Abstract
The epidemiology of autoimmune diseases and helminth infections led to suggestions that helminths could improve inflammatory conditions, which was then tested using animal models. This has translated to clinical investigations aimed at the safe and controlled reintroduction of helminthic exposure to patients suffering from autoimmune diseases (so-called “helminthic therapy”) in an effort to mitigate the inflammatory response. In this review, we will summarize the results of recent clinical trials of helminthic therapy, with particular attention to mechanisms of action. Whereas previous reviews have emphasized immune regulatory mechanisms activated by helminths, we propose that enhancement of mucosal barrier function may have an equally important role in improving conditions of inflammatory bowel diseases.
Introduction: rationale for helminthic therapy
Based on the epidemiology of autoimmune diseases, environmental factors such as helminth infection have long been part of the hygiene hypothesis to explain why autoimmunity may be less prevalent in the developing world. Subsequently, helminth infections in animal models have been shown to improve the conditions of certain inflammatory diseases, leading to clinical trials of “helminthic therapy”. Helminths are large metazoan organisms with the potential to cause significant tissue injury as they mature, migrate, and feed within the host. Due to effective immune evasion strategies, these parasites can persist in the host for many years. The immune response that optimizes host fitness must be well-adapted for 1) expelling large multicellular pathogens, 2) wound healing and tissue repair, and 3) mitigating inflammatory pathology associated with chronic infection. These mechanisms are encompassed within the type-2 immune response elicited by helminth infection and the activation of regulatory networks that dampen effector T cell responses 1,2. Elements of the type-2 immune response, as well the induction of regulatory T cells, may contribute in varying degrees to the benefits of helminth infection in different autoimmune and inflammatory disease settings. The type 2 immune response triggered by gastrointestinal helminths includes cytokines produced by CD4+ TH2 cells (e.g. IL-4, IL-5 and IL-13), activation of alternatively activated macrophages and mast cells, increased goblet cell hyperplasia and mucus production,, and increased turnover of intestinal epithelial cells 3. We propose that these alterations to mucosal barrier function in the gut play a protective role against pathology associated with inflammatory bowel diseases (especially ulcerative colitis) and may potentially be as mechanistically important as immune regulation in modulating the inflammatory response.
Clinical trials with Trichuris suis ova (TSO)
Therapeutic infection with the pig whipworm Trichuris suis was first investigated in 2003 by Summers et al. in an exploratory open-label study of seven patients with inflammatory bowel disease 4. Trichuris suis ova (TSO) was considered to be an ideal agent because it produces a self-limited colonization in humans and remains isolated to the gastrointestinal tract. Additionally, ova can be obtained under pathogen-free conditions, can be stored for approximately two years, and any unexpected long term colonization can be effectively eradicated with short courses of oral anti-helminthic agents.
Subsequent clinical trials of T. suis ova (TSO) reported significant improvement in patient responses for both ulcerative colitis and Crohn’s disease with essentially no adverse effects 5–8. In a landmark randomized placebo-controlled double-blind study of 54 subjects with moderate to severe ulcerative colitis, subjects in the treatment group ingested 2,500 TSO every two weeks for a total of 12 weeks5. Subjects were evaluated with the Ulcerative Colitis Disease Activity Index (UCDAI) at week 0 and week 12 (which requires inspection of the colonic mucosa by endoscopy) in addition to biweekly assessment with a symptom-based index 9. After 12 weeks of therapy, 43.3% of the individuals treated with TSO had improved symptoms (defined as a decrease in UCDAI of ≥ 4 points) compared to 16.7% in the placebo group, which was a statistically significant response rate. Furthermore, TSO treated subjects reported significant improvement in their symptoms (compared to placebo) as early as week six. This was the first trial to define a subgroup of relatively treatment-refractory patients who responded to helminthic therapy in a controlled setting. There was a trend towards improved response in those subjects with extensive colonic involvement and shorter duration of disease activity. A 24-week open label study of TSO in 29 patients with active Crohn’s disease showed an even more robust response rate (79.3%), with an impressive remission rate of 72.4% with no adverse events reported 8. Currently, larger phase II dose-escalation trials of TSO in Crohn’s disease are ongoing in Europe (Dr. Falk Pharma, GmbH; NCT01279577) and the United States (Coronado Biosciences and OvaMed GmbH; NCT01434693). We are currently recruiting moderate to severe ulcerative colitis patients to conduct an exploratory mechanistic trial of TSO in order to better characterize the mucosal immune response at NYU School of Medicine (NCT01433471).
TSO therapy is also being evaluated in extraintestinal diseases such as multiple sclerosis 10,11. A strong body of epidemiological and experimental evidence suggests that parasitic infection may be protective for multiple sclerosis 12. In an uncontrolled prospective double-cohort study of 12 patients with relapsing-remitting multiple sclerosis (RRMS) who presented with infection with different organisms (Hymenolepis nana, Trichuris trichiura, Ascaris lumbricoides, Strongyloides sterocolaris, and Enterobius vermicularis), Correale et al. observed that helminth infected patients had a significantly lower number of exacerbations and fewer magnetic resonance imaging changes compared with uninfected patients 12. Furthermore, increased regulatory cytokine production (e.g. IL-10 and TGF-β) and CD4+CD25+FoxP3+ T cell clones were noted to be significantly enriched in the infected cohort 12. Additional studies by this group demonstrated that helminth infections induced regulatory B cells capable of suppressing the immune response through IL-10 production 13. Interestingly, when some of these patients were ultimately treated with anti-helminthics for worsening parasite-associated symptoms, a flare in clinical and radiologic MS activity occurred that was accompanied by an increase in IFNγ and IL-12 producing cells and a decline in IL-10, TGF-β, and regulatory T cells 14.
In 2011, Fleming et al. published the first prospective use of TSO in five subjects with treatment-naive relapsing-remitting multiple sclerosis (RRMS) in the HINT 1 study 15. The mean number of new gadolinium-enhancing lesions in treated individuals decreased from 6.6 at baseline to 2.0 at the end of 12 weeks of TSO treatment. Lesion incidence increased to a mean of 5.8 two months after the completion of the treatment phase, indicating that any protective effects were transient. TSO treatment was associated with relative increases in TH2 cytokines such as IL-4, IL-5, IL-10, and IL-13 without significant decreases in Th1 cytokines such as IFNγ or IL-2. Acute phase reactants such as hs-CRP rose during the first two months of TSO administration and fell during and after the last four weeks of ova exposure. Anti-T. suis IgG1 antibodies showed a durable response, whereas T. suis-specific IgA returned to baseline after treatment and IgE was undetectable during the study period. Peripheral immunoregulatory T cells (CD4+CD25+Foxp3+ cells) modestly increased in only two of the five subjects under study, but this may be a reflection of the lack of entry of regulatory T cells into the circulation after induction in the gastrointestinal tract.
