation of antibody secretion by B-cells (Matsushima and others 1986). IL-1 is a potent inducer of other cytokines and regulatory proteins, including IL-1RA, IL-6, colony stimulating factors, tumor necrosis factor, and intracellular adhesion molecules (Onozaki and others 1985; Dinarello 2011; Dinarello and others 2012).
IL-1α and IL-1β are integral to the hematopoietic growth factor cascade, acting synergistically with and inducing expression of hematopoietic growth factors, including G-CSF, GM-CSF, IL-3, IL-6, and c-kit ligand (Bartelmez and others 1985; Griffin and others 1987). These interactions improve survival of progenitor cells and increase multipotential colony formation (Bartelmez and others 1985).
Of particular relevance to cancer therapy are the antitumor effects of IL-1. IL-1 has direct antiproliferative activity against certain human tumor cell lines in vitro, including melanoma, bladder, cervical, choriocarcinoma, osteosarcoma, glioblastoma, and breast, pancreatic, lung, and other adenocarcinoma cell lines (Nakamura and others 1986). IL-1 has also demonstrated antitumor effects in vivo against several murine syngeneic tumors (Fibbe and others 1988), including several that were not inhibited in in vitro studies, suggesting a possible requirement for cytotoxic effector cells in the in vivo setting (Fibbe and others 1988).
Therefore, with this multitude of immunological, antiproliferative, and hematopoietic properties, it was deemed appropriate to evaluate the potential of IL-1 in the treatment of human cancer. The goal of this phase I study was to evaluate the immunological and marrow-protective effects of IL-1 in 3 different schedules and 3 different doses in combination with cyclophosphamide. Cyclophosphamide was chosen for both its antitumor and immunomodulatory properties, as noted in both animal and human studies (Castelli and others 1988; Benjamin and others 1989; Mitchell 1992). It was noted that the immunomodulatory effects of cyclophosphamide in humans were observed at lower doses, in which suppressor cells are downregulated (Mitchell 1992, 2003). However, a cytotoxic dose was used to evaluate the hematopoietic properties of IL-1 as well.
At the time that this study was initiated and conducted, several hematopoietic growth factors were undergoing clinical investigation. It was hypothesized that an early acting, pluripotent agent like IL-1 could be more efficacious for general marrow support, compared with a more lineage-specific agent, such as G-CSF. Therefore this trial was initiated. This is one of the few clinical studies to investigate the potential effect of schedule of IL-1 in relationship to myelosuppressive agents. This may have implications for current research on inhibition of IL-1 as a proinflammatory and autoinflammatory cytokine and in cancer (Dinarello 2011; Dinarello and others 2012).
Patients and Methods
Study management
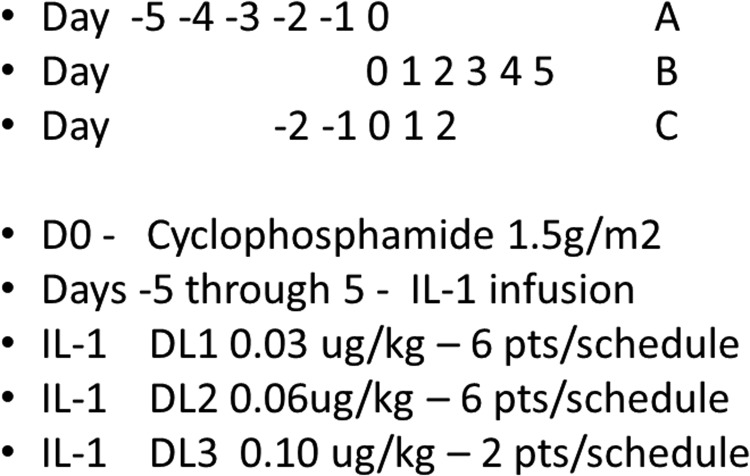
This was a multicenter phase I study, conducted at specific centers participating in the Biologic Response Modifier (BRM) Committee of the Eastern Cooperative Oncology Group (ECOG) and reported early on at the American Society of Clinical Oncology meeting in 1994 (Schuchter and others 1994). The study itself had a complex schema, evaluating 3 dose levels of IL-1α and 3 different schedules of the combination of cyclophosphamide and IL-1α (Fig. 1). This was a 3+3 design at each dose level and in each schedule, to evaluate the potential interactions of dose and schedule. At each dose level, subjects were randomized to a schedule. Three patients at each schedule at each dose were evaluated for toxicity, and the decision to add 3 more subjects was determined after toxicity evaluation for each dose/schedule cohort. Because of the complexity, there were numerous clinical holds to evaluate toxicity before accrual could be resumed. Therefore, the accrual continued from mid-1992 to mid-1996. Accrual to dose levels 1 and 2 were completed, but dose level 3 did not meet accrual goals, in part due to toxicity observed, which reduced enthusiasm. Therefore, the study was closed with 2 patients per schedule in dose level 3. Dose level 2 was considered the maximally tolerated dose, but there was no extension of the trial beyond this dose-finding evaluation. The objectives were to evaluate toxicity of cyclophosphamide in combination with IL-1α, to determine immunological and hematopoietic effects of IL-1α, and to assess any signals of antitumor activity for this combination. The study was approved by the human subjects review committees of the participating institutions.
FIG. 1.
Schedule of treatment.
