Abstract
As a classic type I cytokine, interferon-gamma (IFN-γ) is known to manifest a miscarriage-inducing effect, although the specific mechanism is still unclear. To determine whether immune cells such as regulatory T (Treg) and Th17 cells are involved in these abortions, syngeneically pregnant (BALB/c×BALB/c) mice were subjected to intravaginal IFN-γ administration (5×103 IU/mouse on D3 of gestation). These mice experienced significant fetal loss on D7/D8 of pregnancy, and a remarkable drop in the Treg cell ratio was observed in the peripheral blood and the spleen by flow cytometry. In situ detection of the uterine tissue peri-implantation revealed that IFN-γ treatment also caused statistically significant reductions in forkhead box P3, RAR-related orphan receptor gamma, and IL-17 levels, which indicated local decreases in Treg and Th17 cells at uterine implantation sites. The IFN-γ receptor alpha (IFN-γRα) level was also lowered in the uterus. These results demonstrate that in murine pregnancy, a supraphysiological dose of IFN-γ could induce peri-implantation failure. Moreover, in this study, the decreases in both Treg and Th17-type cells, which may be relevant to the role of IFN-γRα, may be one of the main reasons that IFN-γ causes abortion.
Introduction
Interferon-gamma (IFN-γ) was first identified in phytohemagglutinin-activated lymphocyte supernatants due to the protein's distinctive antiviral activity (Wheelock and others 1965). As a typical type I cytokine, IFN-γ was found in supernatants from the first trimester of pregnancy but was barely detectable in the second trimester and undetectable in the third trimester (Lin and others 1993). The expression of protective immunity against Mycobacterium tuberculosis in mice is mediated by T lymphocytes that secrete cytokines. Among these cytokines, IFN-γ is believed to play a key role (Cooper and others 1993). Previous studies have indicated the existence and positive role of IFN-γ in mammalian pregnancy. In the early 1990s, there were reports of weight loss and a high rate of resorption in IFN-γ-treated mice (Chaouat and others 1990; Mattsson and others 1992). This miscarriage-inducing effect of IFN-γ was both gestational age- and dose-dependent. These observations indicate that IFN-γ may be harmful to normal fetal development. However, the specific mechanism is still unclear.
The fetus is protected by the maternal immune system, rather than rejected as a corpus alienum during pregnancy. The maternal immune system must remodel itself to balance the opposing needs of maintaining adequate immune reactivity to protect both the mother and the fetus from exogenous infections and tolerating the invading fetus to allow healthy development. One elementary step is the establishment of a tolerant microenvironment in early pregnancy when the embryo implants in the uterus, which is the time of peri-implantation (D4–D7 of pregnancy) in mice. Various cells participate in this remodeling program, and Th cells play a key role among these cells.
As the main immunosuppressive cell, regulatory T (Treg) cells are responsible for the establishment of tolerance by modulating the immune responses (Munoz-Suano and others 2011). The specific activity of Treg cells is crucial not only for successful implantation but also in ectopic pregnancy. Even in cases of ovarian endometriosis and ectopic pregnancy, mononuclear immune cells are recruited to the microenvironment of ectopic lesions. Afterward, Treg cells infiltrate the tissue (Basta and others 2010). Arruvito and others (2007) have demonstrated that reproductive failure might result from the inability of Tregs to expand during the preimplantation phase, combined with the cells' lower functional capacity, in women with recurrent spontaneous abortion. These cases highlight the specific activity of Treg cell as crucial for successful implantation.
Beyond Treg cells, another Th subset in the uterus is notable: the newly found Th17 cell, which differs from Treg cells by protecting against autoimmunity and dominating immune tolerance. Th17 cells are believed to be the major proinflammatory cells involved in autoimmunity and are critical for rejection. Treg and Th17 cells are reciprocally regulated by several positive and negative regulatory networks (Jetten 2007). Brigitte Santner-Nanan and Ralph Nanan observed that pre-eclampsia is associated with the absence of normal systemic skewing away from IL-17 production and toward forkhead box P3 (Foxp3+) expression (Santner-Nanan and others 2009). Based on this result, the authors presented the viewpoint that homeostasis between regulatory and proinflammatory CD4+ T cells might be pivotal for the semiallogeneic fetus to be tolerated within the maternal environment (Santner-Nanan and others 2009). There is also evidence that Th17 is pivotal for the induction of the neutrophil-mediated protective immune response against extracellular bacteria and fungal pathogens.
IFN-γ inhibits Th17 cell development in vitro. However, based on the observation of psoriatic blood and lesional skin, IFN-γ programs myeloid antigen-presenting cells (APCs) to induce human IL-17+ T cells via IL-1 and IL-23. IFN-γ also stimulates APC production of CCL20, supporting the migration of IL-17+ T cells and synergizes with IL-17 in the production of human beta-defensin 2, an antimicrobial and chemotactic protein that is highly overexpressed by psoriatic keratinocytes (Kryczek and others 2008). These findings suggest that the relationship between IFN-γ and Th17 cells is more complicated than simple suppression. Th17 cell involvement in IFN-γ-induced miscarriage thus needs further investigation for a deeper understanding of the development of this Th subset.
Previous experimental evidence suggests that IFN-γ exerts its miscarriage-inducing effect in various ways. Both the increased expression of MHC class II antigen (Liu and others 2002; Sun and others 2005) and the promotion of apoptosis at the maternal–fetal interface (Liu and others 2003; Sun and others 2006; Sun and others 2007) may be responsible for the miscarriage caused by IFN-γ. However, these phenomena are still not sufficient to explain the severe miscarriage caused by IFN-γ. Given the severe nature of IFN-γ-induced miscarriage and the important role of immune cells, we speculate that the role of Th cells in miscarriage induced by IFN-γ should not be ignored. The present study was undertaken to investigate another possible mechanism of the IFN-γ activity and to determine whether abnormalities observed in IFN-γ-treated mice are independent of Treg and Th17 cell variations during early pregnancy.
Materials and Methods
Mouse mating strategy and reagent administration
BALB/c mice were obtained from the Experimental Animal Center of the Chinese Academy of Military Medical Sciences. Eight- to 12-week-old female mice were used in all experiments. The mice were housed under conditions of controlled temperature and humidity, with a 12-h/12-h light/dark photoperiod. All of the procedures performed during our study were approved by the Animal Care and Use Committee of the Institute of Zoology, Chinese Academy of Sciences. Female mice were caged overnight with fertile males to induce pregnancy or with vasectomized males of the same strain (2:1). The presence of a vaginal plug by the next morning validated D1 of pregnancy (D1).