As of January 2012, additional clinical trials of TSO in adult autism (NCT01040221), peanut and tree nut allergy (NCT01070498), and multiple sclerosis (NCT01413243) are ongoing.
Safety Concerns of TSO
TSO has been extensively studied in IBD patients on concomitant prednisone, thiopurines, and other immunosuppresants, suggesting relative safety even in immunocompromised hosts 16. In a 2010 randomized double-blind placebo-controlled investigation of 100 subjects with allergic rhinitis (the largest clinical trial of a helminthic agent in a human study population to date), treatment with TSO showed no significant effect on symptom scores or subclinical measures of allergic reactivity compared with placebo 10. However, this was the first clinical trial to detail treatment-emergent symptoms such as diarrhea, excessive flatulance, and upper abdominal pain in the majority of TSO-treated subjects 17–21. These events peaked 30 to 50 days after the first treatment with TSO, were generally transient (median duration of two days) and could be related to the expulsion of T. suis larvae from the gut 21. These symptoms were not observed in earlier studies of TSO in IBD patients, perhaps because they were occurring in the context of already moderate to severe gastrointestinal pathology in the study cohorts. Concerns have been raised about aberrant migration of T. suis in its non-natural host to other organs and tissues 19, but this has not occurred in any of the subjects studied in any trial of TSO to date. Secondary bacterial infection, specifically Campylobacter jejuni, could also be a concern 22,23. A case of life-threatening campylobacterjejunosis leading to toxic megacolon and acute renal failure associated with concomitant T. trichiura infection has been reported 20, but this has also never been observed with TSO treated subjects.
Clinical trials with N. americanus
The only other helminth studied in clinical trials thus far is the hookworm N. americanus, but this has been less successful than its porcine whipworm counterpart. Hookworm infection is highly prevalent in impoverished regions of the world 24 and can also upregulate immunoregulatory molecules such as IL-10, TGF-β, and metalloproteases 25–28. However, hookworm infection can be far from benign, with the most common hookworm-related injuries being a pruritic maculopapular pruritic skin eruption, gastrointestinal symptoms such as diarrhea, increased flatulence, abdominal pain, cough, dyspnea, malaise, and iron deficiency anemia secondary to chronic blood loss 25.
Due to the requisite extraintestinal phase of its lifecycle and the fact that humans are the natural host, there is probably a much narrower therapeutic window between achieving effective immune modulation and causing unacceptable adverse events for N. americanus. Dose-ranging studies of therapeutic infection of N. americanus in humans have shown that doses higher than 10 larvae correlate with more frequent adverse events than low-dose inocula 25,29. This is a very small number compared with the 2500 TSO being used in Phase II trials. In a 2009 randomized double-blind investigation by Feary et al., 32 subjects with asthma were randomized to 10 larvae or placebo for 16 weeks, with the primary outcome being a change in the provocation dose of inhaled adenosine monophosphate (AMP) required to reduce the forced expiratory volume in 1 second by 20% (PD20AMP) from baseline to week 16. Although the absolute mean PD20AMP increased more in the hookworm group, the differences between the treated groups were non-significant 30. Furthermore, gastrointestinal side effects such as abdominal pain, loss of appetite, and nausea were significantly higher in the N. americanus -treated group than in the placebo group.
More recently, a 2011 randomized double-blind clinical trial of N. americanus larvae or placebo in 20 HLA-DQ2 positive patients with well-controlled clinically inactive celiac disease was completed in Australia by Daveson et al 31. After subcutaneous inoculations of 10 and 5 stage three larvae (or placebo mixed with Tabasco sauce in order to simulate the expected hookworm-associated pruritic skin eruption that follows inoculation), subjects underwent a five-day gluten challenge followed by esophagogastroduodenoscopy with duodenal biopsies for determination of histopathologic Marsh score. No significant difference was observed in pathologic grade or systemic inflammatory immune response determined by gluten-specific IFN-γ producing PBMC’s after gluten challenge between the hookworm infected and placebo injected groups, although the adverse reactions were more similar than previously reported between the two groups 32. Subsequent characterization of the circulating and mucosal immune response to N. americanus in these subjects showed significant declines in IFN-γ and IL-17A in supernatents derived from cultured duodenal pinch biopsies, but no significant differences in CD4+CD25+FoxP3+ cells were observed 33. The authors comment that the relatively low inoculation dose of hookworm (15 worms) used in the trial may have been insufficient to induce an immunosuppressive phenotype in this patient population.
Regulatory mechanisms in helminthic therapy
In recent years, immune regulation has been the major mechanism proposed to explain the potential beneficial effects of helminths 34. Since these mechanisms have been reviewed in detail recently 35, we provide here only a brief summary of studies addressing the function of immunoregulatory cell populations during helminth infection.
Regulatory T cells (Tregs) have been by far the most studied immunoregulatory population and clearly expand during a wide range of helminth infections 1,35. Neutralizing antibodies against CTLA-4 enhance cytokine responses 36–40 and in some 37,40,41 but not all 39 cases promote parasite clearance. Anti-GITR antibodies can also heighten lymphocyte proliferation 37 and cytokine responses 42, resulting in reduced parasite burden 42,43 and increased inflammatory pathology 43. Finally, depleting antibodies have demonstrated the importance of CD25+ Tregs in limiting effector responses 42,44–48 and subverting parasite clearance 41,42,44. Recently, the use of a mouse strain engineered for the inducible deletion of FoxP3-expressing Tregs (depletion of regulatory T cell, DEREG) has established a role for this subset in regulating effector responses that mediate parasite killing 39 and inflammatory pathology 49. Thus, helminth-elicited Treg populations appear to benefit both parasite and host.
In murine models of helminthic therapy, functional studies of Treg populations have demonstrated that there is considerable heterogeneity in the role of Treg subsets among helminth infections. Suppression of allergic airway disease by infection with Heligmosomoides polygyrus was lost following depletion of CD25+ cells, and adoptive transfer of CD4+CD25+ T cells from infected mice (of both wild-type and IL-10−/− genetic backgrounds) recapitulated the therapeutic effect of infection 50. Similarly, reduced airway inflammation in response to Schistosoma mansoni eggs was dependent on CD25+ cells, but not IL-10 receptor signaling 51. However, neither the depletion of CD25+ cells nor TGFβ neutralization affected the suppression of airway inflammation mediated by Litomosoides sigmodontis 52. In contrast to the asthma model, H. polygyrus-mediated inhibition of diabetes onset, in NOD mice as well as cyclophosphamide-induced diabetes, was not reversed by CD25 depletion or IL-10 neutralization 53. The anti-diabetic effect of schistosomal egg antigens (SEA) was transferable by adoptive transfer of unfractionated, but not CD25-depleted splenocytes from SEA-exposed mice 54. In contrast, while the suppression of diabetes by L. sigmodontis infection did not appear to be dependent on CD25+, FoxP3+ Tregs, or IL-10 signaling, neutralization of TGFβ reversed the therapeutic effect 55. TGFβ was also shown to be critical in Fasciola hepaticus-mediated protection against experimental autoimmune encephalomyelitis, a model of multiple sclerosis 56. In a study of DSS-induced colitis, CD25 depletion also did not reverse the protective effect of S. mansoni exposure 57.
