Abstract
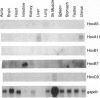
Smooth muscle cell plasticity is considered a prerequisite for atherosclerosis and restenosis following angioplasty and bypass surgery. Identification of transcription factors that specify one smooth muscle cell phenotype over another therefore may be of major importance in understanding the molecular basis of these vascular disorders. Homeobox genes exemplify one class of transcription factors that could govern smooth muscle cell phenotypic diversity. Accordingly, we screened adult and fetal human smooth muscle cell cDNA libraries with a degenerate oligonucleotide corresponding to a highly conserved region of the homeodomain with the idea that homeobox genes, if present, would display a smooth muscle cell phenotype-dependent pattern of expression. No homeobox genes were detected in the adult human smooth muscle cell library; however, five nonparalogous homeobox genes were uncovered from the fetal library (HoxA5, HoxA11, HoxB1, HoxB7, and HoxC9). Northern blotting of adult and fetal tissues revealed low and restricted expression of all five homeobox genes. No significant differences in transcripts of HoxA5, HoxA11, and HoxB1 were detected between adult or fetal human smooth muscle cells in culture. HoxB7 and HoxC9, however, showed preferential mRNA expression in fetal human smooth muscle cells that appeared to correlate with the age of the donor. This phenotype-dependent expression of homeobox genes was also noted in rat pup versus adult smooth muscle cells. While similar differences in gene expression have been reported between subsets of smooth muscle cells from rat vessels of different-aged animals or clones of rat smooth muscle, our findings represent a demonstration of a transcription factor distinguishing two human smooth muscle cell phenotypes.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauters C., de Groote P., Adamantidis M., Delcayre C., Hamon M., Lablanche J. M., Bertrand M. E., Dupuis B., Swynghedauw B. Proto-oncogene expression in rabbit aorta after wall injury. First marker of the cellular process leading to restenosis after angioplasty? Eur Heart J. 1992 Apr;13(4):556–559. doi: 10.1093/oxfordjournals.eurheartj.a060213. [DOI] [PubMed] [Google Scholar]
- Bochaton-Piallat M. L., Gabbiani F., Gabbiani G. Heterogeneity of rat aortic smooth muscle cell replication during development: correlation with replicative activity after experimental endothelial denudation in adults. J Submicrosc Cytol Pathol. 1994 Jan;26(1):1–8. [PubMed] [Google Scholar]
- Bodner M., Castrillo J. L., Theill L. E., Deerinck T., Ellisman M., Karin M. The pituitary-specific transcription factor GHF-1 is a homeobox-containing protein. Cell. 1988 Nov 4;55(3):505–518. doi: 10.1016/0092-8674(88)90037-2. [DOI] [PubMed] [Google Scholar]
- Campbell G. R., Campbell J. H. The phenotypes of smooth muscle expressed in human atheroma. Ann N Y Acad Sci. 1990;598:143–158. doi: 10.1111/j.1749-6632.1990.tb42286.x. [DOI] [PubMed] [Google Scholar]
- Candia A. F., Hu J., Crosby J., Lalley P. A., Noden D., Nadeau J. H., Wright C. V. Mox-1 and Mox-2 define a novel homeobox gene subfamily and are differentially expressed during early mesodermal patterning in mouse embryos. Development. 1992 Dec;116(4):1123–1136. doi: 10.1242/dev.116.4.1123. [DOI] [PubMed] [Google Scholar]
- Cook C. L., Weiser M. C., Schwartz P. E., Jones C. L., Majack R. A. Developmentally timed expression of an embryonic growth phenotype in vascular smooth muscle cells. Circ Res. 1994 Feb;74(2):189–196. doi: 10.1161/01.res.74.2.189. [DOI] [PubMed] [Google Scholar]
- Cserjesi P., Lilly B., Bryson L., Wang Y., Sassoon D. A., Olson E. N. MHox: a mesodermally restricted homeodomain protein that binds an essential site in the muscle creatine kinase enhancer. Development. 1992 Aug;115(4):1087–1101. doi: 10.1242/dev.115.4.1087. [DOI] [PubMed] [Google Scholar]