Patients
Patients with metastatic or locally recurrent solid tumors, for which no other conventional therapy was deemed appropriate, were eligible for this study (Table 1). Patients could have had no more than 2 prior chemotherapy regimens, none within 4 weeks of starting this study, and complete hematologic recovery from previous treatment was required prior to study entry. Prior radiation was allowed, but no greater than 30 Gy to the hemipelvis, skull, or spine. Patients were required to be ECOG performance status 0 or 1, with a life expectancy of at least 3 months. Required hematologic parameters included a white blood cell (WBC) count of >4,000/mm3 and platelet count of >120,000/mm3. Normal renal and hepatic function was required. Patients were required to be free of infection, with no evidence of autoimmune disorders, and negative for human immunodeficiency virus antibody. Patients could not be receiving or possibly require corticosteroids. Patients with active cardiac disease were excluded and all patients were required to have normal pulmonary function due to known potential toxicity from IL-1.
Table 1.
Patient Demographics
| Characteristics | N=42 |
|---|---|
| Median age (range) | 55 (34–76) years |
| Sex: male/female | 26/16 |
| PS 0/1 | 15/27 |
| Tumor type | |
| GI | 12 |
| Lung | 7 |
| Melanoma | 6 |
| Unk primary | 5 |
| Renal | 4 |
| Sarcoma | 4 |
| Ovarian | 2 |
| Head and neck | 2 |
| Prior chemo | 33 |
| Prior XRT | 11 |
| No prior therapy | 4 |
Study drug administration
The design of the study required cohorts of 6 patients to be treated at each dose level in each schedule. The dose of cyclophosphamide was held constant, at 1.5 gm/m2, administered as an intravenous infusion over 1 h on Day 0 of each of the 3 schedules (Fig. 1). Two liters of normal saline was administered with the cyclophosphamide to prevent hemorrhagic cystitis. Antiemetics were administrated as needed, but dexamethasone was not permitted.
IL-1α was administered through a central intravenous line over 30 min for 5 consecutive days. Acetaminophen (650 mg) was administered 1 h prior to each dose of IL-1α, and every 4 h for 1–2 doses following each dose to prevent and treat fever associated with the infusion. Meperidine (25–50 mg) was administered intravenously as needed after IL-1α infusion to treat chills associated with its administration.
Cycles were repeated every 28 days. Dose level 1 of IL-1α was 0.03 μg/kg, dose level 2 was 0.06 μg/kg, and dose level 3 was 0.10 μg/kg. Three different schedules of IL-1α were administered in relationship to the cyclophosphamide infusion, for each dose level of IL-1α. In schedule A, IL-1α was administered prior to cyclophosphamide on day −5 through day −1, with cyclophosphamide on day 0. In schedule B, IL-1α was administered on days 1–5, following cyclophosphamide given on day 0. Schedule C was concurrent administration, with cyclophosphamide on day 0, and IL-1α on days −2, −1, 0, 1, and 2.
All patients were hospitalized for treatment, and vital signs were performed every 15 min for the first hour following IL-1α, then every hour for 3 h, and then every 4 h. At some institutions, patients were treated in an NIH-designated GCRC facility. Patients were discharged from the hospital 24 h after their last study drug administration (either IL-1α or cyclophosphamide depending upon the schedule). There was a period of evaluation for each schedule when a given dose level was completed and fully accrued, to assess toxicity and efficacy, thus putting accrual on hold until complete assessments could be conducted.
Study drugs
Cyclophosphamide was commercially available. The IL-1α used in this study was produced in Escherichia coli by recombinant DNA technology, and was supplied by NCI/DCT and Dainippon. IL-1α was supplied as lyophilized powder in 10 μg/vial and 100 μg/vial with human serum albumin as a stabilizer. The product was reconstituted with 1 mL saline for injection that was further diluted in 150 mL of sterile normal saline.
Laboratory evaluations
Patients were evaluated at baseline, and then daily during IL-1α administration, with complete blood count, platelet count, and differential WBC count. Also daily during IL-1α administration, chemistry profile, including electrolytes, liver function tests (LFTs), blood urea nitrogen (BUN), creatinine, and lactate dehydrogenase (LDH), was obtained. Following 5 days of IL-1α administration, the same laboratory testing was obtained weekly. Thyroid function was evaluated at baseline and monthly. No pharmacokinetic studies were done in this trial.
Toxicity evaluation
Toxicity was evaluated according to the Common Toxicity Criteria of the Cancer Therapy Evaluation Program (CTEP). The study was designed to assess dose-limiting toxicity (DLT) during cycle 1 of treatment and was specifically defined for this study (Table 2).
Table 2.
Defined Dose-Limiting Toxicity
| Any Grade 3 or 4 nonhematologic toxicity |
| Grade 4 myelosuppression |
| ANC <500/mm3 for >7 days |
| Platelet count <25,000/mm3 for >7 days |
| Hypotension during infusion requiring vasopressors |
| Any toxicity requiring interruption or modification of IL-1 dose administration |
DLT was any grade 3 or 4 nonhematologic toxicity; grade 4 myelosuppression, with an absolute neutrophil count (ANC) of <500/mm3, or platelet count <25,000/mm3, for >7 days; hypotension during infusion unresponsive to fluid administration and requiring vasopressor therapy; and any toxicity that required interruption or modification of IL-1α dosing. Patients who developed a DLT were eligible to resume therapy, provided the toxicity resolved to less than grade 2. When IL-1α was resumed, the dose was reduced to the prior dose level. If the DLT occurred at dose level 1, then the dose of IL-1α was reduced to 0.02 μg/kg. Patients who received at least one dose of IL-1α were evaluable for toxicity. All patients who completed one cycle of therapy with IL-1α and cyclophosphamide were considered evaluable.
Statistical evaluation
Laboratory analyses were conducted to assess the timing, depth, and duration of nadir counts by both the doses and schedules of IL-1α administration. Patients with at least 5 days of laboratory data were included in these analyses. Analysis of variance (ANOVA) and t-tests were used to compare doses and schedules. The strategy used was to explore the widest difference for significance, and to stop testing if the most extreme groupings did not achieve statistical significance. Limitations regarding evaluation of the day of the nadir WBC count and ANC were primarily due to the schedule of blood examinations obtained. Blood counts were obtained daily during drug administration, with subsequent laboratory evaluations on days 7, 14, and 21 only. All recorded nadirs were noted on either day 7 or day 14. The study was not designed nor sufficiently powered to evaluate interaction between dose and schedule.