Therefore, distinct Treg subsets induced by the same helminth infection may mediate protection against different inflammatory diseases (for example, TGFβ is essential for L. sigmodontis-mediated suppression of diabetes but not allergic airway disease). Finally, for some models of helminthic therapy (for example, chemically-induced colitis) a role for helminth-elicited Tregs remains to be demonstrated.
Dendritic cells (DCs) and macrophages (MΦs) are also important immune regulatory cells during helminth infection 2. Balb/c mice infected with male worms of S. mansoni are protected from DSS-induced colitis through a macrophage dependent pathway and not through IL-10, TGFβ or Tregs 57. Extracts from the tapeworm H. diminuta can reduce inflammation caused by DNBS-induced colitis, perhaps because it suppresses macrophage activation58. Intestinal DCs of H. polygyrus-infected mice are also diminished in their ability to activate T cells 59 and may be protective in an IL-10−/− T cell transfer model of colitis. Since intestinal DCs and MΦs are important in regulating mucosal homeostasis 60, it is not surprising that changes in their phenotype may occur during helminthic therapy. Since the immunoregulatory alternatively activated macrophages (or M2 cells) are induced by helminths to repair tissue damage61 and have been shown to suppress colitis62, they may play a critical role in helminthic therapy for inflammatory bowel diseases.
Enhancement of the mucosal barrier in helminthic therapy for IBD
The immune response to intestinal helminth infection drives a potent physiologic response with the dual aims of parasite expulsion and mucosal healing 3. Recently, we characterized longitudinally the mucosal response of an individual who self infected with T. trichiura to treat his own symptoms of ulcerative colitis 63. He was able to put his disease into remission twice by self-infection. While we originally speculated that regulatory mechanisms might play an important role in his situation, our detailed analysis of mucosal pinch biopsies collected during colonoscopy suggested to us that the effect of TH2 cytokines and IL-22 on mucosal barrier function may play an even greater role in symptomatic improvement. TH2 cytokines and IL-22 have profound effects on colonic epithelial cell function 64–66, including the stimulation of goblet cell and Paneth cell differentiation with their attendant mucus production and anti-microbial peptide expression, and the activation of anti-apoptotic pathways. Furthermore, accessory cells recruited and activated by type-2 cytokines, most notably alternatively-activated macrophages, can promote mucosal healing 67. Additionally, IL-13 and IL-22 can increase the proliferation and turnover of IECs, which serves the dual function of parasite expulsion and mucosal healing 3,64. Taken together, these functions enhance the epithelial barrier against luminal antigens, and they have demonstrated protective effects in murine models of colitis 67–69. Mucus hypersecretion, a ubiquitous feature of the host response to intestinal worms, may therefore be implicated in the protective effect of helminth infection in the setting of IBD by reinforcing the mucosal barrier.
Intestinal mucus is a carbohydrate-rich gel, approximately 1 millimeter thick, charged with the formidable task of separating the intestinal epithelium from ~1013 commensal bacteria. The scaffolding of the mucus gel is primarily composed of mucins, high molecular weight glycoproteins bearing O-linked oligosaccharides that are commonly decorated with chemical moieties such as sulphate and acetyl groups. Of the nineteen mucins identified in humans, Muc2 is the most important mucin secreted in the intestine 70. Muc2 forms two distinct layers following secretion by goblet cells. The loosely packed outer layer is the main bulk of the mucus gel, and harbors a large number of bacteria. Conversely, the thin inner layer is composed of tightly packed lamellar sheets that are normally impermeable to bacteria 71. Below the Muc2 layers, transmembrane mucins (e.g. Muc3) cover the apical surface of enterocytes. A lipid fraction largely composed of amphipathic phospholipids contributes to the viscosity and hydrophobicity of the mucus gel 72. Phosphatidylcholine (PC) and lyso-PC are the most abundant phospholipids in colonic mucus 73.
Histochemical studies have demonstrated that the mucus gel is abnormal in both quantity and quality in a large fraction of UC patients 74. Muc2 abundance is lower in rectal mucus samples from UC patients 75 and displays altered glycosylation 76 and reduced sulphation 77. A causal role for altered expression and post-translational processing of mucins in the pathogenesis of colitis is supported in several mouse models. Genetic deficiency 78,79 or terminal misfolding 80 of Muc2 precipitates severe, spontaneous colitis in mice. Impaired glycolylation of mucins due to specific glycosyltransferase deficiencies also increases susceptibility to colitis 81,82. More recently, abnormalities in phospholipid species have also been described in UC patients, with a significant decrease in PC 73,83,84. Intriguingly, clinical trials in which the phospholipid content of mucus in UC patients was restored to that of healthy individuals by oral intake of delayed-release PC have shown promising results 83–85.
Helminth infection is also associated with qualitative changes in mucus composition, including increased sulphation of mucins 86,87 and stimulation of bulk mucus production via goblet cell hyperplasia and increased mucin expression 88–91. Muc5ac is a mucin that is specifically induced by helminth infection and is important for the expulsion of these parasites 92. The increased production of resistin-like molecule (RELM) beta by goblets cells after helminth infection may also play a critical role in reinforcing mucosal barrier function 93,94. TH2 cytokines can induce intestinal epithelial cells (IECs) to differentiate into goblets cells producing RELM-beta, which in turn plays a critical role in the expulsion of worms that live in the gut lumen93,94. Interestingly, RELM-beta deficient mice are more sensitive to DSS-induced colitis95 and delivering RELM-beta to the colon can improve TNBS-induced colitis96. RELM beta also regulates expression of antibacterial peptides like REG3 beta/gamma95, which may be associated with alteration in the gut microbiota97–99.
These changes in the quality, quantity and antibacterial peptide composition of mucus during helminth infection is likely to have a major impact on the gut microbial environment. H. polygyrus infection has been shown to have major effects on the microbiota of mice, especially increasing the abundance of the Lactobacillaceae family100. Indeed, successful colonization of the colon with Trichuris muris is dependent on the gut microbiota101. Further studies may reveal the intricate relationship between helminth infection, gut microbiota and the protection against inflammatory bowel diseases. It is conceivable that some of the protective effects of helminth infection may be attributable to indirect effects downstream of alterations in gut microbiota rather than direct effects of helminth infection.