- Desmoulière A., Gabbiani G. The cytoskeleton of arterial smooth muscle cells during human and experimental atheromatosis. Kidney Int Suppl. 1992 Jun;37:S87–S89. [PubMed] [Google Scholar]
- Gehring W. J., Affolter M., Bürglin T. Homeodomain proteins. Annu Rev Biochem. 1994;63:487–526. doi: 10.1146/annurev.bi.63.070194.002415. [DOI] [PubMed] [Google Scholar]
- Giachelli C. M., Bae N., Almeida M., Denhardt D. T., Alpers C. E., Schwartz S. M. Osteopontin is elevated during neointima formation in rat arteries and is a novel component of human atherosclerotic plaques. J Clin Invest. 1993 Oct;92(4):1686–1696. doi: 10.1172/JCI116755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giachelli C. M., Majesky M. W., Schwartz S. M. Developmentally regulated cytochrome P-450IA1 expression in cultured rat vascular smooth muscle cells. J Biol Chem. 1991 Feb 25;266(6):3981–3986. [PubMed] [Google Scholar]
- Giachelli C., Bae N., Lombardi D., Majesky M., Schwartz S. Molecular cloning and characterization of 2B7, a rat mRNA which distinguishes smooth muscle cell phenotypes in vitro and is identical to osteopontin (secreted phosphoprotein I, 2aR). Biochem Biophys Res Commun. 1991 Jun 14;177(2):867–873. doi: 10.1016/0006-291x(91)91870-i. [DOI] [PubMed] [Google Scholar]
- Gordon D., Reidy M. A., Benditt E. P., Schwartz S. M. Cell proliferation in human coronary arteries. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4600–4604. doi: 10.1073/pnas.87.12.4600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorski D. H., LePage D. F., Patel C. V., Copeland N. G., Jenkins N. A., Walsh K. Molecular cloning of a diverged homeobox gene that is rapidly down-regulated during the G0/G1 transition in vascular smooth muscle cells. Mol Cell Biol. 1993 Jun;13(6):3722–3733. doi: 10.1128/mcb.13.6.3722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall K. L., Harding J. W., Hosick H. L. Isolation and characterization of clonal vascular smooth muscle cell lines from spontaneously hypertensive and normotensive rat aortas. In Vitro Cell Dev Biol. 1991 Oct;27A(10):791–798. doi: 10.1007/BF02631245. [DOI] [PubMed] [Google Scholar]
- Hedin U., Holm J., Hansson G. K. Induction of tenascin in rat arterial injury. Relationship to altered smooth muscle cell phenotype. Am J Pathol. 1991 Sep;139(3):649–656. [PMC free article] [PubMed] [Google Scholar]
- Ingraham H. A., Albert V. R., Chen R. P., Crenshaw 3d E. B., Elsholtz H. P., He X., Kapiloff M. S., Mangalam H. J., Swanson L. W., Treacy M. N. A family of POU-domain and Pit-1 tissue-specific transcription factors in pituitary and neuroendocrine development. Annu Rev Physiol. 1990;52:773–791. doi: 10.1146/annurev.ph.52.030190.004013. [DOI] [PubMed] [Google Scholar]
- Ingraham H. A., Chen R. P., Mangalam H. J., Elsholtz H. P., Flynn S. E., Lin C. R., Simmons D. M., Swanson L., Rosenfeld M. G. A tissue-specific transcription factor containing a homeodomain specifies a pituitary phenotype. Cell. 1988 Nov 4;55(3):519–529. doi: 10.1016/0092-8674(88)90038-4. [DOI] [PubMed] [Google Scholar]
- Jonasson L., Bondjers G., Hansson G. K. Lipoprotein lipase in atherosclerosis: its presence in smooth muscle cells and absence from macrophages. J Lipid Res. 1987 Apr;28(4):437–445. [PubMed] [Google Scholar]
- Jones F. S., Chalepakis G., Gruss P., Edelman G. M. Activation of the cytotactin promoter by the homeobox-containing gene Evx-1. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2091–2095. doi: 10.1073/pnas.89.6.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komuro I., Izumo S. Csx: a murine homeobox-containing gene specifically expressed in the developing heart. Proc Natl Acad Sci U S A. 1993 Sep 1;90(17):8145–8149. doi: 10.1073/pnas.90.17.8145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemire J. M., Covin C. W., White S., Giachelli C. M., Schwartz S. M. Characterization of cloned aortic smooth muscle cells from young rats. Am J Pathol. 1994 May;144(5):1068–1081. [PMC free article] [PubMed] [Google Scholar]