Results
Patient characteristics
Forty-five patients were registered and randomized, but 3 were not treated due to rapid progression of disease (Table 1). Forty-two patients received at least one dose of IL-1α and were evaluable for toxicity. All but 4 patients were previously treated with chemotherapy and/or radiation therapy. The median age was 55 years (range 34–76 years), with a male/female ratio of nearly 2:1. All patients were ECOG performance status 0 or 1. Patient characteristics were relatively evenly distributed among the doses and schedules. Dose levels 1 and 2 had 6 patients evaluated on each of the 3 schedules. Dose level 3 only accrued 2 patients each per schedule, and the study was closed due to toxicity observed at dose level 3.
Nonhematologic toxicity of IL-1α
Known toxicities of IL-1α administration are fever and chills and these were observed in all patients, usually occurring 1–2 h following drug infusion (Table 3). The highest temperature recorded was 104.9 F. Fever and chills were not dose limiting in any patient. Other common, less than grade 3 toxicities were headache, nausea, hypertension, hypotension, and LFT abnormalities.
Table 3.
Nonhematologic Toxicity—All Grades
| Grade of toxicity | ||||
|---|---|---|---|---|
| Toxicity | 1 | 2 | 3 | 4 |
| Nausea/emesis | 11 | 14 | 4 | 0 |
| Fever | 3 | 32 | 2 | 0 |
| Hypotension | 24 | 5 | 2 | 0 |
| Hepatic | 16 | 1 | 2 | 3 |
| Headache | 6 | 11 | 0 | 0 |
| Hypertension | 12 | 3 | 0 | 0 |
| Renal | 5 | 5 | 0 | 0 |
| Abdominal pain | 4 | 2 | 1 | 0 |
| Neurologic | 5 | 1 | 0 | 0 |
| Pulmonary | 1 | 3 | 0 | 1 |
| Allergy | 0 | 3 | 2 | 0 |
| Diarrhea | 1 | 2 | 1 | 0 |
| Cardiac | 1 | 1 | 0 | 1 |
| Mucositis | 1 | 1 | 1 | 0 |
| Hypoglycemia | 0 | 0 | 1 | 0 |
Grade 3 toxicity (continuing therapy)
One patient treated at dose level 1 (0.03 μg/kg) required re-hospitalization and intravenous fluids for several days after completing protocol therapy due to dehydration from prolonged nausea and vomiting (Table 4). Six patients complained of transient epigastric/abdominal pain, but in one patient, at dose level 3, the pain was severe and dose limiting (see Grade 3–4 dose-limiting toxicity and Table 4). Significant hypotension only occurred at the highest dose level (0.10 μg/kg). One patient at this dose level required transient vasopressor support following his second dose of IL-1α. Subsequently, this patient was weaned off vasopressors and received his subsequent 3 infusions of IL-1α at a reduced dose (0.06 μg/kg).
Table 4.
Observed Grade 3 and 4 Toxicities
| Grade 3—continuing therapy | |
| 0.03 μg/kg | Re-hospitalization for dehydration secondary to nausea and vomiting post-treatment (preondansetron era) |
| 0.10 μg/kg | Transient vasopressor support for hypotension. Able to receive 3 more doses at 0.06 μg/kg. |
| 0.06 μg/kg | Significant dyspnea with IL-1 administration, but continued treatment |
| Grade 3–4 dose-limiting toxicity | |
| Pulmonary toxicity | 0.03 μg/kg—acute bronchospasm |
| 0.06 μg/kg—acute respiratory distress and bronchospasm | |
| Led to requirement for normal pulmonary function test evaluation prior to study entry | |
| Cardiac toxicity | 0.06 μg/kg—acute myocardial infarction after 2 doses |
| Hepatic toxicity | 0.10 μg/kg—grade 4 bilirubin (had liver metastases) |
| 0.10 μg/kg—grade 4 transaminitis after grade 4 hypotension requiring vasopressor support | |
| Abdominal pain | 0.10 μg/kg—severe abdominal pain and rigors with each of 2 IL-1 infusions, so drug discontinued |
Four of 6 patients at 0.10 μg/kg developed predefined dose-limiting toxicity: 2 hypotension requiring pressors; 3 grade 3 hepatic toxicity; and 1 severe abdominal pain and could not continue. Patients accrued to all schedules simultaneously.
Grade 3–4 dose-limiting toxicity
Six patients were unable to complete the 5-day course of IL-1α due to toxicity (Table 4).
Pulmonary toxicity
Two patients with extensive pulmonary metastases developed acute respiratory symptoms after receiving IL-1α, possibly due to capillary leak syndrome. One patient (55-year-old man) developed acute bronchospasm after the second dose of IL-1α, at a dose of 0.03 μg/kg. Symptoms subsided within several hours. The second patient (42-year-old woman) developed severe respiratory distress following her 4th dose of IL-1α at a dose of 0.06 μg/kg, and responded to interventions with aminophylline, oxygen, and nebulizer treatment. A third patient (68-year-old woman) developed significant dyspnea associated with treatment, but was able to complete 5 doses of IL-1α (0.06 μg/kg per dose). A requirement for baseline pulmonary function tests (PFTs) was established following these pulmonary events. No further pulmonary toxicity occurred in the study once patients with abnormal PFTs were excluded.
Cardiac toxicity
A patient (76-year-old man) with known coronary artery disease experienced a nonfatal myocardial infarction following 2 doses of IL-1α at dose level 2 (0.06 μg/kg).