Concluding remarks
While the therapeutic window for N. americanus may be too narrow for it to be used clinically, TSO could potentially become the first live parasite that is used as a therapeutic agent. Phase II clinical trials for Crohns Disease are in progress and should be completed in 2012. While there has been an extensive body of work on the role that regulatory cells and cytokines may play during helminthic therapy, we propose that more direct effects of helminths on mucosal barrier function may play an equally important role in inflammatory diseases of the intestinal tract. We propose a model whereby the immune response that is triggered to expel gastrointestinal parasites, which includes increased mucus production, changes to the composition of mucus secreted by goblet cells, and increased epithelial cell turnover, may have a beneficial effect in restoring mucosal barrier function during inflammatory bowel disease and reducing inflammation driven by gut bacteria. To test this model, we are conducting a clinical trial that is focused on elucidating the mechanism of action of TSO, rather than evaluating clinical efficacy (NCT 01433471). The further study of host protective mechanisms activated during intestinal helminth infection may identify novel pathways that can bolster mucosal barrier functions without the risks of immunosuppression associated with current treatments for severe IBD.
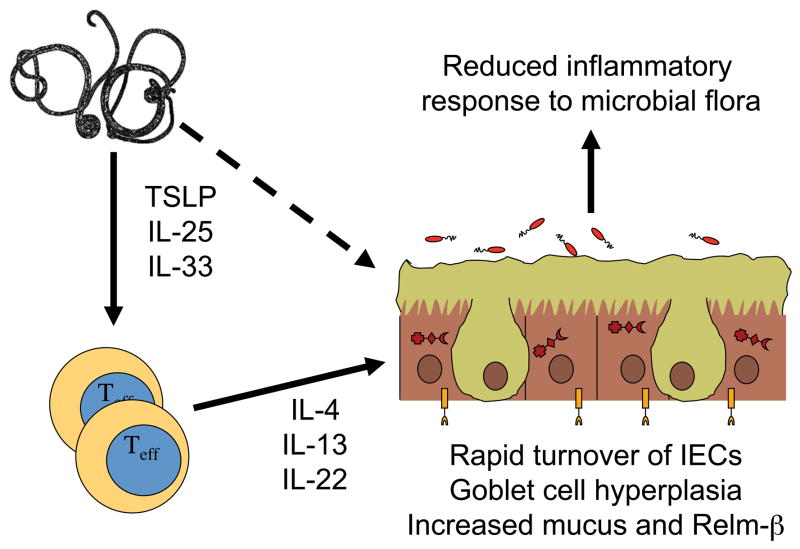
Figure 1. A mechanistic model for improved mucosal barrier function from helminthic therapy with Trichuris worms.
Interactions between the parasites and intestinal epithelial cells leads to the production of cytokines like TSLP, IL-25, IL-33 and other yet unidentified factors that can induce the differentiation of naïve T cells into effector cells that produce TH2 cytokines such as IL-4 and IL-13, as well as IL-22. These cytokines can then increase the turnover of intestinal epithelial cells (IECs) as well as induce goblet cell differentiation, maturation and hyperplasia. Goblet cells increase production of mucus and molecules such as Relm-β, which improves the physical barrier separating the IECs from gut microbiota in the lumen of the colon. This could lead to a reduced inflammatory response to luminal bacteria and improve the conditions of individuals with ulcerative colitis.
Table 1.
Selected Clinical Studies of Helminthic Therapy in Human Disease
| Year of Publication | Patient Population (Institution) | Number of Subjects | Trial Design | Clinical Assessment Measures | Main Observations |
|---|---|---|---|---|---|
| 20034 | Crohn’s Disease and Ulcerative Colitis (University of Iowa) |
Initial phase: 4 (CD) 3 (UC) Maintenance phase: 2 (CD) 2 (UC) |
Open-label phase 1 pilot Initial phase: 2,500 TSO PO x 1 Clinical monitoring q2 week x 12 weeks Maintenance phase: 2,500 TSO q3week x 28 weeks |
Safety assessment Remission: CDAI < 150 IBDQ >170 SSCAI ≤ 4 |
|
| 20058 | Active Crohn’s Disease (CDAI ≥ 220) (University of Iowa) | 29 | 24-week open-label phase 1 study 2,500 TSO PO q3week |
Response: CDAI decrease >100 Remission: CDAI <150 |
|
| 20055 | Moderate-Severe Ulcerative Colitis (University of Iowa) | 54 | 12-week randomized double-blind placebo-controlled trial 2,500 TSO PO q2weeks |
Response: UCDAI ≤ 4 Remission: UCDAI ≤ 2 |
|
| 200632 | Crohn’s Disease (Townsville Hospital, Australia) | 9 | 45-week open-label POC study 25–50 NA L3i SC at 0 and 27 weeks |
Response: CDAI < 150 IBDQ > 170 |
|
| 200930 | AMP-responsive Asthma | 32 | 16-week randomized double-blind placebo-controlled study 10 NA L3i SC |
Change in provocation dose of inhaled AMP required to reduced forced expiratory volume in 1 s by 20% (PD20AMP) |
|
| 201015 | Treatment-naïve Relapsing Remitting Multiple Sclerosis (University of Wisconsin) | 5 | Phase 1 baseline vs. treatment study 2,500 TSO PO q2weeks x 3 months |
Neurological function (MSFC and EDSS) Gadolinium-enhancing lesions on MRI Immunologic assessments (e.g. serum T. suis specific IgG1 and IgA) |
|
| 201010 | Allergic Rhinitis (Statens Serum Institut and University of Copenhagen, Denmark) | 100 | 27-week randomized double-blind placebo-controlled trial 2,500 TSO PO q2weeks x 25 weeks |
Symptom severity score of allergic rhinitis Skin prick testing Immunologic assessments (e.g. serum T suis-specific IgA and IgE, serum grass-specific IgE, total histamine) |
|
| 201063 | Ulcerative Colitis (University of San Francisco) | 1 | Case study 1,500 Trichuris trichiura PO ova ad lib for 5 years |
Mucus production Mucosal gene expression Flow cytometric analysis of effector T helper cell cytokine production |
|
| 201131 | Celiac Disease (Princess Alexandra Hospital, Brisbane, Australia) | 20 | 21-week randomized double-blind placebo-controlled study 10 and 5 L3i NA or placebo SC at 0 and 12 weeks. At week 20, subjects underwent a 5 day 16 gram gluten challenge |
Duodenal histologic Marsh score Systemic IFN-γ measured by QE65-ELISpot |
|
CDAI: Crohn’s Disease Activity Index, EDSS: Expanded Disability Status Scale (EDSS), IBDQ: Inflammatory Bowel Disease Quality of Life Index, IFN: Interferon, L3i: Third stage infective filariform larvae of Necator Americanus, MSFC: Multiple Sclerosis Funcitonal Composite (MSFC), NA: Necator Americanus, PO: Per os, SC: Subcutaneous, SCCAI: Simple Clinical Colitis Activity Index, TSO: Trichuris Suis Ova, UCDAI: Ulcerative Colitis Disease Activity Index
Contributor Information
Martin J. Wolff, Email: Martin.Wolff@nyumc.org.