Recombinant murine IFN-γ (Catalog #315-05; PeproTech) was administered via the vaginal orifice muscle at 5×103 IU/mouse (5×104 IU/mL, 100 μL per mouse) on D3 of pregnancy. Saline controls were mice that received an injection of saline into the vaginal orifice muscle (100 μL per mouse). These mice were sacrificed on D4, D5, D6, D7, or D8 of pregnancy. Uteri were excised from D4 to D8 pregnancy mice and frozen in liquid nitrogen for further protein analysis.
Isolation of mononuclear cells from the spleen and peripheral blood
Blood was drawn from the eyeballs and diluted with a volume of onefold Hank's solution for mononuclear cell isolation. Isolation was performed using Histopaque®-1083 (Sigma-Aldrich) and centrifugation at 200 g for 30 min. The spleens of the same mice were used for splenocyte isolation. Core needle grinding was used to obtain splenic cells, and splenocytes were also isolated using Histopaque-1083 (Sigma-Aldrich) and centrifugation at 200 g for 30 min. All mice used for embryo counting were also used for cell isolation. Fresh peripheral blood was collected from D4 to D8 of pregnancy for flow cytometry analysis no more than 4 h later. Spleens were excised from D4 to D8 of pregnancy for further flow cytometry analysis, also no more than 4 h later.
Surface and intracellular cytokine staining
Surface staining was performed for 15–20 min with the appropriate cocktail of fluorescently labeled antibodies. After surface staining, the cells were resuspended in the fixation/permeabilization solution (88-8823-88; eBioscience), and intracellular cytokine staining was performed according to the manufacturer's protocol.
Flow cytometry and antibodies
Flow cytometry analysis was performed on FACS Calibur (BD Biosciences) instruments and analyzed using FlowJo software 8.7 (Tree Star, Inc.). All antibodies were purchased from eBioscience. The following are the antibodies used in flow cytometry: anti-CD4 (11-0041; eBioscience), anti-Foxp3 (17-5773; eBioscience), and anti-CD25 (12-0251; eBioscience). All mice used for embryo counting were also used for cell isolation and flow cytometry analysis. At least 3 mice were used for flow cytometry analysis at each time point.
Immunohistochemistry
Frozen sections (8 μm) of mouse uterine implantation sites were mounted on 3-aminopropyltriethoxy-silane-coated slides and fixed in 4% PFA for 10 min. The sections were then washed with phosphate-buffered saline (PBS). Endogenous peroxidase activity was blocked by 0.3% H2O2 for 10 min at room temperature. After blocking in horse serum for 1 h at 37°C, the sections were incubated with primary antibody diluted in PBS overnight at 4°C. Normal rat or rabbit IgG was used as a negative control. After washing thoroughly in PBS, the sections were incubated with secondary antibody diluted in PBS at 37°C for 1 h. The color was developed in situ using diaminobenzidine tetrahydrochloride (Sigma-Aldrich). The sections were counterstained with hematoxylin (Sigma-Aldrich). The following primary and secondary antibodies were used in immunohistochemistry: anti-RORγt (17-6988; eBioscience), anti-Foxp3 (17-5773; eBioscience), anti-IL-17 (sc-7929; Santa Cruz), anti-IFN-γRα (sc-700; Santa Cruz), goat anti-rat IgG (112-035-003; Jackson ImmunoResearch), and goat anti-rabbit IgG (074-1506; KPL).
Western blotting
Mouse uterine protein was extracted from implantation sites by nondenaturing lysis buffer, and protein concentrations were determined by a Bio-Rad Protein Assay. Uterine proteins were separated by 12% SDS-PAGE and electroblotted onto a nitrocellulose membrane (Pall). After blocking in 5% nonfat milk at 37°C for 1 h, the membranes were incubated with primary antibody at 4°C overnight. The membranes were then gently washed in TBST buffer, incubated with the appropriate secondary antibodies at 37°C for 1 h, and thoroughly washed in TBST. A chemiluminescence reaction (Pierce) was performed to test each protein. The bands were analyzed using the Quantity One Analysis System (Bio-Rad).
The following primary and secondary antibodies were used in Western blotting: anti-RORγt (17-6988; eBioscience), anti-Foxp3 (17-5773; eBioscience), anti-IL-17 (sc-7929; Santa Cruz), anti-IFN-γRα (sc-700; Santa Cruz), goat anti-rat IgG (112-035-003; Jackson ImmunoResearch), and goat anti-rabbit IgG (074-1506; KPL).
Statistical analysis
All results were reported as the mean±SEM or the mean±SD. One-way ANOVA or a paired t-test was used to assess the significance of differences. A value of P<0.05 represented statistical significance, and P<0.01 represented sufficient statistical significance. The software used for statistical analysis was SPSS 15.0 (SPSS Software).
Results
The abortion-inducing effect of IFN-γ is most evident on D7 of pregnancy at a dose of 5×103 IU/mouse
Based on our experiments, we found that 5×103 IU/mL IFN-γ administered at 100 μL per mouse via the vaginal orifice muscle is the most effective for inducing abortion.
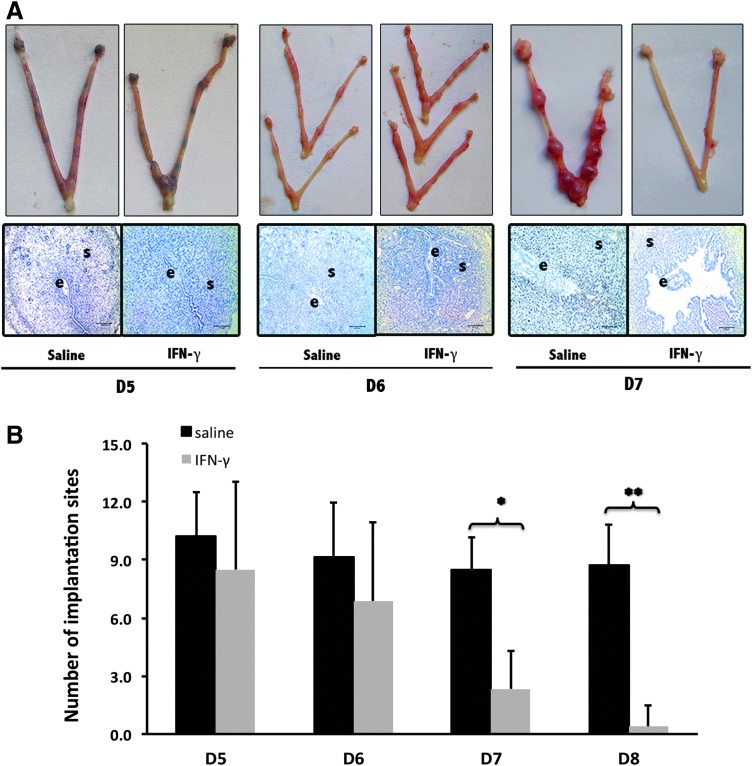
We then affirmed the abortion time following this dose. We analyzed pregnancy outcomes at 4 different time points: D5, D6, D7, and D8 of pregnancy. As indicated in Figure 1, in the group receiving a supraphysiological dose of recombinant murine IFN-γ injection, differences were revealed by both of morphological observation of pregnant uteri and stained slides of implantation sites (Fig. 1A). There was no significant difference in the implantation site numbers between D5 and D6 of pregnancy (Fig. 1). In contrast, a reduction in the number of implantation sites occurred on D7 of pregnancy (Fig. 1). Until D8 of pregnancy, hardly any implantation sites were detected in the uterus following the administration of a supraphysiological dose of recombinant murine IFN-γ (Fig. 2).