- Lewis E. B. A gene complex controlling segmentation in Drosophila. Nature. 1978 Dec 7;276(5688):565–570. doi: 10.1038/276565a0. [DOI] [PubMed] [Google Scholar]
- Lints T. J., Parsons L. M., Hartley L., Lyons I., Harvey R. P. Nkx-2.5: a novel murine homeobox gene expressed in early heart progenitor cells and their myogenic descendants. Development. 1993 Oct;119(2):419–431. doi: 10.1242/dev.119.2.419. [DOI] [PubMed] [Google Scholar]
- Majesky M. W., Benditt E. P., Schwartz S. M. Expression and developmental control of platelet-derived growth factor A-chain and B-chain/Sis genes in rat aortic smooth muscle cells. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1524–1528. doi: 10.1073/pnas.85.5.1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majesky M. W., Giachelli C. M., Reidy M. A., Schwartz S. M. Rat carotid neointimal smooth muscle cells reexpress a developmentally regulated mRNA phenotype during repair of arterial injury. Circ Res. 1992 Oct;71(4):759–768. doi: 10.1161/01.res.71.4.759. [DOI] [PubMed] [Google Scholar]
- Majesky M. W., Lindner V., Twardzik D. R., Schwartz S. M., Reidy M. A. Production of transforming growth factor beta 1 during repair of arterial injury. J Clin Invest. 1991 Sep;88(3):904–910. doi: 10.1172/JCI115393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majesky M. W., Reidy M. A., Bowen-Pope D. F., Hart C. E., Wilcox J. N., Schwartz S. M. PDGF ligand and receptor gene expression during repair of arterial injury. J Cell Biol. 1990 Nov;111(5 Pt 1):2149–2158. doi: 10.1083/jcb.111.5.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maulbecker C. C., Gruss P. The oncogenic potential of deregulated homeobox genes. Cell Growth Differ. 1993 May;4(5):431–441. [PubMed] [Google Scholar]
- Mendel D. B., Crabtree G. R. HNF-1, a member of a novel class of dimerizing homeodomain proteins. J Biol Chem. 1991 Jan 15;266(2):677–680. [PubMed] [Google Scholar]
- Miano J. M., Vlasic N., Tota R. R., Stemerman M. B. Localization of Fos and Jun proteins in rat aortic smooth muscle cells after vascular injury. Am J Pathol. 1993 Mar;142(3):715–724. [PMC free article] [PubMed] [Google Scholar]
- Mikawa T., Fischman D. A. Retroviral analysis of cardiac morphogenesis: discontinuous formation of coronary vessels. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9504–9508. doi: 10.1073/pnas.89.20.9504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. P., McGehee R. E., Jr, Habener J. F. IDX-1: a new homeodomain transcription factor expressed in rat pancreatic islets and duodenum that transactivates the somatostatin gene. EMBO J. 1994 Mar 1;13(5):1145–1156. doi: 10.1002/j.1460-2075.1994.tb06363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelken N. A., Soifer S. J., O'Keefe J., Vu T. K., Charo I. F., Coughlin S. R. Thrombin receptor expression in normal and atherosclerotic human arteries. J Clin Invest. 1992 Oct;90(4):1614–1621. doi: 10.1172/JCI116031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelken N. A., Soifer S. J., O'Keefe J., Vu T. K., Charo I. F., Coughlin S. R. Thrombin receptor expression in normal and atherosclerotic human arteries. J Clin Invest. 1992 Oct;90(4):1614–1621. doi: 10.1172/JCI116031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien E. R., Garvin M. R., Stewart D. K., Hinohara T., Simpson J. B., Schwartz S. M., Giachelli C. M. Osteopontin is synthesized by macrophage, smooth muscle, and endothelial cells in primary and restenotic human coronary atherosclerotic plaques. Arterioscler Thromb. 1994 Oct;14(10):1648–1656. doi: 10.1161/01.atv.14.10.1648. [DOI] [PubMed] [Google Scholar]
- Okamoto E., Imataka K., Fujii J., Kuro-o M., Nakahara K., Nishimura H., Yazaki Y., Nagai R. Heterogeneity in smooth muscle cell population accumulating in the neointimas and the media of poststenotic dilatation of the rabbit carotid artery. Biochem Biophys Res Commun. 1992 May 29;185(1):459–464. doi: 10.1016/s0006-291x(05)81007-1. [DOI] [PubMed] [Google Scholar]