LFT abnormalities
One patient (59-year-old man) with liver metastases developed grade 4 elevation of bilirubin after 2 doses of IL-1α at dose level 3 (0.10 μg/kg). This was suspected to be due to metastatic disease. A second patient (47-year-old woman) who experienced grade 4 hypotension after dose 1 (dose level 0.10 μg/kg), transiently requiring vasopressor support, was noted to have grade 4 LFT abnormalities 12 h following hypotension. IL-1α was discontinued and LFTs normalized without sequelae.
Abdominal pain
A patient with advanced ovarian cancer and ascites developed severe abdominal pain and rigors with the first IL-1α infusion (0.10 μg/kg dose). During the second dose this occurred again, and IL-1α was discontinued. There was no evidence of perforation or obstruction.
In summary, dose-limiting toxicity occurred in 4 of 6 patients treated at dose level 3 (0.10 μg/kg). This included 3 patients with grade 3 or 4 hepatic toxicity, 2 patients with hypotension requiring vasopressors, and 1 patient who could not continue therapy due to severe abdominal pain. Therefore, the maximal tolerated dose (MTD) for this study of IL-1α in combination with cyclophosphamide is 0.06 μg/kg.
At the MTD, 18 patients were treated, and there was one episode of grade 4 cardiac toxicity, one episode of grade 4 pulmonary toxicity, and one grade 3 hepatic toxicity. It is possible that this dose defined by this study may be higher than a true MTD.
Hematologic toxicity
Fourteen episodes of grade 4 neutropenia were reported, including one grade 3 sepsis, and 3 episodes of grade 4 febrile neutropenia. The only death on study was attributed to disease progression. There was no preponderance of neutropenia or febrile neutropenia by dose or schedule.
Hematologic responses
Thirty-eight patients were evaluable for changes in WBC from baseline to a time point after dose 1 of IL-1α. Thirty-five patients completed 5 days of treatment with IL-1α and received cyclophosphamide, 1.5 gm/m2 on day 0, and were considered evaluable for hematologic responses and assessment of nadir WBC and nadir ANC. Preclinical and clinical data suggested that the myeloprotective effects of IL-1α are influenced by both dose of IL-1α and the timing of its administration with relationship to the myelotoxic agent. Thus, both dose and schedule were tested in this phase I study.
Changes in WBC count after initial dose of IL-1α (n=38)
Treatment with IL-1α at all dose levels resulted in a significant increase in the total WBC count above baseline. The rise in the WBC counts occurred within 24 h of receiving IL-1α and lasted for several days with a gradual decline over time. The increased WBC counts consisted of neutrophils and bands, with a marked decrease in absolute lymphocyte count at all 3 dose levels.
Statistical analysis of the WBC count during treatment was available for 6 patients on each schedule at dose level 1, 5 patients on each schedule on dose level 2, and 5 total patients on dose level 3, 2 each on schedules A and B, and 1 on schedule C. This totaled 13 patients on schedule A, 13 on schedule B, and 12 on schedule C. ANOVA suggests that as the dose of IL-1α increased, the magnitude of the rise in WBC also increased (P=0.02). At dose level 1, the average change in WBC between baseline and day 1 was 8,161, at dose level 2, 10,900, and at dose level 3, 16,740.
Nadir WBC by dose level and by schedule (n=34)
Patient data were evaluated for those patients who had at least 5 days of data available for analysis and whose data extended beyond the days in which they received IL-1α (Table 5). A total of 34 patients are included in this analysis. Dose level 1: 17 patients; dose level 2: 14 patients; and dose level 3: 3 patients. Schedule A: 13 patients; schedule B: 12 patients; and schedule C: 9 patients. The nadir WBC counts do not differ significantly by dose level—DL1 1741, DL2 1764, and DL3 733 (P=0.48)—or by schedule—A 1746, B 2009, and C 1078 (P=0.29)—based on ANOVA.
Table 5.
Nadir WBC and ANC by Dose Level and by Schedule
| Dose level(μg/kg) | Mean WBC (sd)(n=34) | P=0.48 | Mean ANC (sd)(n=29) | P=0.52 |
|---|---|---|---|---|
| 0.03 | 1,741 (1,185) | 777 (2,235) | ||
| 0.06 | 1,764 (1,623) | 1,208 (1,536) | ||
| 0.10 | 733 (750) | 226 (269) |
| Schedule | Mean WBC (sd) | P=0.29 | Mean ANC (sd) | P=0.10 |
|---|---|---|---|---|
| A (D−5—D−1) | 1,746 (1,249) | 826 (1,077) | ||
| B (D1–D5) | 2,008 (1,800) | 1,578 (1,721) | ||
| C (D−2—D2) | 1,078 (463) | 158 (105) |
ANC, absolute neutrophil count; WBC, white blood cell.
Nadir ANC by dose level and by schedule (n=29)
Patient data for ANC were based on the same criteria outlined earlier (Table 5). Percentage of neutrophils was not always available with WBC. Data from 29 patients were included in this analysis: 13 at dose level 1, 14 at dose level 2, and 2 at dose level 3. Schedule A: 13 patients; schedule B: 10 patients; and schedule C: 6 patients. The nadir ANCs do not differ significantly by dose level—DL1 777, DL2 1208, and DL3 226 (P=0.10)—or by schedule—A 826, B 1578, and C 158 (P=0.52). Because of the small numbers, a subsequent analysis by pairs was conducted, to explore the widest differences, but no significant differences in nadir ANC by dose or schedule were noted.