FIG. 1.
The miscarriage-inducing effect of interferon-gamma (IFN-γ) in mice peri-implantation (D4–D7 of pregnancy). (A) Overall pregnancy uterine pictures and representative uterine implantation site sections. Trypan blue was administered by mouse tail vein injection to stain the implantation sites of the uterus on D5. The uterine implantation site sections were stained with hematoxylin. The letter e is for embryo and s is for uterine stroma. (B) Histogram of implantation site numbers in the uterus on D5–D7 with the administration of IFN-γ. All results are expressed in terms of the implantation site number in the uterus on different days of pregnancy. The bars represent the SD of the mean. The data at each point were derived from 9 separate samples from pregnant mice. A total of 54 samples from pregnant mice were observed to perform this count. *P<0.05, **P<0.01.
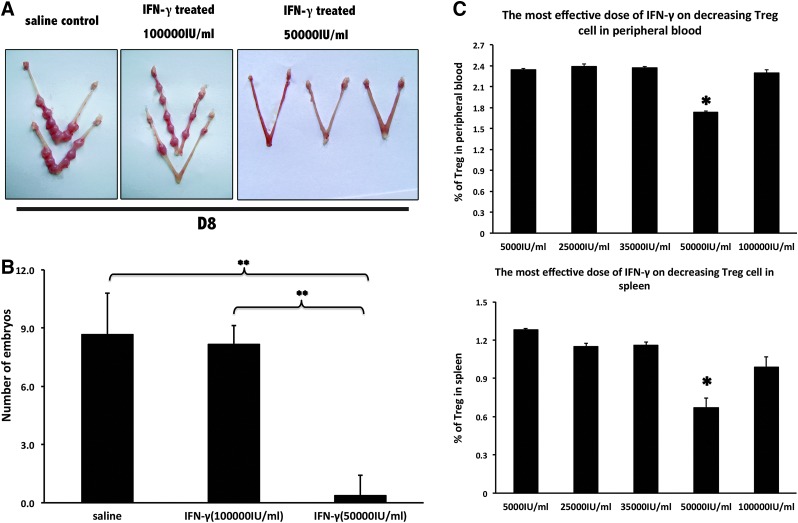
FIG. 2.
Determination of the most effective miscarriage-inducing dose of mIFN-γ. (A) Overall pregnancy uterine pictures on D8 of pregnancy. (B) Histogram of implantation site numbers in the uterus on D8 with the administration of IFN-γ at different doses. The data at each point were derived from at least 7 separate samples from pregnant mice. **P<0.01. (C) Effects of different doses of IFN-γ on the regulatory T (Treg) cell ratios in the peripheral blood and the spleen. All results are expressed in terms of the % of Treg cells (CD4+CD25+Foxp3+) at different IFN-γ doses. The bars represent the SEM. One-way ANOVA was used to assess the significance of differences. *P<0.05. *Also showed significant differences between the dose of 50,000 IU/mL and other doses (5,000, 25,000, 35,000, and 100,000 IU/mL). There was no significant difference between other compared groups (P>0.05).
A higher dose (10,000 IU/mouse) of supraphysiological dose of IFN-γ had no effect on early pregnancy (Fig. 2A,B). In this case, abortion events occurred on D7 of pregnancy. On D8 of pregnancy, the abortion rate of BALB/c×BALB/c mates was significantly augmented compared with normal pregnant mice. Changes in morphology occurred on D6, before fetal loss on D7 (Fig. 1A).
Treg cell numbers are decreased by supraphysiological dose of IFN-γ in the peripheral blood and the spleen of abortion-prone mice
Gradient doses of IFN-γ were tested (5×102 IU/mouse, 2.5×103 IU/mouse, 3.5×103 IU/mouse, 5×103 IU/mouse, and 10×103 IU/mouse), and 5×103 IU/mouse was the most effective dose for inducing abortion. Treg cell ratios in the peripheral blood and the spleen of abortion-prone mice on a gradient dose of IFN-γ were also calculated (Fig. 2C). The most effective dose of IFN-γ for reducing CD4+CD25+Foxp3+ cell numbers in the peripheral blood and the spleen coincided with the most effective abortion-inducing dose of IFN-γ (Fig. 2C). This finding indicates a close connection between the Treg cell role and the pregnancy failure caused by IFN-γ.
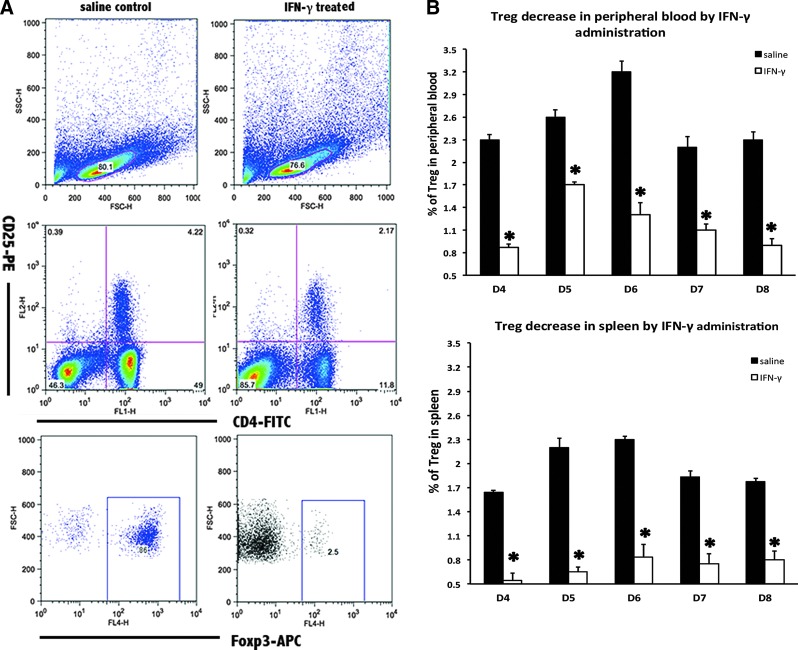
To confirm the role of Treg cells in the pregnancy failure triggered by supraphysiological dose of IFN-γ, flow cytometry was used to analyze the Treg cell ratio in the peripheral blood and the spleen. In the abortion-prone mice, no expansion of the Treg cell subpopulation could be observed in the peripheral blood or the spleen from D4 to D8 of pregnancy (Fig. 3).