Mara J. Broadhurst, Email: Mara.Broadhurst@ucsf.edu.
P’ng Loke, Email: Png.Loke@nyumc.org.
References
- 1.Maizels R, Yazdanbakhsh M. Immune regulation by helminth parasites: cellular and molecular mechanisms. Nature reviews. Immunology. 2003;3:733–744. doi: 10.1038/nri1183. [DOI] [PubMed] [Google Scholar]
- 2.Allen J, Maizels R. Diversity and dialogue in immunity to helminths. Nature reviews. Immunology. 2011;11:375–388. doi: 10.1038/nri2992. [DOI] [PubMed] [Google Scholar]
- 3.Artis D, Grencis RK. The intestinal epithelium: sensors to effectors in nematode infection. Mucosal immunology. 2008;1:252–264. doi: 10.1038/mi.2008.21. [DOI] [PubMed] [Google Scholar]
- 4.Summers R, et al. Trichuris suis seems to be safe and possibly effective in the treatment of inflammatory bowel disease. The American journal of gastroenterology. 2003;98:2034–2041. doi: 10.1111/j.1572-0241.2003.07660.x. [DOI] [PubMed] [Google Scholar]
- 5.Summers R, Elliott D, Urban J, Thompson R, Weinstock J. Trichuris suis therapy for active ulcerative colitis: a randomized controlled trial. Gastroenterology. 2005;128:825–832. doi: 10.1053/j.gastro.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 6.Summers R, Elliott D, Weinstock J. Why Trichuris suis should prove safe for use in inflammatory bowel diseases. Inflammatory bowel diseases. 2005;11:783–784. doi: 10.1097/01.mib.0000179316.50002.f3. [DOI] [PubMed] [Google Scholar]
- 7.Summers R, Elliott D, Weinstock J. Therapeutic colonization with Trichuris suis. Archives of pathology & laboratory medicine. 2006;130:1753–1754. doi: 10.5858/2006-130-1753b-IR. [DOI] [PubMed] [Google Scholar]
- 8.Summers RW, Elliott DE, Urban JF, Thompson R, Weinstock JV. Trichuris suis therapy in Crohn’s disease. Gut. 2005;54:87–90. doi: 10.1136/gut.2004.041749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walmsley RS, Ayres RC, Pounder RE, Allan RN. A simple clinical colitis activity index. Gut. 1998;43:29–32. doi: 10.1136/gut.43.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bager P, et al. Trichuris suis ova therapy for allergic rhinitis: a randomized, double-blind, placebo-controlled clinical trial. Journal of allergy and clinical immunology. 2010;125:123–130.e121. doi: 10.1016/j.jaci.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 11.Fleming J, Cook T. Multiple sclerosis and the hygiene hypothesis. Neurology. 2006;67:2085–2086. doi: 10.1212/01.wnl.0000247663.40297.2d. [DOI] [PubMed] [Google Scholar]
- 12.Correale J, Farez M. Association between parasite infection and immune responses in multiple sclerosis. Annals of neurology. 2007;61:97–108. doi: 10.1002/ana.21067. [DOI] [PubMed] [Google Scholar]
- 13.Correale J, Farez M, Razzitte G. Helminth infections associated with multiple sclerosis induce regulatory B cells. Annals of neurology. 2008;64:187–199. doi: 10.1002/ana.21438. [DOI] [PubMed] [Google Scholar]
- 14.Correale J, Farez M. The impact of parasite infections on the course of multiple sclerosis. Journal of neuroimmunology. 2011;233:6–11. doi: 10.1016/j.jneuroim.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 15.Fleming J, et al. Probiotic helminth administration in relapsing-remitting multiple sclerosis: a phase 1 study. Multiple Sclerosis Journal. 2011;17:743–754. doi: 10.1177/1352458511398054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reddy A, Fried B. The use of Trichuris suis and other helminth therapies to treat Crohn’s disease. Parasitology research. 2007;100:921–927. doi: 10.1007/s00436-006-0416-4. [DOI] [PubMed] [Google Scholar]
- 17.Kradin R, Badizadegan K, Auluck P, Korzenik J, Lauwers G. Iatrogenic Trichuris suis infection in a patient with Crohn disease. Archives of pathology & laboratory medicine. 2006;130:718–720. doi: 10.5858/2006-130-718-ITSIIA. [DOI] [PubMed] [Google Scholar]
- 18.Hsu SJ, Tseng PH, Chen PJ. Trichuris suis therapy for ulcerative colitis: nonresponsive patients may need anti-helminth therapy. Gastroenterology. 2005;129:768–769. doi: 10.1016/j.gastro.2005.06.038. [DOI] [PubMed] [Google Scholar]
- 19.Van Kruiningen H, West AB. Potential danger in the medical use of Trichuris suis for the treatment of inflammatory bowel disease. Inflammatory bowel diseases. 2005;11:515–515. doi: 10.1097/01.mib.0000160369.47671.a2. [DOI] [PubMed] [Google Scholar]
- 20.Shin J, Gardiner G, Deitel W, Kandel G. Does whipworm increase the pathogenicity of Campylobacter jejuni? A clinical correlate of an experimental observation. Canadian journal of gastroenterology. 2004;18:175–177. doi: 10.1155/2004/298064. [DOI] [PubMed] [Google Scholar]
- 21.Bager P, et al. Symptoms after Ingestion of Pig Whipworm Trichuris suis Eggs in a Randomized Placebo-Controlled Double-Blind Clinical Trial. PLoS ONE. 2011;6:e22346–e22346. doi: 10.1371/journal.pone.0022346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mansfield L, et al. Enhancement of disease and pathology by synergy of Trichuris suis and Campylobacter jejuni in the colon of immunologically naive swine. The American journal of tropical medicine and hygiene. 2003;68:70–80. [PubMed] [Google Scholar]
- 23.Abner SR, et al. Response of intestinal epithelial cells to Trichuris suis excretory-secretory products and the influence on Campylobacter jejuni invasion under in vitro conditions. The Journal of parasitology. 2002;88:738–745. doi: 10.1645/0022-3395(2002)088[0738:ROIECT]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 24.de Silva N, et al. Soil-transmitted helminth infections: updating the global picture. Trends in parasitology. 2003;19:547–551. doi: 10.1016/j.pt.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 25.Mortimer K, et al. Dose-ranging study for trials of therapeutic infection with Necator americanus in humans. The American journal of tropical medicine and hygiene. 2006;75:914–920. [PubMed] [Google Scholar]
- 26.Loukas A, Prociv P. Immune responses in hookworm infections. Clinical microbiology reviews. 2001;14:689–703. doi: 10.1128/CMR.14.4.689-703.2001. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilson M, Maizels R. Regulation of allergy and autoimmunity in helminth infection. Clinical reviews in allergy & immunology. 2004;26:35–50. doi: 10.1385/CRIAI:26:1:35. [DOI] [PubMed] [Google Scholar]
