Abstract
Natural killer (NK) cells act as innate immune cells against hepatitis C virus (HCV) infection. Interferon-α (IFN-α) and ribavirin are the standard treatments for patients with HCV infection. This study is aimed at investigating the dynamic changes in the frequency of different subsets of NK cells following treatment in xx chronic hepatitis C (CHC) patients. CHC patients were treated with peg-IFN or IFN-α, and followed up for 72 weeks. The frequency of different subsets of NK in CHC patients was determined longitudinally by flow cytometry. Treatment with the standard therapy increased the percentages of NKp30+, NKp46+, and CD107a+ NK cells, which were positively correlated with the declining of serum HCV-RNA, but not IFN-γ+ NK cells. NKG2A+ and KIR2DL3+ NK cells were associated with an early virological response in CHC patients. Treatment with IFN-α adjusts the balance of activated receptors and inhibitory receptors and enhances the cytotoxic activity of NK cells. Therefore, measuring NK subsets may be valuable for therapeutic responses in CHC patients.
Introduction
Hepatitis C virus (HCV) infection remains a serious concern and affects an estimated 130 million people worldwide (Ahlenstiel and others 2011). Because of the lack of available vaccines for the prevention of HCV spreading, the infection rate is increasing worldwide. After exposure to HCV, about 70%–80% of people progress into chronic hepatitis C (CHC). More importantly, many patients with CHC eventually develop cirrhosis and hepatocellular carcinoma, the major causes of end-stage liver disease. It is well known that liver injury and CHC progression are dependent on host immunity (Ahlenstiel and others 2010; Amadei and others 2010; Beziat and others 2010). Although antigen-specific T cells are crucial for the clearance of HCV, other immunocompetent cells also play an important role in disease progression and control. Therefore, the discovery of the role of other immunocompetent cells, such as natural killer (NK) cells, in the pathogenesis and therapeutic response is of great significance (Amadei and others 2010; Beziat and others 2010).
Currently, combination therapy of pegylated interferon (Peg-IFN) or IFN-α with antiviral ribavirin is a standard protocol for the treatment of patients with CHC in the clinic (Bonorino and others 2009). Its therapeutic efficacy is regulated by several factors, including the levels of viral loads, the genotype of HCV with which the patients infected, gender, age, and the status of immune system. The sustained virological response (SVR) rate is only nearly 40%–45% in patients with genotype 1 HCV infection (Chiesa and others 2005; Brook and others 2010). However, how treatment with IFN could modulate the frequency of NK cells in CHC patients is poorly understood.
NK cells are innate immune cells and play important roles in the defense against viral infection. In humans, NK cells are usually defined as CD3−CD56+ lymphocytes and comprised about 5%–20% of peripheral blood lymphocytes (Cooper and others 2001; Dessouki and others 2010). However, the frequency of NK cells in intrahepatic lymphocytes can reach about 30%–50% (Dominguez-Villar and others 2012). NK cells are a diverse population and their function depends on the expression of activating and inhibitory receptors that recognize various classes of cell surface ligands (Dunn and others 2007). Furthermore, the function of NK cells is regulated positively by proinflammatory cytokines, but negatively by inhibitory cytokines and Tregs (Edlich and others 2012). NK cells can be cytotoxic to their sensitive target cells through perforin-containing cytotoxic granules (Gao and others 2008), and NK cells can also induce target cell apoptosis through surface expression of tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) or FasL (Hadziyannis and others 2004). CD56bright NK cells do not express CD16 and account for about 10% of the peripheral blood NK cell population, and they are considered the main producers of cytokines and TRAIL (Harrison and others 2010). In addition, NK cells may secrete cytokines, such as IFN-γ and TNF-α, or through cell contact to promote target cell apoptosis (Hoofnagle and Seeff 2006). Hence, the degranulation of NK cells is the hallmark of NK cell activation (Brook and others 2010).
Previous studies have shown that following HCV infection, the frequency of NK cells increases, suggesting that NK cells contribute to the initial viral containment (Khakoo and others 2004; Joncker and others 2009; Knapp and others 2010). Furthermore, NK cells appear to be associated with liver injury in CHC patients (Lin and others 2004; Miyagi and others 2007; Lavanchy 2009; McHutchison and others 2009). Earlier studies have reported that treatment with IFN-α2b increases NK cell cytotoxicity and the percentage of perforin+ lymphocytes (Morishima and others 2006), particularly in those with SVR (Nattermann and others 2006). Recent studies suggest that the effect of IFN treatment on the frequency, cytotoxicity, cytokine production, and receptor expression in NK cells remains controversial (Norris and others 1998; Par and others 2006; Joncker and others 2009; Oliviero and others 2009). However, there is no information on the dynamic changes in the frequency, cytotoxicity, cytokine production, and receptor expression in NK cells in Chinese patients with CHC following treatment with IFN and antiviral therapy.
In this study, we recruited a group of CHC patients to examine the frequency of different subsets of NK cells and their function longitudinally following treatment with interferon-α (IFN-α) and antiviral ribavirin, as well as in gender- and age-matched healthy subjects. We found that CHC patients had a lower frequency of NK cells that were dysfunctional and that the standard therapy transiently modulated NK cell function. Characterization of different subsets of NK cells revealed that a high frequency of activating receptor-expressing NK cells and a low percentage of inhibitory receptor-expressing NK cells were associated with liver damage in CHC patients. The IFN-α therapy transiently enhanced NK cell cytotoxicity and triggered NK cell activation. Our findings may provide new insights into understanding the therapeutic effect of antiviral therapy in CHC patients.
Patients and Methods
Patients and healthy subjects
A total of 35 patients with CHC and 13 gender- and age-matched healthy subjects were recruited at the outpatient clinic of the Department of Hepatology of the First Hospital of Jilin University, China, from February 2010 to June 2011. Individual patients with CHC were diagnosed, according to the criteria of detectable HCV virions and positive anti-HCV antibodies as well as clinical symptoms for 6 months (Pelletier and others 2010). Individuals were excluded if she/he had a history of hepatitis B and hepatitis D, positive antibodies against HBV and HDV, human immunodeficiency virus (HIV), or other inflammatory diseases, such as rheumatoid arthritis, diabetes, autoimmune hepatitis, hypertension, kidney disease, or recent infectious diseases. Written informed consent was obtained from all subjects, and the experimental protocol was approved by the Ethics Committee of the First Hospital of Jilin University.