Timing of nadir and recovery of WBC
Following treatment with IL-1α, blood tests were done weekly, obtained at days 7, 14, 21, and 28. All recorded nadirs were on day 7 or 14; the protocol only required reporting of weekly laboratory results. For WBC, 22 nadirs were on day 7 (10 of 17 on dose level 1, 10 of 14 on dose level 2, and 2 of 3 on dose level 3). Seven of 13 were on schedule A, 7 of 12 on schedule B, and 8 of 9 on schedule C. For ANC, 14 nadirs were on day 7 (4 of 13 at dose level 1, 9 of 14 on dose level 2, and 1 of 2 at dose level 3). Six of 13 were on schedule A, 4 of 10 on schedule B, and 4 of 6 on schedule C. All but 2 patients with laboratory values available on day 21 had recovered to a WBC of ≥4,000/mm3. Those 2 patients recovered on day 28. Nine patients who nadired earlier, also recovered earlier, by day 14. One patient nadired above 4,000/mm3.
Antitumor efficacy
Because of the toxicities noted and varying durations of therapy, and the use of a single dose of an alkylating agent in a broad variety of previously treated malignancies, efficacy was in the end not reported. The primary endpoint was assessment of hematologic support.
Discussion
IL-1α as hematopoietic cytokine
IL-1 has the ability to both protect and restore the bone marrow from injury due to chemotherapeutic agents or radiation (Castelli and others 1988). Castelli and others (1988) have demonstrated that IL-1α and IL-1β can increase the colony forming unit (CFU)–culture activity in murine spleens. In addition, IL-1 can rescue animals if administered either before or just after lethal doses of cyclophosphamide or radiation (Castelli and others 1988). Dose, sequence, and timing of IL-1 administration were critical in these experiments. The effects were mainly seen on granulopoiesis, with recovery of myeloid CFUs and total marrow cellularity (Benjamin and others 1989). Additional studies evaluated IL-1's hematopoietic protective effect in murine models of antitumor chemotherapy, including doxorubicin, 5 fluorouracil (5FU), and cisplatin, in addition to cyclophosphamide (Eppstein and others 1989; Damia and others 1992; Lynch and others 1993). In these studies, schedule was also explored. Pretreatment allows dose escalation in one series (Lynch and others 1993), but was not protective in another (Eppstein and others 1993). Postchemotherapy usage was protective in one study (Eppstein and others 1989) but depended upon the chemotherapy utilized in another study, only helping with cyclophosphamide (Damia and others 1992).
Historical data on cyclophosphamide 1.5 gm/m2
In an early study (late 1970s to early 1980s) conducted at the Baltimore Cancer Research Program of the National Cancer Institute, patients with non-Hodgkin's lymphoma were treated with a regimen of cyclophosphamide, vincristine, and prednisone with 1.5 gm/m2 of cyclophosphamide given on a 21-day schedule (Bishop and others 1987). This was conducted prior to the development and usage of hematopoietic growth factor support, and prior to modern antibiotics. In that study, patients experienced the nadir WBC counts between days 7 and 14, and recovered by day 21, including patients having marrow involvement with lymphoma. Febrile neutropenia occurred in 15% of courses, and grade 3–4 neutropenia occurred in 36% of courses. There were fatal infections in 3 patients (Bishop and others 1987). This provides some historical data on the outcome of treatment with this dose level of cyclophosphamide without hematopoietic growth factor support.
Present study
In this study, there was no statistically significant difference among dose levels and schedules on the nadir WBC or nadir ANC. However, there was a numerical decline of WBC and ANC and an increase in toxicity with increasing dose levels of IL-1. All grade 3–4 toxicity occurred at the 2 highest doses, except for one episode of bronchospasm at the 0.03 μg/kg dose level. Additionally schedule C (IL-1 given pre- and post-cyclophosphamide) had the deepest numerical nadirs of WBC and ANC, in this small study. Further attention to schedule may be informative for studies of IL-1 antagonists as they continue in clinical use.
Summary of additional IL-1 clinical trials
The present study was designed to clarify the dose and schedule necessary to optimize hematopoietic support with IL-1α in combination with cyclophosphamide, similar to preclinical studies (Eppstein and others 1989; Damia and others 1992; Lynch and others 1993). Three other phase I studies with similar dose-finding goals and 2 with comparisons of schedule relative to chemotherapy have also been reported (Crown and others 1991; Smith and others 1993; Nemunaitis and others 1994) (Table 6). All seem to demonstrate a dose-response effect for peak WBC but also for toxicity. Effects on WBC nadir and duration of neutropenia were variable. Nonhematologic toxicity included hypotension, bone pain, fever, chills, tachycardia, hypertension, and rare cardiac arrhythmias (Crown and others 1991; Starnes, 1991; Smith and others 1993; Nemunaitis and others 1994).
Table 6.
Summary of Phase I IL1/Chemo Clinical Trials
| Study/pts | Chemo | IL1 doses | Schedules | Heme effect | Tox |
|---|---|---|---|---|---|
| Crown 1991 | 5FU | 0.002–0.1 μg/kg | IL1 alone | Plts OK | Hypotension |
| N=19 | 833 mg/m2 | 5FU alone | Marrow- | Pain | |
| GI malig | Daily×3 | IL1+5FU | No change; higher nadir; dec G-penia duration | ||
| Nemunaitis 1994 | Bu/CY/TBI | 0.01 μg/kg | D1–D5 | 25 D to ANC | Fever/chills |
| N=17 | or Bu/Cy | 0.02 μg/kg | >500 | Hypotension | |
| AML | 0.05 μg/kg | 12% infection | 30% fluids, | ||
| ABMT | Vasopressors | ||||
| Smith 1993 | Carboplatin | 0.03 μg/kg | D−5—D−1 | Prenadir 19k | Hypotension |
| N=43 | 800 mg/m2 | 0.1 μg/kg | D1–D5 | Plts | 2-pressors |
| Solid T | 0.03 μg/kg | D1–D5 | Postnadir | Pain | |
| 91K–332K | Capillary leak | ||||
| (0.3 μg dose) | |||||
| Schuchter 1994 | Cyclophos | 0.03 μg/kg | D−5—D−1 | ANC nadir | Hypotension |
| N=43 | 1.5 mg/m2 | 0.06 μg/kg | D−2—D2 | >500, dose | 2-pressors |
| Solid T | 0.1 μg/kg | D1–D5 | level 1 & 2 | Pain | |
| Schd A, B; | Capillary leak | ||||
| Schd C | |||||
| ANC<200 |
5FU, 5 fluorouracil; Plts, platelets; G-penia, granulocytopenia; AML, acute myeloid leukemia; ABMT, autologous bone marrow transplant; Bu/Cy, busulfan, cytclophosphamide; TBI, total body irradiation; D, day; Schd, schedule.