FIG. 3.
Treg cell decrease in the peripheral blood and the spleen following IFN-γ injection into mice peri-implantation. (A) Flow cytometry scatterplot of Treg cells. Treg cells are CD4+CD25+Foxp3+. (B) The Treg cell proportion decreased significantly in the peripheral blood and the spleen under the influence of supraphysiological dose of mIFN-γ. The CD4+CD25+Foxp3+ Treg cell ratio was evaluated by flow cytometry analysis. The data are expressed as the percent of CD4+CD25+Foxp3+ triple-positive cells. All mIFN-γ administrations were performed at a dose of 5×104 IU/mL, with 100 μL per mouse. The bars represent the SD of the mean. A paired t-test was used to assess the significance of differences. *P<0.05, IFN-γ treatment group versus the corresponding saline control group.
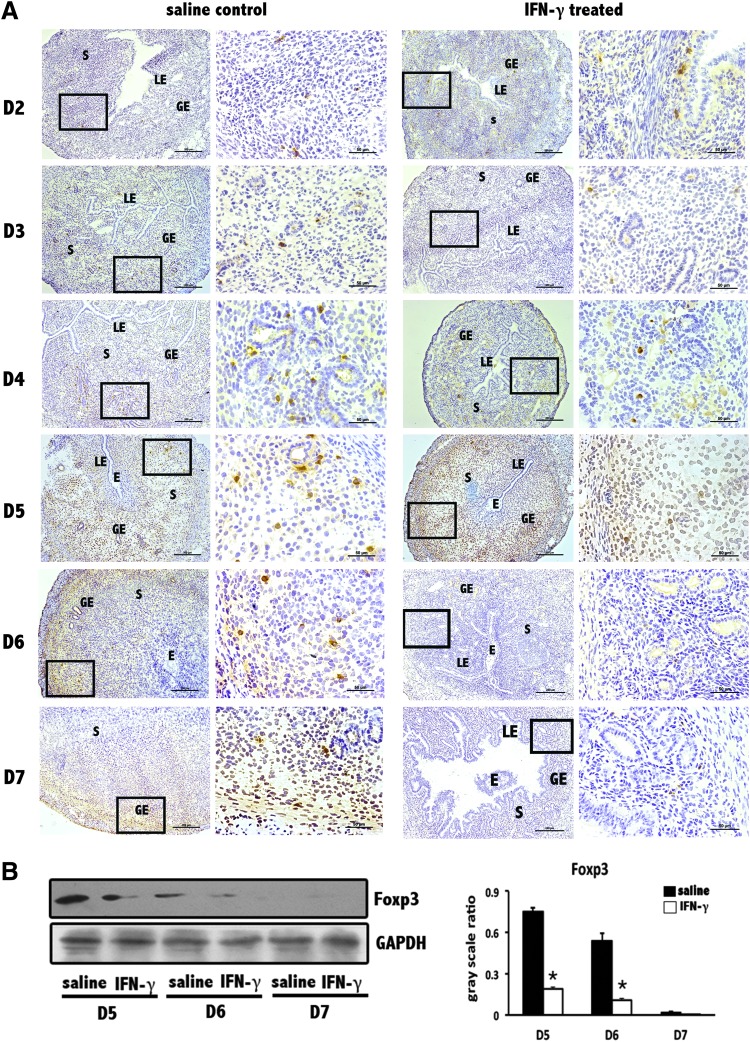
Foxp3+ cell numbers decrease at the implantation sites in abortion-prone mice due to supraphysiological dose of IFN-γ
To gain insight into the unique effect of IFN-γ on Treg cells in the decidua, we performed immunohistochemical localization and Western blotting analyses of Foxp3, the key transcription factor in Treg cells. Compared with the clear staining signal at the normal implantation sites of the saline control group, a less definite signal was observed at the implantation sites of the IFN-γ treatment group (Fig. 4A). Western blotting showed an analogous trend, with a significant decrease in Foxp3 levels at the implantation sites of the IFN-γ treatment group (Fig. 4B). Generalizing the results of Figures 3 and 4, a systemic decrease in Treg cells may be an important reason that supraphysiological doses of IFN-γ lead to severe abortion.
FIG. 4.
Decrease in forkhead box P3 (Foxp3) levels at uterine implantation sites. (A) Foxp3 levels were obviously reduced at implantation sites based on immunohistochemistry. This signal reduction was not obvious on D2 but became more and more remarkable as the pregnancy progresses. Scale bars=200 and 50 μm. At least 3 independent experiments were repeated for each time point. The data at each point were derived from 3 separate samples from pregnant mice. A total of 40 samples from pregnant mice were assessed. (B) Foxp3 levels were significantly reduced at uterine implantation sites based on Western blotting. GAPDH was used as a loading control. The bars represent the SD of the mean of the relative value in gray (Foxp3/GAPDH). A paired t-test was used to assess the significance of differences. *P<0.05, IFN-γ treatment group versus the corresponding saline control group. At least 3 independent experiments were repeated for each time point.
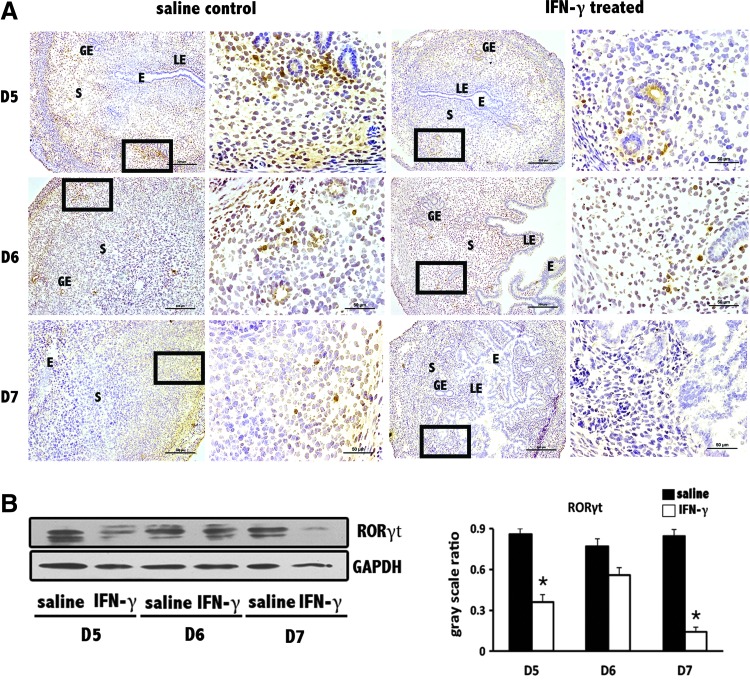
Th17 cells decrease at the implantation sites of abortion-prone mice due to supraphysiological dose of IFN-γ
To investigate whether this Treg cell decrease during IFN-γ-induced abortion is relevant to other lymphocytes, we included Th17 cells, which are reciprocally regulated with Treg cells in many physiological processes, in our study. As observed in Figure 5 A, immunohistochemical localization showed a decrease in levels of RAR-related orphan receptor gamma (RORγt), which is the dominant transcription factor in Th17 cells. This observation was also confirmed by the results of Western blotting (Fig. 5B).