Treatment
Individual patients with CHC were randomized and treated with 15 mg/kg of body weight/day of ribavirin for 12 months plus 180 mg Peg-IFNα-2a (Roche, Basel, Switzerland) weekly or 5×106 IU IFN-α2b (Cain Biotechnology, Beijing, China) every other day for 48 weeks. All the patients met the most recent criteria of the European guideline for treatment of CHC (Pelletier and others 2010) and 24 of them were followed up for 72 weeks. Peripheral blood samples were obtained from individual participants before and after the treatment longitudinally.
Viral genotyping, viral load, and other biochemical measurements
The HCV genotyping was performed by quantitative polymerase chain reaction (PCR) using the specific kit (iQur®, Southampton, United Kingdom), according to the manufacturer's instruction. The concentrations of plasma anti-HCV antibodies were determined by enzyme-linked immunosorbent assay (ELISA). The virus loads in individual plasma samples were measured by quantitative PCR using HCV COBAS Amplicor Monitor version 2.0 (Roche, Burgess Hill, Sussex, United Kingdom), and the limitation of detection for HCV was 15 IU/mL. The levels of serum aspartate aminotransferase (AST) and alanine transaminase (ALT) in individual subjects were detected by Biochemistry Automatic Analyzer (Roche Diagnostics, Branchburg, NJ).
Flow cytometry analysis
To determine the percentages of different subsets of NK cells, anticoagulated blood samples were incubated for 30 min with a cocktail of allophycocyanin (APC)-conjugated CD56 (clone B159), fluorescence isothiocyanate (FITC)-conjugated CD3 (clone UCHT1), and PerCP-Cy™5.5-conjugated CD16 (clone 3G8), and stained with PE-conjugated antibodies against CD158a (clone HP-3E4), CD158b (clone CH-L), NKp30 (clone P30-15), NKp44 (clone p44-8.1), NKp46 (clone 9E2/Nkp46; BD PharMigen, San Diego, CA), NKB1 (clone DX9; BD Biosciences, Erembodegem, Belgium), KIR2DL3 (clone 180701), NKG2D-PE (clone 149810), NKG2A (clone 131411), NKG2C (clone 134591; R&D Systems, St. Paul, MN), TRAIL-PE (clone 550516), FasL-PE (clone 12-9919; eBioscience, San Diego, CA), or isotope controls, respectively. After lysis of red blood cells, the frequency of different subsets of NK cells was characterized by flow cytometry analysis.
The CD107a+ NK cells represent degranulated NK cells (Post and others 2009). To determine the frequency of degranulated NK cells, PBMCs were directly stimulated with K562 cells (E:T=10:1) in RPMI 1640 in 6-well plates for 1 h. The cells were exposed to anti-CD107a in the presence of GolgiStop for another 5 h. Subsequently, the cells were stained with anti-CD3 and anti-CD56, fixed, and permeabilized, followed by staining with anti-IFN-γ. The degranulated and IFN-γ-secreting NK cells were characterized by flow cytometry analysis.
Statistical analysis
Data are expressed as mean values of individual subjects or median and range. The difference between 2 independent groups was analyzed by the Mann–Whitney U-test, and the difference between paired variables by the Wilcoxon matched-pair test using the Prism 5.0 (GraphPad Software, La Jolla, CA). The potential correlation between 2 variables was analyzed by the Spearman's rank correlation coefficient test. A 2-tailed P value of less than 0.05 was considered statistically significant.
Result
Alteration in the frequency of NKp30+ and NKG2C+ NK cells is associated with liver damage in CHC patients
To determine the frequency of NK cells, 35 patients with CHC and 13 healthy subjects were recruited. As expected, there was no significant difference in the distribution of gender and age between CHC patients and healthy subjects, and CHC patients displayed high concentrations of ALT and HCV loads (Table 1). Characterization of HCV genotypes indicated 19 patients infected with genotype 1b HCV, 10 with genotype 2a, and others with neither genotypes 1b nor 2a.
Table 1.
The Characteristics of the Study Population
| Health controls (n=18) | CHC (n=35) | Peg-IFN group (n=13) | IFN group (n=22) | |
|---|---|---|---|---|
| Age, years (mean±SEM) | 43.6±8.8 | 46.3±8.6 | 44.6±8.5 | 47.7±8.2 |
| Gender (female/male) | 8/10 | 14/21 | 7/6 | 7/15 |
| ALT, IU/mL (median [IQ range]) | NA | 42.5 (12–268) | 35 (12–268) | 50.4 (23–171) |
| HCV-RNA (mean log10 IU/mL±SEM) | NA | 6.3±0.89 | 6.41±0.67 | 6.22±1.02 |
| HCV genotype 1b/2a/other | NA | 19/10/6 | 6/4/3 | 13/6/3 |
HCV, hepatitis C virus; CHC, chronic hepatitis C; Peg-IFN, pegylated interferon.
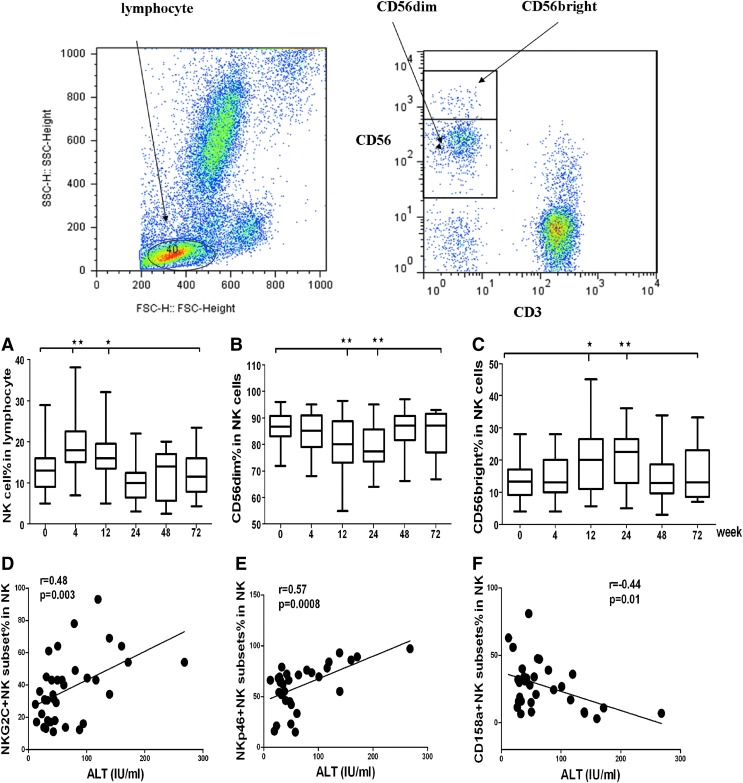
To characterize optimal changes in NK cell phenotype and function, we initially focused on the 24 patients who finished 48 weeks treatment and were followed up to 72 weeks. NK cells were identified as CD3−CD56+ cells by multicolor flow cytometry after gating on single cells (forward scatter height versus forward scatter area) and lymphocytes (forward scatter versus side scatter). For a detailed NK phenotype analysis, PBMCs were stained for activating and inhibitory NK cell receptors, characterization of different subsets of NK cells indicated that after treatment with interferon and antiviral ribavirin. As shown in Figure 1A, the frequency of NK cells in the blood lymphocyte population increased during the early stage (12 week) of therapy. This was paralleled by an increase in CD56bright (Fig. 1C) cells, but not CD56dim cells. Furthermore, CD56bright cells increased further in week 48 (P=0.009) and were maintained at that level for 72 weeks. Interestingly, the concentrations of plasma ALT in CHC patients were positively correlated with the frequency of NKp30+ and NKG2C+ NK cells, but were negatively correlated with the frequency of CD158a+ NK cells in CHC patients (Fig. 1A, B). These data indicated that alteration in the frequency of activating receptor+ cells was associated with liver damage in CHC patients and indicated its function to clear HCV.