- 28.Yazdanbakhsh M, Kremsner P, van Ree R. Allergy, parasites, and the hygiene hypothesis. Science. 2002;296:490–494. doi: 10.1126/science.296.5567.490. [DOI] [PubMed] [Google Scholar]
- 29.Scrivener S, et al. Independent effects of intestinal parasite infection and domestic allergen exposure on risk of wheeze in Ethiopia: a nested case-control study. Lancet. 2001;358:1493–1499. doi: 10.1016/S0140-6736(01)06579-5. [DOI] [PubMed] [Google Scholar]
- 30.Feary JR, et al. Experimental hookworm infection: a randomized placebo-controlled trial in asthma. Clinical and experimental allergy. 2010;40:299–306. doi: 10.1111/j.1365-2222.2009.03433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Daveson AJ, et al. Effect of hookworm infection on wheat challenge in celiac disease--a randomised double-blinded placebo controlled trial. PLoS ONE. 2011;6:e17366–e17366. doi: 10.1371/journal.pone.0017366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Croese J, et al. A proof of concept study establishing Necator americanus in Crohn’s patients and reservoir donors. Gut. 2006;55:136–137. doi: 10.1136/gut.2005.079129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McSorley H, et al. Suppression of inflammatory immune responses in celiac disease by experimental hookworm infection. PLoS ONE. 2011;6:e24092–e24092. doi: 10.1371/journal.pone.0024092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Elliott DE, Weinstock JV. Helminth–host immunological interactions: prevention and control of immune-mediated diseases. Annals of the New York Academy of Sciences. 2012:no–no. doi: 10.1111/j.1749-6632.2011.06292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fallon P, Mangan N. Suppression of TH2-type allergic reactions by helminth infection. Nature reviews. Immunology. 2007;7:220–230. doi: 10.1038/nri2039. [DOI] [PubMed] [Google Scholar]
- 36.Walsh CM, Smith P, Fallon PG. Role for CTLA-4 but not CD25+ T cells during Schistosoma mansoni infection of mice. Parasite immunology. 2007;29:293–308. doi: 10.1111/j.1365-3024.2007.00947.x. [DOI] [PubMed] [Google Scholar]
- 37.Furze R, Culley F, Selkirk M. Differential roles of the co-stimulatory molecules GITR and CTLA-4 in the immune response to Trichinella spiralis. Microbes and infection. 2006;8:2803–2810. doi: 10.1016/j.micinf.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 38.Babu S, Blauvelt C, Kumaraswami V, Nutman T. Regulatory networks induced by live parasites impair both Th1 and Th2 pathways in patent lymphatic filariasis: implications for parasite persistence. The journal of immunology. 2006;176:3248–3256. doi: 10.4049/jimmunol.176.5.3248. [DOI] [PubMed] [Google Scholar]
- 39.Blankenhaus B, et al. Strongyloides ratti infection induces expansion of Foxp3+ regulatory T cells that interfere with immune response and parasite clearance in BALB/c mice. The journal of immunology. 2011;186:4295–4305. doi: 10.4049/jimmunol.1001920. [DOI] [PubMed] [Google Scholar]
- 40.McCoy K, Camberis M, Gros GL. Protective immunity to nematode infection is induced by CTLA-4 blockade. The Journal of experimental medicine. 1997;186:183–187. doi: 10.1084/jem.186.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taylor M, et al. CTLA-4 and CD4+ CD25+ regulatory T cells inhibit protective immunity to filarial parasites in vivo. The journal of immunology. 2007;179:4626–4634. doi: 10.4049/jimmunol.179.7.4626. [DOI] [PubMed] [Google Scholar]
- 42.Taylor M, et al. Removal of regulatory T cell activity reverses hyporesponsiveness and leads to filarial parasite clearance in vivo. The journal of immunology. 2005;174:4924–4933. doi: 10.4049/jimmunol.174.8.4924. [DOI] [PubMed] [Google Scholar]
- 43.D’Elia R, Behnke J, Bradley J, Else K. Regulatory T cells: a role in the control of helminth-driven intestinal pathology and worm survival. The journal of immunology. 2009;182:2340–2348. doi: 10.4049/jimmunol.0802767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taylor M, et al. Early recruitment of natural CD4+ Foxp3+ Treg cells by infective larvae determines the outcome of filarial infection. European Journal of Immunology. 2009;39:192–206. doi: 10.1002/eji.200838727. [DOI] [PubMed] [Google Scholar]
- 45.Beiting D, et al. Coordinated control of immunity to muscle stage Trichinella spiralis by IL-10, regulatory T cells, and TGF-beta. The journal of immunology. 2007;178:1039–1047. doi: 10.4049/jimmunol.178.2.1039. [DOI] [PubMed] [Google Scholar]
- 46.Taylor M, Harris A, Nair M, Maizels R, Allen J. F4/80+ alternatively activated macrophages control CD4+ T cell hyporesponsiveness at sites peripheral to filarial infection. The journal of immunology. 2006;176:6918–6927. doi: 10.4049/jimmunol.176.11.6918. [DOI] [PubMed] [Google Scholar]
- 47.Baumgart M, Tompkins F, Leng J, Hesse M. Naturally occurring CD4+Foxp3+ regulatory T cells are an essential, IL-10-independent part of the immunoregulatory network in Schistosoma mansoni egg-induced inflammation. The journal of immunology. 2006;176:5374–5387. doi: 10.4049/jimmunol.176.9.5374. [DOI] [PubMed] [Google Scholar]
- 48.Gillan V, Devaney E. Regulatory T cells modulate Th2 responses induced by Brugia pahangi third-stage larvae. Infection and immunity. 2005;73:4034–4042. doi: 10.1128/IAI.73.7.4034-4042.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rausch S, et al. Establishment of nematode infection despite increased Th2 responses and immunopathology after selective depletion of Foxp3+ cells. European Journal of Immunology. 2009;39:3066–3077. doi: 10.1002/eji.200939644. [DOI] [PubMed] [Google Scholar]
- 50.Wilson M, et al. Suppression of allergic airway inflammation by helminth-induced regulatory T cells. The Journal of experimental medicine. 2005;202:1199–1212. doi: 10.1084/jem.20042572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pacífico LGG, et al. Schistosoma mansoni antigens modulate experimental allergic asthma in a murine model: a major role for CD4+ CD25+ Foxp3+ T cells independent of interleukin-10. Infection and immunity. 2009;77:98–107. doi: 10.1128/IAI.00783-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dittrich A, et al. Helminth infection with Litomosoides sigmodontis induces regulatory T cells and inhibits allergic sensitization, airway inflammation, and hyperreactivity in a murine asthma model. The journal of immunology. 2008;180:1792–1799. doi: 10.4049/jimmunol.180.3.1792. [DOI] [PubMed] [Google Scholar]