FIG. 5.
Decrease in RAR-related orphan receptor gamma (RORγt) levels at uterine implantation sites. (A) RORγt levels were obviously reduced at implantation sites based on immunohistochemistry. Scale bars=200 and 25 μm. At least 3 independent experiments were repeated for each time point. The data at each point were derived from 3 separate samples from pregnant mice. A total of 18 samples from pregnant mice were assessed. (B) RORγt levels were significantly reduced at uterine implantation sites based on Western blotting. GAPDH was used as a loading control. The bars represent the SD of the mean of the relative value in gray (RORγt/GAPDH). A paired t-test was used to assess the significance of differences. *P<0.05, IFN-γ treatment group versus the corresponding saline control group. At least 3 independent experiments were repeated for each time point.
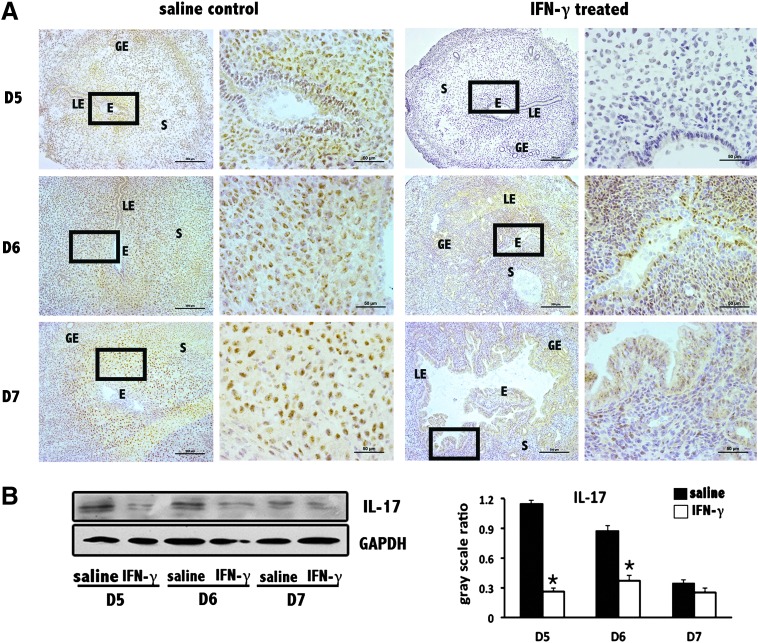
To further confirm Th17 cell functional change in the abortion-prone environment, as the main functional cytokine that Th17 cells secrete, IL-17 was also assessed. Highly similar trends for IL-17 and RORγt at implantation sites were determined by immunohistochemical staining and Western blotting (Fig. 6). One minor difference occurred between the IL-17 and RORγt trends on D7 of pregnancy in the normal controls, which we believe was due to limitations of the test facility and antibodies.
FIG. 6.
Decrease in IL-17 levels at uterine implantation sites. (A) IL-17 levels were obviously reduced at implantation sites based on immunohistochemistry. Scale bars=200 and 50 μm. At least 3 independent experiments were repeated for each time point. The data at each point were derived from 3 separate samples from pregnant mice. A total of 18 samples from pregnant mice were assessed. (B) IL-17 levels were significantly reduced at implantation sites based on Western blotting. GAPDH was used as a loading control. The bars represent the SD of the mean of the relative value in gray (IL-17/GAPDH). A paired t-test was used to assess the significance of differences. *P<0.05, IFN-γ treatment group versus the corresponding saline control group. At least 3 independent experiments were repeated for each time point.
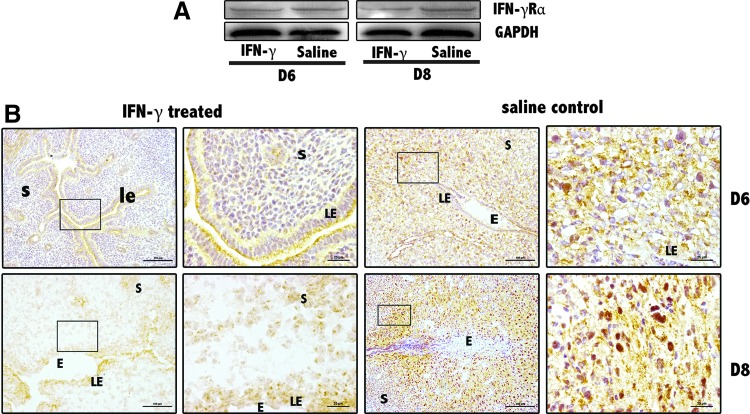
IFN-γ receptor alpha expression decreases at the implantation sites of abortion-prone mice due to supraphysiological dose of IFN-γ
IFN-γ needs to bind to its specific receptor to perform its role. To investigate the possible mechanisms by which IFN-γ regulates Treg and Th17 cells in mice peri-implantation, IFN-γ receptor alpha (IFN-γRα) was also examined by Western blotting and immunohistochemical staining at the implantation sites of the uteri (Fig. 7). Summarizing the detection results, a mIFN-γ dose of 5×103 IU/mouse effectively reduced IFN-γRα expression at uterine implantation sites.
FIG. 7.
Decrease in IFN-γ receptor alpha (IFN-γRα) levels at uterus implantation sites. (A) IFN-γRα levels were significantly reduced at D6 and D8 implantation sites based on Western blotting. GAPDH was used as a loading control. At least 3 independent experiments were repeated for each time point. (B) IFN-γRα levels were obviously reduced at implantation sites based on immunohistochemistry. Scale bars=100 and 25 μm. At least 3 independent experiments were repeated for each time point. The data at each point were derived from 3 separate samples from pregnant mice. A total 12 samples from pregnant mice were assessed.