Crown and others (1991) evaluated IL-1β in patients with gastrointestinal malignancies treated with myelosuppressive doses of 5FU, 833 mg/m2×3 days, comparing the same dose of 5FU alone to IL-1β alone, and to the combination. They observed transient neutropenia and monocytopenia, followed by neutrophil leukocytosis after day 1 of IL-1β administration. Increases in platelet count were seen at a median of 14 days. They described a less-deep nadir neutrophil count and a shorter duration of neutropenia with the combination compared with 5FU alone. They obtained bone marrow specimens and described no change in marrow cellularity with administration of IL-1β, which they interpreted as possibly reflecting a demargination effect, or secondary release of other specific neutrophil growth factors (Crown and others 1991).
Nemunaitis and others evaluated IL-1β in patients with acute myeloid leukemia who underwent autologous bone marrow transplantation (Nemunaitis and others 1994). IL-1β was administered at 3 dose levels, beginning on the day of bone marrow infusion, for a total of 5 daily doses. There appeared to be a shorter nadir compared with historical controls (25 versus 34 days to ANC of ≥500/μL) and less infections between days 0 and 28 (12% versus 23%). The major limitation of this study is the use of historical controls for comparison to IL-1β in an era of overall improved supportive care. Only one patient in that study received the highest dose (0.05 μg/kg) and 9 received 0.01 μg/kg and 7 received 0.02 μg/kg.
Smith and others evaluated the potential benefit of IL-1α on platelet recovery after high-dose carboplatin, well known for its resultant thrombocytopenia (Smith and others 1993). Again, IL-1α was administered at 3 dose levels, 0.03, 0.1, and 0.3 μg/kg for 5 days, either before or after carboplatin, and this was compared with a control group who received the same dose of carboplatin (800 mg/m2) without growth factor support. The median nadir platelet count for carboplatin alone was 19,000/μL, and there was a median of 10 days with platelet count <100,000/μL. All 15 patients who received IL-1α prior to carboplatin had the same nadir level of platelet count and duration of low count as the control group. However, in those who received IL-1α after carboplatin, 5 of 15 patients given the 2 higher doses of IL-1α had a minimal decrease in platelet count, with nadirs ranging from 91,000/μL to 332,000/μL. Escalation of dose above 0.3 μL/kg was limited by hypotension.
Summary of clinical hematopoietic studies
In 4 phase I studies of a range of doses of IL-1 and 3 with varied schedules of IL-1 (2 with IL-1α and 2 with IL-1β), there appeared to be some measurable improvement in neutrophil nadir and duration of neutropenia, and in the Smith study, there was improvement in the degree of nadir thrombocytopenia in some patients. However, these were all small studies and only 2 had concurrent control populations. Nevertheless, there did appear to be a beneficial hematopoietic effect. However, this was all at the price of manageable, but significant systemic toxicities related to the other effects of IL-1 (Crown and others 1991; Starnes, 1991; Smith and others 1993; Nemunaitis and others 1994; Schuchter and others 1994; Veltri and others 1996). These included hypotension, often requiring fluids and in some cases vasopressors; unusual pain syndromes; abnormal liver function; and capillary leak syndrome, with occasional respiratory distress. These have been consistent and dose dependent, as was the effect on hematopoiesis across these studies (Crown and others 1991; Smith and others 1993; Nemunaitis and others 1994; Schuchter and others 1994).
Three studies investigated different dose levels and 2 studies investigated timing of IL-1 with relation to chemotherapy administration. In the first 3, there was a dose response for both maintenance of blood counts and toxicity (Crown and others 1991; Smith and others 1993; Nemunaitis and others 1994). Schedule differences were less apparent in our study (Schuchter and others 1994), but seemed to have a major impact on platelet nadir in the Smith study (Smith and others 1993). These 2 studies were also using different chemotherapy agents.
Implications for current IL-1 research
IL-1, as a broadly acting hematopoietic cytokine, has been shown to have restorative effects on marrow after radiation or chemotherapy. However, the level of toxicity with this agent compared to that seen with more specific, downstream neutrophil growth factors has made its general use prohibitive. The hematopoietic clinical trials utilized short pulses of relatively high doses of IL-1 (Crown and others 1991; Smith and others 1993; Nemunaitis and others 1994; Schuchter and others 1994) and, in addition to hematopoietic support, it produced clinically significant proinflammatory effects that were not tolerable. No adverse effects related to the malignancies were observed.