- 53.Liu Q, et al. Helminth infection can reduce insulitis and type 1 diabetes through CD25− and IL-10-independent mechanisms. Infection and immunity. 2009;77:5347–5358. doi: 10.1128/IAI.01170-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zaccone P, et al. Schistosoma mansoni egg antigens induce Treg that participate in diabetes prevention in NOD mice. European Journal of Immunology. 2009;39:1098–1107. doi: 10.1002/eji.200838871. [DOI] [PubMed] [Google Scholar]
- 55.Hubner M, et al. Helminth Protection against Autoimmune Diabetes in Nonobese Diabetic Mice Is Independent of a Type 2 Immune Shift and Requires TGF-β. The journal of immunology. 2012;188:559–568. doi: 10.4049/jimmunol.1100335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Walsh K, Brady M, Finlay C, Boon L, Mills KHG. Infection with a helminth parasite attenuates autoimmunity through TGF-beta-mediated suppression of Th17 and Th1 responses. The journal of immunology. 2009;183:1577–1586. doi: 10.4049/jimmunol.0803803. [DOI] [PubMed] [Google Scholar]
- 57.Smith P, et al. Infection with a helminth parasite prevents experimental colitis via a macrophage-mediated mechanism. The journal of immunology. 2007;178:4557–4566. doi: 10.4049/jimmunol.178.7.4557. [DOI] [PubMed] [Google Scholar]
- 58.Johnston MJG, et al. Extracts of the rat tapeworm, Hymenolepis diminuta, suppress macrophage activation in vitro and alleviate chemically induced colitis in mice. Infection and immunity. 2010;78:1364–1375. doi: 10.1128/IAI.01349-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hang L, et al. Heligmosomoides polygyrus infection can inhibit colitis through direct interaction with innate immunity. The journal of immunology. 2010;185:3184–3189. doi: 10.4049/jimmunol.1000941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bar On L, Zigmond E, Jung S. Management of gut inflammation through the manipulation of intestinal dendritic cells and macrophages? Seminars in immunology. 2011;23:58–64. doi: 10.1016/j.smim.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 61.Allen J, Wynn T. Evolution of Th2 immunity: a rapid repair response to tissue destructive pathogens. PLOS pathogens. 2011;7:e1002003–e1002003. doi: 10.1371/journal.ppat.1002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hunter M, et al. In vitro-derived alternatively activated macrophages reduce colonic inflammation in mice. Gastroenterology. 2010;138:1395–1405. doi: 10.1053/j.gastro.2009.12.041. [DOI] [PubMed] [Google Scholar]
- 63.Broadhurst M, et al. IL-22+ CD4+ T cells are associated with therapeutic trichuris trichiura infection in an ulcerative colitis patient. Science translational medicine. 2010;2:60ra88–60ra88. doi: 10.1126/scitranslmed.3001500. [DOI] [PubMed] [Google Scholar]
- 64.Sonnenberg G, Fouser L, Artis D. Border patrol: regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by IL-22. Nature Immunology. 2011;12:383–390. doi: 10.1038/ni.2025. [DOI] [PubMed] [Google Scholar]
- 65.Steenwinckel V, et al. IL-9 promotes IL-13-dependent paneth cell hyperplasia and up-regulation of innate immunity mediators in intestinal mucosa. The journal of immunology. 2009;182:4737–4743. doi: 10.4049/jimmunol.0801941. [DOI] [PubMed] [Google Scholar]
- 66.Finkelman FD, et al. Interleukin-4- and interleukin-13-mediated host protection against intestinal nematode parasites. Immunological Reviews. 2004;201:139–155. doi: 10.1111/j.0105-2896.2004.00192.x. [DOI] [PubMed] [Google Scholar]
- 67.Seno H, et al. Efficient colonic mucosal wound repair requires Trem2 signaling. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:256–261. doi: 10.1073/pnas.0803343106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sugimoto K, et al. IL-22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis. The Journal of clinical investigation. 2008;118:534–544. doi: 10.1172/JCI33194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zenewicz L, et al. Innate and adaptive interleukin-22 protects mice from inflammatory bowel disease. Immunity. 2008;29:947–957. doi: 10.1016/j.immuni.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Johansson MEV, et al. Composition and functional role of the mucus layers in the intestine. Cellular and molecular life sciences. 2011;68:3635–3641. doi: 10.1007/s00018-011-0822-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Johansson MEV, et al. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:15064–15069. doi: 10.1073/pnas.0803124105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gibson PR, Muir JG. Reinforcing the mucus: a new therapeutic approach for ulcerative colitis? Gut. 2005;54:900–903. doi: 10.1136/gut.2004.058453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ehehalt R, et al. Phosphatidylcholine and lysophosphatidylcholine in intestinal mucus of ulcerative colitis patients. A quantitative approach by nanoElectrospray-tandem mass spectrometry. Scandinavian journal of gastroenterology. 2004;39:737–742. doi: 10.1080/00365520410006233. [DOI] [PubMed] [Google Scholar]
- 74.Pullan RD, et al. Thickness of adherent mucus gel on colonic mucosa in humans and its relevance to colitis. Gut. 1994;35:353–359. doi: 10.1136/gut.35.3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tytgat KM, van der Wal JW, Einerhand AW, Bller HA, Dekker J. Quantitative analysis of MUC2 synthesis in ulcerative colitis. Biochemical and biophysical research communications. 1996;224:397–405. doi: 10.1006/bbrc.1996.1039. [DOI] [PubMed] [Google Scholar]
- 76.Larsson JMH, et al. Altered O-glycosylation profile of MUC2 mucin occurs in active ulcerative colitis and is associated with increased inflammation. Inflammatory bowel diseases. 2011;17:2299–2307. doi: 10.1002/ibd.21625. [DOI] [PubMed] [Google Scholar]
- 77.Corfield AP, et al. Colonic mucins in ulcerative colitis: evidence for loss of sulfation. Glycoconjugate journal. 1996;13:809–822. doi: 10.1007/BF00702345. [DOI] [PubMed] [Google Scholar]