Discussion
The goals of this study were to explore the mechanism of IFN-γ-induced miscarriage and to determine whether the rapid abortion phenotype caused by exogenous mIFN-γ could be related to Treg or Th17 cells. The apparent abortion phenotype caused by a supraphysiological dose of mIFN-γ can be observed before D7. This fact indicated the time that IFN-γ exerts its effect peri-implantation (D4–D8 of mouse pregnancy). By detecting specific transcription factors and certain cytokines, we revealed that both Treg and Th17 cells are the target cells of IFN-γ during pregnancy, which may be related to the role of the IFN-γ receptor.
In humans, during the implantation period, a significant proportion of embryos are lost, and eventually, less than half of clinically established pregnancies end as full-term pregnancies, without obstetrical complications (Kwak-Kim and others 2010). Although genetic etiologies are often involved in pregnancy losses, a significant proportion of pregnancy losses is related to immunological abnormalities (Kwak-Kim and others 2010). In normal pregnancies in mice, IFN-γ plays critical roles that include the initiation of endometrial vasculature remodeling, the induction of angiogenesis at implantation sites and the maintenance of the decidual (maternal) component of the placenta (Murphy and others 2009). There are also many studies relevant to IFN-γ and pregnancy-related disease. A study including 219 women showed that a severe pre-eclamptic state is associated with high levels of the proinflammatory cytokines IL-8, IL-6, and IFN-γ (Pinheiro and others 2013). Additionally, IFN-γ can modulate placental adiponectin receptors and adiponectin gene expression and secretion (Chen and others 2006). A prominent Th1 response (high IFN-γ levels) to autoantigen stimulation has been observed, especially in babies of T1D fathers and of mothers with perfect diabetes compensation during the third trimester in comparison with control newborns (Stechova and others 2009). Moreover, in a study of pregnant women with a history of miscarriage, the levels of IFN-γ were significantly higher than in the normal pregnant group (Jenkins and others 2000). These patients were all pregnant at the time of sampling, although 50% miscarried later in the first trimester (Jenkins and others 2000).
Given the dose dependence that is characteristic of IFN-γ, to understand the exact role of IFN-γ during early murine pregnancy, it is first necessary to ascertain the most effective supraphysiological dose of IFN-γ for inducing high abortion rates and the exact time that the abortions occur. Vaginal orifice muscle administration was performed to ensure the consistency of local IFN-γ increases in the uterus due to pharmacodynamics. Several gradient doses (5×102 IU/mouse, 2.5×103 IU/mouse, 3.5×103 IU/mouse, 5×103 IU/mouse, and 10×103 IU/mouse) were tested, and 5×103 IU/mouse was most effective in inducing abortion. A higher dose did not lead to a higher abortion rate or more severe miscarriages. In fact, the experimental mice treated with a high dose of IFN-γ showed no symptoms of poisoning and a normal pregnancy outcome (Fig. 2A,B). This finding suggested that the effect of IFN-γ peri-implantation is not due to pure toxicity but due to biological activity. Interestingly, the most effective dose for inducing abortion was also the most effective in reducing CD4+CD25+Foxp3+ Treg cells in the peripheral blood and the spleen (Fig. 2C).
These findings indicate the indispensable role of Treg cells in successful implantation. The crucial role of Treg cells in preventing immunological rejection of the fetus was first proposed after observing a diminished number of and lower function among Treg cells in abortion-prone mice (Zenclussen and others 2006). The adoptive transfer of Treg cells from normal pregnant mice significantly diminished the abortion rate, which further confirmed the protective role of Treg cells in pregnancy (Zenclussen and others 2006). In contrast, CD4+CD25high Treg cell numbers increased in the peripheral blood, and in early pregnant decidua, these Treg cells further increased to 3 times the level found in the peripheral blood in humans (Sasaki and others 2004; Saito and others 2007). Moreover, an expanded Treg cell pool is detectable in lymph nodes draining the uterus from as early as 2 days after mating, whereas elevated blood levels do not become evident until after implantation (Aluvihare and others 2004; Guerin and others 2009). The expansion of Treg cells in pregnancy is even earlier than fetal implantation.
Data from the literature show that IFN-γ is primarily produced by natural killer (NK) cells and thymus-derived natural killer T cells (Hanna and others 2006). Uterine NK (uNK) cells are the most common lymphocytes at the implantation site and are constitutively present throughout the female reproductive system, representing ∼10%–30% of total leukocytes. In the endometrium, this level increases up to 70% during the fertile phase (Munoz-Suano and others 2011). Nevertheless, based on new research and our observation of Treg cells during gestation, we speculate that Treg cells are more likely than uNK cells to lead immune system remodeling initiation for successful pregnancy (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/jir).
Despite years of intense research, what we know about the establishment of tolerance mechanisms in pregnancy is still limited. To explain the complexity of the immune microenvironment of the uterus, beyond the classic “Th1/Th2 balance” described in 1986, another important paradigm (“Treg/Th17”) was proposed to be more important for immune remodeling in early pregnancy in recent years (Supplementary Fig. S2). Given a degree of similarity between chronic inflammation and pregnancy, interest in Th17 cells in pregnancy has emerged. In contrast to the identified importance of Treg cells, research findings on Th17 cells in pregnancy are far from enough. We only know that Th17 cells are necessary for pregnancy (Santner-Nanan and others 2009; Wang and others 2010; Nakashima and others 2010a, 2010b). The significant cytokine that these cells secrete, IL-17, may participate in the invasion of trophoblasts (Pongcharoen and others 2006). Despite the lack of data, Th17 cells have successfully attracted the interest of scientists, several reviews have been published (Firestein 2003; Korn and others 2007; Weaver and others 2007; Dong 2008), and reports on Th17 cells in the reproductive system are accumulating. Additionally, a newly identified family of heterogeneous cell subsets, named ILCs, that are developmentally related and evolutionarily conserved has recently been described (Liu and others 2012). ILCs are mostly enriched in mucosal tissues and are important for innate protection against infectious microorganisms, lymphoid tissue formation, tissue remodeling, and the homeostasis of tissue stromal cells (Liu and others 2012). RORγt is the key transcription factor in Th17 cells and is also essential for the development of RORγt1 ILCs (Eberl and others 2004).
In contrast to previous projections, the balance between Treg and Th17 cells is not simply disturbed in IFN-γ-induced miscarriage; the decrease in both of these Th subsets may be responsible. Here, we provide what we believe is new evidence indicating that increased IFN-γ levels are directly associated with an altered uterine immune microenvironment peri-implantation, which is correlated with remarkably reduced frequency of Treg and Th17 cells at uterine implantation sites. The role of the ligand-dependent transcription factor aryl hydrocarbon receptor in regulating Treg and Th17 cell generation (Quintana and others 2008) provides us with new clues for understanding the development of these 2 Th subsets. We hope that this result supplies useful data regarding the development of Th17 cells during reproduction and is helpful for understanding the complex role of IFN-γ in both the induction and the regulation of immune remodeling processes.