More recent research has focused on low-level chronic inflammatory states in which IL-1 is integrally involved (Balkwill and others 2005; Mantovani 2005). Early studies showed that inflammation increased adherence of tumor cells to endothelium providing a mechanism for metastasis (Giavazzi and others 1990; Lauri and others 1990; Arguello and others 1992). This may still be significant, even with chronic low-level inflammation. Therefore, dose and schedule of intrinsic cytokines (ie, low-dose, chronic exposure) have implications in disease process as well as disease control. Recent studies link inflammation and angiogenesis, and again IL-1 is implicated in these processes (Voronov and others 2003; Carmi and others 2009). IL-1 receptor antagonists are in clinical practice and clinical research—most prominently for autoinflammatory and autoimmune diseases (Dinarello 2011; Dinarello and others 2012). The balance of inhibition and salutary effects continues to be critical in utilizing cytokines and cytokine inhibition for clinical therapy. A potential role for IL-1 inhibition as therapy in some early, cytokine-mediated premalignant situations such as smouldering myeloma has been postulated, through subsequent control of IL-6 (Dinarello 2010; Dinarello 2011).
Therefore, the IL-1 family of cytokines continues to draw major research and clinical interest as key players in oncology, both in understanding disease production and potentially mediating disease control.
Author Disclosure Statement
The authors do not have any conflicts of interest in connection with this article.
References
- Arguello F, Baggs RB, Graves BT, Harwell Se, Cohen HJ, Frantz CN. 1992. Effect of IL-1 on experimental bone/bone marrow metastases. Int J Cancer 52:802–807 [DOI] [PubMed] [Google Scholar]
- Balkwill F, Charles KA, Mantovani A. 2005. Smouldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell 7:211–217 [DOI] [PubMed] [Google Scholar]
- Bartelmez SH, Stanley ER. 1985. Synergism between hemopoietic growth factors (HGSs) detected by their effects on cells bearing receptors for a lineage specific HGF: Assay of hemopoietin-1. J Cell Physiol 22:370–374 [DOI] [PubMed] [Google Scholar]
- Benjamin WR, Tare NS, Hayes TJ, Becker JM, Anderson TD. 1989. Regulation of hemopoiesis in myelosuppressed mice by human interleukin-1 alpha. J Immunol 142:792–799 [PubMed] [Google Scholar]
- Bishop JF, Wiernik PH, Wesley MN, Kaplan RS, Diggs CH, Barcos MP, Sutherland JC. 1987. A randomized trial of high dose cyclophosphamide, vincristine, and prednisone plus or minus doxorubicin with long-term follow-up in advanced non-Hodgkin's lymphoma. Leukemia 1:508–513 [PubMed] [Google Scholar]
- Carmi Y, Voronov E, Dotan S, Lahat N, Rahat MA, Fogel M, Huszar M, White MR, Dinarello CA, Apte RN. 2009. The role of macrophage-derived IL-1 in induction and maintenance of angiogenesis. J Immunol 183:4705–4714 [DOI] [PubMed] [Google Scholar]
- Castelli M, Black P, Schneider M, Pennington R, Fuminori A, Talmadge JE. 1988. Protective, restoration and therapeutic properties of recombinant IL-1 in rodent models. J Immunol 140:3830–3837 [PubMed] [Google Scholar]
- Crown J, Jakubowski A, Kemeny N, Gordon M, Gasparetto C, Wong G, Sheridan C, Toner G, Meisenberg B, Botet J, Applewhite J, Sinha S, Moore M, Kelsen D, Buhles W, Gabrilove J. 1991. A phase I trial of recombinant human Interleukin-1β alone and in combination with myelosuppressive doses of 5-fluorouracil in patients with gastrointestinal cancer. Blood 78:1420–1427 [PubMed] [Google Scholar]
- Damia G, Komschlies KL, Futami H, Back T, Gruys ME, Longo DL, Keller JR, Ruscetti FW, Wiltrout RH. 1992. Prevention of acute chemotherapy-induced death in mice by recombinant human interleukin-1: Protection from hematological and nonhematological toxicities. Cancer Res 52:4082–4089 [PubMed] [Google Scholar]
- Dempsey R, Dinarello CA, Mier JW, Rosenwasser LF, Allegretta M, Brown TE, Parkinson DR. 1982. The differential effects of human leucocyte pyrogen lymphocyte activating factor, T-cell growth factor, and interferon on human natural killer activity. J Immunol 129:2504–2510 [PubMed] [Google Scholar]
- Dinarello CA. 1988. Biology of interleukin-1. FASEB J 2:108–115 [PubMed] [Google Scholar]
- Dinarello CA. 1991. Interleukin-1 and interleukin-1 antagonism. Blood 77:1627–1652 [PubMed] [Google Scholar]
- Dinarello CA. 1996. Biologic basis for interleukin-1 in disease. Blood 87:2095–2147 [PubMed] [Google Scholar]
- Dinarello CA. 2010. Why not treat human cancer with interleukin-1 blockade? Cancer Metastasis Rev 29:317–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello CA. 2011. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood 117:3720–3732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello CA, Cannon JG, Mier JW, Bernheim HA, LoPreste G, Lynn DL, Love RN, Webb AC, Auron PE, Reuben RC. 1986. Multiple biological activities of human recombinant interleukin 1. J Clin Invest 77:1734–1739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello CA, Simon A, van der Meer JWM. 2012. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat Rev Drug Discov 11:633–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eppstein DA, Kurahara CG, Bruno NA, Terrell TG. 1989. Prevention of doxorubicin-induced hematotoxicity in mice by Interleukin-1. Cancer Res 49:3955–3960 [PubMed] [Google Scholar]