FIG. 1.
The dynamic changes of natural killer (NK) cell subsets during treatment. NK subsets were determined by flow cytometry analysis using specific antibodies. Data shown are representative charts of flow cytometry analysis of some subsets of NK cells. In 12 weeks of treatment, total NK cells increased and CD56bright+ cells also increased; furthermore, NKG2C+ and NKp30+ NK were a positive correlation with the level of serum ALT. (A) The dynamic change of total NK cells during the course of treatment. (B) The dynamic change of CD56dim+ NK cells during the course of treatment. (C) The dynamic change of CD56bright+ NK cells during the course of treatment. (D) The correlation between NKG2C+ NK cells and the level of serum ALT. (E) The correlation between NKp30+ NK cells and the level of serum ALT. (F) The correlation between CD158a+ NK cells and the level of serum ALT (*P<0.5; **P<0.01).
Treatment with IFN-α and ribavirin modulates NK subsets in CHC patients
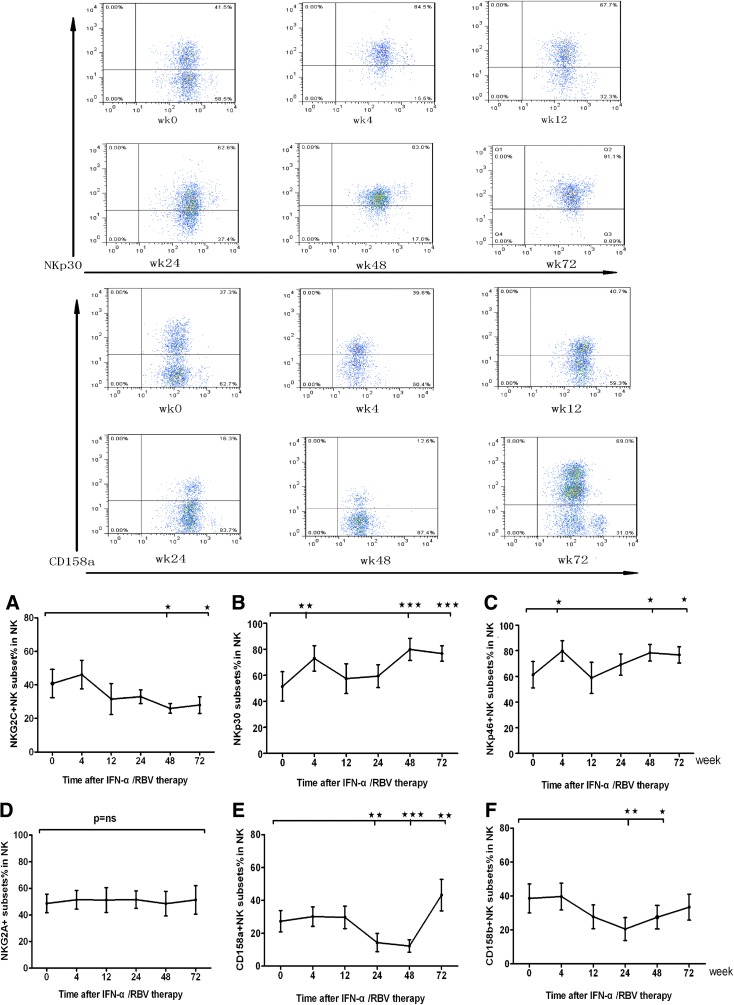
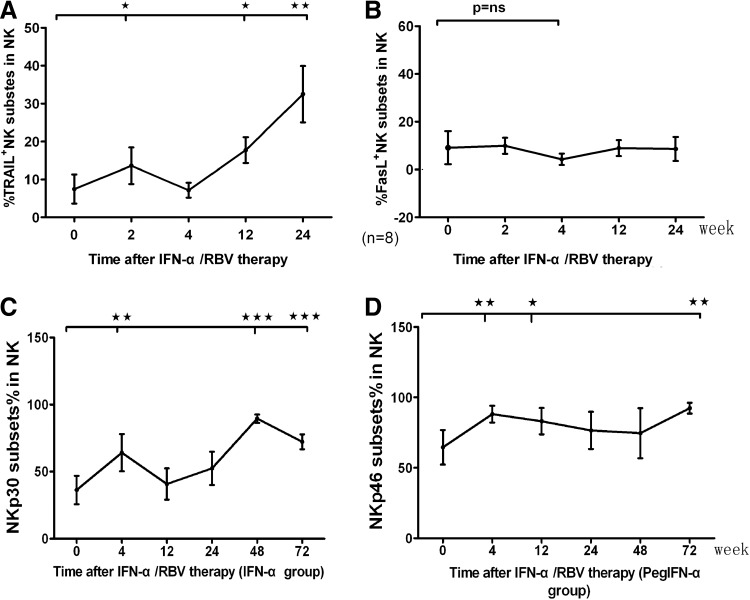
Given that different subsets of NK cells have different functions, we characterized the frequency of different subsets of NK cells longitudinally after treatment. For example, the expression level of NKp30+ and NKp46+ NK cells peaked as early as 4 weeks after initiation of therapy (Fig. 2B, C) and there were significant changes in those cells during the last 48 and 72 weeks of therapy (Fig. 2B, C), specially there were bulk NKp30+ NK cells at terminal treatment (Fig. 2B). Whereas the NKG2C+ subsets (Fig. 2A) were slightly downregulated (P<0.05) and CD158a+ and CD158b+ NK cells decreased significantly at 24 and 48 weeks (Fig. 2D, E). Surprisingly, CD158a+ NK cells increased significantly at 24 weeks after the end of treatment. The percentage of NKp30+ NK cells increased after standard IFN-α therapy, instead of NKp46+ NK cells in the Peg-IFN-α group. Another 8 patients were followed to 24 weeks, PBMCs were stained for TRAIL and FasL, and TRAIL+ NK cells gradually increased after treatment, which reached a peak at 24 weeks; the IFN-α treatment had no effect on FasL+ NK cells (Fig. 3A, B).