- Orlandi A., Ehrlich H. P., Ropraz P., Spagnoli L. G., Gabbiani G. Rat aortic smooth muscle cells isolated from different layers and at different times after endothelial denudation show distinct biological features in vitro. Arterioscler Thromb. 1994 Jun;14(6):982–989. doi: 10.1161/01.atv.14.6.982. [DOI] [PubMed] [Google Scholar]
- Patel C. V., Gorski D. H., LePage D. F., Lincecum J., Walsh K. Molecular cloning of a homeobox transcription factor from adult aortic smooth muscle. J Biol Chem. 1992 Dec 25;267(36):26085–26090. [PubMed] [Google Scholar]
- Rogina B., Coelho C. N., Kosher R. A., Upholt W. B. The pattern of expression of the chicken homolog of HOX1I in the developing limb suggests a possible role in the ectodermal inhibition of chondrogenesis. Dev Dyn. 1992 Jan;193(1):92–101. doi: 10.1002/aja.1001930112. [DOI] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993 Apr 29;362(6423):801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- Scheidereit C., Cromlish J. A., Gerster T., Kawakami K., Balmaceda C. G., Currie R. A., Roeder R. G. A human lymphoid-specific transcription factor that activates immunoglobulin genes is a homoeobox protein. Nature. 1988 Dec 8;336(6199):551–557. doi: 10.1038/336551a0. [DOI] [PubMed] [Google Scholar]
- Schwartz S. M., Campbell G. R., Campbell J. H. Replication of smooth muscle cells in vascular disease. Circ Res. 1986 Apr;58(4):427–444. doi: 10.1161/01.res.58.4.427. [DOI] [PubMed] [Google Scholar]
- Schwartz S. M., Foy L., Bowen-Pope D. F., Ross R. Derivation and properties of platelet-derived growth factor-independent rat smooth muscle cells. Am J Pathol. 1990 Jun;136(6):1417–1428. [PMC free article] [PubMed] [Google Scholar]
- Schwartz S. M., Heimark R. L., Majesky M. W. Developmental mechanisms underlying pathology of arteries. Physiol Rev. 1990 Oct;70(4):1177–1209. doi: 10.1152/physrev.1990.70.4.1177. [DOI] [PubMed] [Google Scholar]
- Scott M. P., Tamkun J. W., Hartzell G. W., 3rd The structure and function of the homeodomain. Biochim Biophys Acta. 1989 Jul 28;989(1):25–48. doi: 10.1016/0304-419x(89)90033-4. [DOI] [PubMed] [Google Scholar]
- Seifert R. A., Schwartz S. M., Bowen-Pope D. F. Developmentally regulated production of platelet-derived growth factor-like molecules. Nature. 1984 Oct 18;311(5987):669–671. doi: 10.1038/311669a0. [DOI] [PubMed] [Google Scholar]
- Singh G., Kaur S., Stock J. L., Jenkins N. A., Gilbert D. J., Copeland N. G., Potter S. S. Identification of 10 murine homeobox genes. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10706–10710. doi: 10.1073/pnas.88.23.10706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stary H. C., Blankenhorn D. H., Chandler A. B., Glagov S., Insull W., Jr, Richardson M., Rosenfeld M. E., Schaffer S. A., Schwartz C. J., Wagner W. D. A definition of the intima of human arteries and of its atherosclerosis-prone regions. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation. 1992 Jan;85(1):391–405. doi: 10.1161/01.cir.85.1.391. [DOI] [PubMed] [Google Scholar]
- Taubman M. B. Tissue factor regulation in vascular smooth muscle: a summary of studies performed using in vivo and in vitro models. Am J Cardiol. 1993 Sep 9;72(8):55C–60C. doi: 10.1016/0002-9149(93)90256-c. [DOI] [PubMed] [Google Scholar]
- Zanellato A. M., Borrione A. C., Tonello M., Scannapieco G., Pauletto P., Sartore S. Myosin isoform expression and smooth muscle cell heterogeneity in normal and atherosclerotic rabbit aorta. Arteriosclerosis. 1990 Nov-Dec;10(6):996–1009. doi: 10.1161/01.atv.10.6.996. [DOI] [PubMed] [Google Scholar]
- Zhao G. Q., Zhou X., Eberspaecher H., Solursh M., de Crombrugghe B. Cartilage homeoprotein 1, a homeoprotein selectively expressed in chondrocytes. Proc Natl Acad Sci U S A. 1993 Sep 15;90(18):8633–8637. doi: 10.1073/pnas.90.18.8633. [DOI] [PMC free article] [PubMed] [Google Scholar]