- Farran W, Mizel S, Farran J. 1980. Participation of lymphocyte activating factor (IL-1) in the induction of cytotoxic T-cell responses. J Immunol 124:1371–1377 [PubMed] [Google Scholar]
- Fibbe W, Damme J, Billiau A, Goselink HM, Voogt PJ, vanEeden G, Ralph P, Altrock BW, Falkenberg JH. 1988. Interleukin-1 induces human marrow stromal cells in long-term culture to produce granulocyte colony-stimulating factors and macrophage colony stimulating factor. Blood 71:430–435 [PubMed] [Google Scholar]
- Giavazzi R, Garofalo A, Bani MR, Abbate M, Ghezzi P, Boraschi D, Mantovani A, Dejana E. 1990. Interleukin-1-induced augmentation of experimental metastases from human melanoma in nude mice. Cancer Res 50:4771–4775 [PubMed] [Google Scholar]
- Griffin JD, Rambaldi A, Vellenga E, Young DC, Ostapovicz D, Cannistra SA. 1987. Secretion of interleukin-1 by acute myeloblastic leukemia cells in vitro induces endothelial cells to secrete colony stimulating factors. Blood 70:1218–1224 [PubMed] [Google Scholar]
- Lauri D, Bertomeu MC, Orr FW, Bastida E, Sauer D, Buchanan MR. 1990. Interleukin-1 increases tumor cell adhesion to endothelial cells through an RGD dependent mechanism: in vitro and in vivo studies. Clin Exp Metastasis 8:27–32 [DOI] [PubMed] [Google Scholar]
- Lynch DH, Rubin AS, Miller RE, Williams DE. 1993. Protective effects of recombinant human Interleukin-1α in doxorubicin-treated normal and tumor–bearing mice. Cancer Res 53:1565–1570 [PubMed] [Google Scholar]
- Mantovani A. 2005. Cancer: Inflammation by remote control. Nature 435:752–753 [DOI] [PubMed] [Google Scholar]
- Matsushima K, Akahoshi T, Yamada M, Furutani Y, Oppenheim JJ. 1986. Properties of a specific interleukin 1 IL1) receptor on human Epstein Barr virus-transformed B lymphocytes: identity of the receptor for IL1-alpha and IL1-beta. J Immunol 136:4496–4502 [PubMed] [Google Scholar]
- Mitchell MS. 1992. Chemotherapy in combination with biomodulation: A 5 year experience with cyclophosphamide and interleukin-2. Sem Oncol 19 (Suppl. 4):80–87 [PubMed] [Google Scholar]
- Mitchell MS. 2003. Combinations of anti-cancer drugs and immunotherapy. Cancer Immunol Immunother 52:686–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura S, Nakata K, Kashimoto S, Yoshida H, Yamada M. 1986. Antitumor effects of recombinant human Interleukin-1 alpha against murine syngeneic tumors. Jpn J Can Res 77:767–773 [PubMed] [Google Scholar]
- Nemunaitis J, Appelbaum FR, Lilleby K, Buhles WC, Rosenfeld C, Zeigler ZR, Shadduck RK, Singer JW, Meyer W, Buckner CD. 1994. Phase I study of recombinant Interleukin-1β in patients undergoing autologous bone marrow transplant for acute myelogenous leukemia. Blood 83:3473–3479 [PubMed] [Google Scholar]
- Numerof RP, Aronson FR, Mier JW. 1988. IL-2 stimulates the production of IL-2 alpha and IL-1 beta by human peripheral blood mononuclear cells. J Immunol 141:4250–4257 [PubMed] [Google Scholar]
- Onozaki K, Matsushima K, Aggarwal B, Oppenheim JJ. 1985. Human Interleukin-1 is a cytocidal factor for several tumor cell lines. J Immunol 135:3962–3968 [PubMed] [Google Scholar]
- Schuchter L, Neuberg D, Atkins M, Recio a, Tester W, Wadler S, Chachoua A, Dutcher JP. 1994. A phase I study of interleukin-1α and high dose cyclophosphamide in patients with advanced cancer. Proc Am Soc Clin Oncol 13:133a [Google Scholar]
- Smith JW, Longo DL, Alvord WG, Janik JE, Sharfman WH, Gause BL, Curti BD, Creekmore SP, Holmlund JT, Fenton RG, Sznol M, Miller LL, Shimzu M, Oppenhein JJ, Fiem SJ, Hursey JC, Powers GC, Urba WJ. 1993. The effects of treatment with Interleukin-1α on platelet recovery after high dose carboplatin. N Engl J Med 328:756–761 [DOI] [PubMed] [Google Scholar]
- Starnes HF., Jr.1991. Biologic effects and possible clinical applications of interleukin-1. Semin Hematol 28:34–40 [PubMed] [Google Scholar]
- Tilg H, Atkins MB, Dinarello CA, Mier JW. 1995. Induction of circulating interleukin 10 by interleukin 1 and interleukin 2, but not interleukin 6 immunotherapy. Cytokine 7:734–739 [DOI] [PubMed] [Google Scholar]
- Tilg H, Shapiro L, Vannier E, Poutsiaka DD, Trehu E, Atkins MB, Dinarello A, Mier JW. 1994. Induction of circulating antagonists to IL-1 and TNF by IL-2 administration and their effects on IL-2 induced cytokine production in vitro. J Immunol 152:3189–3198 [PubMed] [Google Scholar]
- Vannier E, Kaser A, Atkins MB, Fantuzzi G, Dinarello CA, Mier JW, Tilg H. 1999. Elevated circulating levels of soluble interleukins-1 receptor type II during interleukin-2 immunotherapy. Eur Cytokine Netw 10:37–42 [PubMed] [Google Scholar]
- Veltri S, Smith JW. 1996. Interleukin −1 trials in cancer patients; A review of the toxicity, anti-tumor and hematopoietic effects. Stem Cells 14:164–176 [DOI] [PubMed] [Google Scholar]
- Voronov E, Shouval DS, Krelin Y, Cagnano E, Benharroch D, Iwakura Y, Dinarello CA, Apte RN. 2003. IL-1 is required for tumor invasiveness and angiogenesis. PNAS 100:2645–2650 [DOI] [PMC free article] [PubMed] [Google Scholar]