FIG. 2.
The dynamic changes of NK cell receptors during treatment. Furthermore, the cells were stained with APC anti-CD56 and PE-conjugated antibodies against CD158a or CD158b, NKp30, NKp44, NKp46, NKB1, KIR2DL3, NKG2D, NKG2A, NKG2C, or isotope controls, respectively, for characterizing the frequency of different subsets of NK cells. The frequency of activation receptor+ and inhibitory receptor+ NK cells in chronic hepatitis C (CHC) patients (n=24) and healthy subjects (n=13) was determined by flow cytometry analysis. We displayed FACS of NKp30 and CD158a during the treatment from baseline to 72 weeks. (A–C) The dynamic changes of NK cell activating receptors during the course of treatment. (D–F) The dynamic changes of NK cell inhibitory receptors during the course of treatment (*P<0.5; **P<0.01; ***P<0.001).
FIG. 3.
The dynamic changes of NK cell subsets during treatment. Eight patients were followed up to 24 weeks to assess the frequency of TRAIL+ or FasL+ NK cells. Anglicizing affect of different IFN on NK cells, to divide patients into 2 groups [standard interferon-α (IFN-α) groups and pegylated interferon (Peg-IFN-α) groups] and observe the changes of NK receptors. (A, B) The dynamic change of apoptosis-related ligands on NK cells during the treatment. (C, D) The dynamic change of NK cell activating receptors during the course of treatment in different groups (*P<0.5; **P<0.01; ***P<0.001).
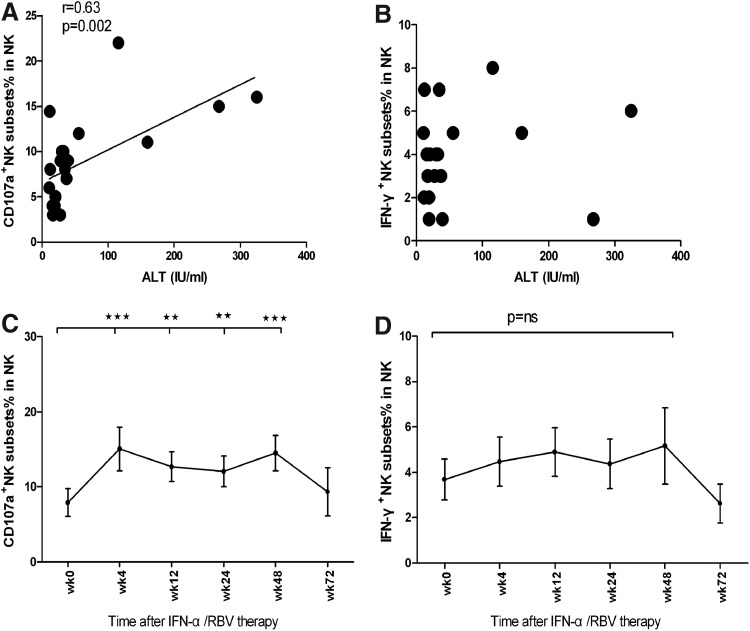
The NK cells reduced the antiviral effects by killing infected hepatocytes and by producing antiviral and immunomodulatory cytokines, such as IFN-γ. To evaluate changes in NK cell cytotoxicity during therapy, we incubated PBMCs with the major histocompatibility complex-negative target K562 and measured degranulation by staining CD107a on the cell surface. A significant increase in CD107a+ subsets was observed within 4 weeks of treatment and was maintained for 48 weeks. (Fig. 4C) Inversely, the frequency of IFN-γ-secreting NK cells in CHC patients was significantly lower than that in healthy controls (data not shown), treatment failed to significantly modulate the frequency of IFN-γ-secreting NK cells. In the correlation analysis, we found that the level of the serum ALT associated with degranulation. These data clearly indicated that treatment with interferon and ribavirin modulated the frequency and function of NK cells, contributing to the inhibition of HCV replication and liver damage in CHC patients.
FIG. 4.
The dynamic change of NK cell functions during the course of treatment. Individual patients were treated with interferon and the frequency of NK and activated NK cells were characterized by flow cytometry analysis. PBMCs separated from patients were incubated with K562 cells, then marked CD107a and IFN-γ to determine the functions. (A) The correlation between CD107a+ NK cell and the level of serum ALT. (B) The correlation between IFN-γ+ NK cell and the level of serum ALT. (C) The dynamic change of CD107a+ NK cells. (D) The dynamic change of IFN-γ+ NK cells (*P<0.5; **P<0.01; ***P<0.001).
Effective viral load reduction upon IFN-α treatment results in increased frequencies of activated NK cells and degranulation
After the combination of interferon and ribavirin treatment, viral loads substantially decreased in most hepatitis C patients at 4 weeks. Accordingly, the treatment remarkably increased the frequency of NKp30+ and NKp46+ NK cells at 4 weeks postinitial treatment in CHC patients. We investigated a possible relationship between the early NK cell response and first-phase virological response to treatment, which is predictive of a SVR.
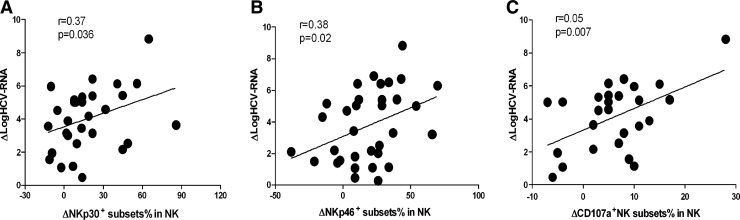
Interestingly, we found that the therapy-induced decrease in HCV-RNA strongly correlated with the therapy-induced change in NKp30-positive NK cells, the same with NKp46-positive NK cells. (Fig. 5A, B). At 4 weeks treatment, the viral load dropped most significantly, and we also found that the function of NK cell degranulating was stronger at 4 weeks, therefore, in the correlation analysis, we found that viral load decline associated with degranulation (Fig. 5C).