Spontaneous abortion is strongly associated with an increase in the activity of the maternal immune system. Many other factors could also cause spontaneous abortion, such as viral infection, embryonic developmental defects, and maternal disease. This type of abortion most often occurs in the middle or late trimester of pregnancy, whereas the abortion caused by IFN-γ in our study rapidly and strenuously occurred in the early trimester of pregnancy, during the peri-implantation period. It is important to note that only cases of IFN-γ-induced abortions in early pregnancy at a specific effective dose were discussed here. The phenomenon is largely due to the downregulation of the IFN-γ receptor. IFN-γ binds to its cell surface receptor to interfere with viral infections. This IFN-γ receptor is composed of 2 chains, IFN-γRα and IFN-γRβ. The decrease in IFN-γRα helps us to understand the way in which IFN-γ plays its role in early pregnancy. We must admit that the decrease in Treg and Th17 cells occurred in an artificially established miscarriage environment, which was similar but not equal to naturally occurring pregnancy failure. Compared with the scored resorbing fetoplacental units on D12 or D14 of pregnancy (Chaouat and others 1995) in naturally occurring pregnancy failure cases, hardly some visible resorbing units were detected in the IFN-γ-induced abortions in our study. However, the abnormal structure of the implantation sites and the contents were clearly visible in histological sections of the drug-treated uterus, which can be traced back to early pregnancy, on D6. That time is much earlier than full placenta formation on D9, and angiogenesis begins on D7. These phenomena suggest that the uterine microenvironment was not yet adapted to healthy embryonic implantation and subsequent development at the time of mIFN-γ administration, not to mention angiogenesis or placenta formation.
More clinical evidence is needed to understand immune cell behavior in healthy pregnancy. Furthermore, lymphocyte behavior in other situations, such as ectopic pregnancy and pre-eclampsia, still needs further study.
We hope that this basic laboratory work will lead to more alarm regarding the utilization of IFN-γ as a therapeutic in the clinic. We also hope that further characterization of IFN-γ functions and IFN-γ-mediated Th subset interactions will provide insights into the involvement of these cells in several gynecological conditions, including pre-eclampsia, ectopic pregnancy, endometrial cancers, and recurrent miscarriages.
Supplementary Material
Acknowledgments
This work was financially supported through grants from the National Basic Research Program of China (No. 2011CB944402), the National Natural Science Foundation of China (No. 31171435), the National Key Technology R&D Program (2012BAI31B07), and the Knowledge Innovation Program of the Chinese Academy of Sciences (KSCX2-EW-R-06).
Author Disclosure Statement
No competing financial interests exist in this article.
References
- Aluvihare VR, Kallikourdis M, Betz AG. 2004. Regulatory T cells mediate maternal tolerance to the fetus. Nat Immunol 5(3):266–271 [DOI] [PubMed] [Google Scholar]
- Arruvito L SM, Banham AH, Fainboim L. 2007. Expansion of CD4+CD25+ and FOXP3+ regulatory T cells during the follicular phase of the menstrual cycle: implications for human reproduction. J Immunol 178(4):2572–2578 [DOI] [PubMed] [Google Scholar]
- Basta P, Majka M, Jozwicki W, Lukaszewska E, Knafel A, Grabiec M, Stasienko E, Wicherek L. 2010. The frequency of CD25+CD4+ and FOXP3+ regulatory T cells in ectopic endometrium and ectopic decidua. Reprod Biol Endocrinol 8:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaouat G, Assal Meliani A, Martal J, Raghupathy R, Elliott JF, Mosmann T, Wegmann TG. 1995. IL-10 prevents naturally occurring fetal loss in the CBA X DBA/2 mating combination, and local defect in IL-10 production in this abortion-prone combination is corrected by in vivo injection of IFN-tau. J Immunol 154(9):4261–4268 [PubMed] [Google Scholar]
- Chaouat G, Menu E, Clark DA, Dy M, Minkowski M, Wegmann TG. 1990. Control of fetal survival in CBA X DBA/2 mice by lymphokine therapy. J Reprod Fertil 89(2):447–458 [DOI] [PubMed] [Google Scholar]
- Chen J, Tan B, Karteris E, Zervou S, Digby J, Hillhouse EW, Vatish M, Randeva HS. 2006. Secretion of adiponectin by human placenta: differential modulation of adiponectin and its receptors by cytokines. Diabetologia 49(6):1292–1302 [DOI] [PubMed] [Google Scholar]
- Cooper AM, Dalton DK, Stewart TA, Griffin JP, Russell DG, Orme IM. 1993. Disseminated tuberculosis in interferon-gamma gene-disrupted mice. J Exp Med 178(6):2243–2247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong C. 2008. TH17 cells in development: an updated view of their molecular identity and genetic programming. Nat Rev Immunol 8(5):337–348 [DOI] [PubMed] [Google Scholar]
- Eberl G, Marmon S, Sunshine MJ, Rennert PD, Choi Y, Littman DR. 2004. An essential function for the nuclear receptor RORgamma(t) in the generation of fetal lymphoid tissue inducer cells. Nat Immunol 5(1):64–73 [DOI] [PubMed] [Google Scholar]
- Firestein GS. 2003. Evolving concepts of rheumatoid arthritis. Nature 423(6937):356–361 [DOI] [PubMed] [Google Scholar]
- Guerin LR, Prins JR, Robertson SA. 2009. Regulatory T-cells and immune tolerance in pregnancy: a new target for infertility treatment? Hum Reprod Update 15(5):517–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna J, Goldman-Wohl D, Hamani Y, Avraham I, Greenfield C, Natanson-Yaron S, Prus D, Cohen-Daniel L, Arnon TI, Manaster I, Gazit R, Yutkin V, Benharroch D, Porgador A, Keshet E, Yagel S, Mandelboim O. 2006. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat Med 12(9):1065–1074 [DOI] [PubMed] [Google Scholar]
- Jenkins C, Roberts J, Wilson R, MacLean MA, Shilito J, Walker JJ. 2000. Evidence of a T(H)1 type response associated with recurrent miscarriage. Fertil Steril 73(6):1206–1208 [DOI] [PubMed] [Google Scholar]