- 78.Van der Sluis M, et al. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology. 2006;131:117–129. doi: 10.1053/j.gastro.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 79.Burger-van Paassen N, et al. Colitis development during the suckling-weaning transition in mucin Muc2-deficient mice. American journal of physiology: Gastrointestinal and liver physiology. 2011;301:G667–G678. doi: 10.1152/ajpgi.00199.2010. [DOI] [PubMed] [Google Scholar]
- 80.Heazlewood C, et al. Aberrant mucin assembly in mice causes endoplasmic reticulum stress and spontaneous inflammation resembling ulcerative colitis. PLoS Medicine. 2008;5:e54–e54. doi: 10.1371/journal.pmed.0050054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fu J, et al. Loss of intestinal core 1-derived O-glycans causes spontaneous colitis in mice. The Journal of clinical investigation. 2011;121:1657–1666. doi: 10.1172/JCI45538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.An G, et al. Increased susceptibility to colitis and colorectal tumors in mice lacking core 3-derived O-glycans. The Journal of experimental medicine. 2007;204:1417–1429. doi: 10.1084/jem.20061929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stremmel W, et al. Retarded release phosphatidylcholine benefits patients with chronic active ulcerative colitis. Gut. 2005;54:966–971. doi: 10.1136/gut.2004.052316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Stremmel W, Hanemann A, Ehehalt R, Karner M, Braun A. Phosphatidylcholine (lecithin) and the mucus layer: Evidence of therapeutic efficacy in ulcerative colitis? Digestive diseases. 2010;28:490–496. doi: 10.1159/000320407. [DOI] [PubMed] [Google Scholar]
- 85.Stremmel W, Ehehalt R, Autschbach F, Karner M. Phosphatidylcholine for steroid-refractory chronic ulcerative colitis: a randomized trial. Annals of Internal Medicine. 2007;147:603–610. doi: 10.7326/0003-4819-147-9-200711060-00004. [DOI] [PubMed] [Google Scholar]
- 86.Koninkx JF, Mirck MH, Hendriks HG, Mouwen JM, van Dijk JE. Nippostrongylus brasiliensis: histochemical changes in the composition of mucins in goblet cells during infection in rats. Experimental Parasitology. 1988;65:84–90. doi: 10.1016/0014-4894(88)90109-9. [DOI] [PubMed] [Google Scholar]
- 87.Soga K, et al. Alteration of the expression profiles of acidic mucin, sialytransferase, and sulfotransferases in the intestinal epithelium of rats infected with the nematode Nippostrongylus brasiliensis. Parasitology research. 2008;103:1427–1434. doi: 10.1007/s00436-008-1152-8. [DOI] [PubMed] [Google Scholar]
- 88.Shekels LL, et al. Coordinated Muc2 and Muc3 mucin gene expression in Trichinella spiralis infection in wild-type and cytokine-deficient mice. Digestive diseases and sciences. 2001;46:1757–1764. doi: 10.1023/a:1010622125040. [DOI] [PubMed] [Google Scholar]
- 89.Karlsson NG, Olson FJ, Jovall PA, Andersch Y, Enerback L, Hansson GC. Identification of transient glycosylation alterations of sialylated mucins oligosaccharides during infection by the rat intestinal parasite Nippostrongylus brasiliensis. Biochem J. 2000;350:805–814. [PMC free article] [PubMed] [Google Scholar]
- 90.Olson F, et al. Blood group A glycosyltransferase occurring as alleles with high sequence difference is transiently induced during a Nippostrongylus brasiliensis parasite infection. Journal of biological chemistry. 2002;277:15044–15052. doi: 10.1074/jbc.M112287200. [DOI] [PubMed] [Google Scholar]
- 91.Yamauchi J, et al. Altered expression of goblet cell- and mucin glycosylation-related genes in the intestinal epithelium during infection with the nematode Nippostrongylus brasiliensis in rat. APMIS. Acta pathologica, microbiologica et immunologica Scandinavica. 2006;114:270–278. doi: 10.1111/j.1600-0463.2006.apm_353.x. [DOI] [PubMed] [Google Scholar]
- 92.Hasnain S, et al. Muc5ac: a critical component mediating the rejection of enteric nematodes. The Journal of experimental medicine. 2011;208:893–900. doi: 10.1084/jem.20102057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Herbert DB, et al. Intestinal epithelial cell secretion of RELM-beta protects against gastrointestinal worm infection. The Journal of experimental medicine. 2009;206:2947–2957. doi: 10.1084/jem.20091268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Artis D, et al. RELMbeta/FIZZ2 is a goblet cell-specific immune-effector molecule in the gastrointestinal tract. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:13596–13600. doi: 10.1073/pnas.0404034101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hogan S, et al. Resistin-like molecule beta regulates innate colonic function: barrier integrity and inflammation susceptibility. Journal of allergy and clinical immunology. 2006;118:257–268. doi: 10.1016/j.jaci.2006.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Krimi R, et al. Resistin-like molecule beta regulates intestinal mucous secretion and curtails TNBS-induced colitis in mice. Inflammatory bowel diseases. 2008;14:931–941. doi: 10.1002/ibd.20420. [DOI] [PubMed] [Google Scholar]
- 97.Hill DA, et al. Metagenomic analyses reveal antibiotic-induced temporal and spatial changes in intestinal microbiota with associated alterations in immune cell homeostasis. Mucosal immunology. 2010;3:148–158. doi: 10.1038/mi.2009.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Johansson MEV, Hansson G. Microbiology. Keeping bacteria at a distance. Science. 2011;334:182–183. doi: 10.1126/science.1213909. [DOI] [PubMed] [Google Scholar]
- 99.Vaishnava S, et al. The antibacterial lectin RegIIIgamma promotes the spatial segregation of microbiota and host in the intestine. Science. 2011;334:255–258. doi: 10.1126/science.1209791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Walk S, Blum A, Ewing S, Weinstock J, Young V. Alteration of the murine gut microbiota during infection with the parasitic helminth Heligmosomoides polygyrus. Inflammatory bowel diseases. 2010;16:1841–1849. doi: 10.1002/ibd.21299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hayes KS, et al. Exploitation of the intestinal microflora by the parasitic nematode Trichuris muris. Science. 2010;328:1391–1394. doi: 10.1126/science.1187703. [DOI] [PMC free article] [PubMed] [Google Scholar]