FIG. 5.
The effect of treatment with IFN on the frequency of activation receptor+ NK cells and affected functions of NK cells in CHC patients. The potential correlation of the net increases in the frequency of NKp30+ and NKp46+ NK cells with the net increases in the amount of hepatitis C virus (HCV) loads in CHC patients at 4 weeks post-treatment was analyzed. Data are expressed as mean values of individual patients at different time points post-treatment. (A) Spearman's rank correlation coefficient between the therapy-induced change in logHCV-RNA and the change in NKp30-positive NK cells was determined. Change was defined as the absolute difference between percentages at baseline and 4 weeks. ΔlogHCV-RNA=logHCV-RNAbaseline−logHCV-RNA4 week; ΔNKp30+NK cell%%=NKp30+NK cell%4 week−NKp30+NK cell%baseline. (B) Spearman's rank correlation coefficient between the therapy-induced change in logHCV-RNA and the change in NKp46-positive NK cells was determined. Change was defined as the absolute difference between percentages at baseline and 4 weeks. ΔlogHCV-RNA=logHCV-RNAbaseline−logHCV-RNA4 week; ΔNKp46+NK cell%%=NKp46+NK cell%4 week−NKp46+NK cell%baseline. (C) Spearman's rank correlation coefficient between the therapy-induced decrease in logHCV-RNA and increase in CD107a-positive NK cells was determined at 4 weeks. ΔlogHCV-RNA=logHCV-RNAbaseline−logHCV-RNA4 week; ΔCD107A+NK cell%=CD107A+NK cell%4 week−CD107A+NK cell%baseline.
A higher frequency of inhibitory receptor+ NK cell at baseline is associated with early virological response in CHC patients
The response of individuals to therapy was evaluated, according to the European guideline criteria for treatment of CHC. Individuals with no detectable HCV RNA (<50 IU/mL) for 24 weeks postinitial treatment were defined as SVR; with no datable HCV RNA within 4 weeks postinitial treatment and maintained up to end of treatment as rapid virological response (RVR); with positive detection of HCV RNA at 4 weeks, but not at 12 weeks, and maintenance up to end of treatment as early virological response (EVR). There were 3 patients with RVR in the Peg-IFNα-2a group, while there were 4 patients with RVR in the IFN-α2b group. There were 6 patients with EVR in the Peg-IFNα-2a group and 8 patients in the IFN-α2b group. There were 4 patients with SVR in the Peg-IFNα-2a group, while there were 6 patients with SVR in the IFN-α2b group.
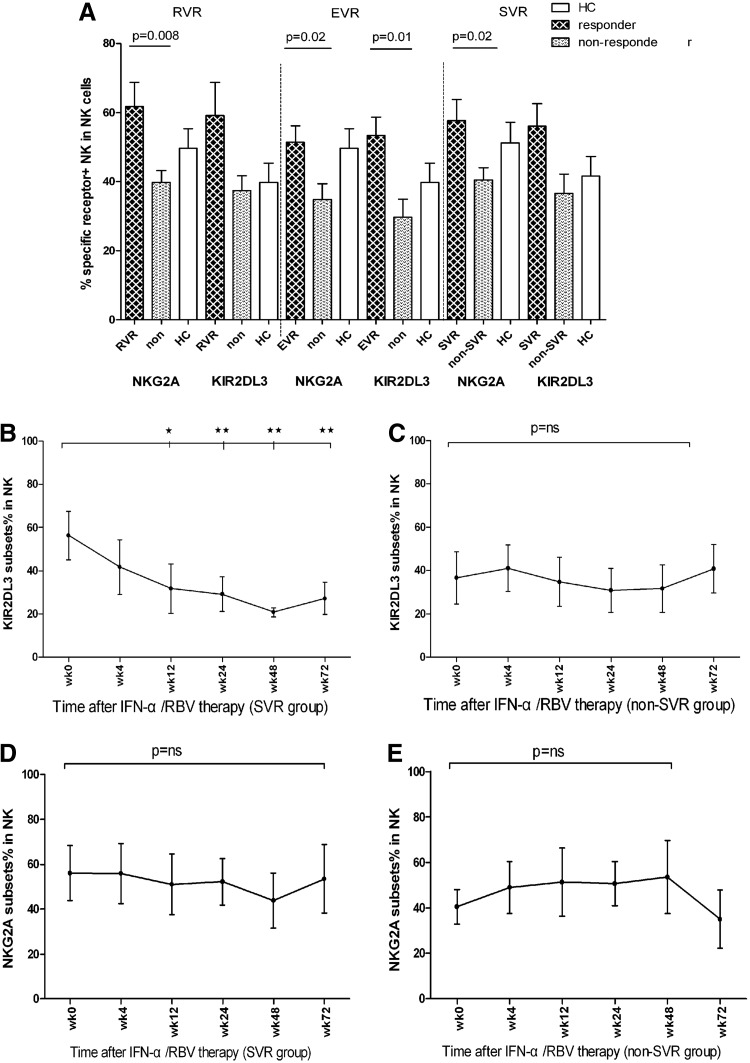
The RVR, EVR, or SVR of CHC patients to therapy is crucial for the control of disease progression (Pelletier and others 2010). We further stratified the patients into the responder and nonresponder groups and characterized the frequency of different subsets of NK cells by flow cytometry analysis (Fig. 6). We found that the frequency of NKG2A+NK cells in the RVR patients was significantly higher than that in the non-RVR patients, while the percentage of KIR2DL3+ NK cells in the RVR patients was slightly higher than that in the non-RVR patients.
FIG. 6.
The association of the frequency of inhibitory receptor+ NK cells with the sustained virological response (SVR) in CHC patients. Following treatment with IFN, according to the respond for treatment, these patients were stratified into the rapid virological response (RVR), early virological response (EVR) or SVR, non-RVR, non-EVR, or non-SVR groups, and their percentages of activation and inhibitory receptor+ NK cells were analyzed. Data are expressed as mean values of individual patients at the indicated time points post-treatment. (A) Comparing inhibitory receptors on NK cell in responders with that in nonresponders; (B, C) the dynamic changes in the percentages of KIR2DL3+ NK cells in the SVR and non-SVR groups of patients; (D, E) the dynamic changes in the percentages of NKG2A+ NK cells in the SVR and non-SVR group of patients (*P<0.5; **P<0.01).