- Jetten. 2007. Retinoid-related orphan receptors (RORs): critical roles in development, immunity, circadian rhythm, and cellular metabolism. Nucl Recept Signal 7:e003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn T, Oukka M, Kuchroo V, Bettelli E. 2007. Th17 cells: Effector T cells with inflammatory properties. Semin Immunol 19(6):362–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kryczek I, Bruce AT, Gudjonsson JE, Johnston A, Aphale A, Vatan L, Szeliga W, Wang Y, Liu Y, Welling TH, Elder JT, Zou WP. 2008. Induction of IL-17(+) T cell trafficking and development by IFN-gamma: mechanism and pathological relevance in psoriasis. J Immunol 181(7):4733–4741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak-Kim J, Park JC, Ahn HK, Kim JW, Gilman-Sachs A. 2010. immunological modes of pregnancy loss. Am J Reprod Immunol 63(6):611–623 [DOI] [PubMed] [Google Scholar]
- Lin H, Mosmann TR, Guilbert L, Tuntipopipat S, Wegmann TG. 1993. Synthesis of T-helper 2-type cytokines at the maternal-fetal interface. J Immunol 151(9):4562–4573 [PubMed] [Google Scholar]
- Liu J, Liu S, Cao X. 2012. Highlights of the advances in basic immunology in 2011. Cell Mol Immunol 9(3):197–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Chen Y, Yang Y, Peng JP. 2002. The effect on MHC class II expression and apoptosis in placenta by IFN-gamma administration. Contraception 65(2):177–184 [DOI] [PubMed] [Google Scholar]
- Liu Z, Sun Q-H, Yang Y, Liu J-M, Peng J-P. 2003. Effect of IFNγ on caspase-3, Bcl-2 and Bax expression, and apoptosis in rabbit placenta. Cytokine 24(5):201–209 [DOI] [PubMed] [Google Scholar]
- Mattsson R, Mattsson A, Holmdahl R, Scheynius A, Vandermeide PH. 1992. In vivo treatment with interferon-gamma during early-pregnancy in mice induces strong expression of major histocompatibility complex class-I and class-II molecules in uterus and decidua but not in extraembryonic tissues. Biol Reprod 46(6):1176–1186 [DOI] [PubMed] [Google Scholar]
- Munoz-Suano A, Hamilton AB, Betz AG. 2011. Gimme shelter: the immune system during pregnancy. Immunol Rev 241(1):20–38 [DOI] [PubMed] [Google Scholar]
- Murphy SP, Tayade C, Ashkar AA, Hatta K, Zhang J, Croy BA. 2009. Interferon gamma in successful pregnancies. Biol Reprod 80(5):848–859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima A, Ito M, Shima T, Bac ND, Hidaka T, Saito S. 2010a. Accumulation of IL-17-positive cells in decidua of inevitable abortion cases. Am J Reprod Immunol 64(1):4–11 [DOI] [PubMed] [Google Scholar]
- Nakashima A, Ito M, Yoneda S, Shiozaki A, Hidaka T, Saito S. 2010b. Circulating and decidual Th17 cell levels in healthy pregnancy. Am J Reprod Immunol 63(2):104–109 [DOI] [PubMed] [Google Scholar]
- Pinheiro MB, Martins-Filho OA, Mota AP, Alpoim PN, Godoi LC, Silveira AC, Teixeira-Carvalho A, Gomes KB, Dusse LM. 2013. Severe preeclampsia goes along with a cytokine network disturbance towards a systemic inflammatory state. Cytokine 62(1):165–173 [DOI] [PubMed] [Google Scholar]
- Pongcharoen S, Niumsup P, Sanguansermsri D, Supalap K, Butkhamchot P. 2006. The effect of interleukin-17 on the proliferation and invasion of JEG-3 human choriocarcinoma cells. Am J Reprod Immunol 55(4):291–300 [DOI] [PubMed] [Google Scholar]
- Quintana FJ, Basso AS, Iglesias AH, Korn T, Farez MF, Bettelli E, Caccamo M, Oukka M, Weiner HL. 2008. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature 453(7191):65–71 [DOI] [PubMed] [Google Scholar]
- Saito S, Shiozaki A, Sasaki Y, Nakashima A, Shima T, Ito M. 2007. Regulatory T cells and regulatory natural killer (NK) cells play important roles in feto-maternal tolerance. Semin Immunopathol 29(2):115–122 [DOI] [PubMed] [Google Scholar]
- Santner-Nanan B, Peek MJ, Khanam R, Richarts L, Zhu E, Fazekas de St Groth B, Nanan R. 2009. Systemic increase in the ratio between Foxp3+ and IL-17-producing CD4+ T cells in healthy pregnancy but not in preeclampsia. J Immunol 183(11):7023–7030 [DOI] [PubMed] [Google Scholar]
- Sasaki Y SM, Miyazaki S, Higuma S, Shiozaki A, Saito S. 2004. Decidual and peripheral blood CD4+CD25+ regulatory T cells in early pregnancy subjects and spontaneous abortion cases. Mol Hum Reprod 10:347–353 [DOI] [PubMed] [Google Scholar]
- Stechova K, Spalova I, Durilova M, Bartaskova D, Cerny M, Cerna M, Pithova P, Chudoba D, Stavikova V, Ulmannova T, Faresjo M. 2009. Influence of maternal hyperglycaemia on cord blood mononuclear cells in response to diabetes-associated autoantigens. Scand J Immunol 70(2):149–158 [DOI] [PubMed] [Google Scholar]
- Sun QH, Peng JP, Xia HF. 2006. IFNgamma pretreatment sensitizes human choriocarcinoma cells to etoposide-induced apoptosis. Mol Hum Reprod 12(2):99–105 [DOI] [PubMed] [Google Scholar]
- Sun QH, Peng JP, Xia HF, Yang Y. 2007. IFN-gamma promotes apoptosis of the uterus and placenta in pregnant rat and human cytotrophoblast cells. J Interferon Cytokine Res 27(7):567–578 [DOI] [PubMed] [Google Scholar]
- Sun QH, Peng JP, Xia HF, Yang Y, Liu ML. 2005. Effect on expression of RT1-A and RT1-DM molecules of treatment with interferon-gamma at the maternal—fetal interface of pregnant rats. Hum Reprod 20(9):2639–2647 [DOI] [PubMed] [Google Scholar]
- Wang WJ, Hao CF, Yi L, Yin GJ, Bao SH, Qiu LH, Lin QD. 2010. Increased prevalence of T helper 17 (Th17) cells in peripheral blood and decidua in unexplained recurrent spontaneous abortion patients. J Reprod Immunol 84(2):164–170 [DOI] [PubMed] [Google Scholar]
- Weaver CT, Hatton RD, Mangan PR, Harrington LE. 2007. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol 25:821–852 [DOI] [PubMed] [Google Scholar]
- Wheelock EF. 1965. Interferon-like virus-inhibitor induced in human leukocytes by phytohemagglutinin. Science 149(3681):310–311 [DOI] [PubMed] [Google Scholar]
- Zenclussen AC, Gerlof K, Zenclussen ML, Ritschel S, Zambon Bertoja A, Fest S, Hontsu S, Ueha S, Matsushima K, Leber J, Volk HD. 2006. Regulatory T cells induce a privileged tolerant microenvironment at the fetal-maternal interface. Eur J Immunol 36(1):82–94 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.