Similarly, stratification of patients into the EVR and non-EVR groups and the frequency of NKG2A+ and KIR2DL3+ NK cells in the EVR patients were significantly higher than that in the non-EVR patients. Longitudinal analysis indicated that the frequency of KIR2DL3+, but not NKG2A+, NK cells was reduced in the SVR patients at 72 weeks postinitial treatment. Collectively, these data suggest that an early higher frequency of inhibitory receptor+ NK cells is associated with EVR in CHC patients to interferon and ribavirin therapy.
Discussion
This study demonstrates a long-term follow-up between NK cell responsiveness, and the whole process (72 weeks) of virological response, as well as the longitudinal (72 weeks) virological response of IFN-α-based therapy.
We examined the frequency of different subsets of NK cells in patients with CHC following standard therapy. We found that CD56birght+ NK cells were significantly elevated after initial treatment, the related research results consistent with ours. In the correlation study, we also found that the NKp30+ and NKG2C+ NK cells and the ALT levels were positively correlated. Hence, standard therapy increased the frequency of NKp30+ and NKp46+ NK cells, but decreased the frequency of CD158a+ and CD158b+ NK cells later posttreatment. More importantly, the increased percentages of NKp30+ and NKp46+ NK cells were positively correlated with the reduced levels of HCV loads in CHC patients early after treatment. These data are consistent with a previous report in CHB patients (Rehermann and Nascimbeni 2005). Standard IFN-α triggered NKp30+ NK cells increasing, but Peg-IFN-α triggered NKp46+ NK cells. These data suggest that NKp30+ and NKp46+ NK cells are crucial for the clearance of HCV in CHC patients and that the frequency of peripheral blood NKp30+ and NKp46+ NK cells may be a valuable biomarker for the evaluation of HCV clearance in CHC patients.
IFN-α is a regulator of NK cell activity (Smyth and others 2005) and the only cytokines currently used for the treatment of viral hepatitis. Previous studies have shown controversial results on the frequency and function of NK cells in CHC patients following standard therapies (Rehermann and Nascimbeni 2005; Miyagi and others 2007; McHutchison and others 2009). We found that treatment with standard therapy increased the frequency and degranulation of NK cells, but not IFN-γ+ NK cells, in CHC patients postinitial treatment. Previous studies showed that an increased TRAIL expression on NK cells had been associated with flares in chronic hepatitis B (Stegmann and others 2010). A recent study gave further evidence that TRAIL expression was inversely correlated with HCV-RNA levels during the early phase of PEG-IFN-α therapy in vitro (Tjwa and others 2011). We found that TRAIL+ NK cells reached a burst at the sixth month during treatment (Fig. 3A). Consistent with our results, recent data show that interferon activates NK cells early after treatment is initiated, and their cytotoxic functions strongly related to the virologic response (Trobonjaca and others 2002; Tjwa and others 2011). Given that treatment with the interferon therapy significantly reduced the levels of plasma HCV loads and serum ALT, the higher frequency of NK cells and activated NK cells suggested that activated NK cells participated in the clearance of HCV in CHC patients.
Previous studies have shown that IFN-γ can inhibit HCV replication in human hepatocytes in vitro, which can be abrogated by anti-IFN-γ antibodies (Post and others 2009), and that a high frequency of IFN-γ-producing T cells is associated with HCV clearance in patients recovered from acute HCV infection (Ahlenstiel and others 2010). We found that treatment with the standard therapy did not significantly modulate the frequency of IFN-γ+ NK cells, consistent with a previous report that the responses of CHC patients to standard therapy are not associated with an increase in the frequency of peripheral HCV-specific IFN-γ-secreting T cells in CHC patients (Rehermann and Nascimbeni 2005). The low frequency of IFN-γ-secreting activated NK cells may reflect a rapid migration of activated NK cells into the liver in CHC patients or stem from the high susceptibility of activated NK cells to apoptosis. Alternatively, the low frequency of IFN-γ-secreting NK cells may be because high doses of IFN-α promote high levels of STAT1 activation and IFN-α-induced modulation of STAT1/4 phosphorylation is the base of the polarization of NK cells, which inhibits IFN-γ expression and signaling in NK cells (Vivier and others 2008; Vidal-Castineira and others 2010). We are interested in further investigating the mechanisms underlying the action of standard therapy in the activation and migration of NK cells.
One important question is how the responses of individual patients to the standard therapy are associated with the change in the frequency of different subsets of NK cells. We stratified the patients into the responder and nonresponder groups (such as RVR, EVR, and SVR) and found that the frequency of NKG2A+ and KIR2DL3+ NK cells in the responder group was significantly higher than that in the nonresponder group, especially in the EVR group. Moreover, KIR2DL3+ NK cells in the SVR group were gradually decreased after treatment, but not NKG2A+ NK cells. These data are consistent with a previous report (Par and others 2006). It is substantiated that inhibitory signals are helpful to activation of NK cells (Yamagiwa and others 2009). Knapp confirmed that patients with KIR2DL3 and group 1 HLA-C were more likely to acquire a SVR (Yoon and others 2009). Interestingly, a recent study has shown that KIR2DL3+ NK cells have a higher degranulation activity in patients with self-limited HCV infection (Khakoo and others 2004). Indeed, interaction of KIR2DL3-specific cells with HLA-C1 influences resolution of HCV infection in some populations (Lavanchy 2009). In addition, the frequency of KIR2DL3+ NK cells is associated with a satisfactory response to treatment in patients with HLA-C1 (Trobonjaca and others 2002). Interestingly, previous evidences support the hypothesis that a fraction of the CD56dimNKG2A+KIR+NK cells might be directly derived from CD56bright cells and CD56dimNKG2A−KIR+NK cells were the NK cell terminal differentiation (Zhu and others 2007). CD56dimNKG2A−KIR+ cells acted as the most differentiated phenotype associated with their particular ability to respond against HLA-E+ target cells. The hypothesis elucidated that CD56dimNKG2A+KIR+ and CD56dimNKG2A+KIR− NK cells are the metaphases of differentiation phenotypes after stimulation, while in our studies, we supposed that NKG2A+KIR2DL3+ NK cells could have greater cytotoxicity, but NKG2A+ KIR2DL3− NK cells would be relatively quiet after treatment. More evidence should be provided to certify the above hypothesis. Nevertheless, the frequency of NKG2A+ and KIR2DL3+ NK cells may be a prognostic marker for the evaluation of individual responses to the standard therapy in the clinic.
In summary, our data indicate that although the frequency of NK cells in CHC patients is similar to that in healthy controls, the percentages of CD56bright NK cells were significantly elevated after immunotherapy. Treatment with the standard therapy increased the frequency of NK, TRAIL+ NK cells, and degranulated NK cells, but not IFN-γ-secreting NK cells, accompanied by the balance of activating receptor+ NK cells and inhibitory receptor+ NK cells in CHC patients; furthermore, NKp30+ and NKp46+ NK cells are crucial for the clearance of HCV. In addition, we found that a high frequency of NKG2A+ and KIR2DL3+ NK cells is associated with SVR to the standard therapy in CHC patients. Therefore, our findings provide new insights into the role of NK cells in the therapeutic responses to the standard treatment in CHC patients. We recognized that our study had limitations of small sample size and the lack of analysis of NK cells in the target tissue. Thus, further studies with a large population combined with liver tissue analysis, to validate the findings, are warranted.
Acknowledgments
We thank Medjaden Bioscience Limited for assisting in the preparation of this article. This study was supported by grants from the National Natural Science Foundation of China (no. 81072347 and 30972611).
Author Disclosure Statement
No competing financial interests exist.
References
- Ahlenstiel G, Edlich B, Hogdal LJ, Rotman Y, Noureddin M, Feld JJ, Holz LE, Titerence RH, Liang TJ, Rehermann B. 2011. Early changes in natural killer cell function indicate virologic response to interferon therapy for hepatitis C. Gastroenterology 141(4):1231–1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahlenstiel G, Titerence RH, Koh C, Edlich B, Feld JJ, Rotman Y, Ghany MG, Hoofnagle JH, Liang TJ, Heller T, et al. 2010. Natural killer cells are polarized toward cytotoxicity in chronic hepatitis C in an interferon-alfa-dependent manner. Gastroenterology 138(1):325–335e1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amadei B, Urbani S, Cazaly A, Fisicaro P, Zerbini A, Ahmed P, Missale G, Ferrari C, Khakoo SI. 2010. Activation of natural killer cells during acute infection with hepatitis C virus. Gastroenterology 138(4):1536–1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beziat V, Descours B, Parizot C, Debre P, Vieillard V. 2010. NK cell terminal differentiation: correlated stepwise decrease of NKG2A and acquisition of KIRs. PLoS One 5(8):e11966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonorino P, Ramzan M, Camous X, Dufeu-Duchesne T, Thelu MA, Sturm N, Dariz A, Guillermet C, Pernollet M, Zarski JP, et al. 2009. Fine characterization of intrahepatic NK cells expressing natural killer receptors in chronic hepatitis B and C. J Hepatol 51(3):458–467 [DOI] [PubMed] [Google Scholar]
- Brook G, Soriano V, Bergin C. 2010. European guideline for the management of Hepatitis B and C virus infections, 2010. Int J STD AIDS 21(10):669–678 [DOI] [PubMed] [Google Scholar]
- Chiesa MD, Sivori S, Castriconi R, Marcenaro E, Moretta A. 2005. Pathogen-induced private conversations between natural killer and dendritic cells. Trends Microbiol 13(3):128–136 [DOI] [PubMed] [Google Scholar]
- Cooper MA, Fehniger TA, Turner SC, Chen KS, Ghaheri BA, Ghayur T, Carson WE, Caligiuri MA. 2001. Human natural killer cells: a unique innate immunoregulatory role for the CD56(bright) subset. Blood 97(10):3146–3151 [DOI] [PubMed] [Google Scholar]
- Dessouki O, Kamiya Y, Nagahama H, Tanaka M, Suzu S, Sasaki Y, Okada S. 2010. Chronic hepatitis C viral infection reduces NK cell frequency and suppresses cytokine secretion: reversion by anti-viral treatment. Biochem Biophys Res Commun 393(2):331–337 [DOI] [PubMed] [Google Scholar]
- Dominguez-Villar M, Garcia-Cozar FJ, Chambers BJ. 2012. The effects of Hepatitis C virus core protein on functional responses in the NK cell line YTS. Scand J Immunol 75(1):54–60 [DOI] [PubMed] [Google Scholar]
- Dunn C, Brunetto M, Reynolds G, Christophides T, Kennedy PT, Lampertico P, Das A, Lopes AR, Borrow P, Williams K, et al. 2007. Cytokines induced during chronic hepatitis B virus infection promote a pathway for NK cell-mediated liver damage. J Exp Med 204(3):667–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlich B, Ahlenstiel G, Azpiroz AZ, Stoltzfus J, Noureddin M, Serti E, Feld JJ, Liang TJ, Rotman Y, Rehermann B. 2012. Early changes in interferon signaling define natural killer cell response and refractoriness to interferon-based therapy of hepatitis C patients. Hepatology 55(1):39–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao B, Jeong WI, Tian Z. 2008. Liver: an organ with predominant innate immunity. Hepatology 47(2):729–736 [DOI] [PubMed] [Google Scholar]
- Hadziyannis SJ, Sette H, Jr., Morgan TR, Balan V, Diago M, Marcellin P, Ramadori G, Bodenheimer H, Jr., Bernstein D, Rizzetto M, et al. 2004. Peginterferon-alpha2a and ribavirin combination therapy in chronic hepatitis C: a randomized study of treatment duration and ribavirin dose. Ann Intern Med 140(5):346–355 [DOI] [PubMed] [Google Scholar]
- Harrison RJ, Ettorre A, Little AM, Khakoo SI. 2010. Association of NKG2A with treatment for chronic hepatitis C virus infection. Clin Exp Immunol 161(2):306–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoofnagle JH, Seeff LB. 2006. Peginterferon and ribavirin for chronic hepatitis C. N Engl J Med 355(23):2444–2451 [DOI] [PubMed] [Google Scholar]
- Joncker NT, Fernandez NC, Treiner E, Vivier E, Raulet DH. 2009. NK cell responsiveness is tuned commensurate with the number of inhibitory receptors for self-MHC class I: the rheostat model. J Immunol 182(8):4572–4580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khakoo SI, Thio CL, Martin MP, Brooks CR, Gao X, Astemborski J, Cheng J, Goedert JJ, Vlahov D, Hilgartner M, et al. 2004. HLA and NK cell inhibitory receptor genes in resolving hepatitis C virus infection. Science 305(5685):872–874 [DOI] [PubMed] [Google Scholar]
- Knapp S, Warshow U, Hegazy D, Brackenbury L, Guha IN, Fowell A, Little AM, Alexander GJ, Rosenberg WM, Cramp ME, et al. 2010. Consistent beneficial effects of killer cell immunoglobulin-like receptor 2DL3 and group 1 human leukocyte antigen-C following exposure to hepatitis C virus. Hepatology 51(4):1168–1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavanchy D. 2009. The global burden of hepatitis C. Liver Int 29 (Suppl 1):74–81 [DOI] [PubMed] [Google Scholar]
- Lin AW, Gonzalez SA, Cunningham-Rundles S, Dorante G, Marshall S, Tignor A, Ha C, Jacobson IM, Talal AH. 2004. CD56(+dim) and CD56(+bright) cell activation and apoptosis in hepatitis C virus infection. Clin Exp Immunol 137(2):408–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHutchison JG, Lawitz EJ, Shiffman ML, Muir AJ, Galler GW, McCone J, Nyberg LM, Lee WM, Ghalib RH, Schiff ER, et al. 2009. Peginterferon alfa-2b or alfa-2a with ribavirin for treatment of hepatitis C infection. N Engl J Med 361(6):580–593 [DOI] [PubMed] [Google Scholar]
- Miyagi T, Gil MP, Wang X, Louten J, Chu WM, Biron CA. 2007. High basal STAT4 balanced by STAT1 induction to control type 1 interferon effects in natural killer cells. J Exp Med 204(10):2383–2396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishima C, Paschal DM, Wang CC, Yoshihara CS, Wood BL, Yeo AE, Emerson SS, Shuhart MC, Gretch DR. 2006. Decreased NK cell frequency in chronic hepatitis C does not affect ex vivo cytolytic killing. Hepatology 43(3):573–580 [DOI] [PubMed] [Google Scholar]
- Nattermann J, Feldmann G, Ahlenstiel G, Langhans B, Sauerbruch T, Spengler U. 2006. Surface expression and cytolytic function of natural killer cell receptors is altered in chronic hepatitis C. Gut 55(6):869–877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris S, Collins C, Doherty DG, Smith F, McEntee G, Traynor O, Nolan N, Hegarty J, O'Farrelly C. 1998. Resident human hepatic lymphocytes are phenotypically different from circulating lymphocytes. J Hepatol 28(1):84–90 [DOI] [PubMed] [Google Scholar]
- Oliviero B, Varchetta S, Paudice E, Michelone G, Zaramella M, Mavilio D, De Filippi F, Bruno S, Mondelli MU. 2009. Natural killer cell functional dichotomy in chronic hepatitis B and chronic hepatitis C virus infections. Gastroenterology 137(3):1151–1160, 1160.e1–7. [DOI] [PubMed] [Google Scholar]
- Par G, Berki T, Palinkas L, Balogh P, Szereday L, Halasz M, Szekeres-Bartho J, Miseta A, Hegedus G, Mozsik G, et al. 2006. [Immunology of HCV infection: the causes of impaired cellular immune response and the effect of antiviral treatment]. Orv Hetil 147(13):591–600 [PubMed] [Google Scholar]
- Pelletier S, Drouin C, Bedard N, Khakoo SI, Bruneau J, Shoukry NH. 2010. Increased degranulation of natural killer cells during acute HCV correlates with the magnitude of virus-specific T cell responses. J Hepatol 53(5):805–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post J, Ratnarajah S, Lloyd AR. 2009. Immunological determinants of the outcomes from primary hepatitis C infection. Cell Mol Life Sci 66(5):733–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehermann B, Nascimbeni M. 2005. Immunology of hepatitis B virus and hepatitis C virus infection. Nat Rev Immunol 5(3):215–229 [DOI] [PubMed] [Google Scholar]
- Smyth MJ, Cretney E, Kelly JM, Westwood JA, Street SE, Yagita H, Takeda K, van Dommelen SL, Degli-Esposti MA, Hayakawa Y. 2005. Activation of NK cell cytotoxicity. Mol Immunol 42(4):501–510 [DOI] [PubMed] [Google Scholar]
- Stegmann KA, Bjorkstrom NK, Veber H, Ciesek S, Riese P, Wiegand J, Hadem J, Suneetha PV, Jaroszewicz J, Wang C, et al. 2010. Interferon-alpha-induced TRAIL on natural killer cells is associated with control of hepatitis C virus infection. Gastroenterology 138(5):1885–1897 [DOI] [PubMed] [Google Scholar]
- Tjwa ET, van Oord GW, Hegmans JP, Janssen HL, Woltman AM. 2011. Viral load reduction improves activation and function of natural killer cells in patients with chronic hepatitis B. J Hepatol 54(2):209–218 [DOI] [PubMed] [Google Scholar]
- Trobonjaca Z, Kroger A, Stober D, Leithauser F, Moller P, Hauser H, Schirmbeck R, Reimann J. 2002. Activating immunity in the liver. II. IFN-beta attenuates NK cell-dependent liver injury triggered by liver NKT cell activation. J Immunol 168(8):3763–3770 [DOI] [PubMed] [Google Scholar]
- Vidal-Castineira JR, Lopez-Vazquez A, Diaz-Pena R, Alonso-Arias R, Martinez-Borra J, Perez R, Fernandez-Suarez J, Melon S, Prieto J, Rodrigo L, et al. 2010. Effect of killer immunoglobulin-like receptors in the response to combined treatment in patients with chronic hepatitis C virus infection. J Virol 84(1):475–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. 2008. Functions of natural killer cells. Nat Immunol 9(5):503–510 [DOI] [PubMed] [Google Scholar]
- Yamagiwa S, Kamimura H, Ichida T. 2009. Natural killer cell receptors and their ligands in liver diseases. Med Mol Morphol 42(1):1–8 [DOI] [PubMed] [Google Scholar]
- Yoon JC, Shiina M, Ahlenstiel G, Rehermann B. 2009. Natural killer cell function is intact after direct exposure to infectious hepatitis C virions. Hepatology 49(1):12–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Dong H, Eksioglu E, Hemming A, Cao M, Crawford JM, Nelson DR, Liu C. 2007. Hepatitis C virus triggers apoptosis of a newly developed hepatoma cell line through antiviral defense system. Gastroenterology 133(5):1649–1659 [DOI] [PubMed] [Google Scholar]